Assessing Golden Tides from Space: Meteorological Drivers in the Accumulation of the Invasive Algae Rugulopteryx okamurae on Coasts
Abstract
1. Introduction
2. Materials and Methods
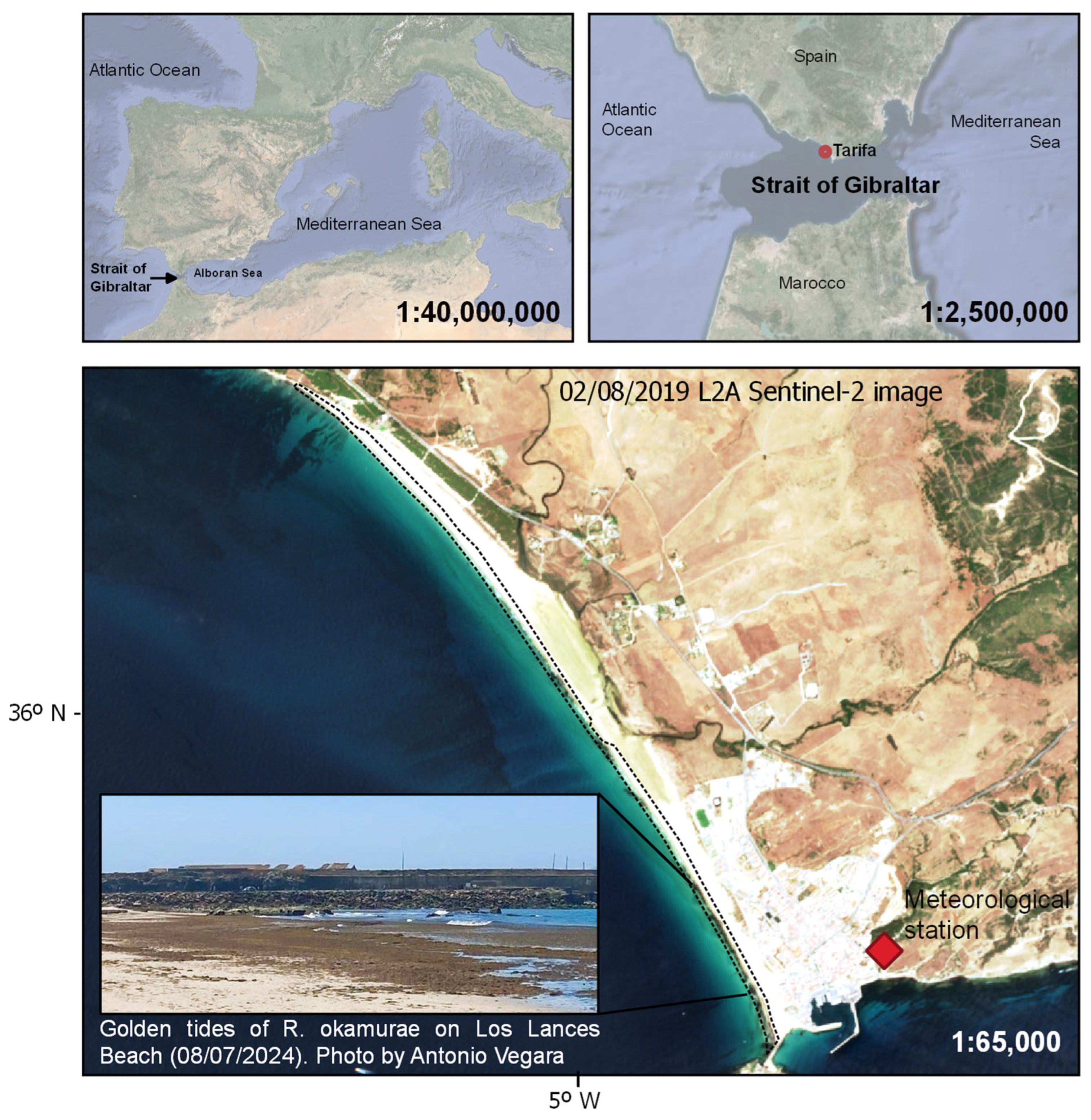
2.1. Study Site
2.2. Sentinel-2 Data Processing
2.3. Temporal and Spatial Dynamics
2.4. Statistical Analysis
3. Results
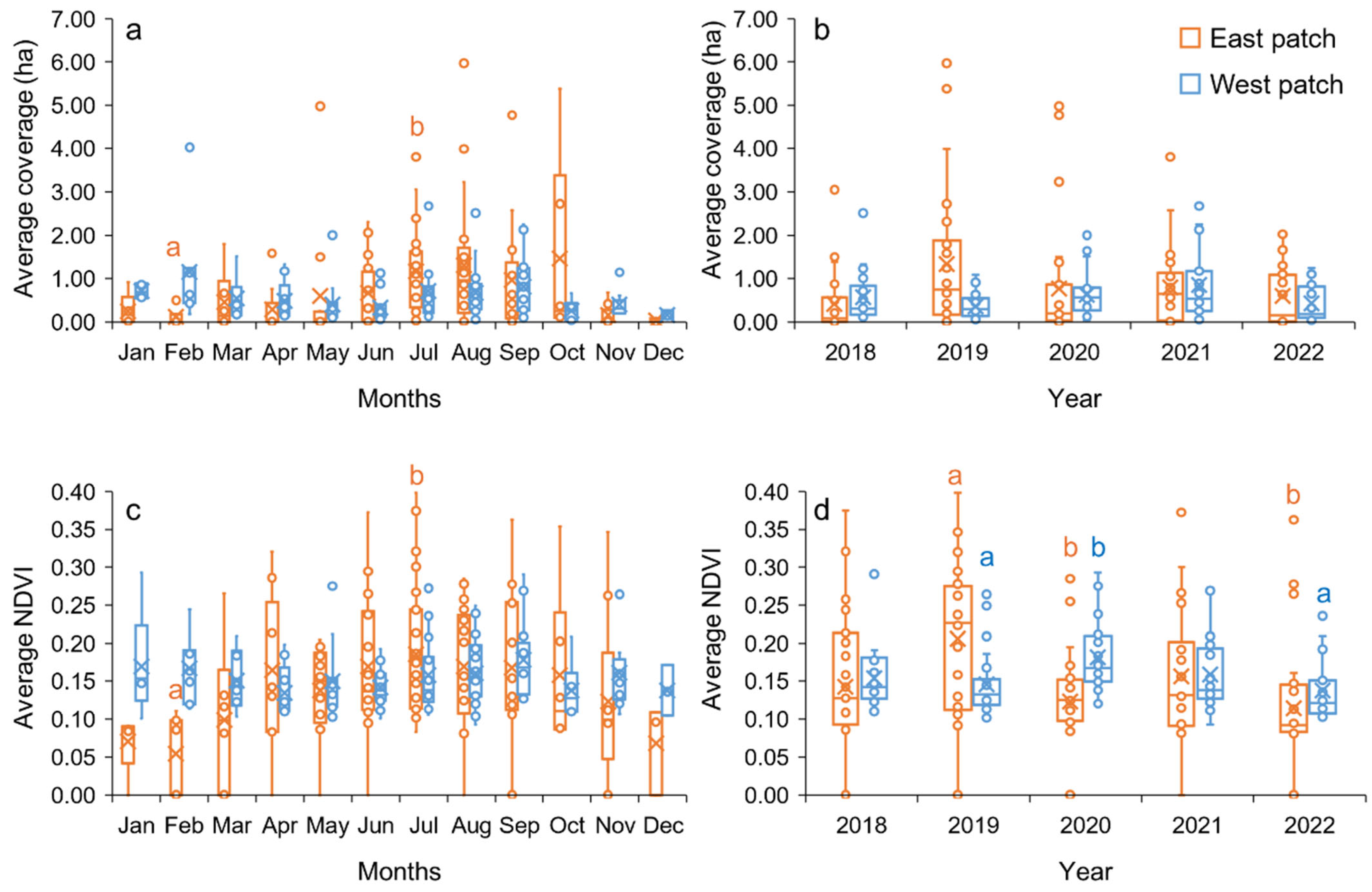
3.1. Monitoring Spatiotemporal Dynamics
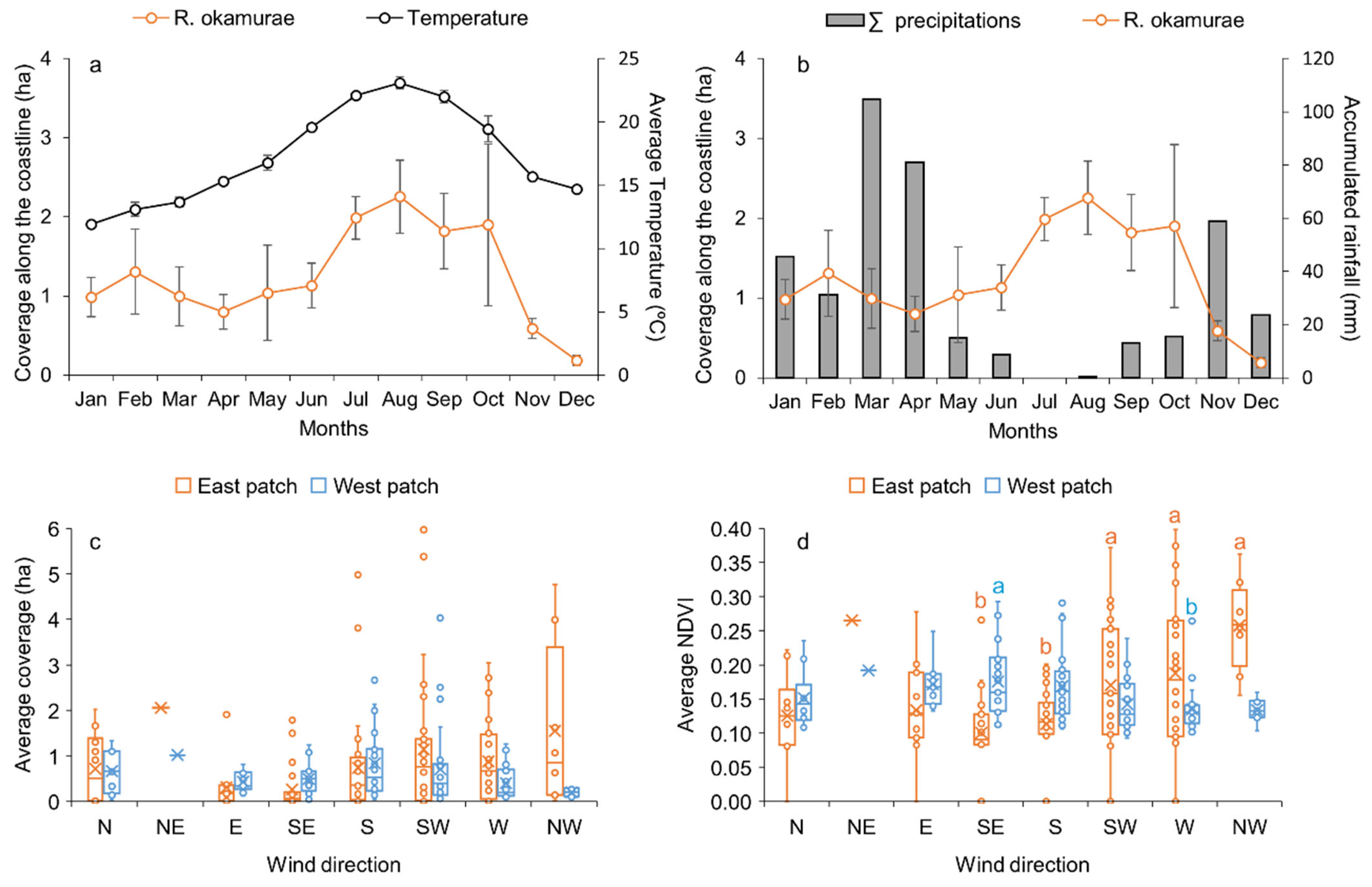
3.2. Spatiotemporal Distribution and Meteorological Variables
4. Discussion
4.1. Impact of Meteorological Conditions on R. okamurae Accumulation
4.2. Strategies for Enhanced Management and Removal of R. okamurae Strandings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smetacek, V.; Zingone, A. Green and Golden Seaweed Tides on the Rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, C. Mapping and Quantifying Sargassum Distribution and Coverage in the Central West Atlantic Using MODIS Observations. Remote Sens. Environ. 2016, 183, 350–367. [Google Scholar] [CrossRef]
- Jeffrey, D.W.; Madden, B.; Rafferty, B. Beach Fouling by Ectocarpus Siliculosus in Dublin Bay. Mar. Pollut. Bull. 1993, 26, 51–53. [Google Scholar] [CrossRef]
- Haro, S.; Bermejo, R.; Wilkes, R.; Bull, L.; Morrison, L. Monitoring Intertidal Golden Tides Dominated by Ectocarpus Siliculosus Using Sentinel-2 Imagery. Int. J. Appl. Earth Obs. Geoinf. 2023, 122, 103451. [Google Scholar] [CrossRef]
- Zárate, R.; Portillo, E.; Teixidó, S.; de Carvalho, M.A.A.P.; Nunes, N.; Ferraz, S.; Seca, A.M.L.; Rosa, G.P.; Barreto, M.C. Pharmacological and Cosmeceutical Potential of Seaweed Beach-Casts of Macaronesia. Appl. Sci. 2020, 10, 5831. [Google Scholar] [CrossRef]
- García-Gómez, J.C.; Sempere-Valverde, J.; González, A.R.; Martínez-Chacón, M.; Olaya-Ponzone, L.; Sánchez-Moyano, E.; Ostalé-Valriberas, E.; Megina, C. From Exotic to Invasive in Record Time: The Extreme Impact of Rugulopteryx okamurae (Dictyotales, Ochrophyta) in the Strait of Gibraltar. Sci. Total Environ. 2020, 704, 135408. [Google Scholar] [CrossRef] [PubMed]
- Schaffelke, B.; Hewitt, C.L. Impacts of Introduced Seaweeds. Bot. Mar. 2007, 50, 397–417. [Google Scholar] [CrossRef]
- Bermejo, R.; Green-Gavrielidis, L.; Gao, G. Editorial: Macroalgal Blooms in a Global Change Context. Front. Mar. Sci. 2023, 10, 1204117. [Google Scholar] [CrossRef]
- Valiela, I.; McClelland, J.; Hauxwell, J.; Behr, P.J.; Hersh, D.; Foreman, K. Macroalgal Blooms in Shallow Estuaries: Controls and Ecophysiological and Ecosystem Consequences. Limnol. Oceanogr. 1997, 42, 1105–1118. [Google Scholar] [CrossRef]
