Winter Durum Wheat Disease Severity Detection with Field Spectroscopy in Phenotyping Experiment at Leaf and Canopy Level
Abstract
1. Introduction
2. Materials and Methods
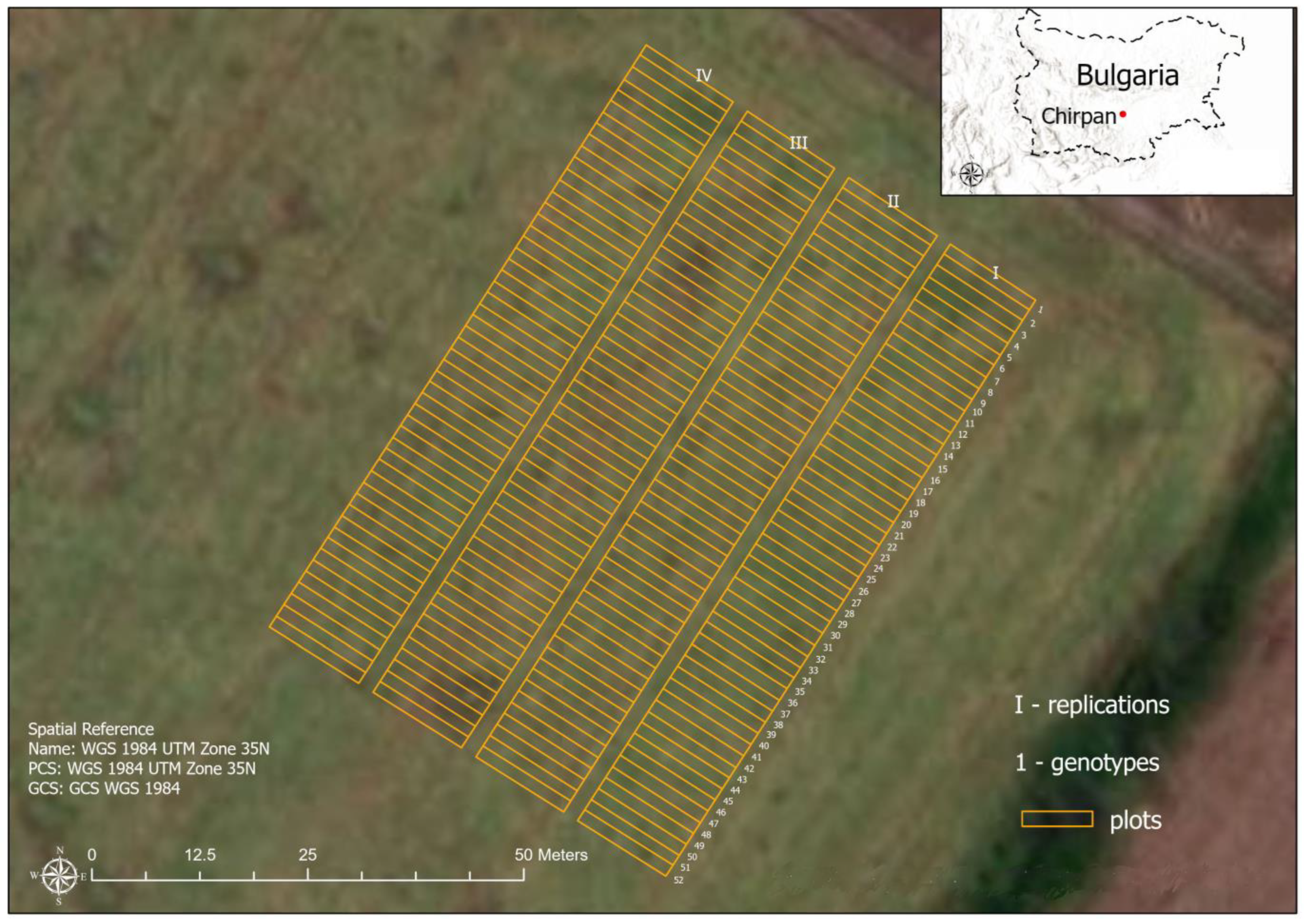
2.1. Investigated Wheat Diseases and Study Area
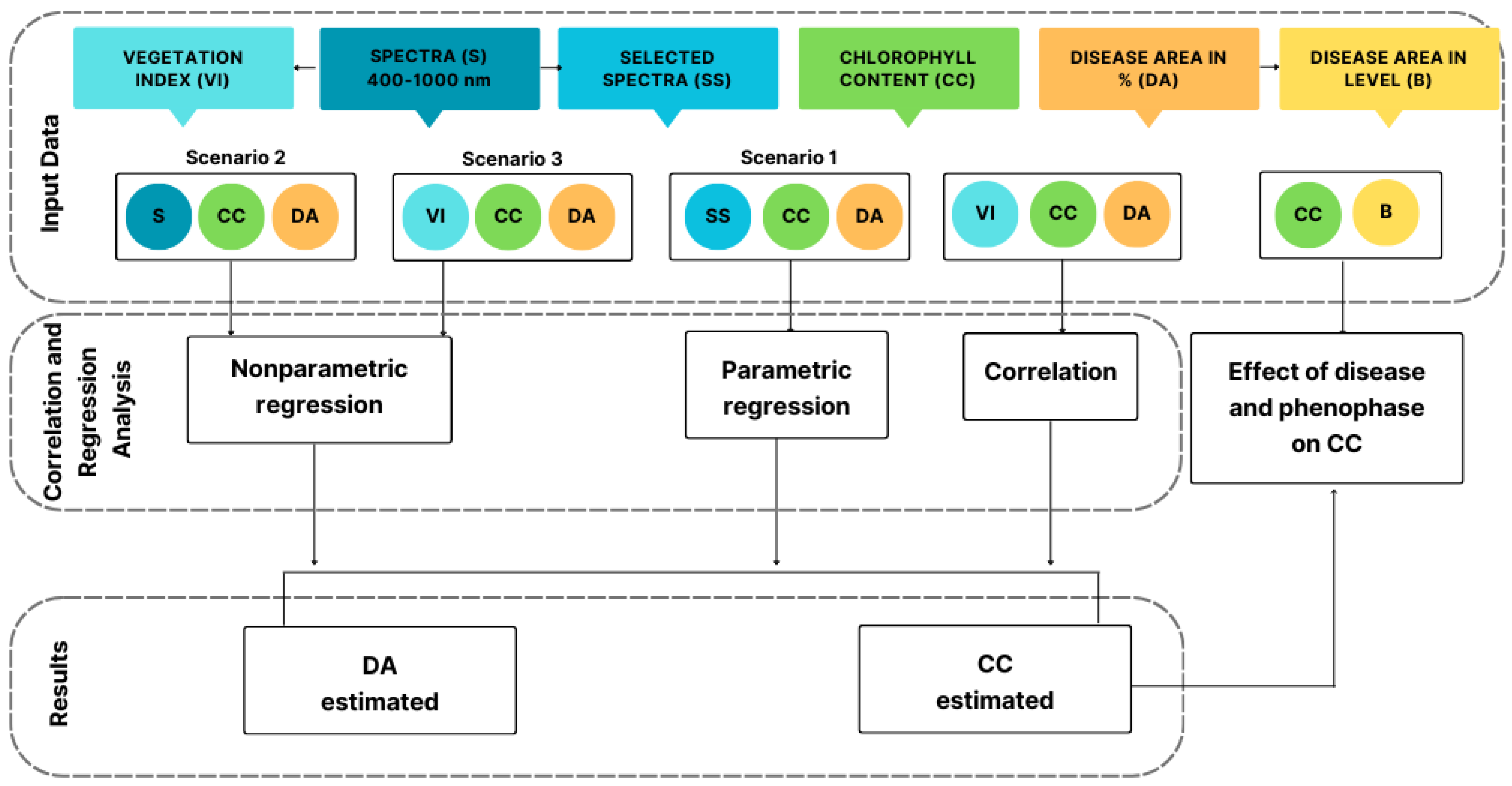
2.2. Input Data
2.2.1. Phenological Observations
2.2.2. Laboratory Measurements, Leaf Level
2.2.3. Field Measurements, Canopy Level
2.3. Spectra Preprocessing
2.4. Effect of Disease Severity and Phenophase on Chlorophyll Content
