Community-Based Monitoring for Rapid Assessment of Nearshore Coral Reefs Amid Disturbances in Teahupo’o, Tahiti
Abstract
1. Introduction
2. Materials and Methods
2.1. Citizens’ Science Education and Training
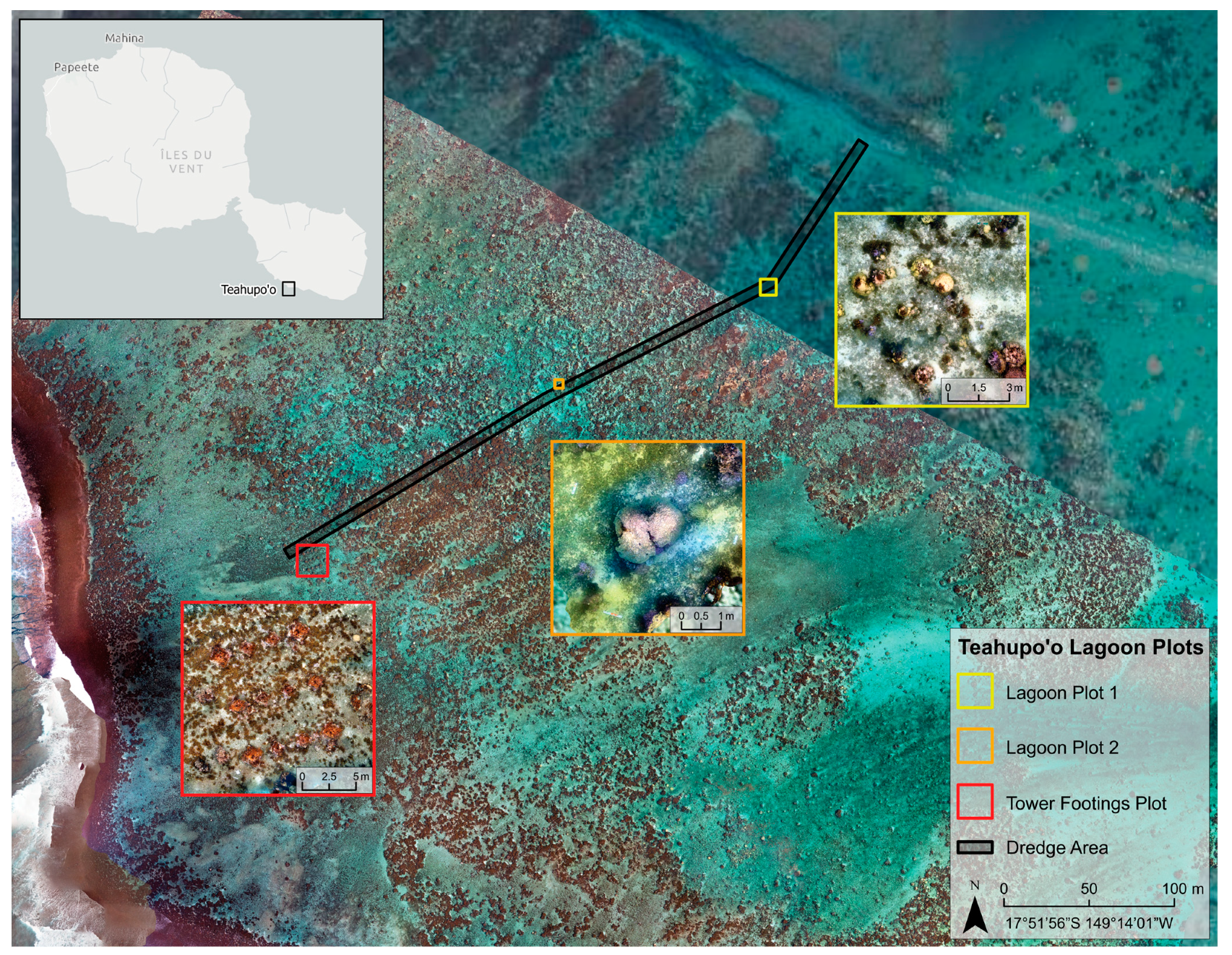
2.2. Three-Dimensional Photogrammetry Surveys
2.3. Three-Dimensional Reconstructions and Annotations
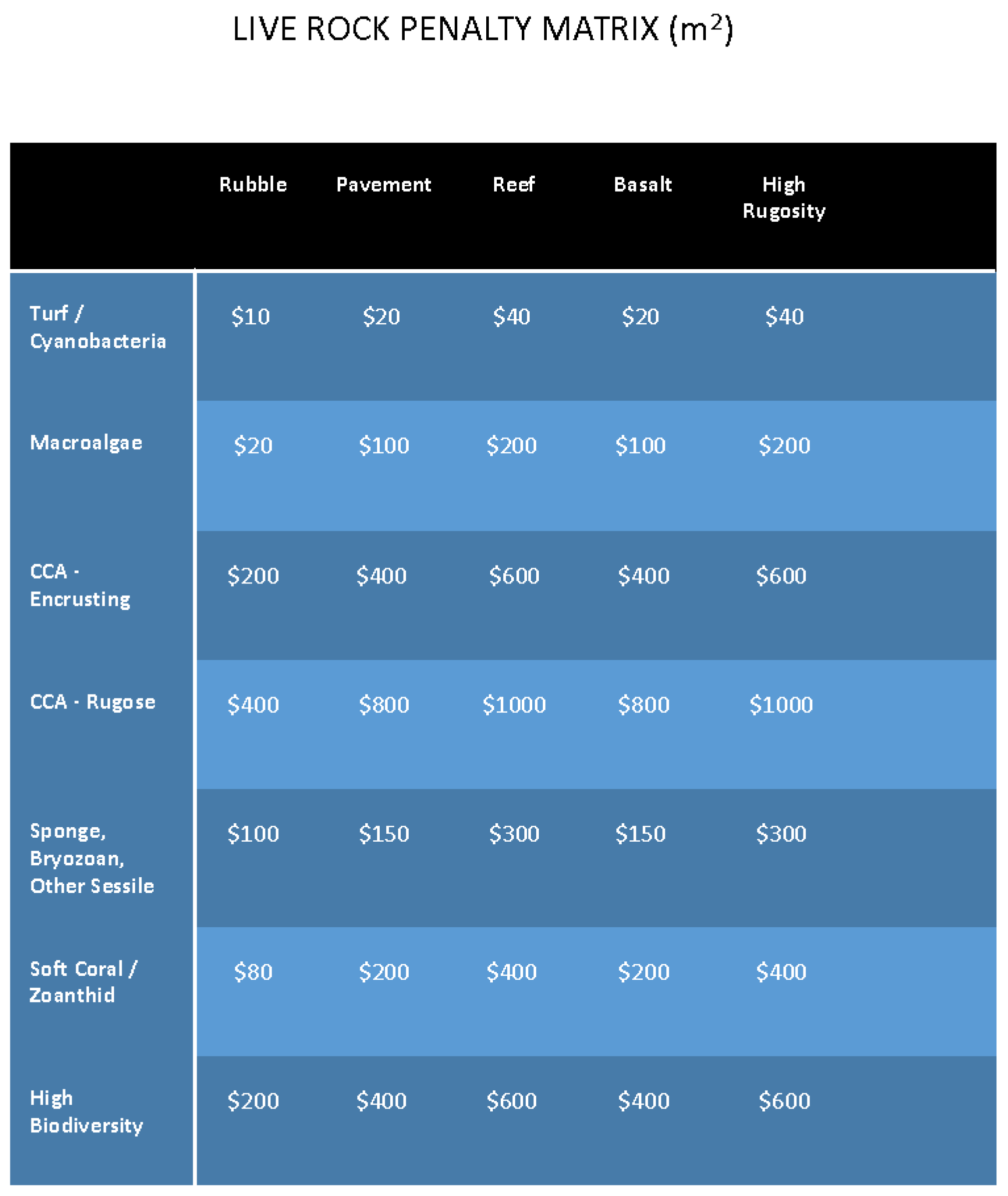
2.4. Valuation of the Living Benthos
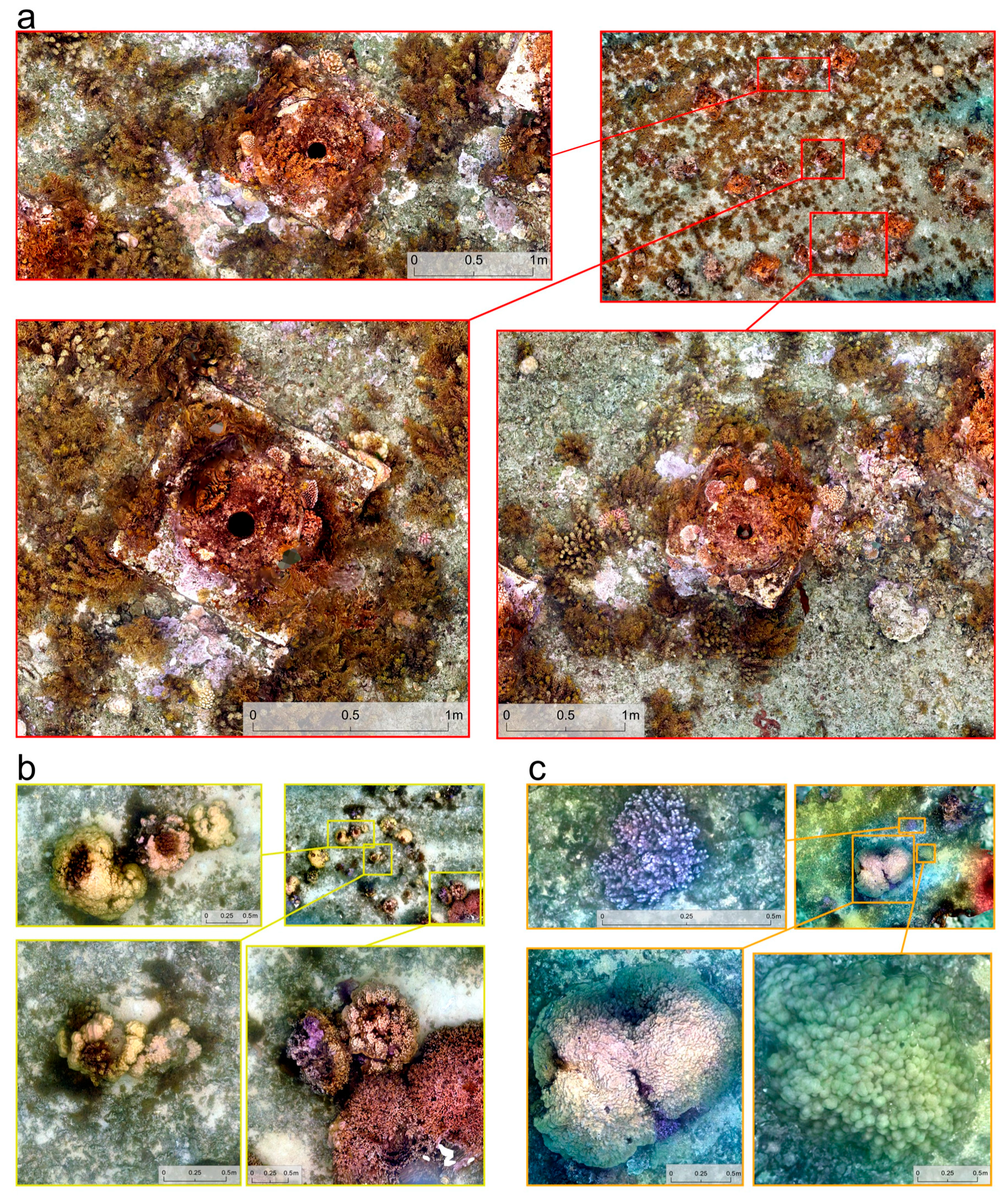
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Muscatine, L.; Porter, J.W. Reef corals: Mutualistic symbioses adapted to nutrient-poor environments. Bioscience 1977, 27, 454–460. [Google Scholar] [CrossRef]
- Weis, V.M.; Davy, S.K.; Hoegh-Guldberg, O.; Rodriguez-Lanetty, M.; Pringle, J.R. Cell biology in model systems as the key to understanding corals. Trends Ecol. Evol. 2008, 23, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Reaka-Kudla, M. The global biodiversity of coral reefs: A comparison with rain forests. In Biodiversity II: Understanding and Protecting our Biological Resources; Reaka-Kudla, M., Wilson, D.E., Wilson, E.O., Eds.; Joseph Henry Press: Washington, DC, USA, 1997; pp. 83–108. [Google Scholar]
- Knowlton, N.; Brainard, R.E.; Fisher, R.; Moews, M.; Plaisance, L.; Caley, M.J. Coral reef biodiversity. In Life in the World’s Ocean: Diversity, Distribution, and Abundance; McIntyre, A., Ed.; Wiley-Blackwell: Oxford, UK, 2010; pp. 65–78. [Google Scholar] [CrossRef]
