A Standardized Procedure to Build a Spectral Library for Hazardous Chemicals Mixed in River Flow Using Hyperspectral Image
Abstract
1. Introduction
2. Materials and Methods
2.1. Target River and Hazardous Chemicals
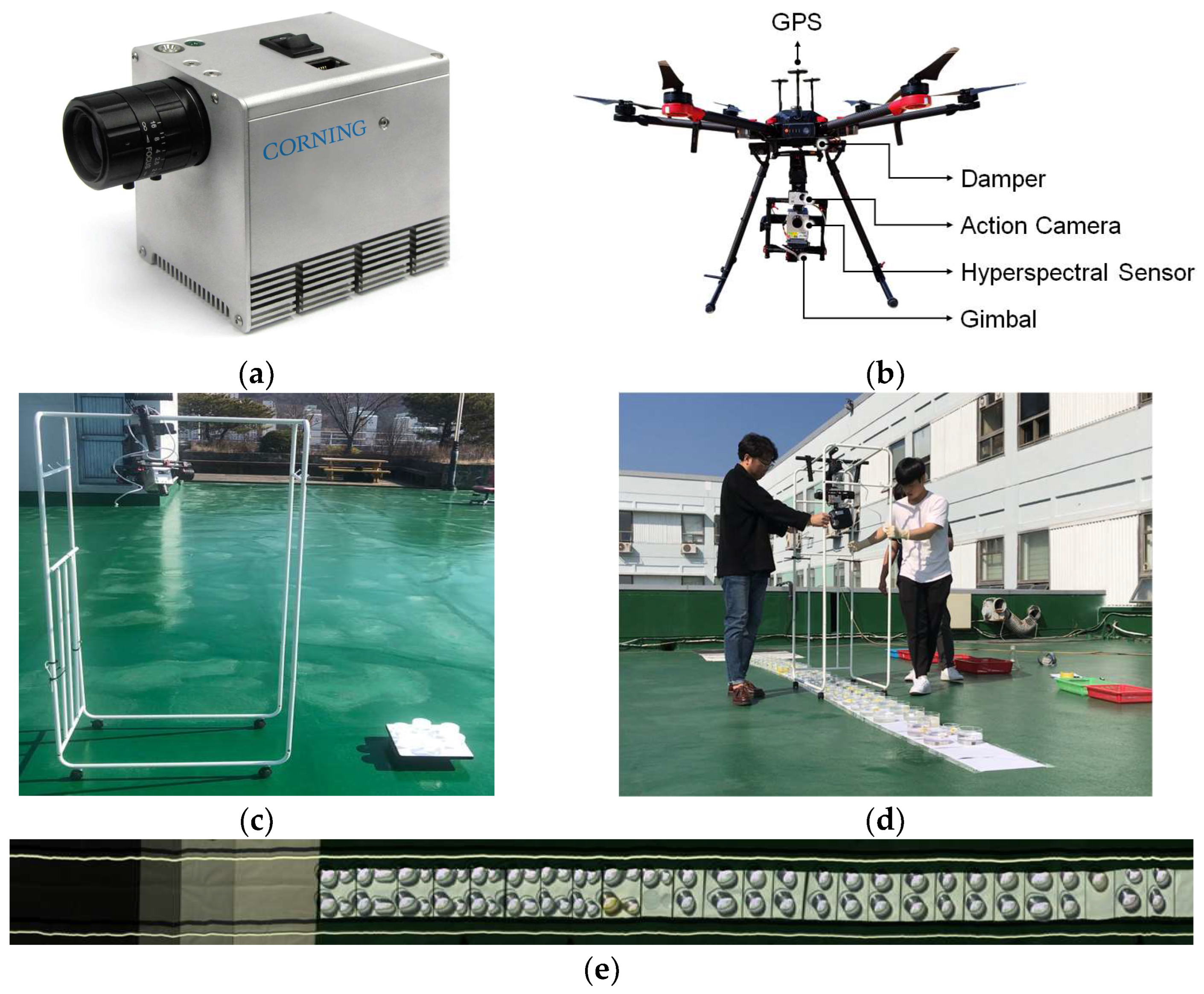
2.2. Hyperspectral Image Collection
3. Results and Discussion
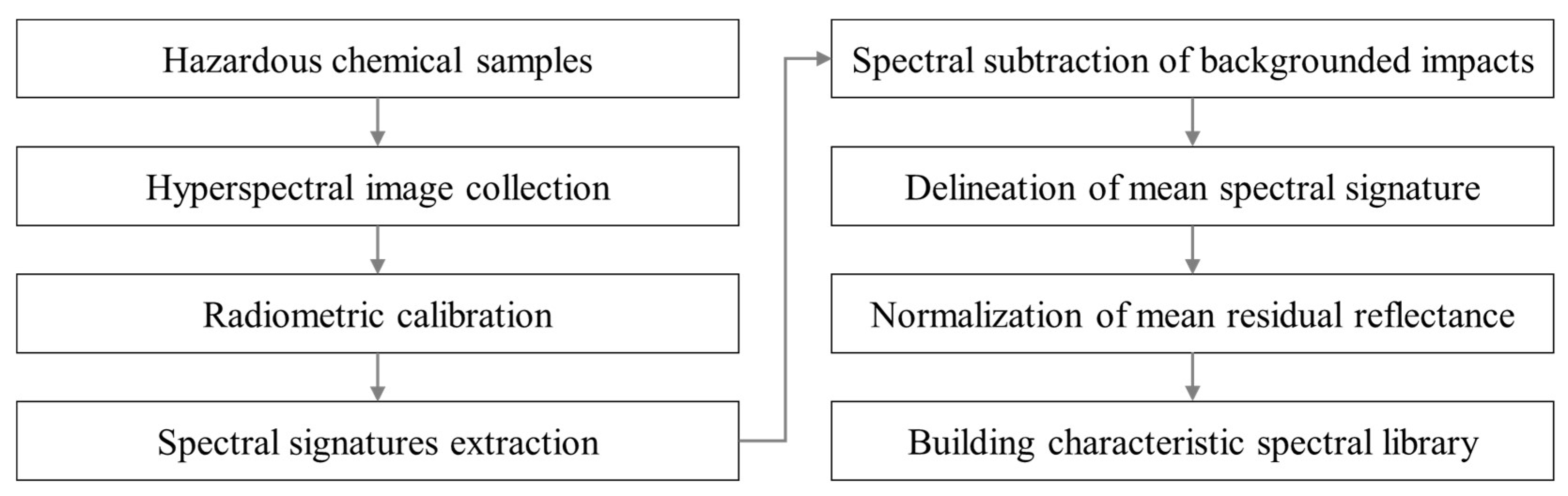
3.1. Procedure for Building a Spectral Library
