Monitoring Saltmarsh Restoration in the Upper Bay of Fundy Using Multi-Temporal Sentinel-2 Imagery and Random Forests Classifier
Abstract
1. Introduction
| Sensor | Classifier * | Classification Accuracy (%) | Number of Classes | Season | Region | Reference |
|---|---|---|---|---|---|---|
| Sentinel-2 | OBIA + RF | 99 | 4 | Summer | Turkey | [40] |
| RF | 91 | 5 | All seasons | China | [41] | |
| Supervised hierarchical classifier | 80 | 8 | Spring, Summer, Fall | Italy | [42] | |
| Sentinel-1 and Sentinel-2 | RF | 92 | 3 | Summer | China | [43] |
| Sentinel-2 and Worldview-2,3 | OBIA + RF | 93 | 10 | Spring | USA | [44] |
| SVM | 93 | 8 | Summer, Fall | Ontario, Canada | [45] | |
| MLC | 75 | 8 | Summer, Fall | Ontario, Canada | [45] | |
| SPOT and IRS | MLC | 68 | 6 | Spring, Summer | UK | [46] |
| AVNIR-2 and Alos PalSAR | OBIA + RF | 82 | 9 | Spring | China | [47] |
| Alos PalSAR | OBIA + RF | 89 | 7 | Fall | China | [48] |
| Sentinel-1 | RF | 87 | 5 | Multiple | China | [49] |
| GF-1, ZY-3 | OBIA + RF | 84 | 6 | Multiple | China | [50] |
2. Materials and Methods
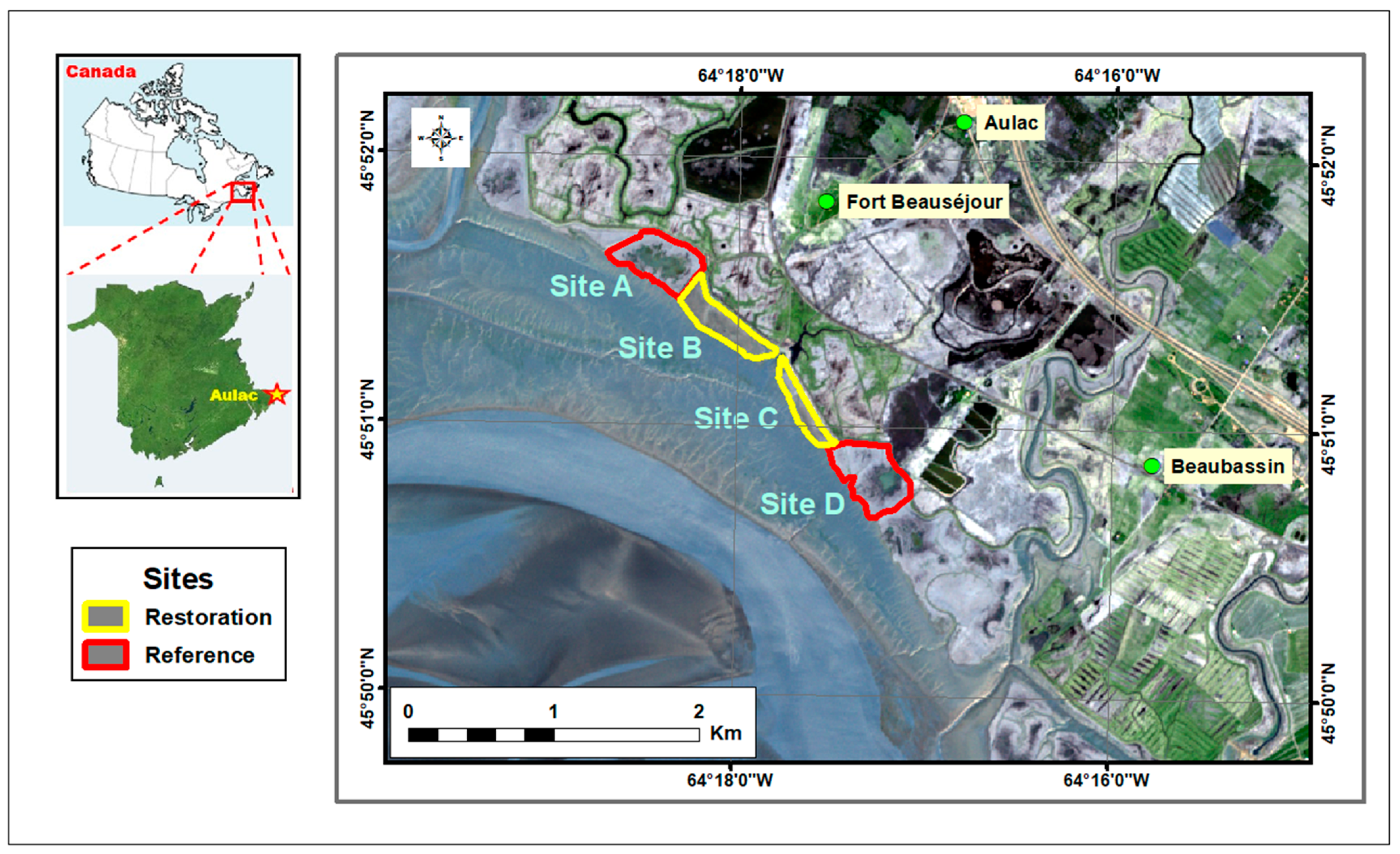
2.1. Study Area
2.2. Sentinel-2 Images
2.3. Ground Truth Data
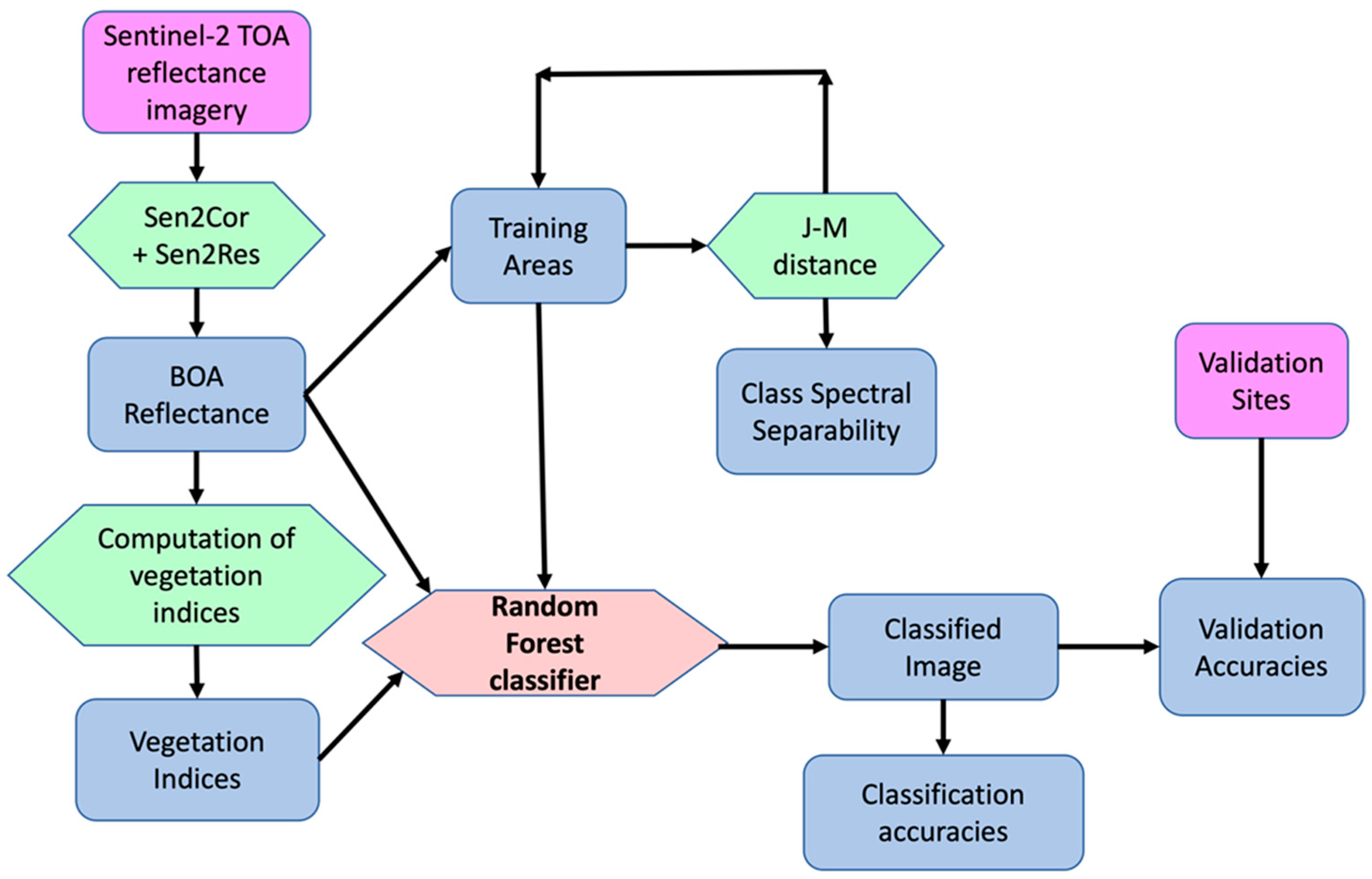
2.4. Image Processing
2.5. Image Classification
2.6. Accuracy Assessment
3. Results
3.1. Class Spectral Separability
3.2. Classification
3.3. Variable Importance
3.4. Validation Accuracy
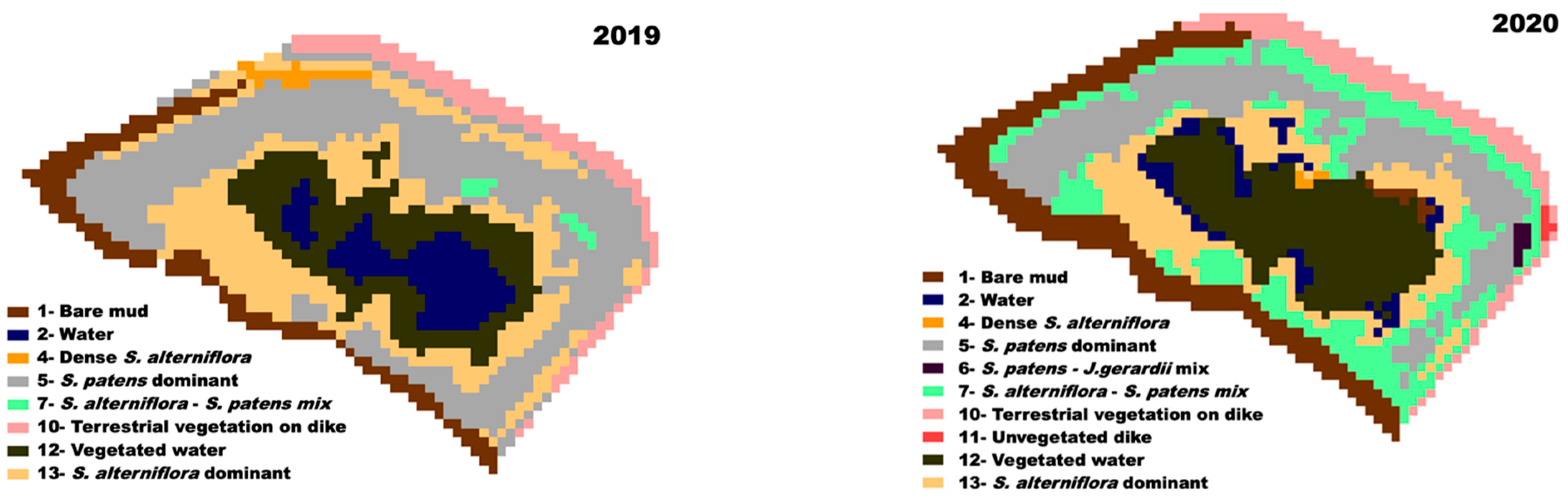
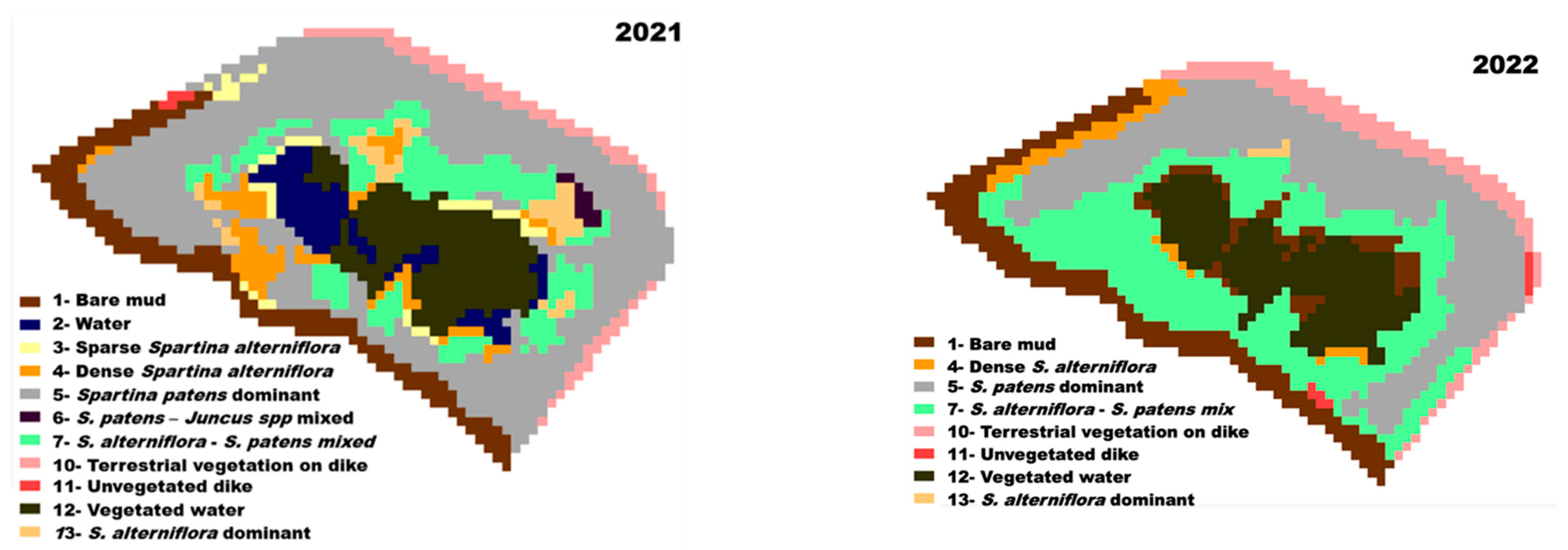
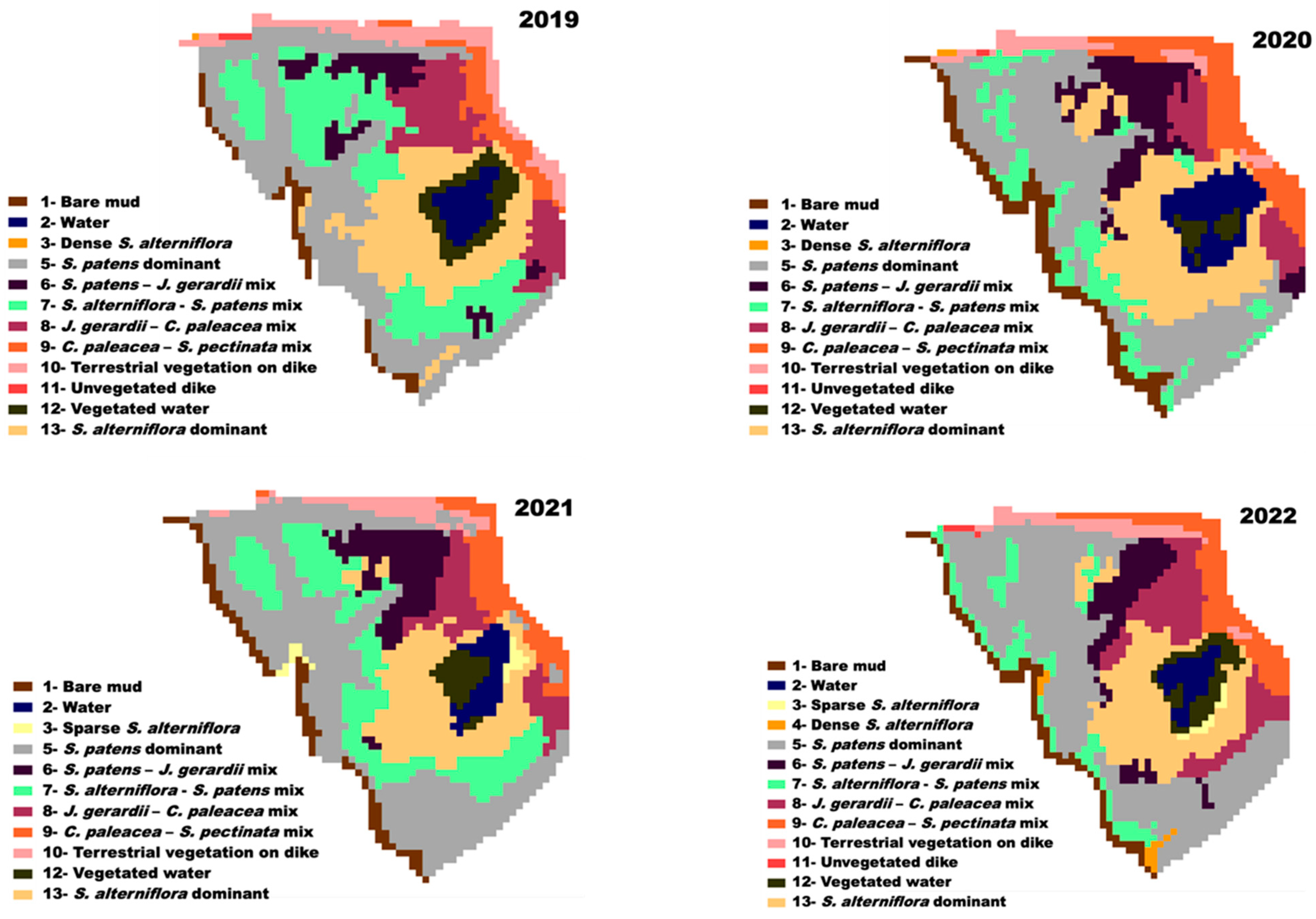
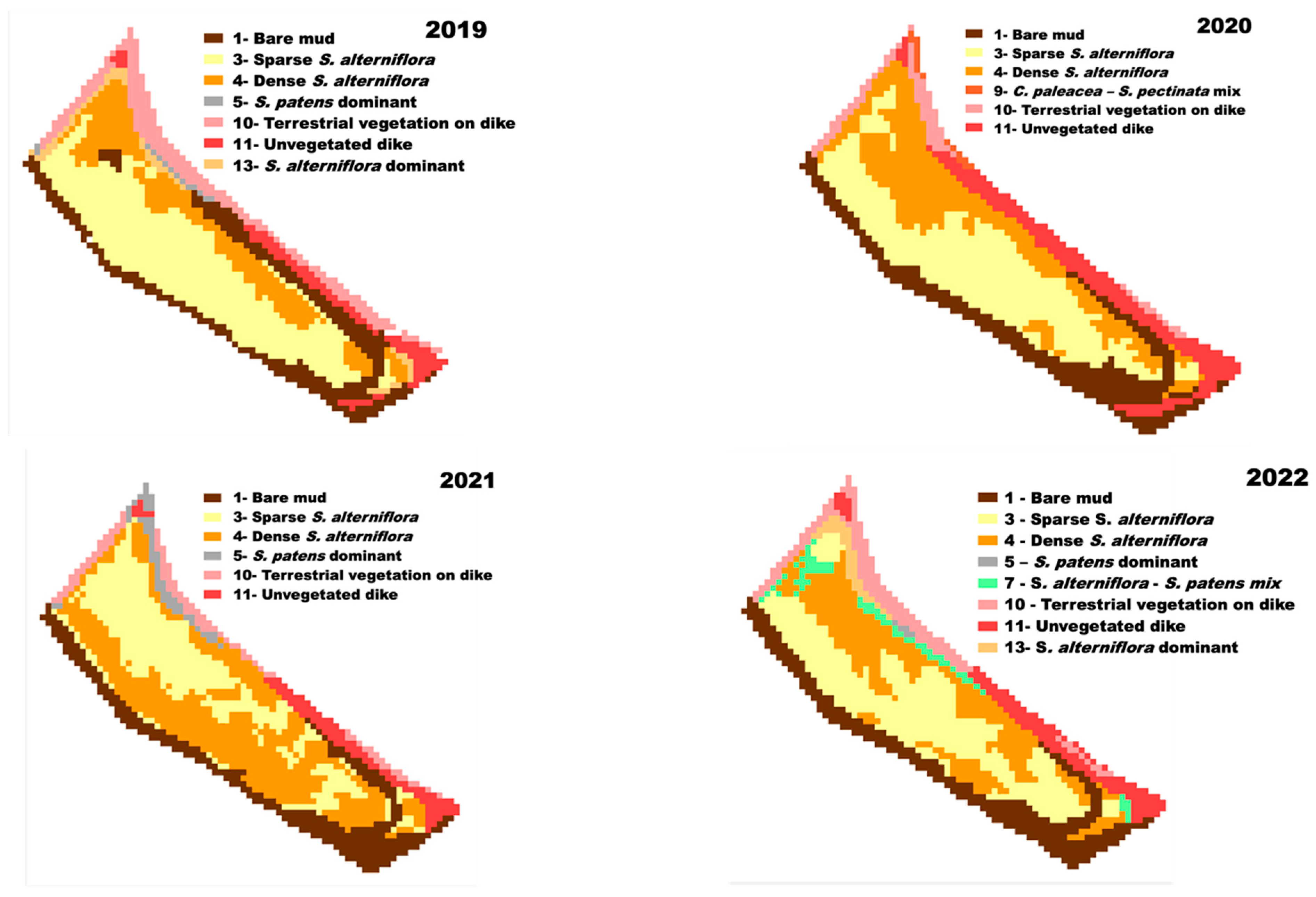
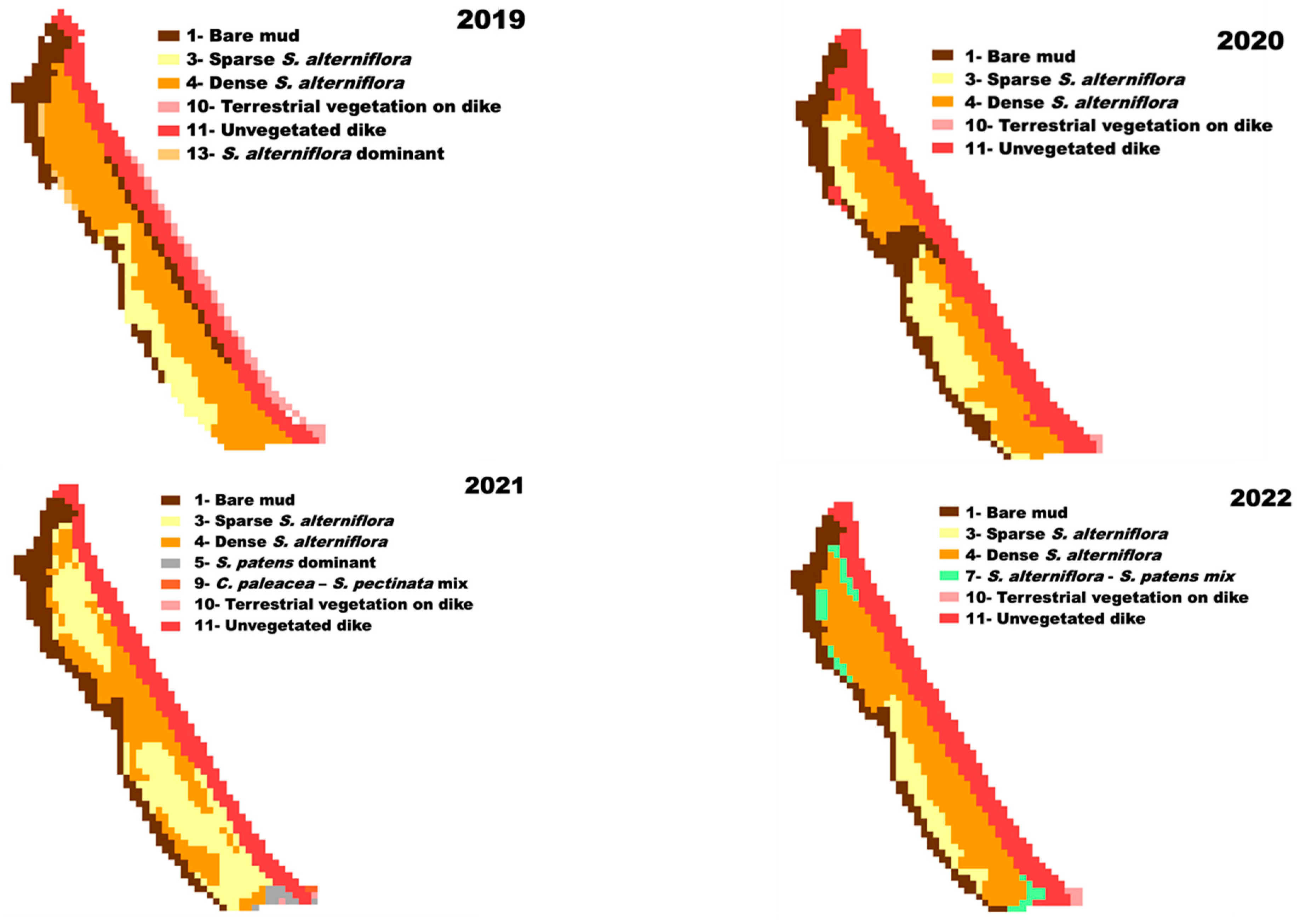
3.5. Landcover Change
4. Discussion
4.1. Classification and Validation Accuracies
4.2. Insights from the Variable Importance Ranking
4.3. Landcover Change and Assessment of Saltmarsh Recovery
4.4. Assessment of Sentinel-2 Imagery for Monitoring Saltmarsh Restoration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitsch, W.J.; Gosselink, J.G. Wetlands, 5th ed.; Van Nostrand Reinhold: New York, NY, USA, 2015; 747p. [Google Scholar]
- Bertness, M.D. Atlantic Shorelines: Natural History and Ecology; Princeton University Press: Princeton, NJ, USA, 2007; 432p. [Google Scholar]
- Redfield, A.C. Development of a New England Salt Marsh. Ecol. Monogr. 1972, 42, 201–237. [Google Scholar] [CrossRef]
- Ford, H.; Garbutt, A.; Ladd, C.; Malarkey, J.; Skov, M.W. Soil Stabilization Linked to Plant Diversity and Environmental Context in Coastal Wetlands. J. Veg. Sci. 2016, 27, 259–268. [Google Scholar] [CrossRef]
- Gracia, A.; Rangel-Buitrago, N.; Oakley, J.A.; Williams, A.T. Use of Ecosystems in Coastal Erosion Management. Ocean Coast. Manag. 2018, 156, 277–289. [Google Scholar] [CrossRef]
- Breckle, S.W. Salinity, Halophytes, and Salt-Affected Natural Ecosystems. In Salinity: Environment—Plants—Molecules; Läuchli, A., Lüttge, U., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 53–77. [Google Scholar] [CrossRef]
- Bertness, M.D.; Ellison, A.M. Determinants of Pattern in a New England Salt Marsh Plant Community. Ecol. Monogr. 1987, 57, 129–147. [Google Scholar] [CrossRef]
