Abstract
Recently, forest management faces new challenges resulting from increasing temperatures and drought occurrences. For sustainable, site-specific management strategies, the availability of up to date soil information is crucial. Proximal soil sensing techniques are a promising approach for rapid and inexpensive collection of data, and could facilitate the provision of the necessary information. This study evaluates the potential of visual and near-infrared spectroscopy (vis-NIRS) for estimating soil parameters relevant for humus mapping in Saxon forests. Therefore, soil samples from the organic layer are included. So far there is little knowledge about the applicability of vis-NIRS in the humus layer of forests. We investigate the spectral behaviour of samples from organic (Oh) and mineral (0–5 cm, Ah) horizons, pointing out differences in the occurring absorption features. Further, we identify and assess the accuracy of selected soil properties based on vis-NIRS for forest sites, compare the outcome of different regression methods, investigate the implications for forest soils due to the presence and different composition of the humus layer and organic horizons and interpret the results regarding their usefulness for soil mapping and monitoring purposes. For this, we used retained humus soil samples of forests from Saxony. Regression models were built with Partial Least Squares Regression, Support Vector Machine and Cubist. Investigated properties were carbon (C) and nitrogen (N) content, C/N ratio, pH value, cation exchange capacity (CEC) and base saturation (BS) due to their importance for assessing humus conditions in forests. In organic Oh horizons, prediction results for C and N content achieved R2 values between 0.44 and 0.58, with corresponding RPIQ ranging from 1.58 to 2.06 depending on the used algorithm. Estimations of C/N ratio were more precise with R2 = 0.65 and RMSE = 2.16. Best results were reported for pH value, with R2 = 0.90 and RMSE = 0.20. Regarding BS, the best model accuracy was R2 = 0.71, with RMSE = 13.97. In mineral topsoil, C and N content models achieved higher values of R2 = 0.59 to 0.72, with RPIQ values between 2.22 and 2.54. However, prediction accuracy was lower for C/N ratio (R2 = 0.50, RMSE = 3.52) and pH values (R2 = 0.62, RMSE = 0.29). Models for CEC achieved R2 = 0.65, with RPIQ = 2.81. In general, prediction precision varied dependent on the used algorithm, without showing clear tendencies. Classification into pH classes was exemplified since this offers a new perspective for humus mapping on forest soils. Balanced accuracy for the defined classes ranged from 0.50 to 0.87. We show that vis-NIR spectroscopy is suitable for assessing humus conditions in Saxon forests (Germany), in particular not only for mineral horizons but also for organic Oh horizons.
1. Introduction
Forests in Europe are highly affected by changing environmental conditions caused by increasing annual temperatures and drought events. As a result, tree mortality increased and forest stands remain vulnerable to further impacts like insect or fungal attacks [1]. This can lead to rapidly altering forest ecosystems [2]. Further, persistently high nitrogen inputs shift the nutrient balance of forests. In order to avoid decreasing resilience of forests and loss of forest ecosystem services, this has to be addressed by management strategies. For sustainable and site-specific forest management, the availability of up to date soil information is crucial. Hereby, information is needed at desired spatial scale [3], as soil properties such as carbon content are spatially highly variable [4].
This information can support management decisions regarding tree species selection, silvicultural treatments, timber harvesting and soil protection measures [5]. However, forest soils are complex systems and it is challenging to properly describe and classify their properties and functions, in particular in the organic and the uppermost mineral soil layer [6,7]. In the methodological approach of forest site mapping in Eastern Germany, it is common to subclassify soil properties in stable and variable components [8]. Soil properties which are stable over a time span of at least one rotation period or 100 years are mainly vinculated to parent material, physical properties and typical sequence of pedogenetic soil horizons. These properties are summarized in so called soil forms.
In contrary to this, variable properties in particular of the organic layers and upper mineral soil are defined by chemical properties, C/N-ratio, pH-value, cation exchange capacity (CEC) and base saturation (BS). Variable soil properties are consolidated in humus forms. Currently, a vegetation scheme consisting of indicator plants is used to map humus properties according to the mapping field guide for the Northeastern Lowland of Germany [8]. Element input, anthropogenic overprint in vegetation as a result of forest management and changing climate cause fast changing base conditions. Thus, the current methods have weaknesses and are increasingly questioned.
There is a substantial need for inexpensive and viable methods for periodic mapping of the variable soil properties in particular [3]. The National Forest Soil Inventory in Germany (NFSI) provides periodic information about the conditions of forest soils [9]. Its focus lies on long term observations and monitoring of the same sampling points. Its sampling raster is too coarse to assess forest stands.
Proximal soil sensing (PSS) is an interesting approach for rapid and inexpensive collection of data, and could facilitate the provision of the necessary information at desired scale. It can be used for a better understanding of soils spatial variability [10], and for meaningful statistical analyses. One PSS technique which arouses widespread interest to provide data for soil mapping purposes is diffuse visual and near-infrared reflectance spectroscopy (vis-NIRS). It is known to be fast, cost efficient and non-destructive. Further, it does not require complex soil preparation and is suitable for estimation of several soil properties [11,12].