- Bermejo, R.; MacMonagail, M.; Heesch, S.; Mendes, A.; Edwards, M.; Fenton, O.; Knöller, K.; Daly, E.; Morrison, L. The Arrival of a Red Invasive Seaweed to a Nutrient Over-Enriched Estuary Increases the Spatial Extent of Macroalgal Blooms. Mar. Environ. Res. 2020, 158, 104944. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, G.; Uchimura, M.; Hiraoka, M. Persistent Occurrence of Floating Ulva Green Tide in Hiroshima Bay, Japan: Seasonal Succession and Growth Patterns of Ulva Pertusa and Ulva spp. (Chlorophyta, Ulvales). Hydrobiologia 2015, 758, 223–233. [Google Scholar] [CrossRef]
- Anderson, L.W.J. Control of Invasive Seaweeds. Bot. Mar. 2007, 50, 418–437. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Ramus, A.P.; Long, Z.T.; Silliman, B.R. A Seaweed Increases Ecosystem Multifunctionality When Invading Bare Mudflats. Biol. Invasions 2019, 21, 27–36. [Google Scholar] [CrossRef]
- Critchley, A.T.; Farnham, W.F.; Morrell, S.L. An Account of the Attempted Control of an Introduced Marine Alga, Sargassum Muticum, in Southern England. Biol. Conserv. 1986, 35, 313–332. [Google Scholar] [CrossRef]
- South, P.M.; Floerl, O.; Forrest, B.M.; Thomsen, M.S. A Review of Three Decades of Research on the Invasive Kelp Undaria Pinnatifida in Australasia: An Assessment of Its Success, Impacts and Status as One of the World’s Worst Invaders. Mar. Environ. Res. 2017, 131, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.W.J. California’s Reaction to Caulerpa Taxifolia: A Model for Invasive Species Rapid Response. Biol. Invasions 2005, 7, 1003–1016. [Google Scholar] [CrossRef]
- Hwang, I.-K.; Lee, W.J.; Kim, H.-S.; De Clerck, O. Taxonomic Reappraisal of Dilophus okamurae (Dictyotales, Phaeophyta) from the Western Pacific Ocean. Phycologia 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Verlaque, M.; Steen, F.; De Clerck, O. Rugulopteryx (Dictyotales, Phaeophyceae), a Genus Recently Introduced to the Mediterranean. Phycologia 2009, 48, 536–542. [Google Scholar] [CrossRef]
- Ruitton, S.; Blanfuné, A.; Boudouresque, C.-F.; Guillemain, D.; Michotey, V.; Roblet, S.; Thibault, D.; Thibaut, T.; Verlaque, M.; Rodríguez, C. Rapid Spread of the Invasive Brown Alga Rugulopteryx okamurae in a National Park in Provence (France, Mediterranean Sea). Water 2021, 13, 2306. [Google Scholar] [CrossRef]
- Altamirano, M.; De la Rosa Álamos, J.; Martínez Medina, F.J. Arribazones de La Especie Exótica Rugulopteryx okamurae (E.Y. Dawson) I.K. Hwang, W.J. Lee & H.S. Kim (Dictyotales, Ochrophyta) En El Estrecho de Gibraltar: Primera Cita Para El Atlántico y España. Algas 2016, 52, 20. [Google Scholar]
- Ocaña, Ó.; Afonso Carrillo, J.; Ballesteros, E. Massive Proliferation of a Dictyotalean Species (Phaeophyceae, Ochrophyta) through the Strait of Gibraltar (Research Note). Rev. la Acad. Canar. Ciencias 2016, 28, 165–170. [Google Scholar]
