Abstract
The increasing extreme weather and climate events have a significant impact on the resistance and resilience of Norway spruce trees. The responses and adaptation of individual trees to certain factors can be assessed through the tree breeding programmes. Tree breeding programmes combined with multispectral unmanned aircraft vehicle (UAV) platforms may assist in acquiring regular information of individual traits from large areas of progeny trials. Therefore, the aim of this study was to investigate the vegetation indices (VI) to detect the early stages of tree stress in Norway spruce stands under prolonged drought and summer heatwave. Eight plots within four stands throughout the vegetation season of 2021 were monitored by assessing spectral differences of tree health classes (Healthy, Crown damage, New crown damage, Dead trees, Stem damage, Root rot). From all tested VI, our models showed a moderate marginal R2 and total explanatory power—for Normalized Difference Red-edge Index (NDRE), marginal R2 was 0.26, and conditional R2 was 0.49 (p < 0.001); for Normalized Difference Vegetation Index (NDVI), marginal R2 was 0.34, and conditional R2 was 0.60 (p < 0.001); for Red Green Index (RGI), marginal R2 was 0.36, and conditional R2 was 0.55 (p < 0.001); while for Chlorophyll Index (CI), marginal R2 was 0.27, and conditional R2 was 0.49 (p < 0.001). The reliability of the identification of tree health classes for selected VI was weak to fair (overall classification accuracy ranged from 34.4% to 56.8%, kappa coefficients ranged from 0.09 to 0.34) if six classes were assessed, and moderate to substantial (overall classification accuracy ranged from 71.1% to 89.6% and kappa coefficient from 0.39 to 0.71) if two classes (Crown damage and Healthy trees) were tested.
1. Introduction
Climate change has enormous effects on various environments, including forests, and has already amplified the economic and ecological impacts caused by damages of biotic and abiotic factors [1]. The vulnerability to climate change is expected to become more challenging for forest management and nature conservation [2]. These factors are particularly important for coniferous tree species, such as Norway spruce (Picea abies (L.) Karst.), whose regeneration on nutrient rich soils or in eutrophic forests is possible only by planting. For example, more than 90% of all young Norway spruce stands on fertile rich forest soils in Latvia have been planted [3], and in Sweden planted spruce makes up more than 40% of the total growing stock [4]. In addition, in many countries spruce is artificially regenerated as pure stands. Spruce monoculture stands are very productive [5] but also are highly susceptible to damaging agents, both biotic and abiotic, including pathogen and insect infestations, wind, droughts during vegetation season, etc. [6]. Moreover, among extreme climatic events, droughts are projected to increase in frequency and severity [7], having a significant impact on the resistance and resilience of individual trees [8].
Previous studies [9,10] show that the resistance to certain factors can be increased with the help of tree breeding studies. However, studies about resilience can include field sampling methods that are very time-consuming, expensive and labour-intensive, because of the large area of progeny trials, and assessment of individual traits requires regular monitoring of frequently repeated measurements [11,12]. Therefore, effective surveillance procedures on tree growth are essential in reaching the defined breeding goals [13].
Rapid advances in technology and increasing affordability over the last decades have promoted the use of remote sensing methods for monitoring vegetation and ecosystems [14,15,16]. Traditionally, remote sensing methods have been extensively used to study forests at various spatial and temporal resolutions based on the data acquired from satellites or aircrafts [17]. However, the use of these data at regional or local levels has been limited due to relatively high acquisition costs and return intervals [18]. One alternative to such platforms is unmanned aircraft vehicle (UAV) platforms [19], which complement the established remote sensing methods and have been proven to provide very accurate forest health observations at stand and even species level [20,21].
UAV-based applications are widely applied in forest research for non-invasive mapping of forest disturbances and for monitoring the dynamics of forest disturbances [22]. Furthermore, when equipped with appropriate (multispectral or hyperspectral) sensors, potentially reliable information about the current plant physiology (linked to the pigments content of leaves (chlorophyll, carotenoid and anthocyanin)) in different electromagnetic spectrums can be obtained in a cost-effective and timely manner [23]. This is particularly important because the initial attack of pests or pathogens is not visible to the human eye [24], while timely removal of infested trees at early stages of the disturbance may protect unaffected trees nearby [25]. Canopy colour is a good indicator to determine the level of damage and is commonly used to detect single-tree diseases [26,27,28,29]. When the duration of plant stress exceeds a critical threshold, a different biochemical and morphological component may change the reflectance spectrum of foliage as adaptation strategies [30,31]. That happens mainly due to the loss of chlorophyll (Chl), which strongly affects the absorption of photosynthetically active radiation [26]. Most often, the reduction of photosynthesis (stomata closure) is a commonly observed response of plants to water stress, as drought results in changes in leaf biochemical properties that may change spectral absorbance properties of leaves [32]. The duration of the drought period determines the impact of water stress and the recovery of a plant when irrigated [33]. The weakened trees may revive and develop without any symptoms visible to the human eye; however, they may exhibit stress impact in the near infrared regions of the electromagnetic spectrum [34,35]. In recent studies, the impact of drought on the health of vegetation has been widely studied by using multispectral remote sensing imagery acquired by the UAV platforms [18]. Nevertheless, high spatial resolution of single spectral bands may contain interfering factors, like atmospheric effects or undesirable background noise, thereby presenting challenges in processing of the data or even leading to misinterpretations of the spectral characteristics of foliage. Therefore, to avoid the impact of interfering factors, the reflectance of foliage can be analysed by using vegetation indices. Vegetation indices are calculated as ratios of two or more spectral bands and are a very effective approach in the early detection of plant stress [36,37,38].
Effective surveillance procedures are essential to detect, quantify and help mitigate the impact of these damaging biotic and abiotic factors. In recent years, significant progress has been made in the detection of early signs of tree stress in forest areas, mostly due to the relatively simplified availability and implications of remote sensing techniques compared to traditional forestry techniques. Consequently, the interest from researchers has also increased. However, there is limited empirical evidence about the early stages of health-related stress of Norway spruce. Therefore, the aim of this study was to investigate the vegetation indices to detect the early stages of tree stress in Norway spruce stands under a prolonged period of drought and summer heatwave. A decision-tree classifier, such as the Random Forest classifier, was also used on UAV images to segment tree health status and provide predictions of vegetation health in forested landscapes. In this study, Norway spruce stands were monitored at the beginning of vegetation period, after a pre-longed period of drought and summer heatwave and at the end of the vegetation period and were analyzed using different tree vitality groups.
