Methodology and Results of Satellite Monitoring of Karenia Microalgae Blooms, That Caused the Ecological Disaster off Kamchatka Peninsula
Abstract
1. Introduction
2. Materials and Methods
3. Results
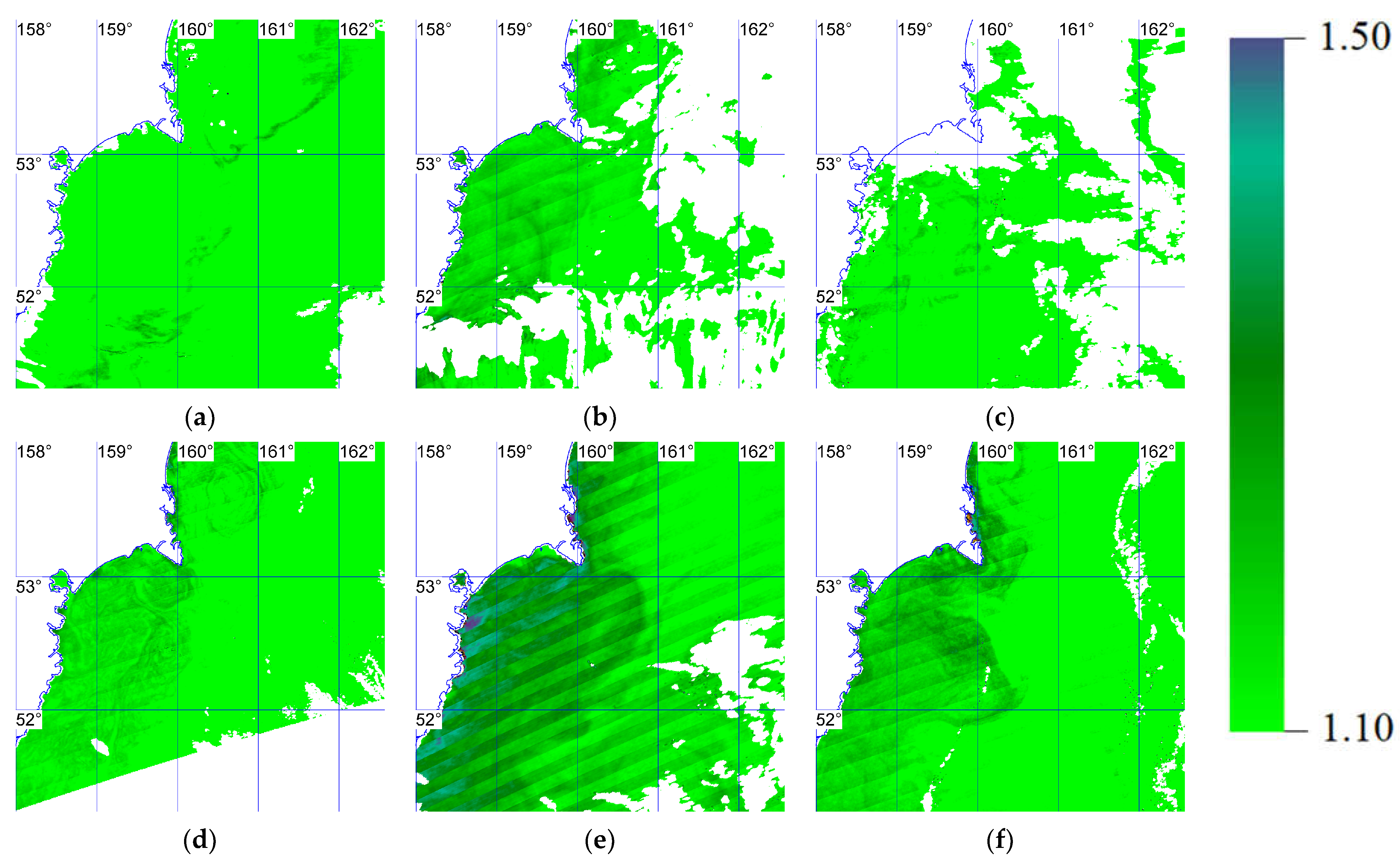
3.1. Bloom Scale
3.2. Karenia spp. Monitoring
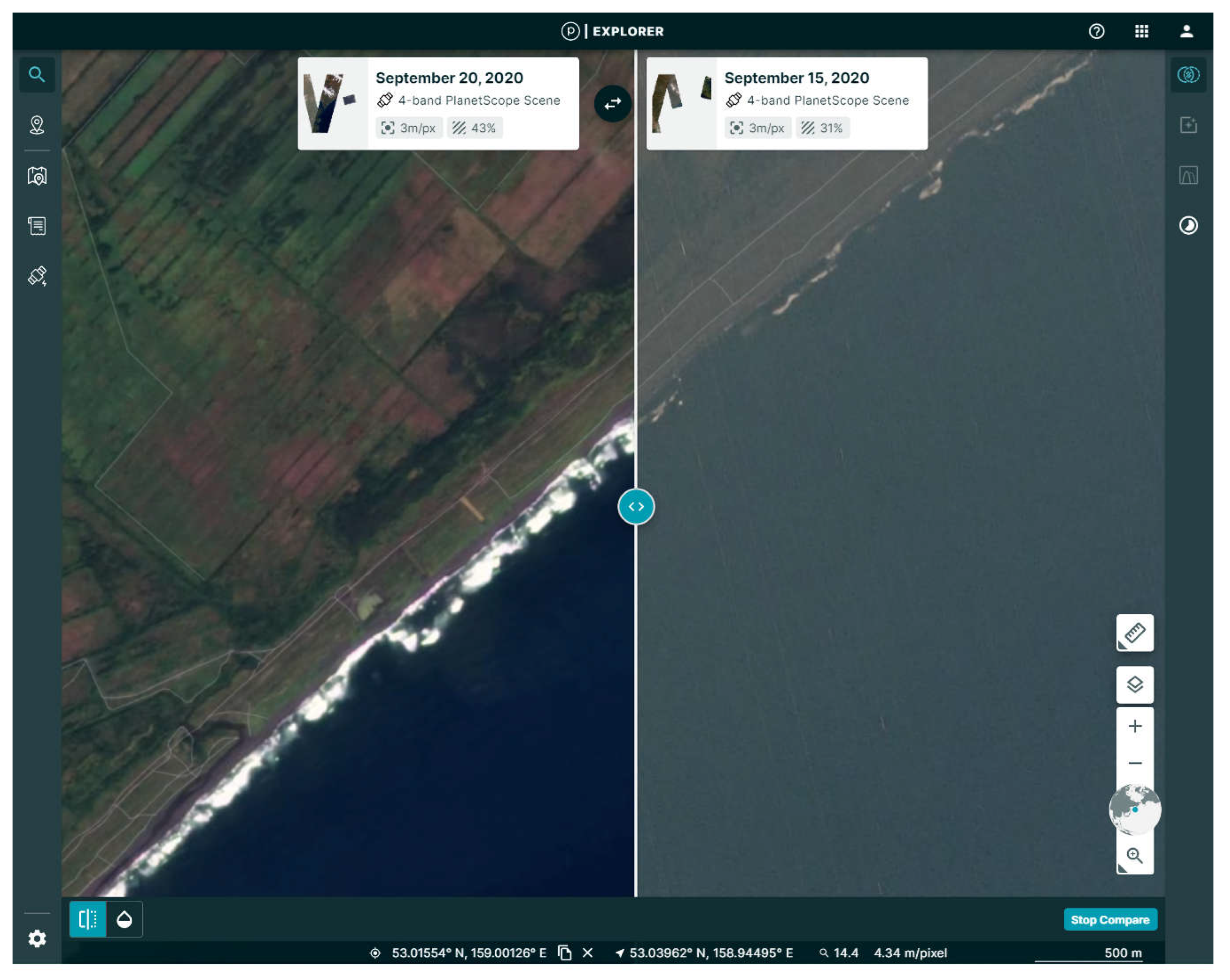
3.3. “Beer Tide” Observations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Orlova, T. Kamchatka Event, Harmful Algal Blooms in the Bering/Chukchi Seas//Looking across the Border: US-Russia Science Corner. Alaska Ocean Observing System& World Wildlife Fund Arctic Program (AOOS&WWF). Available online: https://www.youtube.com/watch?v=wJpq0YE02Qg (accessed on 5 January 2022).
- Harmful Algal Event Database. Available online: http://haedat.iode.org/ (accessed on 20 January 2021).