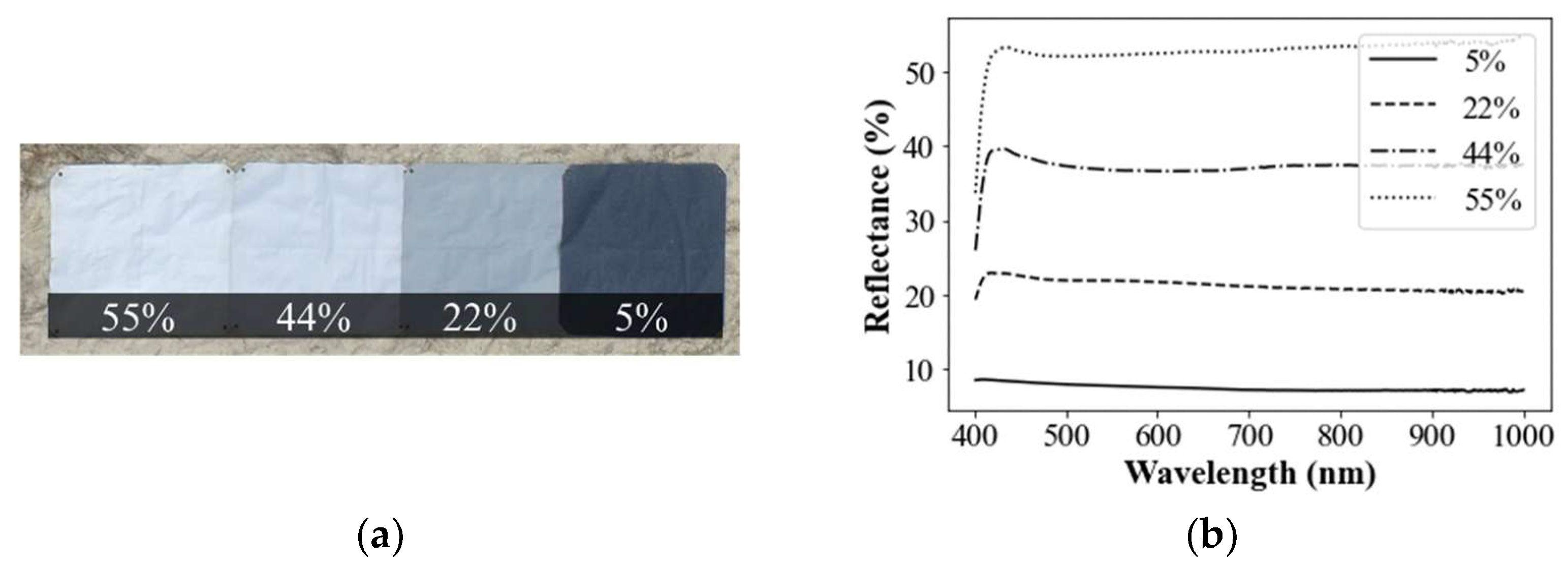
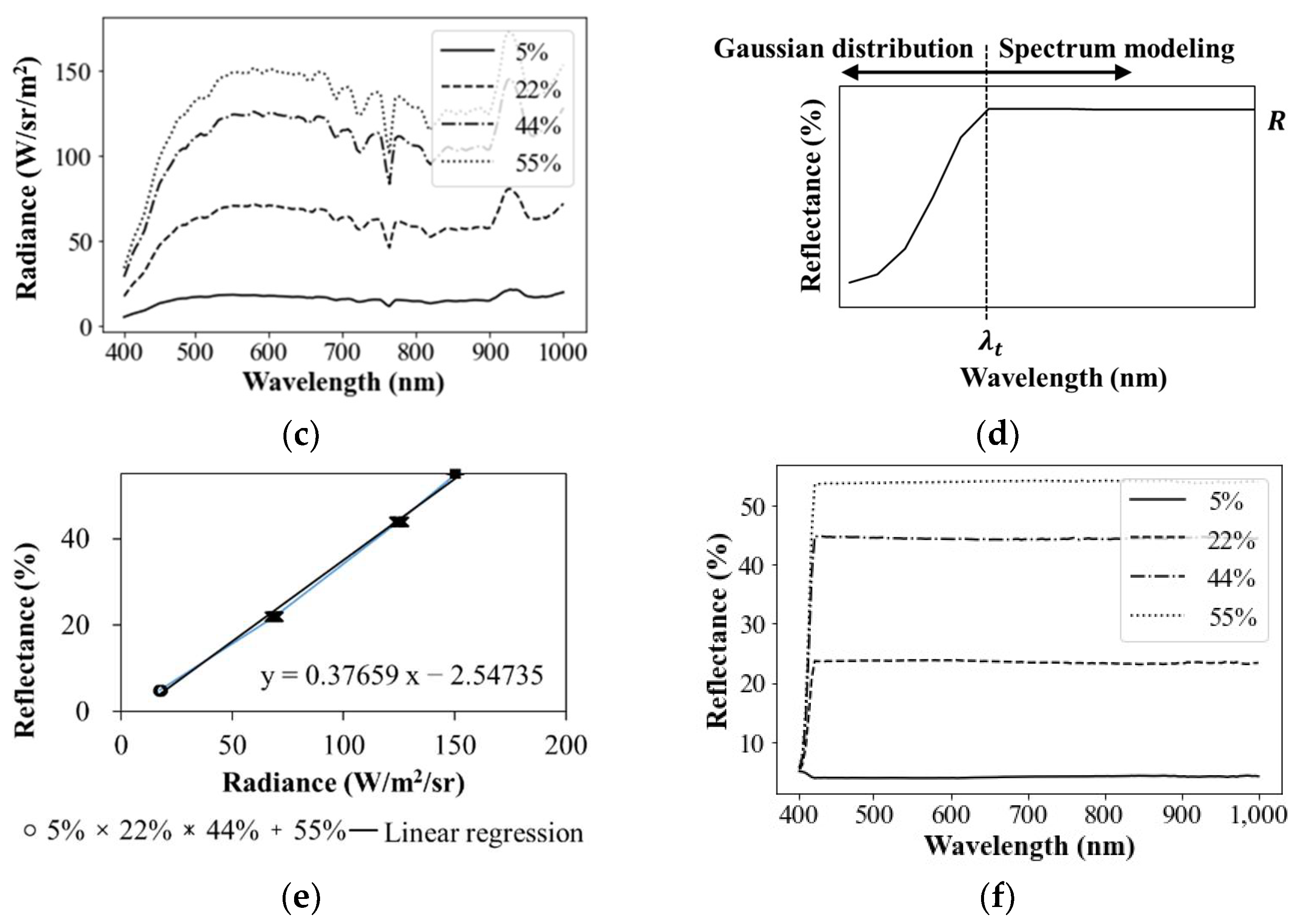
3.1.1. Deriving Radiometric Calibration Equation
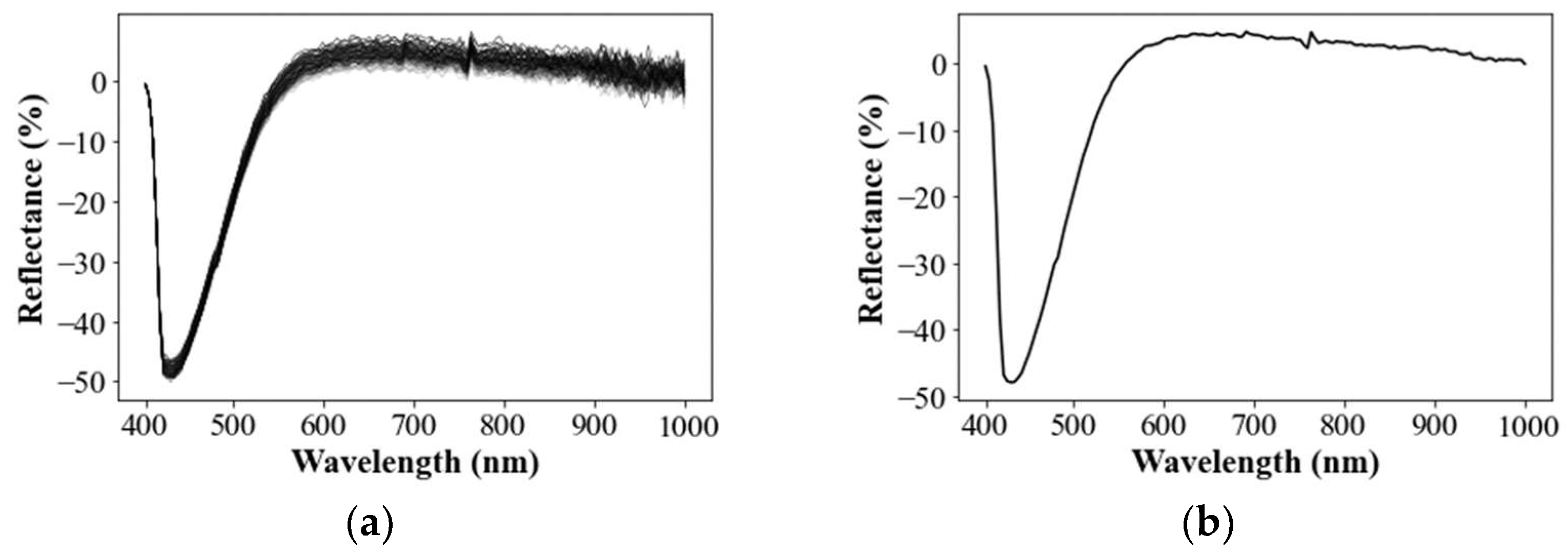
3.1.2. Extraction of Spectral Signatures
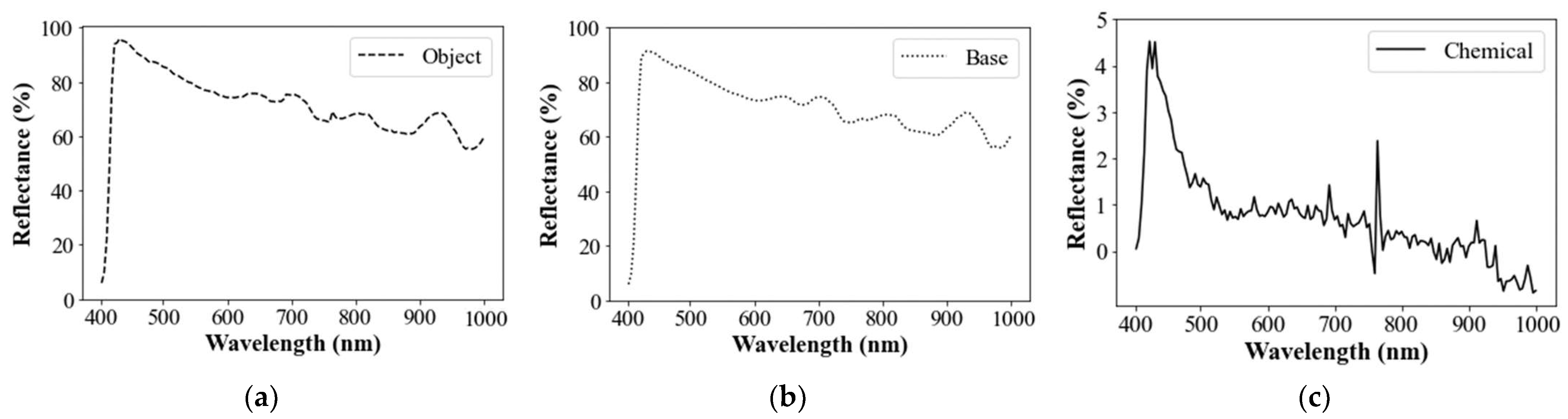
3.1.3. Spectral Subtraction of Backgrounded Impacts
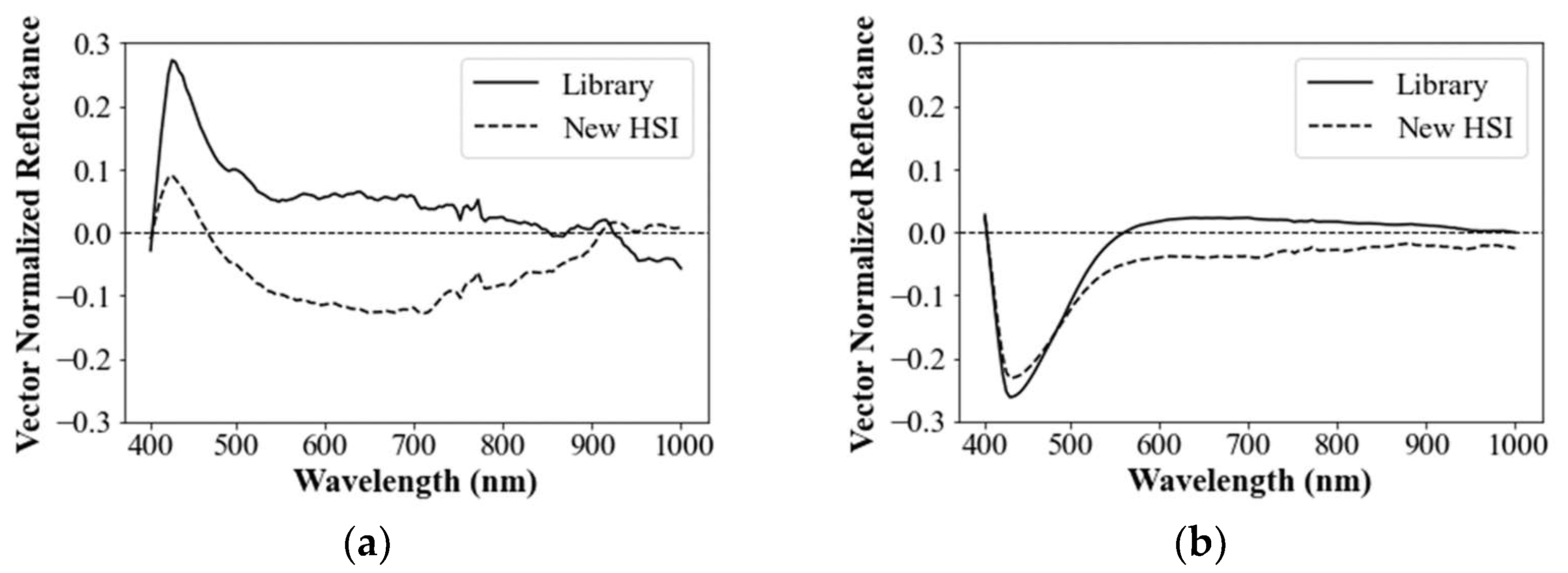
