Assessment of Nitrogen Nutrition Index of Winter Wheat Canopy from Visible Images for a Dynamic Monitoring of N Requirements
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experimental Site
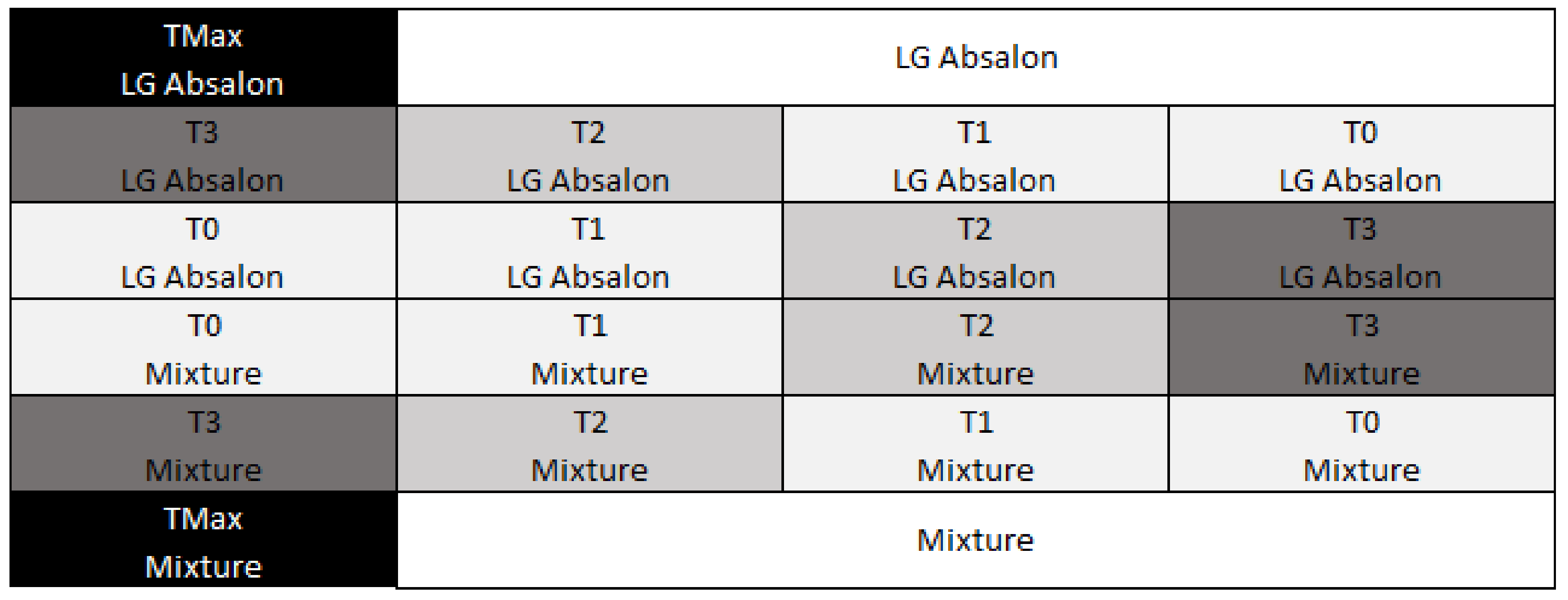
2.2. Nitrogen Gradient in the Experiments
2.3. Sampling and Measurements
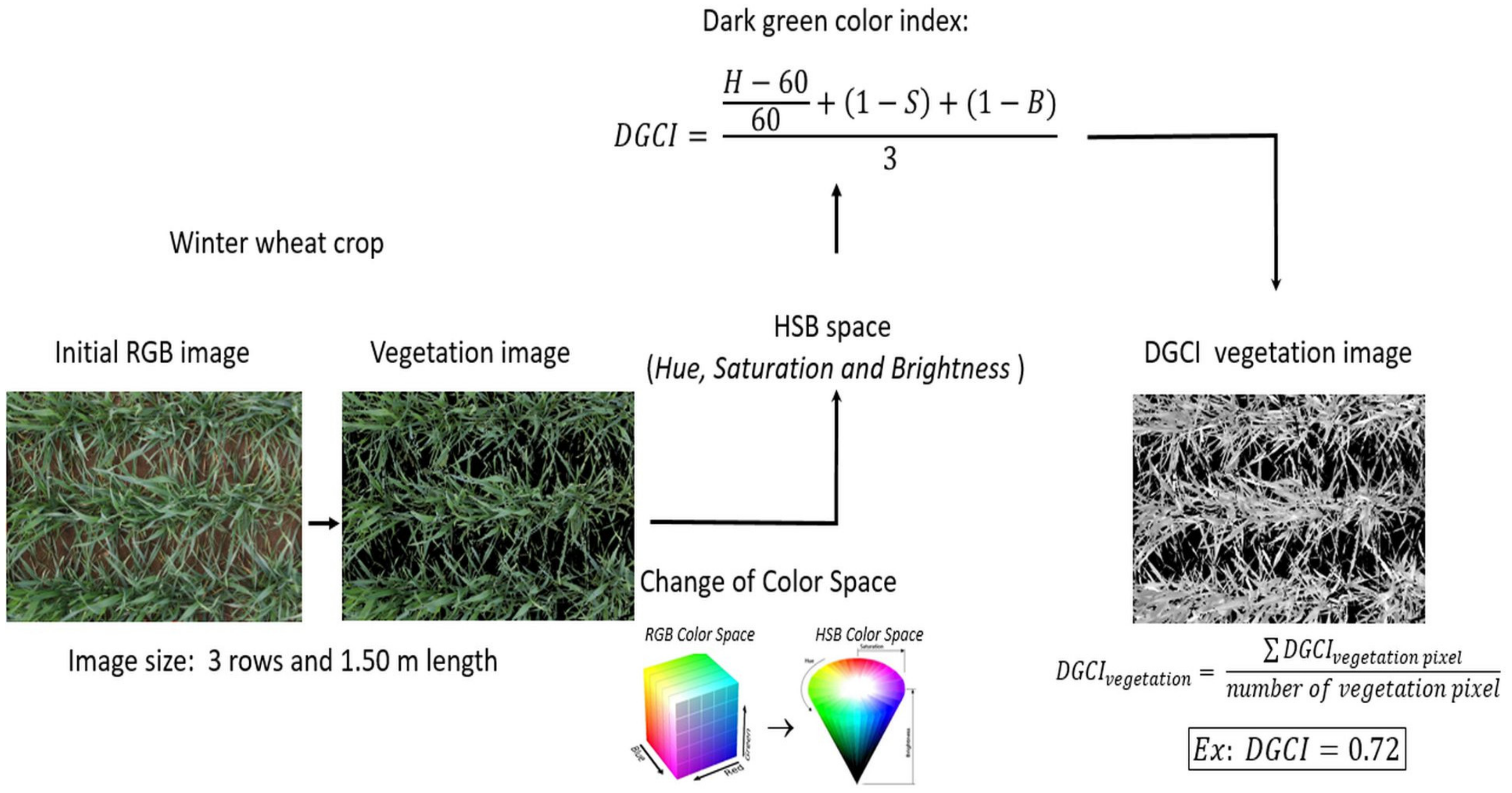
2.4. Image Processing-DGCI Indicator Calculation
2.5. Statistical Analysis
3. Results
3.1. Comparison between DGCI and nDGCI Values
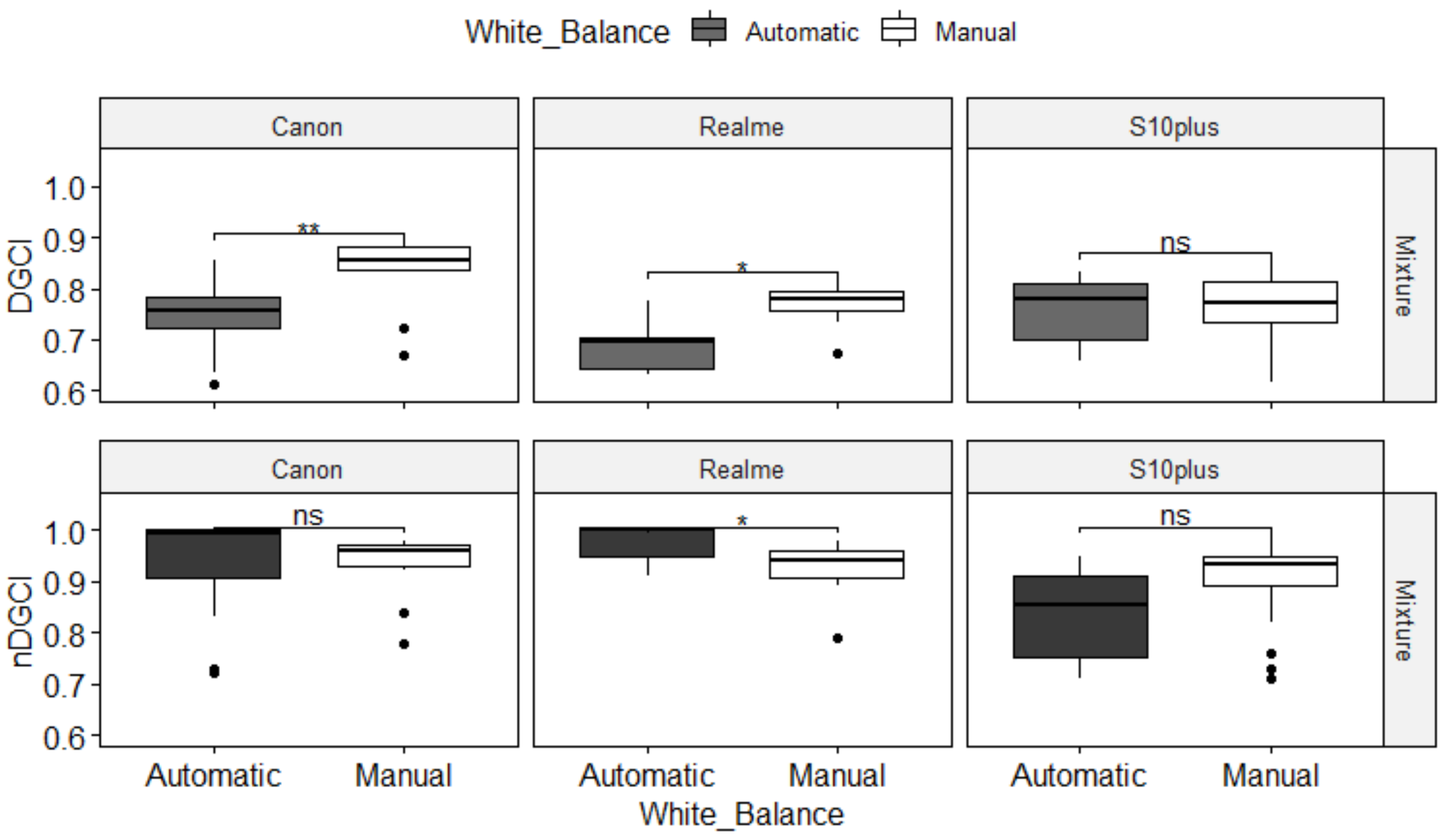
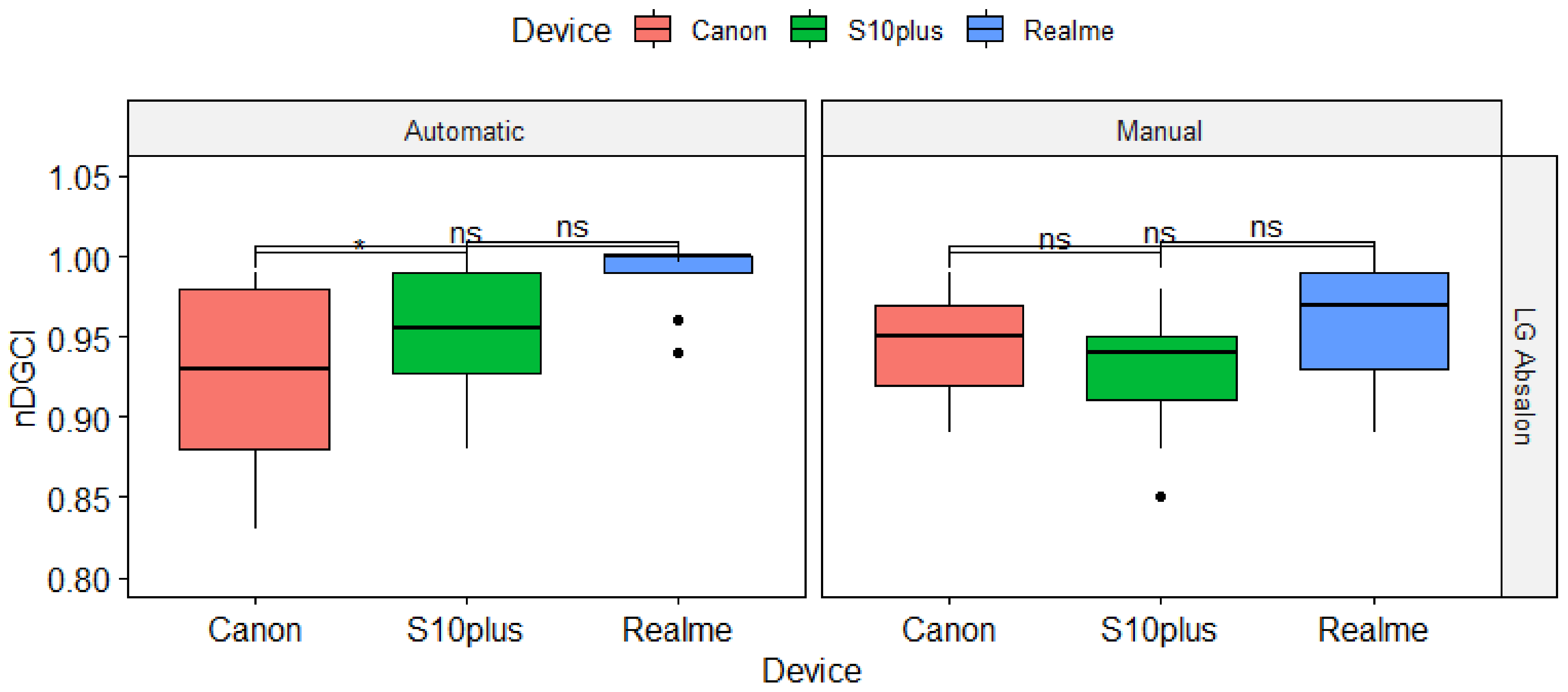
3.2. Impact of the Devices on nDGCI Values
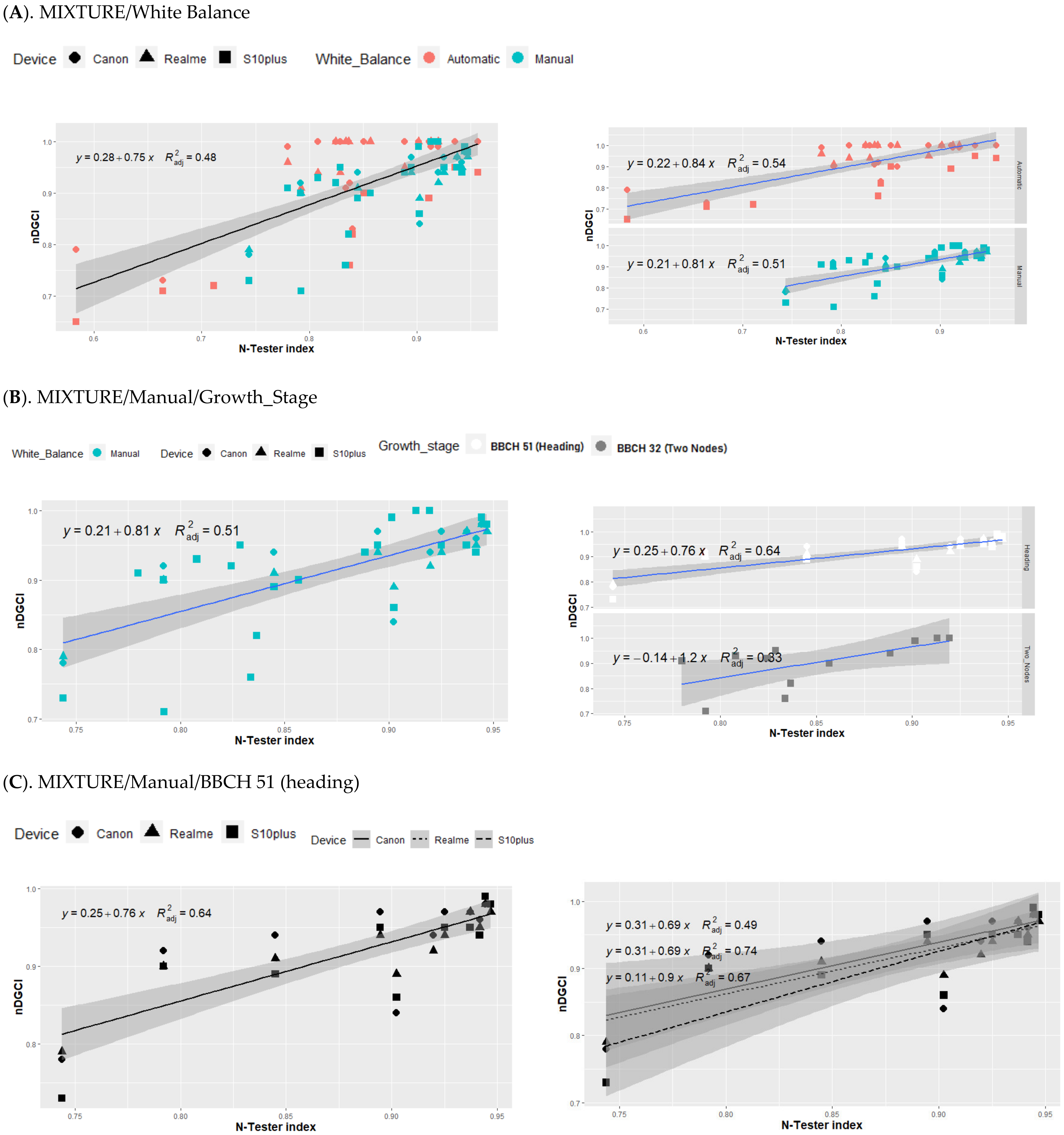
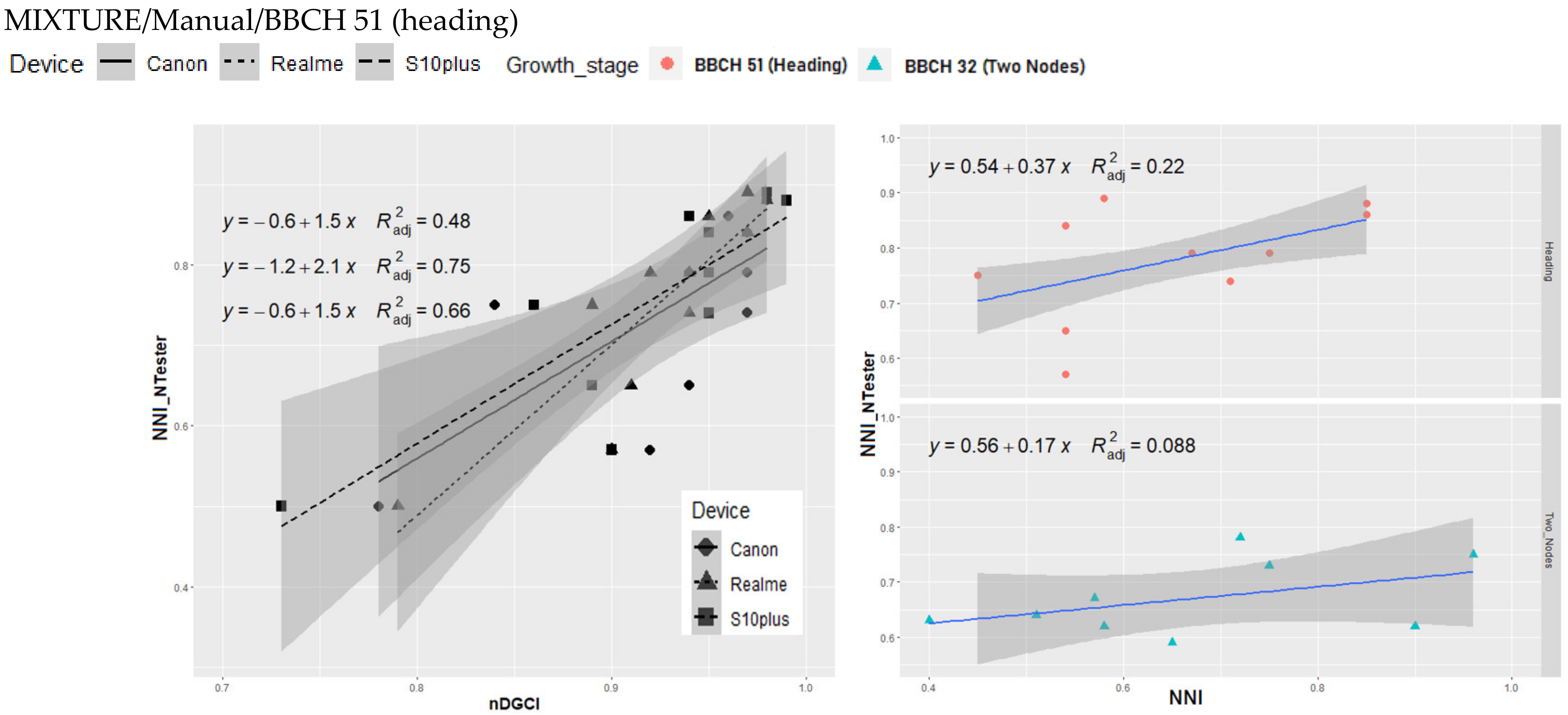
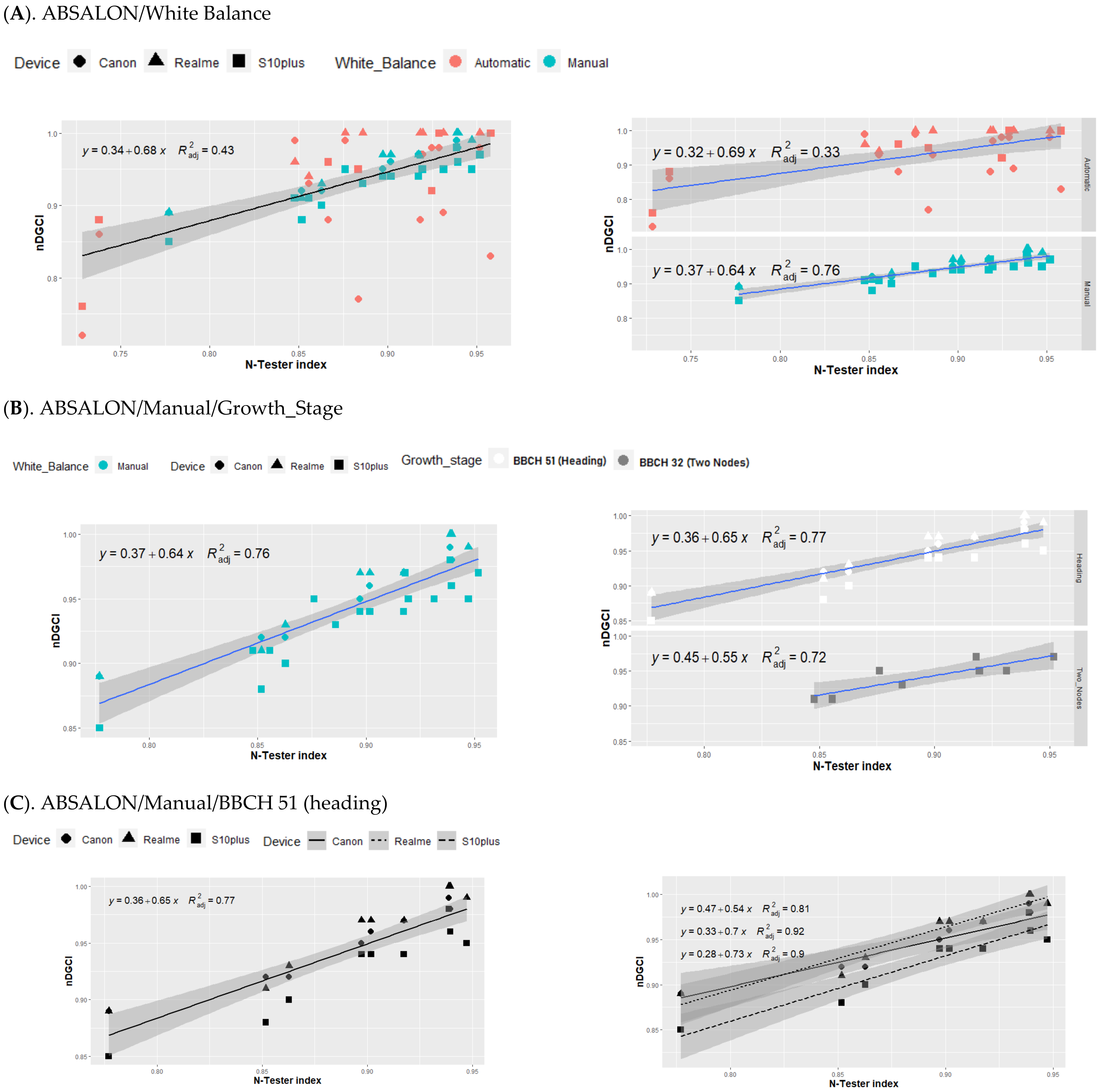
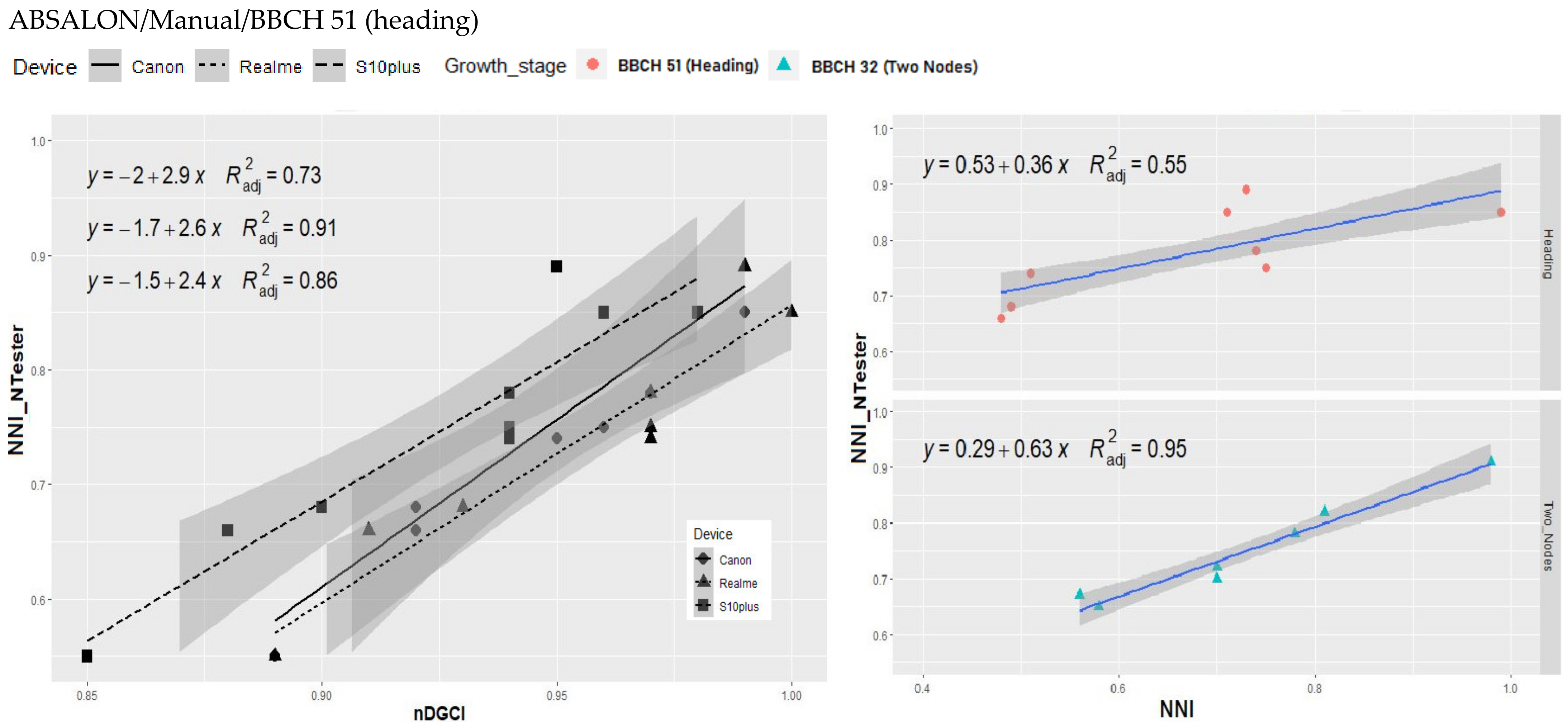
3.3. Relationship between nDGCI and Yara N-Tester
4. Discussion
Response of the nDGCI to Leaf Nitrogen Status
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Comifer. Calcul De La Fertilisation Azotée, Guide Méthodologique Pour L’établissement Des Prescriptions Locales (Calculation of Nitrogen Fertilisation, Methodological Guide for Establishing Local Requirements); Comifer: Paris, France, 2011. [Google Scholar]
