The Minimum Temperature Outweighed the Maximum Temperature in Determining Plant Growth over the Tibetan Plateau from 1982 to 2017
Abstract
1. Introduction
2. Materials and Methods
2.1. Normalized Difference Vegetation Index (NDVI)
2.2. Total Ecosystem Primary Productivity (GPP)
2.3. Air Temperature Data
2.4. Frozen Soil Distribution Data
2.5. Trend Analysis
2.6. Attribution Analysis Methods
3. Results
3.1. Interannual Variation of Plant Growth and Air Temperature Indices in the Growing Season over the TP
3.2. Correlations between Air Temperature Indices and Plant Growth
3.3. Temperature Determinants to Plant Growth
4. Discussion
4.1. Possible Reasons for Tmin in Determining Plant Growth over the TP
4.2. Possible Uncertainty Sources and Future Work
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, F.H.; Ding, L.; Piao, S.L.; Zhou, T.J.; Xu, B.Q.; Yao, T.D.; Li, X. The Tibetan Plateau as the engine for Asian environmental change: The Tibetan Plateau Earth system research into a new era. Sci. Bull. 2021, 66, 1263–1266. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Xu, Q.; Yue, Y.H.; Wang, H.Q.; Cai, F.L.; Li, S. The Andean-type Gangdese Mountains: Paleoelevation record from the Paleocene-Eocene Linzhou Basin. Earth Planet. Sci. Lett. 2014, 392, 250–264. [Google Scholar] [CrossRef]
- Chen, F.H.; Fu, B.J.; Xia, J.; Wu, D.; Wu, S.H.; Zhang, Y.L.; Sun, H.; Liu, Y.; Fang, X.M.; Qin, B.Q.; et al. Major advances in studies of the physical geography and living environment of China during the past 70 years and future prospects. Sci. China Earth Sci. 2019, 62, 1665–1701. [Google Scholar] [CrossRef]
- You, Q.L.; Fraedrich, K.; Ren, G.Y.; Pepin, N.; Kang, S.C. Variability of temperature in the Tibetan Plateau based on homogenized surface stations and reanalysis data. Int. J. Climatol. 2013, 33, 1337–1347. [Google Scholar] [CrossRef]
- Liu, X.D.; Chen, B.D. Climatic warming in the Tibetan Plateau during recent decades. Int. J. Climatol. 2000, 20, 1729–1742. [Google Scholar] [CrossRef]
- You, Q.L.; Kang, S.C.; Pepin, N.; Flugel, W.A.; Yan, Y.P.; Behrawan, H.; Huang, J. Relationship between temperature trend magnitude, elevation and mean temperature in the Tibetan Plateau from homogenized surface stations and reanalysis data. Glob. Planet. Chang. 2010, 71, 124–133. [Google Scholar] [CrossRef]
