1. Introduction
Anomalous aggregation of phytoplankton, known as a bloom event, has negative impacts on the coastal fishing industry and marine ecosystems. According to previous research, bloom frequency has increased in recent decades because of excess nutrients input resulting from human activities [
1,
2,
3,
4]. The East China Sea (ECS), one of China’s most important marginal seas, is abundant in fishery resources. Because of the interaction of freshwater from the Yangtze River, the Taiwan Warm Current, the Kuroshio Current, and monsoons, this region is favorable for growth of phytoplankton and is consequently susceptible to occurrence of blooms [
5,
6]. In the past decade, there have been numerous reports of
Prorocentrum donghaiense blooms, which are known to have harmful impacts on marine ecosystem health by causing mortality of fishes and other marine organisms [
7,
8,
9]. Comprehensive and detailed monitoring, including both spatial and temporal distribution of blooms, can assist in understanding the occurrence and evolution of blooms and also clarify the distribution characteristics of blooms in the ECS.
In general, bloom waters exhibit increased chlorophyll concentrations, with strong reflectance peaks in the green and red wavebands, as well as considerable changes in absorption and scattering properties [
10,
11,
12]. Bloom detection methods based on remote sensing techniques have, therefore, been established using the distinct remote sensing reflectance
characteristic of bloom waters. At present, there are several methods for bloom detection using satellite imagery, including methods based on anomalous chlorophyll a concentration (Chl-a),
spectrum variation, and the inherent optical properties of blooms.
Based on anomalous Chl-a in bloom waters, Stumpf et al. (2003) [
13] detected
Karenia brevis blooms in the Gulf of Mexico using Chl-a data derived from SeaWiFS; Sun et al. (2012) [
14] extracted blooms along the ECS coast using MODIS data by comparing the Chl-a with the monthly averaged value; He et al. (2013) [
1] explored the long-term bloom changes in the ECS using satellite-derived Chl-a data with a threshold of 10µg/L. However, Chl-a-based approaches remain challenging in coastal regions because satellite estimations of Chl-a are often overestimates because of high levels of resuspended sediments and colored dissolved organic matter in nearshore areas [
15,
16]. Further, these approaches may fail if the phytoplankton cells have a low Chl-a content. Consequently, when using Chl-a as a bloom indicator, false-positive detections may occur in coastal regions.
Other studies have been conducted that directly apply the
spectrum rather than the satellite-derived Chl-a. Lou et al. (2014) [
17] developed an improved red tide index (RI) for bloom detection in ECS by utilizing Geostationary Ocean Color Imager (GOCI) imagery; Tao et al. (2015) [
18] successfully extracted
P. donghaiense and diatom bloom waters in the ECS using the algal bloom ratio (
) with Moderate Resolution Imaging Spectroradiometer (MODIS) satellite data; Shin et al. (2018) [
19] extracted
Margalefidinium bloom waters by calculating the spectral slope change at 490 nm and successfully applied to GOCI, Sentinel-3 Ocean, Land Color Instrument (OLCI), and Landsat Operational Land Imager (OLI) data. Other algorithms also detect bloom species by utilizing the inherent optical features of phytoplankton. Shang et al. (2014) [
11] distinguished
P. donghaiense and diatom blooms in the ECS using MODIS data by taking advantage of the higher absorption coefficient at 443 nm in
P. donghaiense bloom waters; Cannizzaro et al. (2008) [
20] detected
Karenia brevis blooms in the Gulf of Mexico using the lower particle backscattering signal of bloom waters on MODIS imagery; Shen et al. (2019) [
9] used Medium Resolution Imaging Spectrometer (MERIS) imagery to differentiate
P. donghaiense and diatom blooms by analyzing the absorption spectrum of algae species at green–red bands; Qi et al. (2019) [
21] detected
Noctiluca scintillans blooms in the ECS based on its distinctive absorption and scattering properties using MODIS data from 2000 to 2017.
In summary, various approaches based on specific ocean color satellites have been developed. However, applicability of different bloom identification methods varies across study regions, as does efficiency of one bloom extraction approach on a different sensor. As a result, it is necessary to validate and analyze input threshold values and extraction results using historical field bloom data when applied on different satellites.
In December 2017, Japan launched the Global Change Observation Mission-Climate (GCOM-C) satellite with the Second-Generation Global Imager (SGLI) sensor, which provides spectral data in 19 channels ranging from near-ultraviolet to thermal infrared (380 nm–12 m) with a bandwidth of 10 nm and a temporal resolution of 1–2 days. The visible bands are 412 nm, 443 nm, 490 nm, 530 nm, 565 nm, and 670 nm, respectively. It is known that various phytoplankton have distinct spectral differences in the blue–green bands of
; thus, species such as diatoms or dinoflagellates can be differentiated based on spectral morphology of satellite
[
12,
22,
23]. Therefore, inclusion of a band at 530 nm is critical for bloom monitoring and algae species classification.
In this study, we evaluated the SGLI data for detecting blooms along the coast of the ECS with three objectives. The first task was to validate satellite data by comparing them to in situ measured data because accuracy of satellite data is crucial to reliability of bloom detection results. The second objective was to evaluate the applicability of bloom extraction using SGLI data by comparing various bloom detection methods. On this basis, the effect of different remote sensing techniques for distinguishing harmful algae in the ECS based on SGLI images was also discussed.