- Mateo-Ramírez, Á.; Iñiguez, C.; Fernández-Salas, L.M.; Sánchez-Leal, R.F.; Farias, C.; Bellanco, M.J.; Gil, J.; Rueda, J.L. Healthy Thalli of the Invasive Seaweed Rugulopteryx okamurae (Phaeophyceae) Being Massively Dragged into Deep-Sea Bottoms by the Mediterranean Outflow Water. Phycologia 2023, 62, 99–108. [Google Scholar] [CrossRef]
- Faria, J.; Prestes, A.C.L.; Moreu, I.; Martins, G.M.; Neto, A.I.; Cacabelos, E. Arrival and Proliferation of the Invasive Seaweed Rugulopteryx okamurae in NE Atlantic Islands. Bot. Mar. 2022, 65, 45–50. [Google Scholar] [CrossRef]
- Terradas-Fernández, M.; Pena-Martín, C.; Valverde-Urrea, M.; Gran, A.; Blanco-Murillo, F.; Leyva, L.; Abellán-Gallardo, E.; Beresaluze, E.; Izquierdo, A.; del Pilar-Ruso, Y.; et al. An Outbreak of the Invasive Macroalgae Rugulopteryx okamurae in Alicante Bay and Its Colonization on Dead Posidonia Oceanica Matte. Aquat. Bot. 2023, 189, 103706. [Google Scholar] [CrossRef]
- El Madany, M.; Hassoun, M.; El Aamri, F.; El Mtili, N. Recent Occurrence and Expansion of the Non-Indigenous Alga Rugulopteryx okamurae in Morocco (Mediterranean and Atlantic Shores). Aquat. Bot. 2024, 190, 103722. [Google Scholar] [CrossRef]
- Bellissimo, G.; Altamirano, M.; Muñoz, A.; De la Rosa, J.; Hung, T.H.; Rizzuto, G.; Vizzini, S.; Tomasello, A. The Invasive Brown Seaweed Rugulopteryx okamurae (Dictyotales, Ochrophyta) Continues to Expand: First Record in Italy. BioInvasions Rec. 2024, 13, 385–401. [Google Scholar] [CrossRef]
- El Aamri, F.; Idhalla, M.; Tamsouri, M.N. Occurrence of the Invasive Brown Seaweed Rugulopteryx okamurae (E.Y.Dawson) I.K.Hwang, W.J.Lee & H.S.Kim (Dictyotales, Phaeophyta) in Morocco (Mediterranean Sea). Mediterr. Fish. Aquac. Res. 2018, 1, 92–96. [Google Scholar]
- Mercado, J.M.; Gómez-Jakobsen, F.; Korbee, N.; Aviles, A.; Bonomi-Barufi, J.; Muñoz, M.; Reul, A.; Figueroa, F.L. Analyzing Environmental Factors That Favor the Growth of the Invasive Brown Macroalga Rugulopteryx okamurae (Ochrophyta): The Probable Role of the Nutrient Excess. Mar. Pollut. Bull. 2022, 174, 113315. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.; Prestes, A.C.L.; Moreu, I.; Cacabelos, E.; Martins, G.M. Dramatic Changes in the Structure of Shallow-Water Marine Benthic Communities Following the Invasion by Rugulopteryx okamurae (Dictyotales, Ochrophyta) in Azores (NE Atlantic). Mar. Pollut. Bull. 2022, 175, 113358. [Google Scholar] [CrossRef] [PubMed]
- Azcárate-García, T.; Beca-Carretero, P.; Brun, F.G. Plant and Meadow Structure Characterisation of Posidonia Oceanica in Its Westernmost Distribution Range. Diversity 2023, 15, 101. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Sesmero, R.; Arijo, S. Interdisciplinar Research (Oceanography, Botany, Ecophysiology and Biotechnology) about the Invasive Exotic Species Rugulopteryx okamurae (Ochrophyta), in the Frame of the Project “BLUEMARO”. Memorias la Real Soc. Española Hist. Nat. 2023, 16, 9–29. [Google Scholar]
