Studying Conditions of Intense Harmful Algal Blooms Based on Long-Term Satellite Data
Abstract
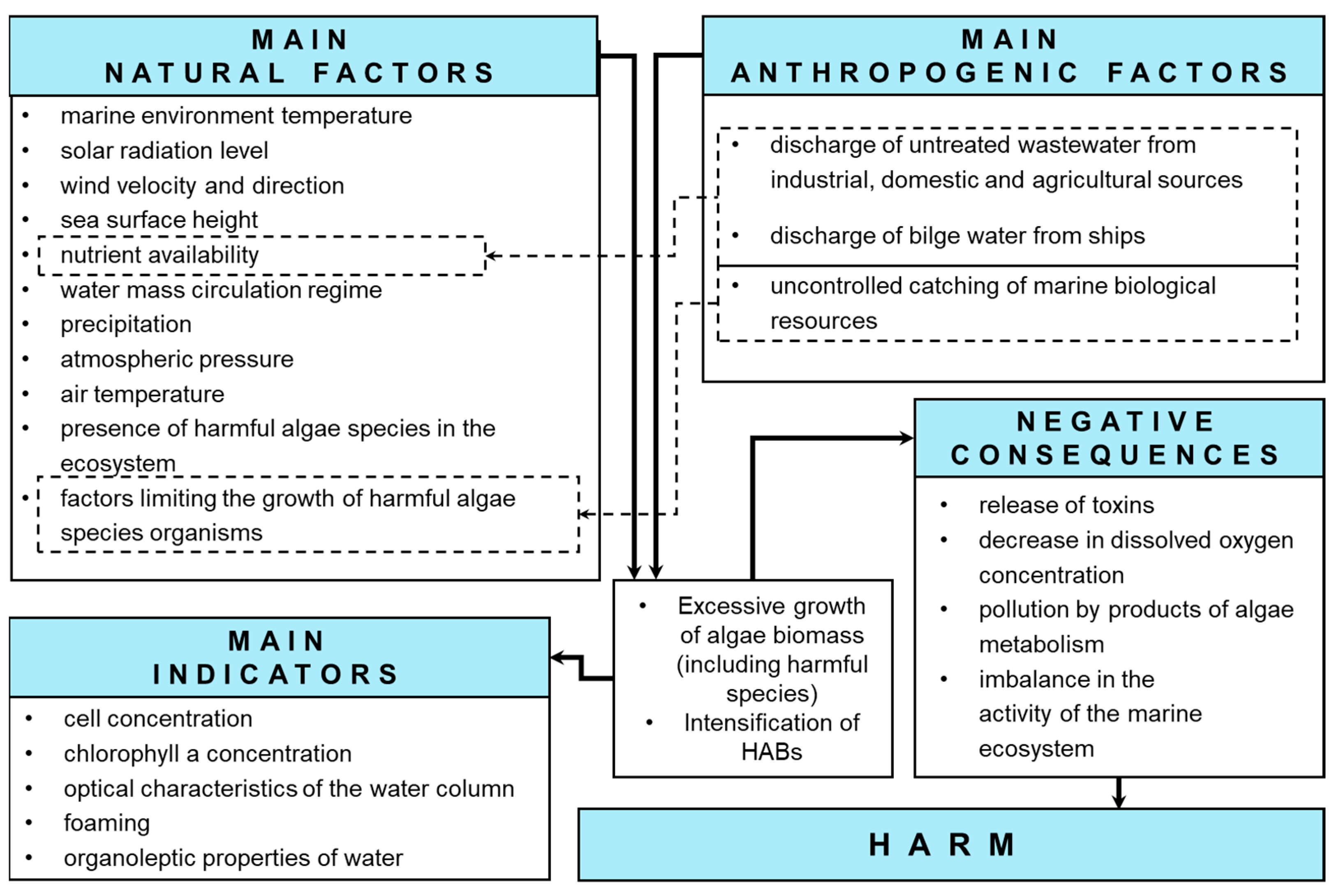
1. Introduction
1.1. HAB Issues and Some of the Most Intense Cases
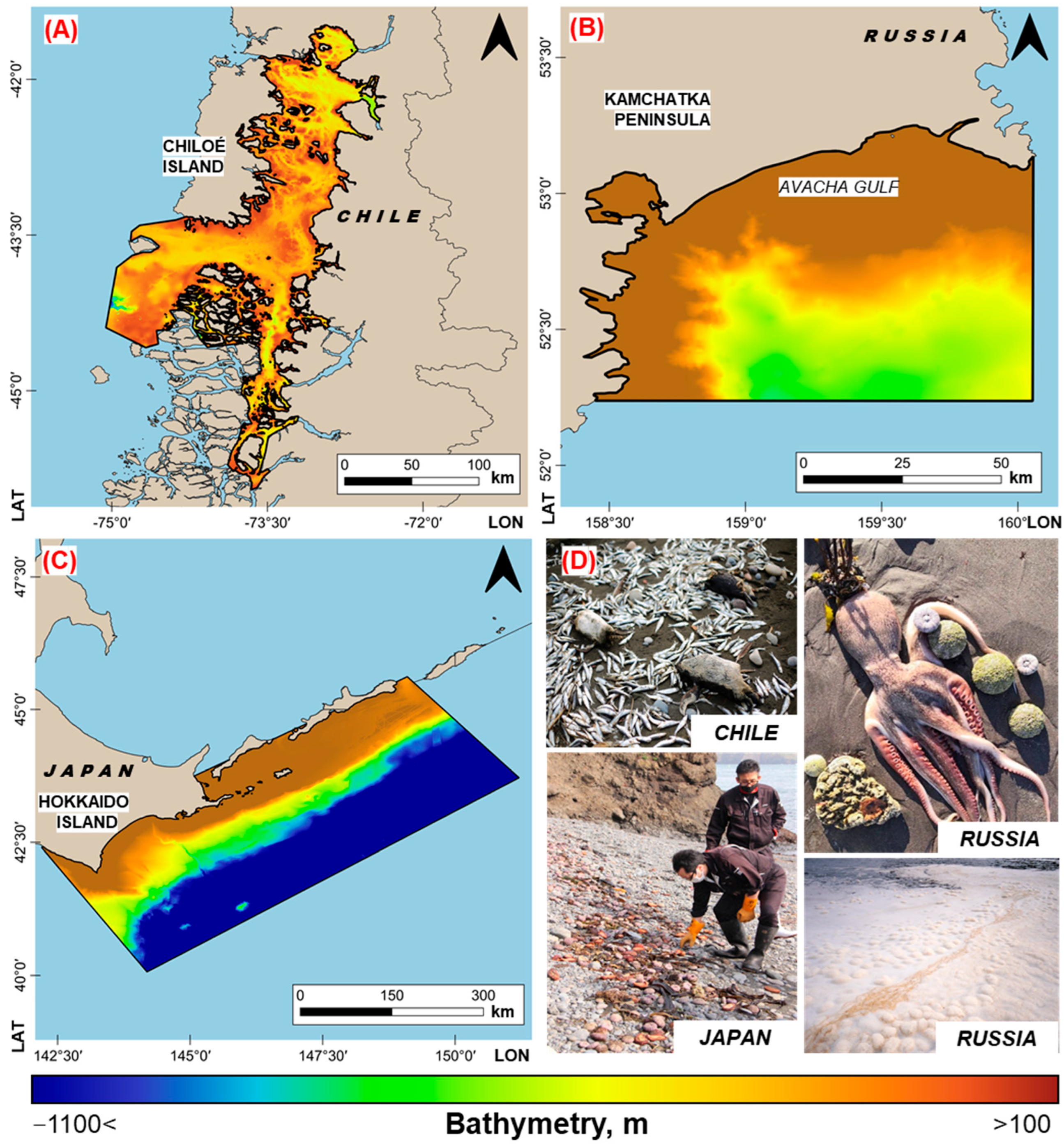
- Harmful algal blooms (genus Pseudochattonella and genus Alexandrium) in the bays of Ancud and Corcovado out of the island of Chiloe (Chile), occurred in February–March 2016 [3]. Mass commercial fish mortality (Atlantic salmon, Coho salmon, and trout) was recorded, which led to a large economic loss (about USD 800 million) [4].
