Potential of Satellite Spectral Resolution Vegetation Indices for Estimation of Canopy Chlorophyll Content of Field Crops: Mitigating Effects of Leaf Angle Distribution
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Field Measurements
2.2. Validation Datasets from the PROSAIL Model Simulation
2.3. Satellite Broadband Reflectance Simulations
2.4. Tested Vegetation Indices
2.5. Statistical Analysis
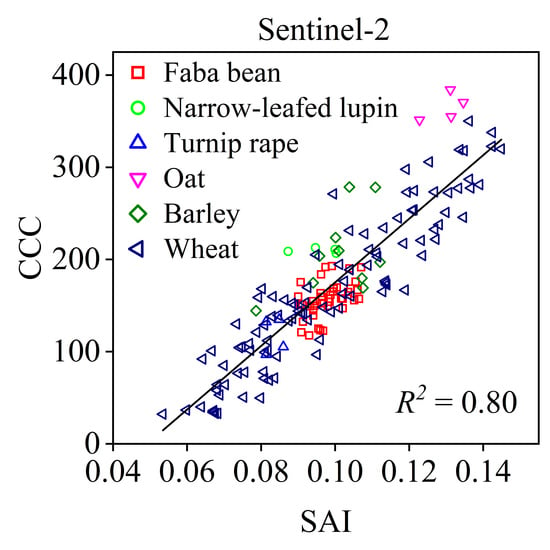
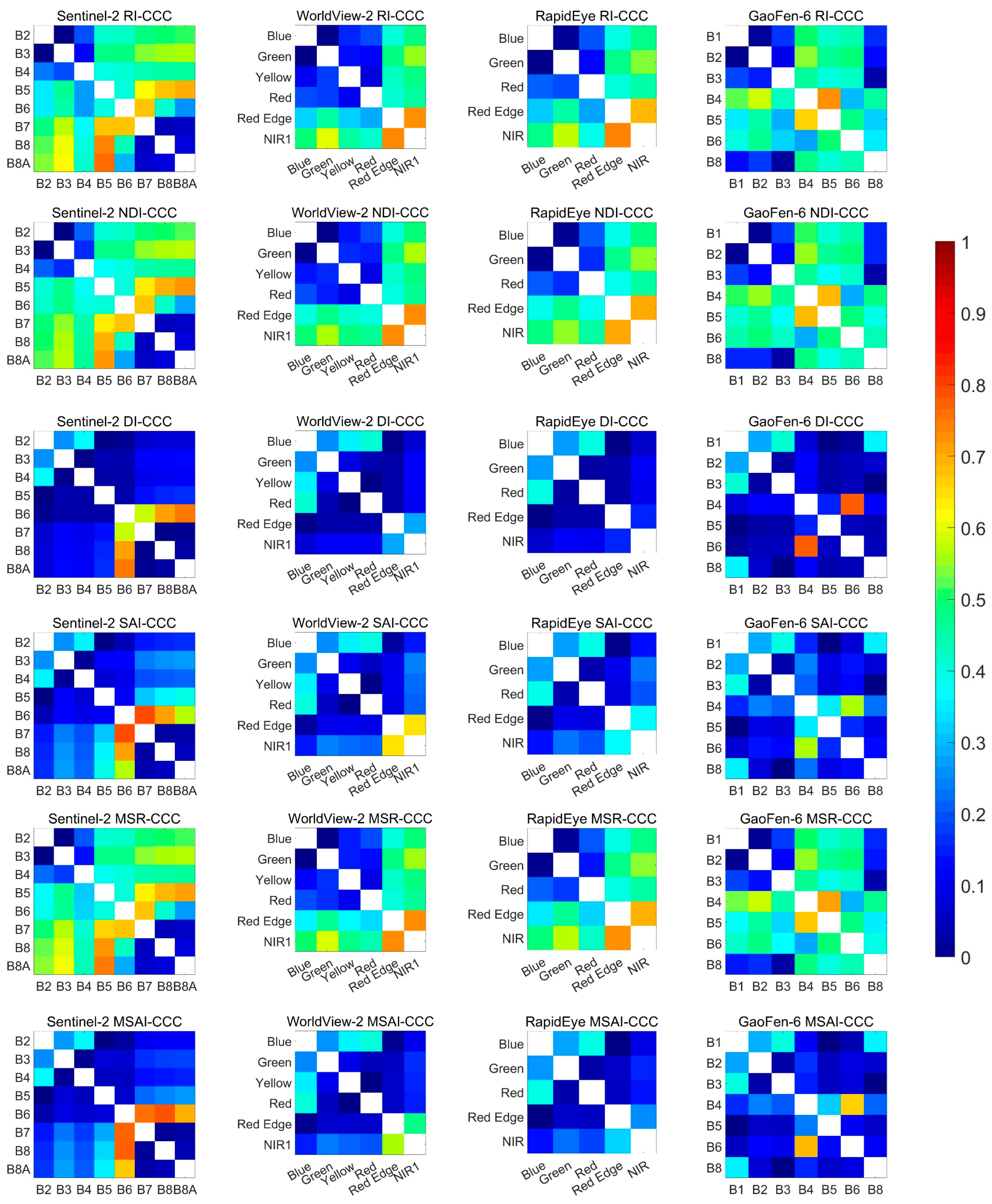
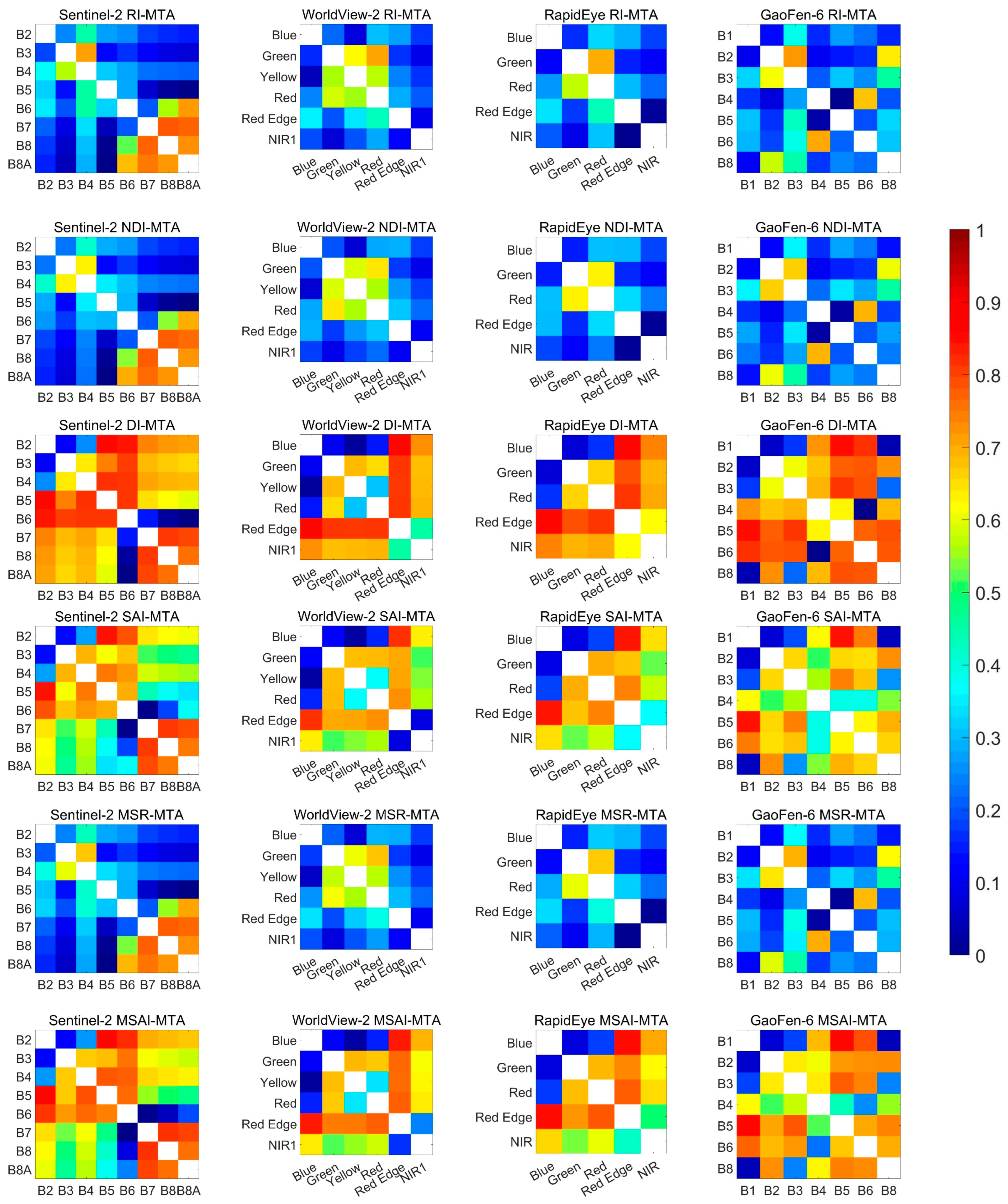
3. Results
3.1. Responses of Satellite Broadband Reflectance to MTA
3.2. Performance of Existing Vegetation Indices
3.3. Identification of New Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sensor | Central Wavelength (nm) | Band/Band Number | Bandwidth (nm) | Spatial Resolution (m) | Measurements | Model | ||
|---|---|---|---|---|---|---|---|---|
| Sentinel-2 | 490 | 2 | 65 | 10 | 0.58 | 0.00 | 0.39 | 0.25 |
| 560 | 3 | 50 | 10 | 0.44 | 0.05 | 0.42 | 0.08 | |
| 665 | 4 | 30 | 10 | 0.53 | 0.08 | 0.54 | 0.07 | |
| 705 | 5 | 15 | 20 | 0.07 | 0.77 | 0.43 | 0.10 | |
| 740 | 6 | 15 | 20 | 0.00 | 0.87 | 0.00 | 0.45 | |
| 783 | 7 | 20 | 20 | 0.04 | 0.78 | 0.27 | 0.39 | |
| 842 | 8 | 115 | 10 | 0.04 | 0.77 | 0.26 | 0.39 | |
| 865 | 8A | 20 | 20 | 0.04 | 0.76 | 0.26 | 0.40 | |
| Worldview-2 | 478 | Blue | 60 | 1.8 | 0.60 | 0.00 | 0.29 | 0.45 |
| 546 | Green | 70 | 1.8 | 0.45 | 0.05 | 0.41 | 0.08 | |
| 608 | Yellow | 40 | 1.8 | 0.49 | 0.01 | 0.51 | 0.05 | |
| 659 | Red | 60 | 1.8 | 0.54 | 0.05 | 0.57 | 0.06 | |
| 724 | Red Edge | 40 | 1.8 | 0.00 | 0.87 | 0.10 | 0.33 | |
| 831 | NIR1 | 125 | 1.8 | 0.04 | 0.77 | 0.26 | 0.39 | |
| RapidEye | 475 | Blue | 70 | 5 | 0.60 | 0.00 | 0.29 | 0.47 |
| 555 | Green | 70 | 5 | 0.45 | 0.04 | 0.42 | 0.08 | |
| 657.5 | Red | 55 | 5 | 0.53 | 0.07 | 0.57 | 0.07 | |
| 710 | Red Edge | 40 | 5 | 0.03 | 0.83 | 0.31 | 0.19 | |
| 805 | NIR | 90 | 5 | 0.04 | 0.78 | 0.26 | 0.39 | |
| GaoFen-6 | 485 | 1 | 70 | 16 | 0.58 | 0.01 | 0.39 | 0.26 |
| 555 | 2 | 70 | 16 | 0.46 | 0.03 | 0.42 | 0.08 | |
| 660 | 3 | 60 | 16 | 0.55 | 0.05 | 0.57 | 0.06 | |
| 830 | 4 | 120 | 16 | 0.04 | 0.77 | 0.26 | 0.39 | |
| 710 | 5 | 40 | 16 | 0.08 | 0.76 | 0.39 | 0.15 | |
| 750 | 6 | 40 | 16 | 0.01 | 0.85 | 0.08 | 0.44 | |
| 610 | 8 | 40 | 16 | 0.49 | 0.01 | 0.51 | 0.05 | |

References
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An Evaluation of Noninvasive Methods to Estimate Foliar Chlorophyll Content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Ustin, S.L.; Gitelson, A.A.; Jacquemoud, S.; Schaepman, M.; Asner, G.P.; Gamon, J.A.; Zarco-Tejada, P. Retrieval of Foliar Information about Plant Pigment Systems from High Resolution Spectroscopy. Remote Sens. Environ. 2009, 113, S67–S77. [Google Scholar] [CrossRef]
- Gitelson, A.A. Remote Estimation of Canopy Chlorophyll Content in Crops. Geophys. Res. Lett. 2005, 32, L08403. [Google Scholar] [CrossRef]
- Bacour, C.; Baret, F.; Béal, D.; Weiss, M.; Pavageau, K. Neural Network Estimation of LAI, FAPAR, FCover and LAI×Cab, from Top of Canopy MERIS Reflectance Data: Principles and Validation. Remote Sens. Environ. 2006, 105, 313–325. [Google Scholar] [CrossRef]
- Wu, C.; Niu, Z.; Gao, S. The Potential of the Satellite Derived Green Chlorophyll Index for Estimating Midday Light Use Efficiency in Maize, Coniferous Forest and Grassland. Ecol. Indic. 2012, 14, 66–73. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll Fluorescence Analysis: A Guide to Good Practice and Understanding Some New Applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Croft, H.; Chen, J.M.; Bartlett, P.; Staebler, R.; Froelich, N. Incorporating Leaf Chlorophyll Content into a Two-Leaf Terrestrial Biosphere Model for Estimating Carbon and Water Fluxes at a Forest Site. Agric. For. Meteorol. 2018, 248, 156–168. [Google Scholar] [CrossRef]