2. Materials and Methods
2.1. Study Area and Bloom Records
The study area covers the northwestern part of the East China Sea, including the mouth of the Yangtze River, Hangzhou Bay, and Zhejiang Coast (27°N–32°N, 120°E–123°E) (see
Figure 1). The study area is located in a subtropical region with frequent monsoon activities, abundant precipitation, and temperature and humidity conditions that make it a "high-risk" location for bloom disasters. Since 2011, outbreaks of
P. donghaiense bloom have occurred frequently along the ECS coast. The deterioration of water quality and the decrease of dissolved oxygen in the water during bloom periods have seriously affected the coastal ecological health. In this study, bloom records for the study area from 2019 to 2020 were collected from the Wenzhou Marine Environmental Monitoring Center Station of State Oceanic Administration (China) and the China Marine Disaster Bulletin (
https://www.nmdis.org.cn/hygb/zghyzhgb/, accessed on 18 January 2018, in Chinese). The bloom records were used as the ground truth for comparison of bloom detection methods. The data include information such as bloom location, occurrence time, and algal species (
Table 1). As a result of a lack of in situ measured
data for bloom waters, satellite
was matched with the bloom locations to assess the performance of the bloom detection methods. Details regarding the matching methods can be found in
Section 2.3.
2.2. In Situ
From September 2019 to September 2021, in situ measured
data were collected from the Dongou Ocean Observing Platform (27.675°N, 121.355°E, indicated by a red star in
Figure 1). The observation platform was constructed as part of the Dongou Comprehensive Ocean Observation Platform Project, which was managed by the Wenzhou Marine Environment Monitoring Center of the State Oceanic Administration (China). The observation platform combines the functions of marine observation, instrument experiments, and scientific research. The implementation of the platform is significant in terms of improving observation ability relating to marine hazards and providing comprehensive coverage of major marine disasters along the ECS.
The offshore observation platform employs a Sea-Viewing Wide Field-of-View Sensor Photometer Revision for Incident Surface Measurements (SeaPRISM) autonomous measurement system, with measurements every 30 min from 8:00 am to 4:00 pm each day. The system collects radiometric data from the water surface and sky. After processing to obtain the normalized water-leaving radiance , it is divided by the solar irradiance () in the corresponding band to obtain the remote sensing reflectance . The SeaPRISM system comprises 11 upper spectrum observation channels ranging from 400 nm to 1020 nm. To assess the accuracy of satellite GCOM-C, local measured data at the central wavelengths of 412 nm, 443 nm, 490 nm, 560 nm, and 667 nm were collected corresponding with the bands of SGLI data.
2.3. SGLI Imagery Processing
The purpose of the Global Change Observation Mission-Climate (GCOM-C) project is to conduct long-term worldwide observations of the Earth’s environment. The GCOM-C is expected to play an important role in monitoring both global water circulation and climate change from space. The Second-Generation Global Imager (SGLI) carried on the GCOM-C conducts observations related to land, ocean, cryosphere, atmosphere, and so on (details of SGLI data are summarized in
Table 2). Of the SGLI visible channels, 490 nm, 565 nm, and 670 nm are high signal-to-noise channels for retrieval of ocean color variables, such as Chl-a, total suspended matter, and gelbstoff [
24].
The SGLI provides three levels of data: L1, L2, and L3. Among them, L2 and L3 can provide radiometer products with a spatial resolution of 250 m, and ocean water color product data with a spatial resolution of 1 km, respectively. The SGLI products were downloaded via FTP from the Satellite Monitoring for Environmental Studies (JASMES) data center of the Japan Aerospace Exploration Agency (
ftp.gportal.jaxa.jp, accessed on 18 January 2018).
Specifically, the L2 data contain three radiometer products: normalized water leaving radiance
at 380–670 nm, aerosol optical thickness (AOT) at 670–865 nm, and photosynthetically available radiation (PAR). The SGLI ocean color atmospheric correction algorithm is basically same as those of MODIS and VIIRS [
25] (the details could be checked at
https://suzaku.eorc.jaxa.jp/GCOM_C/data/, accessed on 18 January 2018). Specifically, the
is derived by correcting the directional dependency of in-water and water-surface reflectance using the Look Up Table (LUT) developed by Morel and Maritorena (2001) [
26]. In the L3 data, the satellite Chl-a product was derived using the OCx algorithm with an empirical exponential power algorithm for the remote sensing reflectance ratio in the blue–green band (details of SGLI Chl-a retrieval can be found in Murakami, 2020 [
27]).
To obtain the satellite
the
data in L2 were converted to
using the following Equation (1):
Here, denotes the remote sensing reflectance of band i; DN denotes the value; and denote the slope and intercept, respectively, when transferring the value to satellite of band i. The conversion parameters for the different bands can be found in the SGLI attributes file.