- Harmful algal bloom (genus Karenia and genus Pseudo-nitzschia) in Avacha Bay out of the Kamchatka Peninsula (Russia) in September–October 2020 [5,6,7]. The main consequences of this HAB were the mass killing of hydrobionts, the deterioration of people’s health, and intensive foaming on the coastline [5,6,8].
- Harmful algal bloom (genus Karenia) in the waters out of the island of Hokkaido (Japan) and the Southern Kuril Islands (Russia) in September 2021, resulting in the deaths of a large number of sea urchins and salmon, as well as general damage to coastal ecosystems [9].
1.2. Summarized HAB Research Approaches
1.3. The Purpose and Key Directions of the Research
- (1)
- identification and interpretation of the dynamics of significant parameters of the marine environment and the near-surface layer of the atmosphere (hereinafter, investigated parameters, significant environmental parameters) before those HABs;
- (2)
- the capability to determine HAB risk levels by analyzing the time series of the significant environmental parameters.
2. Materials and Methods
2.1. Prerequisites for the Research Approach
2.2. Features of the Studied Water Areas
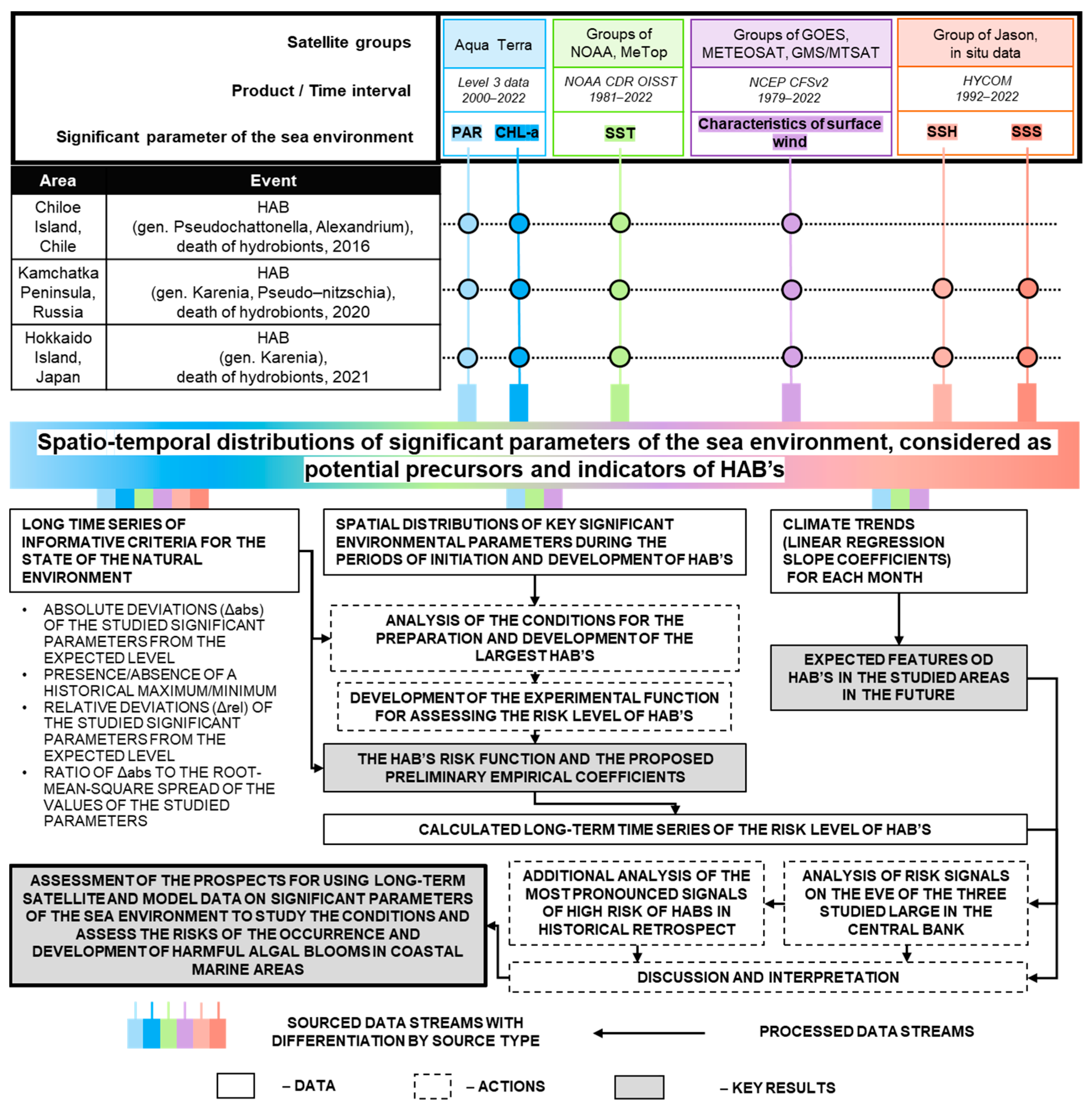
2.3. Used Data
- sea surface temperature (SST) obtained on the basis of AVHRR satellite spectroradiometer data and NOAA OISST model data [56];
- anomalies of the sea surface height (SSH), calculated using the HYCOM hybrid isopycnic ocean model [61];
- salinity of the water column at a depth of 0 m (sea surface salinity, SSS), calculated using the HYCOM hybrid isopycnic ocean model [61];
- latitudinal and meridional components of the near-surface wind vectors, calculated using the NCEP CFSv2 model [62].
2.4. Methodology
2.4.1. An Approach to Informative Criteria Based on a Long-Term Series of Investigated Parameters
- absolute deviation () of the investigated parameter from the expected level (see Equations (1) and (2));
- relative deviation () of the investigated parameter from the expected level (see Equation (3));
- the ratio of to σ, i.e., RMS spread of the investigated parameter () (see Equations (4) and (5)).
2.4.2. Experimental Function for Assessing the HAB Risk Level
- conceptual;
- empirical;
- numerical.