- Sempere-Valverde, J.; Ostalé-Valriberas, E.; Maestre, M.; González Aranda, R.; Bazairi, H.; Espinosa, F. Impacts of the Non-Indigenous Seaweed Rugulopteryx okamurae on a Mediterranean Coralligenous Community (Strait of Gibraltar): The Role of Long-Term Monitoring. Ecol. Indic. 2021, 121, 107135. [Google Scholar] [CrossRef]
- Mogollón, S.L.; Zilio, M.I.; Buitrago, E.M.; Caraballo, M.Á.; Yñiguez, R. Economic Impact of Rugulopteryx okamurae (Dictyotales, Ochrophyta) along the Andalusian Coastline: The Case of Tarifa, Spain. Wetl. Ecol. Manag. 2024, 32, 19–32. [Google Scholar] [CrossRef]
- Ayuntamiento de Tarifa. Informe Sobre Las Actuaciones Realizadas En La Playa de Los Lances Sur y Playa de Atlanterra Ante La Acumulación Masiva de Algas En El Litoral Tarifeño. Verano 2021; Ayuntamiento de Tarifa: Tarifa, Spain, 2021; pp. 1–4. [Google Scholar]
- Wan, A.H.L.; Wilkes, R.J.; Heesch, S.; Bermejo, R.; Johnson, M.P.; Morrison, L. Assessment and Characterisation of Ireland’s Green Tides (Ulva Species). PLoS ONE 2017, 12, e0169049. [Google Scholar] [CrossRef] [PubMed]
- Joniver, C.F.H.; Photiades, A.; Moore, P.J.; Winters, A.L.; Woolmer, A.; Adams, J.M.M. The Global Problem of Nuisance Macroalgal Blooms and Pathways to Its Use in the Circular Economy. Algal Res. 2021, 58, 102407. [Google Scholar] [CrossRef]
- Harb, T.B.; Vega, J.; Bonomi-Barufi, J.; Casas, V.; Abdala-Díaz, R.; Figueroa, F.L.; Chow, F. Brazilian Beach-Cast Seaweeds: Antioxidant, Photoprotection and Cytotoxicity Properties. Waste Biomass Valorization 2023, 14, 2249–2265. [Google Scholar] [CrossRef]
- Pereira, A.G.; Fraga-Corral, M.; Garcia-Oliveira, P.; Lourenço-Lopes, C.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J. The Use of Invasive Algae Species as a Source of Secondary Metabolites and Biological Activities: Spain as Case-Study. Mar. Drugs 2021, 19, 178. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Non-Indigenous Seaweeds in the Iberian Peninsula, Macaronesia Islands (Madeira, Azores, Canary Islands) and Balearic Islands: Biodiversity, Ecological Impact, Invasion Dynamics, and Potential Industrial Applications. Algal Res. 2024, 78, 103407. [Google Scholar] [CrossRef]
- Casal-Porras, I.; Zubía, E.; Brun, F.G. Dilkamural: A Novel Chemical Weapon Involved in the Invasive Capacity of the Alga Rugulopteryx okamurae in the Strait of Gibraltar. Estuar. Coast. Shelf Sci. 2021, 257, 107398. [Google Scholar] [CrossRef]