Despite the widespread use on agricultural sites and mineral soils (e.g., [13,14,15,16]), there are so far only few examples for applications on forest soils and humus layers. Pietrzykowski and Chodak [17] evaluated the potential of vis-NIRS to estimate, amongst other properties, soil organic carbon content (SOC), N content, C/N ratio on mineral soil samples from afforested former mining sites. They report the development of successful models for prediction, even though the studied soils differed from natural conditions in chemical and microbial properties. Ludwig et al. [18] investigated the applicability of spectroscopy to predict SOC, N content and pH value along with enzyme activities on mineral horizons in two forest sites in Germany. They used partial least squares regression (PLSR) approaches and confirmed the usefulness for SOC and N contents, but had variable results for pH-values dependent on the data range. Comparing different sensors for acquiring spectral data, Thomas et al. [19] found useful results for C and N content in Saxon forests.
On a large-scale approach across Chinese forests, Liu et al. [20] built the Chinese forest soil spectral library with high spatial heterogeneity. Samples were taken from different depths, but horizons were not separated during analysis. They were able to accurately predict SOC and confirmed the feasibility of estimating forest soil parameters based on vis-NIR spectra. Pinheiro et al. [21] reported good prediction for SOC and reasonable results for other properties including CEC in a study in the Brazilian Central Amazon. Investigating forest soils of the Czech Republic, Gholizadeh et al. [22] were able to accurately estimate SOC in organic horizons, also using material from whole soil profiles instead of investigating a specific horizon. As separation of horizons can be challenging during sample collection, an approach with sample collection at defined depths might be a solution to face this problem, as done in agriculture.
To our knowledge, there are so far no other studies investigating the applicability of vis-NIRS for prediction of N content, C/N ratio, pH value, CEC and BS on organic surface layers of forest soils. Most studies focus on mineral soil and investigate samples from other land use forms. However in terms of forest soil mapping the organic surface layer has a major role and its condition has impact on management decisions. For humus assessment in forest management, the selected properties, including CEC and BS, are important to assess current soil conditions. Previous studies show that vis-NIR spectra are sensitive to differences in soil organic matter from different land use and vegetation forms [23]. We therefore investigate organic and mineral layers separately.
Nutrient availability is part of the basis for decision making when developing site-specific management strategies. It shall supplement or enhance current methods of vegetation-based mapping of humus properties as well as grid based periodic soil inventory. If successful, vis-NIRS raises the possibility to predict desired soil parameters without expensive chemical analyses of all collected samples. In this study, we focus on using vis-NIRS to predict selected forest soil properties that are relevant for the periodic mapping of humus properties of forest soils in Saxony. We investigate C and N content, C/N ratio, pH, CEC and BS. We apply the method to samples from the organic layer (Oh horizon, mainly humic material and decomposed plant residues with at least 30% organic substance) and mineral soil samples (0–5 cm, Ah horizon with less than 30% organic substance). Within this scope, the objectives are (i) to identify and assess the accuracy of estimations of selected soil properties based on vis-NIRS for forest sites in the state of Saxony (Germany) using different algorithms, (ii) investigate the implications for forest soils due to the different composition of the humus layer and organic horizons and (iii) discuss options for the usage of vis-NIRS predictions for classification and soil monitoring purposes.
2. Methods
2.1. Study Area
For this study, retained soil samples from forest sites in Saxony (Germany, approx. 17,400 km2, with 5209 km2 covered by forests) were used. Data collection took place within the periodic NFSI between 2006 and 2014 in a grid-based approach and during a previous study at forest sites representing the typical natural areas lowland, hilly terrain and middle mountain range [24].
An overview of the locations of the sampling sites and the corresponding natural areas can be seen in Figure 1.
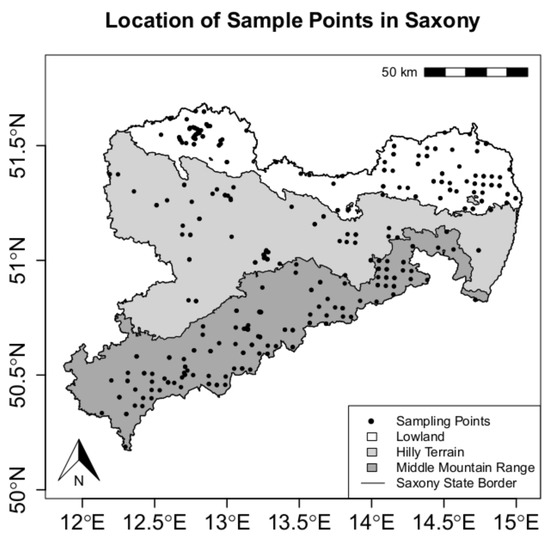
Figure 1.