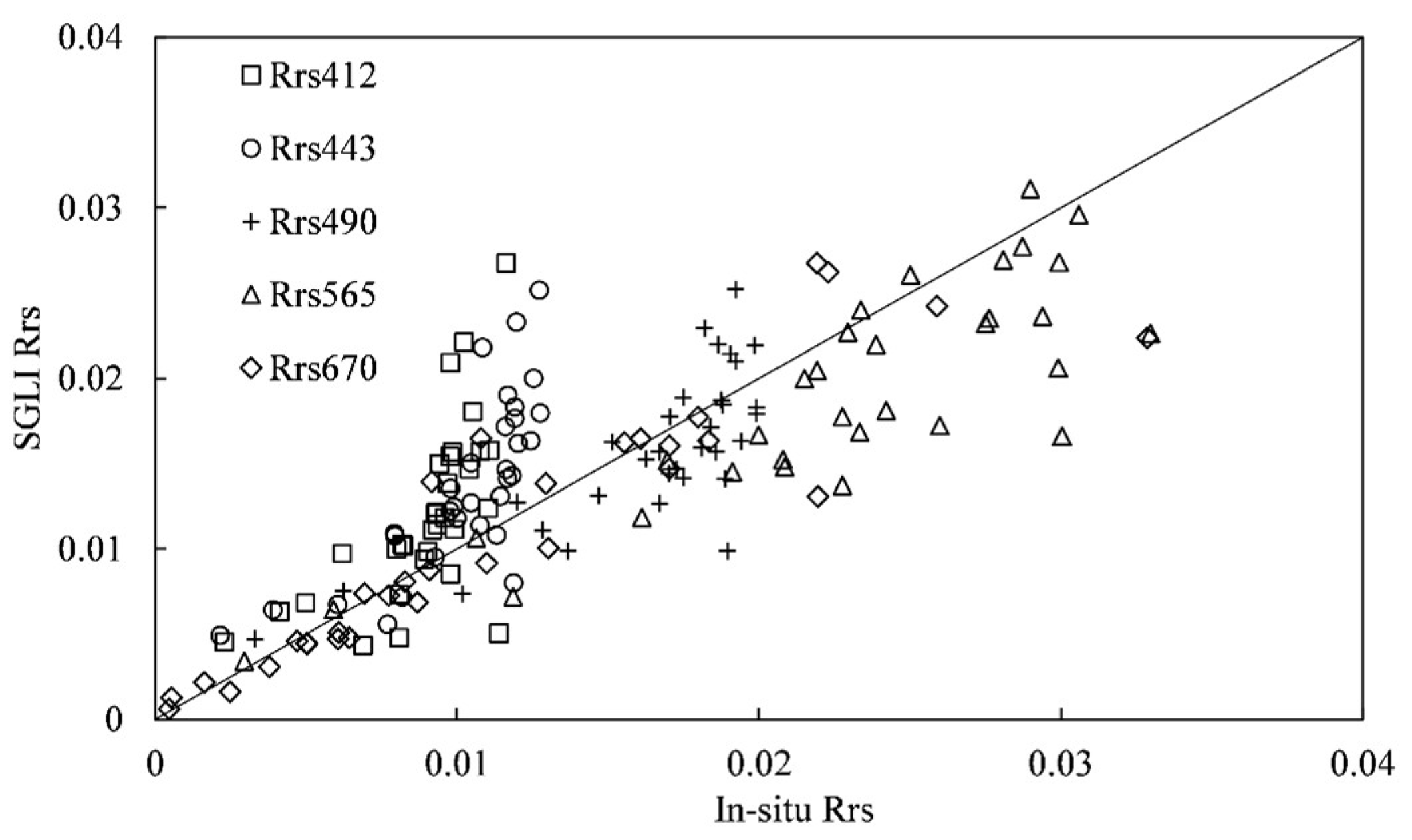
The SGLI satellite images obtained in this study were divided into two parts. The first part of the data was used to verify the satellite accuracy, and the second part was used to compare the effectiveness of bloom detection methods in the ECS.
The first part of the satellite data was chosen based on the in situ measured
by Dongou Observation Platform with a time window of ±1 h. Because the spatial reference of the original SGLI L2 images is a user-defined geographic coordinate system, it cannot be spatially matched with the local measured
data. Therefore, the original image data were converted to a projection of equal longitude and latitude with WGS84 using MATLAB 2021b. In addition, the SGLI data were checked using the satellite flag attribute data to eliminate abnormal or invalid pixels (conditions such as haze cover, atmospheric correction errors, etc.). Then, the nearest neighbor pixels were selected to be matched with the local
data using a 3
3 pixels window. The pixel closest to the target location was chosen if there were invalid satellite pixels in the local measurement. Finally, 32 pairs of satellite
data were matched with the local observations for the satellite
accuracy assessment. To evaluate the accuracy of SGLI
, the coefficient of determination (R
2), root mean square error (RMSE), and mean absolute percentage error (MAPE) were calculated. R
2 was used to measure the consistency of variation between satellite data and local measured data; RMSE was used to evaluate the uncertainty of satellite data; MAPE was used to evaluate the deviation of satellite data from local measured data. R
2 was obtained by linear regression from the matched pairs of satellite and in situ measured data. The RMSE and MAPE were defined as follows:
where
and
denote the satellite-derived and local measured
data, respectively. N represents the number of matched pairs.
