A Geographic Object-Based Image Approach Based on the Sentinel-2 Multispectral Instrument for Lake Aquatic Vegetation Mapping: A Complementary Tool to In Situ Monitoring
Abstract
1. Introduction
2. Materials and Methods
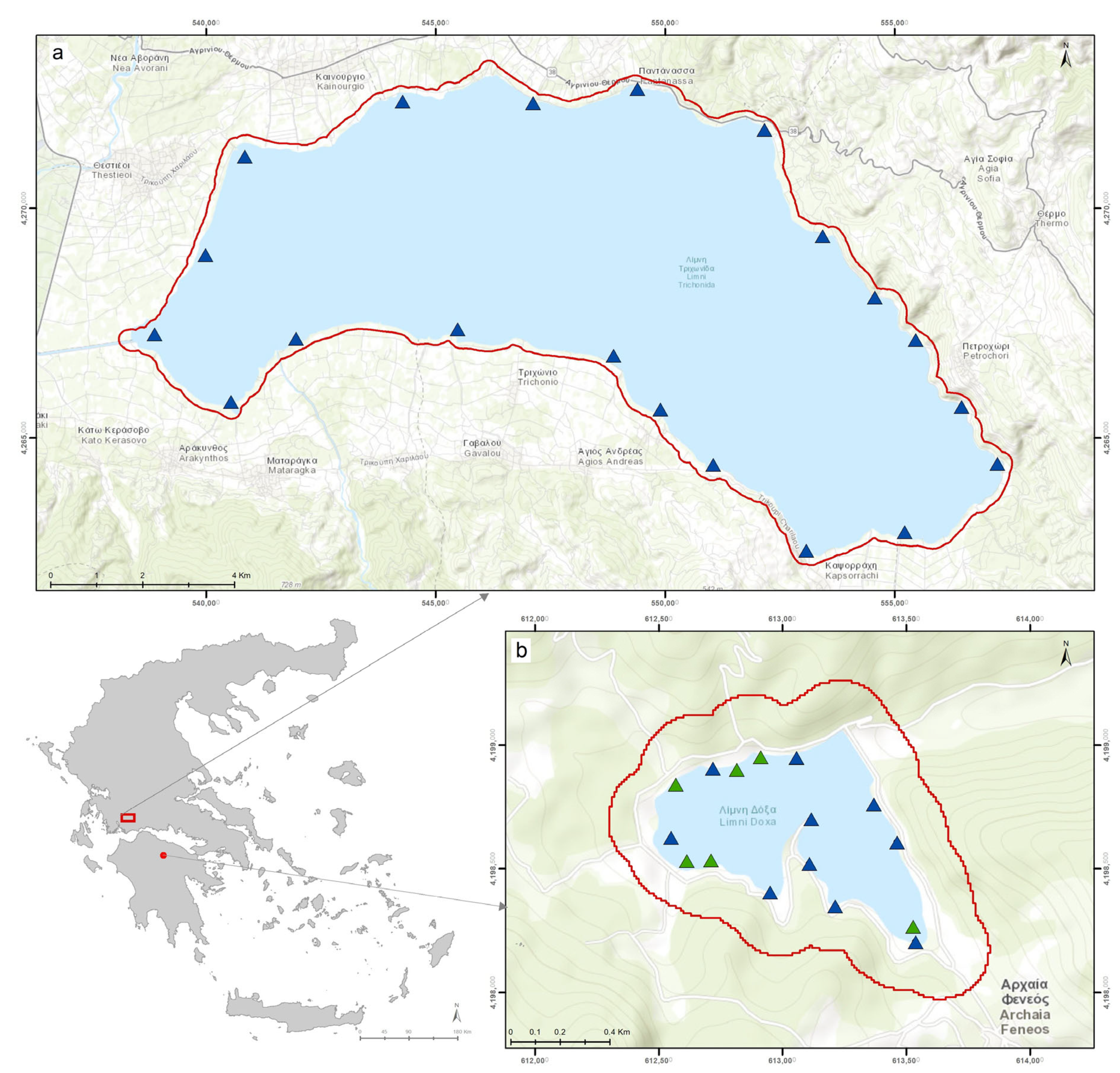
2.1. Site Description
2.2. Datasets
2.2.1. In Situ Monitoring Data of Aquatic Vegetation
2.2.2. Bathymetric Data
2.2.3. Satellite Data
2.3. Study Workflow
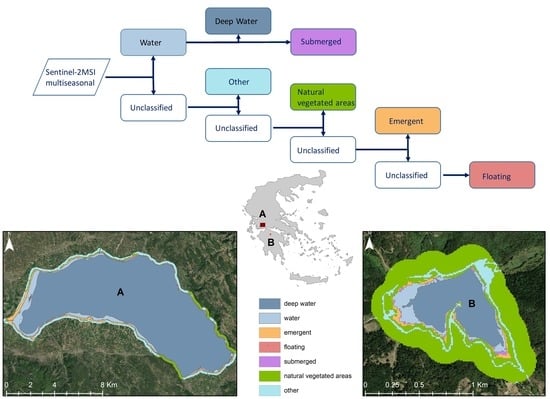
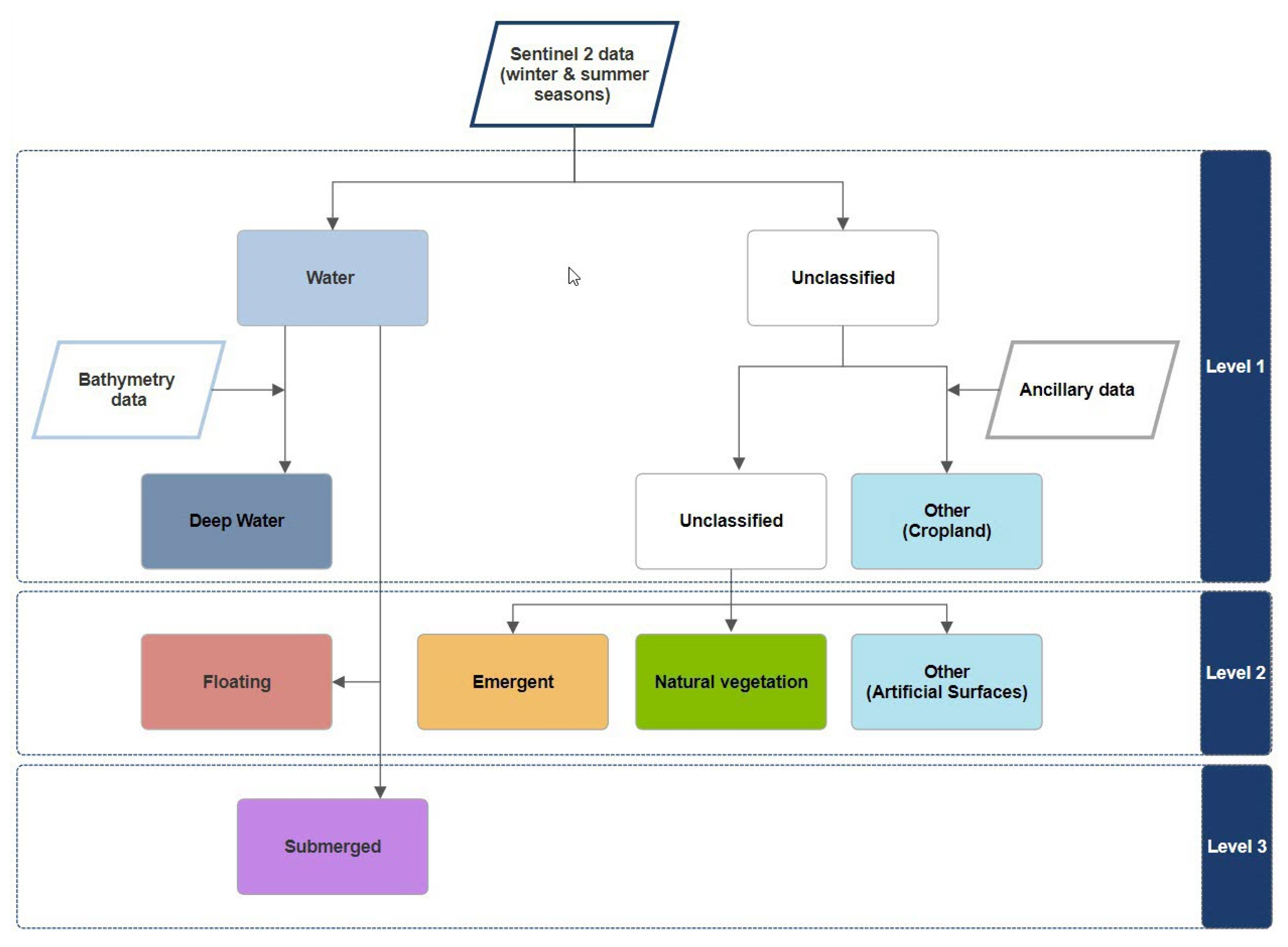
2.3.1. Classification Scheme
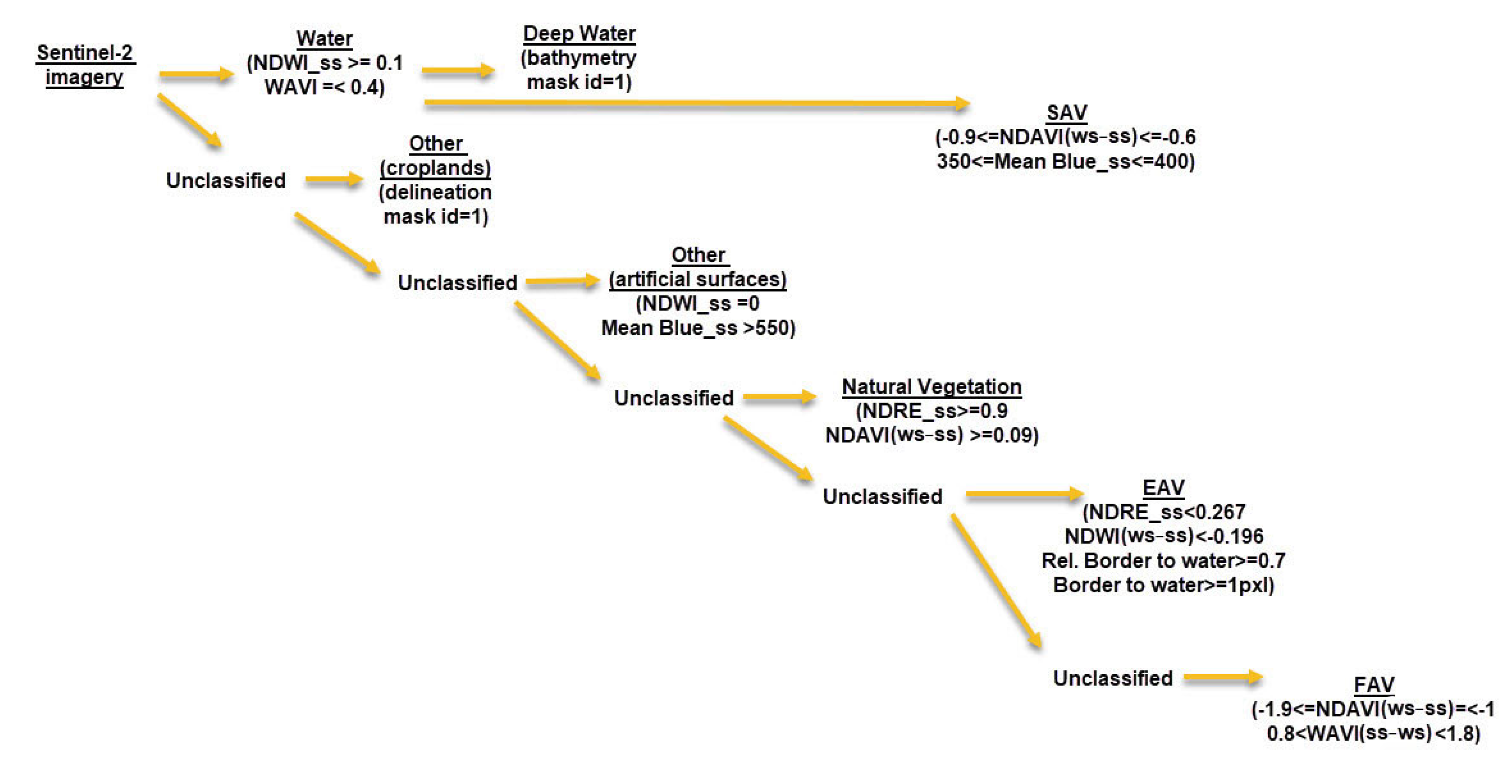
2.3.2. Geographic Object-Based Image Analysis
- Level 1: image layers weighted—all spectral information images of both seasons; thematic layer used—layers for croplands and bathymetry; scale—30; shape—0.7; compactness—0.5.
- Level 2: image layers weighted—all spectral information images of both seasons; scale—9, shape—0.1; compactness—0.5.
- Level 3: applied to “Water” class only. Image layers weighted—all spectral information images of both seasons; scale—2, shape—0.1; compactness—0.5.
2.3.3. Accuracy Assessment
- Πi is the proportion of a population in the ith class out of k classes that has the proportion closest to 50%.
- bi is the desired precision (e.g., 5%) for this class.
- B is the upper (a/k) × 100th percentile of the chi square (χ2) distribution with 1 degree of freedom.
- a is calculated by the confidence level (1−a) (when confidence level is equal to 95%, a is equal to 0.05).
- k is the number of classes.