2.4.3. Generalized Flowchart of the Study
3. Results and Analysis
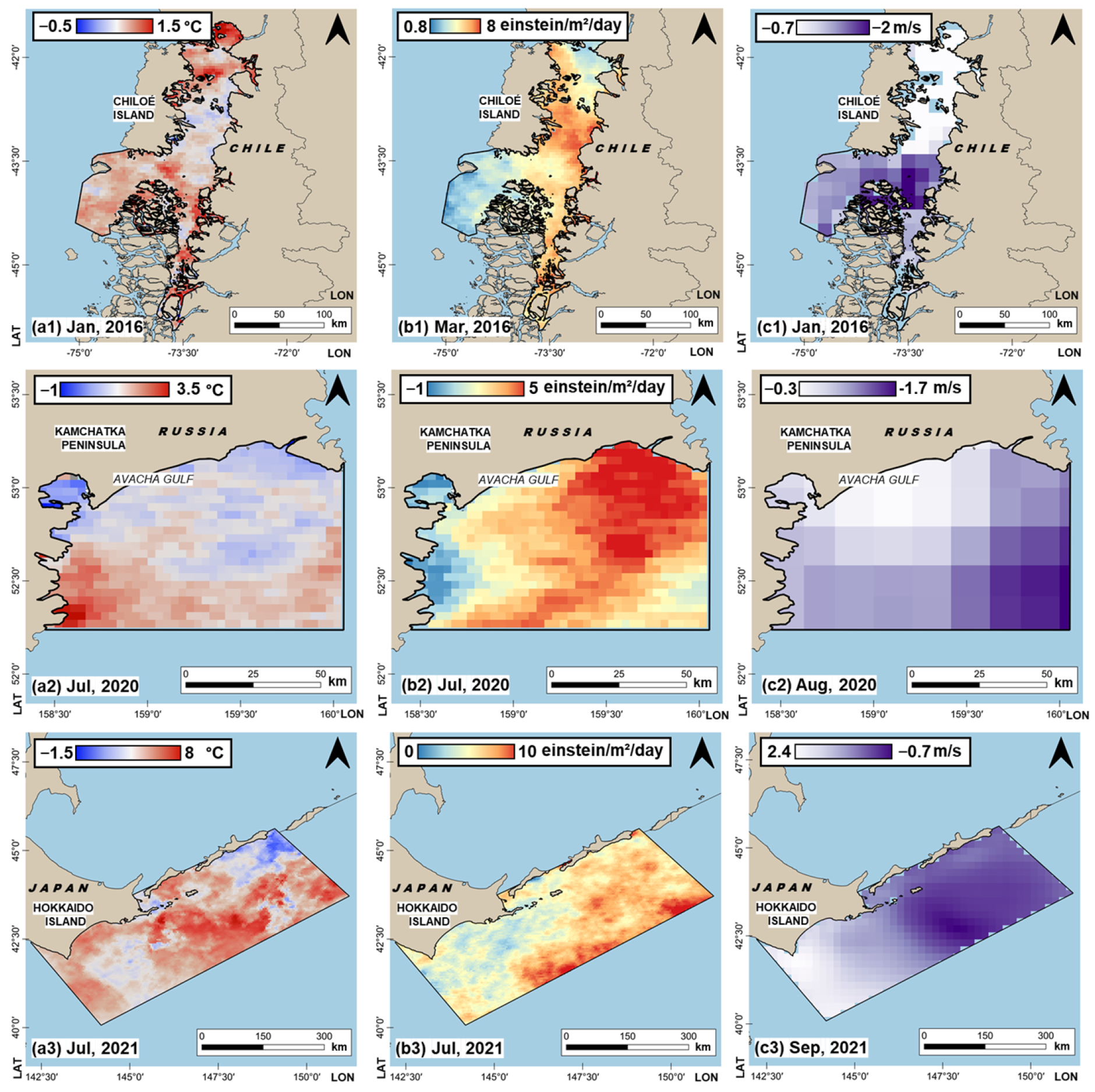
3.1. Features of Significant Environmental Parameters in the Initiation and Development of Studied HABs
- > 1 was used for sea surface temperature (SST) and photosynthetically active radiation (PAR) (increased values of these parameters contribute to the HABs’ intensification; the corresponding cells of Table 4 are marked with red).
- < −1 was used for the near-surface wind velocity (WV) (wind subsiding contributes to HABs’ intensification; the corresponding cells of Table 4 are marked with red).
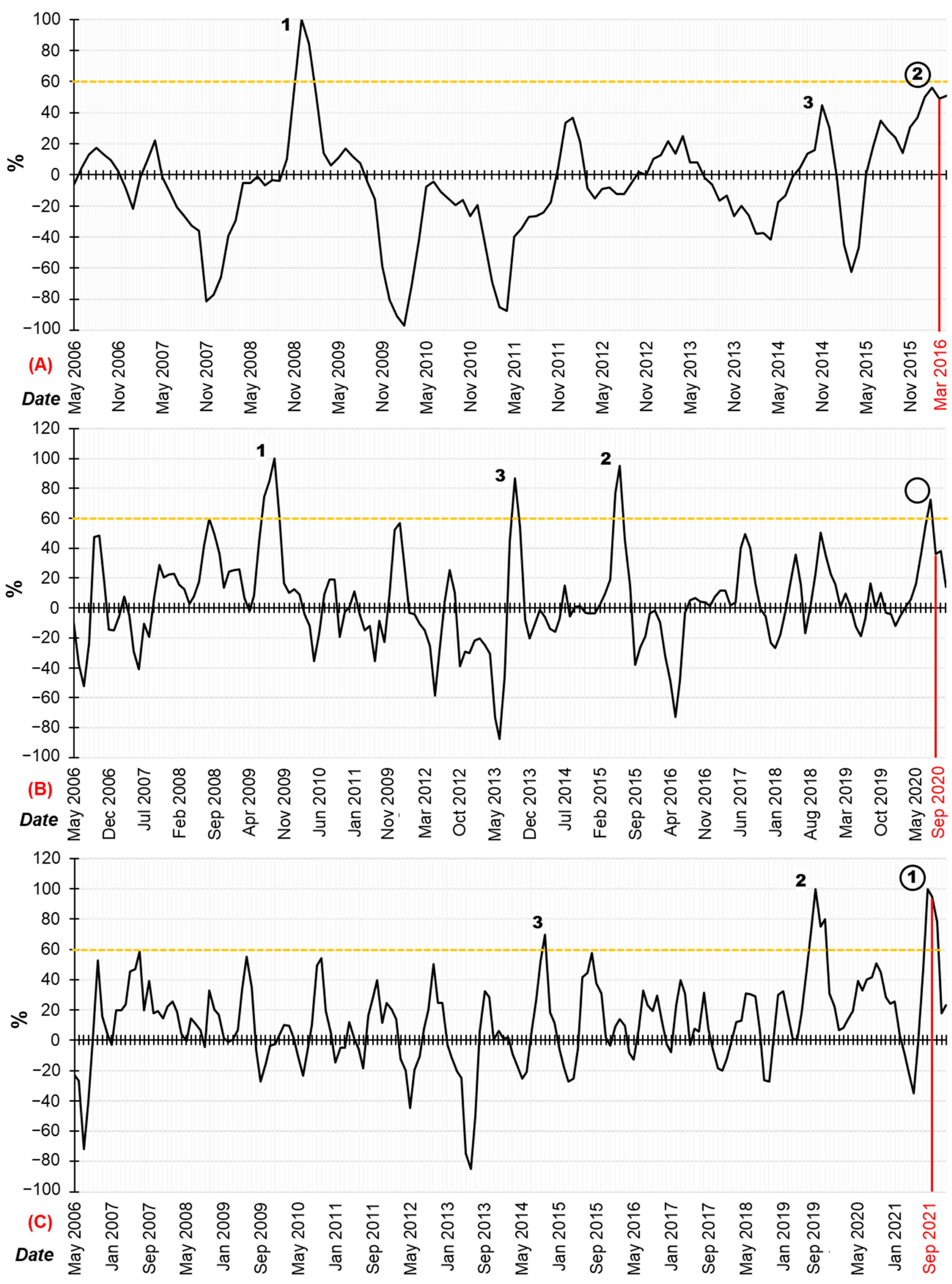
3.2. The HAB Risk
- These maxima are above or at the level of 60% (yellow lines in Figure 5a–c) relative to the previously recorded absolute maxima of HAB risk level over the entire history of observations.
3.3. Long-Term Dynamics of Investigated Parameters in the Studied Water Areas
- The strongest positive SST trends were recorded in the water areas adjacent to the Kamchatka Peninsula (Russia) and the island of Hokkaido (Japan) in the summer months (June−August). In the water area of Avacha Bay (Russia), the values of the linear regression slope coefficients in June, July, and August reached 0.042, 0.054, and 0.046 °C per year, respectively, which, in terms of the entire period of satellite observations (39 years for this area), is equivalent to warming by ~1.63 °C, 2.09 °C, and 1.81 °C, respectively. In the water area off the island of Hokkaido (Japan), the values of the linear regression slope coefficients in June, July, and August reached 0.032, 0.045, and 0.041 °C per year, respectively.
- The strongest negative trends for the WV parameter were manifested in the water area off the island of Chiloe (Chile) in the summer and autumn months (for the Southern Hemisphere, January−May). At this site, the linear regression slope coefficients in January, February, March, and April reached values of −0.013, −0.009, −0.012, and −0.009 m/s per year, which, in terms of the entire period of satellite observations (42 years for this water area), is equivalent to a decrease in wind velocity by ~−0.47 m/s, −0.34, −0.46, and −0.35 m/s, respectively.