- Bertness, M.D. Zonation of Spartina patens and Spartina alterniflora in New England Salt Marsh. Ecology 1991, 72, 138–148. [Google Scholar] [CrossRef]
- Crain, C.M.; Silliman, B.R.; Bertness, S.L.; Bertness, M.D. Physical and Biotic Drivers of Plant Distribution across Estuarine Salinity Gradients. Ecology 2004, 85, 2539–2549. [Google Scholar] [CrossRef]
- Ollerhead, J.; Davidson-Arnott, R.G.D.; Scott, A. Cycles of Saltmarsh Extension and Contraction, Cumberland Basin, Bay of Fundy, Canada. In Geomorphologia Littoral I Quaternari: Homenatge al Professor V. M. Rosselló i Verger; Fleury, M., McCann, S.B., Eds.; Servei de Publicacions, Universitat de València: València, Spain, 2005; pp. 293–305. [Google Scholar]
- Virgin, S.D.S.; Beck, A.D.; Boone, L.K.; Dykstra, A.K.; Ollerhead, J.; Barbeau, M.A.; McLellan, N.R. A Managed Realignment in the Upper Bay of Fundy: Community Dynamics during Saltmarsh Restoration over 8 Years in a Megatidal, Ice-Influenced Environment. Ecol. Eng. 2020, 149, 105713. [Google Scholar] [CrossRef]
- Buth, G.J.C. Decomposition of Roots of Three Plant Communities in a Dutch Salt Marsh. Aquat. Bot. 1987, 29, 123–138. [Google Scholar] [CrossRef]
- Artigas, F.; Shin, J.Y.; Hobble, C.; Marti-Donati, A.; Schäfer, K.V.R.; Pechmann, I. Long-Term Carbon Storage Potential and CO2 Sink Strength of a Restored Salt Marsh in New Jersey. Agric. For. Meteorol. 2015, 200, 313–321. [Google Scholar] [CrossRef]
- Wollenberg, J.T.; Ollerhead, J.; Chmura, G.L. Rapid Carbon Accumulation Following Managed Realignment on the Bay of Fundy. PLoS ONE 2018, 13, e0193930. [Google Scholar] [CrossRef]
- Pétillon, J.; Potier, S.; Carpentier, A.; Garbutt, A. Evaluating the Success of Managed Realignment for the Restoration of SaltMarshes: Lessons from Invertebrate Communities. Ecol. Eng. 2014, 69, 70–75. [Google Scholar] [CrossRef]
- Baker, R.; Taylor, M.D.; Able, K.; Beck, M.W. Fisheries Rely on Threatened Salt Marshes. Science 2020, 370, 670–671. [Google Scholar] [CrossRef] [PubMed]
- Shriver, W.G.; Greenberg, R. Avian Community Responses to Tidal Restoration along the North Atlantic Coast of North America. In Tidal Marsh Restoration: A Synthesis of Science and Management; Roman, C.T., Burdick, D.M., Eds.; Island Press/Center for Resource Economics: Washington, DC, USA, 2012; pp. 119–143. [Google Scholar] [CrossRef]
- Adam, P. Saltmarshes in a Time of Change. Environ. Conserv. 2002, 29, 39–61. [Google Scholar] [CrossRef]
- Gedan, K.B.; Silliman, B.R.; Bertness, M.D. Centuries of Human-Driven Change in Saltmarsh Ecosystems. Annu. Rev. Mar. Sci. 2009, 1, 117–141. [Google Scholar] [CrossRef]
- McOwen, C.J.; Weatherdon, L.V.; van Bochove, J.-W.; Sullivan, E.; Blyth, S.; Zöckler, C.; Stanwell-Smith, D.; Kingston, N.; Martin, C.S.; Spalding, M.; et al. A Global Map of Saltmarshes. Biodivers. Data J. 2017, 5, e11764. [Google Scholar] [CrossRef] [PubMed]
- Ganong, W.F. The Vegetation of the Bay of Fundy Salt and Diked Marshes: An Ecological Study. Bot. Gaz. 1903, 36, 161–186, 280–302, 349–369, 429–455. [Google Scholar]
- Thomas, M.L.H. (Ed.) Marine and Coastal Systems of the Quoddy Region, New Brunswick; Canadian Special Publication of Fisheries and Aquatic Sciences; Dept. of Fisheries and Oceans: Ottawa, ON, Canada, 1983; 306p. [Google Scholar]