2. Materials and Methods
2.1. Study Area
Four pure stands of Norway spruce in the Forest Research station Kalsnava in the eastern part of Latvia (56°41′3″ N, 25°50′28″ E) were selected for the research (Figure 1). The area is characterized by relatively flat terrain with Scots pine (Pinus sylvestris), Norway spruce- and silver birch (Betula pendula)-dominated forest stands. The study area is around 100 m above sea level. The site has a moderately continental climate (the total annual precipitation is about 700 mm; the mean annual temperature is around 5 °C). The mean length of the vegetation period is around 175 days, which usually extends from late April to October. According to the data of the local weather station (~5 km from the study area), the weather conditions (the distribution of precipitation and temperatures) in the vegetation season of 2021 were not typical of those previously observed. The majority of precipitation was recorded at the beginning of the vegetation period in the first six days of May, followed by 41 days without rain and with relatively high temperatures (15.4 °C mean daily temperature with 33.5 °C maximum mean (hour) temperature). The selected stands were similar by age and growing conditions. Stand 1 was 37 years old. Stand 2 and Stand 3 were 26 years old; these stands were located on drained mineral soil (Myrtillosa mel.). Stand 4 was 26 years old and was located on drained peat soil (forest type Myrtillosa turf. mel.). At the beginning of this study, during the first image acquisitions, we observed an intensive spruce flowering in Stand 1, whereas in the other stands the flowering could be rated as average. The ditches alongside all studied stands were recently cleaned.
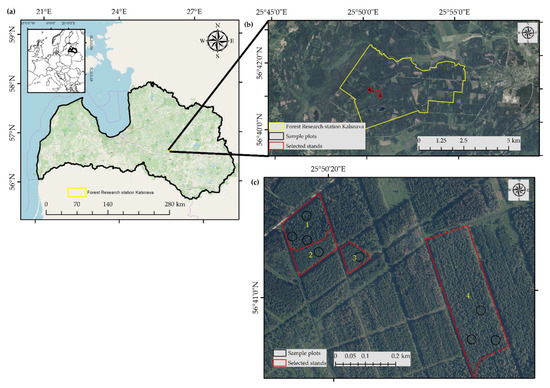
Figure 1.
Location of the study sites in Latvia. (a) The selected sites displayed on a map of Latvia (OpenStreetMap in the background); (b,c) Maps of the locations of the studied Norway spruce stands in the Research forests of Kalsnava; Orthophoto of Latvia in the background [39].
2.2. Field Sampling
The field sampling was conducted in eight round sample plots (500 m2, radius = 12.62 m) in May 2021 (after the first flight campaign). All plots were distributed across stands, ensuring that each plot contained dead trees or trees with visible crown damages, and the distance between the plot centers was at least 26 m. The coordinates of plot centers were measured with the Leica GS16 Global Navigation Satellite System (GNSS) receiver. Precise coordinates of each individual tree within the sample plot were recorded by using a Leica TS06 total station. The total station was positioned in the plot center and pulsed a laser beam to each stem within the plot at 1.3 m height. We excluded smaller trees if they were suppressed by adjacent trees and/or if they were in the shadow of the larger trees. Overall, 800 trees were measured, from which 239 trees were cored.
In each plot, for each tree, the diameter at breast height was measured and tree crown vitality status and types of damage (e.g., ungulate damage, stem, or crown damage (e.g., loss of treetop), and other) were recorded. The vitality of crowns was visually assessed for decolorization and needle density from the ground and divided into three groups—visually healthy tree (individuals with no outward signs of stresses (e.g., drought induced decline), trees with noticeable damage to crown (e.g., yellowish green, yellow needles and brownish treetops) and dead trees (complete loss of green foliage). Each tree was assessed from different angles, and to reduce the bias associated with assessment subjectivity, the same person performed all assessments. The tree age was detected as follows: within each plot, increment cores were taken during the field campaign from 30 trees selected based on the mean NDRE index values for tree crowns after the first flight campaign, (10 trees with the lowest values of NDRE index, 10 trees with medium NDRE index values and 10 trees with the highest NDRE index values). In addition, increment cores from trees with possible root rot infection were also taken (based on visual inspection) Tree increment cores were obtained at breast height (1.3 m) with a Pressler increment borer. The increment cores were processed and measured in the laboratory. Increment cores were air dried and grinded using sandpaper, and a LINTAB 5 (RinnTECH, Heidelberg, Germany) measurement system was used to measure tree ring widths with a precision of 0.01 mm. The second field campaign was performed in October 2021 when we updated the tree crown vitality status and/or other damages.
2.3. Data Acquisition
The weather conditions were mostly sunny and windless during the flights; to minimize shadows, the flights were conducted in mornings before noon when the lighting conditions were the best. The study area was overflown using a DJI Matrice 210 drone equipped with SlantRange 3PX (SlantRange, San Diego, CA, USA) multispectral sensor, which is composed of single-band cameras (Green, Red, Red Edge and NIR) at 1.2-megapixel (1248 × 994 pixels) resolution (Table 1). The camera was equipped with an ambient light (weather and position of sun) sensor to adjust illumination conditions for each frame, an integrated global positioning system (GPS) and an inertial measurement unit (IMU) system with extended Kalman filter. The flight altitude was set to 75 m above ground (174 m above sea level) with 5.0 ± 1 m s−1 ground speed, with a 70% side and 80% frontal overlap for the images. The ground sampling distance or spatial resolution for each pixel was 2.25 cm. UAV image acquisition took place during the vegetation period of 2021 in three flight campaigns—first on 7 May 2021, second in the middle of summer after a prolonged period of drought on 10 July 2021 and at the end of vegetation season on 19 October 2021. Along the edges of the stands and in the openings of stands (if any), at least five ground control points (GCP) were distributed within each stand prior to each flight. The LKS 92 (EPGS:3059) coordinates of GCP were measured by a geodetic global navigation satellite system (GNSS) receiver Leica GS16 with a real-time kinematic (RTK) correction with a position accuracy of 1–2 cm.

Table 1.
Spectral resolution of the SlantRange 3PX sensor used in the study.
2.4. Photogrammetric Processing
The acquired raw images were processed using SlantView software (SlantRange, Inc., San Diego, CA, USA). To derive quantitative information, the pixels were converted from false colours to reflectance values. Each image plane that did not fall within the field of view of all sensors were trimmed to a valid content and exported to the Agisoft Metashape Professional (v. 1.6.4.). The Structure from Motion (SfM) photogrammetric method was implemented for orthophoto production [40]. The green spectrum to align photos, build a dense cloud and generate a digital surface model (DSM) with interpolation and orthomosaics was set as the default band. The GCPs were used to optimize the sparse cloud and to transform image orientation into geodetic coordinate system LKS 92 (EPGS:3059). A 4-band multispectral orthomosaics was created and resampled to pixel size 10 × 10 cm with the nearest-neighbour technique, as studies [41] have shown it can reduce image noise and number of pixels at the outcome.