- Baret, F.; Houlès, V.; Guérif, M. Quantification of Plant Stress Using Remote Sensing Observations and Crop Models: The Case of Nitrogen Management. J. Exp. Bot. 2007, 58, 869–880. [Google Scholar] [CrossRef]
- Wu, C.; Wang, L.; Niu, Z.; Gao, S.; Wu, M. Nondestructive Estimation of Canopy Chlorophyll Content Using Hyperion and Landsat/TM Images. Int. J. Remote Sens. 2010, 31, 2159–2167. [Google Scholar] [CrossRef]
- Watson, D.J. Comparative Physiological Studies on the Growth of Field Crops: I. Variation in Net Assimilation Rate and Leaf Area between Species and Varieties, and within and between Years. Ann. Bot. 1947, 11, 41–76. [Google Scholar] [CrossRef]
- Inoue, Y.; Guérif, M.; Baret, F.; Skidmore, A.; Gitelson, A.; Schlerf, M.; Darvishzadeh, R.; Olioso, A. Simple and Robust Methods for Remote Sensing of Canopy Chlorophyll Content: A Comparative Analysis of Hyperspectral Data for Different Types of Vegetation. Plant Cell Environ. 2016, 39, 2609–2623. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, Z.; Wang, J.; Huang, W.; Guo, T. Early Detection of Canopy Nitrogen Deficiency in Winter Wheat (Triticum aestivum L.) Based on Hyperspectral Measurement of Canopy Chlorophyll Status. N. Z. J. Crop Hortic. Sci. 2011, 39, 251–262. [Google Scholar] [CrossRef]
- Korus, A. Effect of Preliminary and Technological Treatments on the Content of Chlorophylls and Carotenoids in Kale (Brassica oleracea L. Var. Acephala). J. Food Process. Preserv. 2013, 37, 335–344. [Google Scholar] [CrossRef]
- Schlemmer, M.; Gitelson, A.; Schepers, J.; Ferguson, R.; Peng, Y.; Shanahan, J.; Rundquist, D. Remote Estimation of Nitrogen and Chlorophyll Contents in Maize at Leaf and Canopy Levels. Int. J. Appl. Earth Obs. Geoinf. 2013, 25, 47–54. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Verma, S.B.; Rundquist, D.C.; Arkebauer, T.J.; Keydan, G.; Leavitt, B.; Ciganda, V.; Burba, G.G.; Suyker, A.E. Relationship between Gross Primary Production and Chlorophyll Content in Crops: Implications for the Synoptic Monitoring of Vegetation Productivity. J. Geophys. Res. Atmos. 2006, 111, D08S11. [Google Scholar] [CrossRef]
- Peng, Y.; Gitelson, A.A.; Keydan, G.; Rundquist, D.C.; Moses, W. Remote Estimation of Gross Primary Production in Maize and Support for a New Paradigm Based on Total Crop Chlorophyll Content. Remote Sens. Environ. 2011, 115, 978–989. [Google Scholar] [CrossRef]
- Prey, L.; Hu, Y.; Schmidhalter, U. High-Throughput Field Phenotyping Traits of Grain Yield Formation and Nitrogen Use Efficiency: Optimizing the Selection of Vegetation Indices and Growth Stages. Front. Plant Sci. 2020, 10, 1672. [Google Scholar] [CrossRef]
- Dian, Y.; Le, Y.; Fang, S.; Xu, Y.; Yao, C.; Liu, G. Influence of Spectral Bandwidth and Position on Chlorophyll Content Retrieval at Leaf and Canopy Levels. J. Indian Soc. Remote Sens. 2016, 44, 583–593. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Liu, M.; Wang, C.; Xia, X. A Hyperspectral Index Sensitive to Subtle Changes in the Canopy Chlorophyll Content under Arsenic Stress. Int. J. Appl. Earth Obs. Geoinf. 2015, 36, 41–53. [Google Scholar] [CrossRef]
- Darvishzadeh, R.; Matkan, A.A.; Dashti Ahangar, A. Inversion of a Radiative Transfer Model for Estimation of Rice Canopy Chlorophyll Content Using a Lookup-Table Approach. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2012, 5, 1222–1230. [Google Scholar] [CrossRef]
- Ali, A.M.; Darvishzadeh, R.; Skidmore, A.; Heurich, M.; Paganini, M.; Heiden, U.; Mücher, S. Evaluating Prediction Models for Mapping Canopy Chlorophyll Content Across Biomes. Remote Sens. 2020, 12, 1788. [Google Scholar] [CrossRef]
- Dorigo, W.A.; Zurita-Milla, R.; de Wit, A.J.W.; Brazile, J.; Singh, R.; Schaepman, M.E. A Review on Reflective Remote Sensing and Data Assimilation Techniques for Enhanced Agroecosystem Modeling. Int. J. Appl. Earth Obs. Geoinf. 2007, 9, 165–193. [Google Scholar] [CrossRef]