- Butzer, K.W. French Wetland Agriculture in Atlantic Canada and Its European Roots: Different Avenues to Historical Diffusion. Ann. Assoc. Am. Geogr. 2002, 92, 451–470. [Google Scholar] [CrossRef]
- Koohzare, A.; Vaníček, P.; Santos, M. Pattern of Recent Vertical Crustal Movements in Canada. J. Geodyn. 2008, 45, 133–145. [Google Scholar] [CrossRef]
- Boon, J.D. Evidence of Sea Level Acceleration at U.S. and Canadian Tide Stations, Atlantic Coast, North America. J. Coast. Res. 2012, 28, 1437–1445. [Google Scholar] [CrossRef]
- Sherren, K.; Ellis, K.; Guimond, J.A.; Kurylyk, B.; LeRoux, N.; Lundholm, J.; Mallory, M.L.; van Proosdij, D.; Walker, A.K.; Bowron, T.M.; et al. Understanding Multifunctional Bay of Fundy Dykelands and Tidal Wetlands Using Ecosystem Services—A Baseline. FACETS 2021, 6, 1446–1473. [Google Scholar] [CrossRef]
- Waltham, N.J.; Elliott, M.; Lee, S.Y.; Lovelock, C.; Duarte, C.M.; Buelow, C.; Simenstad, C.; Nagelkerken, I.; Classens, L.; Wen, C.C.K.; et al. UN Decade on Ecosystem of Restoration 2021–2030—What Chance for Success in Restoring Coastal Ecosystems? Front. Mar. Sci. 2020, 7, 71. [Google Scholar] [CrossRef]
- Waltham, N.J.; Alcott, C.; Barbeau, M.A.; Cebrian, J.; Connolly, R.M.; Deegan, L.A.; Dodds, K.; Goodridge Gaines, L.A.; Gilby, B.L.; Henderson, C.J. Tidal Marsh Restoration Optimism in a Changing Climate and Urbanizing Seascape. Estuaries Coasts 2021, 44, 1681–1690. [Google Scholar] [CrossRef]
- Naojee, S.M.; Leblon, B.; LaRocque, A.; Norris, G.S.; Barbeau, M.A.; Rowland, M. Saltmarsh vegetation mapping in Atlantic Canada using Sentinel-2 imagery. In Proceedings of the 10th International Conference on Agro-Geoinformatics and 43rd Canadian Symposium on Remote Sensing (CSRS), Quebec City, QC, Canada, 12–14 July 2022; Abstracts. p. 2. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Pal, M. Random Forest Classifier for Remote Sensing Classification. Int. J. Remote Sens. 2005, 26, 217–222. [Google Scholar] [CrossRef]
- Gislason, P.O.; Benediktsson, J.A.; Sveinsson, J.R. Random Forests for Landcover Classification. Pattern Recognit. Lett. 2006, 27, 294–300. [Google Scholar] [CrossRef]
- Waske, B.; Braun, M. Classifier Ensembles for Landcover Mapping Using Multitemporal SAR Imagery. ISPRS J. Photogramm. Remote Sens. 2009, 64, 450–457. [Google Scholar] [CrossRef]
- LaRocque, A.; Leblon, B.; Woodward, R.; Mordini, M.; Bourgeau, L.; Landon, A.; Camill, P. Use of Radarsat-2 and Alos-PalSAR SAR Images for Wetland Mapping in New Brunswick. In Proceedings of the 2014 IEEE International Geoscience and Remote Sensing Symposium (IGARSS 2014), Quebec City, QC, Canada, 13–18 July 2014; pp. 1226–1229. [Google Scholar]
- He, J.; Harris, J.R.; Sawada, M.; Behnia, P.A. Comparison of Classification Algorithms Using Landsat-7 and Landsat-8 Data for Mapping Lithology in Canada’s Arctic. Int. J. Remote Sens. 2015, 36, 2252–2276. [Google Scholar] [CrossRef]