2.5. Individual Tree Crown Masks
There are many methods and software solutions for the detection and segmentation of tree crowns [41]. Even though they are suitable for a variety of forest scenarios, most of them are formed from the data obtained in seed orchards, and when implemented in dense forest stands it is problematic to automatically separate adjacent crowns. Therefore, we combined automated separation of high vegetation from other objects in the image by using the multivariate toolset in the ArcGIS 10.5 [42] and manually adjusted it to separate individual crowns from neighbouring trees.
Initially, the Isodata segmentation technique with two tools in ArcGIS 10.5. (Iso Cluster and Maximum Likelihood Classification) was performed. With the Iso Cluster tool, we created two groups—canopy and ground. The resulting signature file was further processed as an input for the Maximum Likelihood Classification. Accordingly, from the created classified raster, the canopy class was converted into vector-based polygons. However, some treetops needed to be manually separated if a large polygon for certain group of adjacent trees were drawn (see in example in Figure 2). Single polygons were created, and ground survey data were combined based on spatial location. These polygons were used as masks to extract the cells of orthomosaics. The extracted cells as a raster file were converted into a vector-based point feature class. Finally, we extracted pixel values (reflectance values of each band) in a point feature to the attribute table, which was exported as a txt file for calculations of vegetation indices and for further statistical analysis.
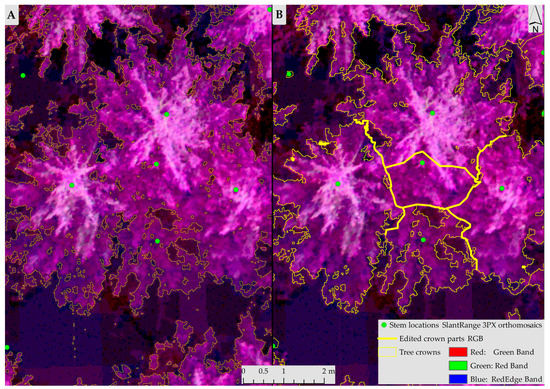
Figure 2.
Detection and delimitation of individual tree crowns from the Isodata segmentation technique (A) Automatic crown classification; (B) Crowns after manual separation and deletion of features (bold yellow represents edited parts).
2.6. Vegetation Indices and Statistical Analysis
The relationship between tree health status and the detected reflectance was characterized by using various vegetation indices. The selection of vegetation indices was based on previous studies [22,43,44,45] and upon the configuration of available sensors. Thus, in this study, the metrics that are based on the greenness and leaf pigment (chlorophyll, anthocyanin) concentrations were used to indicate the early signals of stress and/or level of tree health (Table 2).

Table 2.
Indices and formula for calculation.
To evaluate the early detection of tree stress we observed temporal changes in vegetation indices by using the linear mixed-effect model (LME). The LME, also known as the variance component model, is a statistical method that is widely used to model dependent data structures, such as clustered data and longitudinal data [55]. The LME incorporates two parameters: the fixed effects and random effects [56]. The fixed effects have a common linear relationship for all the data, whereas the random effects can be used to account for the structure of the data [56]. The statistical measures were used to quantify the separability between tree health classes over one vegetation season in four Norway spruce stands. For this purpose, we classified trees into six health classes (Healthy, Crown damage, New crown damage, Dead trees, Stem damage, Root rot) based on our recordings during the field campaigns (Table 3, Figure 3). In the situations when for a single tree two or more classes were possible to be assigned, we preferred Stem damage over Crown damage and Root rot over Stem damage.

Table 3.
Description of allocated tree health classes.
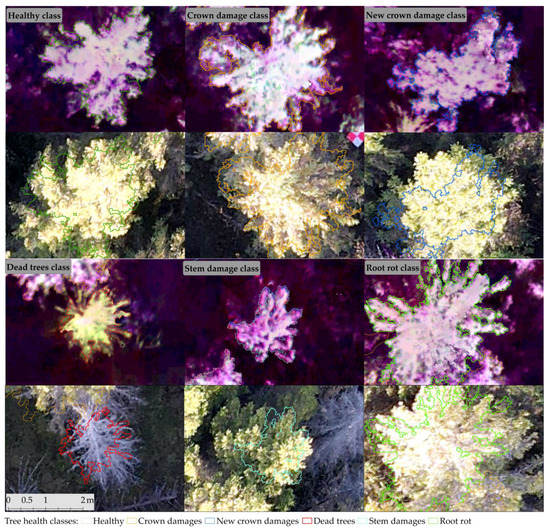
Figure 3.
Examples of the SlantRange 3PX orthomosaics (top row) for classified tree health classes and RGB images captured by DJI Mavic air 2 (bottom row) used to update the tree health status.
In the models, the means of vegetation indices for each crown were used as dependent variables, but tree health class, flight campaign and the interaction of both factors were used as independent variables. The stand was used as a random factor to deal with possible pseudo replication. We fitted a linear mixed-effect model by using maximum likelihood estimation by optimization through the functions “nlminb”. The root mean square error (RMSE) was used to evaluate the predictive accuracy of the models. To quantify the ability of each model to explain observed variation in the response variable, we calculated marginal and conditional R2 values [57], where marginal R2 variance was explained by fixed effects (vegetation indices), and conditional R2 variance was explained by the entire model. The Akaike information criterion (AIC) was calculated to compare and rank the proposed models by different combination of factors [58,59]. The package “lsmeans” [60] was used to calculate the predicted means for tree health classes. The Kenward–Roger approximation was used to estimate the degrees of freedom, and a 95% confidence interval was recorded. The calculation of vegetation indices and the linear mixed-effects models were computed in the R software 3.6.3 [61].
2.7. Validation and Forest Mapping
The classification of vegetation indices (selected basing on the outcome of the “lmer” models) was performed by applying the Random Forest (RF) algorithm, which is a supervised machine-learning technique. RF is a method based on inductive decision trees and can effectively handle high-dimensional, noisy and multi-source datasets without over-fitting [62,63]. The main features of RF include speed and flexibility in creating the relationship between input and output functions [62]. The choice of the classifier was based on the simplicity of the model, as RF allows classification of multiple variables and classes without the need of sophisticated models or parameters [62], and it was proved to be a promising method in estimating tree parameters [64]. RF models were constructed, trained and cross-validated using “randomForest” and “caret” R packages [65,66]. The pixels containing vegetation index values belonging to the tree health classes were classified separately for each stand and flight campaign. We created a balanced training set where 75% of all data was randomly assigned to the training dataset and 25% of all data was used for testing. Due to the fact that Stand 2 and Stand 3 each had only one sample plot, and as the two stands were the same age and located nearby, the data sets of these stands were combined for classification purposes. The model fitting was performed by the default resampling implementation and parameter selection, which provided a quantity of 500 decision trees. We generated confusion matrices along with the Gini index criterion, the out-of-bag (OOB) estimated error rate, overall classification accuracy, Cohen’s kappa coefficient and multi-dimensional scaling (MDS) using the “caret” package in R 3.6.3 [61].