- Piao, S.L.; Ciais, P.; Huang, Y.; Shen, Z.H.; Peng, S.S.; Li, J.S.; Zhou, L.P.; Liu, H.Y.; Ma, Y.C.; Ding, Y.H.; et al. The impacts of climate change on water resources and agriculture in China. Nature 2010, 467, 43–51. [Google Scholar] [CrossRef]
- Descals, A.; Verger, A.; Filella, I.; Baldocchi, D.; Janssens, I.A.; Fu, Y.H.; Piao, S.; Peaucelle, M.; Ciais, P.; Penuelas, J. Soil thawing regulates the spring growth onset in tundra and alpine biomes. Sci. Total Environ. 2020, 742, 140637. [Google Scholar] [CrossRef]
- Piao, S.L.; Cui, M.D.; Chen, A.P.; Wang, X.H.; Ciais, P.; Liu, J.; Tang, Y.H. Altitude and temperature dependence of change in the spring vegetation green-up date from 1982 to 2006 in the Qinghai-Xizang Plateau. Agric. For. Meteorol. 2011, 151, 1599–1608. [Google Scholar] [CrossRef]
- Duan, A.; Xiao, Z. Does the climate warming hiatus exist over the Tibetan Plateau? Sci. Rep. 2015, 5, 13711. [Google Scholar] [CrossRef]
- Wang, K.; Xu, Q.; Li, T. Does recent climate warming drive spatiotemporal shifts in functioning of high-elevation hydrological systems? Sci. Total Environ. 2020, 719, 137507. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Zhang, Y.J.; Zhu, J.T.; Liu, Y.J.; Zu, J.X.; Zhang, J. The Influences of Climate Change and Human Activities on Vegetation Dynamics in the Qinghai-Tibet Plateau. Remote Sens. 2016, 8, 876. [Google Scholar] [CrossRef]
- Li, L.H.; Zhang, Y.L.; Liu, L.S.; Wu, J.S.; Wang, Z.F.; Li, S.C.; Zhang, H.M.; Zu, J.X.; Ding, M.J.; Paudel, B. Spatiotemporal Patterns of Vegetation Greenness Change and Associated Climatic and Anthropogenic Drivers on the Tibetan Plateau during 2000–2015. Remote Sens. 2018, 10, 1525. [Google Scholar] [CrossRef]
- Melaas, E.K.; Friedl, M.A.; Richardson, A.D. Multiscale modeling of spring phenology across Deciduous Forests in the Eastern United States. Glob. Change Biol. 2016, 22, 792–805. [Google Scholar] [CrossRef]
- Wang, Y.J.; Shen, X.J.; Jiang, M.; Lu, X.G. Vegetation Change and Its Response to Climate Change between 2000 and 2016 in Marshes of the Songnen Plain, Northeast China. Sustainability 2020, 12, 3569. [Google Scholar] [CrossRef]
- Wan, S.Q.; Xia, J.Y.; Liu, W.X.; Niu, S.L. Photosynthetic overcompensation under nocturnal warming enhances grassland carbon sequestration. Ecology 2009, 90, 2700–2710. [Google Scholar] [CrossRef]