2.5. Correlation and Regression Analysis
2.5.1. Correlation Analysis
2.5.2. Training and Validation Strategies for the Regression Analysis
2.5.3. Regression Analysis
3. Results
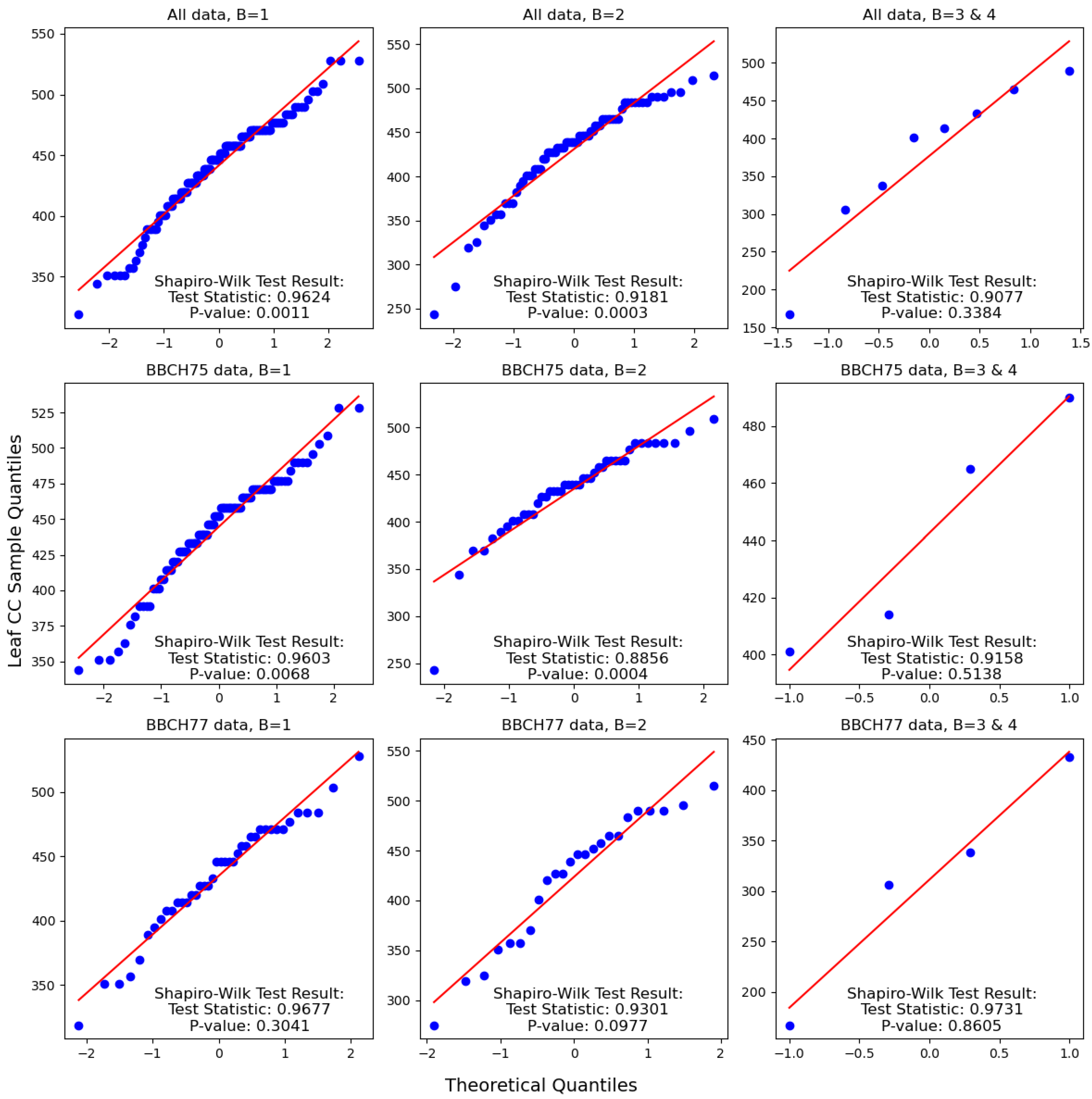
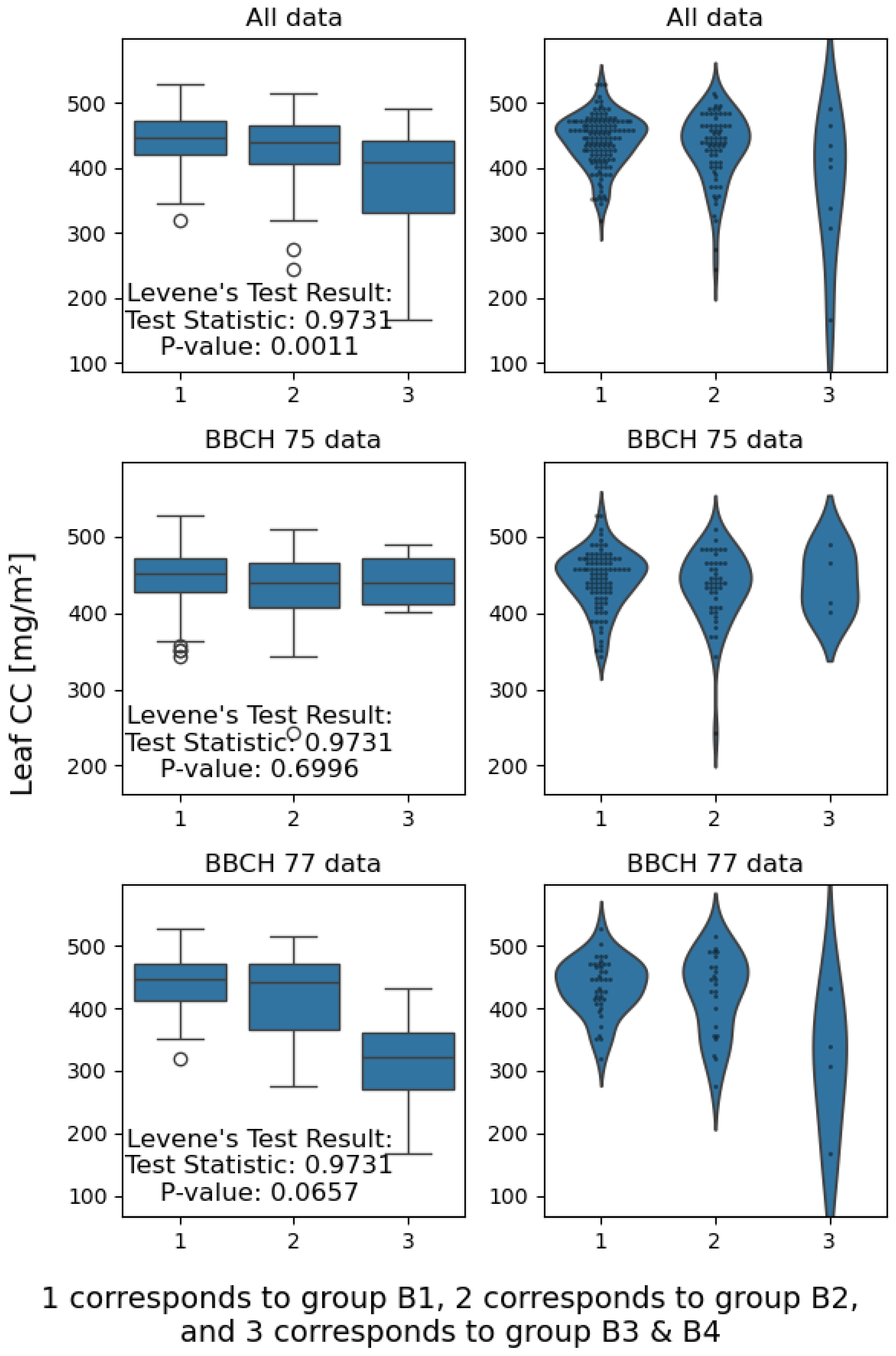
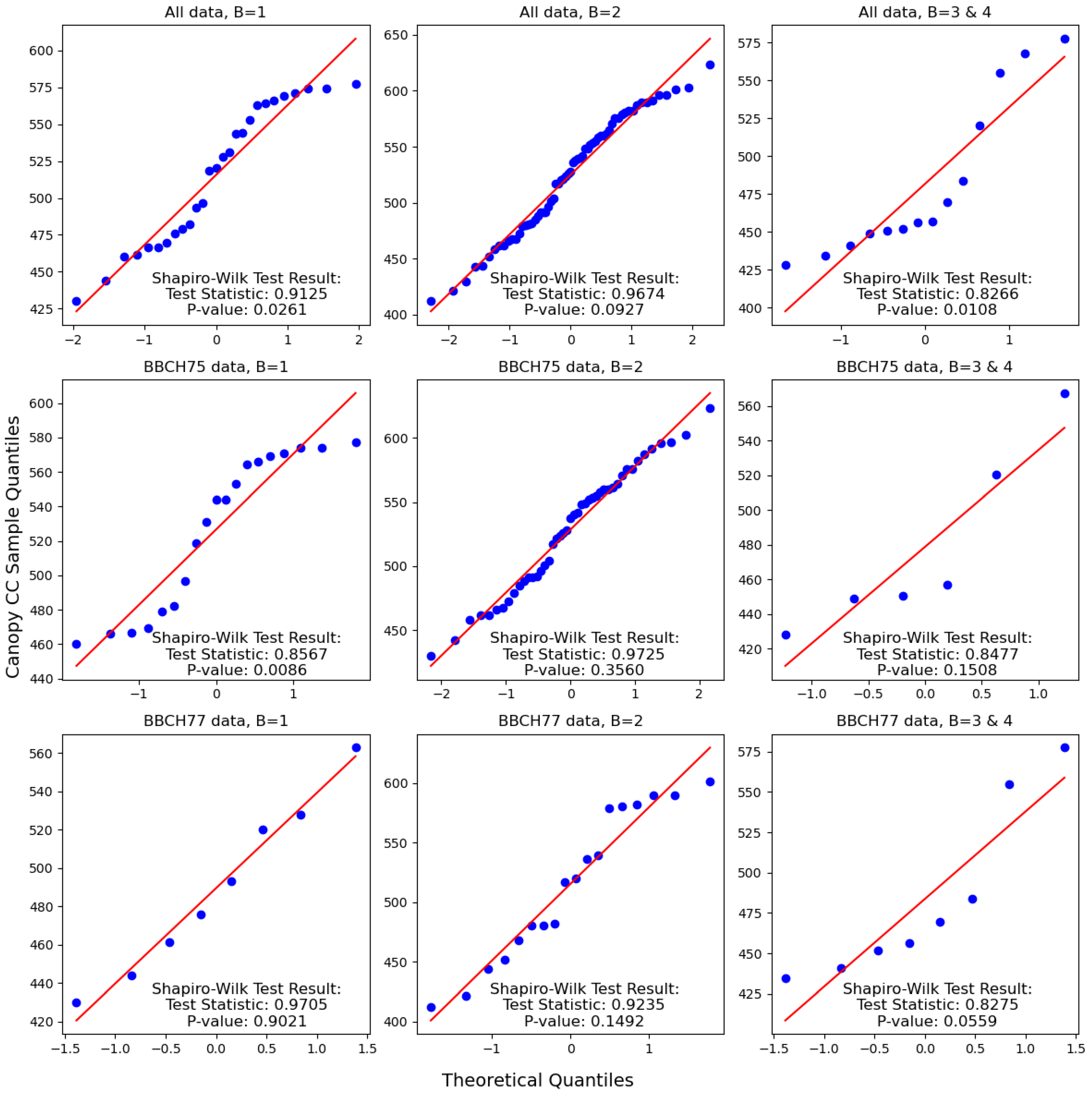
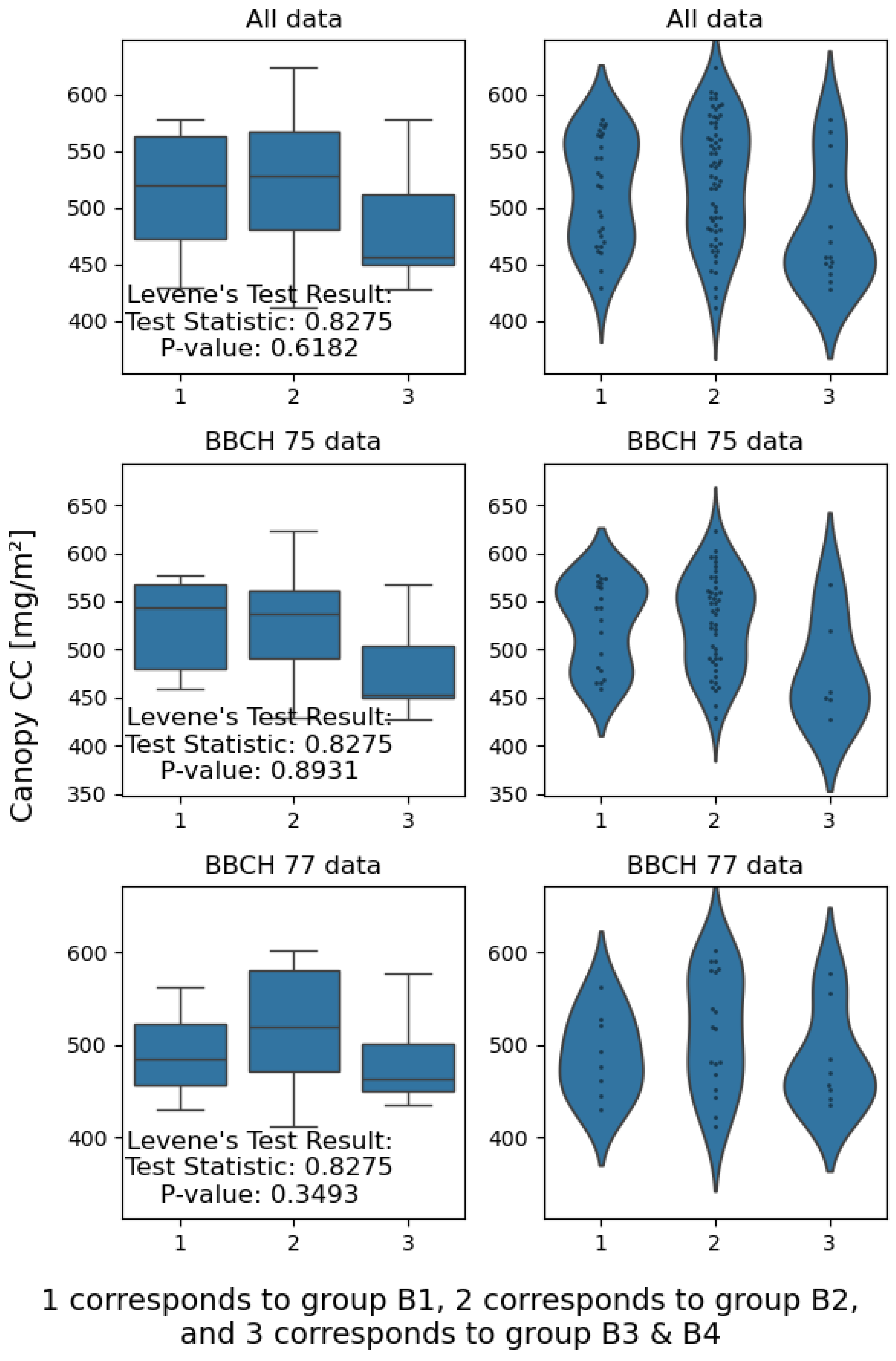
3.1. Effect of Disease Severity and Phenophase on Chlorophyll Content
3.2. Correlation and Regression Analysis
4. Discussion
