Abstract
Wood distillate (WD) has recently emerged as a promising bio-stimulant for sustainable legume crop production, owing to its ability to enhance seed yield and quality. However, no studies exist on the effects of WD on chickpea plants at pre-harvesting stages, hindering the farmers’ ability to acquire valuable knowledge on the early action of WD on the plants’ status and preventing the establishment of proactive measures to optimize WD use in agriculture. In this study, two multispectral, thermographic and spectroradiometric surveys, along with in-situ measurements of specific plant biometric traits, were conducted across the reproductive stage of field-grown chickpea in order to evaluate the early involvement of WD on plant health. The acquired multispectral images were used to calculate the Normalized Difference Vegetation Index (NDVI), revealing a notable ~35% increase in NDVI scores of WD-treated plants at the onset of physiological maturity, and indicating an improved plant status compared to the control (water-treated) plants. Moreover, control and WD-treated plants exhibited distinct spectral signatures across the visible, near-infrared (NIR) and short-wave infrared (SWIR) spectra, suggesting potential changes in their photosynthetic capacity, structural properties and water content both at the leaf and at the pod level. Furthermore, WD-treated plants showed a 25% increase in pod production, particularly at the beginning of seed maturity, suggesting that enhancements in plant status were also reflected in higher pod yields. These results point to a beneficial effect of WD on plant health during the preliminary stages of seed formation and indicate that a combination of both multispectral and spectroradiometric analyses can provide critical insights on the status of chickpea crops at pre-harvesting stages. In addition, these findings emphasize the importance of analyzing pre-harvesting stages to gain insights into the early involvement of WD in promoting plant health and, ultimately, in predicting final crop yields.
1. Introduction
Chickpea (Cicer arietinum L.) holds a prominent position as one of the most widely consumed pulses worldwide, with a total production of 18 million tons harvested over more than 14 million hectares [1]. This pulse contributes significantly to global food security and sustainable agriculture, as it is the third most widely grown legume crop in the world and it can capture atmospheric nitrogen and improve soil structure and fertility [2]. However, the excessive use of synthetic fertilizers in agriculture is urgently demanding for effective and sustainable strategies to improve chickpea production [3]. In this context, the re-valorization of plant waste-derived by-products is increasingly gaining preference over the use of harmful agrochemicals as an eco-friendlier approach to boost crop health and productivity [4]. In particular, wood distillate (WD), a liquid by-product obtained during the production of green energy through the pyrolysis of waste plant biomass, is increasingly being used as a bio-stimulant, owing to its ability to promote crop growth and yield with minimal impact on sensitive non-target organisms [5,6,7]. Studies on the effects of WD on legumes have emerged only recently and have focused on the measurement of specific seed traits at the post-harvesting stages, including biomass production, nutrient content and anatomical features. For example, application of WD through foliar spray and fertigation has shown to increase protein content, mineral content and yield in lentil (L. culinaris L.), chickpea and bean (Phaseolus vulgaris L.) seeds [8,9], while foliar application alone has increased the diameter as well as both the protein content and antioxidant power of chickpea seeds [10].
However, while the effects of WD on seed yield are well documented, the influence of WD on the plant at pre-harvesting stages has not been investigated yet. These stages can indeed provide critical insights into the early involvement of WD in determining final seed yields, ultimately allowing for proactive measures in managing plant health and optimizing growth conditions to ensure crop health. In this context, high-throughput field phenotyping techniques, including multispectral and thermographic analyses, are particularly suitable for the determination of plant health at pre-harvesting stages. These approaches can provide continuous, non-invasive monitoring of plant development and, hence, realistic insights into the dynamic nature of crop performance in agricultural settings [11,12,13]. For example, multispectral analyses and the calculation of vegetation indices, such as the Normalized Difference Vegetation Index (NDVI), have been previously used to phenotype the potential yield of different legume plants, including pea and chickpea [12,14], as well as to assess disease severity in chickpea caused by the fungal pathogen Ascochyta rabiei [15]. Moreover, thermographic analyses have been previously used to evaluate the index of stomatal conductance among different chickpea genotypes [16] as well as to evaluate the germination capacity of pea seeds [17]. Hence, these non-destructive approaches could provide important insights on the bio-stimulatory effects of WD on chickpea at pre-harvesting stages, allowing farmers to optimize field management strategies (e.g., biofertilizer/biopesticide application or irrigation scheduling), especially during periods of harsh conditions.
One of the most critical pre-harvesting steps in chickpea development is the reproductive stage, during which plants develop flowers that give rise to oblong pods containing one or two seeds each. This reproductive process is of outmost importance, as it ensures seed production, which is not only valuable for consumption but also plays a vital role in plant propagation and yield [18]. Considering that there is no evidence on the effect of WD on this critical developmental stage, this study employed a comprehensive approach combining multispectral, thermographic and spectroradiometric analyses, along with in-situ measurements of specific biometric indicators, to evaluate the effects of WD on the health status of chickpea plants during the reproductive stage. The first hypothesis of this study was that the application of WD could improve both the plant health status and the production of pods during the preliminary stages of seed formation. In the second place, it was hypothesized that the combination of multispectral, thermographic and spectroradiometric analyses could be a reliable approach to assess potential differences in the health status of non-treated and WD-treated chickpea plants. Since previous studies were not performed on this application stage, the present work can be considered a precursor in verifying the positive effect of WD on plants’ health and production in the pre-harvesting chickpea stage.
2. Materials and Methods
2.1. Plant Material and Wood Distillate Characteristics
Chickpea seeds were kindly provided by Del Colle Srl. (Bientina, Italy). The wood distillate (WD) tested in this study was provided by BioDea© Srl (Arezzo, Italy) and was obtained from the pyrolysis of sweet chestnut (Castanea sativa Mill.) biomass, using a thermal gradient up to 75 °C and then left to settle for at least three months. Table 1 shows the WD components and their quantities.

Table 1.
List of the wood distillate (WD) components and their quantities.