- Moulin, S. Impacts of Model Parameter Uncertainties on Crop Reflectance Estimates: A Regional Case Study on Wheat. Int. J. Remote Sens. 1999, 20, 213–218. [Google Scholar] [CrossRef]
- Taddeo, S.; Dronova, I.; Depsky, N. Spectral Vegetation Indices of Wetland Greenness: Responses to Vegetation Structure, Composition, and Spatial Distribution. Remote Sens. Environ. 2019, 234, 111467. [Google Scholar] [CrossRef]
- Mao, Z.H.; Deng, L.; Duan, F.Z.; Li, X.J.; Qiao, D.Y. Angle Effects of Vegetation Indices and the Influence on Prediction of SPAD Values in Soybean and Maize. Int. J. Appl. Earth Obs. Geoinf. 2020, 93, 102198. [Google Scholar] [CrossRef]
- Sun, Q.; Jiao, Q.; Qian, X.; Liu, L.; Liu, X.; Dai, H. Improving the Retrieval of Crop Canopy Chlorophyll Content Using Vegetation Index Combinations. Remote Sens. 2021, 13, 470. [Google Scholar] [CrossRef]
- Dash, J.; Curran, P.J. The MERIS Terrestrial Chlorophyll Index. Int. J. Remote Sens. 2004, 25, 5403–5413. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Quantitative Estimation of Chlorophyll-a Using Reflectance Spectra: Experiments with Autumn Chestnut and Maple Leaves. J. Photochem. Photobiol. B 1994, 22, 247–252. [Google Scholar] [CrossRef]
- Clevers, J.G.P.W.; Kooistra, L. Using Hyperspectral Remote Sensing Data for Retrieving Canopy Chlorophyll and Nitrogen Content. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2012, 5, 574–583. [Google Scholar] [CrossRef]
- He, R.; Li, H.; Qiao, X.; Jiang, J. Using Wavelet Analysis of Hyperspectral Remote-Sensing Data to Estimate Canopy Chlorophyll Content of Winter Wheat under Stripe Rust Stress. Int. J. Remote Sens. 2018, 39, 4059–4076. [Google Scholar] [CrossRef]
- Li, L.; Ren, T.; Ma, Y.; Wei, Q.; Wang, S.; Li, X.; Cong, R.; Liu, S.; Lu, J. Evaluating Chlorophyll Density in Winter Oilseed Rape (Brassica napus L.) Using Canopy Hyperspectral Red-Edge Parameters. Comput. Electron. Agric. 2016, 126, 21–31. [Google Scholar] [CrossRef]
- Okuda, K.; Taniguchi, K.; Miura, M.; Obata, K.; Yoshioka, H. Application of Vegetation Isoline Equations for Simultaneous Retrieval of Leaf Area Index and Leaf Chlorophyll Content Using Reflectance of Red Edge Band. In Proceedings of the Remote Sensing and Modeling of Ecosystems for Sustainability XIII, San Diego, CA, USA, 28 August–1 September 2016; Volume 9975, pp. 87–93. [Google Scholar]
- Peng, Y.; Nguy-Robertson, A.; Arkebauer, T.; Gitelson, A.A. Assessment of Canopy Chlorophyll Content Retrieval in Maize and Soybean: Implications of Hysteresis on the Development of Generic Algorithms. Remote Sens. 2017, 9, 226. [Google Scholar] [CrossRef]
- Clevers, J.; Kooistra, L.; van den Brande, M. Using Sentinel-2 Data for Retrieving LAI and Leaf and Canopy Chlorophyll Content of a Potato Crop. Remote Sens. 2017, 9, 405. [Google Scholar] [CrossRef]
- Xie, Q.; Dash, J.; Huete, A.; Jiang, A.; Yin, G.; Ding, Y.; Peng, D.; Hall, C.C.; Brown, L.; Shi, Y.; et al. Retrieval of Crop Biophysical Parameters from Sentinel-2 Remote Sensing Imagery. Int. J. Appl. Earth Obs. Geoinf. 2019, 80, 187–195. [Google Scholar] [CrossRef]
- Gausman, H.W.; Allen, W.A.; Cardenas, R.; Richardson, A.J. Effects of Leaf Nodal Position on Absorption and Scattering Coefficients and Infinite Reflectance of Cotton Leaves, Gossypium hirsutum L. Agron. J. 1971, 63, 87–91. [Google Scholar] [CrossRef]
- Sellers, P.J. Vegetation-Canopy Spectral Reflectance and Biophysical Processes; Asrar, G., Ed.; John Wiley and Sons: New York, NY, USA, 1989. [Google Scholar]
- Asner, G.P. Biophysical and Biochemical Sources of Variability in Canopy Reflectance. Remote Sens. Environ. 1998, 64, 234–253. [Google Scholar] [CrossRef]
- Zou, X.; Hernández-Clemente, R.; Tammeorg, P.; Lizarazo Torres, C.; Stoddard, F.L.; Mäkelä, P.; Pellikka, P.; Mõttus, M. Retrieval of Leaf Chlorophyll Content in Field Crops Using Narrow-Band Indices: Effects of Leaf Area Index and Leaf Mean Tilt Angle. Int. J. Remote Sens. 2015, 36, 6031–6055. [Google Scholar] [CrossRef]