- Ogashawara, I. Advances and Limitations of Using Satellites to Monitor Cyanobacterial Harmful Algal Blooms. Acta Limnol. Bras. 2019, 31, e103. [Google Scholar] [CrossRef]
- Zohdi, E.; Abbaspour, M. Harmful Algal Blooms (Red Tide): A Review of Causes, Impacts and Approaches to Monitoring and Prediction. Int. J. Environ. Sci. Technol. 2019, 16, 1789–1806. [Google Scholar] [CrossRef]
- Hill, P.R.; Kumar, A.; Temimi, M.; Bull, D.R. HABNet: Machine Learning, Remote Sensing-Based Detection of Harmful Algal Blooms. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2020, 13, 3229–3239. [Google Scholar] [CrossRef]
- Dierssen, H.M.; Kudela, R.M.; Ryan, J.P.; Zimmerman, R.C. Red and Black Tides: Quantitative Analysis of Water-Leaving Radiance and Perceived Color for Phytoplankton, Colored Dissolved Organic Matter, and Suspended Sediments. Limnol. Oceanogr. 2006, 51, 2646–2659. [Google Scholar] [CrossRef]
- Blondeau-Patissier, D.; Gower, J.F.R.; Dekker, A.G.; Phinn, S.R.; Brando, V.E. A Review of Ocean Color Remote Sensing Methods and Statistical Techniques for the Detection, Mapping and Analysis of Phytoplankton Blooms in Coastal and Open Oceans. Prog. Oceanogr. 2014, 123, 123–144. [Google Scholar] [CrossRef]
- Wolny, J.L.; Tomlinson, M.C.; Schollaert Uz, S.; Egerton, T.A.; McKay, J.R.; Meredith, A.; Reece, K.S.; Scott, G.P.; Stumpf, R.P. Current and Future Remote Sensing of Harmful Algal Blooms in the Chesapeake Bay to Support the Shellfish Industry. Front. Mar. Sci. 2020, 7, 337. [Google Scholar] [CrossRef]
- Soto, I.M.; Cannizzaro, J.; Muller-Karger, F.E.; Hu, C.; Wolny, J.; Goldgof, D. Evaluation and Optimization of Remote Sensing Techniques for Detection of Karenia Brevis Blooms on the West Florida Shelf. Remote Sens. Environ. 2015, 170, 239–254. [Google Scholar] [CrossRef]
- Aleksanin, A.I.; Kim, V.; Orlova, T.Y.; Stonik, I.V.; Shevchenko, O.G. Phytoplankton of the Peter the Great Bay and Its Remote Sensing Problem. Oceanology 2012, 52, 219–230. [Google Scholar] [CrossRef]
- Seubert, E.L.; Gellene, A.G.; Howard, M.D.A.; Connell, P.; Ragan, M.; Jones, B.H.; Runyan, J.; Caron, D.A. Seasonal and Annual Dynamics of Harmful Algae and Algal Toxins Revealed through Weekly Monitoring at Two Coastal Ocean Sites off Southern California, USA. Environ. Sci. Pollut. Res. 2013, 20, 6878–6895. [Google Scholar] [CrossRef]
- Stonik, I.V.; Orlova, T.Y.; Chikalovets, I.V.; Aizdaicher, N.A.; Aleksanin, A.I.; Kachur, V.A.; Morozova, T.V. Pseudo-Nitzschia Species (Bacillariophyceae) and the Domoic Acid Concentration in Pseudo-Nitzschia Cultures and Bivalves from the Northwestern Sea of Japan, Russia. Nova Hedwig. 2019, 108, 73–93. [Google Scholar] [CrossRef]
- Shen, L.; Xu, H.; Guo, X. Satellite Remote Sensing of Harmful Algal Blooms (HABs) and a Potential Synthesized Framework. Sensors 2012, 12, 7778–7803. [Google Scholar] [CrossRef]
- Al-Ghelani, H.M.; AlKindi, A.Y.A.; Amer, S.; Al-Akhzami, Y.K. Harmful Algal Blooms: Physiology, Behavior, Population Dynamics and Global Impacts- A Review. Sultan Qaboos Univ. J. Sci. 2005, 10, 1–30. [Google Scholar] [CrossRef]
- Li, X.; Yan, T.; Yu, R.; Zhou, M. A Review of Karenia Mikimotoi: Bloom Events, Physiology, Toxicity and Toxic Mechanism. Harmful Algae 2019, 90, 101702. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.L.; Fargion, G.S.; McClain, C.R. Ocean optics protocols for satellite ocean color sensor validation, Rev. 1., Vol. III: Radiometric measurements and data analysis protocols. In NASA/TM-2003-21641/Rev-Vol. III; NASA Goddard Space Flight Center: Greenbelt, MD, USA, 2003; p. 78. Available online: https://oceancolor.gsfc.nasa.gov/docs/technical/protocols_ver4_voliii.pdf (accessed on 11 February 2022).