3. Results and Discussion
3.1. Spectral Reflectance of Vegetation Health and Monitoring of One Vegetation Season
The seasonal patterns of 2021 for different tree health classes have been assessed by using different vegetation indices related to greenness (chlorophyll concentration)—Normalized Difference Red-edge Index (NDRE), Normalized Difference Vegetation Index (NDVI), Chlorophyll Index (CI)—and to leaf pigment (anthocyanins)—Red Green Index (RGI). The reflectance of light from Norway spruce needles can change over the growth season, especially during the active period of growth, due to various physiological processes (e.g., canopy chlorophyll content and plant characteristics (green biomass and leaf water content)) [67,68], which makes it more difficult to distinguish between tree health classes. However, according to results of linear mixed-effect models for all selected indices, we found that tree health class, flight campaign and interaction of both factors had a significant effect (p < 0.001) on the means of vegetation indices of individual tree crowns. The random effect (Stand) of the models explained 29 to 45% of the variance in selected vegetation indices values for different tree health classes (Table 4). Our models showed a moderate marginal R2 and total explanatory power—for the NDRE index, the marginal R2 was 0.26 and the conditional R2 was 0.49 (p < 0.001); for the NDVI index, the marginal R2 was 0.34 and conditional R2 was 0.60 (p < 0.001); for the RGI index, the marginal R2 was 0.36 and conditional R2 was 0.55 (p < 0.001); while for the CI index, the marginal R2 was 0.27 and conditional R2 was 0.49 (p < 0.001) (Figure 4, Table 4). These results are lower than in other studies for detecting stress-induced changes [69,70]. Mostly, studies with good model prediction power are based on lower resolution imagery (approx. 1.25–2.4 m). In our study, a very detailed imagery (0.10 m) was used, but the drawback of such data is the high noise, which can occur from the background (due to the conical form of spruce crown), and/or partial crown damage, which had a negative effect on the outcome of the models.

Table 4.
Summary of results of LMER models.
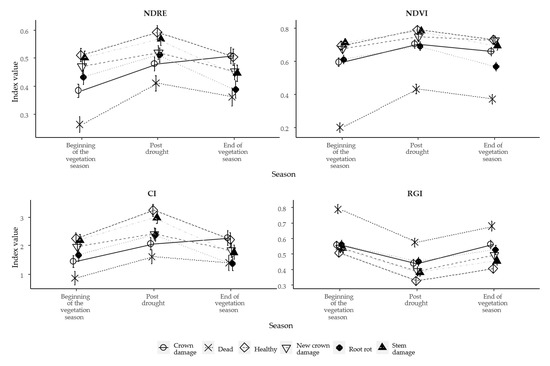
Figure 4.
The mean values of selected vegetation indices and tree health classes over vegetation season.
However, we found that each of these indices had different levels of sensitivity in distinguishing certain tree health classes. The only exception was class Dead, which could be easily distinguished by all indices. This was mostly because of the different levels of leaf pigments over the vegetation season [71]. For example, such high variability of chlorophyll content in needles could be caused by needle aging, as chlorophyll content for needles of the current year is lower and it increases until the middle of summer and stays relatively constant until the end of the vegetation season, in contrast to older needles, which have higher and more stable chlorophyll content [72,73]. In our study, we also found that the NDRE, NDVI and CI indices were differentiated between Healthy trees and Damaged trees already from the beginning of the vegetation season. However, the results of the RGI index, which is sensitive to the anthocyanin pigment content in plant leaves, did not show significant differences between healthy and damaged trees at the beginning of the vegetation season. The RGI index was able to differentiate Root rot class from other classes, indicating significant (p < 0.05) differences. But there are some other limits in the levels of sensitivity of the spectral bands used in calculation of these indices. Moreover, it is possible that the values of vegetation indices in our studied stands at the beginning of the vegetation season were reduced due to the active flowering and pollination of spruces (Figure 5); as a result, trees were covered in pollen, which might have decreased the levels of green vegetation (at least detectable with the sensor), which in turn can increase the reflected visible light in the red spectrum. This could have affected the quality of data, as the reflectance in red and the red-edge spectrum is very sensitive to changes in chlorophyll content [28,74]. However, we found that trees from the New crown damage class, which initially were visually assessed as healthy trees, with no sign of any damages, were possible to be separated from Healthy tree class already at the beginning of the vegetation season by using the NDRE (p < 0.05) and NDVI (p < 0.01) indices, while RGI (p = 0.76) and CI (p = 0.76) did not show any separation. These results suggest that monitoring of Norway spruces with these indices might provide fairly good information on early onset of stress expression.

Figure 5.
(A) Example of tree crowns full of cones and (B) example of spruce flowering in spring.
We noticed that the season of image acquisition was found to have a more relevant impact on the separation of tree health classes. The analysis of the spectral indices showed that in the middle of summer, which coincided with a prolonged period of drought, the values of all indices were significantly (p < 0.001) increased for all chlorophyll-based indices compared to the beginning of the vegetation season. At the same time, we examined the relative differences between the tree health classes, and greater differences between healthy trees and other tree health classes were determined. Our results suggested that the drought impact was more pronounced for trees that were already stressed, and we noticed an increase in relative differences between the Healthy tree class and other classes in the post-drought images compared to what was detected at the beginning of the vegetation season. For example, at the beginning of the vegetation season, the CI index value for Stem damage class was 3% lower than for the Healthy trees, but after the drought period, the difference increased to 8%. An even more pronounced decrease in relative differences was obtained between New crown damages and Healthy trees; for example, the values of CI index for New damage classes were 13% lower than for Healthy trees at the beginning of the vegetation season. However, this index increased to 26% after the drought (Figure 6). There could be several reasons for such differences; however, the most plausible explanation could be linked to the exceptionally dry and hot June of 2021, which could have weakened the spruces, since Norway spruce is very sensitive to the changes in water availability and to high temperatures [75]. This conforms with the well-known fact that trees with any damage, such as root damage [76] or stem damages [77] may have lower resilience to disturbances, such as drought. It is related to the physiological processes of a tree: when the hydraulic conductivity in a tree is reduced [76], it consequently leads to a decrease in photosynthesis [78], and eventual changes in the tree crown can take place as a result of water stress.
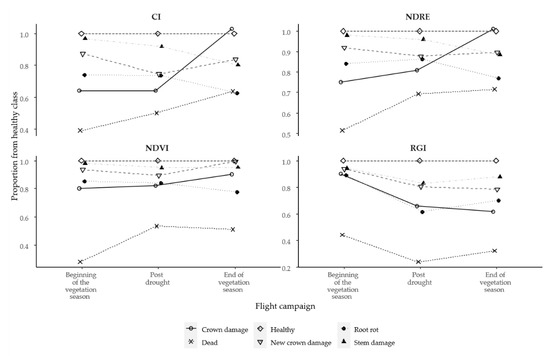
Figure 6.