- Hébert, J. La fumure azotée du blé tendre d’hiver (Nitrogen fertilizsation of soft winter wheat). Bull. Technol. Inform. Min. Agric. 1969, 224, 755–766. [Google Scholar]
- Ravier, C.; Jeuffroy, M.H.; Meynard, J.M. Mismatch between a science-based decision tool and its use: The case of the balance-sheet method for nitrogen fertilization in France. NJAS Wagening J. Life Sci. 2016, 79, 31–40. [Google Scholar] [CrossRef]
- Ravier, C.; Jeuffroy, M.H.; Gate, P.; Cohan, J.P.; Meynard, J.M. Combining user involvement with NNIovative design to develop a radical new method for managing N fertilization. Nutr. Cycl. Agroecosyst. 2018, 110, 117–134. [Google Scholar] [CrossRef]
- Lemaire, G.; Gastal, F.; Salette, J. Analysis of the effect of N nutrition on dry matter yield of a sward by reference to potential yield and optimum N content. In Proceedings of the 16th International Grassland Congress, Nice, France, 4–11 October 1989; pp. 179–180. [Google Scholar]
- Gastal, F.; Lemaire, G. N uptake and distribution in crops: An agronomical and ecophysiological perspective. J. Exp. Bot. 2002, 53, 789–799. [Google Scholar] [CrossRef]
- Lemaire, G.; Ciampitti, I. Crop mass and N status as prerequisite covariables for unravelling nitrogen use efficiency across genotype-by-environment-by-management scenarios: A Review. Plants 2020, 9, 1309. [Google Scholar] [CrossRef]