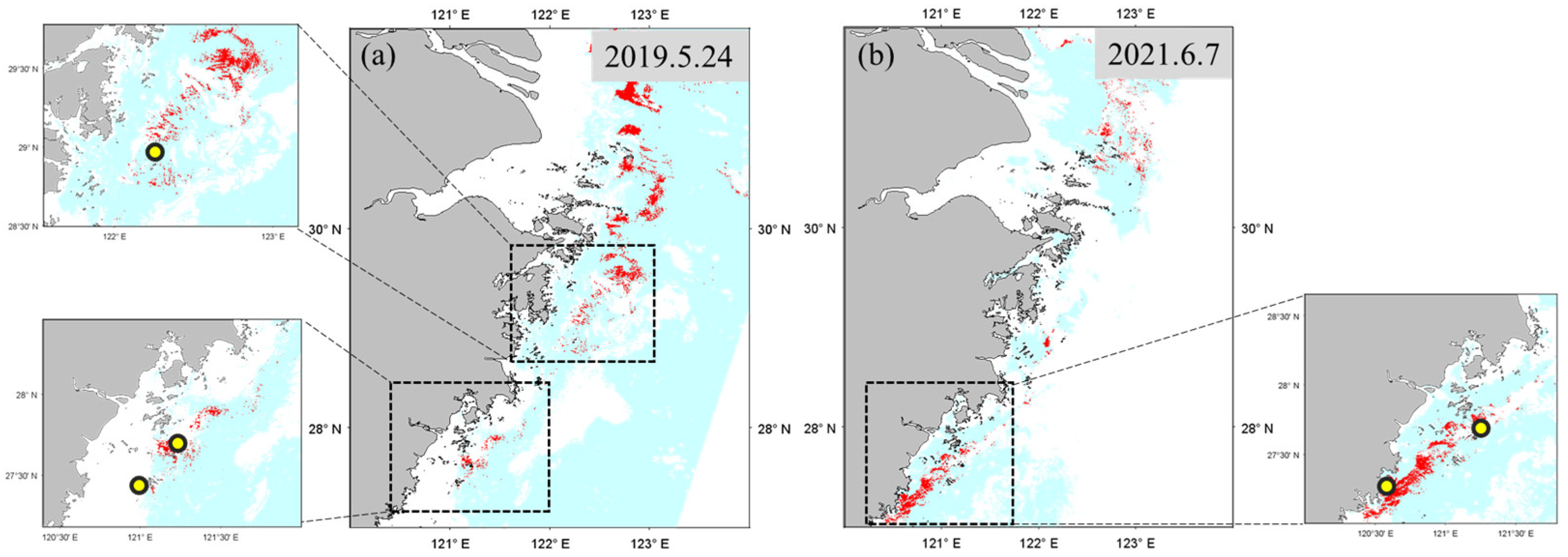
For the comparison of bloom detection methods, another set of SGLI L2 images were processed to collect the satellite
during local bloom surveys in 2020 (
Table 1). The SGLI L2
data were matched with the bloom occurrence locations using a time window of
2 h. Finally, five pairs of matched
from
P. Donghaiense bloom locations were obtained (from four SGLI images). In addition, four pairs of satellite
data on non-bloom days (14 April 2020) were chosen to assess the ability of bloom detection methods to differentiate bloom waters from turbid or clear waters. To analyze the spatial distribution extracted by different bloom detection methods, three additional SGLI images were also processed using the
conversion method mentioned above (including SGLI images on 24 May 2019; 29 April 2020; 7 June 2021).