- Zou, X.; Mõttus, M. Sensitivity of Common Vegetation Indices to the Canopy Structure of Field Crops. Remote Sens. 2017, 9, 994. [Google Scholar] [CrossRef]
- Ross, J. Tasks for Vegetation Science. In The Radiation Regime and Architecture of Plant Stands; Springer: Dordrecht, The Netherlands, 1981; ISBN 978-90-6193-607-7. [Google Scholar]
- McNeil, B.E.; Pisek, J.; Lepisk, H.; Flamenco, E.A. Measuring Leaf Angle Distribution in Broadleaf Canopies Using UAVs. Agric. For. Meteorol. 2016, 218, 204–208. [Google Scholar] [CrossRef]
- Zou, X.; Mõttus, M. Retrieving Crop Leaf Tilt Angle from Imaging Spectroscopy Data. Agric. For. Meteorol. 2015, 205, 73–82. [Google Scholar] [CrossRef]
- Zou, X.; Zhu, S.; Mõttus, M. Estimation of Canopy Structure of Field Crops Using Sentinel-2 Bands with Vegetation Indices and Machine Learning Algorithms. Remote Sens. 2022, 14, 2849. [Google Scholar] [CrossRef]
- Jiao, Q.; Sun, Q.; Zhang, B.; Huang, W.; Ye, H.; Zhang, Z.; Zhang, X.; Qian, B. A Random Forest Algorithm for Retrieving Canopy Chlorophyll Content of Wheat and Soybean Trained with PROSAIL Simulations Using Adjusted Average Leaf Angle. Remote Sens. 2022, 14, 98. [Google Scholar] [CrossRef]
- Zou, X.; Mõttus, M.; Tammeorg, P.; Torres, C.L.; Takala, T.; Pisek, J.; Mäkelä, P.; Stoddard, F.L.; Pellikka, P. Photographic Measurement of Leaf Angles in Field Crops. Agric. For. Meteorol. 2014, 184, 137–146. [Google Scholar] [CrossRef]
- Ryu, Y.; Sonnentag, O.; Nilson, T.; Vargas, R.; Kobayashi, H.; Wenk, R.; Baldocchi, D.D. How to Quantify Tree Leaf Area Index in an Open Savanna Ecosystem: A Multi-Instrument and Multi-Model Approach. Agric. For. Meteorol. 2010, 150, 63–76. [Google Scholar] [CrossRef]
- Pisek, J.; Ryu, Y.; Alikas, K. Estimating Leaf Inclination and G-Function from Leveled Digital Camera Photography in Broadleaf Canopies. Trees 2011, 25, 919–924. [Google Scholar] [CrossRef]
- Pisek, J.; Sonnentag, O.; Richardson, A.D.; Mõttus, M. Is the Spherical Leaf Inclination Angle Distribution a Valid Assumption for Temperate and Boreal Broadleaf Tree Species? Agric. For. Meteorol. 2013, 169, 186–194. [Google Scholar] [CrossRef]
- Campbell, G.S. Derivation of an Angle Density Function for Canopies with Ellipsoidal Leaf Angle Distributions. Agric. For. Meteorol. 1990, 49, 173–176. [Google Scholar] [CrossRef]
- Markwell, J.; Osterman, J.C.; Mitchell, J.L. Calibration of the Minolta SPAD-502 Leaf Chlorophyll Meter. Photosynth. Res. 1995, 46, 467–472. [Google Scholar] [CrossRef]
- Vohland, M.; Mader, S.; Dorigo, W. Applying Different Inversion Techniques to Retrieve Stand Variables of Summer Barley with PROSPECT+SAIL. Int. J. Appl. Earth Obs. Geoinf. 2010, 12, 71–80. [Google Scholar] [CrossRef]
- Feret, J.-B.; François, C.; Asner, G.P.; Gitelson, A.A.; Martin, R.E.; Bidel, L.P.R.; Ustin, S.L.; le Maire, G.; Jacquemoud, S. PROSPECT-4 and 5: Advances in the Leaf Optical Properties Model Separating Photosynthetic Pigments. Remote Sens. Environ. 2008, 112, 3030–3043. [Google Scholar] [CrossRef]
- Verhoef, W. Light Scattering by Leaf Layers with Application to Canopy Reflectance Modeling: The SAIL Model. Remote Sens. Environ. 1984, 16, 125–141. [Google Scholar] [CrossRef]
- Kuusk, A. The Hot Spot Effect in Plant Canopy Reflectance. In Photon-Vegetation Interactions; Springer: Berlin/Heidelberg, Germany, 1991; pp. 139–159. [Google Scholar] [CrossRef]
- Hosgood, B.; Jacquemoud, S.; Andreoli, G.; Verdebout, J.; Pedrini, G.; Schmuck, G. Leaf Optical Properties EXperiment 93 (LOPEX93); Office for Official Publications of the European Communities: Luxembourg, 1994. [Google Scholar]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral Vegetation Indices and Novel Algorithms for Predicting Green LAI of Crop Canopies: Modeling and Validation in the Context of Precision Agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Mäkelä, P.; Kleemola, J.; Jokinen, K.; Mantila, J.; Pehu, E.; Peltonen-Sainio, P. Growth Response of Pea and Summer Turnip Rape to Foliar Application of Glycinebetaine. Acta Agric. Scand. Sect. B Soil Plant Sci. 1997, 47, 168–175. [Google Scholar] [CrossRef]