- Patón, D.; García-Gómez, J.C.; Loring, J.; Torres, A. Composting the Invasive Toxic Seaweed Rugulopteryx okamurae Using Five Invertebrate Species, and a Mini-Review on Composting Macroalgae. Waste Biomass Valorization 2023, 14, 167–184. [Google Scholar] [CrossRef]
- Manca, E.; Pascucci, V.; Deluca, M.; Cossu, A.; Andreucci, S. Shoreline Evolution Related to Coastal Development of a Managed Beach in Alghero, Sardinia, Italy. Ocean Coast. Manag. 2013, 85, 65–76. [Google Scholar] [CrossRef]
- Kiirikki, M.; Blomster, J. Wind Induced Upwelling as a Possible Explanation for Mass Occurrences of EpiphyticEctocarpus Siliculosus (Phaeophyta) in the Northern Baltic Proper. Mar. Biol. 1996, 127, 353–358. [Google Scholar] [CrossRef]
- Robledo, D.; Vázquez-Delfín, E.; Freile-Pelegrín, Y.; Vásquez-Elizondo, R.M.; Qui-Minet, Z.N.; Salazar-Garibay, A. Challenges and Opportunities in Relation to Sargassum Events Along the Caribbean Sea. Front. Mar. Sci. 2021, 8, 699664. [Google Scholar] [CrossRef]
- Finch, D.M.; Butler, J.L.; Runyon, J.B.; Fettig, C.J.; Kilkenny, F.F.; Jose, S.; Frankel, S.J.; Cushman, S.A.; Cobb, R.C.; Dukes, J.S.; et al. Effects of Climate Change on Invasive Species. In Invasive Species in Forests and Rangelands of the United States; Springer International Publishing: Cham, Switzerland, 2021; pp. 57–83. ISBN 978-3-030-45366-4. [Google Scholar]
- Barcellos, L.; Pham, C.K.; Menezes, G.; Bettencourt, R.; Rocha, N.; Carvalho, M.; Felgueiras, H.P. A Concise Review on the Potential Applications of Rugulopteryx okamurae Macroalgae. Mar. Drugs 2023, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Vega, J.; Catalá, T.S.; García-Márquez, J.; Speidel, L.G.; Arijo, S.; Cornelius Kunz, N.; Geisler, C.; Figueroa, F.L. Molecular Diversity and Biochemical Content in Two Invasive Alien Species: Looking for Chemical Similarities and Bioactivities. Mar. Drugs 2022, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wang, Z.; Liu, D.; Fu, M.; Yuan, C.; Yan, T. Harmful Macroalgal Blooms (HMBs) in China’s Coastal Water: Green and Golden Tides. Harmful Algae 2021, 107, 102061. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Hu, C. To What Extent Can Ulva and Sargassum Be Detected and Separated in Satellite Imagery? Harmful Algae 2021, 103, 102001. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, R.; Kim, K.; Zhang, J.; Cui, T. A Random Forest-Based Algorithm to Distinguish Ulva prolifera and Sargassum From Multispectral Satellite Images. IEEE Trans. Geosci. Remote Sens. 2022, 60, 4201515. [Google Scholar] [CrossRef]
- Wang, M.; Hu, C. Satellite Remote Sensing of Pelagic Sargassum Macroalgae: The Power of High Resolution and Deep Learning. Remote Sens. Environ. 2021, 264, 112631. [Google Scholar] [CrossRef]