2.4. Bloom Detection Methods
Algal blooms are commonly accompanied by accumulation of pigments, resulting in strong absorption in the range of blue to green bands. In contrast, turbid water scatters light at all visible bands, whereas clear water absorbs light at all wavebands [
28,
29]. These features enable detection of bloom waters via satellite
. In this study, three existing approaches, the spectral shape (SS) algorithms [
30], the red tide index (RI) [
17], and the algal bloom ratio (
) [
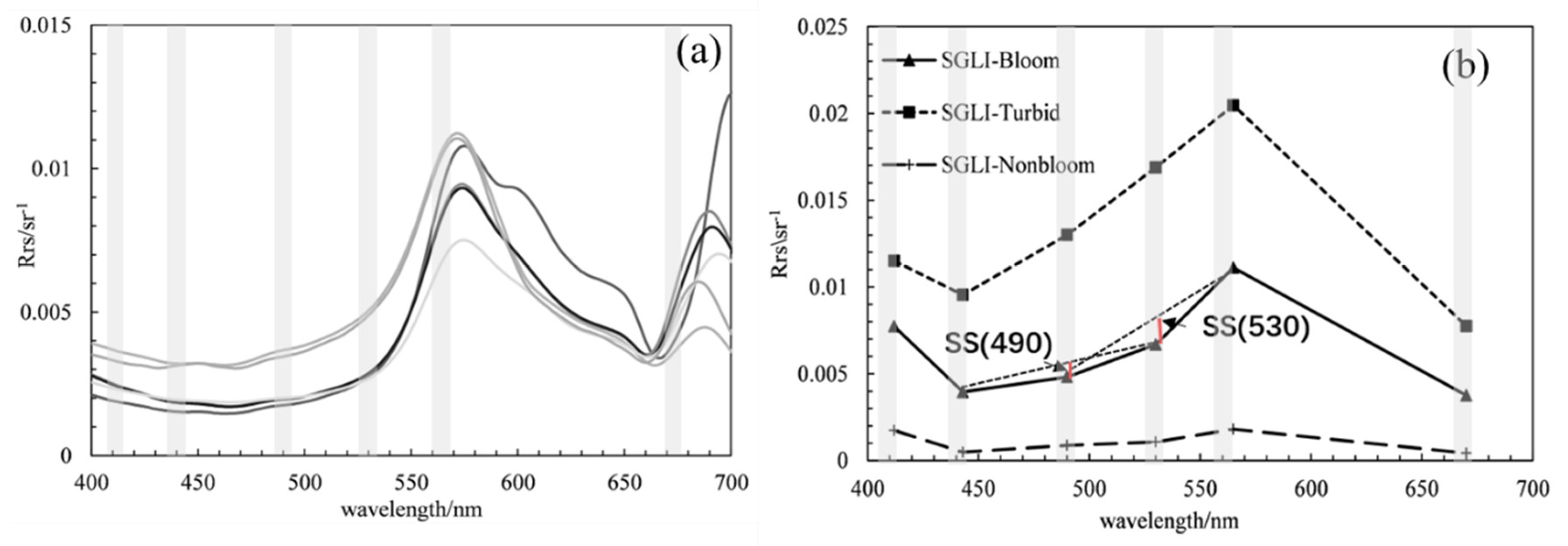
18], were compared for their ability to detect blooms using SGLI data. The SS algorithms are defined methods as shown below:
where λ denotes the position of the central band; λ
− and λ+ denote the positions of the two bands closest to the central band; and
,
, and
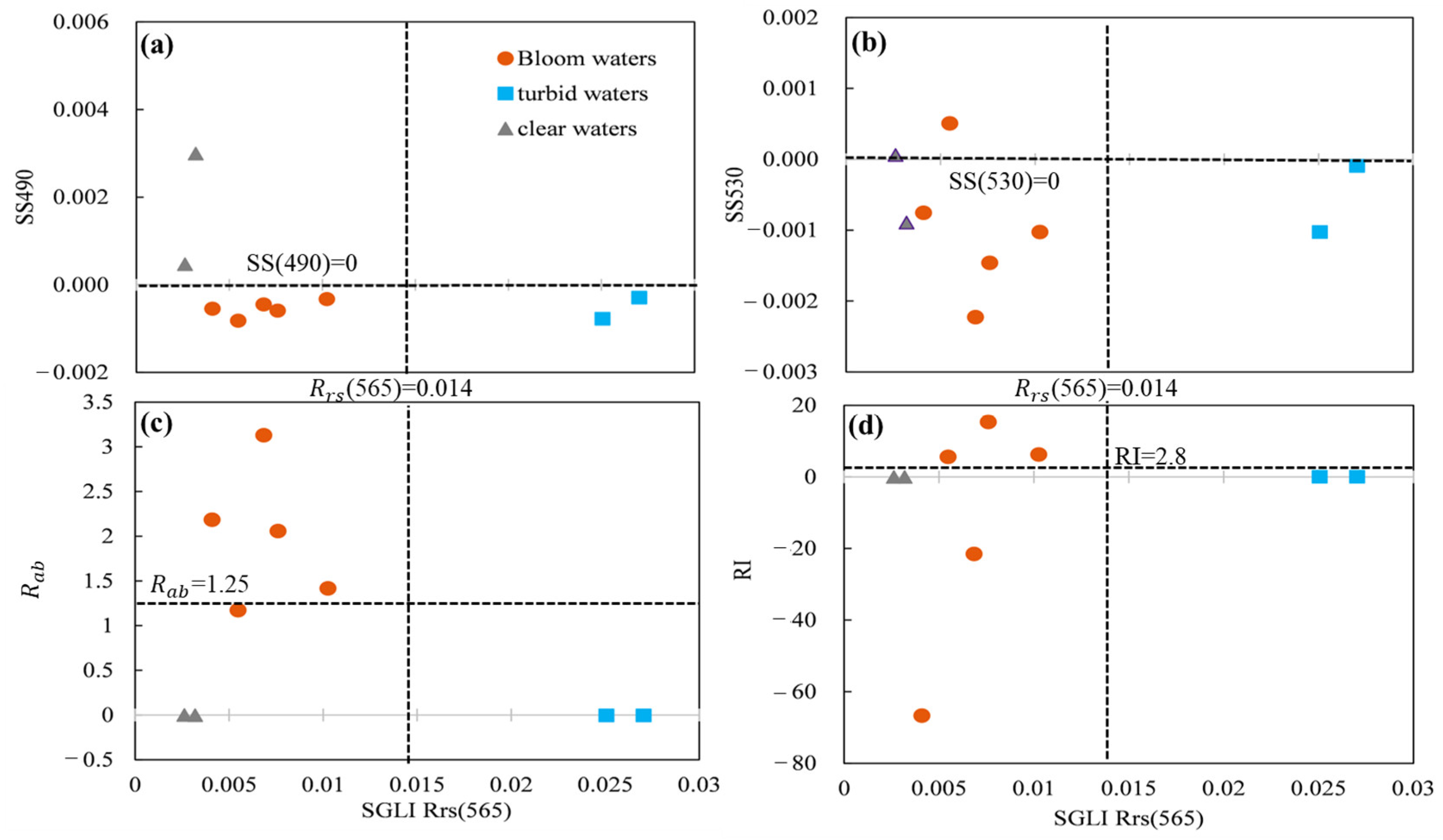
correspond to the remotely sensed reflectance on band positions. Based on the spectral characteristics of the SGLI data in the blue–green band, calculation of spectral shapes was performed on 490 nm and 530 nm. A pixel was flagged as bloom water when SS(490) < 0 or SS(530) < 0.