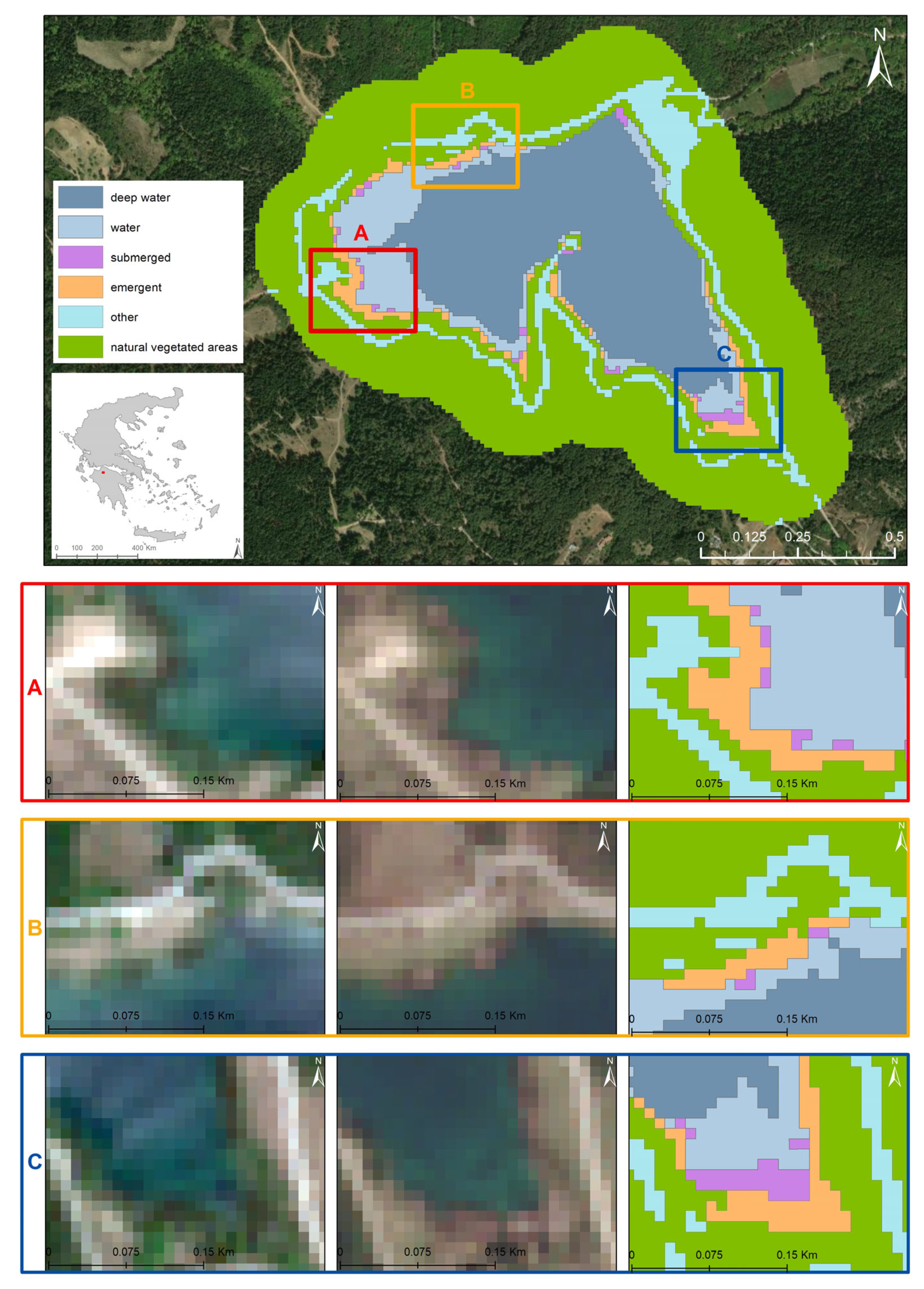
3. Results
3.1. Hierarchical Image Classification Model
3.2. Thematic Accuracies
3.3. Spatial Extent per Class
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collen, B.; Whitton, F.; Dyer, E.E.; Baillie, J.E.M.; Cumberlidge, N.; Darwall, W.R.T.; Pollock, C.; Richman, N.I.; Soulsby, A.-M.; Böhm, M. Global patterns of freshwater species diversity, threat and endemism. Glob. Ecol. Biogeogr. 2014, 23, 40–51. [Google Scholar] [CrossRef]
- Garcia-Moreno, J.; Harrison, I.J.; Dudgeon, D.; Clausnitzer, V.; Darwall, W.; Farrell, T.; Savy, C.; Tockner, K.; Tubbs, N. Sustaining Freshwater Biodiversity in the Anthropocene. In The Global Water System in the Anthropocene: Challenges for Science and Governance; Bhaduri, A., Bogardi, J., Leentvaar, J., Marx, S., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 247–270. [Google Scholar]
- Likens, G.E. Lake Ecosystem Ecology: A Global Perspective; Elsevier Science: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Ormerod, S.J.; Dobson, M.; Hildrew, A.G.; Townsend, C.R. Multiple stressors in freshwater ecosystems. Freshw. Biol. 2010, 55, 1–4. [Google Scholar] [CrossRef]
- Almond, R.E.A.; Grooten, M.; Peterson, T. (Eds.) Living Planet Report 2020—Bending the Curve of Biodiversity Loss; World Wildlife Fund: Gland, Switzerland, 2020. [Google Scholar]
- Revenga, C.; Campbell, I.C.; Abell, R.; Villiers, P.D.; Bryer, M. Prospects for monitoring freshwater ecosystems towards the 2010 targets. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 397–413. [Google Scholar] [CrossRef]
- ISO 5667-4:1987; Guidance on Sampling from Lakes, Natural and Man-Made. International Organization for Standardization: Geneva, Switzerland, 1987.
- ISO 5667-4:2016; Guidance on Sampling from Lakes, Natural and Man-Made. International Organization for Standardization: Geneva, Switzerland, 2016.
- ILNAS-EN 15204:2006; Water Quality—Guidance Standard on the Enumeration of Phytoplankton Using Inverted Microscopy (Utermohl Method). Institut Luxembourgeois de la Normalisation, de l’Accréditation, de la Sécurité et qualité des produits et services: Luxembourg, 2006.