4.1. Effect of Disease Severity and Phenophase on Chlorophyll Content
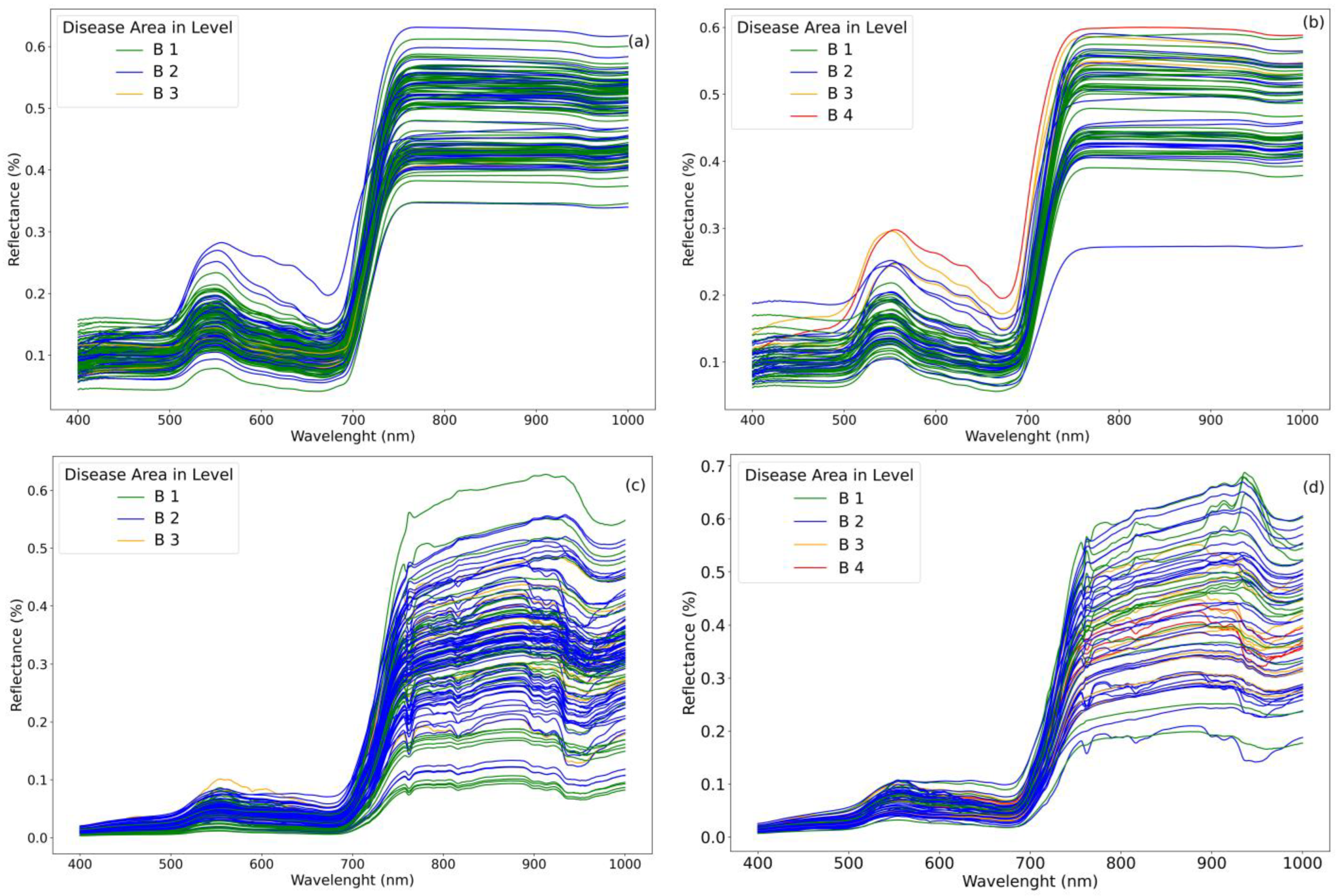
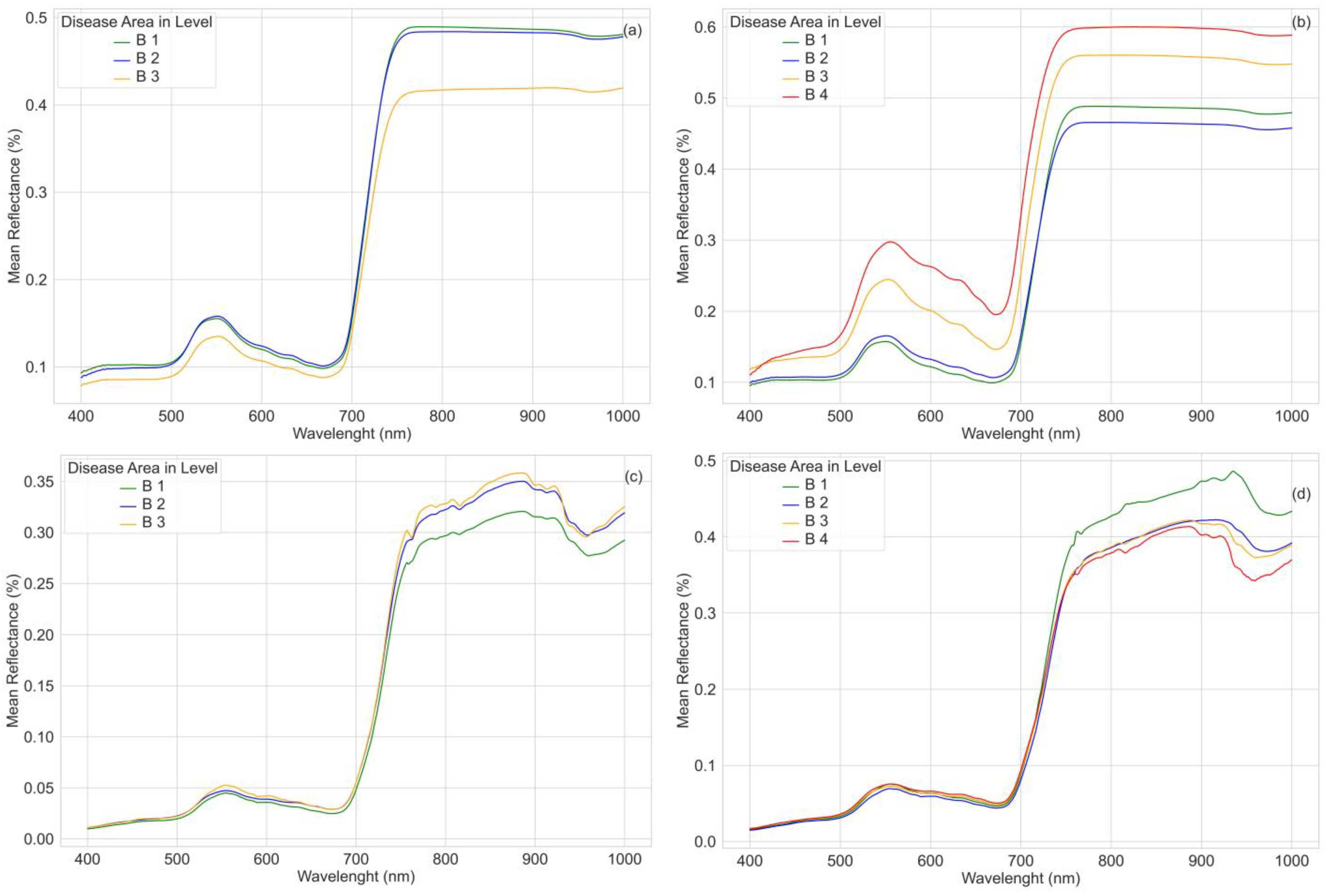
4.2. Spectral Reflectance of Wheat Disease at Different Growth Stages
4.3. Disease Severity Assessment
4.3.1. Leaf Level
4.3.2. Canopy Level
4.4. Limitations and Future Work
4.4.1. Challenges in Disease Data Collection for Hyperspectral Disease Severity Assessment
4.4.2. Chlorophyll Content as a Proxy for Detecting Disease Severity
4.4.3. Phenological Crop Growth Stages on Monitoring Crop Disease
4.4.4. Texture Information, Short-Wave Infrared Regions, and Application to Hyperspectral Airborne or Space-Borne Imagery
4.4.5. Replicability of the Proposed Approach
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Global Durum Wheat Use Trending Upward|World-Grain.Com|11 October 2017 18:22|World Grain. Available online: https://www.world-grain.com/articles/8777-global-durum-wheat-use-trending-upward (accessed on 18 December 2023).