The RI and
are defined by the satellite
in the blue and green bands as follows:
where
,
,
and
denote the remote sensing reflectance at 443 nm, 490 nm, 530 nm, and 550 nm of satellite data, respectively. It should be noted that
and
were used instead of
and
to match the wavebands in SGLI data. A pixel was flagged as bloom water when RI > 2.8 or
> 1.25 [
17,
18]. Furthermore, the
algorithm excluded turbid water by judging the distribution range of the green band as follows: (1) if
< 0.014 sr
−1, the
is calculated to determine the location of blooms; (2) if
> 0.014 sr
−1, it is directly flagged as turbid water. Because SGLI lacks the 555 nm band, the
was replaced by
from the SGLI data.
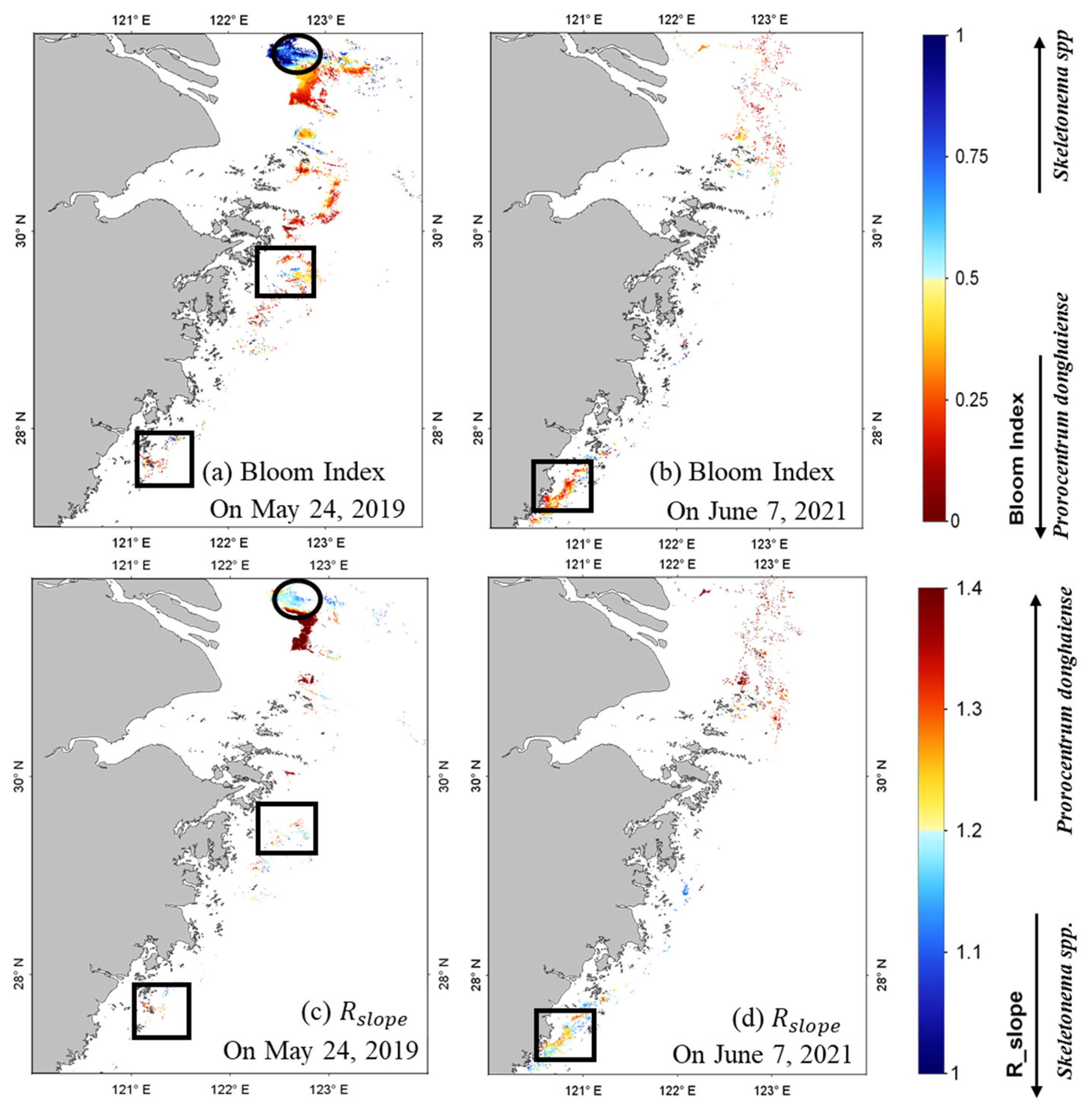
Following the comparison of bloom detection methods, species identification of
P. donghaiense and diatom blooms using SGLI data were investigated using the bloom index (BI) and a green-red spectral shape (
) algorithms (developed by Shang et al. (2014) [
11] and Shen et al. (2019) [
9], respectively). The algorithms were described as follows:
- (1)
If 0 < BI = < 0.3, the pixel was classified as P. donghaiense bloom water or diatom bloom water.