- Ruddick, K.G.; De Cauwer, V.; Park, Y.-J.; Moore, G. Seaborne Measurements of near Infrared Water-Leaving Reflectance: The Similarity Spectrum for Turbid Waters. Limnol. Oceanogr. 2006, 51, 1167–1179. [Google Scholar] [CrossRef]
- Aleksanin, A.I.; Kachur, V.A. Specificity of Atmospheric Correction of Satellite Data on Ocean Color in the Far East. Izv. Atmos. Ocean. Phys. 2017, 53, 996–1006. [Google Scholar] [CrossRef]
- Palmer, S.C.J.; Kutser, T.; Hunter, P.D. Remote Sensing of Inland Waters: Challenges, Progress and Future Directions. Remote Sens. Environ. 2015, 157, 1–8. [Google Scholar] [CrossRef]
- Bailey, S.W.; Werdell, P.J. A Multi-Sensor Approach for the on-Orbit Validation of Ocean Color Satellite Data Products. Remote Sens. Environ. 2006, 102, 12–23. [Google Scholar] [CrossRef]
- Concha, J.; Mannino, A.; Franz, B.; Kim, W. Uncertainties in the Geostationary Ocean Color Imager (GOCI) Remote Sensing Reflectance for Assessing Diurnal Variability of Biogeochemical Processes. Remote Sens. 2019, 11, 295. [Google Scholar] [CrossRef]
- Kopelevich, O.V.; Burenkov, V.I.; Sheberstov, S.V. Development and use of regional algorithms for calculating the bio-optical characteristics of the seas of Russia according to the data of satellite color scanners. Mod. Probl. Remote Sens. Earth Space 2006, 2, 99–105. [Google Scholar]
- Bramich, J.M.; Christopher, J.S.; Fischer, B.A.M. Evaluation of atmospheric correction and high-resolution processing on SeaDAS-derived chlorophyll-a: An example from mid-latitude mesotrophic waters. Int. J. Remote Sens. 2018, 39, 2119–2138. [Google Scholar] [CrossRef]
- Ma, L.L.; Liu, Y.; Zhang, B.; Lu, l.; Sun, G.; Wang, D.; Liu, Z.; Li, B.; Wang, Y.; Zhang, Y.; et al. Remotely sensed short-term changes in noctilucent algae blooms in the Bohai Sea. Int. J. Remote Sens. 2021, 42, 8661–8674. [Google Scholar] [CrossRef]
- Carder, K.L.; Chen, F.R.; Lee, Z.P.; Hawes, S.K.; Cannizzaro, J.P.; MODIS Ocean Science Team Algorithm Theoretical Basis Document. ATBD 19, Case 2 Chlorophyll a, Version 7. 2003. Available online: http://modis.gsfc.nasa.gov/data/atbd/atbd_mod19.pdf (accessed on 5 January 2022).