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A Review of Wheat Diseases—A Field Perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Espino, J.; Singh, R.P.; Germán, S.; McCallum, B.D.; Park, R.F.; Chen, W.Q.; Bhardwaj, S.C.; Goyeau, H. Global Status of Wheat Leaf Rust Caused by Puccinia triticina. Euphytica 2011, 179, 143–160. [Google Scholar] [CrossRef]
- Rodeva, R.; Stoyanova, Z.; Nedyalkova, S.; Pastirčák, M.; Hudcovicova, M. Vertical Distribution of Foliar Pathogens on Wheat. Agric. Sci. Technol. 2014, 6, 341–345. [Google Scholar]
- Rodeva, R. Septoria Diseases on Wheat—Cause Agents, Symptoms, Epidemiology, and Disease Control. Field Crops Stud. 2004, 1, 328–335. [Google Scholar]
- Shipton, W.A.; Boyd, W.R.J.; Rosielle, A.A.; Shearer, B.I. The Common Septoria Diseases of Wheat. Bot. Rev. 1971, 37, 231–262. [Google Scholar] [CrossRef]
- King, J.E.; Cook, R.J.; Melville, S.C. A Review of Septoria Diseases of Wheat and Barley. Ann. Appl. Biol. 1983, 103, 345–373. [Google Scholar] [CrossRef]
- Eyal, Z.; Scharen, A.L.; Prescott, J.M.; van Ginkel, M. The Septoria Diseases of Wheat: Concepts and Methods of Disease Management; CIMMYT: Mexico City, Mexico, 1987; ISBN 978-968-6127-06-5. [Google Scholar]
- Cunfer, B.M.; Ueng, P.P. Taxonomy and Identification of Septoria and Stagonospora Species on Small-Grain Cereals. Annu. Rev. Phytopathol. 1999, 37, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Todorova, M. First Report of Tan Spot Caused by Pyrenophora tritici-Repentis (Anamorph Drechslera tritici-Repentis) in Bulgaria. Plant Pathol. 2006, 55, 305. [Google Scholar] [CrossRef]
- Nedyalkova, S.; Stoyanova, Z.; Georgieva, V.; Rodeva, R. Occurrence and Relative Prevalence of Fungal Pathogens on Durum Wheat. Int. J. Innov. Approaches Agric. Res. 2019, 3, 442–454. [Google Scholar] [CrossRef]
- Fernandez, M.R.; Clarke, M.; DePauw, R.M. Comparison of Durum and Common Wheat Cultivars for Reaction to Leaf Spotting Fungi in the Field. Plant Dis. 1996, 80, 793–797. [Google Scholar] [CrossRef]
- Gilbert, J.; Woods, S.M.; Tekauz, A. Relationship between Environmental Variables and the Prevalence and Isolation Frequency of Leaf-Spotting Pathogens in Spring Wheat. Can. J. Plant Pathol. 1998, 20, 158–164. [Google Scholar] [CrossRef]
- Bohnenkamp, D.; Kuska, M.T.; Mahlein, A.-K.; Behmann, J. Hyperspectral Signal Decomposition and Symptom Detection of Wheat Rust Disease at the Leaf Scale Using Pure Fungal Spore Spectra as Reference. Plant Pathol. 2019, 68, 1188–1195. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, N.; Yuan, L.; Chen, F.; Wu, K. Discrimination of Winter Wheat Disease and Insect Stresses Using Continuous Wavelet Features Extracted from Foliar Spectral Measurements. Biosyst. Eng. 2017, 162, 20–29. [Google Scholar] [CrossRef]
- Vélez, S.; Barajas, E.; Rubio, J.A.; Pereira-Obaya, D.; Rodríguez-Pérez, J.R. Field-Deployed Spectroscopy from 350 to 2500 Nm: A Promising Technique for Early Identification of Powdery Mildew Disease (Erysiphe necator) in Vineyards. Agronomy 2024, 14, 634. [Google Scholar] [CrossRef]
- Prechsl, U.E.; Mejia-Aguilar, A.; Cullinan, C.B. In Vivo Spectroscopy and Machine Learning for the Early Detection and Classification of Different Stresses in Apple Trees. Sci. Rep. 2023, 13, 15857. [Google Scholar] [CrossRef]
- Ren, Y.; Huang, W.; Ye, H.; Zhou, X.; Ma, H.; Dong, Y.; Shi, Y.; Geng, Y.; Huang, Y.; Jiao, Q.; et al. Quantitative Identification of Yellow Rust in Winter Wheat with a New Spectral Index: Development and Validation Using Simulated and Experimental Data. Int. J. Appl. Earth Obs. Geoinf. 2021, 102, 102384. [Google Scholar] [CrossRef]
- Koc, A.; Odilbekov, F.; Alamrani, M.; Henriksson, T.; Chawade, A. Predicting Yellow Rust in Wheat Breeding Trials by Proximal Phenotyping and Machine Learning. Plant Methods 2022, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.N.; Krishnan, P.; Singh, V.K.; Sah, S.; Das, B. Combining Biophysical Parameters with Thermal and RGB Indices Using Machine Learning Models for Predicting Yield in Yellow Rust Affected Wheat Crop. Sci. Rep. 2023, 13, 18814. [Google Scholar] [CrossRef]
- Heidarian Dehkordi, R.; El Jarroudi, M.; Kouadio, L.; Meersmans, J.; Beyer, M. Monitoring Wheat Leaf Rust and Stripe Rust in Winter Wheat Using High-Resolution UAV-Based Red-Green-Blue Imagery. Remote Sens. 2020, 12, 3696. [Google Scholar] [CrossRef]
- He, R.; Li, H.; Qiao, X.; Jiang, J. Using Wavelet Analysis of Hyperspectral Remote-Sensing Data to Estimate Canopy Chlorophyll Content of Winter Wheat under Stripe Rust Stress. Int. J. Remote Sens. 2018, 39, 4059–4076. [Google Scholar] [CrossRef]
- Devadas, R.; Lamb, D.W.; Simpfendorfer, S.; Backhouse, D. Evaluating Ten Spectral Vegetation Indices for Identifying Rust Infection in Individual Wheat Leaves. Precis. Agric. 2009, 10, 459–470. [Google Scholar] [CrossRef]
- Wójtowicz, A.; Piekarczyk, J.; Czernecki, B.; Ratajkiewicz, H. A Random Forest Model for the Classification of Wheat and Rye Leaf Rust Symptoms Based on Pure Spectra at Leaf Scale. J. Photochem. Photobiol. B Biol. 2021, 223, 112278. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Huang, W.; Cui, X.; Dong, Y.; Shi, Y.; Ma, H.; Liu, L. Identification of Wheat Yellow Rust Using Optimal Three-Band Spectral Indices in Different Growth Stages. Sensors 2019, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yuan, L.; Luo, J.; Du, S.; Huang, L.; Huang, W. Spectral Differences of Opposite Sides of Stripe Rust Infested Winter Wheat Leaves Using ASD’s Leaf Clip. In Proceedings of the 2012 IEEE International Geoscience and Remote Sensing Symposium, Munich, Germany, 22–27 July 2012; pp. 6585–6588. [Google Scholar]