2.2. Study Site and Experimental Set Up
The experiment was conducted in a chickpea crop field at Meristema Srl (located in Buti, Tuscany, Italy, Figure 1), where no mineral fertilizers, pesticides nor herbicides were added. The same applies for the surrounding proximal areas, meaning that the possibilities of external contamination are to be considered very negligible. Chickpea seeds were sown on 15 April 2023 in a soil characterized by 26% clay, 37% sand and 3.3% organic matter [8]. When plants reached the fifth node stage (approximately 30 days after sowing), 6 experimental plots (1.5 m × 7.5 m) were selected, containing 20 plants each (Figure 1 and Figure 2). Following a randomized complete block design, in half of the plots, plants were both foliar sprayed weekly and fertigated every 2 weeks with WD. The other half were treated in the same way but using tap water only (i.e., control, C). Before application, WD was diluted in tap water at 0.2% (v/v) for foliar treatments and at 0.3% (v/v) for fertigation ones, according to the producer’s instructions. The volume of treatment applied varied according to the plant growth stage, ranging from 100 to 250 mL per plant. Plots were separated by a row of untreated plants.
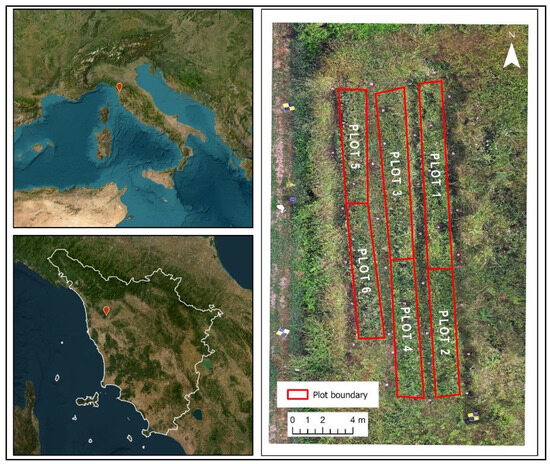
Figure 1.
Site location in Italy (upper left) and Tuscany (lower left) represented by the red dots; the image on the (right) shows the position and size of the six analyzed plots.

Figure 2.
Picture of one of the chickpea plots on the first day of WD treatment.
2.3. Multispectral-Thermal Surveys and Image Processing
The multispectral and thermographic surveys were carried out during the reproductive stage of chickpea: the first one on 14 July 2023, corresponding to the early beginning of seed formation (i.e., when a fully expanded green pod was present and seed cotyledon growth was visible); the second one on 4 August 2023, corresponding to the first stage of plant maturity (i.e., when the first pods reach maturity and thus become dry, turning into a light-yellow color), approximately three weeks before harvesting (i.e., when ~90% of pods reach maturity) (Figure 3). This approach was chosen given that both techniques allowed for efficient phenotyping in different legume crops and have been used for detecting changes in plant status, which are not visible to the naked eye [12,14,16]. The multispectral survey was conducted using a rod armed at its end with a ParrotTM Sequoia sensor (four bands within the spectral range from 0.55 to 0.79 µm) equipped with a 16 MP camera (Figure 4).
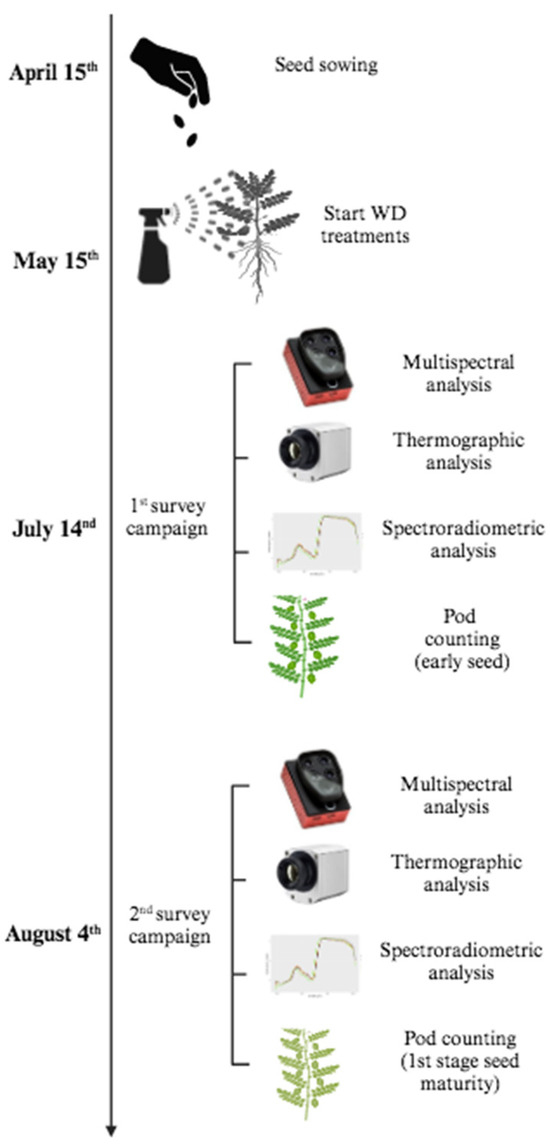
Figure 3.
Flowchart illustrating the different tasks carried out throughout the field experiment, including seed sowing, WD treatments and the two survey campaigns.

Figure 4.
Setting of the ParrotTM Sequoia multispectral camera and OPTRISTM PI450 thermal camera on the support.
The frames obtained from the survey were georeferenced using 31 targets, operating as ground control points (GCP), which were measured through a LeicaTM GS15 dual-frequency geodetic GNSS (Global Navigation Satellite System) receiver in NRTK (Network Real Time Kinematic) mode. Measurements were executed, with acquisition times ranging from 20 s to a few minutes, to achieve positional accuracy values of about ±1 cm for planimetry and about ±1.2 cm for altimetry. The correction service, used in NRTK, is the HxGNTM Smartnet, while LeicaTM Infinity software [19] version 3.4.2, was used to visualize and validate the measured points in the laboratory. Using the Convergo application [20] and related geodetic grids, the points’ elevation was transferred from the WGS84 ellipsoid to sea level. Results from the topographic survey were used to georeference each image in the ETRF2000/UTM32N reference system.