- Maritorena, S.; Siegel, D.A.; Peterson, A.R. Optimization of a semianalytical ocean color model for global-scale applications. Appl. Opt. 2002, 41, 2705–2714. [Google Scholar] [CrossRef]
- Lee, Z.; Carder, K.L.; Arnone, R.A. Deriving Inherent Optical Properties from Water Color: A Multiband Quasi-Analytical Algorithm for Optically Deep Waters. Appl. Opt. 2002, 41, 5755–5772. [Google Scholar] [CrossRef]
- Salyuk, P.; Bukin, O.; Alexanin, A.; Pavlov, A.; Mayor, A.; Shmirko, K.; Akmaykin, D.; Krikun, V. Optical Properties of Peter the Great Bay Waters Compared with Satellite Ocean Color Data. Int. J. Remote Sens. 2010, 31, 4651–4664. [Google Scholar] [CrossRef]
- Feng, C.; Ishizaka, J.; Saitoh, K.; Mine, T.; Yamashita, H. A Novel Method Based on Backscattering for Discriminating Summer Blooms of the Raphidophyte (Chattonella Spp.) and the Diatom (Skeletonema Spp.) Using MODIS Images in Ariake Sea, Japan. Remote Sens. 2020, 12, 1504. [Google Scholar] [CrossRef]
- Wynne, T.T.; Stumpf, R.P.; Tomlinson, M.C.; Warner, R.A.; Tester, P.A.; Dyble, J.; Fahnenstiel, G.L. Relating Spectral Shape to Cyanobacterial Blooms in the Laurentian Great Lakes. Int. J. Remote Sens. 2008, 29, 3665–3672. [Google Scholar] [CrossRef]
- Gower, J.; King, S.; Goncalves, P. Global Monitoring of Plankton Blooms Using MERIS MCI. Int. J. Remote Sens. 2008, 29, 6209–6216. [Google Scholar] [CrossRef]
- Amin, R.; Zhou, J.; Gilerson, A.; Gross, B.; Moshary, F.; Ahmed, S. Novel Optical Techniques for Detecting and Classifying Toxic Dinoflagellate Karenia Brevis Blooms Using Satellite Imagery. Opt. Express 2009, 17, 9126–9144. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Stumpf, R.P.; McGillicuddy, D.J., Jr.; He, R. Dynamics of an Intense Alexandrium Catenella Red Tide in the Gulf of Maine: Satellite Observations and Numerical Modeling. Harmful Algae 2020, 99, 101927. [Google Scholar] [CrossRef] [PubMed]
- Mcgillicuddy, D.J.; Signell, R.P.; Stock, C.A.; Keafer, B.A.; Keller, M.D.; Hetland, R.D.; Anderson, D.M. A Mechanism for Offshore Initiation of Harmful Algal Blooms in the Coastal Gulf of Maine. J. Plankton Res. 2003, 25, 1131–1138. [Google Scholar] [CrossRef]
- Hetland, R.D.; Campbell, L. Convergent Blooms of Karenia Brevis along the Texas Coast. Geophys. Res. Lett. 2007, 34, 474. [Google Scholar] [CrossRef]
- Hillebrand, H.; Dürselen, C.-D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Orlova, T.Y.; Aleksanin, A.I.; Lepskaya, E.V.; Efimova, K.V.; Selina, M.S.; Morozova, T.V.; Stonik, I.V.; Kachur, V.A.; Karpenko, A.A.; Vinnikov, K.A.; et al. A massive bloom of Karenia species (Dinophyceae) off the Kamchatka coast, Russia, in the fall of 2020. Harmful Algae 2022, 120, 102337. [Google Scholar] [CrossRef]
- IOCCG. Atmospheric Correction for Remotely-Sensed Ocean-Color Products; Reports of the International Ocean-Color Coordinating Group, No. 10; Wang, M., Ed.; IOCCG: Dartmouth, NS, Canada, 2010; 78p. [Google Scholar] [CrossRef]
- Abbott, M.R.; Ricardo, M.; Letelier, R.M. Algorithm Theoretical Basis Document Chlorophyll Fluorescence (MODIS Product Number 20). 1999. Available online: https://eospso.nasa.gov/sites/default/files/atbd/atbd_mod22.pdf (accessed on 5 January 2022).