- For the investigated PAR parameter, there is an increase in trends in the summer season (in January for the area off Chiloe Island (Chile), in June−July for the water areas of Avacha Bay near the Kamchatka Peninsula (Russia) and Hokkaido Island (Japan)). This may indicate the predominance of cloudless days in these summer months and the subsequent increase in the amount of incoming solar radiation. In the water area off Chiloe Island (Chile), the value of the PAR trend in January (summer in the Southern Hemisphere) reached 0.55 einstein/m2/day per year, which is equivalent to an increase in the amount of incoming radiation by 8.83 einstein/m2/day for the entire period of satellite observations (16 years for this water area). In the water area of Avacha Bay near the Kamchatka Peninsula (Russia), the values of the PAR trend in June reached 0.05 einstein/m2/day per year, which, in terms of the entire period of satellite observations (20 years for this water area), is equivalent to an increase in the amount of incoming radiation by ~1.0 einstein/m2/day. In the water area off Hokkaido (Japan), the values of the PAR trend in July reached 0.15 einstein/m2/day, which, in terms of the entire period of satellite observations (21 years), is equivalent to an increase in the amount of incoming radiation by ~3.15 einstein/m2/day.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anderson, D.M. Toxic Algal Blooms and Red Tides: A Global Perspective; Elsevier: Amsterdam, The Netherlands, 1989; pp. 11–16. [Google Scholar]
- Konovalova, G.V. Red Tides in the Far Eastern Seas of Russia and Adjacent Aquatic Areas of the Pacific Ocean (A Review). Algologiya 1992, 2, 87–93. [Google Scholar]
- Trainer, V.L.; Moore, S.K.; Hallegraeff, G.M.; Kudela, R.M.; Clément, A.; Mardones, J.I.; Cochlan, W.P. Pelagic harmful algal blooms and climate change: Lessons from nature’s experiments with extremes. Harmful Algae 2020, 91, 101591. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.; Alvarez, G.; Varela, D.; Santos, I.E.; Diaz, M.; Molinet, C.; Seguel, M.; Aguilera, B.A.; Guzmán, L.; Uribe, E.; et al. Impacts of harmful algal blooms on the aquaculture industry: Chile as a case study. Perspect. Phycol. 2019, 6, 39–50. [Google Scholar] [CrossRef]
- Bondur, V.G.; Zamshin, V.V.; Chvertkova, O.I. Space Study of a Red Tide-Related Environmental Disaster near Kamchatka Peninsula in September–October 2020. Dokl. Earth Sci. 2021, 497, 255–260. [Google Scholar] [CrossRef]
- Orlova, T.Y.; Aleksanin, A.I.; Lepskaya, E.V.; Efimova, K.V.; Selina, M.S.; Morozova, T.V.; Stonik, I.V.; Kachur, V.A.; Karpenko, A.A.; Vinnikov, K.A.; et al. A massive bloom of Karenia species (Dinophyceae) off the Kamchatka coast, Russia, in the fall of 2020. Harmful Algae 2022, 120, 102337. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Lim, W.A.; Lu, D.; Dai, X.; Orlova, T.; Iwataki, M. Harmful algal blooms and associated fisheries damage in East Asia: Current status and trends in China, Japan, Korea and Russia. Harmful Algae 2021, 102, 101787. [Google Scholar] [CrossRef] [PubMed]
- Bondur, V.G.; Zamshin, V.V.; Chvertkova, O.I.; Matrosova, E.R.; Khodaeva, V.N. Analyzing Causes for the Environmental Disaster in Kamchatka in Autumn 2020 Due to a Red Tide Based on Satellite Data. Izv. Atmos. Ocean. Phys. 2021, 57, 937–949. [Google Scholar] [CrossRef]
- Kuroda, H.; Taniuchi, Y.; Watanabe, T.; Azumaya, T.; Hasegawa, N. Distribution of Harmful Algae (Karenia spp.) in October 2021 Off Southeast Hokkaido, Japan. Front. Mar. Sci. 2022, 9, 841364. [Google Scholar] [CrossRef]
- Bondur, V.G. Aerospace Methods and Technologies for Monitoring Oil and Gas Areas and Facilities. Izv. Atmos. Ocean. Phys. 2011, 47, 1007–1018. [Google Scholar] [CrossRef]
- Keeler, R.N.; Bondur, V.G.; Vithanage, D. Sea truth measurements for remote sensing of littoral water. Sea Technol. 2004, 45, 53–58. [Google Scholar]
- Bondur, V.G.; Filatov, N.N.; Grebenyuk, Y.V.; Dolotov, Y.S.; Zdorovennov, R.E.; Petrov, M.P.; Tsidilina, M.N. Studies of hydrophysical processes during monitoring of the anthropogenic impact on coastal basins using the example of Mamala Bay of Oahu Island in Hawaii. Oceanology 2007, 47, 769–787. [Google Scholar] [CrossRef]
- Kartushinsky, A.V. Numerical Modeling Of The Hydrophysical Influence Effects on the Phytoplankton Distribution. Math. Biol. Bioinf. 2012, 7, 112–124. [Google Scholar] [CrossRef]
- Bondur, V.G. Satellite monitoring and mathematical modelling of deep runoff turbulent jets in coastal water areas. In Waste Water—Evaluation and Management; InTechOpen: London, UK, 2011; pp. 155–180. ISBN 978-953-307-233-3. [Google Scholar] [CrossRef]
- Zhao, J.; Ghedira, H. Monitoring red tide with satellite imagery and numerical models: A case study in the Arabian Gulf. Mar. Pollut. Bull. 2014, 79, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Bondur, V.G.; Zhurbas, V.M.; Grebenyuk, Y.V. Mathematical Modeling of Turbulent Jets of Deep-Water Sewage Discharge into Coastal Basins. Oceanology 2006, 46, 757–771. [Google Scholar] [CrossRef]
- Bondur, V.G.; Vorobjev, V.E.; Grebenjuk, Y.V.; Sabinin, K.D.; Serebryany, A.N. Study of fields of currents and pollution of the coastal waters on the Gelendzhik Shelf of the Black Sea with space data. Izv. Atmos. Ocean. Phys. 2013, 49, 886–896. [Google Scholar] [CrossRef]
- Binding, C.E.; Greenberg, T.A.; McCullough, G.; Watson, S.B.; Page, E. An analysis of satellite-derived chlorophyll and algal bloom indices on Lake Winnipeg. J. Great Lakes Res. 2018, 44, 436–446. [Google Scholar] [CrossRef]
- Bondur, V.G.; Zamshin, V.V.; Chvertkova, O.I. Study of Anomalous Biogenic Pollution of the Marmara Sea Based on Satellite Data. Dokl. Earth Sc. 2022, 507, 968–976. [Google Scholar] [CrossRef]
- Aleksanin, A.I.; Kim, V.; Orlova, T.Y.; Stonik, I.V.; Shevchenko, O.G. Phytoplankton of the Peter the Great Bay and Its Remote Sensing Problem. Oceanology 2012, 52, 219–230. [Google Scholar] [CrossRef]
- Blondeau-Patissier, D.; Gower, J.F.R.; Dekker, A.G.; Phinn, S.R.; Brando, V.E. A review of ocean color remote sensing methods and statistical techniques for the detection, mapping and analysis of phytoplankton blooms in coastal and open oceans. Prog. Oceanogr. 2014, 123, 123–144. [Google Scholar] [CrossRef]
- Aleksanin, A.I.; Orlova, T.Y.; Fomin, E.V.; Shevchenko, O.G. Prospects for Determining the Species Composition of Phytoplankton According to the MODIS Radiometer Data. Sovr. Probl. Distants. Zondir. Zemli Kosm. 2008, 2, 22–29. [Google Scholar]
- Bondur, V.; Zamshin, V.; Chvertkova, O.; Matrosova, E.; Khodaeva, V. Detection and Analysis of the Causes of Intensive Harmful Algal Bloom in Kamchatka Based on Satellite Data. J. Mar. Sci. Eng. 2021, 9, 1092. [Google Scholar] [CrossRef]
- Stumpf, R.P.; Tomlinson, M.C. Remote Sensing of Harmful Algal Blooms: Remote Sensing of Coastal Aquatic Environments; Springer: Dordrecht, The Netherlands, 2008; pp. 277–296. ISBN 978-1-4020-3099-4. [Google Scholar]
- Kim, D.-W.; Jo, Y.-H.; Choi, J.-K.; Choi, J.-G.; Bi, H. Physical processes leading to the development of an anomalously large Cochlodinium polykrikoides bloom in the East sea/Japan sea. Harmful Algae 2016, 55, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, I.N.; Flint, M.V. Anomalous blooming of coccolithophorids over the eastern Bering Sea shelf. Oceanology 1998, 38, 502–505. [Google Scholar]
- Orlova, T.Y. Red tides and toxic microalgae in the Far Eastern seas of Russia. Vestn. DVO RAN 2005, 1, 27–31. [Google Scholar]
- Vershinin, A.O.; Orlova, T.Y. Toxic and harmful algae in the coastal waters of Russia. Oceanology 2008, 48, 524–537. [Google Scholar] [CrossRef]
- Shoman, N.Y. The Combined Effect of Light, Temperature and Nitrogen Availability on the Growth Rate and Chlorophyll a Content in Marine Diatoms. Available online: https://www.dissercat.com/content/sovmestnoe-deistvie-sveta-temperatury-i-obespechennosti-azotom-na-skorost-rosta-i-soderzhani (accessed on 20 May 2023).