- Sharma, L.K.; Bali, S.K. A review of methods to improve nitrogen use efficiency in agriculture. Sustainability 2018, 10, 51. [Google Scholar] [CrossRef]
- Gastal, F.; Lemaire, G.; Durand, J.L.; Louarn, G. Chap. 8: Quantifying crop responses to nitrogen and avenues to improve nitrogen-use efficiency. In Crop Physiology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 161–206. [Google Scholar] [CrossRef]
- Justes, E.; Mary, B.; Meynard, J.M.; Machet, J.M.; Thelier-Huché, L. Determination of a critical nitrogen dilution curve for winter wheat crops. Ann. Bot. 1994, 74, 397–407. [Google Scholar] [CrossRef]
- Chen, P. A comparison of two approaches for estimating the wheat nitrogen nutrition index using remote sensing. Remote Sens. 2015, 7, 4527–4548. [Google Scholar] [CrossRef]
- Padilla, F.M.; Gallardo, M.; Pena-Fleitas, M.T.; De Souza, R.; Thompson, R. Proximal optical sensors for nitrogen management of vegetable crops: A review. Sensors 2018, 18, 2083. [Google Scholar] [CrossRef]
- Baret, F.; Fourty, T. Radiometric estimates of nitrogen status of leaves and canopies. In Diagnosis of the Nitrogen Status in Crops; Lemaire, G., Ed.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 201–227. [Google Scholar] [CrossRef]