- Forsey, D.; LaRocque, A.; Leblon, B.; Skinner, M.; Douglas, A. Refinements in Eelgrass Mapping at Tabusintac Bay (New Brunswick, Canada): A Comparison between Random Forest and the Maximum Likelihood Classifier. Can. J. Remote Sens. 2020, 46, 640–659. [Google Scholar] [CrossRef]
- Martínez-Prentice, R.; Villoslada Peciña, M.; Ward, R.D.; Bergamo, T.F.; Joyce, C.B.; Sepp, K. Machine Learning Classification and Accuracy Assessment from High-Resolution Images of Coastal Wetlands. Remote Sens. 2021, 13, 3669. [Google Scholar] [CrossRef]
- Desplanque, C.; Mossman, D.J. Tides and Their Seminal Impact on the Geology, Geography, History, and Socio-Economics of the Bay of Fundy, Eastern Canada. Atl. Geosci. 2004, 40, 1–65. [Google Scholar] [CrossRef]
- Boone, L.K.; Ollerhead, J.; Barbeau, M.A.; Beck, A.D.; Sanderson, B.G.; McLellan, N.R. Returning the Tide to Dikelands in a Macrotidal and Ice-Influenced Environment: Challenges and Lessons Learned. In Coastal Wetlands: Alteration and Remediation; Finkl, C., Makowski, C., Eds.; Coastal Research Library; Springer: Cham, Switzerland, 2017; Volume 21, pp. 705–749. [Google Scholar] [CrossRef]
- Kaplan, G.; Avdan, U. Mapping and Monitoring Wetlands Using Sentinel-2 Satellite Imagery. ISPRS Ann. Photogramm. Remote Sens. Spat. Inf. Sci. 2017, 4, 271–277. [Google Scholar] [CrossRef]
- Sun, C.; Li, J.; Liu, Y.; Liu, Y.; Liu, R. Plant Species Classification in Salt Marshes Using Phenological Parameters Derived from Sentinel-2 Pixel-Differential Time-Series. Remote Sens. Environ. 2021, 256, 112320. [Google Scholar] [CrossRef]
- Villa, P.; Giardino, C.; Mantovani, S.; Tapete, D.; Vecoli, A.; Braga, F. Mapping Coastal and Wetland Vegetation Communities Using Multi-Temporal Sentinel-2 Data. ISPRS Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2021, 43, 639–644. [Google Scholar] [CrossRef]
- Zhang, C.; Gong, Z.; Qiu, H.; Zhang, Y.; Zhou, D. Mapping Typical Saltmarsh Species in the Yellow River Delta Wetland Supported by Temporal-Spatial-Spectral Multidimensional Features. Sci. Total Environ. 2021, 783, 147061. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.; Wang, Y. High Spatial Resolution Remote Sensing for Salt Marsh Mapping and Change Analysis at Fire Island National Seashore. Remote Sens. 2019, 11, 1107. [Google Scholar] [CrossRef]
- Rupasinghe, P.A.; Chow-Fraser, P. Mapping Phragmites Cover Using WorldView 2/3 and Sentinel 2 Images at Lake Erie Wetlands, Canada. Biol. Invasions 2021, 23, 1231–1247. [Google Scholar] [CrossRef]
- Cawkwell, F.G.; Dwyer, N.; Bartlett, D.; Ameztoy, I.; O’Connor, B.; O’Dea, L.; Hills, J.; Brown, A.; Cross, N.; O’Donnell, M.; et al. Saltmarsh Habitat Classification from Satellite Imagery. In Proceedings of 3rd EARSeL Workshop Remote Sensing of the Coastal Zone, Bolzano, Italy, 7–9 June 2007; p. 12. [Google Scholar]
- Chen, Y.; He, X.; Xu, J.; Guo, L.; Lu, Y.; Zhang, R. Decision Tree-Based Classification in Coastal Area Integrating Polarimetric SAR and Optical Data. Data Technol. Appl. 2021, 56, 342–357. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, J.; Chen, Y.; Xu, K.; Wang, D. Coastal Wetland Classification with GF-3 Polarimetric SAR Imagery by Using Object-Oriented Random Forest Algorithm. Sensors 2021, 21, 3395. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Tian, B.; Yuan, L.; Li, X.; Huang, Y.; Shi, R.; Jiang, X.; Wang, L.; Sun, C. Mapping Coastal Salt Marshes in China Using Time Series of Sentinel-1 SAR. ISPRS J. Photogramm. Remote Sens. 2021, 173, 122–134. [Google Scholar] [CrossRef]