Location of sampling points in Saxony, Germany.
2.2. Vis-NIR Spectroscopy
Vis-NIR spectra are sensitive to mineral and organic soil substances [25] and reflectance spectroscopy is a well-known method in soil science to predict chemical and physical soil properties, even on a global scale [26]. Reflectance spectra are obtained by measuring scattered light from an illuminated soil sample. The light causes molecular vibrations, which absorb parts of the radiated energy. A comparison of emitted and reflected light holds information about molecules present in the sample [27]. As molecules consists of chemically bonded atoms, the exposition to electromagnetic radiation results in excited atoms and therefore in vibrational processes, e.g., in changing length (stretching) or changing angle of the bonds. As this absorbs energy, the absorptions are visible in the measured spectra [28]. In the visual range, the spectra is mainly related to iron oxides [29] and soil organic matter due to the darkness of organic compounds and humic acid which is caused by absorption of organic compounds in the visual range [25,30]. The predominant absorbers in the NIR region are overtones of the C–H, N–H and O–H functional groups, making the NIR region ideal for quantifying forms of carbon and nitrogen [27,31]. A short overview of spectrally active molecules can be seen in Table 1.

Table 1.
Spectral Assignments.
It is important to notice that especially C-H, N-H and O-H molecules imply correlations to organic compounds present in upper soil layers, like hemicellulose, cellulose, lignin, proteins and sugar [32]. Regarding our selected properties, Xu et al. [35] states that the spectral active wavelength ranges for C and N content are similar. For pH value, e.g., Xu et al. [35] found high correlations in the ranges of 480, 780, 1120, 1910, 2200 and 2390 nm. However, Stenberg et al. noted that pH value has no direct relationship to vis-NIR spectra, but can be predicted through co-variations with buffering capacity of organic matter, clay and mineralogy [34]. For CEC, Ben-Dor and Banin [33] suggest important spectral assignments around 2000 as well as around 1400 nm. As they also mention a high correlation of CEC to clay minerals, these assignments are probably caused by the correlation and therefore indirect. BS has no direct spectral assignment as well. However, Xu et al. [35] found good prediction results, suggesting indirect assignments due to its strong correlations to clay, salt and iron oxide.
2.3. Soil Samples and Laboratory Analysis
All soil material was collected based on the German sampling standard for the NFSI [9]. In brief, this method consists of the sampling on eight points around each sampling center point with a radius of 10 m. A schematic presentation of the sampling design is shown in Figure 2. If one or more points could not be probed due to specific site conditions, they were shifted two meters in- or outwards the circle. At all points, soil material from organic layer (Oh) as well as mineral soil (0–5 cm, Ah) was collected and then mixed, resulting in one organic and one mineral sample per point. The separation of the organic and mineral horizons was carried out via visual assessment (see Figure 2 on the right). In this procedure, the soil horizons are separated manually based on the distinguishable colour and structure.
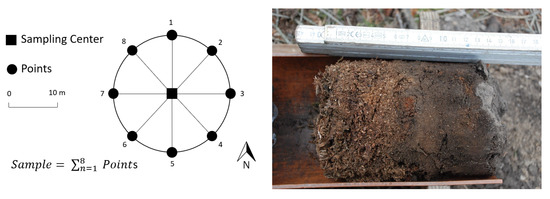
Figure 2.
Schematic presentation of the sampling design (left) and humus sample during data collection (right).
Chemical analysis of all samples was performed by the laboratory of Sachsenforst public enterprise, following German-wide standards of forest soil chemical analysis [36]. The pH value was analyzed on the basis of the norm DIN-ISO-10390 (2005) [37] using solution, as recommended for soils in Germany [38]. To quantify total carbon content the dry combustion method with elementary analysis was applied (DIN10694, 1996) [39]. As we focused on humus properties, we used the soil samples and legacy lab data from both Oh-horizon and Ah-horizon.
From a total of 727 retained samples, we stratified the population into the dominant two classes: spruce (Picea abies) and pine (Pinus sylvestris) stands. Within these classes we performed conditioned Latin hypercube sampling (clhs) in order to optimize the number of samples. Samples from mixed stands were then added manually due to their smaller number. In order to cover the full range of observed values and represent humus conditions in Saxony, the minima, maxima and median values of C/N ratio, pH value and CEC were added per horizon and vegetation type as these properties are also used for humus assessment. A graphical illustration of the sample selection procedure can be seen in Figure 3.
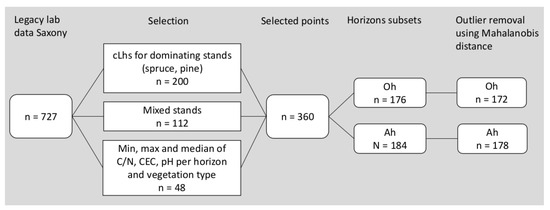
Figure 3.
Graphical illustration of the sample selection procedure.