- Falkowski, P.G.; Kolber, Z. Variation of chlorophyll fluorescence yields in the phytoplankton in the world oceans. Aust. J. Plant Physiol. 1995, 22, 341–355. [Google Scholar] [CrossRef]
- Lin, H.; Kuzminov, F.I.; Park, J.; Lee, S.; Falkowski, P.G.; Gorbunov, M.Y. The Fate of Photons Absorbed by Phytoplankton in the Global Ocean. Science 2016, 351, 264–267. [Google Scholar] [CrossRef]
- Wolanin, A.; Rozanov, V.V.; Dinter, T.; Noël, S.; Vountas, M.; Burrows, J.P.; Bracher, A. Global Retrieval of Marine and Terrestrial Chlorophyll Fluorescence at Its Red Peak Using Hyperspectral Top of Atmosphere Radiance Measurements: Feasibility Study and First Results. Remote Sens. Environ. 2015, 166, 243–261. [Google Scholar] [CrossRef]
- El-Habashi, A.; Duran, C.M.; Lovko, V.; Tomlinson, M.C.; Stumpf, R.P.; Ahmed, S. Satellite Retrievals of Karenia Brevis Harmful Algal Blooms in the West Florida Shelf Using Neural Networks and Impacts of Temporal Variabilities. J. Appl. Remote Sens. 2017, 11, 032408. [Google Scholar] [CrossRef]
- Tomlinson, M.C.; Wynne, T.T.; Stumpf, R.P. An evaluation of remote sensing techniques for enhanced detection of the toxic dinoflagellate, Karenia brevis. Remote Sens. Environ. 2009, 113, 598–609. [Google Scholar] [CrossRef]
- Tskhay, Z.R.; Shevchenko, G.V. Distribution features of chlorophyll a concentration off the east coast of Kamchatka in autumn 2020 from satellite data. Sovremenie Probl. Distancionnogo Zondirovaniya Zemli Cosm. 2022, 19, 226–238. [Google Scholar] [CrossRef]
- Vandersea, M.; Tester, P.; Holderied, K.; Hondolero, D.; Kibler, S.; Powell, K.; Baird, S.; Doroff, A.; Dugan, D.; Meredith, A.; et al. An Extraordinary Karenia Mikimotoi “Beer Tide” in Kachemak Bay Alaska. Harmful Algae 2020, 92, 101706. [Google Scholar] [CrossRef]
- Robin, R.S.; Kanuri, V.V.; Muduli, P.R.; Mishra, R.K.; Jaikumar, M.; Karthikeyan, P.; Suresh Kumar, C.; Saravana Kumar, C. Dinoflagellate Bloom of Karenia Mikimotoi along the Southeast Arabian Sea, Bordering Western India. J. Ecosyst. 2013, 2013, 463720. [Google Scholar] [CrossRef]
- Bondur, V.; Zamshin, V.; Chvertkova, O.; Matrosova, E.; Khodaeva, V. Detection and Analysis of the Causes of Intensive Harmful Algal Bloom in Kamchatka Based on Satellite Data. J. Mar. Sci. Eng. 2021, 9, 1092. [Google Scholar] [CrossRef]