- Birk, S.; Bonne, W.; Borja, A.; Brucet, S.; Courrat, A.; Poikane, S.; Solimini, A.; van de Bund, W.; Zampoukas, N.; Hering, D. Three hundred ways to assess Europe’s surface waters: An almost complete overview of biological methods to implement the Water Framework Directive. Ecol. Indic. 2012, 18, 31–41. [Google Scholar] [CrossRef]
- European Communities. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a framework for Community Action in the Field of Water Policy. Eur. Communities 2000, L327, 1–73. [Google Scholar]
- European Communities. Commission Decision (EU) 2018/229 of 12 February 2018 establishing, pursuant to Directive 2000/60/EC of the European Parliament and of the Council, the values of the Member State monitoring system classifications as a result of the intercalibration exercise and repealing Commission Decision 2013/480/EU. Off. J. Eur. Communities 2018, L47, 1–91. [Google Scholar]
- Carpenter, S.R.; Lodge, D.M. Effects of submersed macrophytes on ecosystem processes. Aquat. Bot. 1986, 26, 341–370. [Google Scholar] [CrossRef]
- Jeppesen, E.; Søndergaard, M.; Søndergaard, M.; Christoffersen, K. (Eds.) The Structuring Role of Submerged Macrophytes in Lakes; Springer: New York, NY, USA, 1998; Volume 131, pp. 381–427. [Google Scholar]
- Barko, J.W.; Gunnison, D.; Carpenter, S.R. Sediment interactions with submersed macrophyte growth and community dynamics. Aquat. Bot. 1991, 41, 41–65. [Google Scholar] [CrossRef]
- Gumbricht, T. Nutrient removal processes in freshwater submersed macrophyte systems. Ecol. Eng. 1993, 2, 1–30. [Google Scholar] [CrossRef]
- Chambers, P.; Lacoul, P.; Murphy, K.; Thomaz, S. Global diversity of aquatic macrophytes in freshwater. Hydrobiologia 2008, 595, 9–26. [Google Scholar] [CrossRef]
- Penning, W.E.; Dudley, B.; Mjelde, M.; Hellsten, S.; Hanganu, J.; Kolada, A.; van den Berg, M.; Poikane, S.; Phillips, G.; Willby, N.; et al. Using aquatic macrophyte community indices to define the ecological status of European lakes. Aquat. Ecol. 2008, 42, 253–264. [Google Scholar] [CrossRef]
- Penning, W.E.; Mjelde, M.; Dudley, B.; Hellsten, S.; Hanganu, J.; Kolada, A.; Van Den Berg, M.; Poikane, S.; Phillips, G.; Willby, N.; et al. Classifying aquatic macrophytes as indicators of eutrophication in European lakes. Aquat. Ecol. 2008, 42, 237–251. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Qin, B.; Shi, K.; Deng, J.; Zhou, Y. Aquatic vegetation in response to increased eutrophication and degraded light climate in Eastern Lake Taihu: Implications for lake ecological restoration. Sci. Rep. 2016, 6, 23867. [Google Scholar] [CrossRef]
- Poikane, S.; Portielje, R.; Denys, L.; Elferts, D.; Kelly, M.; Kolada, A.; Mäemets, H.; Phillips, G.; Søndergaard, M.; Willby, N.; et al. Macrophyte assessment in European lakes: Diverse approaches but convergent views of ‘good’ecological status. Ecol. Indic. 2018, 94, 185–197. [Google Scholar] [CrossRef]
- Phillips, G.; Willby, N.; Moss, B. Submerged macrophyte decline in shallow lakes: What have we learnt in the last forty years? Aquat. Bot. 2016, 135, 37–45. [Google Scholar] [CrossRef]
- Zervas, D.; Tsiaoussi, V.; Tsiripidis, I. HeLM: A macrophyte-based method for monitoring and assessment of Greek lakes. Environ. Monit. Assess. 2018, 190, 326. [Google Scholar] [CrossRef]
- Geist, J.; Hawkins, S.J. Habitat recovery and restoration in aquatic ecosystems: Current progress and future challenges. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 942–962. [Google Scholar] [CrossRef]
- Rohal, C.; Reynolds, L.; Adams, C.; Martin, C.; Latimer, E.; Walsh, S.; Slater, J. Biological and practical tradeoffs in planting techniques for submerged aquatic vegetation. Aquat. Bot. 2021, 170, 103347. [Google Scholar] [CrossRef]
- Carvalho, L.; Mackay, E.B.; Cardoso, A.C.; Baattrup-Pedersen, A.; Birk, S.; Blackstock, K.L.; Borics, G.; Borja, A.; Feld, C.K.; Ferreira, M.T.; et al. Protecting and restoring Europe’s waters: An analysis of the future development needs of the Water Framework Directive. Sci. Total. Environ. 2019, 658, 1228–1238. [Google Scholar] [CrossRef]
- Free, G.; Bresciani, M.; Trodd, W.; Tierney, D.; O’Boyle, S.; Plant, C.; Deakin, J. Estimation of lake ecological quality from Sentinel-2 remote sensing imagery. Hydrobiologia 2020, 847, 1423–1438. [Google Scholar] [CrossRef]
- Rowan, G.S.L.; Kalacska, M. A Review of Remote Sensing of Submerged Aquatic Vegetation for Non-Specialists. Remote Sens. 2021, 13, 623. [Google Scholar] [CrossRef]
- Visser, F.; Wallis, C.; Sinnott, A.M. Optical remote sensing of submerged aquatic vegetation: Opportunities for shallow clearwater streams. Limnologica 2013, 43, 388–398. [Google Scholar] [CrossRef]
- Silva, T.S.F.; Costa, M.P.F.; Melack, J.M.; Novo, E.M.L.M. Remote sensing of aquatic vegetation: Theory and applications. Environ. Monit. Assess. 2008, 140, 131–145. [Google Scholar] [CrossRef]
- Dörnhöfer, K.; Oppelt, N. Remote sensing for lake research and monitoring–Recent advances. Ecol. Indic. 2016, 64, 105–122. [Google Scholar] [CrossRef]
- Knipling, E.B. Physical and physiological basis for the reflectance of visible and near-infrared radiation from vegetation. Remote Sens. Environ. 1970, 1, 155–159. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Shi, K.; Zhou, Y.; Tang, X.; Zhu, G.; Qin, B. Mapping Aquatic Vegetation in a Large, Shallow Eutrophic Lake: A Frequency-Based Approach Using Multiple Years of MODIS Data. Remote Sens. 2015, 7, 10295–10320. [Google Scholar] [CrossRef]