In total, the procedure resulted in a data set consisting of 360 soil samples (176 from Oh and 184 from Ah, with 287 samples originating from NFSI and 73 from Wellbrock et al. [24]). The most abundant parent materials were sand, loess loam, gneiss, granite, shale and phyllite, main soil types were cambisols, gley and podsols. Prior to the spectral analysis, the samples have been dried. To avoid changes or loss of structures or volatile compounds, all soil material was dried according to laboratory standards [36]. Samples from Oh were dried at 60 °C, mineral samples at 40 °C. Further, the dry samples were sieved with a two mm mesh in order to homogenize the soil material.
2.4. Spectral Measurements and Pre-Processing
For data collection, we used a Veris vis-NIR Spectrophotometer by Veris Technologies Inc. (Salina, KS, USA). It contains two sensors, an Ocean Optics USB4000 (340 to 1100 nm) and a Hamamatsu Minispectrometer TG series (1100 to 2220 nm), working with a spectral resolution of five and six nanometres. Calibration of the spectrometer was done using four Fluorillon grey scale standards as external references [40]. Based on the protocol, two petri dishes have been filled per soil sample, and each dish was measured five times in direct contact. To homogenize the measured surface, it was softly pressed using the lid of the dishes. Samples were rotated and shifted between the measures to capture more of the sample variability. This procedure also balances the values within the measured area [41]. The chosen method results in ten spectra for each soil sample. Vis-NIR spectra can be influenced by light scatter during data acquisition, causing, e.g., baseline shifts and non-linearities. Suitable preprocessing is able to largely eliminate these unwanted effects [42]. Selected methods for spectral preprocessing were applied to the spectra. To smooth the data without distorting the signal trend, the Savitzky–Golay filter [43] was applied, using a window size of 11 and a polynomial order of three. It operates as a weighted sum over a given window, computed as follows in Equation (1):
where is the new signal value, the old signal value, N is a normalizing coefficient, k is the gap size on each side of i and are pre-computed coefficients, that depend on the chosen polynomial order and degree [44].
Light scatter and multiplicative interference correction as well as adjusting base line shifts was done using standard normal variate (SNV) [42,45]. It is calculated as shown in Equation (2):
where is the signal of a sample i, is its mean and its standard deviation. All preprocessing steps were carried out by means of the R package prospectr [46].
Measurements taken below 430 nm and above 2205 nm and data between the ranges of the sensors (1000 to 1100 nm) were removed due to occurring noise at the sensors edges. We then formed the mean of ten single measurements per sample. Outlier detection was performed on the data set by means of the R package mvoutlier [47] for both horizons separately. In this approach, a principal component analysis (PCA) builds the basis for the outlier detection. The first two principal components are used to identify outliers based on Mahalanobis distance. Filzmoser et al. [48] states that the Mahalanobis distance of gaussian distributed data follows a chi-square distribution. Data points laying beyond a defined threshold of the chi-square distribution were flagged and removed from the data set. After this procedure, ten samples (four Oh and six Ah) were removed from the data set. The R language for Statistical Computing was used for all processing and calculation steps in this study [49].
2.5. Regression Approaches
Numerous studies use PLSR to calibrate regression models to estimate soil properties based on vis-NIR spectra (e.g., [50,51,52]). However, new methods from the field of machine learning are applied recently. Stevens et al. [44] and Shi et al. [53] used Support Vector Machine (SVM) to estimate organic C and total N, respectively. Cubist regression was also already successfully used, e.g., for prediction of SOC, total N and pH value [54].
PLSR was introduced by Wold et al. [55] as one way to solve multivariate calibration problems. Today, PLSR is widely known as a standard algorithm to calibrate models for predicting soil properties [56]. PLSR constructs a set of linear combinations of the input data by producing a sequence of derived, orthogonal directions [57]. These combinations have high variance as well as high correlation with the response variable. For the regression, these components are used instead of the original data, resulting in a remarkable data reduction.
SVM is based on the idea of non-linearly mapping the data into very high dimensional feature space. A linear decision boundary is then constructed in this space, ensuring high generalization ability [58]. The data points lying on the edge between classes are important for the creation of the boundary. These are called support vectors. For predicting on new data, the distance to the support vectors is calculated and used as basis for the decision [59]. In this study, a radial basis function kernel was used for the calculation of the distances.
As another algorithm from the field of machine learning, we calibrated Cubist regression models. This algorithm was introduced by Quinlan [60] and can be used for learning tasks with high dimensionality. It is based on a regression tree, where intermediate linear models at each step are the basis of the predictions. Contrary to other tree based models, it retrieves a set of rules associated with sets of multivariate models [61]. The rules are then connected using if/else statements. If a condition is fulfilled, the regression rule for this subset is applied, otherwise, the next rule is probed [62]. The predictions can further be improved by creating several models (“committees”), where each new model corrects the previous one to reduce the error [63]. It was already used for soil property estimations in previous studies [19].