The relationships between vegetation indices and tree health classes; differences are shown as a relative difference to Healthy trees.
Although the effect of drought continued to be unclear at the end of the vegetation season, there was evidence that trees have begun to adapt to the disturbance. We observed that the relative differences for the Healthy and Crown damage class pairing and also between the Healthy and New crown damage class were reduced, whereas the difference between Healthy and other classes remained at the previous level. The exception was the RGI index, which showed a continuous drop for all classes if looked at as a relative difference to Healthy trees (Figure 6). A distinct variation in relative differences in values for Crown damage class might be related to other factors that might have affected the quality of the captured images, especially in the NIR spectral band. Namely, in the acquired images at the end of the vegetation season, we found that treetops of Crown damage class were somewhat illuminated, which was reflected in the relatively high values of indices for Crown damage class in comparison to other classes. There might be various factors causing such noise in the data, such as sun-angle dependency, the side-effect of lower needle water content and, as well, higher canopy transmittance, resulting in higher reflectance value at the end of the vegetation season. An alternative explanation for such results may be explained with many bright pixels in the NIR spectrum comprised of trees with crown damages as an indirect side effect of the drought for weakened trees due to the limited water absorption. This may have resulted in an increase in the reflectance of the NIR spectrum [79]. Similarly, researchers [64] found that the mean spectral values of the NIR band for defoliated trees were brighter than for healthy ones. Descriptive statistics of the reference trees are shown in Table 5.

Table 5.
Descriptive statistics of stands.
3.2. Validation and Mapping of the Vegetation Indices
In further analysis, we used vegetation indices that were identified by the “lmer” models and showed significant possibilities to distinguish between different tree health classes. The forest health classification using the Random Forest algorithm was performed for each stand and field campaign separately to provide a proper comparative analysis. The validation of tree health classes of random tree classification against vegetation indices suggested that the reliability of identifying tree health classes was weak to fair if six classes were assessed, and moderate to substantial if two classes (Crown damage and Healthy trees) were tested (Table 6). The RF classified the six tree health classes with an overall classification accuracy ranging from 34.4% to 56.8%; the respective kappa coefficients ranged from 0.09 to 0.34, depending on stand and flight campaign (Table 6). For two classes, the overall classification accuracy ranged from 71.1% to 89.6% and the kappa coefficient from 0.39 to 0.71. Similarly, Kantola et al., 2010 [64] achieved a high accuracy (up to 87.3%) when two classes were used and poorer (38%) accuracy when nine classes were used in feature extraction from an aerial study. The estimated OOB error from 56.2% on average for all tree health classes was reduced to 19.2% on average for two classes only (Crown damage and Healthy trees). According to RF sensitivity, we identified that the tree health classes Root rot, New crown damage and Stem damage had a high impact on classification accuracy (Table 7). The RF correctly classified only 14% on average for class New crown damages, 18% on average for class Stem damage and 4% on average of class Root rot. Similarly, in other studies [37,80], when predicting the discolouration, the model accuracy was the greatest when distinct classes of physiological classes were used. Our results are consistent with these findings, suggesting that the RF sensitivity might be affected due to the problematic distinction between similar classes (Stem damage, Root rot). In general, the main difficulties to distinguish between these two classes derive from the fact that the stem wounds are known as the most common entry for fungal infections; consequently, in some cases they are perceived as an initial stage of Root rot. Another study has suggested that such classification problems could arise when classes are imbalanced [81], thus resulting in biased classification accuracy. Evans et al., 2011 [82] explains this with the bootstrap over-representing the majority class (in our study Crown damage and Healthy trees), resulting in a deceptive model fit and exhibiting high cross classification error from the minority classes (in our study Root rot, New crown damage, Stem damage and Dead trees). As a result, this can also affect the majority classes (such as Crown damage and Healthy trees) by giving a forecast bias to the majority classes [82]. In our study, this manifested as increased sensitivity values when only two classes were used. Our results suggested that the RF sensitivity for Crown damage class increased from 49% on average to 63% of correctly classified trees (Table 5). It may initially appear that the same issue with the bootstrap approach may also arise in the “lmer” regression model, but as reported by [82], such problems have not been seen in other modelling approaches.

Table 6.
The calculated classification accuracy of Random Forest classifier and OOB error grouped by six tree health classes. * the OOB error for two tree health classes is shown in brackets; Kappa coefficient interpretation: 0.00–0.20 as weak, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial, 0.81–1.00 as almost perfect.

Table 7.
Sensitivity and class errors for RF classifier. * the sensitivity and class errors are shown in brackets when two classes (Crown damage and Healthy tree class) were used in the RF classifier.
4. Conclusions
In this study we assessed the separability of tree health classes affected over time by a prolonged drought period in the middle of the vegetation season. UAV-based monitoring has potential in early detection of Norway spruce crown discoloration. Our models showed good results in identifying the indices that can separate tree health classes and, in the best cases, also displayed an early detection of tree stress, but at the same time, models were problematic due to the considerable uncertainties in canopy phenology.
When trying to classify with the RF model, our results were not encouraging when six classes were used. However, the results of the RF model were more accurate when only two classes (Healthy trees and Crown damage) were used. We considered that the overall classification accuracy of the RF model was affected by imbalanced representation of trees within tree health classes, therefore exaggerating the majority classes. Moreover, the overlapping of classes and other factors, such as timing of image acquisition, most likely complicated the classification and separability of early stressed trees from the data of UAV remote sensing. Therefore, future research should address the main challenges to solve limitations of the current study, focusing on how to increase the stability of the RF classifier by reducing the imbalance of the representation between classes. Future research also needs to deal with the damage progression over time in long-term observations. Moreover, uneven distributions of the affected needles within crowns are another set of challenges for future research.
Author Contributions
Conceptualization, E.B. and Ā.J.; methodology, E.B., I.D. and Ā.J.; software, E.R., A.S.; validation, E.B. and O.K.; formal analysis, E.B., I.D.; investigation, E.B.; resources, E.B., Ā.J.; data curation, E.B. and I.D.; writing—original draft preparation, E.B.; writing—review and editing, J.D., I.D., O.K., and Ā.J.; visualization, E.R. and I.D.; supervision, Ā.J.; project administration, E.B.; funding acquisition, Ā.J. and E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Forest Competence Centre (ERDF) project “Technology for early diagnostics of genetically determined variation of resilience-related trait for Norway spruce” (1.2.1.1/18/A/004).
Data Availability Statement
Data sharing is not applicable in this article.