The end of images pre-processing was followed by the manual selection for each frame of chickpea plants, using the polygon selection tool provided by NIH ImageJ software [21] version 1.54g. The generated polygons were loaded into the QGIS software [22] version 3.38, and their Normalized Difference Vegetation Index (NDVI) values were calculated by the Raster Calculator tool, according to Equation (1):
where RED stands for the spectral reflectance measurements acquired in the red region (wavelength range~0.62–0.75 µm) and NIR stands for the spectral reflectance measurements acquired in the near-infrared region (wavelength range~0.75–1.4 µm), respectively. The NDVI values can range from −1 to 1. Values below 0 indicate non-vegetated areas, while values closer to 1 indicate greater amounts and healthier vegetation. This index was selected since it was previously used to evaluate the health status of field-grown chickpea [12,23].
To remove the remaining soil background, a threshold value for pixels with NDVI values lower than 0.75 was applied to create a binary mask separating the soil from the plants and resulting in a new raster layer showing only NDVI values compatible with the presence of chickpea (Figure 5).
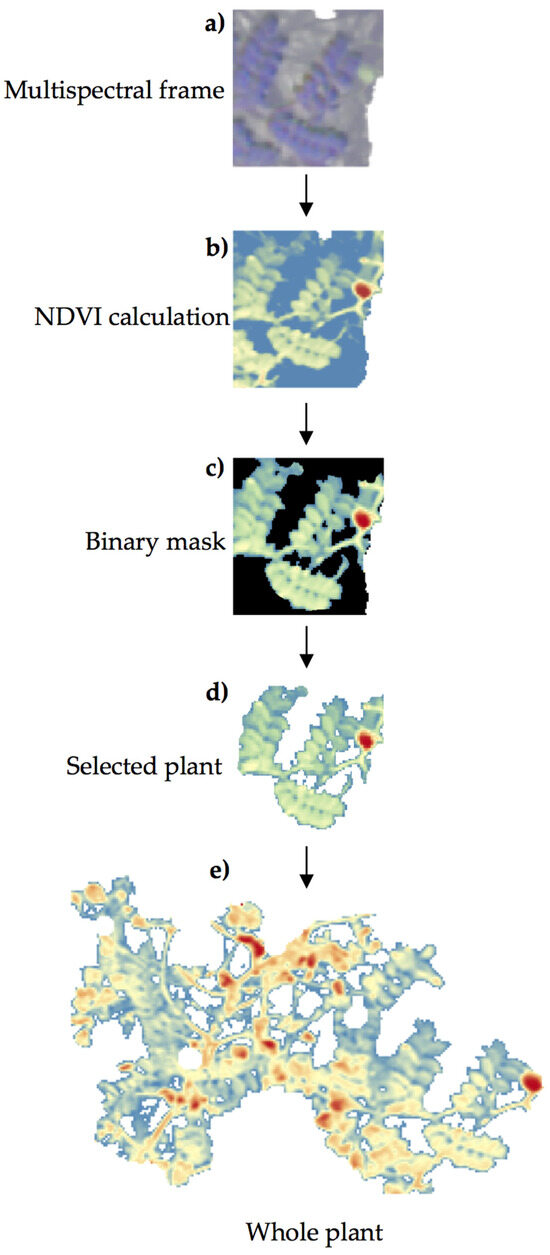
Figure 5.
Procedure for NDVI calculation in chickpea plants: (a) detail of a selected chickpea plant from the multispectral image; (b) calculation of NDVI in the selected plant; (c) application of a threshold to create a binary mask; (d) generation of a new raster layer containing the selected plant and its associated NDVI value; (e) aspect of the whole plant at the end of the image processing.
The same aforementioned support, i.e., metal rod, was equipped simultaneously with an OPTRISTM PI450 thermal camera (Figure 4), which allows video acquisitions in the temperature range between −20 °C and 900 °C, with a thermal sensitivity of 0.1° K and a frame rate of 80 Hz in a spectral range between 7.5 and 13 μm. This type of camera primarily measures the surface temperature of an object and its subtle variations.
While the multispectral camera acquires single images, the thermal camera records a video, from which single frames can be extracted to be processed in a photogrammetric workflow. An example of this output frame is shown in Figure 6, where lower values are indicated by blue shades (i.e., minimum temperature of 26 °C), while higher values are indicated by red shades (i.e., maximum temperature of 52 °C).
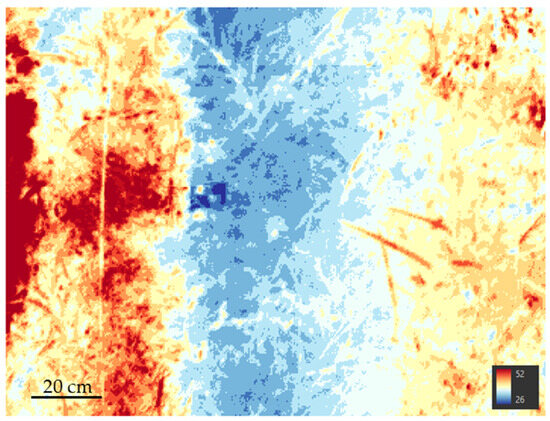
Figure 6.
Examples of thermographic frame as extracted from the recorded video.
The same image processing for geolocating, as described for the multispectral images, was applied to the frames chosen from the thermal sensor surveys, utilizing the same polygon extraction and visualization techniques.
2.4. Acquisition and Processing of Spectral Signatures
In each plot, out of the 20 plants present, 10 plants from control plots and 10 from WD-treated plots were randomly selected for the acquisition of spectral signatures using an ASD™ FieldSpec 3 portable spectroradiometer (Figure 7).

Figure 7.
Acquisition of spectral signatures during fieldwork. For each plant, an adaxial side of the terminal leaflet of the five youngest fully expanded leaves and the surface of a fully developed pod (green in color and full of seeds) were considered.
The instrument is equipped with an optic fiber, which operates in the 0.3–2.5 µm spectral range, and it acquires spectra relating to the visible light (~0.35–0.7 µm), to the NIR (~0.75–1.4 µm) and to the short-wave infrared (SWIR, ~1.8–2.5 µm). In particular, the spectral resolution is 3 nm (full-width-half-maximum) at 700 nm, 10 nm (full-width-half-maximum) at 1400 nm and 10 nm (full-width-half-maximum) at 2100 nm; meanwhile, the sampling interval is 1.4 nm for a spectral interval of 350–1000 nm and 2 nm for a spectral interval of 1000–2500 nm.