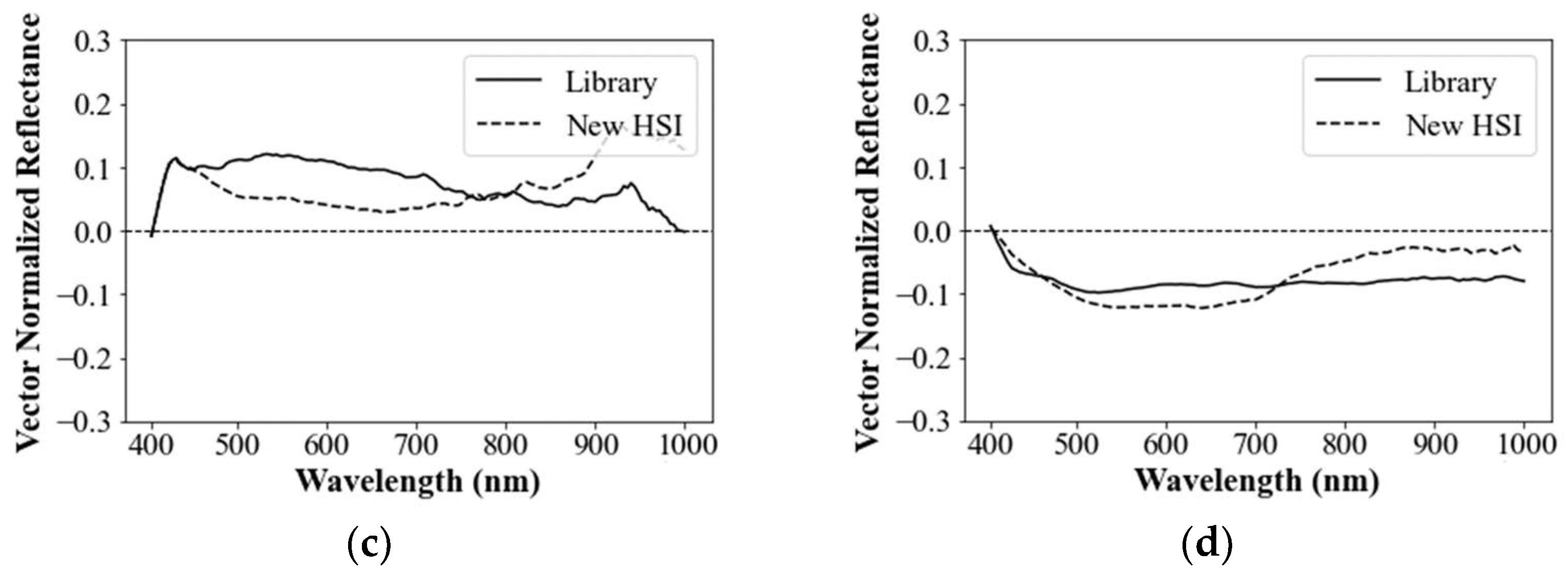
3.1.4. Delineation of Mean Spectral Signature
3.1.5. Normalization of Mean Residual Reflectance
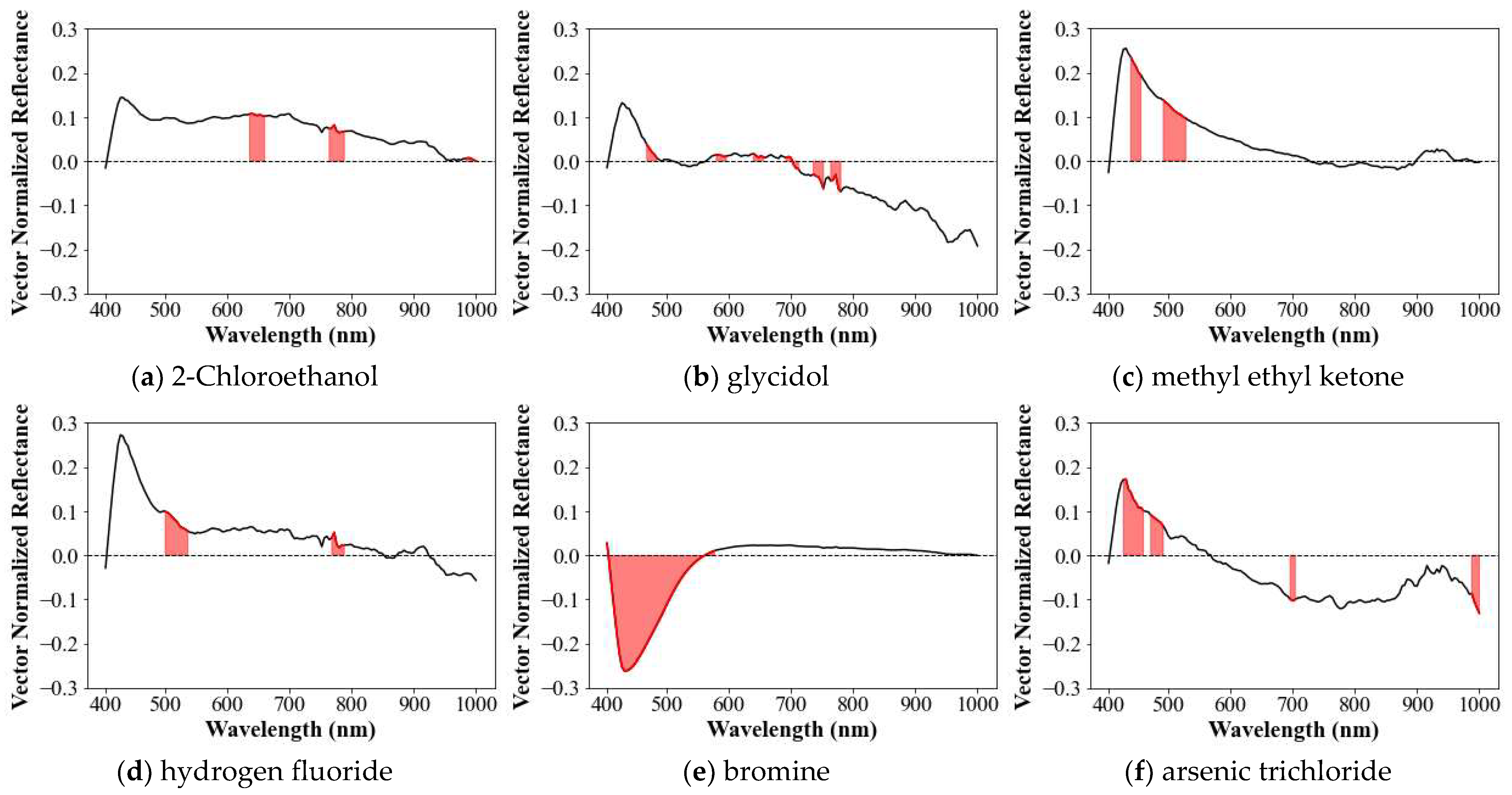
3.1.6. Characteristics of Spectral Library
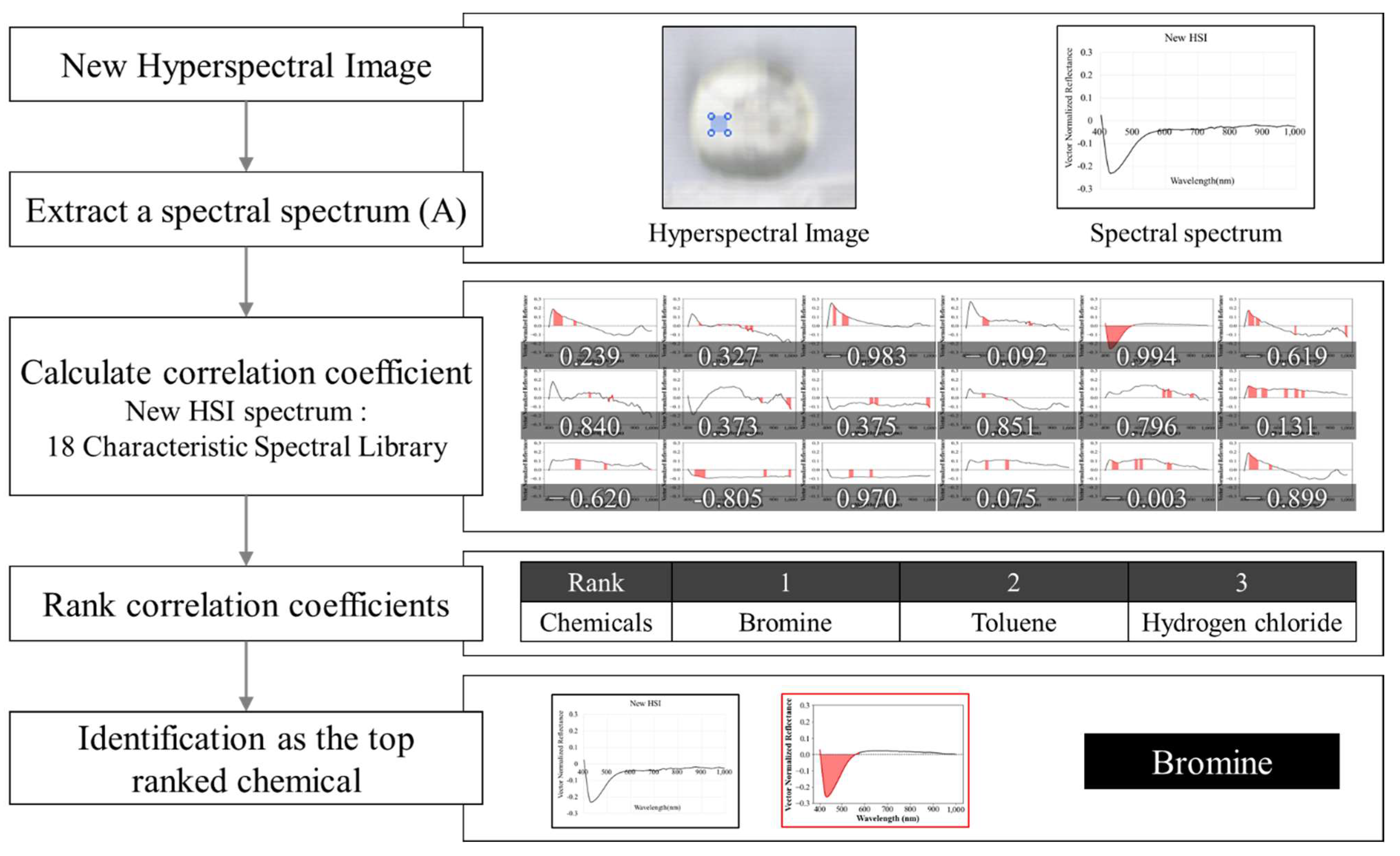
3.2. Recognition Process
4. Conclusions
- (1)
- Hyperspectral-based detection of solvent hazardous chemicals in the riverine environment was scarce, thus very little former research was referable. In this regard, though our initial effort proposed in this study is preliminary and should be more verified, such a library will be referenceable for further research. Practically, the library can be useful for the real-time detection and distinction between hazardous chemicals by facilitating a response to the occurrence of chemical accidents. We highly anticipate that more subtle aspects of enhancing performance of the spectral library will continue to be investigated. From this perspective, further research efforts as well as appropriate guidelines to synthesize various water environment indices such as the concentration of organic matters, concentration of algae, turbidity and water depth are required.