2.6. Spectral Model Tuning and Validation
To predict forest soil properties relevant for humus mapping, we calibrated regression models based on samples from Oh and Ah horizon separately. However, the separation of the horizons was challenging during the sampling of soils. Disturbances can alter the sequential arrangement of the horizons.
Model calibration and validation was done using a nested cross validation. In a cross-validation procedure, the data is randomly split into n subsets of approximately same size. The model is calibrated with folds and then used to predict on the holdout data. This procedure is repeated until every fold was treated as validation set once. In total, n models are built with different parts of the data being used to train and test the performance, resulting in n estimates of the model performance and in predicted values for every sample in the data set. Then, the mean of the n individual prediction error estimates is formed [57].
In a nested cross-validation, there is an inner and an outer loop over the splits of the data into training and test sets. For each of the inner splits, a grid search is done and the inner test set performance using the best tuning parameters is reported for the outer left out validation set with the final model [59]. For the calibration part using the inner splits and a grid search, the models were built using a 10-fold inner cross validation to ensure a robust tuning of hyperparameters. In a grid search procedure, models are trained using different combinations of values of the hyperparameters. The best combination resulting in the lowest prediction error is then used to predict on the test set from the outer cross validation. PLSR was tuned using one up to 20 components. For SVM, sigma values lay between 0.00001 and one, cost values between one and 10,000. Regarding the Cubist models, we probed values between one and 50 for committees and between zero and seven neighbours. The evaluation during the model tuning process was based on the root mean square error (RMSE) of the output of each parameter combination. Model tuning and validation procedure was performed by means of the R package caret [64]. We were working in a closed system as validation does not include prediction on new sampling sites. For all properties except BS, logarithmized values were used.
We computed different error measures to evaluate the model performance: RMSE, coefficient of determination (R2), and ratio of performance to interquartile (RPIQ).
The RMSE is computed as described in Equation (3):
where are the predicted, are the observed values. Apart from the deviations of the predictions to the actual observed values, overall model performance was assessed using the model efficiency coefficient developed by Nash [65]. It is shown in Equation (4).
where are the observed values, are the predicted values and is the observed mean value.
As complementary quality criteria for performance assessment for data without Gaussian distribution, the RPIQ was calculated as described below in Equation (5) [66]:
where and are the first and the third quantile of the observed values. In order to rank the RPIQ values, we used the system suggested by Chang et al. [67] for ratio of performance to deviation (RPD) values. Transformation was done by multiplying the values with 1.34896 (as the interquartile range of a Gaussian distribution equals 1.34896 × SD). Thus, the threshold for good models is >2.70, moderate models are between 1.89 and 2.69 and poor performance is for values < 1.89. Nevertheless, the usefulness of a model should be assessed regarding its context. Generally, large values for R2 and RPIQ and low RMSE values are desired.
We further calibrated PLSR models using all points as training data in order to identify the most important wavelength ranges for the regression models for each property. The cross-validation procedure results in numerous models per property and is therefore not suitable to identify important wavelengths as they can differ between the different splits.
2.7. Evaluate Feasibility for Soil Mapping
Apart from calculating soil parameter estimations based on spectral data, the results are evaluated with respect to their usefulness for soil mapping purposes. For practical applications, simple classes of values are desirable, as they are more easy to interpret as basis for decision making when compared to continuous values. Therefore, we classify our samples to see how well the predictions can be used for such classification purposes.
Schulze and Kopp [8] proposed a classification scheme for humus of forest soils. It is to be used for evaluating nutrients availability. We use this example to present a possible way to classify the soil samples and evaluate the usefulness of the predicted values. The measured and predicted pH values build the basis for the classification. As examples of application of vis-NIRS for organic layers are scarce, the samples from Oh horizon and predictions from SVM are used as basis for the classification.
A confusion matrix is then calculated to compare the outcome of the classifications based on chemically measured and predicted values. This opens up the possibility to see which classes are robust in both classifications and where confusion between defined classes is present. The defined class thresholds can be see in Table 2 (modified after Schulze and Kopp [8]).

Table 2.
Classes and corresponding pH values, (modified from Schulze and Kopp (2013) [8]).
For each class, balanced accuracy is used to assess the outcome of the classification. It is calculated as shown in Equation (6):
where sensitivity is the true positive rate while specificity is the true negative rate. The calculation of the confusion matrix and their evaluation was done as well by means of the R package caret [64].
3. Results
3.1. Descriptive Statistics of Soil Properties
A summary of descriptive statistics for the observed soil parameters investigated in this study, separated by horizon, is presented in Table 3. In the organic layer, values for C content range from 8.60 to 49.43%. The mean is 27.47%, representing large variation due to the use of sampling locations across whole Saxony. The standard deviation is 7.83%. For 18 samples from Oh horizon, C content lay below 17.5%. Therefore, these samples do theoretically not contain enough organic matter to be counted to the organic horizon.