- Beardall, J.; Raven, J.A. The potential effects of global climate change on microalgal photosynthesis, growth and ecology. Phycologia 2004, 43, 26–40. [Google Scholar] [CrossRef]
- León-Muñoz, J.; Urbina, M.A.; Garreaud, R.; Iriarte, J.L. Hydroclimatic conditions trigger record harmful algal bloom in western Patagonia (summer 2016). Sci. Rep. 2018, 8, 1330. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef]
- Moradi, M.; Kabiri, K. Red tide detection in the Strait of Hormuz (east of the Persian Gulf) using MODIS fluorescence data. Int. J. Remote Sens. 2012, 33, 1015–1028. [Google Scholar] [CrossRef]
- Wei, G.; Tang, D.; Wang, S. Distribution of chlorophyll and harmful algal blooms (HABs): A review on space based studies in the coastal environments of Chinese marginal seas. Adv. Space Res. 2008, 41, 12–19. [Google Scholar] [CrossRef]
- Ahn, Y.H.; Shanmugam, P. Detecting the red tide algal blooms from satellite ocean color observations in optically complex Northeast-Asia Coastal waters. Remote Sens. Environ. 2006, 103, 419–437. [Google Scholar] [CrossRef]
- Hu, C.; Feng, L. Modified MODIS fluorescence line height data product to improve image interpretation for red tide monitoring in the eastern Gulf of Mexico. J. Appl. Remote Sens. 2016, 11, 012003. [Google Scholar] [CrossRef]
- Tang, D.L.; Kawamura, H.; Doan-Nhu, H.; Takahashi, W. Remote sensing oceanography of a harmful algal bloom off the coast of southeastern Vietnam. J. Geophys. Res. Ocean. 2004, 109, C03014. [Google Scholar] [CrossRef]
- Pugach, S.P.; Pipko, I.I.; Shakhova, N.E.; Shirshin, E.A.; Perminova, I.V.; Gustafsson, O.; Bondur, V.G.; Ruban, A.S.; Semiletov, I.P. Dissolved organic matter and its optical characteristics in the Laptev and East Siberian seas: Spatial distribution and interannual variability (2003–2011). Ocean. Sci. 2018, 14, 87–103. [Google Scholar] [CrossRef]
- Xiao, X.; Agustí, S.; Pan, Y.; Yu, Y.; Li, K.; Wu, J.; Duarte, C.M. Warming Amplifies the Frequency of Harmful Algal Blooms with Eutrophication in Chinese Coastal Waters. Environ. Sci. Technol. 2019, 53, 13031–13041. [Google Scholar] [CrossRef] [PubMed]
- Laabir, M.; Jauzein, C.; Genovesi, B.; Masseret, E.; Grzebyk, D.; Cecchi, P.; Vaquer, A.; Perrin, Y.; Collos, Y. Influence of temperature, salinity and irradiance on the growth and cell yield of the harmful red tide dinoflagellate Alexandrium catenella colonizing Mediterranean waters. J. Plankton Res. 2011, 33, 1550–1563. [Google Scholar] [CrossRef]
- Kopelevich, O.V.; Vazyulya, S.V.; Grigoriev, A.V.; Khrapko, A.N.; Sheberstov, S.V.; Sahling, I.V. Penetration of visible solar radiation in waters of the Barents Sea depending on cloudiness and coccolithophore blooms. Oceanology 2017, 57, 445–453. [Google Scholar] [CrossRef]
- Ranjbar, M.H.; Hamilton, D.P.; Etemad-Shahidi, A.; Helfer, F. Impacts of atmospheric stilling and climate warming on cyanobacterial blooms: An individual-based modelling approach. Water Res. 2022, 221, 118814. [Google Scholar] [CrossRef]
- Bondur, V.G.; Grebenyuk, Y.V.; Sabinin, K.D. Variability of internal tides in the coastal water area of Oahu Island (Hawaii). Oceanology 2008, 48, 611–621. [Google Scholar] [CrossRef]
- Bondur, V.G.; Grebenyuk, Y.V.; Sabynin, K.D. The spectral characteristics and kinematics of short-period internal waves on the Hawaiian shelf. Izv. Atmos. Ocean. Phys. 2009, 45, 598–607. [Google Scholar] [CrossRef]
- Wilson, C.; Adamec, D. Correlations between surface chlorophyll and sea surface height in the tropical Pacific during the 1997–1999 El Niño-Southern Oscillation event. J. Geophys. Res. Ocean. 2001, 106, 31175–31188. [Google Scholar] [CrossRef]
- Ocheretyana, S.O.; Klochkova, N.G.; Klochkova, T.A. Seasonal species composition of “green tide”-forming algae from Avacha Bay and effect of anthropogenic pollution on physiology and growth of some green algae. Vestn. KamchatGTU 2015, 33, 30–36. [Google Scholar] [CrossRef]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Bakhtiar, M.; Rezaee Mazyak, A.; Khosravi, M. Ocean Circulation to Blame for Red Tide Outbreak in the Persian Gulf and the Sea of Oman. Int. J. Marit. Technol. 2020, 13, 31–39. [Google Scholar]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef]
- Konovalova, G.V. Krasnyye Prilivy u Vostochnoy Kamchatki (Atlas-Spravochnik) [Red Tides Near Eastern Kamchatka (Atlas-Reference Book)]; Kamshat: Petropavlovsk-Kamchatsky, Russia, 1995; 57p. [Google Scholar]
- Jacques-Coper, M.; Segura, C.; de la Torre, M.B.; Valdebenito Muñoz, P.; Vásquez, S.I.; Narváez, D.A. Synoptic-to-intraseasonal atmospheric modulation of phytoplankton biomass in the inner sea of Chiloé, Northwest Patagonia (42.5°–43.5°S, 72.5°–74°W), Chile. Front. Mar. Sci. 2023, 10, 1160230. [Google Scholar] [CrossRef]
- Narváez, D.A.; Vargas, C.A.; Cuevas, L.A.; García-Loyola, S.A.; Lara, C.; Segura, C.; Tapia, F.J.; Broitman, B.R. Dominant scales of subtidal variability in coastal hydrography of the Northern Chilean Patagonia. J. Mar. Syst. 2019, 193, 59–73. [Google Scholar] [CrossRef]
- Lomtev, V.L. To The Structure And History Of Kamchatka Canyon (Eastern Kamchatka). Geol. Miner. Resour. World Ocean 2018, 3, 37–61. [Google Scholar] [CrossRef]
- Luchin, V.A.; Kruts, A.A. Properties of cores of the water masses in the Okhotsk Sea. Izv. TINRO 2016, 184, 204–218. [Google Scholar] [CrossRef]
- Mekler, G.K. Hokkaido, 2nd ed.; Popov, K.M., Kovyzhenko, V.V., Eds.; USSR Academy of Sciences, Institute of Oriental Studies: Moscow, Russia, 1986; p. 163. [Google Scholar]
- Reynolds, R.W.; Banzon, V.F.; NOAA CDR Program. NOAA Optimum Interpolation 1/4 Degree Daily Sea Surface Temperature (OISST) Analysis, 2nd ed.; National Centers for Environmental Information: Asheville, NC, USA, 2008. [Google Scholar]
- NASA Goddard Space Flight Center; Ocean Ecology Laboratory; Ocean Biology Processing Group. Moderate-resolution Imaging Spectroradiometer (MODIS) Aqua Level-3 Mapped Photosynthetically Available Radiation, Version 2022 Data; NASA OB.DAAC: Greenbelt, MD, USA. [CrossRef]
- NASA Goddard Space Flight Center; Ocean Ecology Laboratory; Ocean Biology Processing Group. Moderate-resolution Imaging Spectroradiometer (MODIS) Terra Level-3 Binned Photosynthetically Available Radiation, Version 2022 Data; NASA OB.DAAC: Greenbelt, MD, USA. [CrossRef]
- NASA Goddard Space Flight Center; Ocean Ecology Laboratory; Ocean Biology Processing Group. Moderate-resolution Imaging Spectroradiometer (MODIS) Aqua Level-3 Binned Chlorophyll, Version 2022 Data; NASA OB.DAAC: Greenbelt, MD, USA. [CrossRef]
- NASA Goddard Space Flight Center; Ocean Ecology Laboratory; Ocean Biology Processing Group. Moderate-resolution Imaging Spectroradiometer (MODIS) Terra Level-3 Binned Chlorophyll, Version 2022 Data; NASA OB.DAAC: Greenbelt, MD, USA. [CrossRef]
- Chassignet, E.; Hurlburt, H.; Metzger, E.; Smedstad, O.; Cummings, J.; Halliwell, G.; Bleck, R.; Baraille, R.; Wallcraft, A.; Lozano, C.; et al. Global Ocean Prediction with the Hybrid Coordinate Ocean Model (HYCOM). Oceanography 2009, 22, 64–75. [Google Scholar] [CrossRef]
- Saha, S.; Moorthi, S.; Wu, X.; Wang, J.; Nadiga, S.; Tripp, P.; Behringer, D.; Hou, Y.-T.; Chuang, H.; Iredell, M.; et al. NCEP Climate Forecast System Version 2 (CFSv2) 6-Hourly Products. Available online: https://rda.ucar.edu/datasets/ds094.0/ (accessed on 28 June 2023).