- Laval, M.; Belmouhcine, A.; Courtrai, L.; Descloitres, J.; Salazar-Garibay, A.; Schamberger, L.; Minghelli, A.; Thibaut, T.; Dorville, R.; Mazoyer, C.; et al. Detection of Sargassum from Sentinel Satellite Sensors Using Deep Learning Approach. Remote Sens. 2023, 15, 1104. [Google Scholar] [CrossRef]
- Lazcano-Hernandez, H.E.; Arellano-Verdejo, J.; Rodríguez-Martínez, R.E. Algorithms Applied for Monitoring Pelagic Sargassum. Front. Mar. Sci. 2023, 10, 1216426. [Google Scholar] [CrossRef]
- Roca, M.; Dunbar, M.B.; Román, A.; Caballero, I.; Zoffoli, L.M.; Gernez, P.; Navarro, G. Monitoring the Marine Invasive Alien Species Rugulopteryx okamurae Using Unmanned Aerial Vehicles and Satellites. Front. Mar. Sci. 2022, 9, 1004012. [Google Scholar] [CrossRef]
- Rudin, C. Stop Explaining Black Box Machine Learning Models for High Stakes Decisions and Use Interpretable Models Instead. Nat. Mach. Intell. 2019, 1, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Rueda, J.L.; Mena-Torres, A.; Gallardo-Núñez, M.; González-García, E.; Martín-Arjona, A.; Valenzuela, J.; García-Ruiz, C.; González-Aguilar, M.; Mateo-Ramírez, Á.; García, M.; et al. Spatial Distribution and Potential Impact of Drifted Thalli of the Invasive Alga Rugulopteryx okamurae in Circalittoral and Bathyal Habitats of the Northern Strait of Gibraltar and the Alboran Sea. Diversity 2023, 15, 1206. [Google Scholar] [CrossRef]
- Zoffoli, L.M.; Gernez, P.; Rosa, P.; Le, A.; Brando, V.E.; Barillé, A.; Harin, N.; Peters, S.; Poser, K.; Spaias, L.; et al. Remote Sensing of Environment Sentinel-2 Remote Sensing of Zostera Noltei-Dominated Intertidal Seagrass Meadows. Remote Sens. Environ. 2020, 251, 112020. [Google Scholar] [CrossRef]
- Haro, S.; Jesus, B.; Oiry, S.; Papaspyrou, S.; Lara, M.; González, C.J.; Corzo, A. Microphytobenthos Spatio-Temporal Dynamics across an Intertidal Gradient Using Random Forest Classification and Sentinel-2 Imagery. Sci. Total Environ. 2022, 804, 149983. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Bermejo, R.; Wilkes, R.; Monagail, M.M.; Daly, E.; Healy, M.; Hanafin, J.; McKinstry, A.; Mellander, P.-E.; Fenton, O.; et al. Mapping Spatial Distribution and Biomass of Intertidal Ulva Blooms Using Machine Learning and Earth Observation. Front. Mar. Sci. 2021, 8, 633128. [Google Scholar] [CrossRef]
- Herrero, J.J.; Simes, D.C.; Abecasis, R.; Relvas, P.; Garel, E.; Ventura Martins, P.; Santos, R. Monitoring Invasive Macroalgae in Southern Portugal: Drivers and Citizen Science Contribution. Front. Environ. Sci. 2023, 11, 1324600. [Google Scholar] [CrossRef]
- García-Gómez, J.C.; Florido, M.; Olaya-Ponzone, L.; Rey Díaz de Rada, J.; Donázar-Aramendía, I.; Chacón, M.; Quintero, J.J.; Magariño, S.; Megina, C. Monitoring Extreme Impacts of Rugulopteryx okamurae (Dictyotales, Ochrophyta) in El Estrecho Natural Park (Biosphere Reserve). Showing Radical Changes in the Underwater Seascape. Front. Ecol. Evol. 2021, 9, 639161. [Google Scholar] [CrossRef]