- Haboudane, D.; Tremblay, N.; Miller, J.R. Remote Estimation of Crop Chlorophyll Content Using Spectral Indices Derived From Hyperspectral Data. IEEE Trans. Geosci. Remote Sens. 2008, 46, 423–437. [Google Scholar] [CrossRef]
- Samborski, S.M.; Gozdowski, D.; Walsh, O.S.; Lamb, D.W.; Stępień, M.; Gacek, E.S.; Drzazga, T. Winter Wheat Genotype Effect on Canopy Reflectance: Implications for Using NDVI for In-Season Nitrogen Topdressing Recommendations. J. Agron. 2015, 107, 2097–2106. [Google Scholar] [CrossRef]
- Debaeke, P.; Rouet, P.; Justes, E. Relationship between the normalized SPAD index and the nitrogen nutrition index: Application to Durum Wheat. J. Plant Nutr. 2006, 29, 75–92. [Google Scholar] [CrossRef]
- Bavec, F.; Bavec, M. Chlorophyll meter readings of winter wheat cultivars and grain yield prediction. Commun. Soil Sci. Plan. 2001, 32, 2709–2719. [Google Scholar] [CrossRef]
- Varvel, G.E.; Schepers, J.S.; Francis, D.D. Ability for In-Season Correction of Nitrogen Deficiency in Corn Using Chlorophyll Meters. Soil Sci. Soc. Am. J. 1997, 61, 1233–1239. [Google Scholar] [CrossRef]
- Blackmer, T.M.; Schepers, J.S. Use of a Chlorophyll Meter to Monitor Nitrogen Status and Schedule Fertigation for Corn. J. Prod. Agr. 1995, 8, 56–60. [Google Scholar] [CrossRef]