- Hobday, A.J.; Oliver, E.C.J.; Sen Gupta, A.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Holbrook, N.J.; Moore, P.J.; Thomsen, M.S.; Wernberg, T.; et al. Categorizing and naming marine heatwaves. Oceanography 2018, 31, 162–173. [Google Scholar] [CrossRef]
- Jiang, L.; Zhao, X.; Wang, L. Long-Range Correlations of Global Sea Surface Temperature. PLoS ONE 2016, 11, e0153774. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hobday, A.J.; Alexander, L.V.; Perkins, S.E.; Smale, D.A.; Straub, S.C.; Oliver, E.C.J.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Feng, M.; et al. A hierarchical approach to defining marine heatwaves. Prog. Oceanogr. 2016, 141, 227–238. [Google Scholar] [CrossRef]
- McGillicuddy, D.J. Models of harmful algal blooms: Conceptual, empirical, and numerical approach. J. Mar. Syst. 2010, 83, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.R.; Siegel, D.A.; Kudela, R.M.; Brzezinski, M.A. Empirical models of toxigenic Pseudo-nitzschia blooms: Potential use as a remote detection tool in the Santa Barbara Channel. Harmful Algae 2009, 8, 478–492. [Google Scholar] [CrossRef]
- Hamilton, G.; McVinish, R.; Mengersen, K. Bayesian model averaging for harmful algal bloom prediction. Ecol. Appl. A Publ. Ecol. Soc. Am. 2009, 19, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Hongwon, Y. Prediction model of algal blooms using logistic regression and confusion matrix. Int. J. Electr. Comput. Eng. 2021, 11, 2407. [Google Scholar] [CrossRef]
- Izadi, M.; Sultan, M.; Kadiri, R.E.; Ghannadi, A.; Abdelmohsen, K.A. Remote Sensing and Machine Learning-Based Approach to Forecast the Onset of Harmful Algal Bloom. Remote Sens. 2021, 13, 3863. [Google Scholar] [CrossRef]
- Raine, R.; McDermott, G.; Silke, J.; Lyons, K.; Nolan, G.; Cusack, C. A simple short range model for the prediction of harmful algal events in the bays of southwestern Ireland. J. Mar. Syst. 2010, 83, 150–157. [Google Scholar] [CrossRef]
- Rousso, B.Z.; Bertone, E.; Stewart, R.; Hamilton, D.P. A systematic literature review of forecasting and predictive models for cyanobacteria blooms in freshwater lakes. Water Res. 2020, 182, 115959. [Google Scholar] [CrossRef]
- Vasilenko, N.V.; Medvedeva, A.V.; Aleskerova, A.A.; Kubryakov, A.A.; Stanichny, S.V. Features of cyanobacteria blooms in the central part of the Sea of Azov from satellite data. Sovr. Probl. DZZ Kosm. 2021, 18, 166–180. [Google Scholar] [CrossRef]
- Gavrilenko, G.G.; Zdorovennova, G.E.; Zdorovennov, R.E.; Palshin, N.I.; Efremova, T.V.; Terzhevik, A.Y. Spatio-temporal variability of photosynthetically active solar radiation flow in a shallow lake during the open water period. Obshchest. Sreda Razvit. 2015, 3, 186–192. [Google Scholar]
- Horn, H.; Paul, L. Interactions between Light Situation, Depth of Mixing and Phytoplankton Growth during the Spring Period of Full Circulation. Int. Rev. Der Gesamten Hydrobiol. Und Hydrogr. 1984, 69, 507–519. [Google Scholar] [CrossRef]
- Clement, A.; Lincoqueo, L.; Saldivia, M.; Brito, C.G.; Muñoz, F.; Fernández, C.; Pérez, F.; Maluje, C.P.; Correa, N.; Moncada, V.; et al. Exceptional summer conditions and HABs of Pseudochattonella in Southern Chile create record impacts on salmon farms. Harmful Algae News 2016, 53, 1–23. [Google Scholar]
- Band-Schmidt, C.J.; Morquecho, L.; Lechuga-Devéze, C.H.; Anderson, D.M. Effects of growth medium, temperature, salinity and seawater source on the growth of Gymnodinium catenatum (Dinophyceae) from Bahía Concepción, Gulf of California, Mexico. J. Plankton Res. 2004, 26, 1459–1470. [Google Scholar] [CrossRef]
- Xu, N.; Duan, S.; Li, A.; Zhang, C.; Cai, Z.; Hu, Z. Effects of temperature, salinity and irradiance on the growth of the harmful dinoflagellate Prorocentrum donghaiense Lu. Harmful Algae 2010, 9, 13–17. [Google Scholar] [CrossRef]
- Alexanin, A.; Kachur, V.; Khramtsova, A.; Orlova, T. Methodology and Results of Satellite Monitoring of Karenia Microalgae Blooms, That Caused the Ecological Disaster off Kamchatka Peninsula. Remote Sens. 2023, 15, 1197. [Google Scholar] [CrossRef]
- Xing, Q.; Hu, C.; Tang, D.; Tian, L.; Tang, S.; Wang, X.; Lou, M.; Gao, X. World’s Largest Macroalgal Blooms Altered Phytoplankton Biomass in Summer in the Yellow Sea: Satellite Observations. Remote Sens. 2015, 7, 12297–12313. [Google Scholar] [CrossRef]
- Mardones, J.; Clement, A.; Rojas, X.; Aparicio, C. Alexandrium catenella during 2009 in Chilean waters, and recent expansion to coastal ocean. Harmful Algae News 2010, 41, 8–9. [Google Scholar]
- Harmful Algal Event Database. Available online: http://haedat.iode.org/viewEvent.php?eventID=5752/ (accessed on 18 June 2023).