- Luo, J.; Li, X.; Ma, R.; Li, F.; Duan, H.; Hu, W.; Qin, B.; Huang, W. Applying remote sensing techniques to monitoring seasonal and interannual changes of aquatic vegetation in Taihu Lake, China. Ecol. Indic. 2016, 60, 503–513. [Google Scholar] [CrossRef]
- Marshall, T.R.; Lee, P. Mapping aquatic macrophytes through digital image analysis of aerial photographs: An assessment. J. Aquat. Plant Manag. 1994, 32, 61–66. [Google Scholar]
- Pu, R.; Bell, S. Mapping seagrass coverage and spatial patterns with high spatial resolution IKONOS imagery. Int. J. Appl. Earth Obs. Geoinf. 2017, 54, 145–158. [Google Scholar] [CrossRef]
- Villa, P.; Pinardi, M.; Tóth, V.R.; Hunter, P.D.; Bolpagni, R.; Bresciani, M. Remote sensing of macrophyte morphological traits: Implications for the management of shallow lakes. J. Limnol. 2017, 76, 109–126. [Google Scholar] [CrossRef]
- Welch, R.; Remillard, M.; Slack, R. Remote sensing and geographic information system techniques for aquatic resource evaluation. In (American Society for Photogrammetry and Remote Sensing and ACSM, Annual Convention, Baltimore, MD, USA, 29 March–3 April 1987) Photogrammetric Engineering and Remote Sensing; American Society for Photogrammetry and Remote Sensing and ACSM: Reno, NV, USA, 1988; pp. 177–185. [Google Scholar]
- Luo, J.; Duan, H.; Ma, R.; Jin, X.; Li, F.; Hu, W.; Shi, K.; Huang, W. Mapping species of submerged aquatic vegetation with multi-seasonal satellite images and considering life history information. Int. J. Appl. Earth Obs. Geoinf. 2017, 57, 154–165. [Google Scholar] [CrossRef]
- Elijah, W.R., III; Laine, S.C. Comparison of Landsat Thematic Mapper and High Resolution Photography to Identify Change in Complex Coastal Wetlands. J. Coast. Res. 1997, 13, 281–292. [Google Scholar]
- Ade, C.; Khanna, S.; Lay, M.; Ustin, S.L.; Hestir, E.L. Genus-Level Mapping of Invasive Floating Aquatic Vegetation Using Sentinel-2 Satellite Remote Sensing. Remote Sens. 2022, 14, 3013. [Google Scholar] [CrossRef]
- Farmer, A.M.; Adams, M.S. A consideration of the problems of scale in the study of the ecology of aquatic macrophytes. Aquat. Bot. 1989, 33, 177–189. [Google Scholar] [CrossRef]
- Vis, C.; Hudon, C.; Carignan, R. An evaluation of approaches used to determine the distribution and biomass of emergent and submerged aquatic macrophytes over large spatial scales. Aquat. Bot. 2003, 77, 187–201. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, R.; Hao, Y.; Wu, L.; Zhang, W.; Zhang, Q.; Bu, X. A New Method for Mapping Aquatic Vegetation Especially Underwater Vegetation in Lake Ulansuhai Using GF-1 Satellite Data. Remote Sens. 2018, 10, 1279. [Google Scholar] [CrossRef]
- Davranche, A.; Lefebvre, G.; Poulin, B. Wetland monitoring using classification trees and SPOT-5 seasonal time series. Remote Sens. Environ. 2010, 114, 552–562. [Google Scholar] [CrossRef]
- Rodríguez-Garlito, E.C.; Paz-Gallardo, A.; Plaza, A. Mapping Invasive Aquatic Plants in Sentinel-2 Images Using Convolutional Neural Networks Trained With Spectral Indices. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2023, 16, 2889–2899. [Google Scholar] [CrossRef]
- Zhao, D.; Lv, M.; Jiang, H.; Cai, Y.; Xu, D.; An, S. Spatio-Temporal Variability of Aquatic Vegetation in Taihu Lake over the Past 30 Years. PLoS ONE 2013, 8, e66365. [Google Scholar] [CrossRef]
- Luo, J.; Ma, R.; Duan, H.; Hu, W.; Zhu, J.; Huang, W.; Lin, C. A New Method for Modifying Thresholds in the Classification of Tree Models for Mapping Aquatic Vegetation in Taihu Lake with Satellite Images. Remote Sens. 2014, 6, 7442–7462. [Google Scholar] [CrossRef]
- Villa, P.; Bresciani, M.; Bolpagni, R.; Pinardi, M.; Giardino, C. A rule-based approach for mapping macrophyte communities using multi-temporal aquatic vegetation indices. Remote Sens. Environ. 2015, 171, 218–233. [Google Scholar] [CrossRef]
- de Grandpré, A.; Kinnard, C.; Bertolo, A. Open-Source Analysis of Submerged Aquatic Vegetation Cover in Complex Waters Using High-Resolution Satellite Remote Sensing: An Adaptable Framework. Remote Sens. 2022, 14, 267. [Google Scholar] [CrossRef]
- Visser, F.; Buis, K.; Verschoren, V.; Schoelynck, J. Mapping of submerged aquatic vegetation in rivers from very high-resolution image data, using object-based image analysis combined with expert knowledge. Hydrobiologia 2018, 812, 157–175. [Google Scholar] [CrossRef]
- Chabot, D.; Dillon, C.; Shemrock, A.; Weissflog, N.; Sager, E.P.S. An Object-Based Image Analysis Workflow for Monitoring Shallow-Water Aquatic Vegetation in Multispectral Drone Imagery. ISPRS Int. J. Geo-Inf. 2018, 7, 294. [Google Scholar] [CrossRef]
- Husson, E.; Ecke, F.; Reese, H. Comparison of Manual Mapping and Automated Object-Based Image Analysis of Non-Submerged Aquatic Vegetation from Very-High-Resolution UAS Images. Remote Sens. 2016, 8, 724. [Google Scholar] [CrossRef]
- Hay, G.J.; Castilla, G. Geographic Object-Based Image Analysis (GEOBIA): A new name for a new discipline. In Object-Based Image Analysis: Spatial Concepts for Knowledge-Driven Remote Sensing Applications; Springer: Berlin/Heidelberg, Germany, 2008; pp. 75–89. [Google Scholar]
- Mavromati, E.; Kagalou, I.; Kemitzoglou, D.; Apostolakis, A.; Seferlis, M.; Tsiaoussi, V. Relationships Among Land Use Patterns, Hydromorphological Features and Physicochemical Parameters of Surface Waters: WFD Lake Monitoring in Greece. Environ. Process. 2018, 5, 139–151. [Google Scholar] [CrossRef]
- Apostolakis, A.P.D.; Demertzi, K. Isobaths of Lake Trichonida. Greek Biotope Wetland Centre EKBY. 2021. Available online: http://ekbygis.biodiversity-info.gr/geonetwork/srv/eng/main.home (accessed on 15 September 2022).