- Lou, P.; Fu, B.; He, H.; Li, Y.; Tang, T.; Lin, X.; Fan, D.; Gao, E. An Optimized Object-Based Random Forest Algorithm for Marsh Vegetation Mapping Using High-Spatial-Resolution GF-1 and ZY-3 Data. Remote Sens. 2020, 12, 1270. [Google Scholar] [CrossRef]
- Millard, K.; Redden, A.M.; Webster, T.; Stewart, H. Use of GIS and High-Resolution LiDAR in Salt Marsh Restoration Site Suitability Assessments in the Upper Bay of Fundy, Canada. Wetl. Ecol. Manag. 2013, 21, 243–262. [Google Scholar] [CrossRef]
- Peterson, P.M.; Romaschenko, K.; Arrieta, Y.H.; Saarela, J.M. A Molecular Phylogeny and New Subgeneric Classification of Sporobolus (Poaceae: Chloridoideae: Sporobolinae). Taxon 2014, 63, 1212–1243. [Google Scholar] [CrossRef]
- Bortolus, A.; Adam, P.; Adams, J.B.; Ainouche, M.L.; Ayres, D.; Bertness, M.D. Supporting Spartina: Interdisciplinary Perspective Shows Spartina as a Distinct Solid Genus. Ecology 2019, 100, e02863. [Google Scholar] [CrossRef] [PubMed]
- Norris, G.S.; Virgin, S.D.S.; Schneider, D.W.; McCoy, E.M.; Wilson, J.M.; Morrill, K.L.; Hayter, L.; Hicks, M.E.; Barbeau, M.A. Patch-Level Processes of Vegetation Underlying Site-Level Restoration Patterns in a Megatidal Salt Marsh. Front. Ecol. Evol. 2022, 10, 1000075. [Google Scholar] [CrossRef]
- Sentinel-2 ESA. Sentinel-2 Mission. European Space Agency (ESA). 2022. Available online: https://sentinel.esa.int/web/sentinel/missions/sentinel-2 (accessed on 25 July 2022).
- Forkuor, G.; Dimobe, K.; Serme, I.; Tondoh, J.E. Landsat-8 vs. Sentinel-2: Examining the Added Value of Sentinel-2′s Red-Edge Bands to Land-Use and Land-Cover Mapping in Burkina Faso. GISci. Remote Sens. 2018, 55, 331–354. [Google Scholar] [CrossRef]
- Cui, Z.; Kerekes, J.P. Potential of Red Edge Spectral Bands in Future Landsat Satellites on Agroecosystem Canopy Green Leaf Area Index Retrieval. Remote Sens. 2018, 10, 1458. [Google Scholar] [CrossRef]
- Claverie, M. Evaluation of Surface Reflectance Bandpass Adjustment Techniques. ISPRS J. Photogramm. Remote Sens. 2023, 198, 210–222. [Google Scholar] [CrossRef]
- Norris, G.S.; LaRocque, A.; Leblon, B.; Barbeau, M.A.; Hanson, A. Comparing Pixel- and Object-Based Approaches for Classifying Multispectral Drone Imagery of a Salt Marsh Restoration and Reference Site. Remote Sens. 2024, 16, 1049. [Google Scholar] [CrossRef]
- SNAP. ESA Sentinel Application Platform, Version 8.0. 2022. Available online: https://step.esa.int/main/download/snap-download/ (accessed on 23 June 2022).
- S1TBX. ESA Sentinel-2 Toolbox, Version 8.0.7. 2022. Available online: https://step.esa.int/main/download/snap-download/ (accessed on 23 June 2022).
- Pignatale, F.C. Sen2Cor Configuration and User Manual, Ref. S2-PDGS-MPC-L2A-SUM-V2.10 Issue 1. 2021. Available online: https://step.esa.int/thirdparties/sen2cor/2.10.0/docs/S2-PDGS-MPC-L2A-SUM-V2.10.0.pdf (accessed on 22 April 2022).