- Dennett, M.D.; Ishag, K.H.M. Use of the Expolinear Growth Model to Analyse the Growth of Faba Bean, Peas and Lentils at Three Densities: Predictive Use of the Model. Ann. Bot. 1998, 82, 507–512. [Google Scholar] [CrossRef]
- Pinheiro, C.; Rodrigues, A.P.; de Carvalho, I.S.; Chaves, M.M.; Ricardo, C.P. Sugar Metabolism in Developing Lupin Seeds Is Affected by a Short-Term Water Deficit. J. Exp. Bot. 2005, 56, 2705–2712. [Google Scholar] [CrossRef]
- Vile, D.; Garnier, É.; Shipley, B.; Laurent, G.; Navas, M.L.; Roumet, C.; Lavorel, S.; Díaz, S.; Hodgson, J.G.; Lloret, F.; et al. Specific Leaf Area and Dry Matter Content Estimate Thickness in Laminar Leaves. Ann. Bot. 2005, 96, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Vermote, E.F.; Tanre, D.; Deuze, J.L.; Herman, M.; Morcette, J.-J. Second Simulation of the Satellite Signal in the Solar Spectrum, 6S: An Overview. IEEE Trans. Geosci. Remote Sens. 1997, 35, 675–686. [Google Scholar] [CrossRef]
- Rouse, J.W. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation; NASA/GSFC, Type II; NASA: Greenbelt, MD, USA, 1973. [Google Scholar]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the Radiometric and Biophysical Performance of the MODIS Vegetation Indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Jiang, Z.; Huete, A.R.; Didan, K.; Miura, T. Development of a Two-Band Enhanced Vegetation Index without a Blue Band. Remote Sens. Environ. 2008, 112, 3833–3845. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of Soil-Adjusted Vegetation Indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Roujean, J.-L.; Breon, F.-M. Estimating PAR Absorbed by Vegetation from Bidirectional Reflectance Measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Blackburn, G.A. Quantifying Chlorophylls and Caroteniods at Leaf and Canopy Scales: An Evaluation of Some Hyperspectral Approaches. Remote Sens. Environ. 1998, 66, 273–285. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; de Colstoun, E.B.; McMurtrey, J.E. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Wu, C.; Niu, Z.; Tang, Q.; Huang, W. Estimating Chlorophyll Content from Hyperspectral Vegetation Indices: Modeling and Validation. Agric. For. Meteorol. 2008, 148, 1230–1241. [Google Scholar] [CrossRef]
- Barnes, E.; Clarke, T.R.; Richards, S.E.; Colaizzi, P.; Haberland, J.; Kostrzewski, M.; Waller, P.; Choi, C.; Riley, E.; Thompson, T.L. Coincident Detection of Crop Water Stress, Nitrogen Status, and Canopy Density Using Ground Based Multispectral Data. In Proceedings of the Fifth International Conference on Precision Agriculture, Bloomington, MN, USA, 16–19 July 2000. [Google Scholar]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between Leaf Chlorophyll Content and Spectral Reflectance and Algorithms for Non-Destructive Chlorophyll Assessment in Higher Plant Leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Rouse, J.W.; Haas, R.H.; Deering, D.W.; Schell, J.A.; Harlan, J.C. Monitoring the Vernal Advancement of Retrogradation (Green Wave Effect) of Natural Vegetation; NASA/GSFC, Type III, Final Report; NASA: Greenbelt, MD, USA, 1974. [Google Scholar]
- Tucker, C.J. Red and Photographic Infrared Linear Combinations for Monitoring Vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Huete, A.R. A Soil-Adjusted Vegetation Index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H.; Sorooshian, S. A Modified Soil Adjusted Vegetation Index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing Prediction Power and Stability of Broadband and Hyperspectral Vegetation Indices for Estimation of Green Leaf Area Index and Canopy Chlorophyll Density. Remote Sens. Environ. 2001, 76, 156–172. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-Band Model for Noninvasive Estimation of Chlorophyll, Carotenoids, and Anthocyanin Contents in Higher Plant Leaves. Geophys. Res. Lett. 2006, 33, 026457. [Google Scholar] [CrossRef]