- Shen, M.G.; Piao, S.L.; Chen, X.Q.; An, S.; Fu, Y.S.H.; Wang, S.P.; Cong, N.; Janssens, I.A. Strong impacts of daily minimum temperature on the green-up date and summer greenness of the Tibetan Plateau. Glob. Chang. Biol. 2016, 22, 3057–3066. [Google Scholar] [CrossRef]
- Harrington, C.A.; Gould, P.J.; St Clair, J.B. Modeling the effects of winter environment on dormancy release of Douglas-fir. For. Ecol. Manag. 2010, 259, 798–808. [Google Scholar] [CrossRef]
- Hanninen, H.; Kramer, K. A framework for modelling the annual cycle of trees in boreal and temperate regions. Silva Fenn. 2007, 41, 167–205. [Google Scholar] [CrossRef]
- Shen, X.J.; Liu, Y.W.; Zhang, J.Q.; Wang, Y.J.; Ma, R.; Liu, B.H.; Lu, X.G.; Jiang, M. Asymmetric Impacts of Diurnal Warming on Vegetation Carbon Sequestration of Marshes in the Qinghai Tibet Plateau. Glob. Biogeochem. Cycles 2022, 36, e2022GB007396. [Google Scholar] [CrossRef]
- Li, X.; Zhang, K.; Li, X. Responses of vegetation growth to climate change over the Tibetan Plateau from 1982 to 2018. Environ. Res. Commun. 2022, 4, 045007. [Google Scholar] [CrossRef]
- Zhang, K.; Kimball, J.S.; Nemani, R.R.; Running, S.W.; Hong, Y.; Gourley, J.J.; Yu, Z.B. Vegetation Greening and Climate Change Promote Multidecadal Rises of Global Land Evapotranspiration. Sci. Rep. 2015, 5, 15956. [Google Scholar] [CrossRef] [PubMed]
- Songhan, W.; Yongguang, Z. Long-Term (1982–2018) Global Gross Primary Production Dataset Based on NIRv; National Tibetan Plateau Data Center: Beijing, China, 2020. [Google Scholar] [CrossRef]
- Wang, S.H.; Zhang, Y.G.; Ju, W.M.; Qiu, B.; Zhang, Z.Y. Tracking the seasonal and inter-annual variations of global gross primary production during last four decades using satellite near-infrared reflectance data. Sci. Total Environ. 2021, 755, 142569. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.X.; Peng, S.Z. Spatiotemporal Trends and Attribution of Drought across China from 1901–2100. Sustainability 2020, 12, 477. [Google Scholar] [CrossRef]
- Peng, S.Z.; Ding, Y.X.; Liu, W.Z.; Li, Z. 1 km monthly temperature and precipitation dataset for China from 1901 to 2017. Earth Syst. Sci. Data 2019, 11, 1931–1946. [Google Scholar] [CrossRef]
- Zou, D.F.; Zhao, L.; Sheng, Y.; Chen, J.; Hu, G.J.; Wu, T.H.; Wu, J.C.; Xie, C.W.; Wu, X.D.; Pang, Q.Q.; et al. A new map of permafrost distribution on the Tibetan Plateau. Cryosphere 2017, 11, 2527–2542. [Google Scholar] [CrossRef]