- Huang, W.; Lamb, D.W.; Niu, Z.; Zhang, Y.; Liu, L.; Wang, J. Identification of Yellow Rust in Wheat Using In-Situ Spectral Reflectance Measurements and Airborne Hyperspectral Imaging. Precis. Agric. 2007, 8, 187–197. [Google Scholar] [CrossRef]
- Khan, I.H.; Liu, H.; Li, W.; Cao, A.; Wang, X.; Liu, H.; Cheng, T.; Tian, Y.; Zhu, Y.; Cao, W.; et al. Early Detection of Powdery Mildew Disease and Accurate Quantification of Its Severity Using Hyperspectral Images in Wheat. Remote Sens. 2021, 13, 3612. [Google Scholar] [CrossRef]
- Wang, H.; Qin, F.; Ruan, L.; Wang, R.; Liu, Q.; Ma, Z.; Li, X.; Cheng, P.; Wang, H. Identification and Severity Determination of Wheat Stripe Rust and Wheat Leaf Rust Based on Hyperspectral Data Acquired Using a Black-Paper-Based Measuring Method. PLoS ONE 2016, 11, e0154648. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Huang, Y.; Loraamm, R.W.; Nie, C.; Wang, J.; Zhang, J. Spectral Analysis of Winter Wheat Leaves for Detection and Differentiation of Diseases and Insects. Field Crops Res. 2014, 156, 199–207. [Google Scholar] [CrossRef]
- Ashourloo, D.; Mobasheri, M.R.; Huete, A. Developing Two Spectral Disease Indices for Detection of Wheat Leaf Rust (Pucciniatriticina). Remote Sens. 2014, 6, 4723–4740. [Google Scholar] [CrossRef]
- Huang, W.; Guan, Q.; Luo, J.; Zhang, J.; Zhao, J.; Liang, D.; Huang, L.; Zhang, D. New Optimized Spectral Indices for Identifying and Monitoring Winter Wheat Diseases. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 2516–2524. [Google Scholar] [CrossRef]
- Bravo, C.; Moshou, D.; West, J.; McCartney, A.; Ramon, H. Early Disease Detection in Wheat Fields Using Spectral Reflectance. Biosyst. Eng. 2003, 84, 137–145. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Z.; Xu, Z.; Li, J. Wheat Yellow Rust Severity Detection by Efficient DF-UNet and UAV Multispectral Imagery. IEEE Sens. J. 2022, 22, 9057–9068. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Y.; Huang, W.; Dong, Y.; Ma, H.; Wu, K.; Guo, A. Combining Random Forest and XGBoost Methods in Detecting Early and Mid-Term Winter Wheat Stripe Rust Using Canopy Level Hyperspectral Measurements. Agriculture 2022, 12, 74. [Google Scholar] [CrossRef]
- Devadas, R.; Lamb, D.W.; Backhouse, D.; Simpfendorfer, S. Sequential Application of Hyperspectral Indices for Delineation of Stripe Rust Infection and Nitrogen Deficiency in Wheat. Precis. Agric. 2015, 16, 477–491. [Google Scholar] [CrossRef]
- Ashourloo, D.; Aghighi, H.; Matkan, A.A.; Mobasheri, M.R.; Rad, A.M. An Investigation into Machine Learning Regression Techniques for the Leaf Rust Disease Detection Using Hyperspectral Measurement. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2016, 9, 4344–4351. [Google Scholar] [CrossRef]
- Zhang, J.-C.; Yuan, L.; Wang, J.; Huang, W.; Chen, L.; Zhang, D. Spectroscopic Leaf Level Detection of Powdery Mildew for Winter Wheat Using Continuous Wavelet Analysis. J. Integr. Agric. 2012, 11, 1474–1484. [Google Scholar] [CrossRef]
- Huang, W.; Lu, J.; Ye, H.; Kong, W.; Hugh Mortimer, A.; Shi, Y. Quantitative Identification of Crop Disease and Nitrogen-Water Stress in Winter Wheat Using Continuous Wavelet Analysis. Int. J. Agric. Biol. Eng. 2018, 11, 145–152. [Google Scholar] [CrossRef]
- Ma, H.; Huang, W.; Dong, Y.; Liu, L.; Guo, A. Using UAV-Based Hyperspectral Imagery to Detect Winter Wheat Fusarium Head Blight. Remote Sens. 2021, 13, 3024. [Google Scholar] [CrossRef]
- Roy, S.; Ray, R.; Dash, S.R.; Giri, M.K. Plant Disease Detection Using Machine Learning Tools with an Overview on Dimensionality Reduction. In Data Analytics in Bioinformatics; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 109–144. ISBN 978-1-119-78562-0. [Google Scholar]
- Lapajne, J.; Knapič, M.; Žibrat, U. Comparison of Selected Dimensionality Reduction Methods for Detection of Root-Knot Nematode Infestations in Potato Tubers Using Hyperspectral Imaging. Sensors 2022, 22, 367. [Google Scholar] [CrossRef]
- Verrelst, J.; Rivera, J.P.; Gitelson, A.; Delegido, J.; Moreno, J.; Camps-Valls, G. Spectral Band Selection for Vegetation Properties Retrieval Using Gaussian Processes Regression. Int. J. Appl. Earth Obs. Geoinf. 2016, 52, 554–567. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. Update 2015; FAO, Ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Meier, U. Growth Stages of Mono and Dicotyledonous Plants. BBCH Monograph, 2nd ed.; Federal Biological Research Centre for Agriculture and Forestry: Bonn, Germany, 2001. [Google Scholar]
- CCM-300. Available online: https://www.optisci.com/ccm-300.html (accessed on 1 May 2024).
- Lichtenthaler, H.K. [34] Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Plant Cell Membranes; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- ASD FieldSpec 4—High Resolution Spectroradiometer|Malvern Panalytical. Available online: https://www.malvernpanalytical.com/en/products/product-range/asd-range/fieldspec-range/fieldspec4-hi-res-high-resolution-spectroradiometer (accessed on 1 May 2024).
- Spectralon® Diffuse Reflectance Targets—Durable Reflectance Panels. Available online: https://www.labsphere.com/product/spectralon-reflectance-targets/ (accessed on 27 March 2024).
- R: Splice Correction of a Spectral Matrix Acquired with an ASD. Available online: https://search.r-project.org/CRAN/refmans/prospectr/html/spliceCorrection.html (accessed on 1 May 2024).