Table 3.
Summary statistics of investigated soil properties for Oh (n = 172) and Ah (n = 178) horizon, Pctl = percentile.
In the Ah samples, C content ranged from 0.40 to 12.27% with a mean 5.05%, showing the big differences in C content between the horizons. Similar remarks can be done on the N percentages, ranging from 0.32 to 2.08% in the Oh horizon and from 0.02 to 0.58% in the Ah. The C/N ratio is more evenly distributed, with mean values of 22.77 (Oh) and 24.55 (Ah). The mean of the samples pH-values is 3.39 (Oh) and 3.32 (Ah). It is notable that the majority of the samples have acidic pH-values below 4 and that samples from basic soils are not present in the data set. Values for CEC range from 59.07 to 1065.50 µeq/g in the Oh and are much higher than in the Ah (18.16 to 239.60 µeq/g). Measured values for BS are higher in the Oh as well, with a mean of 46.63% compared to 20.08% in the Ah.
A correlation matrix of the soil parameters for both horizons is shown in Figure 4. Significant correlations are marked. In Oh horizons, C and N show the strongest positive correlation. C is moreover correlated with CEC only. N content is positively correlated with CEC and BS and shows negative correlation to C/N ratio. The C/N ratio is moreover negatively correlated with pH, CEC and BS. CEC shows correlations to all other properties and BS only shows no dependence to C content. In comparison, results for Ah horizons differ from these observations. Here, C content shows positive correlations to N content and CEC, but also negative correlation to C/N and pH value. N has dependence to all other properties, also including pH value. In contradiction to Oh samples, C/N shows no correlation to pH value, and pH value is correlated with C content. CEC correlation with pH is negative. The correlations regarding BS do not differ from the Oh samples, even though they are less strong. The different observations regarding the correlations between the selected soil properties for Oh and Ah horizons reveal differences between organic and mineral horizon.
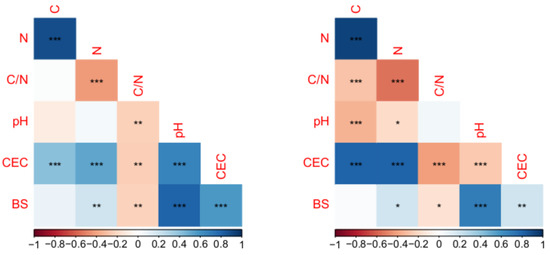
Figure 4.
Significance of Pearsons correlation coefficients between investigated soil properties for Oh (left) and Ah (right) horizon (*, ** and *** indicates a p-value < 0.05, <0.01 and <0.001, respectively).
3.2. Spectral Differences between Horizons
The raw spectra, the mean and the 1st derivative of preprocessed spectra, separated by horizon can be seen in Figure 5. The mean absorbance is shown on the left, the 1st derivative on the right. The absorbance shows the characteristic decline through the visible range up to 1000 nm. The raw spectra show that samples from Oh horizons have higher absorbance values through all measured wavelengths. Further, the most obvious features can clearly be seen in the absorbance around 1400 and 1900 nm in both horizons. However, the 1st derivative allows a more detailed qualitative analysis. Smaller features in the visual range as well as around 1550 and 1700–1800 nm become more visible. Further, differences in the absorption of both horizons were more clear. We identified four ranges with remarkable differences, and marked them with letters inside the plot. In the visual range (A), mineral horizons have two peaks at around 470 and 600 nm, the Oh samples behaved differently here and show several smaller peaks inside a general decline of values. The next difference (B) can be detected at the dip following the peak at 1400 nm, which is stronger for the mineral samples. On the other hand, the peak and dip at 1900 nm are stronger for samples form Oh horizons (C). The last distinction takes place above 2000 nm. Here, the Oh samples show another higher peak with a following decline, while Ah samples show no corresponding dip.
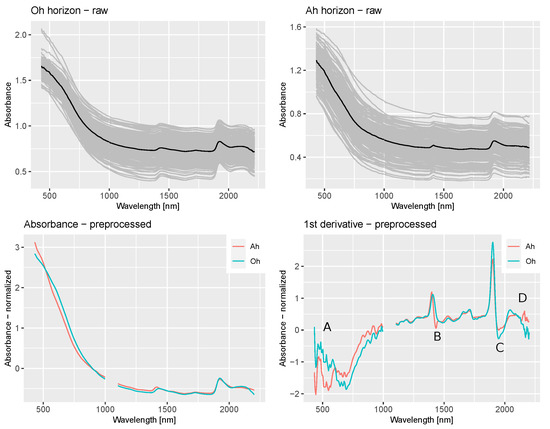
Figure 5.
Raw spectra (black line represents the mean) and preprocessed absorbance and 1st derivative spectra of samples from Oh and Ah samples, ranges with remarkable differences are marked by letters A–D.