The ASD™ RS spectral acquisition software, version 3, allows for the measurement of a mediate spectrum. In fact, over 10 s, it acquires a spectrum per second and then returns a single mediate spectrum based on 10 measurements.
In each selected plant, spectra were collected in the adaxial side of the terminal leaflet of the five youngest fully expanded leaves and in the surface of five fully developed pods (green color and seed-filled) from the same leaves. Thus, considering that 2 measurements were acquired for each of the 10 plants inside the 6 plots, a total of 120 spectral signatures were acquired in each of the 2 spectroradiometric surveys (i.e., 60 for the leaves and 60 for the pods). At the end of the spectral signatures collection, the total number of measurements amounts to 240.
Before starting the acquisition, spectral signatures of a reference white Spectralon panel made of polytetrafluoroethylene (PTFE) were acquired to have a reference for high radiance values (i.e., 95–99% reflectance over the entire spectrum) to be used for computing the spectral reflectance of every leaf and pod. In fact, the reflectance values of plant elements were computed by rationing their radiance mean value to that of contemporary acquisitions on the white Spectralon panel. This procedure was repeated for every five measured plants, respecting the suggested maximum time of 10–15 min, to avoid errors due to solar lightening variations (i.e., passing clouds on a sunny day). Mean spectrum values were calculated to obtain a single spectrum at the leaf and pod level and plotted to illustrate the average spectral signatures of C and WD-treated plots. The reflectance ratios between the measured sample over the white reference were calculated by the ViewSpec Pro™ software, version 6.2, which allows for the performance of spectroradiometric data processing.
2.5. Plant Growth and Pod Production
In the August survey campaign, both the total number of pods and the number of mature (dry) pods were counted and recorded, and plant height was obtained by measuring the main stem height from the soil surface to the tip of the uppermost fully developed leaf extended vertically.
2.6. Statistical Analysis
Since the data did not approach a normal distribution (Shapiro–Wilk test, p < 0.05), a generalized linear mixed-effect model (GLMM) was fitted for each variable and run using the Gamma distribution, with treatment as fixed effect and plot as random effect [24,25]. Results were presented as median ± experimental error, where the latter was expressed as the interquartile range divided by the square root of the number of observations. Analyses were performed using R software, version 4.3.1 [26].
3. Results
3.1. Multispectral and Thermal Scores
The average spatial accuracy of GCPs, measured through the GNSS surveys carried out in July and August 2023, was about 3 cm, and it allowed for the alignment and georeferencing of the photogrammetric blocks. Thanks to this procedure, the precise location of the image acquisition perspective centers in the chosen reference system (i.e., ETRF2000/UTM32N) was obtained, as shown in Figure 8.
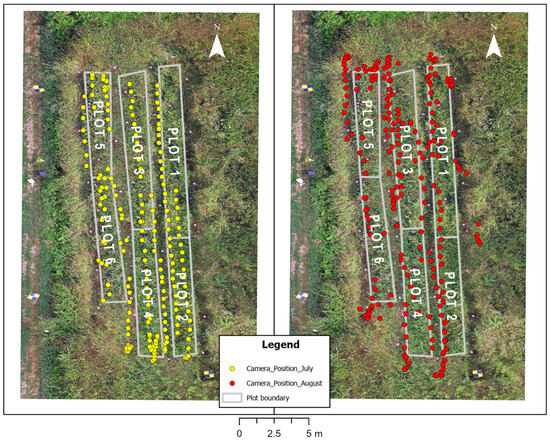
Figure 8.
Location of the image acquisition perspective centers as acquired during the multispectral-thermal surveys of July and August 2023. The image in the background is an orthophoto that was acquired during the August 2023 survey by a drone photogrammetric flight.
The generated NDVI images from the multispectral frames and their associated average scores (Figure 9a,b) unraveled both time- and treatment-dependent differences in the status of chickpea plants. During the July campaign, the NDVI scores were similar between the control and WD-treated plants (0.55 ± 0.05 vs. 0.61 ± 0.04; p < 0.05), as shown by the generated NDVI images, indicating no potential effects of WD on plant status in the initial stages of seed formation. However, at the beginning of physiological maturity (August campaign), the NDVI scores of the control plants significantly decreased by 25% (p < 0.05) compared to July, while those of the WD-treated plants decreased only slightly. As a result, the NDVI scores of the control plants in August were significantly lower compared to the WD-treated ones (0.41 ± 0.03 vs. 0.55 ± 0.02; p < 0.05).
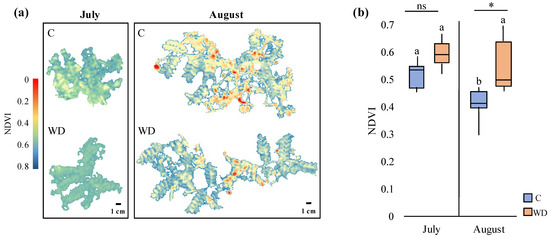
Figure 9.
(a) Generated NDVI images from the most representative control (C) and WD-treated (WD) plants in July and August and (b) associated average NDVI scores (median ± error). Different letters indicate statistically significant differences between the same treatment in July and August, while asterisks (*) indicate statistically significant differences (p < 0.05) between C and WD-treated plants in July and in August; ns: not significant.
Furthermore, generated thermal images and their associated temperature values (Figure 10a,b) showed that plant temperature decreased significantly in August compared to July (from 33 ± 2 °C to 24 ± 2 °C), independently of the treatment analyzed. Accordingly, differences in the environmental temperature were also recorded between the two survey campaigns, with temperatures ranging from 33 °C to 21 °C and from 25 °C to 17 °C in the July and the August campaigns, respectively (Figure 11). On the other hand, no significant differences among treatments were observed in July or in August, indicating similar plant surface temperatures and hence, similar heat emission levels.