- Bermejo, R.; Golden, N.; Schrofner, E.; Knöller, K.; Fenton, O.; Serrão, E.; Morrison, L. Biomass and Nutrient Dynamics of Major Green Tides in Ireland: Implications for Biomonitoring. Mar. Pollut. Bull. 2022, 175, 113318. [Google Scholar] [CrossRef]
- Bermejo, R.; de la Fuente, G.; Vergara, J.J.; Hernández, I. Application of the CARLIT Index along a Biogeographical Gradient in the Alboran Sea (European Coast). Mar. Pollut. Bull. 2013, 72, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, J.J.; Byers, J.E.; Bierwagen, B.G.; Dukes, J.S. Five Potential Consequences of Climate Change for Invasive Species. Conserv. Biol. 2008, 22, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.-R.; Roques, A.; Hulme, P.E.; Sykes, M.T.; Pyšek, P.; Kühn, I.; Zobel, M.; Bacher, S.; Botta-Dukát, Z.; Bugmann, H. Alien Species in a Warmer World: Risks and Opportunities. Trends Ecol. Evol. 2009, 24, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.-C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity Redistribution under Climate Change: Impacts on Ecosystems and Human Well-Being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, T.M.; Pyšek, P.; Bacher, S.; Carlton, J.T.; Duncan, R.P.; Jarošík, V.; Wilson, J.R.U.; Richardson, D.M. A Proposed Unified Framework for Biological Invasions. Trends Ecol. Evol. 2011, 26, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Wesselmann, M.; Hendriks, I.E.; Johnson, M.; Jordà, G.; Mineur, F.; Marbà, N. Increasing Spread Rates of Tropical Non-native Macrophytes in the Mediterranean Sea. Glob. Chang. Biol. 2024, 30, e17249. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, F.L.; Vega, J.V.; Valderrama, M.G.; Flores-Moya, A. Invasión de La Especie Exótica Rugulopteryx okamurae En Andalucía I: Estudios Preliminares de La Actividad Fotosintética. Algas 56. Boletín la Soc. Española Ficología 2020, 56, 35–46. [Google Scholar]
- García-Lafuente, J.; Nadal, I.; Sammartino, S.; Korbee, N.; Figueroa, F.L. Could Secondary Flows Have Made Possible the Cross-Strait Transport and Explosive Invasion of Rugulopteryx okamurae Algae in the Strait of Gibraltar? PLoS ONE 2023, 18, e0285470. [Google Scholar] [CrossRef] [PubMed]
- Bolado-Penagos, M.; González, C.J.; Chioua, J.; Sala, I.; Jesús Gomiz-Pascual, J.; Vázquez, Á.; Bruno, M. Submesoscale Processes in the Coastal Margins of the Strait of Gibraltar. The Trafalgar—Alboran Connection. Prog. Oceanogr. 2020, 181, 102219. [Google Scholar] [CrossRef]