- Jinru, X.; Su, B. Significant Remote Sensing Vegetation Indices: A Review of Developments and Applications. J. Sens. 2017, 2017, 1353691. [Google Scholar] [CrossRef]
- Gabriel, J.L.; Zarco-Tejada, P.J.; Lopez-Herrera, P.J.; Perez-Martin, E.; Alonso-Ayuso, M.; Quemada, M. Airborne and ground level sensors for monitoring nitrogen status in a maize crop. Biosyst. Eng. 2017, 160, 124–133. [Google Scholar] [CrossRef]
- Saberioon, M.M.; Amin MS, M.; Gholizadeh, A.; Ezri, M.H. A Review of Optical Methods for Assessing Nitrogen Contents During Rice Growth. Appl. Eng. Agric. 2014, 30, 657–669. [Google Scholar] [CrossRef]
- Rouse, J.; Haas, R.; Schell, J.; Deering, D. Monitoring vegetation systems in the Great Plains with ERTS. In Third Earth Resources Technology Satellite-1 Symposium—Volume I: Technical Presentations; NASA: Washington, DC, USA, 1974; p. 309. [Google Scholar]
- Huete, A.R. A Soil—Adjusted Vegetation Index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Ghozlen, N.B.; Milhade, C.; Obert, M.; Debuisson, S.; Le Moigne, M. Nondestructive diagnostic test for nitrogen nutrition of grapevine (Vitis vinifera L.) based on dualex leaf-clip measurements in the field. J. Agric. Food Chem. 2015, 63, 3669–3680. [Google Scholar] [CrossRef]