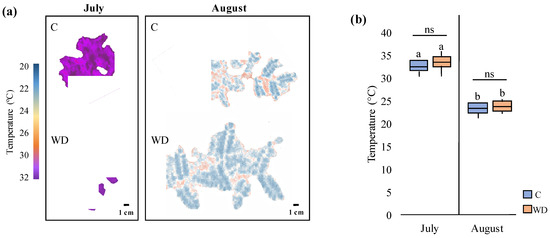
Figure 10.
Thermal analyses of control (C) and WD-treated (WD) chickpea plants in both July and August survey campaigns. (a) Thermal images of the most representative C and WD-treated plants in July and August and (b) associated temperature average values (median ± error). Different letters indicate statistically significant differences between the same treatment in July and August; ns: not significant.
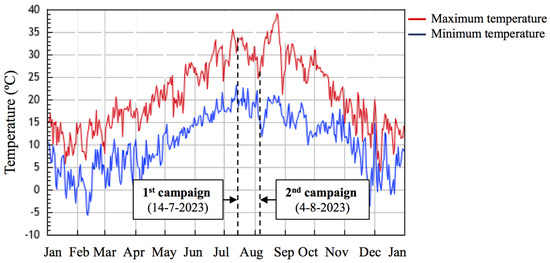
Figure 11.
Minimum (blue line) and maximum (red line) temperatures in Buti in 2023. Dotted lines indicate the dates of the spectral, termographic and spectroradiometric campaigns carried out (data retrieved from [27].
3.2. Leaf and Pod Spectral Signatures
The spectral signatures obtained at the leaf and pod level through spectroradiometric analyses showed a similar pattern compared to what was observed at the whole plant level in the multispectral analyses. While both treatments showed similar spectral signatures in July (Figure 12a and Figure 12c, respectively), several differences were observed in August (Figure 12b,d) across the entire spectrum. In fact, regarding the visible wavelength, WD application led to lower reflectance values compared to control plants at the leaf level (0.06–0.19% vs. 0.04–0.12%), while similar reflectance values were recorded at the pod level (~0.06–0.23%). Moreover, WD-treated plants exhibited higher reflectance values compared to the control in the NIR band, both at the leaf and at the pod level (0.68–0.73% vs. 0.47–0.56% and 0.65–0.76% vs. 0.48–0.62%, respectively) as well as in the SWIR band (0.18–0.42% vs. 0.067–0.22% in leaves and 0.1–0.26% vs. 0.04–0.16%, respectively).
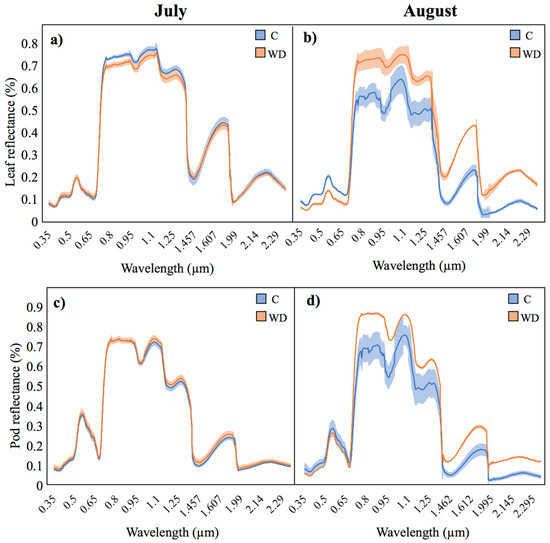
Figure 12.
Mean reflectance values (lines) and associated standard error (shades) from control (C) and WD-treated chickpea leaves and pods in the two survey campaigns: (a) leaves in July; (b) leaves in August; (c) pods in July; (d) pods in August.
3.3. Plant Growth and Pod Production
Regarding the number of pods produced per plant, no significant differences were found between the control and WD-treated plants in the July campaign (beginning of seed formation). However, in the August campaign (first stage of plant maturity), the number of pods in WD-treated plants was almost 25% higher compared to control ones (28.5 ± 4.9 and 43.9 ± 6.3, respectively; p < 0.05). In addition, a similar number of mature (dry) pods was counted in both treatments (5 ± 2 and 7 ± 3, respectively, p > 0.05) (Figure 13), indicating no significant effects of WD on the initiation of pod maturation. As a result, in the August campaign, WD-treated plants showed a lower proportion of dry pods per plant compared to control ones (13% and 18%, respectively).
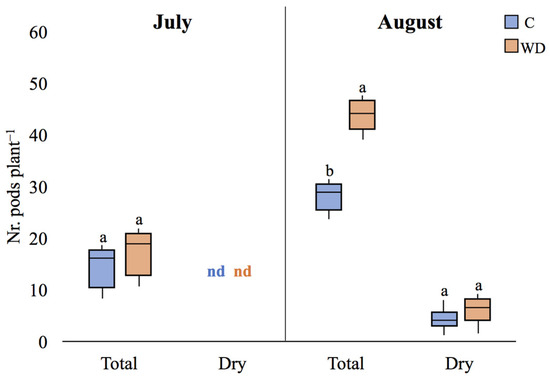
Figure 13.
Total number of pods and number of dry pods, (median plant-1 ± error) of control (C) and WD-treated (WD) plants in both July and August campaigns. For each parameter, different letters indicate statistically significant (p < 0.05) differences between treatments. ns: not significant; nd: not determined.
Regarding plant height, the application of WD did not have a significant effect on this parameter, neither in July nor in August (p > 0.05 in both cases; Figure 14).
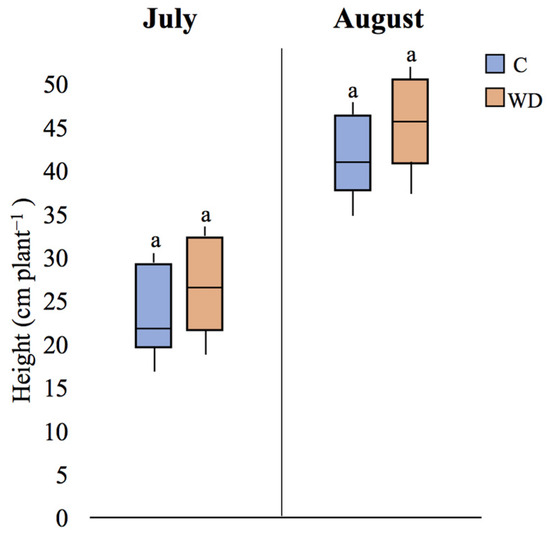
Figure 14.