- Bruno, M.; Chioua, J.; Romero, J.; Vázquez, A.; Macías, D.; Dastis, C.; Ramírez-Romero, E.; Echevarria, F.; Reyes, J.; García, C.M. The Importance of Sub-Mesoscale Processes for the Exchange of Properties through the Strait of Gibraltar. Prog. Oceanogr. 2013, 116, 66–79. [Google Scholar] [CrossRef]
- Haro, S.; Jimenez-Reina, J.; Bermejo, R.; Morrison, L. SoftwareX BioIntertidal Mapper Software: A Satellite Approach for NDVI-Based Intertidal Habitat Mapping. SoftwareX 2023, 24, 101520. [Google Scholar] [CrossRef]
- Bayındır, C.; Namlı, B. Efficient Sensing of von Kármán Vortices Using Compressive Sensing. Comput. Fluids 2021, 226, 104975. [Google Scholar] [CrossRef]
- Bai, L.-H.; Xu, H. Accurate Estimation of Tidal Level Using Bidirectional Long Short-Term Memory Recurrent Neural Network. Ocean Eng. 2021, 235, 108765. [Google Scholar] [CrossRef]




| Wind Direction | 2018 | 2019 | 2020 | 2021 | 2022 | Average |
|---|---|---|---|---|---|---|
| N | 0 | 1 | 1 | 1 | 20 | 5 |
| NE | 2 | 1 | 11 | 12 | 2 | 5 |
| E | 29 | 31 | 31 | 29 | 32 | 31 |
| SE | 4 | 5 | 7 | 6 | 7 | 6 |
| S | 6 | 7 | 8 | 8 | 6 | 7 |
| SW | 13 | 13 | 9 | 12 | 9 | 11 |
| W | 33 | 36 | 25 | 26 | 22 | 28 |
| NW | 4 | 3 | 3 | 5 | 2 | 4 |
| Coverage for the Coastline | Average NDVI for the Coastline | East Patch Size | West Patch Size | Average NDVI for East Patch | Average NDVI for West Patch | |
|---|---|---|---|---|---|---|
| Precipitation | −0.312 | −0.214 | −0.333 | −0.241 | ||
| Temperature | 0.301 | 0.332 | 0.184 | 0.223 | ||
| Wind direction | 0.238 | 0.216 | −0.215 | 0.345 | −0.323 | |
| Wind speed | 0.209 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haro, S.; Morrison, L.; Caballero, I.; Figueroa, F.L.; Korbee, N.; Navarro, G.; Bermejo, R. Assessing Golden Tides from Space: Meteorological Drivers in the Accumulation of the Invasive Algae Rugulopteryx okamurae on Coasts. Remote Sens. 2024, 16, 2689. https://doi.org/10.3390/rs16152689
Haro S, Morrison L, Caballero I, Figueroa FL, Korbee N, Navarro G, Bermejo R. Assessing Golden Tides from Space: Meteorological Drivers in the Accumulation of the Invasive Algae Rugulopteryx okamurae on Coasts. Remote Sensing. 2024; 16(15):2689. https://doi.org/10.3390/rs16152689
Chicago/Turabian StyleHaro, Sara, Liam Morrison, Isabel Caballero, Félix L. Figueroa, Nathalie Korbee, Gabriel Navarro, and Ricardo Bermejo. 2024. "Assessing Golden Tides from Space: Meteorological Drivers in the Accumulation of the Invasive Algae Rugulopteryx okamurae on Coasts" Remote Sensing 16, no. 15: 2689. https://doi.org/10.3390/rs16152689
APA StyleHaro, S., Morrison, L., Caballero, I., Figueroa, F. L., Korbee, N., Navarro, G., & Bermejo, R. (2024). Assessing Golden Tides from Space: Meteorological Drivers in the Accumulation of the Invasive Algae Rugulopteryx okamurae on Coasts. Remote Sensing, 16(15), 2689. https://doi.org/10.3390/rs16152689