- Costanza, R.; d’Arge, R.; de Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J.; et al. The value of the world’s ecosystem services and natural capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshw. Res. 1999, 50, 839–866. [Google Scholar] [CrossRef]
- Obura, D.O.; Aeby, G.; Amornthammarong, N.; Appeltans, W.; Bax, N.; Bishop, J.; Brainard, R.E.; Chan, S.; Fletcher, P.; Gordon, T.A.; et al. Coral reef monitoring, reef assessment technologies, and ecosystem-based management. Front. Mar. Sci. 2019, 6, 580. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O. Reviving the Ocean Economy: The Case for Action—2015; WWF International: Gland, Switzerland, 2015; p. 60. [Google Scholar]
- Hatcher, B.G.; Hatcher, G.H. Question of mutual security: Exploring interactions between the health of coral reef ecosystems and coastal communities. EcoHealth 2004, 1, 229–235. [Google Scholar] [CrossRef]
- Cinner, J. Coral reef livelihoods. Curr. Opin. Environ. Sustain. 2014, 7, 65–71. [Google Scholar] [CrossRef]
- Gregg, T.M.; Mead, L.; Burns, J.H.R.; Takabayashi, M. Puka mai he ko ‘a: The significance of corals in Hawaiian culture. In Ethnobiology of Corals and Coral Reefs; Narchi, N., Price, L., Eds.; Springer: Cham, Switzerland, 2015; pp. 103–115. [Google Scholar]
- Harvell, D.; Jordán-Dahlgren, E.; Merkel, S.; Rosenberg, E.; Raymundo, L.; Smith, G.; Weil, E.; Weil, B.L. Coral disease, environmental drivers, and the balance between coral and microbial associates. Oceanography 2007, 20, 172–195. [Google Scholar] [CrossRef]