- (2)
If > 0.4, the pixel was classified as P. donghaiense bloom water or diatom bloom water.
Note that the , , and in the equations of BI and were replaced as , , and , respectively. The BI method was developed to describe the steep slope of in the blue–green bands based on the strong pigment absorption of P. donghaiense bloom waters. Satellite pixels with a BI range of 0–0.3 were classified as P. donghaiense bloom waters. The was calculated to represent the (λ) variation in spectral morphology in the green–red band, and a pixel with > 0.4 was considered to have a high probability of P. donghaiense bloom occurrence.
4. Discussion
In this study, three kinds of bloom detection algorithms were compared to explore their ability in bloom extraction on SGLI data along the ECS coast. Bloom detection results from local match-up pairs and SGLI imagery showed that the SS(490) outperformed the the SS(530), , and RI methods. The reasons for the unsatisfactory bloom detection results could be different among the methods.
Figure 10 depicts the in situ
associated with
P. donghaiense blooms (provided by the data in [
18]) and the
extracted spectrum from the SGLI image on 29 April 2020. It is known that outbreaks of bloom are often accompanied by an increase in pigment concentration, resulting in a reflectance valley in the blue–green band range. Such a feature is indicated by a negative SS(490) or SS(530) value in the spectrum shape method. Although the SS(530) could detect bloom by the local match-up pairs, an unexpected bloom area was found in the offshore region by SGLI image (
Figure 7b). Uncertainties in satellite
may account for the unsatisfactory results obtained by SS(530) and
. Despite the fact that
was also used in SS(490), it was only employed as a baseline to describe spectrum curvature at 490 nm. Further accuracy assessment of satellite
is recommended when sufficient in situ
data are available. With regard to the failure of the RI method, it only considers the height difference of satellite
between blue–green bands rather than the variation in spectral curvature in this range. Although subtraction of
could eliminate the influence of non-algal sediments, uncertainty regarding atmospheric correction might still result in high RI values in the non-bloom waters. Thus, utilization of Rayleigh-corrected reflectance could be a preferred choice when dealing with unfavorable sky conditions [
17]. In short, uncertainties in atmospheric correction in the offshore regions might be the main reason for the failure in SS(530),
, and RI. Even though validation of SGLI
was evaluated using in situ measurement from the Dongou Ocean Observing Platform (located in the nearshore region), the accuracy of satellite
in the offshore region remains unknown. Further efforts are required to explore the accuracy of satellite data in the region.
In further steps, an empirical threshold of
0.0005 of SS(490) was established for bloom detection on SGLI data. Although
Figure 5a shows that all bloom waters can be accurately determined with a negative SS(490), the local measured
from bloom waters showed a smaller SS(490) value of −0.00047
0.00011 (calculated from the in situ
of bloom waters in
Figure 10a, provided by Tao et al. (2015)). In fact, it was found that coastal waters with high concentrations of dissolved organic matter will also show negative SS(490) by absorbing strongly in blue–green bands [
31]. Therefore, a refined threshold for SS(490) is recommended when applying SS(490) in coastal regions for bloom detection.
In summary, the spectral shape algorithms are outperformed in bloom detection compared to the other two methods in this study when considering the challenge of atmospheric correction in coastal regions. In fact, Blondeau-Patissier et al. (2014) [
32] also concluded that band ratio methods related to blue–green bands could be unreliable in coastal waters because of increasing concentrations of sediments and dissolved organic matter. In contrast, the spectral shape method is robust in analyzing satellite
by capturing increasing pigments absorption from accumulated phytoplankton in bloom waters. Previous methods such as fluorescence line height (FLH), maximum chlorophyll index (MCI), and floating algae index (FAI), which described spectral curvature in red to near-red bands, were also found to work well in detection of bloom waters [
33,
34,
35].