- (2)
- At the initial stages of this study, we considered more than 100 hazardous chemicals, but only covering a small portion of them given the danger of handling them since several of them were forbidden to be treated without permission from national security. In addition, practical abundance to fulfill a solid library even for available chemicals was therefore limited, because it was impossible to obtain sufficient in-situ samples. Instead, the mobile carrier mounting hyperspectral camera was useful for mimicking line-scanning hyperspectral imaging process in outdoor hyperspectral sampling of very low concentration of hazardous chemical samples.
- (3)
- We arbitrarily assigned a criterion for the maximum number of characteristic bands (or coverage) as five. Similarly, ranking criteria to define recognition success was set up as less than third rank. Though we verified that these criteria are suitable to build and utilize a spectral library, such ambiguity in properly defining the optimal criteria remained and should be resolved. For example, we speculate that spectral libraries can better work if developed with the greater number of spectral images, reflecting diverse measurement environments with applying advanced AI techniques to train the capacity to recognize chemicals’ signature. Also, the rank in the recognition process was provided in this study as correlation coefficient, rather than considered to suggest probability.
- (4)
- Characterizing distinctive features among the full spectrum was effective in addition to excluding the ambient spectral impact, such as river water, and normalizing to reduce the impacts from concentration. However, other factors influencing spectral signature should be considered.
- (5)
- Beyond identifying hazardous chemicals, the approach proposed herein for building a characteristic spectral library can also be applied for recognizing other types of solvent materials.
- (6)
- For the better recognition rate and practical uses, various water environment indices, such as the concentration, weather conditions, turbidity, water depth and water color, should be considered ensuring they contain a great number of in-situ spectral images. In those cases, artificial intelligence techniques might be a good solution.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- You, J.S.; Chung, Y.J. Case analysis of the harmful chemical substances’ spill. Fire Sci. Eng. 2014, 28, 90–98. [Google Scholar] [CrossRef]
- Lee, J.S.; Jung, M.S.; Cho, M.S.; Ahn, S.Y.; Kim, K.J.; Yoon, Y.S.; Yoon, J.H.; Seok, K.S. Review on detection analysis and environmental impacts for nitric acid spill response. Korean J. Hazard. Mater. 2013, 1, 25–30. [Google Scholar]
- Kisevic, M.; Morovic, M.; Andricevic, R. The use of hyperspectral data for evaluation of water quality parameters in the river Sava. Fresenius Environ. Bull. 2016, 25, 4814–4822. [Google Scholar]
- Fan, C.L. Spectral Analysis of Water Reflectance for Hyperspectral Remote Sensing of Water Quality in Estuarine Water. J. Geosci. Environ. Prot. 2014, 2, 19–27. [Google Scholar]
- Pokrzywinski, K.; Johansen, R.; Reif, M.; Bourne, S.; Hammond, S.; Fernando, B. Remote sensing of the cyanobacteria life cycle: A mesocosm temporal assessment of a Microcystis sp. bloom using coincident unmanned aircraft system (UAS) hyperspectral imagery and ground sampling efforts. Harmful Algae 2022, 117, 102268. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Si, W.; Wei, L.; Li, Z.; Xia, Z.; Ye, S.; Xia, Y. Retrieval of Water Quality from UAV-Borne Hyperspectral Imagery: A Comparative Study of Machine Learning Algorithms. Remote Sens. 2021, 13, 3928. [Google Scholar] [CrossRef]
- Kwon, S.; Shin, J.; Seo, I.; Noh, H.; Jung, S.; You, H. Measurement of suspended sediment concentration in open channel flows based on hyperspectral imagery from UAVs. Adv. Water Resour. 2022, 159, 104076. [Google Scholar] [CrossRef]
- You, H.J. Development of riverine bathymetry survey technique using drone-based hyperspectral image. Ph.D. Dissertation, Dankook University, Yongin, Republic of Korea, 2018. [Google Scholar]
- Warren, R.; Cohn, D. Chemical detection on surfaces by hyperspectral imaging. J. Appl. Remote Sen. 2017, 11, 015013. [Google Scholar] [CrossRef]
- Nakamura, T. Non-Destructive Trace Detection of Explosives Using Pushbroom Scanning Hyperspectral Imaging System. Sensors 2019, 19, 97. [Google Scholar]
- Zhou, S.; Kaufmann, H.; Bohn, N.; Bochow, M.; Kuester, T.; Segl, K. Identifying distinct plastics in hyperspectral experimental lab-, aircraft-, and satellite data using machine/deep learning methods trained with synthetically mixed spectral data. Remote Sens. Environ. 2022, 281, 113263. [Google Scholar] [CrossRef]
- Yang, J.W.; Cho, G.S. Construction and evaluation of spectral library for SWIR hyperspectral image analysis. J. KOGSIS 2019, 27, 3–12. [Google Scholar] [CrossRef]
- Kokaly, R.F.; Clark, R.N.; Swayze, G.A.; Livo, K.E.; Hoefen, T.M.; Pearson, N.C.; Wise, R.A.; Benzel, W.M.; Lowers, H.A.; Driscoll, R.L.; et al. USGS Spectral Library Version 7; U.S. Geological Survey: Reston, VA, USA, 2017.