- Burns, J.H.R.; Weyenberg, G.; Mandel, T.; Ferreira, S.B.; Gotshalk, D.; Kinoshita, C.K.; Marshall, M.J.; Del Moral, N.A.V.; Murphy, S.J.; Pascoe, K.H.; et al. A Comparison of the Diagnostic Accuracy of in-situ and Digital Image-Based Assessments of Coral Health and Disease. Front. Mar. Sci. 2020, 7, 304. [Google Scholar] [CrossRef]
- Ward, J.R.; Kim, K.; Harvell, C.D. Temperature affects coral disease resistance and pathogen growth. Mar. Ecol. Prog. Ser. 2007, 329, 115–121. [Google Scholar] [CrossRef]
- Burns, J.H.R.; Delparte, D.; Gates, R.D.; Takabayashi, M. Integrating structure-from-motion photogrammetry with geospatial software as a novel technique for quantifying 3D ecological characteristics of coral reefs. PeerJ 2015, 3, e1077. [Google Scholar] [CrossRef]
- Ferrari, R.; McKinnon, D.; He, H.; Smith, R.N.; Corke, P.; González-Rivero, M.; Mumby, P.J.; Upcroft, B. Quantifying multiscale habitat structural complexity: A cost-effective framework for underwater 3D modelling. Remote Sens. 2016, 8, 113. [Google Scholar] [CrossRef]
- Fukunaga, A.; Kosaki, R.K.; Pascoe, K.H.; Burns, J.H.R. Fish Assemblage Structure in the Northwestern Hawaiian Islands Is Associated with the Architectural Complexity of Coral-Reef Habitats. Diversity 2020, 12, 430. [Google Scholar] [CrossRef]
- Pascoe, K.H.; Fukunaga, A.; Kosaki, R.K.; Burns, J.H.R. 3D assessment of a coral reef at Lalo Atoll reveals varying responses of habitat metrics following a catastrophic hurricane. Sci. Rep. 2021, 11, 12050. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.H.R.; Pascoe, K.H.; Ferreira, S.B.; Kane, H.; Kapono, C.A.; Carrell, T.L.; Reyes, A.; Fukunaga, A. How Do Underwater Cultural Heritage Sites Affect Coral Assemblages? Remote Sens. 2023, 15, 2130. [Google Scholar] [CrossRef]