- Mardones, J. Screening of Chilean fish-killing microalgae using a gill cell-based assay. Lat. Am. J. Aquat. Res. 2020, 48, 329–335. [Google Scholar] [CrossRef]
- Harmful Algal Event Database. Available online: http://haedat.iode.org/viewEvent.php?eventID=5976 (accessed on 18 June 2023).
- Harmful Algal Event Database. Available online: http://haedat.iode.org/viewEvent.php?eventID=5740 (accessed on 18 June 2023).
- Ocheretyana, S.O.; Klochkova, N.G. Late Autumn Composition Of Green Ephemeral Algae In The Bunkering Areas Of The Fleet In Avacha Bay (Southeastern Kamchatka). Vestn. KamchatGTU 2010, 11, 58–64. [Google Scholar]
- Lepskaya, E.V.; Tepnin, O.B.; Kolomeitsev, V.V.; Ustimenko, N.V.; Sergeenko, N.V.; Vinogradova, D.S.; Sviridenko, V.D.; Pokhodina, M.A.; Schegolkova, V.A.; Maksimenkov, V.V.; et al. Historical Review Of Studies Of Avachinskaya Bay And Principle Results Of Complex Ecological Monitoring 2013. Res. Aquat. Biol. Resour. Kamchatka North-West Part Pac. Ocean. 2014, 34, 5–21. [Google Scholar]
- Anderson, P. Design and Implementation of Some Harmful Algal Monitoring Systems; IOC Technical Series; Intergovernmental Oceanographic Commission: Paris, France, 1996; 102p. [Google Scholar]
- Weitkamp, L. Recent ocean conditions and trends of Pacific salmon from Alaska to California. Probl. Fish. 2021, 22, 27–34. [Google Scholar] [CrossRef]
- McCabe, R.M.; Hickey, B.M.; Kudela, R.M.; Lefebvre, K.A.; Adams, N.G.; Bill, B.D.; Gulland, F.M.D.; Thomson, R.E.; Cochlan, W.P.; Trainer, V.L. An unprecedented coastwide toxic algal bloom linked to anomalous ocean conditions. Geophys. Res. Lett. 2016, 43, 10366–10376. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Sakamoto, S.; Yamaguchi, M.; Imai, I. First record of two warm-water HAB species Chattonella marina (Raphidophyceae) and Cochlodinium polykrikoides (Dinophyceae) on the west coast of Hokkaido, northern Japan in summer 2014. Reg. Stud. Mar. Sci. 2016, 7, 111–117. [Google Scholar] [CrossRef]
- Inaba, N.; Kodama, I.; Nagai, S.; Mori, K.; Imai, I. Distribution of Growth-Limiting Bacteria Against Harmful Algal Bloom Species at Shinori Fishing Port and Surrounding Environments. Civil Engineering Research Institute for Cold Region (CERI) 2023, 841, 2–10. [Google Scholar]
- Shimada, H. Long-term fluctuation of red tide and shellfish toxin along the coast of Hokkaido (Review). Sci. Rep. Hokkaido Fish. Res. Inst. 2021, 100, 1–12. [Google Scholar]
| Parameter | Type of Relationship with HABs | Time Range | Time Interval of the Initial Data | Data Source |
|---|---|---|---|---|
| Sea surface temperature (SST) | Intensification factor | 1 September 1981–31 December 2021 | Daily | https://www.ncei.noaa.gov/products/climate-data-records/sea-surface-temperature-optimum-interpolation (accessed on 22 June 2023) |
| Photosynthetically active radiation (PAR) | Intensification factor | 24 February 2000–31 December 2021 | Monthly | https://oceancolor.gsfc.nasa.gov (accessed on 27 June 2023) |
| Chlorophyll-a concentration (CHL-a) | Indicator | 24 February 2000–31 December 2021 | Daily | https://oceancolor.gsfc.nasa.gov (accessed on 27 June 2023) |
| Sea surface salinity (SSS) | Intensification factor | 2 October 1992–31 December 2021 | Daily | https://hycom.org/ (accessed on 6 June 2023) |
| Anomaly of sea surface height (SSH) | Intensification factor | 2 October 1992–31 December 2021 | Daily | https://hycom.org/ (accessed on 6 June 2023) |
| Wind velocity (WV) | Intensification factor | 1 January 1991–31 December 2021 | 4 times a day | https://www.cpc.ncep.noaa.gov/products/CFSv2/CFSv2_body.html (accessed on 28 June 2023) |
| Potential Impact on HAB Intensification | Rating of the Factor Significance in HAB Research | Number of Publications Identifying the Factor as Influencing HAB Intensification | The Nature of the Relationship between the Factor and Harmful Bloom |
|---|---|---|---|
| SST | 3 | 39 | Positive |
| PAR | 2 | 9 | Positive |
| WV | 1 | 7 | Negative |
| Input Variable | Values Used | Comment |
|---|---|---|
| n | 3 | SST, PAR, and WV were chosen as the significant environmental parameters, since these parameters are available for all three studied water areas |
| Calculated by Equation (5), the ratio of deviations of the actually observed values of the j-th parameter from the expected level to their standard deviation for the z-th time interval (for each month and for each parameter). | ||
| δ | 3 | 3-month signal accumulation was used |
| 1 | In case of one of the three historical maxima of the current parameter that is characterized by a positive relationship with the HABs (SST, PAR) | |
| 1 | In case of one of the three historical minima of the current parameter that is characterized by a negative relationship with the HABs (VW) | |
| 0 | In all other cases | |
| Taking into account the data given in Table 2 | ||
| 3 | For SST | |
| 2 | For PAR | |
| −1 | For VW | |
| 1 | For the autumn–winter season in the Northern Hemisphere (November–April) and in the Southern Hemisphere (May–October) | |
| 2 | For the spring–summer season in the Northern Hemisphere (May–October) and in the Southern Hemisphere (November–April) | |
| z | A cyclically variable index corresponding to the time grid interval number: 1, 2, 3, etc. | One-month time grid discreteness |
| Time Shift from the HAB (month) | The Island of Chiloe (Chile) Water Area | Avacha Bay (Russia) Water Area | The Island of Hokkaido (Japan) Water Area | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Criteria | Max/ min | Max/ min | Max/ min | |||||||||||
| Parameter | ||||||||||||||
| −4 | Sea surface temperature. SST (for —°C, for —%) | 0.35 | 3.03 | 0.84 | 0.37 | 11.61 | 0.54 | 0.84 | 15.03 | 1.34 | Max-2 | |||
| −3 | 0.30 | 2.36 | 0.54 | 1.05 | 15.06 | 1.11 | Max-2 | 1.08 | 11.21 | 1.43 | Max-2 | |||
| −2 | 0.90 | 6.74 | 1.99 | 1.31 | 12.11 | 1.38 | Max-2 | 1.66 | 12.01 | 1.89 | Max-1 | |||
| −1 | 0.83 | 6.30 | 1.79 | −0.36 | −2.85 | −0.45 | 0.28 | 1.66 | 0.33 | |||||
| 0 | 0.57 | 4.57 | 1.16 | 1.16 | 10.68 | 1.37 | Max-3 | −0.86 | −5.13 | −1.06 | ||||