- Carswell, T.; Costa, M.; Young, E.; Komick, N.; Gower, J.; Sweeting, R. Evaluation of MODIS-Aqua atmospheric correction and chlorophyll products of Western North American coastal waters based on 13 years of data. Remote Sens. 2017, 9, 1063. [Google Scholar] [CrossRef]



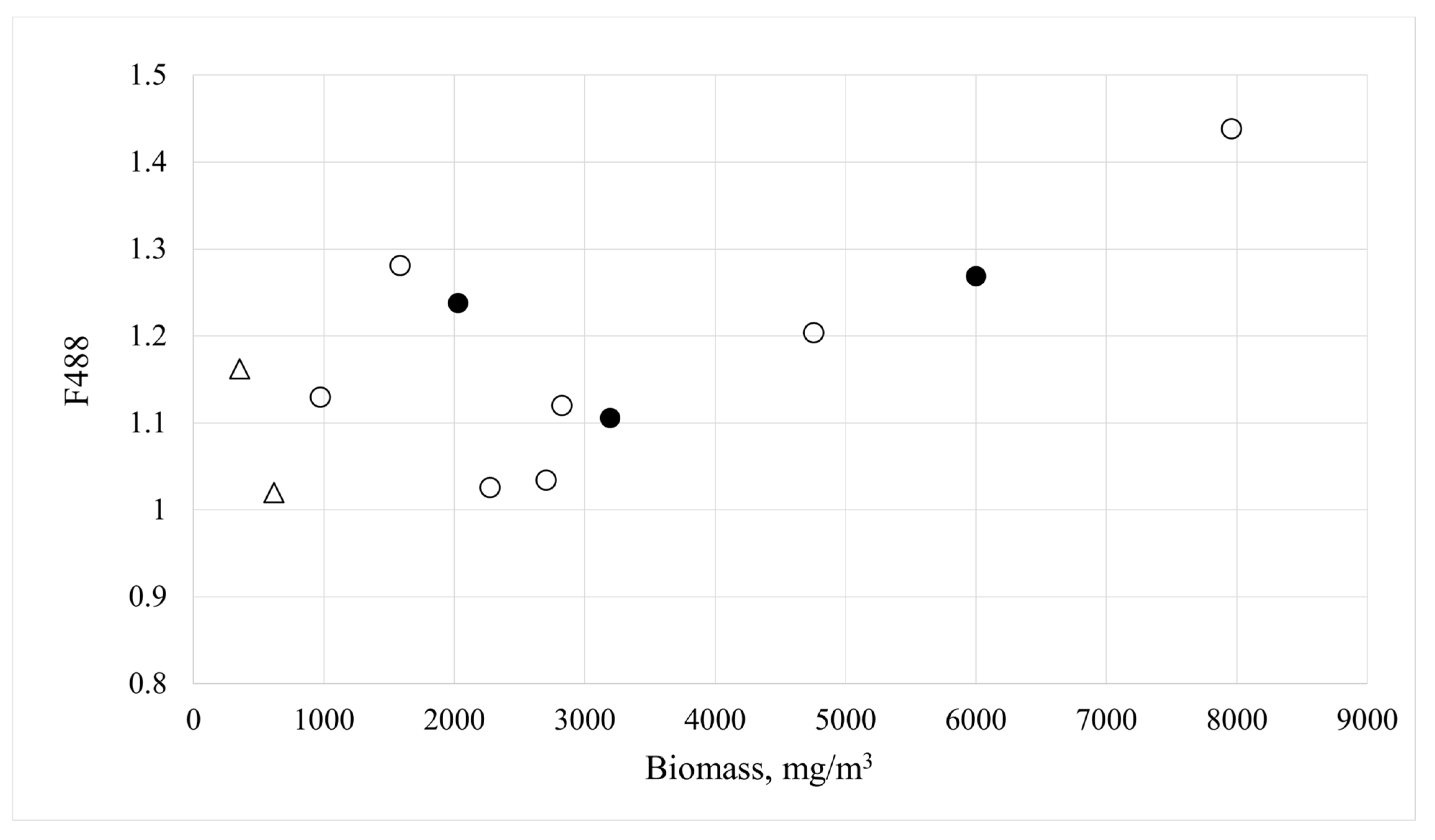
| Station | Date | Coordinates | F488 | Total Phytoplankton | Karenia spp. | |||
|---|---|---|---|---|---|---|---|---|
| Abundance (cells L−1) | Biomass (mg m−3) | Abundance (cells L−1) | Biomass (mg m−3) | Species 1 | ||||
| 2 | September 22 | 52°51.095′N, 159°32.190′E | 1.20 | 434,000 | 4755 | 369,000 (85%) | 4551 (96%) | KA (98%), Kp (1%), Ks (0.5%), Km (0.5%) |
| 3 | September 22 | 53°01.531′N, 158°57.354′E | 1.12 | 647,000 | 2825 | 203,000 (31%) | 2499 (88%) | Kp (98%), Km (1.5%), KA (0.5%) |
| 4 | September 22 | 52°59.095′N, 158°51.594′E | 1.03 | 299,000 | 2705 | 187,000 (63%) | 2319 (86%) | Km (98%), Kp (1.5%), KA (0.5%) |
| 5 | October 4 | 52°59.717′N, 158°57.450′E | 1.28 | 125,000 | 1585 | 105,000 (84%) | 1302 (82%) | Kl (90%), Kp (10%) |
| 6 | October 4 | 52°55.600′N, 158°41.333′E | 1.02 | 73,000 | 617 | 20,000 (27%) | 248 (40%) | Kl (100%) |
| 7 | October 4 | 52°55.991′N, 158°41.151′E | 1.03 | 247,000 | 2274 | 152,000 (61%) | 1885 (83%) | Kl (98%), KC (1.5%), KB (0.5%) |
| 8 | October 4 | 52°49.737′N, 158°36.567′E | 1.13 | 83,000 | 974 | 66,000 (79%) | 819 (84%) | Kl (100%) |
| 12 | October 9 | 53°12.900′N, 159°33.420′E | 0.86 | 8320 | 181.5 | 3039 (36%) | 24.3 (13%) | Km (5%), Ks (95%) |
| 13 | October 9 | 53°11.220′N, 159°26.040′E | 1.16 | 27,512 | 354.9 | 11,477 (42%) | 94.7 (27%) | Km (92%), Kp (8%) |
| 14 | October 12 | 50°51.067′N, 156°41.100′E | 1.24 | 165,000 | 2028 | 162,000 (98%) | 2004 (99%) | Ks (100%) |
| 15 | October 12 | 51°06.117′N, 157°04.067′E | 1.27 | 485,000 | 5999 | 482,000 (99%) | 5981 (99%) | Ks (100%) |
| 16 | October 12 | 51°30.730′N, 157°44.870′E | 1.11 | 262,000 | 3194 | 254,000 (97%) | 3146 (98%) | Ks (99%), KD (1%) |
| 17 | October 13 | 52°43.930′N, 158°32.653′E | 1.4 | 968,000 | 7959 | 622,000 (64%) | 7715 (97%) | Ks (99%), KC (1%) |