- Ferreira, S.B.; Burns, J.H.R.; Pascoe, K.H.; Kapono, C.A.; Reyes, A.J.; Fukunaga, A. Prediction of habitat complexity using a trait-based approach on coral reefs in Guam. Sci. Rep. 2023, 13, 11095. [Google Scholar] [CrossRef]
- Javernick, L.; Brasington, J.; Caruso, B. Modeling the topography of shallow braided rivers using structure-from-motion photogrammetry. Geomorphology 2014, 213, 166–182. [Google Scholar] [CrossRef]
- Figueira, W.; Ferrari, R.; Weatherby, E.; Porter, A.; Hawes, S.; Byrne, M. Accuracy and precision of habitat structural complexity metrics derived from underwater photogrammetry. Remote Sens. 2015, 7, 16883–16900. [Google Scholar] [CrossRef]
- Fukunaga, A.; Burns, J.H.R. Metrics of Coral Reef Structural Complexity Extracted from 3D Mesh Models and Digital Elevation Models. Remote Sens. 2020, 12, 2676. [Google Scholar] [CrossRef]
- Theobald, E.J.; Ettinger, A.; Burgess, H.; DeBey, L.; Schmidt, N.; Froehlich, H.; Wagner, C.; HilleRisLambers, J.; Tewksbury, J.; Harsch, M.A.; et al. Global change and local solutions: Tapping the unrealized potential of citizen science for biodiversity research. Biol. Conserv. 2015, 181, 236–244. [Google Scholar] [CrossRef]
- Bonney, R.; Phillips, T.B.; Ballard, H.L.; Enck, J.W. Can citizen science enhance public understanding of science? Public Underst. Sci. 2016, 25, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Kapono, C.A.; Burns, J.H.R.; Kane, H.H. Branding the MEGA Lab; methods that support science communication and citizen science engagement. Front. Environ. Sci. 2023, 11, 1208683. [Google Scholar] [CrossRef]