- Sen, P.K. Estimates of the regression coefficient based on Kendall’s tau. J. Am. Stat. Assoc. 1968, 63, 1379–1389. [Google Scholar] [CrossRef]
- Kalisa, W.; Igbawua, T.; Henchiri, M.; Ali, S.; Zhang, S.; Bai, Y.; Zhang, J.H. Assessment of climate impact on vegetation dynamics over East Africa from 1982 to 2015. Sci. Rep. 2019, 9, 16865. [Google Scholar] [CrossRef]
- Wetzels, R.; Wagenmakers, E.J. A default Bayesian hypothesis test for correlations and partial correlations. Psychon. B Rev. 2012, 19, 1057–1064. [Google Scholar] [CrossRef]
- Yuan, W.P.; Zheng, Y.; Piao, S.L.; Ciais, P.; Lombardozzi, D.; Wang, Y.P.; Ryu, Y.; Chen, G.X.; Dong, W.J.; Hu, Z.M.; et al. Increased atmospheric vapor pressure deficit reduces global vegetation growth. Sci. Adv. 2019, 5, eaax1396. [Google Scholar] [CrossRef]
- Wang, J.F.; Li, X.H.; Christakos, G.; Liao, Y.L.; Zhang, T.; Gu, X.; Zheng, X.Y. Geographical Detectors-Based Health Risk Assessment and its Application in the Neural Tube Defects Study of the Heshun Region, China. Int. J. Geogr. Inf. Sci. 2010, 24, 107–127. [Google Scholar] [CrossRef]
- Duan, H.C.; Xue, X.; Wang, T.; Kang, W.P.; Liao, J.; Liu, S.L. Spatial and Temporal Differences in Alpine Meadow, Alpine Steppe and All Vegetation of the Qinghai-Tibetan Plateau and Their Responses to Climate Change. Remote Sens. 2021, 13, 669. [Google Scholar] [CrossRef]
- Teng, H.; Luo, Z.; Chang, J.; Shi, Z.; Chen, S.; Zhou, Y.; Ciais, P.; Tian, H. Climate change-induced greening on the Tibetan Plateau modulated by mountainous characteristics. Environ. Res. Lett. 2021, 16, 064064. [Google Scholar] [CrossRef]
- Yang, J.L.; Yang, D.; Lu, W.Q.; Zhang, X.; Ma, M.M.; Liu, G.F.; Jiang, J.; Li, C.H. Somatic embryogenesis and plant regeneration in Betula platyphalla. J. For. Res. 2021, 32, 937–944. [Google Scholar] [CrossRef]
- Thorne, P.W.; Donat, M.G.; Dunn, R.J.H.; Williams, C.N.; Alexander, L.V.; Caesar, J.; Durre, I.; Harris, I.; Hausfather, Z.; Jones, P.D.; et al. Reassessing changes in diurnal temperature range: Intercomparison and evaluation of existing global data set estimates. J. Geophys. Res.-Atmos. 2016, 121, 5138–5158. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Gouveia, C.; Camarero, J.J.; Begueria, S.; Trigo, R.; Lopez-Moreno, J.I.; Azorin-Molina, C.; Pasho, E.; Lorenzo-Lacruz, J.; Revuelto, J.; et al. Response of vegetation to drought time-scales across global land biomes. Proc. Natl. Acad. Sci. USA 2013, 110, 52–57. [Google Scholar] [CrossRef]