3.3. Predicting Oh Properties
The results of the selected model approaches for all investigated soil properties can be seen in Table 4. The models results for the Oh samples are shown on the left, performance measures for Ah horizon on the right side. Predicted vs. observed values for all investigated soil properties and both horizons are shown in Figure 6. The plots show the prediction results of the best performing algorithm per property and horizon.

Table 4.
Model results for selected soil properties and algorithms for Oh (left) and Ah (right) samples from Saxony.
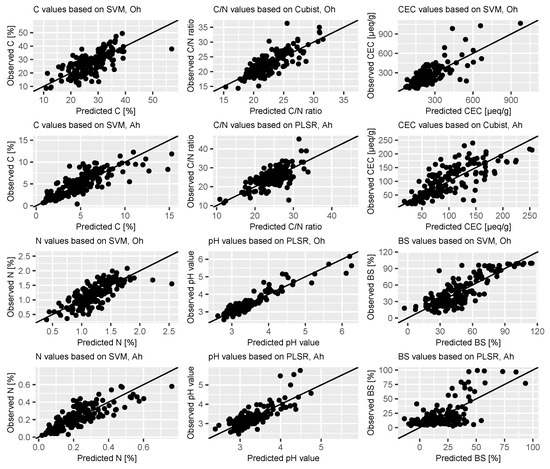
Figure 6.
Predicted vs. observed plots for the investigated properties separated by horizon, results of the most accurate algorithm per property.
For C content, values for R2 lay between 0.46 and 0.54, with corresponding RMSE values ranging from 5.59% to 6.16% and RPIQ between 1.58 and 1.75. Wavelengths important for model building lay between 500 and 600 nm.
The prediction of N content showed similar accuracy and achieved R2 values ranging from 0.48 to 0.58, with RPIQ values between 1.82 and 2.06.
For both C and N content, the plot showed no patterns as the points cluster around the x = y line. Regarding the C/N ratio of the Oh horizon, the predictions resulted in higher R2 values of 0.64–0.66. For this property, RMSE lay between 2.16 and 2.26, with Cubist yielding the most accurate results. RPIQ was calculated between 1.73 and 1.81. The features around 2200 and 1900 nm were most important.
For pH value, we found little differences between the used algorithms. R2 were between 0.88 and 0.9 and RMSE ranged from 0.2 to 0.21, with corresponding RPIQ values of 2.39–2.47. The scatterplot of the predictions also shows that even the underrepresented high pH values show small deviation and cluster tightly to the x = y line. Again wavelength ranges around 1900 and 2200 nm were most important for model calibration.
The predictions derived for CEC were less precise, with R2 being 0.54. Relevant spectral ranges were identified between 570 and 690 nm.
Regarding BS, again little differences between the used algorithms are reported. R2 values ranged from 0.69 to 0.71, RPIQ lay between 2.51 and 2.61. Important spectral features lay around 1900 and 2200 nm.
The scatter plot reveals that especially high BS values close to 100% seem to have bigger deviation from the observed values as they form a visible cluster.
Regarding the used algorithms, none of the chosen methods was superior among all investigated properties, while SVM resulted in the most accurate predictions for C and N content, the differences were minor for the other properties, with no clear trends in favour for one of the algorithms.
3.4. Predicting Ah Properties
Models results for samples from Ah horizon showed different trends. Prediction of C and N content was more precise for mineral samples. For C content we achieved R2 values between 0.59 and 0.72 and corresponding RPIQ values of 2.22–2.54. The calculated RMSE ranged from 1.56% to 1.77%. SVM regression outperformed PLSR and Cubist. The models for N content reached R2 values between 0.63 and 0.71 and RPIQ values from 2.27 to 2.44. Again, SVM regression yielded the most accurate predictions. Important features for C and N content lay between 2150 and 2200 nm as well as in the visual range. Unlike for the Oh models, prediction for C/N ratio was less precise, with R2 ranging from 0.45 to 0.5. The predicted values mostly lay between a C/N ratio of 20 and 30. Relevant spectral ranges were around 450 and 2050 nm.
Compared to predictions for Oh samples, accuracy decreased for pH values, with R2 values between 0.45 and 0.56 and RMSE ranging from 0.29 to 0.34. For this property, PLSR outperformed the other algorithms. The visual interpretation reveals that some high values were underestimated. In contrary to Oh samples, most important wavelengths were in the visual range, but also around 1900 nm.
In contrast, results for CEC in Ah horizons achieved better model predictions. Depending on the algorithm, R2 values ranged from 0.58 to 0.67, with RMSE values between 33.16 and 39.04 µeq/g. RPIQ was calculated from 2.34 to 2.81. The spectral feature around 1900 nm was identified to be most important.
Prediction of BS based on mineral samples resulted in lowest R2 and RPIQ values. The scatter plot shows that the model has difficulties in detecting a relationship between measured spectra and chemically obtained BS values.
Similar to the results for Oh horizon, SVM showed the most accurate predictions for C and N content. For the other investigated properties, again no clear trend was visible. Cubist was most accurate for CEC values. On the other hand, SVM and Cubist showed poorer results for pH, where PLSR was most precise.