- Pocock, M.J.O.; Tweddle, J.C.; Savage, J.; Robinson, L.D.; Roy, H.E. The diversity and evolution of ecological and environmental citizen science. PLoS ONE 2017, 12, e0172579. [Google Scholar] [CrossRef] [PubMed]
- Tauginienė, L.; Butkevičienė, E.; Vohland, K.; Heinisch, B.; Daskolia, M.; Suškevičs, M.; Portela, M.; Balázs, B.; Prūse, B. Citizen science in the social sciences and humanities: The power of interdisciplinarity. Palgrave Commun. 2020, 6, 89. [Google Scholar] [CrossRef]
- Verhoeven, G. Getting computer vision airborne: Using structure from motion for accurate orthophoto production. In Proceedings of the RSPSoc Archaeology Special Interest Group Meeting, Greenwich, UK, 12–14 September 2012; pp. 4–6. [Google Scholar]
- Westoby, M.J.; Brasington, J.; Glasser, N.F.; Hambrey, M.J.; Reynolds, J.M. ‘Structure-from-Motion’photogrammetry: A low-cost, effective tool for geoscience applications. Geomorphology 2012, 179, 300–314. [Google Scholar] [CrossRef]
- Beijbom, O.; Edmunds, P.J.; Roelfsema, C.; Smith, J.; Kline, D.I.; Neal, B.P.; Dunlap, M.J.; Moriarty, V.; Fan, T.-Y.; Tan, C.-J.; et al. Towards automated annotation of benthic survey images: Variability of human experts and operational modes of automation. PLoS ONE 2015, 10, e0130312. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Richardson, L.E.; Graham, N.A.; Hoey, A.S. Coral species composition drives key ecosystem function on coral reefs. Proc. R. Soc. B 2020, 287, 20192214. [Google Scholar] [CrossRef]