- Zhang, G.L.; Zhang, Y.J.; Dong, J.W.; Xiao, X.M. Green-up dates in the Tibetan Plateau have continuously advanced from 1982 to 2011. Proc. Natl. Acad. Sci. USA 2013, 110, 4309–4314. [Google Scholar] [CrossRef]
- Piao, S.L.; Tan, J.G.; Chen, A.P.; Fu, Y.H.; Ciais, P.; Liu, Q.; Janssens, I.A.; Vicca, S.; Zeng, Z.Z.; Jeong, S.J.; et al. Leaf onset in the northern hemisphere triggered by daytime temperature. Nat. Commun. 2015, 6, 6911. [Google Scholar] [CrossRef]
- Gao, Y.H.; Zhou, X.; Wang, Q.; Wang, C.Z.; Zhan, Z.M.; Chen, L.F.; Yan, J.X.; Qu, R. Vegetation net primary productivity and its response to climate change during 2001–2008 in the Tibetan Plateau. Sci. Total Environ. 2013, 444, 356–362. [Google Scholar] [CrossRef]
- Sharma, N.; Yadav, A.; Khetarpal, S.; Anand, A.; Sathee, L.; Kumar, R.R.; Singh, B.; Soora, N.K.; Pushkar, S. High day-night transition temperature alters nocturnal starch metabolism in rice (Oryza sativa L.). Acta Physiol. Plant 2017, 39, 74. [Google Scholar] [CrossRef]
- Liu, X.D.; Yin, Z.Y.; Shao, X.M.; Qin, N.S. Temporal trends and variability of daily maximum and minimum, extreme temperature events, and growing season length over the eastern and central Tibetan Plateau during 1961–2003. J. Geophys. Res.-Atmos. 2006, 111, D19109. [Google Scholar] [CrossRef]
- Atkin, O.K.; Turnbull, M.H.; Zaragoza-Castells, J.; Fyllas, N.M.; Lloyd, J.; Meir, P.; Griffin, K.L. Light inhibition of leaf respiration as soil fertility declines along a post-glacial chronosequence in New Zealand: An analysis using the Kok method. Plant Soil. 2013, 367, 163–182. [Google Scholar] [CrossRef]
- Kim, Y.; Kimball, J.S.; Zhang, K.; McDonald, K.C. Satellite detection of increasing Northern Hemisphere non-frozen seasons from 1979 to 2008: Implications for regional vegetation growth. Remote Sens. Environ. 2012, 121, 472–487. [Google Scholar] [CrossRef]
- Korner, C. Paradigm shift in plant growth control. Curr. Opin. Plant Biol. 2015, 25, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Vitasse, Y.; Lenz, A.; Korner, C. The interaction between freezing tolerance and phenology in temperate deciduous trees. Front. Plant Sci. 2014, 5, 541. [Google Scholar] [CrossRef]