- Woebbecke, D.M.; Meyer, G.E.; Von Bargen, K.; Mortensen, D.A. Color indices for weed identification under various soil, residue, and lighting conditions. Trans. ASAE 1995, 38, 259–269. [Google Scholar] [CrossRef]
- Hunt, E.; Daughtry, C.; Eitel, J.; Long, D. Remote Sensing Leaf Chlorophyll Content Using a Visible Band Index. Agron. J. 2011, 103, 1090–1099. [Google Scholar] [CrossRef]
- Vollmann, J.; Walter, H.; Sato, T.; Schweiger, P. Digital image analysis and chlorophyll metering for phenotyping the effects of nodulation in soybean. Comput. Electron. Agric. 2011, 75, 190–195. [Google Scholar] [CrossRef]
- Rigon, J.; Silvia Capuani, S.; Fernandes, D.; Guimarães, T. A novel method for the estimation of soybean chlorophyll content using a smartphone and image analysis. Photosynthetica 2016, 54, 559–566. [Google Scholar] [CrossRef]
- Karcher, D.E.; Richardson, M.D. Quantifying turfgrass color using digital image analysis. Crop Sci. 2003, 43, 943–951. [Google Scholar] [CrossRef]
- Hassanijalilian, O.; Igathinathane, C.; Doetkott, C.; Bajwa, S.; Nowatzki, J.; Esmaeili, S.A.J. Chlorophyll estimation in soybean leaves infield with smartphone digital imaging and machine learning. Comput. Electron. Agric. 2020, 174, 105433. [Google Scholar] [CrossRef]