- Goreau, T.F. Calcium carbonate deposition by coralline algae and corals in relation to their roles as reef-builders. Ann. N. Y. Acad. Sci. 1963, 109, 127–167. [Google Scholar] [CrossRef]
- Nelson, W.A. Calcified macroalgae–critical to coastal ecosystems and vulnerable to change: A review. Mar. Freshw. Res. 2009, 60, 787–801. [Google Scholar] [CrossRef]
- Brown, B.E.; Le Tissier, M.D.A.; Scoffin, T.P.; Tudhope, A.W. Evaluation of the environmental impact of dredging on intertidal coral reefs at Ko Phuket, Thailand, using ecological and physiological parameters. Mar. Ecol. Prog. Ser. 1990, 65, 273–281. [Google Scholar] [CrossRef]
- Cunning, R.; Silverstein, R.N.; Barnes, B.B.; Baker, A.C. Extensive coral mortality and critical habitat loss following dredging and their association with remotely-sensed sediment plumes. Mar. Pollut. Bull. 2019, 145, 185–199. [Google Scholar] [CrossRef]
- Gintert, B.E.; Precht, W.F.; Fura, R.; Rogers, K.; Rice, M.; Precht, L.L.; D’Alessandro, M.; Croop, J.; Vilmar, C.; Robbart, M.L. Regional coral disease outbreak overwhelms impacts from a local dredge project. Environ. Monit. Assess. 2019, 191, 630. [Google Scholar] [CrossRef]
- Pollock, F.J.; Lamb, J.B.; Field, S.N.; Heron, S.F.; Schaffelke, B.; Shedrawi, G.; Bourne, D.G.; Willis, B.L. Sediment and turbidity associated with offshore dredging increase coral disease prevalence on nearby reefs. PLoS ONE 2014, 9, e102498. [Google Scholar] [CrossRef] [PubMed]
- Fezzi, C.; Ford, D.J.; Oleson, K.L. The economic value of coral reefs: Climate change impacts and spatial targeting of restoration measures. Ecol. Econ. 2023, 203, 107628. [Google Scholar] [CrossRef]