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Zeitschrif 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Kagalou, I.; Ntislidou, C.; Latinopoulos, D.; Kemitzoglou, D.; Tsiaoussi, V.; Bobori, D.C. Setting the Phosphorus Boundaries for Greek Natural Shallow and Deep Lakes for Water Framework Directive Compliance. Water 2021, 13, 739. [Google Scholar] [CrossRef]
- Gourgouletis, N.; Baltas, E. Investigating Hydroclimatic Variables Trends on the Natural Lakes of Western Greece Using Earth Observation Data. Sensors 2023, 23, 2056. [Google Scholar] [CrossRef]
- Dimitriou, E.; Zacharias, I. Quantifying the rainfall-water level fluctuation process in a geologically complex lake catchment. Environ. Monit. Assess. 2006, 119, 491–506. [Google Scholar]
- Seguin, J.; Avramidis, P.; Dörfler, W.; Emmanouilidis, A.; Unkel, I. A 2600-year high-resolution climate record from Lake Trichonida (SW Greece). EG Quat. Sci. J. 2020, 69, 139–160. [Google Scholar] [CrossRef]
- Albrecht, C.; Hauffe, T.; Schreiber, K.; Trajanovski, S.; Wilke, T. Mollusc biodiversity and endemism in the potential ancient lake Trichonis, Greece. Malacologia 2009, 51, 357–375. [Google Scholar] [CrossRef]
- Doulka, E.; Kehayias, G. Spatial and temporal distribution of zooplankton in Lake Trichonis (Greece). J. Nat. Hist. 2008, 42, 575–595. [Google Scholar] [CrossRef]
- Obolewski, K.; SkorbiŁowicz, E.; SkorbiŁowicz, M.; Glińska-Lewczuk, K.; Astel, A.M.; Strzelczak, A. The effect of metals accumulated in reed (Phragmites australis) on the structure of periphyton. Ecotoxicol. Environ. Saf. 2011, 74, 558–568. [Google Scholar] [CrossRef]
- Petriki, O.; Moutopoulos, D.K.; Tsagarakis, K.; Tsionki, I.; Papantoniou, G.; Mantzouni, I.; Barbieri, R.; Stoumboudi, M.T. Assessing the Fisheries and Ecosystem Structure of the Largest Greek Lake (Lake Trichonis). Water 2021, 13, 3329. [Google Scholar] [CrossRef]
- Zervas, D.; Tsiripidis, I.; Bergmeier, E.; Tsiaousi, V. A phytosociological survey of aquatic vegetation in the main freshwater lakes of Greece. Veg. Classif. Surv. 2020, 1, 53–75. [Google Scholar] [CrossRef]
- European Environment Agency: NATURA 2000—Standard Data Form: Limnes Trichonida Kai Lysimacheia, C., Denmark. Available online: https://natura2000.eea.europa.eu/Natura2000/SDF.aspx?site=GR2310009&release=10#7 (accessed on 1 February 2023).
- Koussouris, T. Plankton observations in three lakes of Western Greece. Thalassographica 1978, 2, 115–123. [Google Scholar]
- Overbeck, J.; Anagnostidis, K.; Economou-Amilli, A. Limnological Survey of Three Greek Lakes: Trichonis, Lyssimachia and Amvrakia(Ein Limnologischer Uberblick von drei Griechischen Seen: Trichonis, Lyssimachia und Amvrakia). Arch. Fur Hydrobiol. Vol. 1982, 95, 365–394. [Google Scholar]
- Koumpli-Sovantzi, L. The Aquatic Flora of Aetoloakarnania (W Greece). Willdenowia 1989, 18, 377–385. [Google Scholar]
- Palmer, M.; Bell, S.; Butterfield, I. A botanical classification of standing waters in Britain: Applications for conservation and monitoring. Aquat. Conserv. Mar. Freshw. Ecosyst. 1992, 2, 125–143. [Google Scholar] [CrossRef]
- EN 15460:2007; Water Quality—Guidance Standard for the Surveying of Aquatic Macrophytes in Lakes. European Committee for Standardization: Bruxelles, Belgium, 2007.
- EKBY. GeoServer. Available online: http://ekbygis.biodiversity-info.gr/geoserver/web/ (accessed on 15 November 2022).
- Hutchinson, M. A locally adaptive approach to the interpolation of digital elevation models. In Proceedings of the Third International Conference/Workshop on Integrating GIS and Environmental Modeling, Sante Fe, NM, USA, 21–25 January 1996; pp. 21–26. [Google Scholar]
- Hutchinson, M.; Xu, T.; Stein, J. Recent Progress in the ANUDEM Elevation Gridding Procedure. Geomorphometry 2011, 2011, 19–22. [Google Scholar]
- Hutchinson, M.F. Calculation of hydrologically sound digital elevation models. In Proceedings of the Third International Symposium on Spatial Data Handling, Sydney, Australia, 17–19 August 1988; pp. 117–133. [Google Scholar]
- Hutchinson, M.F. A new procedure for gridding elevation and stream line data with automatic removal of spurious pits. J. Hydrol. 1989, 106, 211–232. [Google Scholar] [CrossRef]
- Hutchinson, M. Optimising the degree of data smoothing for locally adaptive finite element bivariate smoothing splines. ANZIAM J. 2000, 42, C774–C796. [Google Scholar] [CrossRef]
- Ecosystem, C.D.S. Available online: https://dataspace.copernicus.eu/ (accessed on 15 November 2022).
- Drusch, M.; Del Bello, U.; Carlier, S.; Colin, O.; Fernandez, V.; Gascon, F.; Hoersch, B.; Isola, C.; Laberinti, P.; Martimort, P.; et al. Sentinel-2: ESA’s Optical High-Resolution Mission for GMES Operational Services. Remote Sens. Environ. 2012, 120, 25–36. [Google Scholar] [CrossRef]
- Warren, M.A.; Simis, S.G.H.; Martinez-Vicente, V.; Poser, K.; Bresciani, M.; Alikas, K.; Spyrakos, E.; Giardino, C.; Ansper, A. Assessment of atmospheric correction algorithms for the Sentinel-2A MultiSpectral Imager over coastal and inland waters. Remote Sens. Environ. 2019, 225, 267–289. [Google Scholar] [CrossRef]
- Toming, K.; Kutser, T.; Laas, A.; Sepp, M.; Paavel, B.; Nõges, T. First Experiences in Mapping Lake Water Quality Parameters with Sentinel-2 MSI Imagery. Remote Sens. 2016, 8, 640. [Google Scholar] [CrossRef]
- Kuhwald, K.; Schneider von Deimling, J.; Schubert, P.; Oppelt, N. How can Sentinel-2 contribute to seagrass mapping in shallow, turbid Baltic Sea waters? Remote Sens. Ecol. Conserv. 2022, 8, 328–346. [Google Scholar] [CrossRef]
- Mederos-Barrera, A.; Marcello, J.; Eugenio, F.; Hernández, E. Seagrass mapping using high resolution multispectral satellite imagery: A comparison of water column correction models. Int. J. Appl. Earth Obs. Geoinf. 2022, 113, 102990. [Google Scholar] [CrossRef]
- Fornes, A.; Basterretxea, G.; Orfila, A.; Jordi, A.; Alvarez, A.; Tintore, J. Mapping Posidonia oceanica from IKONOS. ISPRS J. Photogramm. Remote Sens. 2006, 60, 315–322. [Google Scholar] [CrossRef]
- Phinn, S.; Roelfsema, C.; Dekker, A.; Brando, V.; Anstee, J. Mapping seagrass species, cover and biomass in shallow waters: An assessment of satellite multi-spectral and airborne hyper-spectral imaging systems in Moreton Bay (Australia). Remote Sens. Environ. 2008, 112, 3413–3425. [Google Scholar] [CrossRef]
- Topouzelis, K.; Charalampis Spondylidis, S.; Papakonstantinou, A.; Soulakellis, N. The use of Sentinel-2 imagery for seagrass mapping: Kalloni Gulf (Lesvos Island, Greece) case study. In Proceedings of the Fourth International Conference on Remote Sensing and Geoinformation of the Environment, Pafos, Cyprus, 4–8 April 2016; SPIE: Bellingham, WA, USA, 2016; Volume 9688, pp. 460–466. [Google Scholar]
- QGIS.org. QGIS Geographic Information System. QGIS Association. Available online: https://qgis.org/en/site/ (accessed on 15 November 2022).