- Baldridge, A.M.; Hook, S.J.; Grove, C.I.; Rivera, G. The ASTER spectral library version 2.0. Remote Sens. Environ. 2009, 113, 711–715. [Google Scholar] [CrossRef]
- Meerdink, S.K.; Hook, S.J.; Roberts, D.A.; Abbott, E.A. The ECOSTRESS spectral library version 1.0. Remote Sens. Environ. 2019, 203, 111–196. [Google Scholar] [CrossRef]
- Corning. Corning microHSI 410 SHARK: Integrated, Coherent, Airborne Hyperspectral Imaging System. 2017. Available online: https://www.corning.com/microsites/coc/oem/documents/hyperspectral-imaging/Corning-MicroHSI-410-SHARK-Brochure.pdf (accessed on 23 December 2022).
- Fowler, J.E. Compressive pushbroom and whiskbroom sensing for hyperspectral remote-sensing imaging. In Proceedings of the 2014 IEEE International Conference on Image Processing (ICIP), Paris, France, 27–30 October 2014. [Google Scholar]
- Mustard, J.F.; Staid, M.I.; Fripp, W.J. A semianalytical approach to the calibration of AVIRIS data to reflectance over water. Remote Sens. Environ. 2001, 75, 335–349. [Google Scholar] [CrossRef]
- McCoy, R.M. Field Methods in Remote Sensing; The Guilford Press: New York, NY, USA, 2005. [Google Scholar]
- Friedman, J.H. A Variable Span Scatterplot Smoother; Laboratory for Computational Statistics, Stanford University Technical Report No. 5; Stanford University: Stanford, CA, USA, 1984. [Google Scholar]
- Cleveland, W.S.; Devlin, S.J. Locally Weighted Regression: An Approach to Regression Analysis by Local Fitting. J. Am. Stat. Assoc. 1988, 83, 596–610. [Google Scholar] [CrossRef]
- Fox, S.; Southam, P.; Pantin, F.; Kennaway, R.; Robinson, S.; Castorina, G.; Sánchez-Corrales, Y.E.; Sablowski, R.; Chan, J.; Grieneisen, V.; et al. Spatiotemporal coordination of cell division and growth during organ morphogenesis. Environ. Sci. Biol. 2018, 16, e2005952. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.H. SMART User’s Guide; Laboratory for Computational Statistics, Stanford University Technical Report No. 1; Stanford University: Stanford, CA, USA, 1984. [Google Scholar]
- Kriegler, F.J.; Malila, W.A.; Nalepka, R.F.; Richardson, W. Preprocessing Transformations and Their Effects on Multispectral Recognition. Proc. Sixth Int. Symp. Remote Sens. Environ. 1969, 2, 13–16. [Google Scholar]
- McFeeters, S.K. The Use of Normalized Difference Water Index (NDWI) in the Delineation of Open Water Features. Int. J. Remote Sens. 1996, 17, 1425–1432. [Google Scholar] [CrossRef]



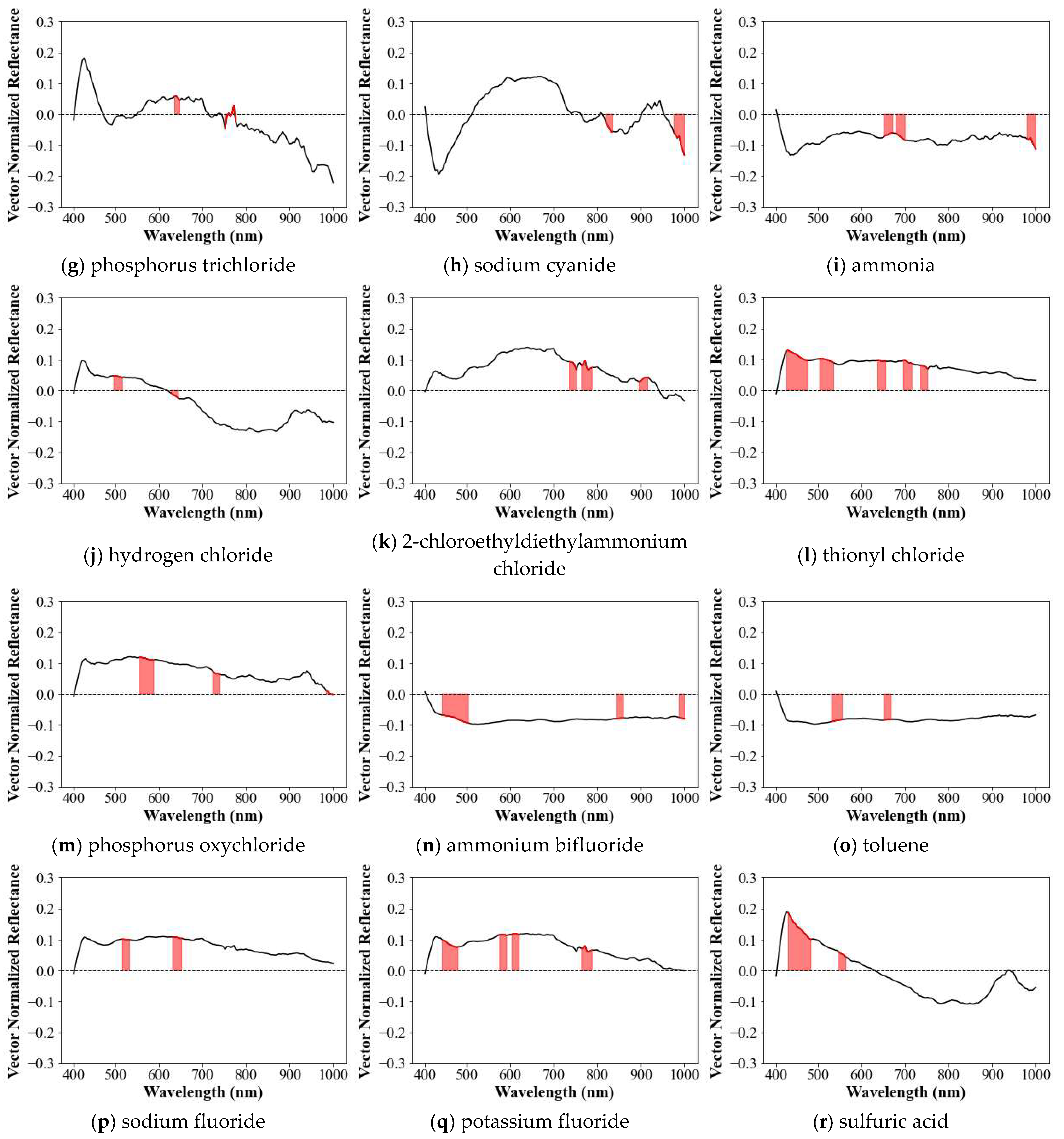
| No. | CAS No. | Name | Chemical Formula | Color | Organic | pH |
|---|---|---|---|---|---|---|
| 1 | 107-07-3 | 2-Chloroethanol | C2H5ClO | Colorless | Organic | 7.42 |
| 2 | 556-52-5 | Glycidol | C3H8O2 | Colorless | Organic | 8.29 |
| 3 | 78-93-3 | Methyl ethyl ketone | C4H8O | Colorless | Organic | 8.22 |
| 4 | 7664-39-3 | Hydrogen fluoride | HF | Colorless | Inorganic | 2.18 |
| 5 | 7726-95-6 | Bromine | Br2 | Dark reddish-brown, Dark red | Inorganic | 5.8 |
| 6 | 7784-34-1 | Arsenic trichloride | AsCl3 | Colorless, Yellow oily fuming liquid | Inorganic | 6.69 |
| 7 | 7719-12-2 | Phosphorus trichloride | PCl3 | Colorless, Yellow oily fuming liquid | Inorganic | 1.53 |
| 8 | 143-33-9 | Sodium cyanide | NaCN | White crystalline solid, Colorless liquid | Inorganic | 10.90 |