- Kobori, H.; Dickinson, J.L.; Washitani, I.; Sakurai, R.; Amano, T.; Komatsu, N.; Kitamura, W.; Takagawa, S.; Koyama, K.; Ogawara, T.; et al. Citizen science: A new approach to advance ecology, education, and conservation. Ecol. Res. 2016, 31, 1–19. [Google Scholar] [CrossRef]
- Branchini, S.; Pensa, F.; Neri, P.; Tonucci, B.M.; Mattielli, L.; Collavo, A.; Sillingardi, M.E.; Piccinetti, C.; Zaccanti, F.; Goffredo, S. Using a citizen science program to monitor coral reef biodiversity through space and time. Biodivers. Conserv. 2015, 24, 319. [Google Scholar] [CrossRef]
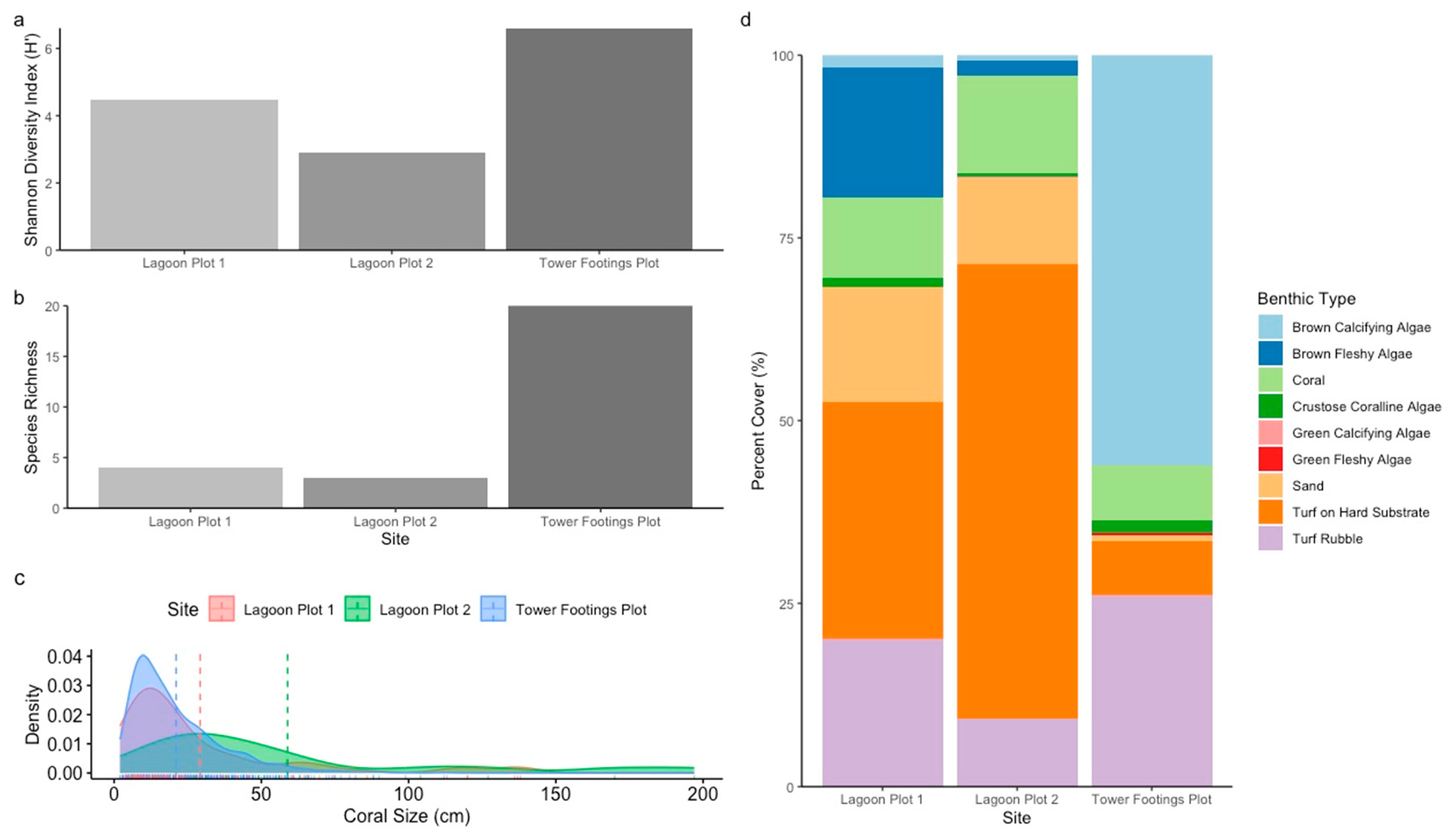
| Site | |||
|---|---|---|---|
| Benthic Type | Lagoon Plot 1 | Lagoon Plot 2 | Tower Footings Plot |
| Brown Calcifying Algae | 1.90% | 3.13% | 48.32% |
| Brown Fleshy Algae | 17.32% | 3.67% | 0.0% |
| Coral | 8.51% | 15.66% | 7.43% |
| Crustose Coralline Algae | 1.20% | 1.08% | 2.14% |
| Green Calcifying Algae | 0.0% | 0.32% | 0.0% |
| Green Fleshy Algae | 0.0% | 0.0% | 1.42% |
| Sand | 14.31% | 13.82% | 0.51% |
| Turf on Hard Substrate | 36.74% | 51.40% | 11.19% |
| Turf Rubble | 20.02% | 10.91% | 28.99% |
| Site | Total Area (m2) | No. of Corals | Species Richness | Shannon–Wiener Diversity Index (H′) | Coral Penalty Value (USD) | Live Rock Penalty Value (USD) | Total Penalty Value (USD) |
|---|---|---|---|---|---|---|---|
| Lagoon Plot 1 | 66 | 87 | 4 | 4.46 | 18,380.00 | 4114.59 | 22,494.59 |
| Lagoon Plot 2 | 16 | 18 | 3 | 2.89 | 7440.00 | 678.19 | 8118.19 |