Plant height (median plant-1 ± error) of control (C) and WD-treated (WD) plants in both July and August campaigns. For each parameter, different letters indicate statistically significant (p < 0.05) differences between treatments.
4. Discussion
In the present study, multispectral, thermographic and spectroradiometric analyses were used to evaluate the effects of WD on the status of chickpea plants during the reproductive stage. Considering the multispectral analyses, the general decrease in the NDVI scores in August in comparison to July was expected for two main reasons. In the first place, variations in temperature can critically affect this index, with higher temperature conditions yielding higher NDVI values [28]. Thus, the lower temperatures recorded during the August campaign could have partly affected the plants’ reflectance properties, ultimately decreasing the NDVI scores. In the second place, during the August campaign, the plants’ dry matter content increased because of the beginning of the pod maturation process, likely decreasing the overall plants’ reflectance in specific bands, particularly in the NIR region, and hence yielding lower NDVI values [29]. Notably, this NDVI decrease from July to August was significantly less pronounced in WD-treated plants compared to control ones. This observation suggests that the application of WD could have delayed the pod maturation process while extending the plants’ vegetative growth, providing more space and resource allocation towards the production of green biomass, including both new branches and pods. This hypothetical WD-mediated vegetative growth-promotion effect could fit well with the indeterminate growth habit of chickpea plants, characterized by a prolonged vegetative growth even after the plant switches to reproductive mode. Consistently with our findings, previous studies have shown positive effects of WD on the vegetative growth of different plant species, displaying either indeterminate or determinate growth, including tomato (Solanum lycopersicum L.) and peanut (Arachis hypogaea L.) as well as eggplant (Solanum melongena L.) and green gram (Vigna radiata L.), respectively [30,31,32]. However, it is important to consider that, in the present study, both the NDVI scores and the number of pods were evaluated during the reproductive stage, and hence, data on the final seed yield (i.e., seed number, seed weight and/or seed diameter) would be required to confirm whether the selected parameters at pre-harvesting stages can be reliable indicators of the final plant productivity.
Furthermore, the different spectral signatures of control and WD-treated plants retrieved from the August campaign suggest that the application of WD could have altered the content of pigments, water and/or other biochemical constituents at the leaf and pod level, potentially affecting their photosynthetic activity and overall health. Considering the visible bands, the lower reflectance at the leaf level may indicate higher absorption of light by chlorophyll, suggesting a higher chlorophyll content and a potential increase in photosynthetic activity in WD-treated leaves [33,34]. In support of this hypothesis, application of WD has been previously shown to increase chlorophyll content in both legume and non-legume species [9,10]. Moreover, previous studies have shown a negative relationship between leaf chlorophyll concentration and reflectance in the visible light in different plant species, including Camphor tree (Cinnamomum camphora L.), Norway maple (Acer platanoides L.), horse chestnut (Aesculus hippocastanum L.), tomato (Solanum lycopersicum L.) and cabbage (Brassica oleracea L.) [35,36,37]. However, it is important to consider that higher concentration of chlorophyll at the leaf and pod level does not necessarily imply higher chlorophyll concentration at the canopy level, and hence, the described changes associated to the spectral signatures remain hypothetical. In this context, measures of other biometric traits (e.g., leaf fresh/dry weight or leaf area) as well as direct measurements of chlorophyll using chlorophyll measurement tools (e.g., chlorophyll content meter) may help to understand the effects of WD on chlorophyll content.
Furthermore, a general increase in reflectance in both NIR and, to a lesser degree, in the SWIR spectra was expected, given that leaves typically contain many air pockets that cause internal reflection and scattering of NIR radiation [38]. The observed changes in reflectance in these regions are related to modifications in both the internal leaf cell structure as well as the water absorption of vegetation [39,40,41]. Hence, the observed stronger reflectance signals in these regions may indicate lower water content in the WD-treated leaves and pods. Yet, it is important to note that lower plant water content can be tolerated by plants quite well, as several plant species, including chickpea, have evolved natural adaptations to efficiently utilize water and maintain their physiological functions in low water environments [42]. Contrary to the spectral results, thermographic analyses did not show any treatment-dependent differences on the status of chickpea plants. Therefore, the information provided by the combination of both multispectral imaging and spectroradiometric analyses may be comparatively more suitable to obtain accurate insights on the plant status under the tested conditions.
Overall, the use of both multispectral and spectroradiometric analyses for the evaluation of the status of chickpea crops could have significant implications for agricultural practices and the management of leguminous crops. In fact, by leveraging these advanced sensing technologies, farmers and researchers could make more informed decisions to optimize crop productivity and sustainability through the application of novel bio-stimulants like WD. For example, by providing detailed information about crop health and variability at the field scale, farmers could implement site-specific management strategies (e.g., concentration and rate of WD to be applied) to optimize resource allocation and to maximize yields. In addition, these techniques could support breeding efforts towards crop improvement by enabling the early detection of diseases and stress, facilitating precision agriculture practices and supporting breeding efforts for crop improvement. Nevertheless, although initial results are encouraging, there is not yet an experimental basis that has proven the mechanisms of action of WD, and hence, future studies are necessary to dig into the processes influenced by WD that generate the observed differences in plant status both at the leaf and at the whole plant level.
5. Conclusions
To the best of our knowledge, this is the first study combining multispectral, thermographic and spectroradiometric analyses to comprehensively evaluate the status of chickpea plants during the reproductive growth stage.
The observed increase in NDVI scores at the whole plant level, the changes in the spectral signatures at the leaf and pod level, and the increased production of pods collectively point to a positive impact of WD on plant health during the preliminary stages of seed formation.
By harnessing the power of advanced sensing technologies, farmers and researchers could make smarter choices to enhance crop productivity and sustainability. This can be achieved by using innovative bio-stimulants such as WD. By obtaining precise information about the health and variations of crops on a field level, farmers can implement tailored management strategies. These strategies may include determining the ideal concentration and rate of WD application, resulting in optimized resource allocation and increased yields.