- Rorie, R.L.; Purcell, L.C.; Mozaffari, M.; Karcher, D.E.; King, C.A.; Marsh, M.C.; Longer, D.E. Association of “greenness” in corn with yield and leaf nitrogen concentration. Agron. J. 2011, 103, 529–535. [Google Scholar] [CrossRef]
- Rorie, R.L.; Purcell, L.C.; Karcher, D.E.; King, C.A. The assessment of leaf nitrogen in corn from digital images. Crop Sci. 2011, 51, 2174–2180. [Google Scholar] [CrossRef]
- Rhezali, A.; Lahlali, R. Nitrogen (N) Mineral Nutrition and Imaging Sensors for Determining N Status and Requirements of Maize. J. Imaging 2017, 3, 51. [Google Scholar] [CrossRef]
- Furuya, S. Growth diagnosis of rice plants by means of leaf colour. Jpn. Agric. Res. Q. 1987, 20, 147–153. [Google Scholar]
- Singh, B.; Singh, Y.; Ladha, J.K.; Bronson, K.F.; Balasubramanian, V.; Singh, J.; Khind, C.S. Chlorophyll meter-and leaf colour chart-based nitrogen management for rice and wheat in Northwestern India. Agron. J. 2002, 94, 821–829. [Google Scholar] [CrossRef]
- Yang, W.H.; Peng, S.; Huang, J.; Sanico, A.L.; Bures, R.J.; Witt, C. Using leaf colour charts to estimate leaf nitrogen status of rice. Agron. J. 2003, 95, 212–217. [Google Scholar] [CrossRef]
- Petiole Pro. Available online: https://www.petiolepro.com/blog/dark-green-colour-index-dgci-a-new-measurement-of-chlorophyll/ (accessed on 20 March 2023).