- Castilla, G.; Hay, G.J. Image objects and geographic objects. In Object-Based Image Analysis: Spatial Concepts for Knowledge-Driven Remote Sensing Applications; Blaschke, T., Lang, S., Hay, G.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 91–110. [Google Scholar]
- Smith, A. Image segmentation scale parameter optimization and land cover classification using the Random Forest algorithm. J. Spat. Sci. 2010, 55, 69–79. [Google Scholar] [CrossRef]
- Townshend, J.R.G.; Justice, C.O. Analysis of the dynamics of African vegetation using the normalized difference vegetation index. Int. J. Remote Sens. 1986, 7, 1435–1445. [Google Scholar] [CrossRef]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- McFeeters, S.K. The use of the Normalized Difference Water Index (NDWI) in the delineation of open water features. Int. J. Remote Sens. 1996, 17, 1425–1432. [Google Scholar] [CrossRef]
- Barnes, E.M.; Clarke, T.R.; Richards, S.E.; Colaizzi, P.D.; Haberland, J.; Kostrzewski, M.; Waller, P.; Choi, C.; Riley, E.; Thompson, T.; et al. Coincident detection of crop water stress, nitrogen status and canopy density using ground-based multispectral data. In Proceedings of the Fifth International Conference on Precision Agriculture, Bloomington, MN, USA, 16–19 July 2000; pp. 1–15. [Google Scholar]
- Villa, P.; Bresciani, M.; Braga, F.; Bolpagni, R. Comparative Assessment of Broadband Vegetation Indices Over Aquatic Vegetation. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 3117–3127. [Google Scholar] [CrossRef]
- Blaschke, T.; Feizizadeh, B.; Hölbling, D. Object-based image analysis and digital terrain analysis for locating landslides in the Urmia Lake Basin, Iran. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 4806–4817. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.; Ohlsen, R.; Stone, C. Classification and Regression Trees; Wadsworth International Group: Franklin, TN, USA, 1984. [Google Scholar]
- Congalton, R.; Green, K. Assessing the Accuracy of Remotely Sensed Data: Princples and Practices; CRC Press: Boca Raton, FL, USA, 2009; pp. 1–200. [Google Scholar]
- Tortora, R.D. A Note on Sample Size Estimation for Multinomial Populations. Am. Stat. 1978, 32, 100–102. [Google Scholar] [CrossRef]
- Hay, A.M. The derivation of global estimates from a confusion matrix. Int. J. Remote Sens. 1988, 9, 1395–1398. [Google Scholar] [CrossRef]
- Qing, S.; Runa, A.; Shun, B.; Zhao, W.; Bao, Y.; Hao, Y. Distinguishing and mapping of aquatic vegetations and yellow algae bloom with Landsat satellite data in a complex shallow Lake, China during 1986–2018. Ecol. Indic. 2020, 112, 106073. [Google Scholar] [CrossRef]
- Chauhan, K.; Patel, J.; Shukla, S.H.; Kalubarme, M.H. Monitoring Water Spread and Aquatic Vegetation using Spectral Indices in Nalsarovar, Gujarat State-India. Int. J. Environ. Geoinform. 2021, 8, 49–56. [Google Scholar] [CrossRef]
- Jaskuła, J.; Sojka, M. Assessing Spectral Indices for Detecting Vegetative Overgrowth of Reservoirs. Pol. J. Environ. Stud. 2019, 28, 4199–4211. [Google Scholar] [CrossRef]
- Klemas, V. Remote sensing of emergent and submerged wetlands: An overview. Int. J. Remote Sens. 2013, 34, 6286–6320. [Google Scholar] [CrossRef]
- Fitoka, E.; Tompoulidou, M.; Hatziiordanou, L.; Apostolakis, A.; Höfer, R.; Weise, K.; Ververis, C. Water-related ecosystems’ mapping and assessment based on remote sensing techniques and geospatial analysis: The SWOS national service case of the Greek Ramsar sites and their catchments. Remote Sens. Environ. 2020, 245, 111795. [Google Scholar] [CrossRef]
- Luo, J.; Ni, G.; Zhang, Y.; Wang, K.; Shen, M.; Cao, Z.; Qi, T.; Xiao, Q.; Qiu, Y.; Cai, Y.; et al. A new technique for quantifying algal bloom, floating/emergent and submerged vegetation in eutrophic shallow lakes using Landsat imagery. Remote Sens. Environ. 2023, 287, 113480. [Google Scholar] [CrossRef]
- Madsen, J.D.; Wersal, R.M. A review of aquatic plant monitoring and assessment methods. J. Aquat. Plant Manag. 2017, 55, 1–12. [Google Scholar]
- Cheng, Q.; Wu, K.; Bai, Y.; Hu, Y. Research on the relationship between the fractional coverage of the submerged plant Vallisneria spiralis and observed spectral parameters. Environ. Monit. Assess. 2013, 185, 5401–5409. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, L. Identification of the spectral characteristics of submerged plant Vallisneria spiralis. Acta Ecol. Sin. 2006, 26, 1005–1010. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, L.-Q. The spectral responses of a submerged plant Vallisneria spiralis with varying biomass using spectroradiometer. Hydrobiologia 2007, 579, 291–299. [Google Scholar] [CrossRef]
- Alagialoglou, L.; Manakos, I.; Papadopoulou, S.; Chadoulis, R.-T.; Kita, A. Mapping Underwater Aquatic Vegetation Using Foundation Models With Air-and Space-Borne Images: The Case of Polyphytos Lake. Remote Sens. 2023, 15, 4001. [Google Scholar] [CrossRef]
- Lu, L.; Luo, J.; Xin, Y.; Xu, Y.; Sun, Z.; Duan, H.; Xiao, Q.; Qiu, Y.; Huang, L.; Zhao, J. A novel strategy for estimating biomass of submerged aquatic vegetation in lake integrating UAV and Sentinel data. Sci. Total. Environ. 2024, 912, 169404. [Google Scholar] [CrossRef]


| Vegetation Type | Dominant Species | |
|---|---|---|
| Trichonida Lake | Feneos Lake | |
| EAV (emergent aquatic vegetation) | Phragmites australis Bolboschoenus maritimus Schoenoplectus litoralis | Typha angustifolia Phragmites australis |