- Elmer, K.; Soffer, R.J.; Arroyo-Mora, J.P.; Kalacska, M. ASDToolkit: A Novel MATLAB Processing Toolbox for ASD Field Spectroscopy Data. Data 2020, 5, 96. [Google Scholar] [CrossRef]
- Beal, D.; Eamon, M. Dynamic, Parabolic Linear Transformations of ‘Stepped’Radiometric Data; Analytical Spectral Devices Inc.: Boulder, CO, USA, 1996. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Levene, H. Robust Tests for Equality of Variances. In Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling; Standford Univerdity Press: Redwood City, CA, USA, 1960; pp. 278–292. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- McKight, P.E.; Najab, J. Kruskal-Wallis Test. In The Corsini Encyclopedia of Psychology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; p. 1. ISBN 978-0-470-47921-6. [Google Scholar]
- Ostertagová, E.; Ostertag, O.; Kováč, J. Methodology and Application of the Kruskal-Wallis Test. Appl. Mech. Mater. 2014, 611, 115–120. [Google Scholar] [CrossRef]
- Gamon, J.A.; Peñuelas, J.; Field, C.B. A Narrow-Waveband Spectral Index That Tracks Diurnal Changes in Photosynthetic Efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Penuelas, J.; Baret, F.; Filella, I. Semi-Empirical Indices to Assess Carotenoids/Chlorophyll a Ratio from Leaf Spectral Reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical Properties and Nondestructive Estimation of Anthocyanin Content in Plant Leaves. Photochem. Photobiol 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing Carotenoid Content in Plant Leaves with Reflectance Spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar] [CrossRef]
- Odilbekov, F.; Armoniené, R.; Henriksson, T.; Chawade, A. Proximal Phenotyping and Machine Learning Methods to Identify Septoria tritici Blotch Disease Symptoms in Wheat. Front. Plant Sci. 2018, 9, 685. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Berjón, A.; López-Lozano, R.; Miller, J.R.; Martín, P.; Cachorro, V.; González, M.R.; de Frutos, A. Assessing Vineyard Condition with Hyperspectral Indices: Leaf and Canopy Reflectance Simulation in a Row-Structured Discontinuous Canopy. Remote Sens. Environ. 2005, 99, 271–287. [Google Scholar] [CrossRef]
- Geladi, P.; Kowalski, B.R. Partial Least-Squares Regression: A Tutorial. Anal. Chim. Acta 1986, 185, 1–17. [Google Scholar] [CrossRef]
- Suykens, J.A.K.; Vandewalle, J. Least Squares Support Vector Machine Classifiers. Neural Process. Lett. 1999, 9, 293–300. [Google Scholar] [CrossRef]
- Rivera, J.P.; Verrelst, J.; Delegido, J.; Veroustraete, F.; Moreno, J. On the Semi-Automatic Retrieval of Biophysical Parameters Based on Spectral Index Optimization. Remote Sens. 2014, 6, 4927–4951. [Google Scholar] [CrossRef]
- Verrelst, J.; Rivera, J.P.; Veroustraete, F.; Muñoz-Marí, J.; Clevers, J.G.P.W.; Camps-Valls, G.; Moreno, J. Experimental Sentinel-2 LAI Estimation Using Parametric, Non-Parametric and Physical Retrieval Methods—A Comparison. ISPRS J. Photogramm. Remote Sens. 2015, 108, 260–272. [Google Scholar] [CrossRef]
- Spitters, C.J.T.; Van Roermund, H.J.W.; Van Nassau, H.G.M.G.; Schepers, J.; Mesdag, J. Genetic Variation in Partial Resistance to Leaf Rust in Winter Wheat: Disease Progress, Foliage Senescence and Yield Reduction. Neth. J. Plant Pathol. 1990, 96, 3–15. [Google Scholar] [CrossRef]
- Shtienberg, D. Effects of Foliar Diseases on Gas Exchange Processes: A Comparative Study. Phytopathology 1992, 82, 760. [Google Scholar] [CrossRef]
- Carretero, R.; Bancal, M.O.; Miralles, D.J. Effect of Leaf Rust (Puccinia triticina) on Photosynthesis and Related Processes of Leaves in Wheat Crops Grown at Two Contrasting Sites and with Different Nitrogen Levels. Eur. J. Agron. 2011, 35, 237–246. [Google Scholar] [CrossRef]
- Yahya, M.; Saeed, N.A.; Nadeem, S.; Hamed, M.; Saleem, K. Effect of Leaf Rust Disease on Photosynthetic Rate, Chlorophyll Contents and Grain Yield of Wheat. Arch. Phytopathol. Plant Prot. 2020, 53, 425–439. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, T.; Wen, X.; Zeng, M.; Chen, J. Detecting the Minimum Limit on Wheat Stripe Rust in the Latent Period Using Proximal Remote Sensing Coupled with Duplex Real-Time PCR and Machine Learning. Plants 2023, 12, 2814. [Google Scholar] [CrossRef] [PubMed]
- Blasch, G.; Anberbir, T.; Negash, T.; Tilahun, L.; Belayineh, F.Y.; Alemayehu, Y.; Mamo, G.; Hodson, D.P.; Rodrigues, F.A. The Potential of UAV and Very High-Resolution Satellite Imagery for Yellow and Stem Rust Detection and Phenotyping in Ethiopia. Sci. Rep. 2023, 13, 16768. [Google Scholar] [CrossRef]
- Franke, J.; Menz, G.; Oerke, E.-C.; Rascher, U. Comparison of Multi- and Hyperspectral Imaging Data of Leaf Rust Infected Wheat Plants. In Proceedings of the Remote Sensing for Agriculture, Ecosystems, and Hydrology VII, Bruges, Belgium, 19 October 2005; Volume 5976, pp. 349–359. [Google Scholar]
- European and Mediterranean Plant Protection Organization. Efficacy Evaluation of Fungicides: Uncinula Necator. EPPO Bulletin 2002, 32, 315–318. [Google Scholar] [CrossRef]
- Sankaran, S.; Mishra, A.; Ehsani, R.; Davis, C. A Review of Advanced Techniques for Detecting Plant Diseases. Comput. Electron. Agric. 2010, 72, 1–13. [Google Scholar] [CrossRef]



| Name | Formula | The Author Who First Introduced the SVI | Selected Studies Utilizing the Index for Wheat Disease at the Leaf and Canopy Level |
|---|---|---|---|
| Photochemical Reflectance Index (PRI) | [58] | Canopy: [18,19,25,27,35,36,58] Leaf: [18,23,32] | |
| Structure insensitive pigment index (SIPI) | [59] | Canopy: [18,35] Leaf: [18,23,32,59] | |
| Yellow rust index (YRI) | [32] | Canopy: [32,35] | |
| Anthocyanin Reflectance Index (ARI) | [60] | Canopy: [18,22,25,35,36] Leaf: [18,23,32,60] | |
| Carotenoid Reflectance Index 550 (CRI550) | [61] | Leaf: [24,61] | |
| Leaf rust disease severity index 1 (LRDSI_1) | [31] | Canopy: [35] Leaf: [31,62] | |
| Leaf Rust Disease Severity Index 2 (LRDSI_2) | [31] | Leaf: [31,62] | |
| Yellow Rust Optimal Index (YROI) | [18] | Leaf and Canopy: [18] | |