- Griffin, K.L.; Turnbull, M.; Murthy, R.; Lin, G.H.; Adams, J.; Farnsworth, B.; Mahato, T.; Bazin, G.; Potasnak, M.; Berry, J.A. Leaf respiration is differentially affected by leaf vs. stand-level night-time warming. Glob. Chang. Biol. 2002, 8, 479–485. [Google Scholar] [CrossRef]
- Shen, M.G.; Piao, S.L.; Cong, N.; Zhang, G.X.; Janssens, I.A. Precipitation impacts on vegetation spring phenology on the Tibetan Plateau. Glob. Chang. Biol. 2015, 21, 3647–3656. [Google Scholar] [CrossRef]
- Wan, G.N.; Yang, M.X.; Wang, X.J. Variations in soil temperature at BJ site on the central Tibetan Plateau. J. Mt. Sci. 2012, 9, 274–285. [Google Scholar] [CrossRef]
- Yang, K.; Qin, J.; Zhao, L.; Chen, Y.Y.; Tang, W.J.; Han, M.L.; Lazhu; Chen, Z.Q.; Lv, N.; Ding, B.H.; et al. A Multiscale Soil Moisture and Freeze-Thaw Monitoring Network on the Third Pole. Bull. Am. Meteorol. Soc. 2013, 94, 1907–1916. [Google Scholar] [CrossRef]
- Yi, S.; Li, N.; Xiang, B.; Wang, X.; Ye, B.; McGuire, A.D. Representing the effects of alpine grassland vegetation cover on the simulation of soil thermal dynamics by ecosystem models applied to the Qinghai-Tibetan Plateau. J. Geophys. Res.-Biogeosci. 2013, 118, 1186–1199. [Google Scholar] [CrossRef]
- Yang, Z.L.; Jiang, L.; Su, F.L.; Zhang, Q.; Xia, J.Y.; Wan, S.Q. Nighttime warming enhances drought resistance of plant communities in a temperate steppe. Sci. Rep. 2016, 6, 23267. [Google Scholar] [CrossRef] [PubMed]
- Alward, R.D.; Detling, J.K.; Milchunas, D.G. Grassland vegetation changes and nocturnal global warming. Science 1999, 283, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.R.; Vincent, J.R.; Auffhammer, M.; Moya, P.F.; Dobermann, A.; Dawe, D. Rice yields in tropical/subtropical Asia exhibit large but opposing sensitivities to minimum and maximum temperatures. Proc. Natl. Acad. Sci. USA 2010, 107, 14562–14567. [Google Scholar] [CrossRef]
- Morita, S.; Yonemaru, J.; Takanashi, J. Grain growth and endosperm cell size under high night temperatures in rice (Oryza sativa L.). Ann. Bot. 2005, 95, 695–701. [Google Scholar] [CrossRef]
- Huang, X.L.; Zhang, T.B.; Yi, G.H.; He, D.; Zhou, X.B.; Li, J.J.; Bie, X.J.; Miao, J.Q. Dynamic Changes of NDVI in the Growing Season of the Tibetan Plateau During the Past 17 Years and Its Response to Climate Change. Int. J. Environ. Res. Public Health 2019, 16, 3452. [Google Scholar] [CrossRef]