- Sripada, R.P.; Heiniger, R.W.; White, J.G.; Meijer, A.D. Aerial Color Infrared Photography for Determining Early In-Season Nitrogen Requirements in Corn. Agron. J. 2006, 98, 968–977. [Google Scholar] [CrossRef]
- Birth, G.S.; McVey, G.R. Measuring the Color of Growing Turf with a Reflectance Spectrophotometer. Agron. J. 1968, 60, 640–643. [Google Scholar] [CrossRef]
- Pôças, I.; Calera, A.; Campos, I.; Cunha, M. Remote Sensing for Estimating and Mapping Single and Basal Crop Coefficients: A Review on Spectral Vegetation Indices Approaches. Agric. Water Manag. 2020, 233, 106081. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and Photographic Infrared Linear Combinations for Monitoring Vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Buschmann, C.; Nagel, E. In Vivo Spectroscopy and Internal Optics of Leaves as Basis for Remote Sensing of Vegetation. Int. J. Remote Sens. 1993, 14, 711–722. [Google Scholar] [CrossRef]
- Villa, P.; Mousivand, A.; Bresciani, M. Aquatic Vegetation Indices Assessment through Radiative Transfer Modeling and Linear Mixture Simulation. Int. J. Appl. Earth Obs. Geoinf. 2014, 30, 113–127. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. In Proceedings of the Third Earth Resources Technology Satellite-1 Symposium, Washington, DC, USA, 10–14 December 1973; NASA SP-351; NASA: Washington, DC, USA, 1974; Volume 1, pp. 309–317. [Google Scholar]
- Cao, Q.; Miao, Y.; Shen, J.; Yu, W.; Yuan, F.; Cheng, S.; Hang, S.; Wang, H.; Yang, W.; Liu, F. Improving In-Season Estimation of Rice Yield Potential and Responsiveness to Topdressing Nitrogen Application with Crop Circle Active Crop Canopy Sensor. Precis. Agric. 2016, 17, 136–154. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. randomForest: Breiman and Cutler’s Random Forests for Classification and Regression. Version 4.6-10. 2019. Available online: https://rdrr.io/rforge/randomForest/ (accessed on 25 March 2020).
- Horning, N. Random Forests: An Algorithm for Image Classification and Generation of Continuous Fields Data Sets. In Proceedings of the International Conference on Geoinformatics for Spatial Infrastructure Development in Earth and Allied Sciences, Osaka, Japan, 9 December 2010; p. 6. [Google Scholar]
- Strobl, C.; Boulesteix, A.L.; Kneib, T.; Augustin, T.; Zeileis, A. Conditional Variable Importance for Random Forests. BMC Bioinform 2008, 9, 307. [Google Scholar] [CrossRef]
- Louppe, G.; Wehenkel, L.; Sutera, A.; Geurts, P. Understanding Variable Importances in Forests of Randomized Trees. Adv. Neural Inf. Process. Syst. 2013, 26, 431–439. [Google Scholar]
- Congalton, R.G. A Review of Assessing the Accuracy of Classifications of Remotely Sensed Data. Remote Sens. Environ. 1991, 37, 35–46. [Google Scholar] [CrossRef]
- Wang, R.; Gamon, J.A.; Montgomery, R.A.; Townsend, P.A.; Zygielbaum, A.I.; Bitan, K.; Tilman, D.; Cavender-Bares, J. Seasonal Variation in the NDVI–Species Richness Relationship in a Prairie Grassland Experiment (Cedar Creek). Remote Sens. 2016, 8, 128. [Google Scholar] [CrossRef]
- He, W.; Ju, W.; Jiang, F.; Parazoo, N.; Gentine, P.; Wu, X.; Zhang, C.; Zhu, J.; Viovy, N.; Jain, A.K.; et al. Peak Growing Season Patterns and Climate Extremes-Driven Responses of Gross Primary Production Estimated by Satellite and Process-Based Models over North America. Agric. For. Meteorol. 2021, 298–299, 108292. [Google Scholar] [CrossRef]
- Duan, H.; Qi, Y.; Kang, W.; Zhang, J.; Wang, H.; Jiang, X. Seasonal Variation of Vegetation and Its Spatiotemporal Response to Climatic Factors in the Qilian Mountains, China. Sustainability 2022, 14, 4926. [Google Scholar] [CrossRef]
- Blaschke, T. Object-Based Image Analysis for Remote Sensing. ISPRS J. Photogramm. Remote Sens. 2010, 65, 2–16. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, D.; Yu, L.; Wang, X.; Chen, Y.; Bai, Y.; Hernández, H.J.; Galleguillos, M.; Estades, C.; Biging, G.S.; et al. Detailed Dynamic Land Cover Mapping of Chile: Accuracy Improvement by Integrating Multi-Temporal Data. Remote Sens. Environ. 2016, 183, 254–271. [Google Scholar] [CrossRef]
- Henits, L.; Jürgens, C.; Mucsi, L. Seasonal Multitemporal Land-Cover Classification and Change Detection Analysis of Bochum, Germany, Using Multitemporal Landsat TM Data. Int. J. Remote Sens. 2016, 37, 3439–3454. [Google Scholar] [CrossRef]
- Li, N.; Lu, D.; Wu, M.; Zhang, Y.; Lu, L. Coastal Wetland Classification with Multiseasonal High-Spatial Resolution Satellite Imagery. Int. J. Remote Sens. 2018, 39, 8963–8983. [Google Scholar] [CrossRef]
- Leblon, B.; LaRocque, A.; Gallant, E.; Clyne, K.; Douglas, A. Eelgrass Bed Mapping with Multispectral UAV Imagery in Atlantic Canada. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2022, 43, 649–656. [Google Scholar] [CrossRef]
- Bärlocher, F.; Moulton, V.D. Spartina alterniflora in Two New Brunswick Salt Marshes. I. Growth and Decomposition. Bull. Mar. Sci. 1999, 64, 299–309. Available online: https://www.ingentaconnect.com/content/umrsmas/bullmar/1999/00000064/00000002/art00010 (accessed on 20 November 2024).
- Vicaire, L.; Stack Mills, A.M.E.; Barbeau, M.A. Developing Knowledge for Salt Marsh Restoration and Salt Marsh Creation in New Brunswick Using Spartina alterniflora Seedlings. New Brunswick Environmental Trust Fund Final Report. Department of Biology, University of New Brunswick: Fredericton, NB, Canada, 2022; 69p, (Unpublished report). [Google Scholar]
- Anderson, C.M.; Treshow, M. A Review of Environmental and Genetic Factors That Affect Height in Spartina alterniflora Loisel. (Salt Marsh Cord Grass). Estuaries 1980, 3, 168. [Google Scholar] [CrossRef]
- van Proosdij, D.; Ross, C.; Matheson, G. Nova Scotia Dyke Vulnerability Assessment; Nova Scotia Federation of Agriculture: Truro, NS, Canada, 2018; 35p, Available online: https://nsfa-fane.ca/wp-content/uploads/2018/08/Nova-Scotia-Dyke-Vulnerability-Assessment.pdf (accessed on 15 September 2023).