Acknowledgments
We thank Didzis Elferts for assistance with statistical calculation and data processing in R.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schelhaas, M.J.; Nabuurs, G.J.; Hengeveld, G.; Reyer, C.; Hanewinkel, M.; Zimmermann, N.E.; Cullmann, D. Alternative forest management strategies to account for climate change-induced productivity and species suitability changes in Europe. Reg. Environ. Chang. 2015, 15, 1581–1594. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.R.; Watt, M.S. Modelling the influence of predicted future climate change on the risk of wind damage within New Zealand’s planted forests. Glob. Chang. Biol. 2015, 21, 3021–3035. [Google Scholar] [CrossRef] [PubMed]
- State Forest Service. State Forest Register Data; Dauagvpils universitātes akadēmiskais apgāds: Daugavpils, Latvia, 2018. [Google Scholar]
- SUAS 2018 Forest Statistics 2018—Official Statistics of Sweden; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2018; 143p.
- Lībiete, Z.; Donis, J.; Jansons, J.; Zālītis, P. Egļu vienvecuma tīraudžu augšanas potenciāls un tā izmaiņas. In Vienvecuma Egļu Meži Latvijā; Jansons, J., Ed.; Dauagvpils universitātes akadēmiskais apgāds “Saule”: Daugavpils, Latvia, 2019; pp. 11–54. ISBN 978-9984-14-853-3. [Google Scholar]
- Jansons, Ā.; Bāders, E.; Zeltiņš, P.; Gailis, A.; Šņepsts, G.; Katrevičs, J. Meža selekcijas potenciāls egļu audžu ražības paaugstināšanā. In Vienvecuma Egļu Meži Latvijā; Jansons, J., Ed.; Dauagvpils Universitātes Akadēmiskais Apgāds “Saule”: Daugavpils, Latvia, 2019; pp. 99–118. ISBN 978-9984-14-853-3. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2014: Synthesis Report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- McDowell, N.G.; Michaletz, S.T.; Bennett, K.E.; Solander, K.C.; Xu, C.; Maxwell, R.M.; Middleton, R.S. Predicting chronic climate-driven disturbances and their mitigation. Trends Ecol. Evol. 2018, 33, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Rieksts-Riekstiņš, R.; Zeltiņš, P.; Baliuckas, V.; Brūna, L.; Zaļuma, A.; Kāpostiņš, R. Pinus sylvestris Breeding for Resistance against Natural Infection of the Fungus Heterobasidion annosum. Forests 2020, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Jansons, A.; Zeltins, P.; Donis, J.; Neimane, U. Long-term effect of Lophodermium needle cast on the growth of Scots pine and implications for financial outcomes. Forests 2020, 11, 718. [Google Scholar] [CrossRef]
- Shakoor, N.; Lee, S.; Mockler, T.C. High throughput phenotyping to accelerate crop breeding and monitoring of diseases in the field. Curr. Opin. Plant Biol. 2017, 38, 184–192. [Google Scholar] [CrossRef]
- Gano, B.; Dembele, J.S.B.; Ndour, A.; Luquet, D.; Beurier, G.; Diouf, D.; Audebert, A. Using UAV Borne, Multi-Spectral Imaging for the Field Phenotyping of Shoot Biomass, Leaf Area Index and Height of West African Sorghum Varieties under Two Contrasted Water Conditions. Agronomy 2021, 11, 850. [Google Scholar] [CrossRef]
- Tao, X.; Li, Y.; Yan, W.; Wang, M.; Tan, Z.; Jiang, J.; Luan, Q. Heritable variation in tree growth and needle vegetation indices of slash pine (Pinus elliottii) using unmanned aerial vehicles (UAVs). Ind. Crops Prod. 2021, 173, 114073. [Google Scholar] [CrossRef]
- Anderson, K.; Gaston, K.J. Lightweight unmanned aerial vehicles will revolutionize spatial ecology. Front. Ecol. Environ. 2013, 11, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Emery, W.; Camps, A. Introduction to Satellite Remote Sensing: Atmosphere, Ocean, Land and Cryosphere Applications; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-12-809259-0. [Google Scholar]
- Roy, P.S.; Behera, M.D.; Srivastav, S.K. Satellite Remote Sensing: Sensors, Applications and Techniques. Proc. Natl. Acad. Sci. USA India Sect. A Phys. Sci. 2017, 87, 465–472. [Google Scholar] [CrossRef] [Green Version]
- White, J.C.; Coops, N.C.; Wulder, M.A.; Vastaranta, M.; Hilker, T.; Tompalski, P. Remote Sensing Technologies for Enhancing Forest Inventories: A Review. Can. J. Remote Sens. 2016, 42, 619–641. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, N.; Pádua, L.; Marques, P.; Silva, N.; Peres, E.; Sousa, J.J. Forestry Remote Sensing from Unmanned Aerial Vehicles: A Review Focusing on the Data, Processing and Potentialities. Remote Sens. 2020, 12, 1046. [Google Scholar] [CrossRef] [Green Version]
- Chianucci, F.; Disperati, L.; Guzzi, D.; Bianchini, D.; Nardino, V.; Lastri, C.; Rindinella, A.; Corona, P. Estimation of Canopy Attributes in Beech Forests Using True Colour Digital Images from a Small Fixed-Wing UAV. Int. J. Appl. Earth Obs. Geoinform. 2016, 47, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Kovacs, J.M. The application of small unmanned aerial systems for precision agriculture: A review. Precis. Agric. 2012, 13, 693–712. [Google Scholar] [CrossRef]
- Lehmann, J.R.K.; Nieberding, F.; Prinz, T.; Knoth, C. Analysis of unmanned aerial system-based CIR images in forestry—A new perspective to monitor pest infestation levels. Forests 2015, 6, 594–612. [Google Scholar] [CrossRef] [Green Version]
- Minařík, R.; Langhammer, J. Use of a multispectral UAV photogrammetry for detection and tracking of forest disturbance dynamics. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci.-ISPRS Arch. 2016, 41, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Modica, G.; Messina, G.; De Luca, G.; Fiozzo, V.; Praticò, S. Monitoring the vegetation vigor in heterogeneous citrus and olive orchards. A multiscale object-based approach to extract trees’ crowns from UAV multispectral imagery. Comput. Electron. Agric. 2020, 175, 105500. [Google Scholar] [CrossRef]
- Ortiz, S.; Breidenbach, J.; Kändler, G. Early detection of bark beetle green attack using TerraSAR-X and RapidEye data. Remote Sens. 2013, 5, 1912–1931. [Google Scholar] [CrossRef] [Green Version]
- Fahse, L.; Heurich, M. Simulation and analysis of outbreaks of bark beetle in-festations and their management at the stand level. Ecol. Modell. 2011, 222, 1833–1846. [Google Scholar] [CrossRef]
- Carter, G.A.; Knapp, A.K. Leaf optical properties in higher plants: Linking spectral characteristics to stress and chlorophyll concentration. Am. J. Bot. 2001, 88, 677–684. [Google Scholar] [CrossRef] [Green Version]
- Wulder, M.A.; White, J.C.; Benz, B.; Alvarez, M.F.; Coops, N.C. Estimating the probability of mountain pine beetle red-attack damage. Remote Sens. Environ. 2006, 101, 150–166. [Google Scholar] [CrossRef]
- Masaitis, G.; Mozgeris, G.; Augustaitis, A. Spectral reflectance properties of healthy and stressed coniferous trees. iForest 2013, 6, 30–36. [Google Scholar] [CrossRef]