| −4 | Photosynthetically active radiation. PAR (for —einstein/m2/day, for —%) | 3.26 | 9.68 | 1.02 | 8.51 | 22.22 | 2.28 | Max-3 | −3.46 | −8.05 | −1.14 | |||
| −3 | 1.89 | 4.59 | 0.56 | 2.33 | 5.48 | 0.51 | 5.29 | 12.73 | 2.25 | Max-2 | ||||
| −2 | 2.03 | 3.96 | 0.65 | Max-2 | 3.36 | 8.52 | 1.02 | 4.85 | 12.21 | 2.19 | Max-1 | |||
| −1 | 0.27 | 0.64 | 0.06 | −3.69 | −10.76 | −1.11 | 1.40 | 3.95 | 0.57 | |||||
| 0 | 3.17 | 10.28 | 1.29 | Max-2 | 2.21 | 8.69 | 0.96 | 3.07 | 9.69 | 1.56 | Max-1 | |||
| −4 | Chlorophyll-a concentration. CHL-a (for —mg/, for —%) | 2.25 | 69.29 | 1.47 | Max-2 | 0.09 | 5.24 | 0.06 | 0.36 | 16.52 | 0.51 | |||
| −3 | 0.11 | 2.65 | 0.06 | 1.09 | 64.22 | 0.81 | 0.25 | 24.24 | 0.52 | |||||
| −2 | 2.34 | 57.69 | 2.09 | Max-1 | −0.29 | −19.67 | −0.24 | − | − | − | ||||
| −1 | 0.29 | 6.64 | 0.14 | −0.77 | −29.13 | −0.71 | 0.35 | 42.50 | 1.54 | Max-2 | ||||
| 0 | 0.77 | 20.96 | 0.41 | 9.82 | 179.94 | 3.74 | Max-1 | 0.59 | 58.30 | 2.13 | Max-2 | |||
| −4 | Sea surface salinity. SSS (for —PSU, for —%) | × | × | × | × | −0.04 | −0.11 | −0.42 | −0.02 | −0.05 | −0.13 | |||
| −3 | × | × | × | × | −0.18 | −0.53 | −1.56 | 0.06 | 0.18 | 0.58 | Max-2 | |||
| −2 | × | × | × | × | −0.15 | −0.45 | −1.08 | 0.00 | 0.00 | 0.01 | ||||
| −1 | × | × | × | × | 0.00 | −0.01 | −0.22 | 0.05 | 0.15 | 0.43 | ||||
| 0 | × | × | × | × | −0.18 | −0.55 | −1.13 | 0.04 | 0.13 | 0.40 | ||||
| −4 | Anomaly of sea surface height. SSH (for m, for —%) | × | × | × | × | 0.01 | −6.70 | 0.33 | 0.01 | −8.80 | 0.58 | |||
| −3 | × | × | × | × | 0.0 | −5.39 | 0.40 | 0.0 | −30.02 | 1.51 | ||||
| −2 | × | × | × | × | 0.0 | −5.54 | 0.65 | −0.02 | 14.10 | −0.59 | ||||
| −1 | × | × | × | × | 0.03 | −17.72 | 1.46 | −0.06 | 74.98 | −1.54 | ||||
| 0 | × | × | × | × | 0.03 | −18.83 | 1.14 | −0.07 | 92.95 | −1.78 | Min-1 | |||
| −4 | Wind velocity. WV (for m/s, for —%) | −0.01 | −0.11 | −0.01 | 0.61 | 12.99 | 1.01 | −0.11 | −1.87 | −0.22 | ||||
| −3 | −0.72 | −14.50 | −0.94 | 0.14 | 3.01 | 0.25 | −0.14 | −2.83 | −0.30 | |||||
| −2 | −0.88 | −20.03 | −1.83 | Min-2 | −0.04 | −0.77 | −0.08 | −0.26 | −5.80 | −0.64 | ||||
| −1 | −0.16 | −3.83 | −0.31 | 1.25 | 25.53 | 2.43 | Max-1 | 0.73 | 14.83 | 1.32 | ||||
| 0 | −0.19 | −4.59 | −0.42 | −0.76 | −12.70 | −1.37 | −0.60 | −10.80 | −1.28 | |||||
| Studied Water Area | HAB Risk Levels, % (3 Historical Maximums) | Time Interval for Registering the Extreme Value of HAB Risk Levels | HAB Registration Time Interval | Brief Description of the Registered HABs | References |
|---|---|---|---|---|---|
| Chiloe Island water area (Chile) | 100.00 | December 2008 | January 2009 |
| [81,82] |
| 45.05 | November 2014 | December 2014 |
| [83,84] | |
| 56.08 | February 2016 | March 2016 |
| [76,85] | |
| Avacha Bay water area (Russia) | 100.00 | September 2009 | Intensive development of green algae throughout the year | [46,86] | |
| 86.94 | September 2013 | October 2013 |
| [87,88] | |
| 95.02 | June 2015 | Information about the HAB was not found | [89,90] | ||
| 72.49 | August 2020 | September 2020 |
| [8,23,79] | |
| The island of Hokkaido water area (Japan) | 69.89 | October 2014 | October 2014 |
| [91,92] |
| 99.86 | August 2019 | August 2019 |
| [93] | |
| 100.00 | August 2021 | September 2021 |
| [10] | |
| Sea Surface Temperature (SST), °C/Year | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Month | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | |
| Area | |||||||||||||
| Chiloe Island (Chile), 1981–2016 | −0.0167 | −0.0157 | −0.0203 | −0.007 | −0.0028 | 0.0039 | 0.0073 | 0.0026 | 0.001 | −0.0048 | −0.0001 | −0.0059 | |
| Kamchatka Peninsula (Russia), 1981–2020 | 0.0017 | 0.002 | 0.0009 | 0.0026 | 0.0193 | 0.0418 | 0.0537 | 0.0463 | 0.0196 | 0.0076 | 0.0076 | 0.0027 | |
| Hokkaido Island (Japan), 1981–2021 | 0.0074 | −0.0032 | −0.007 | 0.0039 | 0.0209 | 0.0332 | 0.0496 | 0.0407 | 0.0368 | 0.022 | 0.0227 | 0.0169 | |
| Photosynthetically active radiation (PAR) einstein/m2/day/year | |||||||||||||
| Month | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | |
| Area | |||||||||||||
| Chiloe Island (Chile), 2000–2016 | 0.5518 | 0.162 | 0.2624 | −0.0186 | −0.0565 | 0.0172 | −0.0235 | −0.1209 | 0.2002 | 0.2342 | 0.0332 | −0.0111 | |
| Kamchatka Peninsula (Russia), 2000–2020 | −0.0166 | −0.0255 | 0.0154 | 0.0304 | 0.0039 | 0.0462 | 0.0093 | −0.0354 | −0.0749 | 0.0554 | −0.0126 | 0.0006 | |
| Hokkaido Island (Japan), 2000–2021 | 0.014 | −0.0649 | −0.0925 | 0.0279 | 0.1031 | −0.0008 | 0.1493 | −0.0462 | 0.163 | −0.027 | −0.0164 | −0.001 | |
| Wind velocity (WV), m/s/year | |||||||||||||
| Month | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | |
| Area | |||||||||||||
| Chiloe Island (Chile), 1979–2016 | −0.0126 | −0.0093 | −0.0124 | −0.0094 | −0.0214 | 0.0164 | 0.0153 | 0.0029 | −0.022 | −0.0033 | −0.01 | 0.0013 | |
| Kamchatka Peninsula (Russia), 1979–2020 | 0.0271 | 0.0377 | 0.0262 | 0.0143 | 0.0018 | 0.0144 | 0.0155 | 0.0241 | 0.0325 | 0.0287 | 0.0433 | 0.0398 | |
| Hokkaido Island (Japan), 1979–2021 | 0.0005 | 0.0188 | 0.003 | 0.0045 | 0.0087 | 0.0025 | −0.0065 | −0.0034 | −0.0027 | −0.0046 | −0.0117 | 0.0212 | |
 | |||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondur, V.; Chvertkova, O.; Zamshin, V. Studying Conditions of Intense Harmful Algal Blooms Based on Long-Term Satellite Data. Remote Sens. 2023, 15, 5308. https://doi.org/10.3390/rs15225308
Bondur V, Chvertkova O, Zamshin V. Studying Conditions of Intense Harmful Algal Blooms Based on Long-Term Satellite Data. Remote Sensing. 2023; 15(22):5308. https://doi.org/10.3390/rs15225308
Chicago/Turabian StyleBondur, Valery, Olga Chvertkova, and Viktor Zamshin. 2023. "Studying Conditions of Intense Harmful Algal Blooms Based on Long-Term Satellite Data" Remote Sensing 15, no. 22: 5308. https://doi.org/10.3390/rs15225308
APA StyleBondur, V., Chvertkova, O., & Zamshin, V. (2023). Studying Conditions of Intense Harmful Algal Blooms Based on Long-Term Satellite Data. Remote Sensing, 15(22), 5308. https://doi.org/10.3390/rs15225308