| Date and Time (UTC) Month.Day (Hours:Minutes) | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | 09.09 (02:40) | 09.21 (1:30) | 09.25 (2:40) | 10.03 (01:55) | 10.09 (01:15) | 10.12 (01:45) | |
| Zone 1 | Chl-a (mg m−3) | 1.60 ± 10−3 | 1.14 ± 4 × 10−4 | 1.80 ± 4 × 10−3 | 1.62 ± 9 × 10−4 | 1.82 ± 10−3 | 1.45 ± 7 × 10−4 |
| Kd490 (m−1) | 0.14 ± 10−4 | 0.14 ± 4 × 10−5 | 0.17 ± 3 × 10−4 | 0.17 ± 5 × 10−5 | 0.18 ± 2 × 10−4 | 0.17 ± 4 × 10−5 | |
| F488 | 0.84 ± 10−4 | 1.17 ± 6 × 10−5 | 1.07 ± 2 × 10−4 | 1.16 ± 7 × 10−5 | 1.37 ± 2 × 10−4 | 1.22 ± 9 × 10−5 | |
| flh/chl-a (mW cm−2 µm−1 sr−1 mg−1∙m3) | 0.06 ± 8*10−5 | 0.15 ± 10−4 | 0.16 ± 3 × 10−4 | 0.23 ± 10−4 | 0.17 ± 7 × 10−5 | 0.20 ± 9 × 10−5 | |
| Zone 2 | Chl-a (mg m−3) | 0.58 ± 10−4 | 0.74 ± 3 × 10−4 | 0.74 ± 10−4 | 0.89 ± 2 × 10−4 | 0.74 ± 9 × 10−5 | 0.81 ± 10−4 |
| Kd490 (m−1) | 0.08 ± 2 × 10−5 | 0.10 ± 2 × 10−5 | 0.10 ± 10−5 | 0.11 ± 10−5 | 0.10 ± 10−5 | 0.09 ± 8 × 10−6 | |
| F488 | 0.89 ± 4 × 10−5 | 0.98 ± 9 × 10−5 | 0.96 ± 6 × 10−5 | 0.95 ± 7 × 10−5 | 1.01 ± 7 × 10−5 | 0.92 ± 8 × 10−5 | |
| flh/chl-a (mW cm−2 µm−1 sr−1 mg−1∙m3) | 0.14 ± 3 × 10−5 | 0.09 ± 8 × 10−5 | 0.16 ± 3 × 10−5 | 0.11 ± 3 × 10−5 | 0.09 ± 6 × 10−5 | 0.09 ± 3 × 10−5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexanin, A.; Kachur, V.; Khramtsova, A.; Orlova, T. Methodology and Results of Satellite Monitoring of Karenia Microalgae Blooms, That Caused the Ecological Disaster off Kamchatka Peninsula. Remote Sens. 2023, 15, 1197. https://doi.org/10.3390/rs15051197
Alexanin A, Kachur V, Khramtsova A, Orlova T. Methodology and Results of Satellite Monitoring of Karenia Microalgae Blooms, That Caused the Ecological Disaster off Kamchatka Peninsula. Remote Sensing. 2023; 15(5):1197. https://doi.org/10.3390/rs15051197
Chicago/Turabian StyleAlexanin, Anatoly, Vasilii Kachur, Anastasiya Khramtsova, and Tatiana Orlova. 2023. "Methodology and Results of Satellite Monitoring of Karenia Microalgae Blooms, That Caused the Ecological Disaster off Kamchatka Peninsula" Remote Sensing 15, no. 5: 1197. https://doi.org/10.3390/rs15051197
APA StyleAlexanin, A., Kachur, V., Khramtsova, A., & Orlova, T. (2023). Methodology and Results of Satellite Monitoring of Karenia Microalgae Blooms, That Caused the Ecological Disaster off Kamchatka Peninsula. Remote Sensing, 15(5), 1197. https://doi.org/10.3390/rs15051197