| FAV (floating aquatic vegetation) | Ludwigia peploides Nymphaea alba | - |
| SAV (submerged aquatic vegetation) | Vallisneria spiralis Ceratophyllum demersum Myriophyllum spicatum Najas marina | Chara vulgaris Myriophyllum spicatum Nitella flexilis Nitella furcata Vallisneria spiralis |
| Sentinel 2_MSI Bands | Central Wavelength (μm) | Resolution (m) | Trichonida Lake | Feneos Lake |
|---|---|---|---|---|
| Band 1—Coastal aerosol | 0.443 | 60 | 17-February-21 (ws) 12-July-21 (ss) | 27-February-21 (ws) 27-July-21 (ss) |
| Band 2—Blue | 0.490 | 10 | ||
| Band 3—Green | 0.560 | 10 | ||
| Band 4—Red | 0.665 | 10 | ||
| Band 5—Veg. Red Edge | 0.705 | 20 | ||
| Band 6—Veg. Red Edge | 0.740 | 20 | ||
| Band 7—Veg. Red Edge | 0.783 | 20 | ||
| Band 8—NIR | 0.842 | 10 | ||
| Band 8A—Veg. Red Edge | 0.865 | 20 | ||
| Band 9—Water vapour | 0.945 | 60 | ||
| Band 10—SWIR Cirrus | 1.375 | 60 | ||
| Band 11—SWIR1 | 1.610 | 20 | ||
| Band 12—SWIR2 | 2.190 | 20 |
| Feature Categories | Features | Description | Number of Features |
|---|---|---|---|
| Customized (Spectral indices) | NDVI (ws, ss) | 18 | |
| NDWI (ws, ss) | |||
| NDRE (ws, ss) | |||
| SAVI (ws, ss) | |||
| WAVI (ws, ss) | |||
| NDAVI (ws, ss) | |||
| Ratios | NDAVI (ss)/(ws), Blue/Green (ws, ss) | ||
| Subtractions | NDWI (ws) − (ss), NDWI (ss) − (ws), NDAVI (ws) − (ss), WAVI (ss) − (ws) | ||
| Layer Values (Spectral Features) | Mean (for all image layers) | The mean value represents the mean brightness of an image object within a single band. | 42 |
| Brightness | Sum of mean values in all bands divided by the number of bands. | ||
| Max. Difference | For each image object, Max.Diff is defined as the absolute difference between the minimum object mean values and the maximum object mean values in the visible bands divided by the mean object brightness [96] | ||
| Standard Deviation (for all image layers both ss and ws) | The standard deviation of all pixels which form an image object within a band | ||
| Thematic attributes | One mask generated through photo-interpretation and one from bathymetry data | For the classification of a part of the “Other” class representing the agricultural areas and the bathymetry data for the discrimination of the “Deep Water” class | 2 |
| Class-related | Relations to super objects | Existence to “Water” class | 2 |
| Relation to neighbor objects | Border to, Relative border to features | 2 | |
| Total | 66 |
| Study Area | Number of Thematic Classes | Total Number of Points | Number of In Situ Points |
|---|---|---|---|
| Trichonida Lake | 7 (Emergent, Floating, Submerged, Natural vegetation, Other, Water, Deep Water) | 262 | 64 |
| Feneos Lake | 6 (absence of “Floating” class) | 193 | 46 |
| Trichonida Lake | Feneos Lake | |||
|---|---|---|---|---|
| PA | UA | PA | UA | |
| Water | 100.00% | 60.66% | 100.00% | 60.00% |
| Deep Water | 100.00% | 100.00% | 100.00% | 100.00% |
| Other | 100.00% | 100.00% | 92.31% | 100.00% |
| Natural vegetated areas | 100.00% | 94.74% | 98.53% | 97.10% |
| Emergent | 91.67% | 97.06% | 91.30% | 80.77% |
| Floating | 85.71% | 85.71% | - | - |
| Submerged | 63.64% | 97.67% | 63.83% | 100.00% |
| Overall Accuracy (OA) | 89.31% | 89.12% | ||
| Kappa index of Agreement (KIA) | 0.8716 | 0.8613 | ||
| Trichonida Lake | Feneos Lake | |||
|---|---|---|---|---|
| PA | UA | PA | UA | |
| Emergent | 92.31% | 92.31% | 100.00% | 91.67% |
| Floating | 50.71% | 100.00% | - | - |
| Submerged | 70.45% | 96.88% | 71.43% | 100.00% |
| Overall Accuracy (OA) | 76.56% | 82.61% | ||
| Kappa index of Agreement (KIA) | 0.7046 | 0.7225 | ||
| Trichonida Lake | Feneos Lake | |||
|---|---|---|---|---|
| Spatial Extent (ha) | % | Spatial Extent (ha) | % | |
| Water | 638 | 6.08 | 9.67 | 7.53 |
| Deep Water | 8545 | 81.42 | 32.58 | 25.38 |
| Other | 673 | 6.42 | 11.01 | 8.58 |
| Natural vegetated areas | 263 | 2.51 | 71.13 | 55.41 |
| Emergent | 285 | 2.72 | 3.10 | 2.42 |
| Floating | 1 | 0.01 | - | - |
| Submerged | 89 | 0.85 | 0.89 | 0.69 |
| Total (ha) | 10,495 | 128.38 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tompoulidou, M.; Karadimou, E.; Apostolakis, A.; Tsiaoussi, V. A Geographic Object-Based Image Approach Based on the Sentinel-2 Multispectral Instrument for Lake Aquatic Vegetation Mapping: A Complementary Tool to In Situ Monitoring. Remote Sens. 2024, 16, 916. https://doi.org/10.3390/rs16050916
Tompoulidou M, Karadimou E, Apostolakis A, Tsiaoussi V. A Geographic Object-Based Image Approach Based on the Sentinel-2 Multispectral Instrument for Lake Aquatic Vegetation Mapping: A Complementary Tool to In Situ Monitoring. Remote Sensing. 2024; 16(5):916. https://doi.org/10.3390/rs16050916
Chicago/Turabian StyleTompoulidou, Maria, Elpida Karadimou, Antonis Apostolakis, and Vasiliki Tsiaoussi. 2024. "A Geographic Object-Based Image Approach Based on the Sentinel-2 Multispectral Instrument for Lake Aquatic Vegetation Mapping: A Complementary Tool to In Situ Monitoring" Remote Sensing 16, no. 5: 916. https://doi.org/10.3390/rs16050916
APA StyleTompoulidou, M., Karadimou, E., Apostolakis, A., & Tsiaoussi, V. (2024). A Geographic Object-Based Image Approach Based on the Sentinel-2 Multispectral Instrument for Lake Aquatic Vegetation Mapping: A Complementary Tool to In Situ Monitoring. Remote Sensing, 16(5), 916. https://doi.org/10.3390/rs16050916