- Tian, Y.; Gu, K.; Chu, X.; Yao, X.; Cao, W.; Zhu, Y. Comparison of Different Hyperspectral Vegetation Indices for Canopy Leaf Nitrogen Concentration Estimation in Rice. Plant Soil 2014, 376, 193–209. [Google Scholar] [CrossRef]
- Verrelst, J.; Rivera, J.P.; Veroustraete, F.; Muñoz-Marí, J.; Clevers, J.G.P.W.; Camps-Valls, G.; Moreno, J. Experimental Sentinel-2 LAI Estimation Using Parametric, Non-Parametric and Physical Retrieval Methods—A Comparison. ISPRS J. Photogramm. Remote Sens. 2015, 108, 260–272. [Google Scholar] [CrossRef]
- Wang, W.; Yao, X.; Yao, X.; Tian, Y.; Liu, X.; Ni, J.; Cao, W.; Zhu, Y. Estimating Leaf Nitrogen Concentration with Three-Band Vegetation Indices in Rice and Wheat. Field Crops Res. 2012, 129, 90–98. [Google Scholar] [CrossRef]
- le Maire, G.; François, C.; Soudani, K.; Berveiller, D.; Pontailler, J.Y.; Bréda, N.; Genet, H.; Davi, H.; Dufrêne, E. Calibration and Validation of Hyperspectral Indices for the Estimation of Broadleaved Forest Leaf Chlorophyll Content, Leaf Mass per Area, Leaf Area Index and Leaf Canopy Biomass. Remote Sens. Environ. 2008, 112, 3846–3864. [Google Scholar] [CrossRef]
- Dong, T.; Liu, J.; Shang, J.; Qian, B.; Ma, B.; Kovacs, J.M.; Walters, D.; Jiao, X.; Geng, X.; Shi, Y. Assessment of Red-Edge Vegetation Indices for Crop Leaf Area Index Estimation. Remote Sens. Environ. 2019, 222, 133–143. [Google Scholar] [CrossRef]
- Zeng, Y.; Hao, D.; Huete, A.; Dechant, B.; Berry, J.; Chen, J.M.; Joiner, J.; Frankenberg, C.; Bond-Lamberty, B.; Ryu, Y.; et al. Optical Vegetation Indices for Monitoring Terrestrial Ecosystems Globally. Nat. Rev. Earth Environ. 2022, 3, 477–493. [Google Scholar] [CrossRef]

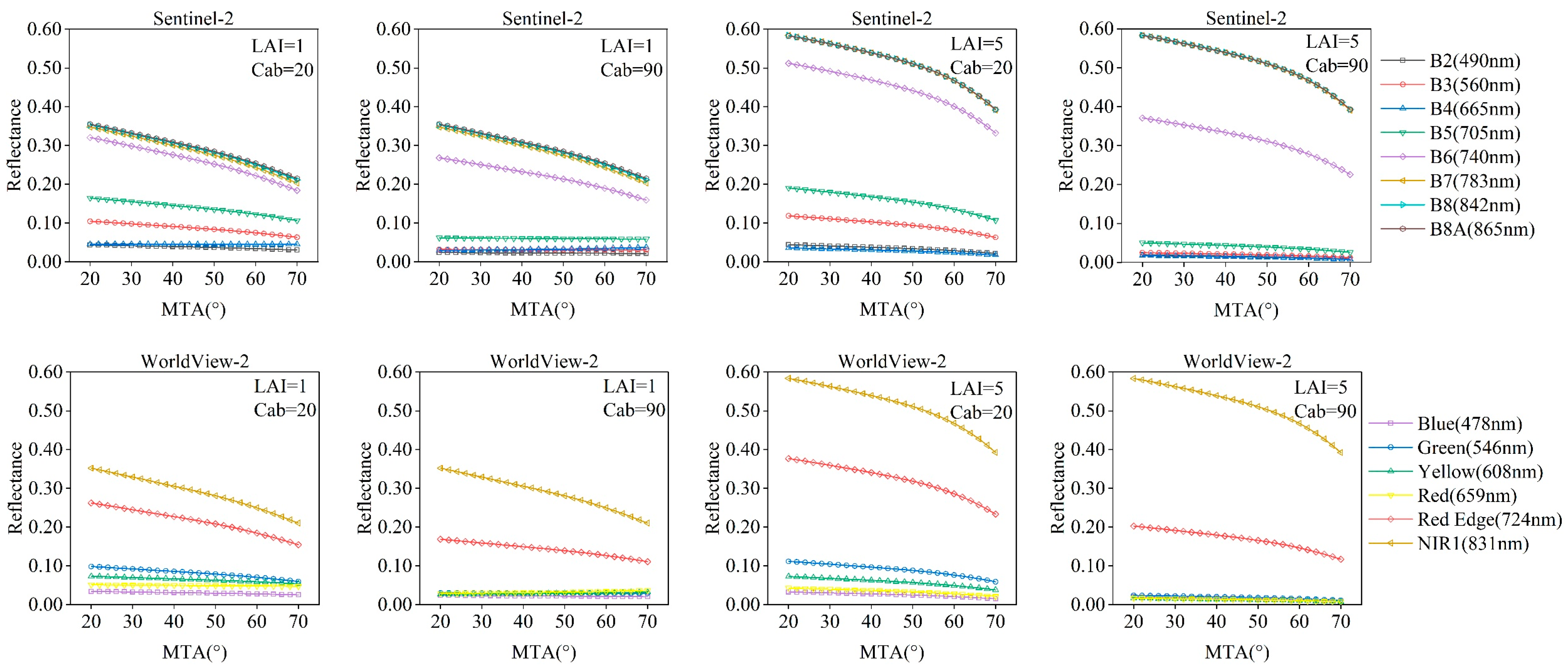


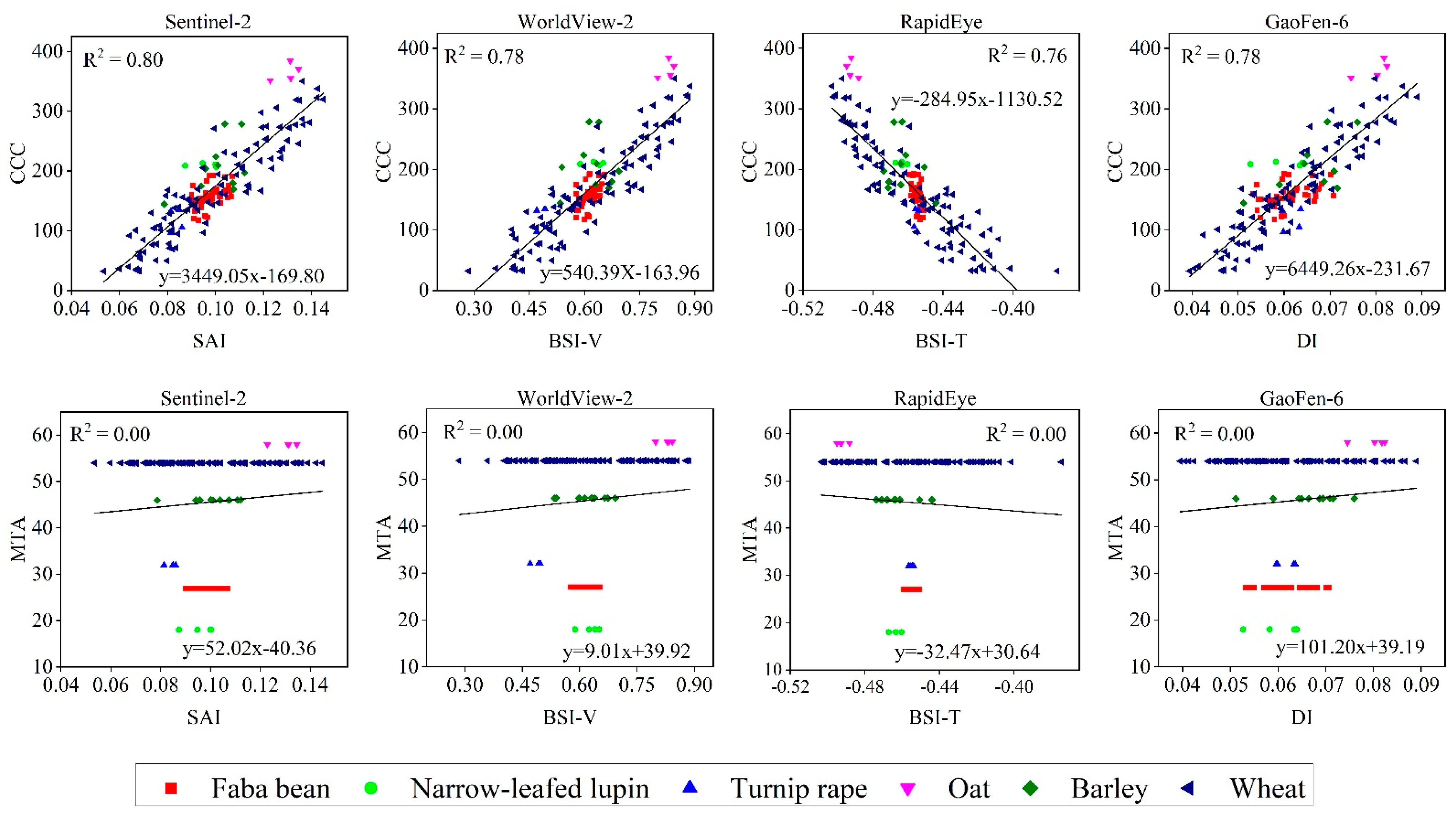
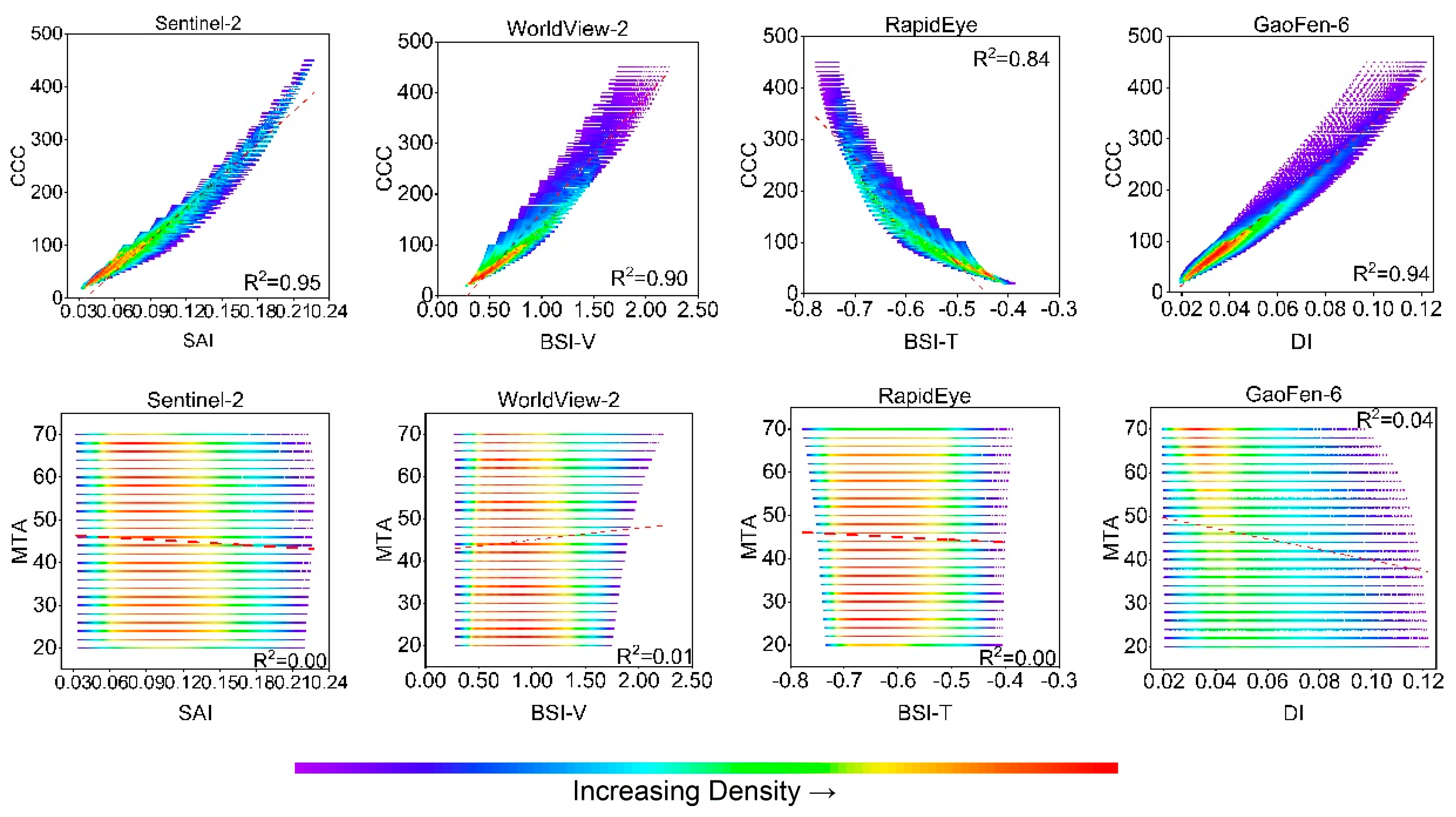
| Model | Variable | Value or Range |
|---|---|---|
| PROSPECT | Leaf structure parameter (N) | 1.55 |
| Leaf chlorophyll content (Cab) | 20:5:90 μg cm−2 | |
| Equivalent water thickness (Cw) | 0.001 cm | |
| Dry matter content (Cm) | 0.005 g cm−2 | |
| Brown pigment content (Cbp) | 0 μg cm−2 | |
| Carotenoid content (Ccar) | Linked to Cab (0.2 × Cab) μg cm−2 | |
| SAIL | Leaf area index (LAI) | 1, 1.1, …, 5.0 |
| Leaf mean tilt angle (MTA) | 20, 22, …, 70° | |
| Hot spot size | 0.01 | |
| Solar zenith angle (ts) | 49.4° | |
| Observer zenith angle (to) | 9° | |
| Azimuth angle (φ) | 90° | |
| Fraction of diffuse radiation (skyl) | 6S model (Wm−2 nm−1) | |
| Soil reflectance | ASD measurement |
| No | Index | Abbreviation | Formulation | Reference |
|---|---|---|---|---|
| 1 | Normalized difference vegetation index | NDVI | [63] | |
| 2 | Enhanced vegetation index | EVI | [64] | |
| 3 | Two-band enhanced vegetation index | EVI2 | [65] | |
| 4 | Optimized soil-adjusted vegetation index | OSAVI | [66] | |
| 5 | Renormalized difference vegetation index | RDVI | [67] | |
| 6 | Pigment-specific normalized difference index | PSND | [68] | |
| 7 | Transformed chlorophyll absorption reflectance index/OSAVI | TCARI/OSAVI | [66,69] | |
| 8 | Red-edge Transformed chlorophyll absorption reflectance index/OSAVI | TCARI/OSAVIred edge | [70] | |
| 9 | MERIS terrestrialchlorophyll index | MTCI | [27] | |
| 10 | Normalized difference red-edge version 1 | NDRE1 | [28] | |
| 11 | Normalized difference red-edge version 2 | NDRE2 | [71] | |
| 12 | Red-edge chlorophyll index | CIred edge | [72] | |
| 13 | Ratio index | RI | [57] | |
| 14 | Normalized difference index | NDI | [73] | |
| 15 | Difference index | DI | [74] | |
| 16 | Soil adjusted index | SAI | [75] | |
| 17 | Modified simple ratio index | MSR | [57] | |
| 18 | Modified soil adjusted index | MSAI | [76] | |