| Band | Band Name | Wavelength (nm) | Spatial Resolution (m) | |
|---|---|---|---|---|
| Sentinel-2A | Sentinel-2b | |||
| B1 | Coastal | 433–453 | 442–452 | 60 |
| B2 | Blue | 460–525 | 460–525 | 10 |
| B3 | Green | 542–577 | 526–591 | 10 |
| B4 | Red | 650–680 | 649–680 | 10 |
| B5 | Red-Edge 1 | 697–711 | 696–711 | 20 |
| B6 | Red-Edge 2 | 734–748 | 733–746 | 20 |
| B7 | Red-Edge 3 | 773–792 | 770–789 | 20 |
| B8 | NIR | 780–885 | 781–885 | 10 |
| B8a | Narrow NIR (NIRn) | 854–875 | 854–875 | 20 |
| B9 | Water Vapor | 926–964 | 923–963 | 60 |
| B11 | SWIR1 | 1569–1659 | 1563–1657 | 20 |
| B12 | SWIR2 | 2115–2289 | 2094–2278 | 20 |
| Index | Index Name | Formula | Reference |
|---|---|---|---|
| DVI | Difference vegetation index | NIR - R | [66] |
| GDVI | Green difference vegetation index | NIR - G | [63] |
| GNDVI | Green normalized difference vegetation index | (NIR - G) / (NIR + G) | [67] |
| GRVI | Green ratio vegetation index | NIR / G | [63] |
| NDAVI | Normalized difference aquatic vegetation index | (NIR - B) / (NIR + B) | [68] |
| NDVI | Normalized difference vegetation index | (NIR - R) / (NIR + R) | [69] |
| NDRE | Normalized difference red-edge vegetation index | (NIR - RE) / NIR + RE) | [70] |
| NG | Normalized green vegetation index | G / (NIR + R + G) | [63] |
| NNIR | Normalized near-infrared vegetation index | NIR / (NIR + R + G) | [63] |
| NR | Normalized red vegetation index | R / (NIR + R + G) | [63] |
| RVI | Red ratio vegetation index | NIR / R | [64] |
| REVI | Red-edge simple ratio vegetation index | NIR / RE | [70] |
| WAVI | Water-adjusted vegetation index | 1.5 × (NIR - B) / (NIR + B + 0.5) | [68] |
| Class Number | Name | Description |
|---|---|---|
| 1 | Bare mud | Areas with no vegetation to minimal vegetation (<5%), covered in mud, such as the tidal flats. |
| 2 | Water | Wet areas visible throughout the year in salt pools (specifically in the reference marshes). |
| 3 | Sparse S. alterniflora | Monoculture of saltwater cordgrass Spartina alterniflora occurring in the restoration sites, with sparse foliage and visible ground mud on imagery. |
| 4 | Dense S. alterniflora | Monoculture of S. alterniflora occurring in the restoration sites, with denser foliage with no vegetation to minimal mud visibility on imagery. |
| 5 | S. patens dominant | Community of saltmarsh vegetation dominated by saltmarsh hay Spartina patens that typically grow at higher elevation. Other species include sea lavender (Limonium carolinianum), sea plantain (Plantago maritima), orach (Atriplex spp.), and seaside goldenrod (Solidago sempervirens). |
| 6 | S. patens−J. gerardii mix | A mix of S. patens and black rush Juncus gerardii at about a 50/50 ratio. |
| 7 | S. alterniflora−S. patens mix | A mix of S. alterniflora and S. patens occurring at about a 50/50 ratio. |
| 8 | J. gerardii−C. paleacea mix | A mix of J. gerardii and scaly sedge Carex paleacea occurring at about a 50/50 ratio. |
| 9 | C. paleacea− S. pectinata mix | A mix of C. paleacea and freshwater cordgrass Spartina pectinata occurring at about a 50/50 ratio. |
| 10 | Terrestrial vegetation on dike | Saline-intolerant terrestrial vegetation growing over and along the dike. |
| 11 | Dike | Non-vegetated path made of soil and gravel that separates the farmland from the saltmarshes. |
| 12 | Vegetated water | Water from salt pools of the reference sites that have algae and other sub-surface aquatic vegetation. |
| 13 | S. alterniflora dominant | Areas in the reference sites that are high in moisture content due to proximity to salt pools, creeks, and similar depressions where S. alterniflora grows. |
| Class Number | Landcover Classes | Training | Validation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2021 | 2022 | 2019 | 2020 | 2021 | 2022 | ||
| 1 | Bare mud | 94 | 48 | 85 | 81 | 37 | 35 | 34 | 18 |
| 2 | Water | 22 | 26 | 28 | 22 | 21 | 19 | 15 | 7 |
| 3 | Sparse S. alterniflora | 49 | 46 | 63 | 36 | 19 | 25 | 24 | 26 |
| 4 | Dense S. alterniflora | 47 | 49 | 54 | 53 | 19 | 15 | 30 | 18 |
| 5 | S. patens dominant | 65 | 67 | 67 | 68 | 35 | 29 | 48 | 28 |
| 6 | S. patens−J. gerardii mix | 23 | 18 | 28 | 23 | 12 | 22 | 16 | 21 |
| 7 | S. alterniflora−S. patens mix | 42 | 32 | 45 | 45 | 13 | 22 | 30 | 29 |
| 8 | J. gerardii−C. paleacea mix | 37 | 33 | 33 | 36 | 18 | 9 | 14 | 12 |
| 9 | C. paleacea−S. pectinata mix | 16 | 27 | 26 | 25 | 10 | 17 | 14 | 15 |
| 10 | Terrestrial vegetation on dike | 59 | 45 | 39 | 45 | 33 | 22 | 15 | 11 |
| 11 | Dike | 67 | 54 | 55 | 55 | 24 | 30 | 24 | 13 |
| 12 | Vegetated water | 45 | 46 | 36 | 48 | 21 | 24 | 20 | 15 |
| 13 | S. alterniflora dominant | 39 | 33 | 29 | 30 | 39 | 32 | 17 | 11 |
| TOTAL | 605 | 524 | 588 | 567 | 301 | 301 | 301 | 224 | |
| Year | Date | Mean | Minimum | Class Pair with the Minimum J-M Distance |
|---|---|---|---|---|
| 2019 | 2019/06/16 | 1.9925 | 1.8688 | 3 vs. 4 |
| 2019/07/18 | 1.9920 | 1.9018 | 5 vs. 7 | |
| 2019/08/30 | 1.9921 | 1.8113 | 5 vs. 7 | |
| 2019/09/19 | 1.9949 | 1.8477 | 5 vs. 7 | |
| 2019/10/21 | 1.9860 | 1.6745 | 5 vs. 7 | |
| 2020 | 2020/05/11 | 1.9948 | 1.8643 | 5 vs. 7 |
| 2020/06/17 | 1.9894 | 1.6788 | 5 vs. 7 | |
| 2020/07/22 | 1.9931 | 1.7982 | 5 vs. 7 | |
| 2020/08/19 | 1.9955 | 1.8733 | 5 vs. 7 | |
| 2020/09/25 | 1.9986 | 1.9742 | 5 vs. 7 | |
| 2021 | 2021/05/03 | 1.9850 | 1.4413 | 3 vs. 4 |
| 2021/06/07 | 1.9759 | 1.6058 | 5 vs. 7 | |
| 2021/07/25 | 1.9944 | 1.8890 | 8 vs. 13 | |
| 2021/08/14 | 1.9838 | 1.4567 | 3 vs. 4 | |
| 2021/09/13 | 1.9851 | 1.5879 | 3 vs. 4 | |
| 2022 | 2022/05/03 | 1.9902 | 1.8326 | 3 vs. 4 |
| 2022/06/15 | 1.9903 | 1.8583 | 5 vs. 7 | |
| 2022/07/10 | 1.9955 | 1.9005 | 5 vs. 7 | |
| 2022/08/21 | 1.9903 | 1.8335 | 8 vs. 9 | |
| 2022/09/10 | 1.9882 | 1.8116 | 5 vs. 7 |
| Class Number | Landcover Classes | 2019 | 2020 | 2021 | 2022 | ||||
|---|---|---|---|---|---|---|---|---|---|
| UA | PA | UA | PA | UA | PA | UA | PA | ||
| 1 | Bare mud | 98.9 | 98.9 | 95.8 | 95.8 | 100.0 | 98.8 | 97.6 | 98.8 |
| 2 | Water | 95.5 | 95.5 | 79.2 | 73.1 | 100.0 | 100.0 | 95.5 | 95.5 |
| 3 | Sparse S. alterniflora | 100.0 | 98.0 | 97.7 | 93.5 | 96.9 | 98.4 | 97.2 | 97.2 |
| 4 | Dense S. alterniflora | 97.9 | 100.0 | 92.3 | 98.0 | 98.1 | 94.4 | 98.1 | 96.2 |
| 5 | S. patens dominant | 100.0 | 92.3 | 96.7 | 86.6 | 98.5 | 100.0 | 97.1 | 97.1 |
| 6 | S. patens−J. gerardii mix | 83.3 | 87.0 | 90.0 | 100.0 | 92.9 | 92.9 | 95.0 | 82.6 |
| 7 | S. alterniflora−S. patens mix | 81.8 | 85.7 | 77.8 | 87.5 | 97.5 | 86.7 | 90.9 | 88.9 |
| 8 | J. gerardii−C. paleacea mix | 94.4 | 91.9 | 93.8 | 90.9 | 90.6 | 87.9 | 91.7 | 91.7 |
| 9 | C. paleacea−S. pectinata mix | 80.0 | 100.0 | 92.9 | 96.3 | 89.3 | 96.2 | 85.7 | 96.0 |