- Bacenetti, J.; Paleari, L.; Tartarini, S.; Vesely, F.M.; Foi, M.; Movedi, E.; Ravasi, R.A.; Bellopede, V.; Durello, S.; Ceravolo, C.; et al. May smart technologies reduce the environmental impact of nitrogen fertilization? A case study for paddy rice. Sci. Total Environ. 2020, 715, 136956. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, F.; Qian, X.; Zhu, Y.; Shen, G. Quantification of rice canopy nitrogen balance index with digital imagery from unmanned aerial vehicle. Remote Sens. Lett. 2015, 6, 183–189. [Google Scholar] [CrossRef]
- Saberioon, M.M.; Amin, M.S.M.; Anuar, A.R.; Gholizadeh, A.; Wayayok, A.; Khairunniza-Bejo, S. Assessment of rice leaf chlorophyll content using visible bands at different growth stages at both the leaf and canopy scale. Int. J. Appl. Earth Obs. Geoinf. 2014, 32, 35–45. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono-and Dicotyledonous Plants. In BBCH Monograph, 2nd ed.; Federal Biological Research Centre for Agriculture and Forestry: Quedlinburg, Germany, 2001; p. 158. [Google Scholar]
- YARA, Nanterre, France. N-Tester, Mode D’emploi. Blé, Orge de Printemps, Pomme de Terre, Maïs. (N-Tester, Instructions for use. Wheat, Spring Barley, Potato, Maize). Available online: https://www.yara.fr/contentassets/89e0f28207c14d8e84aceae75cfc1437/n-tester-manuel-mais.pdf/ (accessed on 20 March 2023).
- Prost, L.; Jeuffroy, M.H. Replacing the nitrogen nutrition index by the chlorophyll meter to assess wheat N status. Agron. Sustain. Dev. 2007, 27, 321–330. [Google Scholar] [CrossRef]
- Aranguren, M.; Castellon, A.; Aizpurua, A. Crop sensor based non-destructive estimation of nitrogen nutritional status, yield, and grain protein content in wheat. Agriculture 2020, 10, 148. [Google Scholar] [CrossRef]
- Gée, C.; Denimal, E. Two new non-destructive image-derived indicators for spatial assessment of the impact of broadleaf weeds on wheat biomass. Remote Sens. 2020, 12, 2982. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org (accessed on 20 March 2023).
- RStudio Team. RStudio: Integrated Development Environment for R. Posit Team; Posit Software, PBC: Boston, MA, USA, 2022; Available online: http://www.posit.co/ (accessed on 20 March 2023).
- Vouillot, M.O.; Huet, P.; Boissard, P. Early detection of N deficiency in a wheat crop using physiological and radiometric methods. Agronomie 1998, 18, 117–130. [Google Scholar] [CrossRef]

| N Fertilizer Inputs through 50 kg N.ha−1 Dose | 14 February | 7 March | 28 March | 18 April | Total | |
|---|---|---|---|---|---|---|
| T0 | 0 dose | 0 | 0 | 0 | 0 | 0 |
| T1 | 1 dose | 0 | 50 | 0 | 0 | 50 |
| T2 | 2 doses | 0 | 50 | 50 | 0 | 100 |
| T3 | 3 doses | 0 | 50 | 50 | 50 | 150 |
| TMax | Over-N fertilization | 80 | 80 | 80 | 80 | 320 |
| Growth Stage | Date | Camera Type | Camera Set-Up | Datset Number of Images | Cultivar | |
|---|---|---|---|---|---|---|
| White Balance | ||||||
| BBCH 32 (two-nodes) | 22nd April | Canon | Automatic | 16 | 32 | LG Abs (7)/Mixture (9) |
| Samsung | Automatic | 16 | LG Abs (7)/Mixture (9) | |||
| 3rd May | Canon | Automatic | 20 | 60 | LG Abs (8)/Mixture (12) | |
| Realme | Automatic | 20 | LG Abs (8)/Mixture (12) | |||
| Samsung | Manual | 20 | LG Abs (8)/Mixture (12) | |||
| BBCH 51 (heading) | 17th May | Canon | Manual | 20 | 59 | LG Abs (9)/Mixture (11) |
| Realme | Manual | 20 | LG Abs (9)/Mixture (11) | |||
| Samsung | Manual | 19 | LG Abs (9)/Mixture (10) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gée, C.; Denimal, E.; de Yparraguirre, M.; Dujourdy, L.; Voisin, A.-S. Assessment of Nitrogen Nutrition Index of Winter Wheat Canopy from Visible Images for a Dynamic Monitoring of N Requirements. Remote Sens. 2023, 15, 2510. https://doi.org/10.3390/rs15102510
Gée C, Denimal E, de Yparraguirre M, Dujourdy L, Voisin A-S. Assessment of Nitrogen Nutrition Index of Winter Wheat Canopy from Visible Images for a Dynamic Monitoring of N Requirements. Remote Sensing. 2023; 15(10):2510. https://doi.org/10.3390/rs15102510
Chicago/Turabian StyleGée, Christelle, Emmanuel Denimal, Maël de Yparraguirre, Laurence Dujourdy, and Anne-Sophie Voisin. 2023. "Assessment of Nitrogen Nutrition Index of Winter Wheat Canopy from Visible Images for a Dynamic Monitoring of N Requirements" Remote Sensing 15, no. 10: 2510. https://doi.org/10.3390/rs15102510
APA StyleGée, C., Denimal, E., de Yparraguirre, M., Dujourdy, L., & Voisin, A.-S. (2023). Assessment of Nitrogen Nutrition Index of Winter Wheat Canopy from Visible Images for a Dynamic Monitoring of N Requirements. Remote Sensing, 15(10), 2510. https://doi.org/10.3390/rs15102510