Hence, these findings underscore the significance of evaluating pre-harvesting stages to gain valuable insights into the early contributions of WD in promoting plant health, providing evidence-based strategies to optimize chickpea cultivation and contributing to the advancement of sustainable crop management in precision agriculture.
As agriculture faces increasing challenges due to climate change and resource limitations, the adoption of more sustainable and eco-friendly products, such as plant waste-derived byproducts, holds promise for enhancing resilience and productivity in agricultural systems worldwide.
Author Contributions
Conceptualization, P.C. and S.L.; methodology, P.C., I.C., R.S., L.B., A.R., A.E. and S.L.; software, P.C., R.S., L.B., A.R. and A.E.; validation, P.C., I.C., R.S., L.B., A.R., A.E., R.S., A.G. and S.L.; formal analysis, P.C.; investigation, P.C.; resources, S.L.; data curation, P.C., R.S., L.B., A.R. and A.E.; writing—original draft preparation, P.C.; writing—review and editing, P.C., I.C., R.S., L.B., A.R., A.E., A.G., C.G. and S.L; visualization, P.C.; supervision, S.L.; project administration, S.L.; funding acquisition, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by project CLASS (“Ceci, distillato di legno, biochar e intelligenza artificiale per un sistema agrifood smart e sostenibile”), project number B69J21027970008. A.E. contributed to this publication while attending the PhD program PhD in Space Science and Technology at the University of Trento, Cycle XXXIX, with the support of a scholarship financed by the Ministerial Decree no. 118 of 2nd March 2023, based on the NRRP-funded by the European Union-NextGenerationEU-Mission 4 “Education and Research”, Component 1 “Enhancement of the offer of educational services: from nurseries to universities”-Investment 4.1 “Extension of the number of research doctorates and innovative doctorates for public administration and cultural heritage”-CUP E66E23000110001”.
Data Availability Statement
All available data can be found in this manuscript.
Acknowledgments
The authors are grateful to Francesco Barbagli (BioDea© and BioEsperia Srl.) for providing the wood distillate and to Alessia Papi and Miriam Negussu for their help in the fieldwork.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vargas-Blandino, D.; Cardenas-Travieso, R.M.; San José de las Lajas, M. Chickpea cultivation, a possible solution to climate change. Cultiv. Trop. 2021, 42, 12. [Google Scholar]
- Zhang, J.; Wang, J.; Zhu, C.; Singh, R.P.; Chen, W. Chickpea: Its Origin, Distribution, Nutrition, Benefits, Breeding, and Symbiotic Relationship with Mesorhizobium Species. Plants 2022, 13, 429. [Google Scholar] [CrossRef]
- Patil, S.B.; Goyal, A.; Chitgupekar, S.S.; Kumar, S.; El-Bouhssini, M. Sustainable management of chickpea pod borer. A review. Agron. Sustain. Dev. 2017, 37, 20. [Google Scholar] [CrossRef]
- Nyambo, P.; Zhou, L.; Chuma, T.; Sokombela, A.; Malobane, M.E.; Musokwa, M. Prospects of vermicompost and biochar in climate smart agriculture. In Vermicomposting for Sustainable Food Systems in Africa; Springer Nature Singapore: Singapore, 2023; pp. 145–159. [Google Scholar]
- Fačkovcová, Z.; Vannini, A.; Monaci, F.; Grattacaso, M.; Paoli, L.; Loppi, S. Effects of wood distillate (pyroligneous acid) on sensitive bioindicators (lichen and moss). Ecotoxicol. Environ. Saf. 2020, 204, 111117. [Google Scholar] [CrossRef]
- Filippelli, A.; Ciccone, V.; Loppi, S.; Morbidelli, L. Characterization of the safety profile of sweet chestnut wood distillate employed in agriculture. Safety 2021, 7, 79. [Google Scholar] [CrossRef]
- Fedeli, R.; Vannini, A.; Guarnieri, M.; Monaci, F.; Loppi, S. Bio-based solutions for agriculture: Foliar application of wood distillate alone and in combination with other plant-derived corroborants results in different effects on lettuce (Lactuca sativa L.). Biology 2022, 11, 404. [Google Scholar] [CrossRef]
- Carril, P.; Bianchi, E.; Cicchi, C.; Coppi, A.; Dainelli, M.; Gonnelli, C.; Loppi, S.; Pazzagli, L.; Colzi, I. Effects of Wood Distillate (Pyroligneous Acid) on the Yield Parameters and Mineral Composition of Three Leguminous Crops. Environments 2023, 10, 126. [Google Scholar] [CrossRef]
- Becagli, M.; Arduini, I.; Cantini, V.; Cardelli, R. Soil and foliar applications of wood distillate differently affect soil properties and field bean traits in preliminary field tests. Plants 2023, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, R.; Vannini, A.; Celletti, S.; Maresca, V.; Munzi, S.; Cruz, C.; Alexandrov, D.; Guarnieri, M.; Loppi, S. Foliar application of wood distillate boosts plant yield and nutritional parameters of chickpea. Ann. App. Biol. 2023, 182, 57–64. [Google Scholar] [CrossRef]
- Candiago, S.; Remondino, F.; De Giglio, M.; Dubbini, M.; Gattelli, M. Evaluating multispectral images and vegetation indices for precision farming applications from UAV images. Remote Sens. 2015, 7, 4026–4047. [Google Scholar] [CrossRef]
- Quirós, J.J.; McGee, R.J.; Vandemark, G.J.; Romanelli, T.; Sankaran, S. Field phenotyping using multispectral imaging in pea (Pisum sativum L.) and chickpea (Cicer arietinum L.). Eng. Agric. Environ. Food 2019, 12, 404–413. [Google Scholar] [CrossRef]
- Demir, S.; Dursun, İ. Determining burned areas using different threshold values of NDVI with Sentinel-2 satellite images on GEE platform: A case study of Muğla province. Uluslararası Sürdürülebilir Mühendislik Teknol. Derg. 2023, 7, 117–130. [Google Scholar]
- Mkhabela, M.S.; Bullock, P.; Raj, S.; Wang, S.; Yang, Y. Crop yield forecasting on the Canadian Prairies using MODIS NDVI data. Agric. For. Meteorol. 2011, 151, 385–393. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, W.; Sankaran, S. High-throughput field phenotyping of Ascochyta blight disease severity in chickpea. Crop Prot. 2019, 125, 104885. [Google Scholar] [CrossRef]
- Pushpavalli, R.; Zaman-Allah, M.; Turner, N.C.; Baddam, R.; Rao, M.V.; Vadez, V. Higher flower and seed number leads to higher yield under water stress conditions imposed during reproduction in chickpea. Funct. Plant Biol. 2014, 42, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, P.; Mazurek, W.; Walczak, R.T. The use of thermography for pre-sowing evaluation of seed germination capacity. In Proceedings of the International Conference on Quality in Chains. An Integrated View on Fruit and Vegetable Quality, Wageningen, The Netherlands, 6–9 July 2003; Volume 604, pp. 459–465. [Google Scholar]
- Lake, L.; Sadras, V.O. The critical period for yield determination in chickpea (Cicer arietinum L.). Field Crops Res. 2014, 168, 1–7. [Google Scholar] [CrossRef]
- Leica Geosystems AG. Leica InfinityTM, Version 3.4.2; Leica Geosystems AG—Part of Hexagon. 2021. Available online: https://leica-geosystems.com/products/gnss-systems/software/leica-infinity (accessed on 20 June 2024).