| 19 | Triangular index | TI | 0.5 [)()-)()] | [77] |
| 20 | Gitelson’s three-band | Git | [78] | |
| 21 | Tian’s three-band spectral index | BSI-T | [79] | |
| 22 | Verrelts’s three-band spectral index | BSI-V | [80] | |
| 23 | Wang’s three-band spectral index | BSI-W | [81] |
| Dataset | Index | Sentinel-2 | WorldView2 | RapidEye | GaoFen-6 | ||||
|---|---|---|---|---|---|---|---|---|---|
| R2CCC | R2MTA | R2CCC | R2MTA | R2CCC | R2MTA | R2CCC | R2MTA | ||
| Measurement | NDVI | 0.46 | 0.24 | 0.47 | 0.23 | 0.46 | 0.24 | 0.47 | 0.23 |
| EVI | 0.16 | 0.65 | 0.18 | 061 | 0.17 | 0.63 | 0.17 | 0.62 | |
| EVI2 | 0.19 | 0.63 | 0.19 | 0.60 | 0.18 | 0.62 | 0.19 | 0.60 | |
| OSAVI | 0.32 | 0.46 | 0.32 | 0.43 | 0.31 | 0.45 | 0.32 | 0.43 | |
| RDVI | 0.22 | 0.56 | 0.23 | 0.55 | 0.22 | 0.57 | 0.23 | 0.55 | |
| PSND | 0.52 | 0.17 | 0.50 | 0.18 | 0.49 | 0.19 | 0.52 | 0.17 | |
| TCARI/OSAVI | 0.31 | 0.40 | 0.32 | 0.38 | 0.29 | 0.41 | 0.33 | 0.37 | |
| TCARI/OSAVIred edge | 0.31 | 0.18 | 0.20 | 0.48 | 0.27 | 0.31 | 0.36 | 0.08 | |
| MTCI | 0.12 | 0.14 | — | — | — | — | 0.48 | 0.21 | |
| NDRE1 | 0.41 | 0.30 | — | — | — | — | 0.49 | 0.21 | |
| NDRE2 | 0.64 | 0.07 | — | — | — | — | — | — | |
| CIred edge | 0.68 | 0.05 | — | — | — | — | — | — | |
| Model | NDVI | 0.50 | 0.01 | 0.57 | 0.01 | 0.56 | 0.01 | 0.56 | 0.01 |
| EVI | 0.26 | 0.33 | 0.37 | 0.31 | 0.36 | 0.32 | 0.31 | 0.33 | |
| EVI2 | 0.36 | 0.28 | 0.39 | 0.28 | 0.38 | 0.28 | 0.39 | 0.28 | |
| OSAVI | 0.41 | 0.18 | 0.46 | 0.17 | 0.45 | 0.17 | 0.46 | 0.17 | |
| RDVI | 0.37 | 0.26 | 0.40 | 0.26 | 0.39 | 0.26 | 0.40 | 0.26 | |
| PSND | 0.67 | 0.00 | 0.57 | 0.01 | 0.56 | 0.01 | 0.68 | 0.00 | |
| TCARI/OSAVI | 0.82 | 0.01 | 0.88 | 0.01 | 0.87 | 0.01 | 0.87 | 0.01 | |
| TCARI/OSAVIred edge | 0.51 | 0.05 | 0.35 | 0.04 | 0.42 | 0.00 | 0.54 | 0.03 | |
| MTCI | 0.76 | 0.00 | — | — | — | — | 0.82 | 0.00 | |
| NDRE1 | 0.76 | 0.00 | — | — | — | — | 0.79 | 0.00 | |
| NDRE2 | 0.76 | 0.00 | — | — | — | — | — | — | |
| CIred edge | 0.90 | 0.00 | — | — | — | — | — | — | |
| Index | Sentinel-2 | WorldView-2 | RapidEye | GaoFen-6 | |||||
|---|---|---|---|---|---|---|---|---|---|
| B1, B2, B3 | R2CCC, R2MTA | B1, B2, B3 | R2CCC, R2MTA | B1, B2, B3 | R2CCC, R2MTA | B1, B2, B3 | R2CCC, R2MTA | ||
| TI | 1 | B7, B4, B5 | 0.79, 0.05 | NIR1, Green, Red Edge | 0.77, 0.02 | Blue, Green, Red Edge | 0.22, 0.32 | B1, B3, B8 | 0.14, 0.02 |
| 2 | B2, B6, B7 | 0.78, 0.06 | NIR1, Blue, Red Edge | 0.72, 0.03 | Blue, Green, NIR | 0.26, 0.45 | B5, B1, B2 | 0.24, 0.20 | |
| 3 | B3, B6, B7 | 0.66, 0.27 | Red, Blue, Yellow | 0.13, 0.06 | Red Edge, Blue, NIR | 0.25, 0.52 | B4, B5, B8 | 0.31, 0.39 | |
| Git | 1 | B5, B8, B8A | 0.76, 0.00 | Green, Red Edge, NIR1 | 0.58, 0.10 | Green, Red Edge, NIR | 0.55, 0.11 | B5, B6, B4 | 0.66, 0.07 |
| 2 | B5, B8A, B8 | 0.75, 0.00 | Yellow, Red Edge, Red | 0.46, 0.02 | Green, Red Edge, Blue | 0.38, 0.00 | B2, B5, B8 | 0.55, 0.06 | |
| 3 | B5, B7, B8A | 0.74, 0.01 | Green, Red Edge, Blue | 0.33, 0.00 | Green, NIR, Red Edge | 0.48, 0.17 | B2, B6, B4 | 0.58, 0.10 | |
| BSI-T | 1 | B7, B6, B2 | 0.78, 0.00 | NIR1, Blue, Red Edge | 0.76, 0.00 | Red Edge, Green, NIR | 0.76, 0.00 | B5, B3, B4 | 0.78, 0.01 |
| 2 | B7, B5, B6 | 0.77, 0.00 | NIR1, Green, Red Edge | 0.73, 0.00 | Red Edge, Blue, NIR | 0.74, 0.00 | B5, B4, B8 | 0.77, 0.00 | |
| 3 | B8, B6, B4 | 0.76, 0.00 | NIR1, Yellow, Red Edge | 0.70, 0.02 | Red Edge, Red, NIR | 0.76, 0.09 | B4, B3, B6 | 0.74, 0.00 | |
| BSI-V | 1 | B8, B6, B2 | 0.78, 0.02 | NIR1, Red, Red Edge | 0.78, 0.00 | NIR, Blue, Red Edge | 0.72, 0.03 | B4, B6, B1 | 0.77, 0.01 |
| 2 | B8, B6, B5 | 0.78, 0.01 | NIR1, Yellow, Red Edge | 0.78, 0.01 | NIR, Green, Red Edge | 0.71, 0.03 | B4, B6, B5 | 0.77, 0.00 | |
| 3 | B2, B6, B8 | 0.76, 0.01 | Red Edge, Red, NIR1 | 0.76, 0.00 | Red Edge, Green, NIR | 0.67, 0.04 | B1, B6, B4 | 0.75, 0.00 | |