| Red-Green Pigment Index (RGI) | [63] | Canopy: [19] Leaf: [63] |
| Total Numbers of Samples/Numbers of Samples in Cross-Validation/Number of Samples in Test/K-Fold | |||
|---|---|---|---|
| BBCH75 & BBCH77 | BBCH75 | BBCH77 | |
| Canopy level DA | 194/129/65/10-fold | 129/86/43/6-fold | 65/43/22/3-fold |
| Canopy level CC | 96/64/32/5-fold | 65/43/22/3-fold | 31/21/10/2-fold |
| Leaf Level DA & CC | 208/138/70/10-fold | 140/93/47/7-fold | 68/46/22/3-fold |
| Group | Early Group Leaf Level 1st Repetition | Late Group Leaf Level 1st Repetition | Samples at Leaf Level | Early Group Canopy Level 1st and 2nd Repetitions | Late Group Canopy Level 1st and 2nd Repetitions | Samples at Canopy Level |
|---|---|---|---|---|---|---|
| BBCH | 75 | 77 | 75 | 77 | ||
| Number of samples | 140 | 68 | 208 | 70 | 34 | 104 |
| B1 | 92 | 40 | 132 | 19 | 8 | 27 |
| B2 | 44 | 24 | 68 | 45 | 18 | 63 |
| B3 & B4 | 4 | 4 | 8 | 6 | 8 | 14 |
| Average CC (range) | 441.36 (243–528) | 423.49 (167–528) | 523.79 (428–623.5) | 501.79 (412.5–601) | ||
| Average DA (range) | 19 (5–55) | 22 (5–80) | 27 (15–50) | 33 (15–65) | ||
| CC-1 1 | 444.39 (344–528) | 434.7 (319–528) | 526.64 (460–577.25) | 489.47 (430–562.75) | ||
| CC-2 2 | 434.93 (243–509) | 423.54 (275–515) | 528.6 (429.75–623.5) | 515.29 (412.5–601) | ||
| CC-3_4 3 | 442.5 (401–490) | 302.0 (167–433) | 478.71 (428.25–567.5) | 468.4 (434.5–577.5) | ||
| % Reduction in CC-3_4 over CC-1 | 0.43 | 30.53 | 9.10 | 4.31 | ||
| % Reduction in CC-3_4 over CC-2 | −1.74 | 28.70 | 9.44 | 9.10 |
| Leaf Level | Canopy Level | |||||
|---|---|---|---|---|---|---|
| All Data | Early Group | Late Group | All Data | Early Group | Late Group | |
| Kruskal–Wallis Statistic | 4.208 | 0.929 | 0.066 | 8.167 | 5.426 | 2.165 |
| Kruskal–Wallis p-Value | 0.122 | 0.629 | 0.049 | 0.017 | 0.066 | 0.339 |
| Conover-Iman p-value between B1/B2 | 0.824 | 0.419 | ||||
| Conover-Iman p-value between B1/B3_4 | 0.043 | 0.081 | ||||
| Conover-Iman p-value between B2/B3_4 | 0.045 | 0.012 | ||||
| Parameter | SVI | Leaf Level, 1st Repetition | Canopy Level, 1st and 2nd Repetitions | ||||
|---|---|---|---|---|---|---|---|
| All Data | Early Group | Late Group | All Data | Early Group | Late Group | ||
| CC | PRI | −0.45 * | −0.26 * | −0.59 * | −0.26 * | ns | ns |
| SIPI | −0.33 * | −0.19 * | −0.57 * | 0.2 * | 0.28 * | ns | |
| YRI | −0.27 * | ns | −0.49 * | −0.23 * | ns | ns | |
| ARI | ns | ns | ns | 0.42 * | 0.42 * | 0.45 * | |
| CRI1 | ns | ns | ns | ns | −0.28 * | ns | |
| LRDSI_1 | −0.54 * | −0.34 * | −0.72 * | −0.21 * | ns | ns | |
| LRDSI_2 | −0.49 * | −0.29 * | −0.68 * | 0.2 * | 0.26 * | 0.38 * | |
| YROI | −0.52 * | −0.31 * | −0.73 * | ns | 0.24 * | 0.4 * | |
| RGI | ns | ns | ns | 0.43 * | 0.51 * | 0.5 * | |
| DA | PRI | 0.46 * | 0.33 * | 0.51 * | ns | ns | ns |
| SIPI | 0.33 * | 0.25 * | 0.49 * | ns | ns | ns | |
| YRI | ns | ns | ns | ns | ns | ns | |
| ARI | 0.26 * | 0.26 * | 0.31 * | ns | ns | ns | |
| CRI1 | ns | ns | ns | ns | ns | ns | |
| LRDSI_1 | 0.39 * | 0.30 * | 0.48 * | ns | ns | ns | |
| LRDSI_2 | 0.38 * | 0.31 * | 0.46 * | ns | ns | ns | |
| YROI | 0.39 * | 0.29 * | 0.5 * | ns | ns | ns | |
| RGI | 0.19 * | 0.17 * | 0.24 | ns | ns | ns | |
| Level/ Phenophase | Model/ Bands | Parameter | Cross-Validation | Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | nRMSE (%) | rRMSE (%) | NSE | R2 | RMSE | nRMSE (%) | rRMSE (%) | NSE | |||
| Leaf/ Late group | YROI *, Linear/611, 452, 550 | Leaf CC | 0.40 | 55.83 | 15.46 | 13.36 | 0.35 | 0.44 | 37.18 | 23.53 | 8.54 | 0.29 |
| Leaf/ Late group | LRDSI_1 *, Polynomial/605, 455 | Leaf CC | 0.36 | 60.78 | 16.84 | 14.55 | 0.23 | 0.45 | 36.22 | 22.92 | 8.32 | 0.33 |
| Level/ Phenophase | Model/ Bands | Parameter | Cross-Validation | Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | nRMSE (%) | rRMSE (%) | NSE | R2 | RMSE | nRMSE (%) | rRMSE (%) | NSE | |||
| Leaf/ Late group | SIPI, Linear /800, 690, 700 | Leaf CC | 0.63 | 42.20 | 11.63 | 10.10 | 0.63 | 0.42 | 38.32 | 24.25 | 8.80 | 0.25 |
| Canopy/Late group | SIPI, Polynomial/445, 550, 531 | Canopy CC | 0.61 | 34.50 | 18.30 | 6.84 | 0.60 | 0.37 | 53.27 | 31.71 | 10.45 | 0.25 |
| Canopy/Early group | SIPI, Polynomial /570, 445, 455 | Canopy CC | 0.48 | 31.59 | 19.53 | 6.04 | 0.48 | 0.50 | 42.77 | 21.90 | 8.04 | 0.47 |
| Phenophase | Model/ Number of Bands | Parameter | Cross-Validation | Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | nRMSE (%) | rRMSE (%) | NSE | R2 | RMSE | nRMSE (%) | rRMSE (%) | NSE | |||
| Leaf/ Late group | KRR/6 1 | Leaf CC | 0.54 | 48.25 | 13.37 | 11.55 | 0.51 | 0.33 | 51.49 | 32.59 | 11.83 | −0.35 |
| Canopy/Late group | KRR/30 2 | Canopy DA | 0.51 | 9.36 | 18.73 | 28.86 | 0.50 | 0.36 | 12.47 | 24.94 | 34.72 | 0.36 |
| Canopy/ Early group | KRR/28 3 | Canopy DA | 0.53 | 7.17 | 20.48 | 25.58 | 0.50 | 0.35 | 8.65 | 24.72 | 32.78 | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganeva, D.; Filchev, L.; Roumenina, E.; Dragov, R.; Nedyalkova, S.; Bozhanova, V. Winter Durum Wheat Disease Severity Detection with Field Spectroscopy in Phenotyping Experiment at Leaf and Canopy Level. Remote Sens. 2024, 16, 1762. https://doi.org/10.3390/rs16101762
Ganeva D, Filchev L, Roumenina E, Dragov R, Nedyalkova S, Bozhanova V. Winter Durum Wheat Disease Severity Detection with Field Spectroscopy in Phenotyping Experiment at Leaf and Canopy Level. Remote Sensing. 2024; 16(10):1762. https://doi.org/10.3390/rs16101762
Chicago/Turabian StyleGaneva, Dessislava, Lachezar Filchev, Eugenia Roumenina, Rangel Dragov, Spasimira Nedyalkova, and Violeta Bozhanova. 2024. "Winter Durum Wheat Disease Severity Detection with Field Spectroscopy in Phenotyping Experiment at Leaf and Canopy Level" Remote Sensing 16, no. 10: 1762. https://doi.org/10.3390/rs16101762
APA StyleGaneva, D., Filchev, L., Roumenina, E., Dragov, R., Nedyalkova, S., & Bozhanova, V. (2024). Winter Durum Wheat Disease Severity Detection with Field Spectroscopy in Phenotyping Experiment at Leaf and Canopy Level. Remote Sensing, 16(10), 1762. https://doi.org/10.3390/rs16101762