- Abdulridha, J.; Batuman, O.; Ampatzidis, Y. UAV-based remote sensing technique to detect citrus canker disease utilizing hyperspectral imaging and machine learning. Remote Sens. 2019, 11, 1373. [Google Scholar] [CrossRef] [Green Version]
- Feret, J.-B.; François, C.; Asner, G.P.; Gitelson, A.A.; Martin, R.E.; Bidel, L.P.; Ustin, S.L.; Le Maire, G.; Jacquemoud, S. PROSPECT-4 and 5: Advances in the leaf optical properties model separating photosynthetic pigments. Remote Sens. Environ. 2008, 112, 3030–3043. [Google Scholar] [CrossRef]
- Du, L.; Gong, W.; Shi, S.; Yang, J.; Sun, J.; Zhu, B.; Song, S. Estimation of rice leaf nitrogen contents based on hyperspectral LIDAR. Int. J. Appl. Earth Obs. Geoinf. 2016, 44, 136–143. [Google Scholar] [CrossRef]
- Dawson, T.P.; Curran, P.J.; Plummer, S.E. Liberty—Modeling the effects of leaf biochemical concentration on reflectance spectra. Remote Sens. Environ. 1998, 65, 50–60. [Google Scholar] [CrossRef]
- Chaves, M. Effects of water deficits on carbon assimilation. J. Exp. Bot. 1991, 42, 1–16. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. The stress concept in plants: An introduction. Ann. N. Y. Acad. Sci. 1998, 851, 187–198. [Google Scholar] [CrossRef]
- Niemann, K.O.; Visintini, F. Assessment of potential for remote sensing detection of bark beetle-infested areas during green attack: A literature review. In Mountain Pine Beetle Initiative Working Paper 2005–2; Natural Resources Canada, Canadian Forest Service: Ottawa, ON, Canada, 2005. [Google Scholar]
- Panigada, C.; Rossini, M.; Meroni, M.; Cilia, C.; Busetto, L.; Amaducci, S.; Boschetti, M.; Cogliati, S.; Picchi, V.; Pinto, F. Fluorescence, PRI and canopy temperature for water stress detection in cereal crops. Int. J. Appl. Earth Obs. Geoinform. 2014, 30, 167–178. [Google Scholar] [CrossRef]
- Dash, J.P.; Watt, M.S.; Pearse, G.D.; Heaphy, M.; Dungey, H.S. Assessing very high resolution UAV imagery for monitoring forest health during a simulated disease outbreak. ISPRS J. Photogramm. Remote Sens. 2017, 131, 1–14. [Google Scholar] [CrossRef]
- Xing, N.; Huang, W.; Xie, Q.; Shi, Y.; Ye, H.; Dong, Y.; Wu, M.; Sun, G.; Jiao, Q. A transformed triangular vegetation index for estimating winter wheat leaf area index. Remote Sens. 2020, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Latvia’s State Forests Map Server (LVM GEO 2022). Ortofoto Map 7 Edition. Available online: https://www.lvmgeo.lv/dati (accessed on 23 December 2021).
- Westoby, M.J.; Brasington, J.; Glasser, N.F.; Hambrey, M.J.; Reynolds, J.M. “Structure-from-motion” photogrammetry: A low-cost, effective tool for geoscience applications. Geomorphology 2012, 179, 300–314. [Google Scholar] [CrossRef] [Green Version]
- Ke, Y.; Quackenbush, L.J. A review of methods for automatic individual tree-crown detection and delineation from passive remote sensing. Int. J. Remote Sens. 2011, 32, 4725–4747. [Google Scholar] [CrossRef]
- ESRI. ArcGIS Desktop: Release 10; Environmental Systems Research Institute: Redlands, CA, USA, 2021. [Google Scholar]
- Tuominen, J.; Haapanen, R.; Lipping, T.; Kuosmanen, V. Remote Sensing of Forest Health; INTECH Open Access Publisher: Rijeka, Croatia, 2009. [Google Scholar]
- Adamczyk, J.; Osberger, A. Red-edge vegetation indices for detecting and assessing disturbances in Norway spruce dominated mountain forests. Int. J. Appl. Earth Obs. Geoinform. 2015, 37, 90–99. [Google Scholar] [CrossRef]
- Junttila, S.; Näsi, R.; Koivumäki, N.; Imangholiloo, M.; Saarinen, N.; Raisio, J.; Holopainen, M.; Hyyppä, H.; Hyyppä, J.; Lyytikäinen-Saarenmaa, P.; et al. Multispectral Imagery Provides Benefits for Mapping Spruce Tree Decline Due to Bark Beetle Infestation When Acquired Late in the Season. Remote Sens. 2022, 14, 909. [Google Scholar] [CrossRef]
- Lyon, J.G.; Yuan, D.; Lunetta, R.S.; Elvidge, D. A change detection experiment using vegetation indices. Photogramm. Eng. Remote Sens. 1998, 64, 143–150. [Google Scholar]
- Motohka, T.; Nasahara, K.N.; Oguma, H.; Tsuchida, S. Applicability of Green-Red Vegetation Index for Remote Sensing of Vegetation Phenology. Remote Sens. 2010, 2, 2369–2387. [Google Scholar] [CrossRef] [Green Version]
- Cicek, H.; Sunohara, M.; Wilkes, G.; McNairn, H.; Pick, F.; Topp, E.; Lapen, D.R. Using vegetation indices from satellite remote sensing to assess corn and soybean response to controlled tile drainage. Agric. Water Manag. 2010, 98, 261–270. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical properties and non-destructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Chivkunova, O.B.; Merzlyak, M.N. Nondestructive estimation of anthocyanins and chlorophylls in anthocyanic leaves. Am. J. Bot. 2009, 96, 1861–1868. [Google Scholar] [CrossRef]
- Ramoelo, A.; Skidmore, A.; Cho, M.A.; Schlerf, M.; Mathieu, R.; Heitkonig, I. Regional estimation of savanna grass nitrogen using the red-edge band of the spaceborne RapidEye sensor. Int. J. Appl. Earth Obs. Geoinf. 2012, 19, 151–162. [Google Scholar] [CrossRef]
- Tilling, A.K.; O’Leary, G.J.; Ferwerda, J.G.; Jones, S.D.; Fitzgerald, G.J.; Rodriguez, D.; Belford, R. Remote sensing of nitrogen and water stress in wheat. Field Crops Res. 2007, 104, 77–85. [Google Scholar] [CrossRef]
- Albetis, J.; Jacquin, A.; Goulard, M.; Poilvé, H.; Rousseau, J.; Clenet, H.; Dedieu, G.; Duthoit, S. On the Potentiality of UAV Multispectral Imagery to Detect Flavescence dorée and Grapevine Trunk Diseases. Remote Sens. 2019, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Barati, S.; Rayegani, B.; Saati, M.; Sharifi, A.; Nasri, M. Comparison the accuracies of different spectral indices for estimation of vegetation cover fraction in sparse vegetated areas. Egypt. J. Remote Sens. Space Sci. 2011, 14, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Laird, N.M.; Ware, J.H. Random-Effects Models for Longitudinal Data. Biometrics 1982, 38, 963–974. [Google Scholar] [CrossRef]
- Bennington, C.C.; Thayne, W.V. Use and Misuse of Mixed Model Analysis of Variance in Ecological Studies. Ecology 1994, 75, 717–722. [Google Scholar] [CrossRef]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-Plus. In Statistics and Computing; Springer: New York, NY, USA, 2000. [Google Scholar]
- Burnham, K.; Anderson, D. Model Selection and Inference: A Practical Information Theoretic Approach; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1998. [Google Scholar] [CrossRef] [Green Version]
- Lenth, R.V.; Hervé, M. lsmeans: Least-Squares Means. Available online: http://CRAN.R-project.org/package=lsmeans (accessed on 20 December 2021).