- Cima, V.; Carroccio, M.; Maseroli, R. Corretto utilizzo dei Sistemi Geodetici di Riferimento all’interno dei software GIS. In Proceedings of the Atti 18th ASITA National Conference, Florence, Italy, 14–16 October 2014; pp. 359–363. [Google Scholar]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- QGIS.org. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2024. Available online: http://qgis.org (accessed on 6 July 2023).
- Srivastava, A.K.; Mondal, B.; Jha, U.C.; Singh, A.; Biradar, R.S.; Praween, N.; Kumar, Y. Selecting stable chickpea genotypes under rainfed cultivation using GGE biplot analysis. Legume Res. Int. J. 2022. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- McCullagh, P. Generalized Linear Models; Routledge: London, UK, 2019. [Google Scholar]
- R Core Team R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 15 July 2023).
- Servizio Idrologico Regionale. Available online: http://www.sir.toscana.it (accessed on 11 September 2023).
- Hao, F.; Zhang, X.; Ouyang, W.; Skidmore, A.K.; Toxopeus, A.G. Vegetation NDVI linked to temperature and precipitation in the upper catchments of Yellow River. Environ. Model. Assess. 2012, 17, 389–398. [Google Scholar] [CrossRef]
- Wang, L.; Qu, J.J.; Hao, X.; Hunt Jr, E.R. Estimating dry matter content from spectral reflectance for green leaves of different species. J. Photogramm. Remote Sens. 2011, 32, 7097–7109. [Google Scholar] [CrossRef]
- Travero, J.T.; Mihara, M. Effects of pyroligneous acid to growth and yield of soybeans (Glycine max). Int. J. Environ. Rural Dev. 2016, 7, 50–54. [Google Scholar]
- Mungkunkamchao, T.; Kesmala, T.; Pimratch, S.; Toomsan, B.; Jothityangkoon, D. Wood vinegar and fermented bioextracts: Natural products to enhance growth and yield of tomato (Solanum lycopersicum L.). Sci. Hort. 2013, 154, 66–72. [Google Scholar] [CrossRef]
- Ofoe, R.; Qin, D.; Gunupuru, L.R.; Thomas, R.H.; Abbey, L. Effect of pyroligneous acid on the productivity and nutritional quality of greenhouse tomato. Plants 2022, 11, 1650. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Yang, L. Deriving leaf chlorophyll content of green-leafy vegetables from hyperspectral reflectance. ISPRS J. Photogramm. Remote Sens. 2009, 64, 97–106. [Google Scholar] [CrossRef]
- Chou, S.; Chen, B.; Chen, J.; Wang, M.; Wang, S.; Croft, H.; Shi, Q. Estimation of leaf photosynthetic capacity from the photochemical reflectance index and leaf pigments. Ecol. Ind. 2020, 110, 105867. [Google Scholar] [CrossRef]
- Lin, C.; Popescu, S.C.; Huang, S.C.; Chang, P.T.; Wen, H.L. A novel reflectance-based model for evaluating chlorophyll concentrations of fresh and water-stressed leaves. Biogeosciences 2015, 12, 49–66. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Ofori, S.; Abebrese, D.K.; Klement, A.; Provazník, D.; Tomášková, I.; Růžičková, I.; Wanner, J. Impact of treated wastewater on plant growth: Leaf fluorescence, reflectance, and biomass-based assessment. Water Sci. Technol. 2024, 89, 1647–1664. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Ustin, S.L. Modeling leaf optical properties. Photobiol. Sci. Online 2008, 736, 737. [Google Scholar]
- Carter, G.A. Primary and secondary effects of water content on the spectral reflectance of leaves. Am. J. Bot. 1991, 78, 916–924. [Google Scholar] [CrossRef]
- Ustin, S.L.; Jacquemoud, S. How the optical properties of leaves modify the absorption and scattering of energy and enhance leaf functionality. In Remote Sensing of Plant Biodiversity; Springer: Berlin/Heidelberg, Germany, 2020; pp. 349–384. [Google Scholar]
- Ge, Y.; Atefi, A.; Zhang, H.; Miao, C.; Ramamurthy, R.K.; Sigmon, B.; Schnable, J.C. High-throughput analysis of leaf physiological and chemical traits with VIS–NIR–SWIR spectroscopy: A case study with a maize diversity panel. Plant Methods 2019, 15, 66. [Google Scholar] [CrossRef] [PubMed]
- Maphosa, L.; Richards, M.F.; Norton, S.L.; Nguyen, G.N. Breeding for abiotic stress adaptation in chickpea (Cicer arietinum L.): A comprehensive review. Crop Breed. Genet. Genom. 2020, 4, e200015. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).