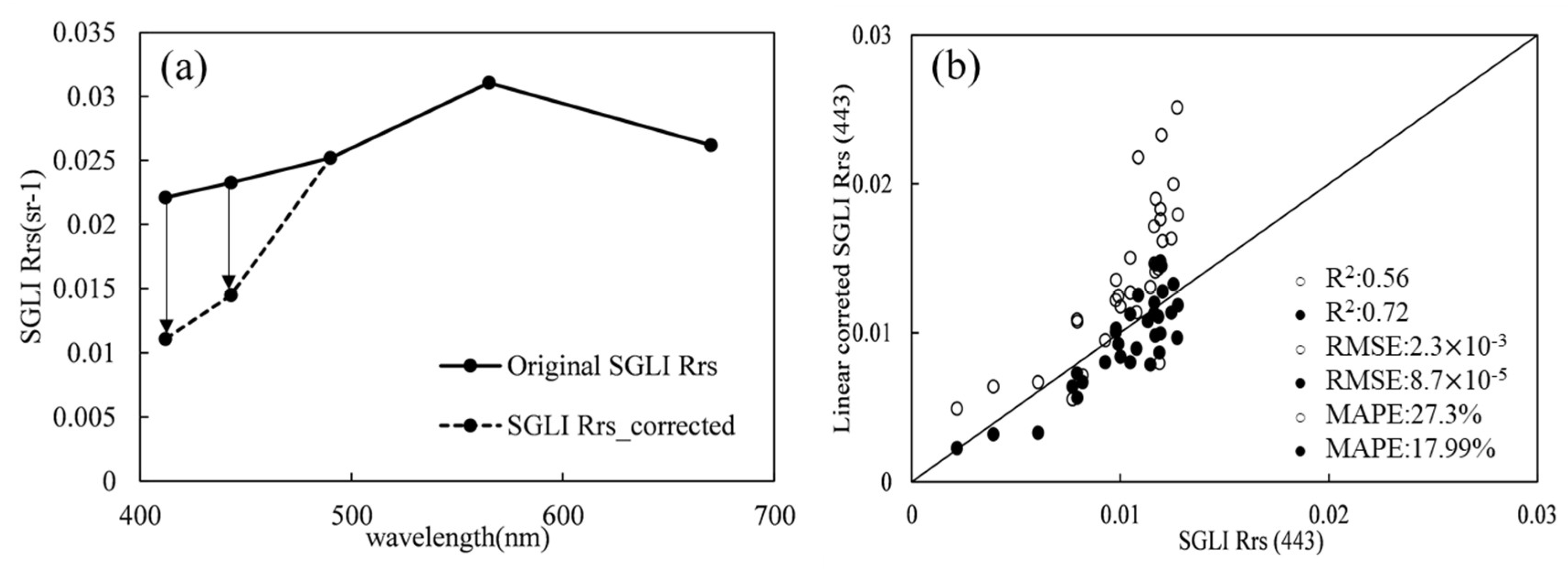
Furthermore, it is suggested that correction for
is required when applying certain bloom detection approaches to SGLI images. The linear correction method can assist in reducing overestimation of bloom areas without the need to repeat the atmospheric correction processes. The linear correction approach on satellite
was first developed for correction of underestimation from MODIS images in the research area of Ise Bay in Japan [
36]. The results of this study demonstrated that the linear correction method could also be applied to correct overestimation of satellite
(similar results found in [
37]). Notably, the parameter used in Equation (7), which describes the relationships between in situ
and
, is an empirical value. In future studies, the unexpectedly overestimated
at short bands was supposed to be more effectively corrected using the locally measured
from bloom waters to improve the utility on bloom detection by SGLI images.
In the final steps, classification of P. donghaiense and diatom blooms was achieved using both the BI and approaches on SGLI images. Inclusion of 530 nm in the SGLI data enhances their ability to distinguish harmful algal species in the ECS by using the multiple phytoplankton discrimination method. Although the bands use in calculation differs from that described by Shen et al. (2019), the method characterizing the spectral shape in green–red bands of bloom waters remain effective for phytoplankton discrimination. Note that the difference in thresholds of BI and might not only be from bands difference between SGLI and MODIS but also from uncertainties in satellite (λ) retrieval. Further thresholds modifications in BI and are recommended when local measured data of P. donghaiense and diatom blooms in ECS are available.
5. Conclusions
In this study, performance of different bloom extraction methods using SGLI imagery was evaluated via comparative analysis. First, the accuracy of SGLI was assessed by comparing matched pairs to the locally measured . Compared with in situ measured , the precision of satellite differed between wavebands. Accuracies of 490 nm, 565 nm, and 670 nm were adequate for bloom observation in the ECS, while overestimation was found at 412 nm and 443 nm. By utilizing a linear correction approach, overestimation of at 443 nm was reduced. Furthermore, based on local matched data and SGLI images, the spectral shape (SS) method, the red tide index (RI), and the algal bloom ratio () were examined for bloom identification accuracy. The bloom extraction results from SS(490) were better than those obtained using SS(530), , and RI. To remove the potential influence of turbid waters, an empirical criterion for was required. In addition, it was notable that the SGLI data could also help in distinguishing P. donghaiense from diatom blooms. Inclusion of 530 nm accurately described the discrepancy in blue–green absorption between the two species. On the other hand, according to China Marine Disaster Bulletin reports, harmful species other than diatoms and P. donghaiense, such as Akashiwo sanguinea and Ceratium furca, have also emerged in recent years. Therefore, more efforts will be required to establish the ability of current phytoplankton discrimination algorithms to detect other harmful species using SGLI data.