| BSI-W | 1 | B6, B8, B2 | 0.74, 0.01 | Red Edge, Blue, NIR1 | 0.74, 0.03 | Red Edge, Blue, NIR | 0.64, 0.04 | B6, B4, B1 | 0.72, 0.00 |
| 2 | B6, B5, B7 | 0.73, 0.01 | Red Edge, Green, NIR1 | 0.72, 0.01 | Red Edge, Green, NIR | 0.62, 0.04 | B5, B6, B4 | 0.68, 0.00 | |
| 3 | B6, B3, B7 | 0.73, 0.01 | Red Edge, NIR1, Blue | 0.71, 0.00 | Red Edge, NIR, Blue | 0.62, 0.07 | B6, B4, B2 | 0.65, 0.01 | |
| Index | Sentinel-2 | WorldView-2 | RapidEye | GaoFen-6 | ||||
|---|---|---|---|---|---|---|---|---|
| B1, B2 | R2CCC, R2MTA | B1, B2 | R2CCC, R2MTA | B1, B2 | R2CCC, R2MTA | B1, B2 | R2CCC, R2MTA | |
| RI | B5, B8A | 0.77, 0.00 | NIR1, Red Edge | 0.73, 0.10 | Red Edge, NIR | 0.74, 0.01 | B5, B4 | 0.73, 0.02 |
| NDVI | B5, B8A | 0.73, 0.00 | Red Edge, NIR1 | 0.74, 0.11 | Red Edge, NIR | 0.71, 0.02 | B5, B4 | 0.69, 0.03 |
| DI | B6, B8A | 0.76, 0.00 | Blue, Yellow | 0.36, 0.03 | Blue, Red | 0.40, 0.18 | B6, B4 | 0.78, 0.00 |
| SAI | B6, B7 | 0.80, 0.00 | Red Edge, NIR1 | 0.65, 0.09 | Blue, Red | 0.39, 0.19 | B8, B1 | 0.36, 0.05 |
| MSR | B5, B8A | 0.75, 0.00 | NIR1, Red Edge | 0.74, 0.11 | Red Edge, NIR | 0.73, 0.01 | B5, B4 | 0.72, 0.02 |
| MSAI | B6, B7 | 0.78, 0.00 | Red Edge, NIR1 | 0.56, 0.17 | Blue, Red | 0.40, 0.18 | B4, B6 | 0.69, 0.23 |
| Index | Sentinel-2 | WorldView-2 | RapidEye | GaoFen-6 | ||||
|---|---|---|---|---|---|---|---|---|
| Bands | R2CCC, R2MTA | Bands | R2CCC, R2MTA | Bands | R2CCC, R2MTA | Bands | R2CCC, R2MTA | |
| RI | B5, B8A | 0.89, 0.00 | NIR1, Red Edge | 0.80, 0.00 | Red Edge, NIR | 0.90, 0.00 | B5, B4 | 0.90, 0.00 |
| NDVI | B5, B8A | 0.76, 0.00 | Red Edge, NIR1 | 0.83, 0.01 | Red Edge, NIR | 0.80, 0.00 | B5, B4 | 0.79, 0.00 |
| DI | B6, B8A | 0.93, 0.04 | Blue, Yellow | 0.51, 0.00 | Blue, Red | 0.61, 0.05 | B6, B4 | 0.94, 0.04 |
| SAI | B6, B7 | 0.95, 0.00 | Red Edge, NIR1 | 0.90, 0.02 | Blue, Red | 0.62, 0.06 | B8,B1 | 0.57, 0.00 |
| MSR | B5, B8A | 0.87, 0.00 | NIR1, Red Edge | 0.82, 0.00 | Red Edge, NIR | 0.87, 0.00 | B5, B4 | 0.88, 0.00 |
| MSAI | B6, B7 | 0.96, 0.01 | Red Edge, NIR1 | 0.90, 0.04 | Blue, Red | 0.61, 0.06 | B4, B6 | 0.95, 0.00 |
| TI | B7, B4, B5 | 0.82, 0.05 | NIR1, Green, Red Edge | 0.92, 0.02 | Blue, Green, Red Edge | 0.43, 0.05 | B1, B3, B8 | 0.36, 0.01 |
| Git | B5, B8, B8A | 0.89, 0.00 | Green, Red Edge, NIR1 | 0.88, 0.00 | Green, Red Edge, NIR | 0.88, 0.00 | B5, B6, B4 | 0.91, 0.00 |
| BSI-T | B7, B6, B2 | 0.90, 0.01 | NIR1, Blue, Red Edge | 0.85, 0.01 | Red Edge, Green, NIR | 0.84, 0.00 | B5, B3, B4 | 0.79, 0.00 |
| BSI-V | B8, B6, B2 | 0.90, 0.01 | NIR1, Red, Red Edge | 0.90, 0.01 | NIR, Blue, Red Edge | 0.91, 0.01 | B4, B6, B1 | 0.87, 0.02 |
| BSI-W | B6, B8, B2 | 0.87, 0.01 | Red Edge, Blue, NIR1 | 0.76, 0.00 | Red Edge, Blue, NIR | 0.72, 0.00 | B6, B4, B1 | 0.83, 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, X.; Jin, J.; Mõttus, M. Potential of Satellite Spectral Resolution Vegetation Indices for Estimation of Canopy Chlorophyll Content of Field Crops: Mitigating Effects of Leaf Angle Distribution. Remote Sens. 2023, 15, 1234. https://doi.org/10.3390/rs15051234
Zou X, Jin J, Mõttus M. Potential of Satellite Spectral Resolution Vegetation Indices for Estimation of Canopy Chlorophyll Content of Field Crops: Mitigating Effects of Leaf Angle Distribution. Remote Sensing. 2023; 15(5):1234. https://doi.org/10.3390/rs15051234
Chicago/Turabian StyleZou, Xiaochen, Jun Jin, and Matti Mõttus. 2023. "Potential of Satellite Spectral Resolution Vegetation Indices for Estimation of Canopy Chlorophyll Content of Field Crops: Mitigating Effects of Leaf Angle Distribution" Remote Sensing 15, no. 5: 1234. https://doi.org/10.3390/rs15051234
APA StyleZou, X., Jin, J., & Mõttus, M. (2023). Potential of Satellite Spectral Resolution Vegetation Indices for Estimation of Canopy Chlorophyll Content of Field Crops: Mitigating Effects of Leaf Angle Distribution. Remote Sensing, 15(5), 1234. https://doi.org/10.3390/rs15051234