- Liang, E.; Lu, X.M.; Ren, P.; Li, X.X.; Zhu, L.P.; Eckstein, D. Annual increments of juniper dwarf shrubs above the tree line on the central Tibetan Plateau: A useful climatic proxy. Ann. Bot. 2012, 109, 721–728. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Ma, Y.M.; Li, H.X.; Yuan, L. Carbon and water fluxes and their coupling in an alpine meadow ecosystem on the northeastern Tibetan Plateau. Theor. Appl. Climatol. 2020, 142, 1–18. [Google Scholar] [CrossRef]
- Cheng, M.; Jin, J.X.; Zhang, J.M.; Jiang, H.; Wang, R.Z. Effect of climate change on vegetation phenology of different land-cover types on the Tibetan Plateau. Int. J. Remote Sens. 2018, 39, 470–487. [Google Scholar] [CrossRef]
- Jiang, H.R.; Zhang, W.J.; Yi, Y.H.; Yan, K.; Li, G.C.; Wang, G.X. The impacts of soil freeze/thaw dynamics on soil water transfer and spring phenology in the Tibetan Plateau. Arct. Antarct. Alp. Res. 2018, 50, e1439155. [Google Scholar] [CrossRef]
- Xia, H.M.; Li, A.N.; Feng, G.; Li, Y.; Qin, Y.C.; Lei, G.B.; Cui, Y.P. The Effects of Asymmetric Diurnal Warming on Vegetation Growth of the Tibetan Plateau over the Past Three Decades. Sustainability 2018, 10, 1103. [Google Scholar] [CrossRef]
- Adams, H.D.; Guardiola-Claramonte, M.; Barron-Gafford, G.A.; Villegas, J.C.; Breshears, D.D.; Zou, C.B.; Troch, P.A.; Huxman, T.E. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proc. Natl. Acad. Sci. USA 2009, 106, 7063–7066. [Google Scholar] [CrossRef]
- Peng, S.S.; Piao, S.L.; Ciais, P.; Myneni, R.B.; Chen, A.P.; Chevallier, F.; Dolman, A.J.; Janssens, I.A.; Penuelas, J.; Zhang, G.X.; et al. Asymmetric effects of daytime and night-time warming on Northern Hemisphere vegetation. Nature 2013, 501, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Luo, Y.; Shafeeque, M. Interpretation of vegetation phenology changes using daytime and night-time temperatures across the Yellow River Basin, China. Sci. Total Environ. 2019, 693, 133553. [Google Scholar] [CrossRef]
- Wu, C.Y.; Wang, X.Y.; Wang, H.J.; Ciais, P.; Penuelas, J.; Myneni, R.B.; Desai, A.R.; Gough, C.M.; Gonsamo, A.; Black, A.T.; et al. Contrasting responses of autumn-leaf senescence to daytime and night-time warming. Nat. Clim. Chang. 2019, 9, 177. [Google Scholar] [CrossRef]