| 10 | Terrestrial vegetation on dike | 98.2 | 93.2 | 95.5 | 93.3 | 95.0 | 97.4 | 93.2 | 91.1 |
| 11 | Dike | 100.0 | 100.0 | 94.6 | 98.1 | 98.2 | 100.0 | 98.2 | 100.0 |
| 12 | Vegetated water | 95.7 | 97.8 | 87.5 | 91.3 | 100.0 | 100.0 | 97.9 | 95.8 |
| 13 | S. alterniflora dominant | 92.5 | 94.9 | 96.9 | 93.9 | 84.4 | 93.1 | 93.8 | 100.0 |
| Average accuracy | 93.71 | 91.58 | 95.49 | 94.74 | |||||
| Overall accuracy | 95.54 | 92.37 | 96.43 | 95.41 | |||||
| Rank | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|
| 1 | SWIR-1_July | Blue_Sept | SWIR-1_July | Water vapour_June |
| 2 | SWIR-2_July | NR_Sept | SWIR-2_July | NNIR_July |
| 3 | Coastal_Aug | NNIR_Sept | NDAVI_July_ | SWIR-1_May |
| 4 | NR_Aug | Green_Sept | NNIR_July | Green_Sept |
| 5 | Water vapour_July | NDVI_Sept | NR_july | SWIR-2_May |
| 6 | Red-Edge 2_June | SWIR-2_Aug | NDVI_July | Coastal_Aug |
| 7 | Water vapour_Aug | SWIR-2_June | GRVI_July | Red_July |
| 8 | GDVI_June | SWIR-1_Aug | DVI_May | Water vapour_Aug |
| 9 | Red-Edge 1_July | RVI_Sept | RVI_July | Coastal_May |
| 10 | Coastal_June | SWIR-2_Sept | GNDVI_July | Blue_Sept |
| 11 | SWIR-1_Oct | Red_Aug | GDVI_July_ | SWIR-2_Aug |
| 12 | RVI_Aug | NDAVI_Sept | Coastal_Sept | SWIR-1_July |
| 13 | NNIR_Aug | Red-Edge 1_June | Red-Edge 3_May | NR_Aug |
| 14 | NIR_Narrow_Oct | Coastal_Aug | Red_July | Coastal_June |
| 15 | Red-Edge 1_Sept | Red-Edge 1_Aug | Red-Edge 1_July | Blue_July |
| 16 | Red-Edge 3_June | Red-Edge 2_June | Water vapour_July | NR_Sept |
| 17 | DVI_June | Green_Aug | Red-Edge 1_Sept | SWIR-2_July |
| 18 | Blue_July | Coastal_May | Red-Edge 3_June | NDRE_July |
| 19 | REVI_Sept | GRVI_July | Red-Edge 1_May | Red-Edge 1_Aug |
| 20 | NR_July | Coastal_July | Coastal_june | NNIR_July |
| 21 | SWIR-2_Sept | Blue_Aug | Coastal_July | Blue_Aug |
| 22 | NIR_Narrow_June | GNDVI_July | SWIR-2_Aug | SWIR-1_Sept |
| 23 | Coastal_July | Coastal_Sept | NG-July | Red-Edge 3_June |
| 24 | NIR_June | NR_Aug | SWIR-1_June | Red_Aug |
| 25 | NG_July | Water vapour_Sept | Water vapour_May | Red_Sept |
| Class Number | Landcover Classes | 2019 | 2020 | 2021 | 2022 | ||||
|---|---|---|---|---|---|---|---|---|---|
| UA | PA | UA | PA | UA | PA | UA | PA | ||
| 1 | Bare mud | 92.5 | 100.0 | 92.1 | 100.0 | 94.4 | 100.0 | 100.0 | 94.4 |
| 2 | Water | 95.5 | 100.0 | 94.4 | 89.5 | 100.0 | 93.3 | 87.5 | 100.0 |
| 3 | Sparse S. alterniflora | 100.0 | 94.7 | 100.0 | 80.0 | 95.5 | 87.5 | 95.5 | 80.8 |
| 4 | Dense S. alterniflora | 100.0 | 89.5 | 87.5 | 93.3 | 93.8 | 100.0 | 83.3 | 83.3 |
| 5 | S. patens dominant | 87.2 | 97.1 | 96.2 | 86.2 | 91.5 | 89.6 | 90.3 | 100.0 |
| 6 | S. patens−J. gerardii mix | 100.0 | 58.3 | 95.5 | 95.5 | 75.0 | 93.8 | 100.0 | 76.2 |
| 7 | S. alterniflora−S. patens mix | 76.5 | 100.0 | 94.7 | 81.8 | 87.5 | 70.0 | 76.5 | 89.7 |
| 8 | J. gerardii−C. paleacea mix | 85.7 | 100.0 | 100.0 | 100.0 | 100.0 | 85.7 | 73.3 | 91.7 |
| 9 | C. paleacea−S. pectinata mix | 88.9 | 80.0 | 77.3 | 100.0 | 87.5 | 100.0 | 93.8 | 100.0 |
| 10 | Dike terrestrial vegetation | 100.0 | 90.9 | 100.0 | 77.3 | 100.0 | 100.0 | 100.0 | 90.9 |
| 11 | Dike | 100.0 | 91.7 | 93.8 | 100.0 | 100.0 | 100.0 | 92.9 | 100.0 |
| 12 | Vegetated water | 95.0 | 90.5 | 95.8 | 95.8 | 100.0 | 95.0 | 100.0 | 80.0 |
| 13 | S. alterniflora dominant | 92.3 | 91.7 | 84.2 | 100.0 | 80.0 | 94.1 | 90.9 | 90.9 |
| Average accuracy | 93.35 | 93.19 | 92.70 | 91.07 | |||||
| Overall accuracy | 93.02 | 92.36 | 92.36 | 89.73 | |||||
| Class Number | Site A | Site D | ||||||
|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2021 | 2022 | 2019 | 2020 | 2021 | 2022 | |
| 1 | 8.39 | 14.98 | 9.59 | 15.44 | 2.56 | 6.71 | 4.33 | 4.18 |
| 2 | 7.92 | 3.55 | 5.55 | 0.00 | 3.28 | 6.31 | 3.27 | 2.84 |
| 3 | 0.00 | 0.00 | 2.48 | 0.00 | 0.00 | 0.03 | 1.36 | 0.64 |
| 4 | 1.18 | 0.27 | 5.01 | 3.06 | 0.24 | 0.14 | 0.03 | 0.78 |
| 5 | 35.91 | 20.90 | 44.26 | 29.93 | 27.94 | 29.00 | 33.62 | 36.51 |
| 6 | 0.00 | 0.43 | 0.65 | 0.00 | 5.23 | 9.86 | 7.95 | 6.80 |
| 7 | 0.75 | 19.39 | 12.28 | 28.71 | 19.12 | 10.04 | 16.48 | 6.83 |
| 8 | 0.00 | 0.00 | 0.00 | 0.00 | 9.91 | 5.14 | 6.34 | 10.51 |
| 9 | 0.00 | 0.00 | 0.00 | 0.00 | 4.60 | 8.99 | 6.26 | 7.83 |
| 10 | 6.22 | 7.19 | 4.15 | 6.76 | 6.36 | 3.33 | 3.02 | 3.56 |
| 11 | 0.00 | 0.27 | 0.26 | 0.54 | 0.25 | 0.11 | 0.01 | 0.25 |
| 12 | 15.35 | 18.80 | 12.82 | 15.24 | 4.01 | 1.61 | 2.49 | 3.52 |
| 13 | 24.29 | 14.22 | 2.96 | 0.32 | 16.48 | 18.73 | 14.83 | 15.75 |
| TOTAL | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Class Number | Site B | Site C | ||||||
|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2021 | 2022 | 2019 | 2020 | 2021 | 2022 | |
| 1 | 18.72 | 18.82 | 17.63 | 18.59 | 16.55 | 19.23 | 18.20 | 15.16 |
| 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 3 | 40.58 | 35.58 | 30.01 | 33.07 | 11.44 | 17.75 | 30.78 | 9.78 |
| 4 | 18.26 | 26.45 | 37.26 | 24.66 | 43.07 | 29.81 | 26.60 | 41.17 |
| 5 | 0.87 | 0.00 | 3.40 | 0.33 | 0.00 | 0.00 | 1.50 | 0.00 |
| 6 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 7 | 0.00 | 0.00 | 0.00 | 4.33 | 0.00 | 0.00 | 0.00 | 4.75 |
| 8 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 9 | 0.00 | 0.78 | 0.00 | 0.00 | 0.00 | 0.00 | 0.18 | 0.00 |
| 10 | 11.9 | 5.04 | 5.51 | 8.74 | 6.13 | 0.43 | 0.31 | 0.72 |
| 11 | 6.38 | 13.34 | 6.18 | 7.77 | 22.68 | 32.78 | 22.43 | 28.43 |
| 12 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 13 | 3.22 | 0.00 | 0.00 | 2.51 | 0.14 | 0.00 | 0.00 | 0.00 |
| TOTAL | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naojee, S.M.; LaRocque, A.; Leblon, B.; Norris, G.S.; Barbeau, M.A.; Rowland, M. Monitoring Saltmarsh Restoration in the Upper Bay of Fundy Using Multi-Temporal Sentinel-2 Imagery and Random Forests Classifier. Remote Sens. 2024, 16, 4667. https://doi.org/10.3390/rs16244667
Naojee SM, LaRocque A, Leblon B, Norris GS, Barbeau MA, Rowland M. Monitoring Saltmarsh Restoration in the Upper Bay of Fundy Using Multi-Temporal Sentinel-2 Imagery and Random Forests Classifier. Remote Sensing. 2024; 16(24):4667. https://doi.org/10.3390/rs16244667
Chicago/Turabian StyleNaojee, Swarna M., Armand LaRocque, Brigitte Leblon, Gregory S. Norris, Myriam A. Barbeau, and Matthew Rowland. 2024. "Monitoring Saltmarsh Restoration in the Upper Bay of Fundy Using Multi-Temporal Sentinel-2 Imagery and Random Forests Classifier" Remote Sensing 16, no. 24: 4667. https://doi.org/10.3390/rs16244667
APA StyleNaojee, S. M., LaRocque, A., Leblon, B., Norris, G. S., Barbeau, M. A., & Rowland, M. (2024). Monitoring Saltmarsh Restoration in the Upper Bay of Fundy Using Multi-Temporal Sentinel-2 Imagery and Random Forests Classifier. Remote Sensing, 16(24), 4667. https://doi.org/10.3390/rs16244667