| Tower Footings Plot | 240 | 898 | 20 | 6.60 | 113,760.00 | 28,724.31 | 142,484.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burns, J.H.R.; Pascoe, K.H.; Kane, H.H.; Nakoa, J.W.P., III; Pascoe, M.; Pierucci, S.R.; Sokol, R.E.; Golgotiu, K.A.; Cortes, M.; Hacker, A.; et al. Community-Based Monitoring for Rapid Assessment of Nearshore Coral Reefs Amid Disturbances in Teahupo’o, Tahiti. Remote Sens. 2024, 16, 853. https://doi.org/10.3390/rs16050853
Burns JHR, Pascoe KH, Kane HH, Nakoa JWP III, Pascoe M, Pierucci SR, Sokol RE, Golgotiu KA, Cortes M, Hacker A, et al. Community-Based Monitoring for Rapid Assessment of Nearshore Coral Reefs Amid Disturbances in Teahupo’o, Tahiti. Remote Sensing. 2024; 16(5):853. https://doi.org/10.3390/rs16050853
Chicago/Turabian StyleBurns, John H. R., Kailey H. Pascoe, Haunani H. Kane, Joseph W. P. Nakoa, III, Makoa Pascoe, Sophia R. Pierucci, Riley E. Sokol, Krista A. Golgotiu, Manuela Cortes, Aralyn Hacker, and et al. 2024. "Community-Based Monitoring for Rapid Assessment of Nearshore Coral Reefs Amid Disturbances in Teahupo’o, Tahiti" Remote Sensing 16, no. 5: 853. https://doi.org/10.3390/rs16050853
APA StyleBurns, J. H. R., Pascoe, K. H., Kane, H. H., Nakoa, J. W. P., III, Pascoe, M., Pierucci, S. R., Sokol, R. E., Golgotiu, K. A., Cortes, M., Hacker, A., Villela, L., Ninomoto, B. K., Steward, K., Otcenasek, C., & Kapono, C. (2024). Community-Based Monitoring for Rapid Assessment of Nearshore Coral Reefs Amid Disturbances in Teahupo’o, Tahiti. Remote Sensing, 16(5), 853. https://doi.org/10.3390/rs16050853