- Liu, Q.; Piao, S.L.; Campioli, M.; Gao, M.D.; Fu, Y.S.H.; Wang, K.; He, Y.; Li, X.Y.; Janssens, I.A. Modeling leaf senescence of deciduous tree species in Europe. Glob. Chang. Biol. 2020, 26, 4104–4118. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Ho, C.H.; Park, T.W.; Kim, J.; Levis, S. Impact of vegetation feedback on the temperature and its diurnal range over the Northern Hemisphere during summer in a 2 × CO2 climate. Clim. Dyn. 2011, 37, 821–833. [Google Scholar] [CrossRef]
- Sedlacek, J.; Wheeler, J.A.; Cortes, A.J.; Bossdorf, O.; Hoch, G.; Lexer, C.; Wipf, S.; Karrenberg, S.; van Kleunen, M.; Rixen, C. The Response of the Alpine Dwarf Shrub Salix herbacea to Altered Snowmelt Timing: Lessons from a Multi-Site Transplant Experiment. PLoS ONE 2015, 10, e012239510. [Google Scholar] [CrossRef]
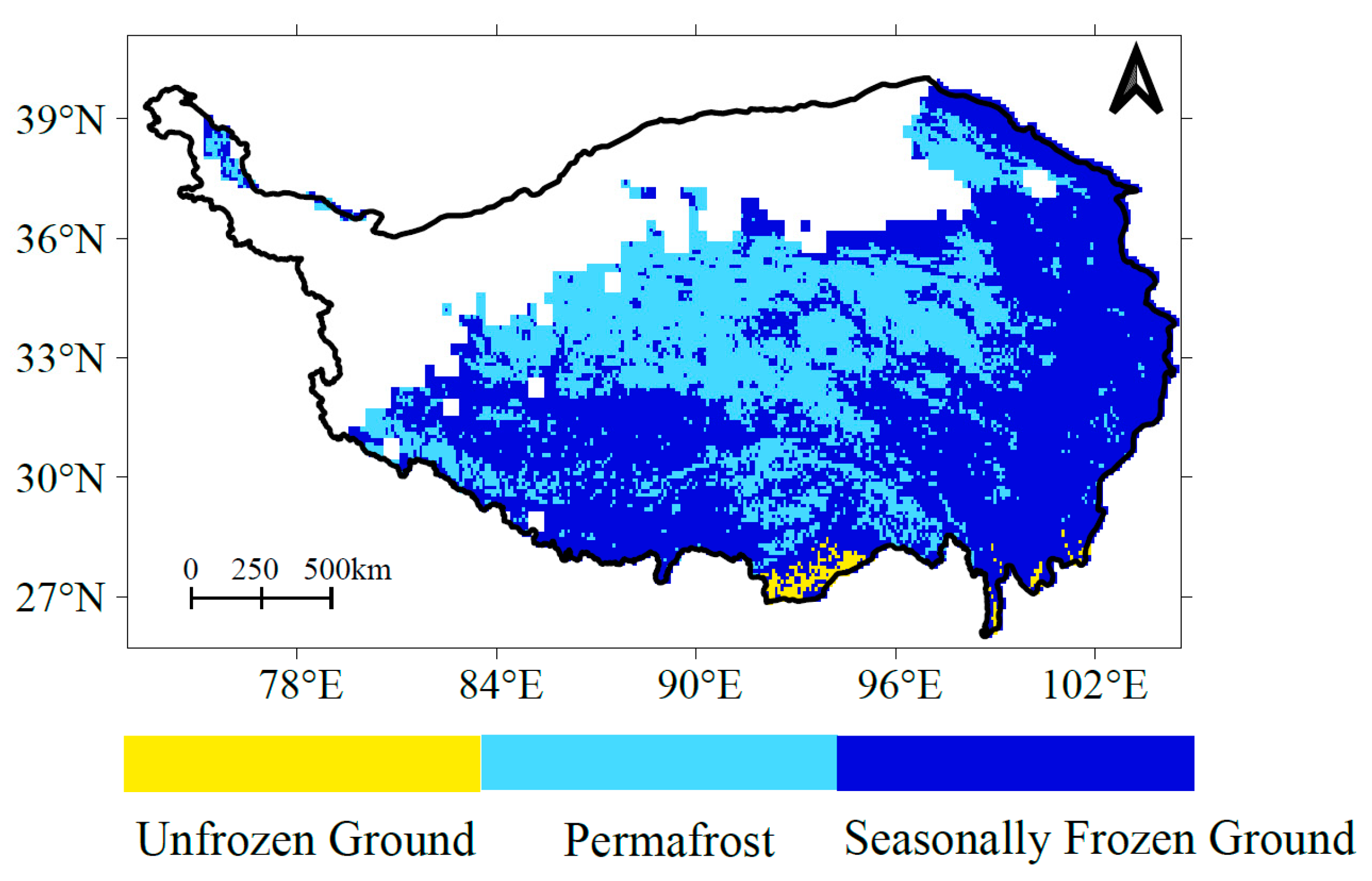
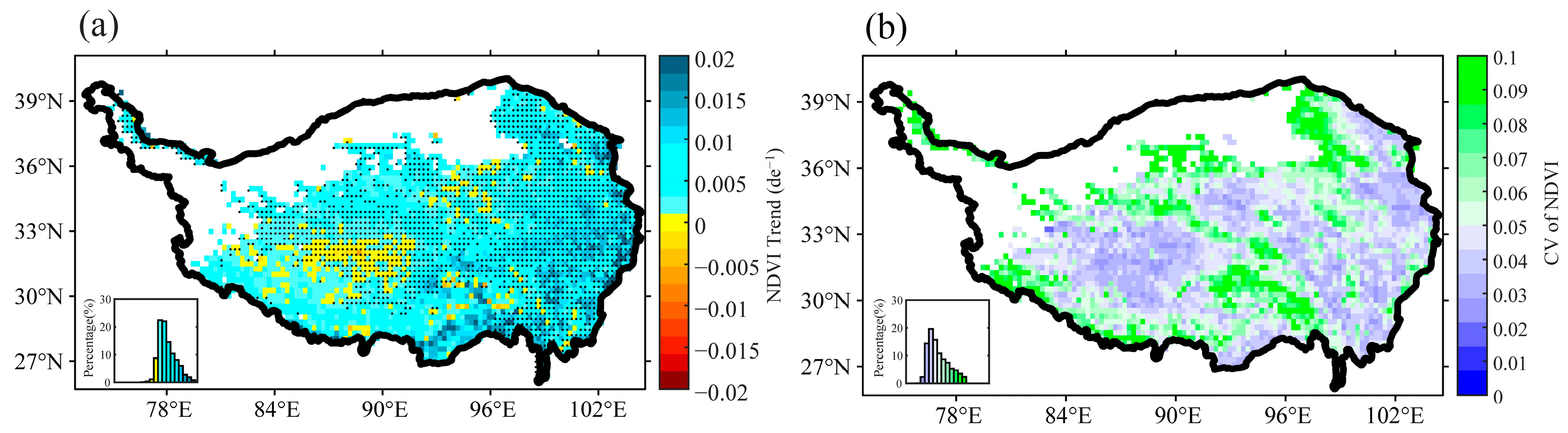
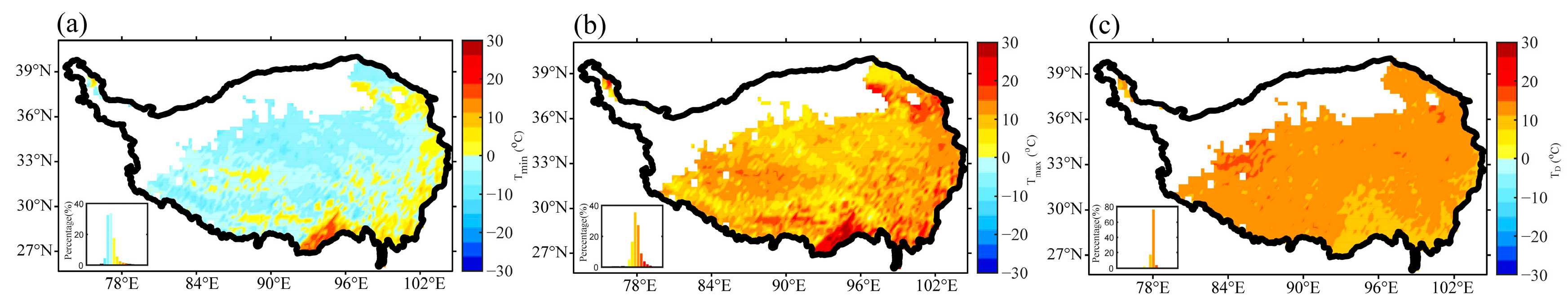
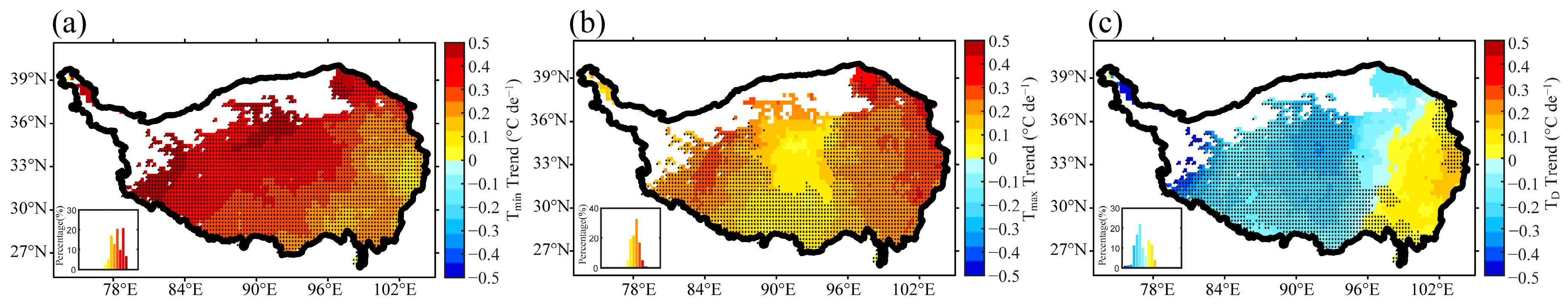
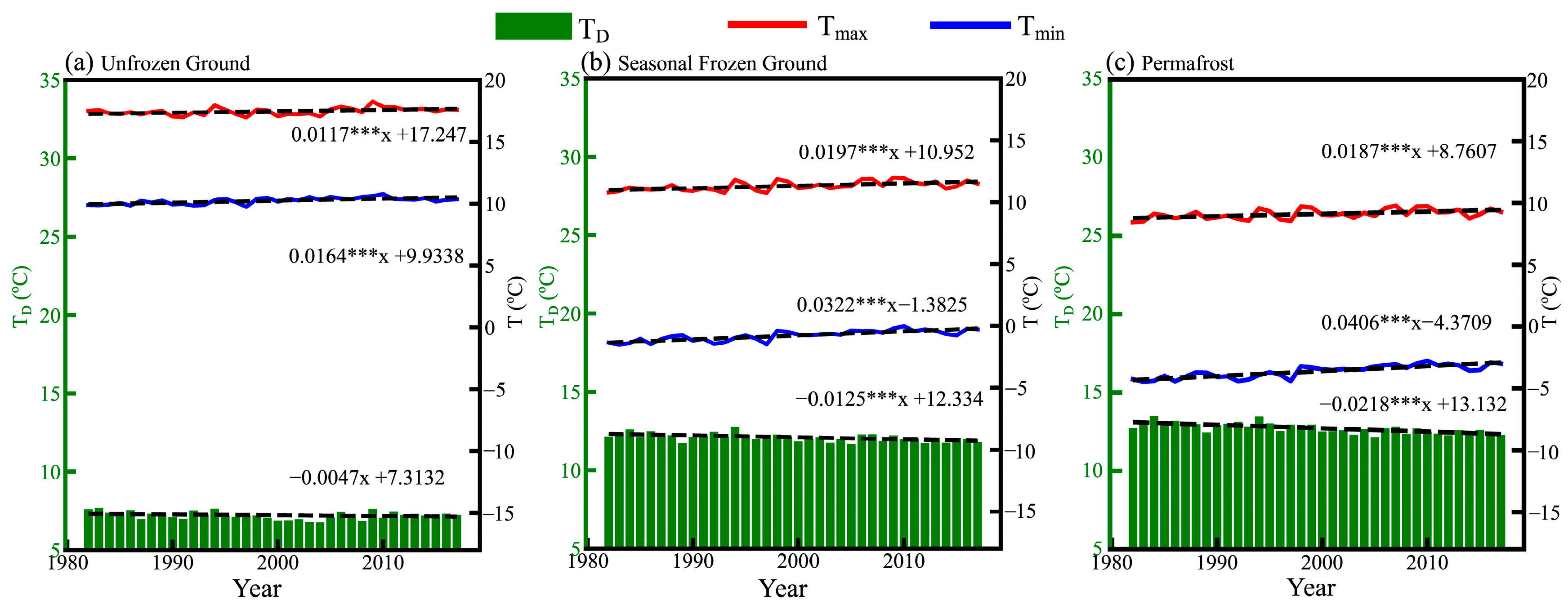
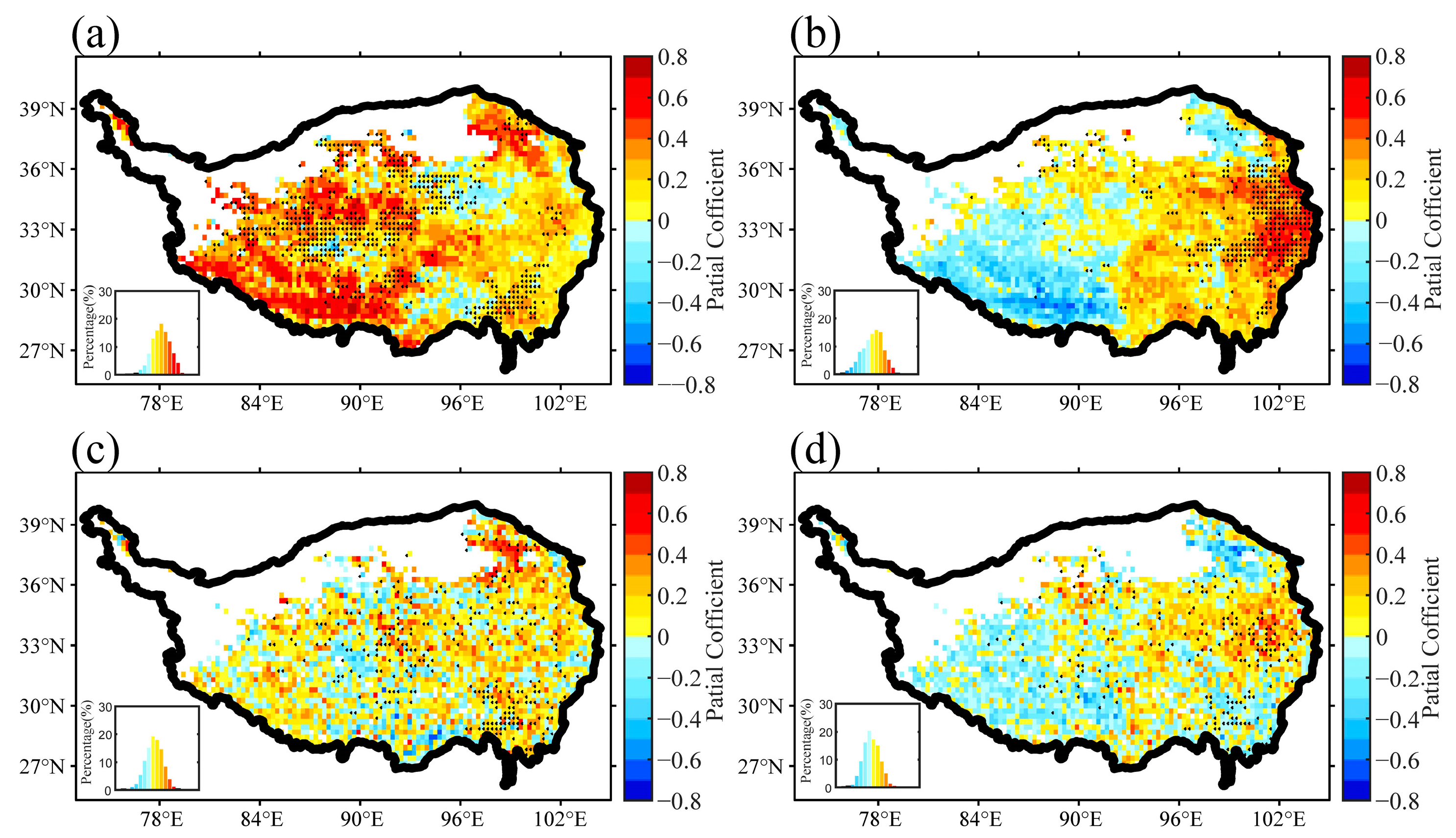

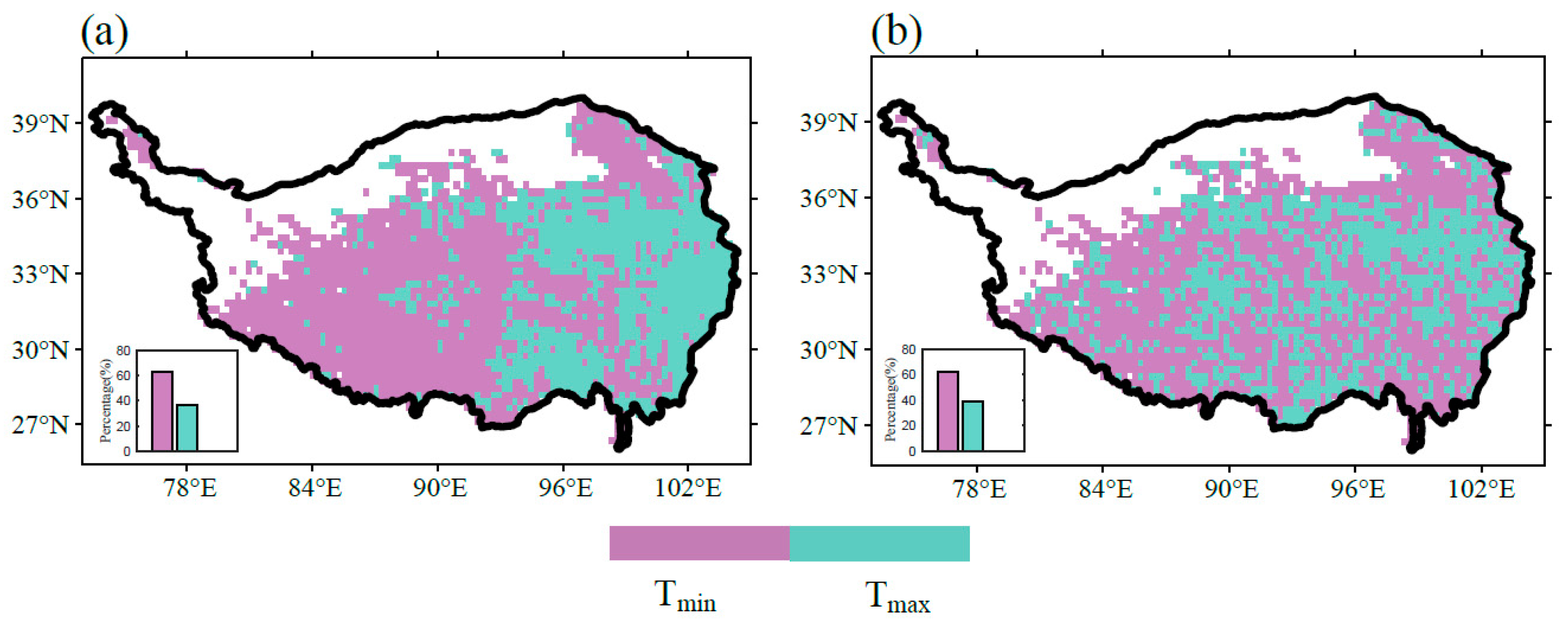
| q Statistic | Unfrozen Ground | Seasonal Frozen Ground | Permafrost | |
|---|---|---|---|---|
| Tmin | NDVI | 0.48 ** | 0.56 ** | 0.53 ** |
| GPP | 0.21 * | 0.44 ** | 0.36 ** | |
| Tmax | NDVI | 0.34 ** | 0.49 ** | 0.40 ** |
| GPP | 0.16 | 0.35 ** | 0.34 ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, K.; Li, X. The Minimum Temperature Outweighed the Maximum Temperature in Determining Plant Growth over the Tibetan Plateau from 1982 to 2017. Remote Sens. 2023, 15, 4032. https://doi.org/10.3390/rs15164032
Li X, Zhang K, Li X. The Minimum Temperature Outweighed the Maximum Temperature in Determining Plant Growth over the Tibetan Plateau from 1982 to 2017. Remote Sensing. 2023; 15(16):4032. https://doi.org/10.3390/rs15164032
Chicago/Turabian StyleLi, Xi, Ke Zhang, and Xin Li. 2023. "The Minimum Temperature Outweighed the Maximum Temperature in Determining Plant Growth over the Tibetan Plateau from 1982 to 2017" Remote Sensing 15, no. 16: 4032. https://doi.org/10.3390/rs15164032
APA StyleLi, X., Zhang, K., & Li, X. (2023). The Minimum Temperature Outweighed the Maximum Temperature in Determining Plant Growth over the Tibetan Plateau from 1982 to 2017. Remote Sensing, 15(16), 4032. https://doi.org/10.3390/rs15164032