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 123–140. [Google Scholar] [CrossRef] [Green Version]
- Dietterich, T.G. An experimental comparison of three methods for constructing ensembles of decision trees: Bagging, boosting, and randomization. Mach. Learn. 2000, 40, 139–157. [Google Scholar] [CrossRef]
- Kantola, T.; Vastaranta, M.; Yu, X.; Lyytikainen-Saarenmaa, P.; Holopainen, M.; Talvitie, M.; Kaasalainen, S.; Solberg, S.; Hyyppa, J. Classification of defoliated trees using tree-level airborne laser scanning data combined with aerial images. Remote Sens. 2010, 2, 2665–2679. [Google Scholar] [CrossRef] [Green Version]
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Middleton, E.M.; Chan, S.S.; Rusin, R.J.; Mitchell, S.K. Optical Properties of Black Spruce and Jack Pine Needles at BOREAS Sites in Saskatchewan, Canada. Can. J. Remote Sens. 1997, 23, 108–119. [Google Scholar] [CrossRef]
- Glenn, E.P.; Huete, A.R.; Nagler, P.L.; Nelson, S.G. Relationship between remotely-sensed vegetation indices, canopy attributes and plant physiological processes: What vegetation indices can and cannot tell us about the landscape? Sensors 2008, 8, 2136–2160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dash, J.P.; Pearse, G.D.; Watt, M.S. UAV Multispectral Imagery Can Complement Satellite Data for Monitoring forest Health. Remote Sens. 2018, 10, 1216. [Google Scholar] [CrossRef] [Green Version]
- Eitel, J.U.H.; Vierling, L.A.; Litvak, M.E.; Long, D.S.; Schulthess, U.; Ager, A.A.; Krofcheck, D.J.; Stoscheck, L. Broadband, red-edge information from satellites improves early stress detection in a New Mexico conifer woodland. Remote Sens. Environ. 2011, 115, 3640–3646. [Google Scholar] [CrossRef]
- Demarez, V. Seasonal variation of leaf chlorophyll content of a temperate forest. Inversion of the PROSPECT model. Int. J. Remote Sens. 1999, 20, 879–894. [Google Scholar] [CrossRef]
- Rautiainen, M.; Lukeš, P.; Homolová, L.; Hovi, A.; Pisek, J.; Mõttus, M. Spectral Properties of Coniferous Forests: A Review of In Situ and Laboratory Measurements. Remote Sens. 2018, 10, 207. [Google Scholar] [CrossRef] [Green Version]
- Moorthy, I.; Miller, J.R.; Noland, T.L. Estimating chlorophyll concentration in conifer needles with hyperspectral data: An assessment at the needle and canopy level. Remote Sens. Environ. 2008, 112, 2824–2838. [Google Scholar] [CrossRef]
- Xie, Q.; Dash, J.; Huang, W.; Peng, D.; Qin, Q.; Mortimer, H.; Casa, R.; Pignatti, S.; Laneve, G.; Pascucci, S.; et al. Vegetation Indices Combining the Red and Red-Edge Spectral Information for Leaf Area Index Retrieval. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2018, 11, 1482–1493. [Google Scholar] [CrossRef] [Green Version]
- Kharuk, V.I.; Im, S.T.; Dvinskaya, M.L.; Golukov, A.S.; Ranson, K.J. Climate-induced mortality of spruce stands in Belarus. Environ. Res. Lett. 2015, 10, 125006. [Google Scholar] [CrossRef] [Green Version]
- Seidl, R.; Blennow, K. Pervasive growth reduction in Norway Spruce forests following wind disturbance. PLoS ONE 2012, 7, e33301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krisans, O.; Saleniece, R.; Rust, S.; Elferts, D.; Kapostins, R.; Jansons, A.; Matisons, R. Effect of Bark-Stripping on Mechanical Stability of Norway Spruce. Forests 2020, 11, 357. [Google Scholar] [CrossRef] [Green Version]
- Flexas, J.; Bota, J.; Galmes, J.; Medrano, H.; Ribas-Carbo, M. Keeping a posi-tive carbon balance under adverse conditions: Responses ofphotosynthesis and respiration to water stress. Physiol. Plant. 2006, 127, 343–352. [Google Scholar] [CrossRef]
- Gates, D.M. Biophysical Ecology; Springer: Berlin/Heidelberg, Germany, 1980. [Google Scholar]
- Näsi, R.; Honkavaara, E.; Lyytikäinen-Saarenmaa, P.; Blomqvist, M.; Litkey, P.; Hakala, T.; Viljanen, N.; Kantola, T.; Tanhuanpää, T.; Holopainen, M. Using UAV-Based Photogrammetry and Hyperspectral Imaging for Mapping Bark Beetle Damage at Tree-Level. Remote Sens. 2015, 7, 15467–15493. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Jönsson, P.; Tamura, M.; Gu, Z.; Matsushita, B.; Eklundh, L. A simple method for reconstructing a high-quality NDVI time-series data set based on the Savitzky-Golay filter. Remote Sens. Environ. 2004, 91, 332–344. [Google Scholar] [CrossRef]
- Evans, J.S.; Murphy, M.A.; Holden, Z.A.; Cushman, S.A. Modeling Species Distribution and Change Using Random Forest. In Predictive Species and Habitat Modeling in Landscape Ecology; Drew, C., Wiersma, Y., Huettmann, F., Eds.; Springer: New York, NY, USA, 2011; pp. 139–159. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).