| 9 | 7664-41-7 | Ammonia | NH3 | Colorless liquid | Inorganic | 10.47 |
| 10 | 7647-01-0 | Hydrogen chloride | HCl | Colorless | Inorganic | 1.05 |
| 11 | 869-24-9 | 2-Chloroethyldiethylammonium chloride | C6H14ClN·HCl | Colorless liquid | Organic | 6.50 |
| 12 | 7719-09-7 | Thionyl chloride | SOCl2 | Colorless to yellow to reddish liquid | Inorganic | 1.41 |
| 13 | 10025-87-3 | Phosphorus oxychloride | POCl3 | Colorless to yellow, oily liquid | Inorganic | 1.46 |
| 14 | 1341-49-7 | Ammonium bifluoride | (NH4)HF2 | White crystals, Colorless liquid | Inorganic | 3.49 |
| 15 | 108-88-3 | Toluene | C7H8 | Colorless | Organic | (basic) |
| 16 | 7681-49-4 | Sodium fluoride | NaF | White powder or colorless crystals, colorless liquid | Inorganic | 6.85 |
| 17 | 7789-23-3 | Potassium fluoride | KF | White crystalline, colorless liquid | Inorganic | 7.27 |
| 18 | 7664-93-9 | Sulfuric acid | H2SO4 | Colorless to dark-brown, oily liquid | Inorganic | 1.34 |
| No. | Chemicals | Correlation Coefficient | No. | Chemicals | Correlation Coefficient |
|---|---|---|---|---|---|
| 1 | 2-Chloroethanol | 0.871 | 10 | Hydrogen chloride | 0.910 |
| 2 | Glycidol | 0.926 | 11 | 2-Chloroethyldiethylammonium chloride | 0.934 |
| 3 | Methyl ethyl ketone | 0.989 | 12 | Thionyl chloride | 0.977 |
| 4 | Hydrogen fluoride | 0.948 | 13 | Phosphorus oxychloride | 0.912 |
| 5 | Bromine | 0.994 | 14 | Ammonium bifluoride | 0.861 |
| 6 | Arsenic trichloride | 0.814 | 15 | Toluene | 0.717 |
| 7 | Phosphorus trichloride | 0.881 | 16 | Sodium fluoride | 0.906 |
| 8 | Sodium cyanide | 0.948 | 17 | Potassium fluoride | 0.881 |
| 9 | Ammonia | 0.962 | 18 | Sulfuric acid | 0.977 |
| Rank | Number | Chemicals |
|---|---|---|
| 1 | 10 | 2-Chloroethanol, glycidol, methyl ethyl ketone, hydrogen fluoride, bromine, ammonia, thionyl chloride, ammonium bifluoride, potassium fluoride, and sulfuric acid |
| 2 | 2 | 2-Chloroethyldiethylammonium chloride and phosphorus oxychloride |
| 3 | 1 | Arsenic trichloride |
| 4 | 2 | Hydrogen chloride and Sodium fluoride |
| 5 | 1 | Sodium cyanide |
| 6 | 1 | Phosphorus trichloride |
| 10 | 1 | Toluene |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwon, Y.; Kim, D.; You, H.; Nam, S.-H.; Kim, Y.D. A Standardized Procedure to Build a Spectral Library for Hazardous Chemicals Mixed in River Flow Using Hyperspectral Image. Remote Sens. 2023, 15, 477. https://doi.org/10.3390/rs15020477
Gwon Y, Kim D, You H, Nam S-H, Kim YD. A Standardized Procedure to Build a Spectral Library for Hazardous Chemicals Mixed in River Flow Using Hyperspectral Image. Remote Sensing. 2023; 15(2):477. https://doi.org/10.3390/rs15020477
Chicago/Turabian StyleGwon, Yeonghwa, Dongsu Kim, Hojun You, Su-Han Nam, and Young Do Kim. 2023. "A Standardized Procedure to Build a Spectral Library for Hazardous Chemicals Mixed in River Flow Using Hyperspectral Image" Remote Sensing 15, no. 2: 477. https://doi.org/10.3390/rs15020477
APA StyleGwon, Y., Kim, D., You, H., Nam, S.-H., & Kim, Y. D. (2023). A Standardized Procedure to Build a Spectral Library for Hazardous Chemicals Mixed in River Flow Using Hyperspectral Image. Remote Sensing, 15(2), 477. https://doi.org/10.3390/rs15020477