3.5. Classification for Mapping Purposes
For a better assessment of the usefulness of the predicted soil property values, we classified both measured and estimated values based on pH value. We then calculated a confusion matrix to see if the defined classes are robust. This classification was done using the Oh samples and the prediction results from SVM.
The results of the classification based on chemically determined and predicted pH values in form of a confusion matrix can be seen in Table 5. Based on the chemical values, 107 samples were classified as strongly acidic, 40 as very acidic, 17 as moderate acidic, seven as weakly acidic and one as neutral. When classified on basis of predictions, 107 samples ended up strongly acidic, 48 as very acidic, 11 as moderately acidic, 6 as weakly acidic and none as neutral. Balanced accuracy is highest for strongly acidic class with 0.87, while the accuracy for the other classes range from 0.5 to 0.79. In total, a value of 0.73 was reached.

Table 5.
Error matrix of predicted and observed pH-classes.
4. Discussion
4.1. Data Ranges of Soil Properties
The data ranges of the investigated properties show typical conditions for Saxon forests. Remarkable are the acidic conditions. Reasons for the low measured pH values lie in the parent materials. Alkaline parent materials are scarce and many forest sites were further acidified by historical coniferous usage on unsuitable sites. In addition, industrial element input (mainly sulfur and nitrogen) also led to soil acidification.
4.2. Spectral Behaviour
The investigation of the spectra obtained from Oh and Ah horizons showed divergent absorbance features. The most remarkable differences occurred in the visual range between 500 and 700 mn, at 1400 nm, at 1900 nm and above 2000 nm. We assume that the differences in spectral behaviour are a result of the distinct composition of Oh horizons when compared to mineral Ah horizons. As the differences occurred in wavelength ranges that are known to be sensitive for organic matter, it is reasonable to assume that the much higher percentage of organic matter in organic layers leads to the observed differences. It also explains higher absorbance values through the whole spectrum and especially the differences in the visual range and above 2000 nm. The feature differences at 1400 nm and 1900 nm can be caused by differences in organic matter, but also due to divergent percentages of clay, which is active in this regions. The differences arelikeweise pronounced in the variable importance of the PLSR models. For example, relevant features for C content prediction for Oh samples lay in the visual range while features around 2200 nm were most important for Ah samples. Regarding pH values, features around 1900 and 2200 nm were most important for Oh samples. On the other hand, the visual range was relevant as well in Ah horizons. Similar observations could be made for CEC, were the visual range was important in Oh samples, but models for Ah horizons mostly relied on the feature around 1900 nm.
4.3. Predicting Oh Properties
Prediction results for soil properties based on samples from Oh horizon yielded diverse results. Accuracy for C content was weak, as no algorithm was able to reach the proposed threshold of RPIQ = 1.89. However, keeping in mind that C content values were up to 49.43% in the Oh horizon, the RMSE values of 5.59 and R2 of 0.54 could still indicate useful results. The identified important spectral ranges in the visual range point to humic acids.
Due to the numerous examples of successful C content prediction in various settings, we expected better results. Possible reasons for the low accuracy could lie in the complex and heterogeneous structure of humic material compared to mineral soil material. A more intensive soil preparation in form of milling could help to address this issue. In an approach using soil samples from all over Europe and PLSR, Nocita et al. [68] reported R2 values of 0.76 for organic samples. However, they did not collect samples explicit from organic horizon, but selected samples with C content > 18% from a larger data set. Further, the sample location also included other land use forms such as cropland and grassland. These differences may explain the differences in prediction accuracy. Investigating forest soils in the Czech Republic, Gholizadeh et al. [22] reported R2 = 0.78 for combined organic horizons. They used material originating from L and F horizons as well. For Oh horizons, they reported R2 = 0.63 and RMSE = 4.2. Using samples originating from forests across China, Liu et al. [20] reached R2 = 0.75 for PLSR and R2 = 0.93 using Cubist. In this case, samples were taken from different depths without separating the horizons and thus covering a much wider range of carbon content values (0.2–99.6%).
We achieved moderate prediction results for N content using SVM and Cubist regression, with R2 up to 0.58 and RPIQ > 1.98 for SVM and Cubist. Thus, N content in humus samples can be estimated using vis-NIRS at sufficient accuracy. Relevant features in the visual range and around 2200 suggest humic acids and organic matter as spectral response. Vohland et al. [69] found similar spectral assignments for C and N content.
The model results of the used algorithms were quite similar for C/N ratio. However, no models reached results classified as moderate as RPIQ values were slightly below the proposed threshold.
Highest accuracy amongst investigated soil properties was found for pH value of Oh samples. Our models reached R2 values of 0.88 to 0.9 and RPIQ > 2.38. The relevant wavelengths around 1900 and 2200 nm point to indirect assignments via O-H and organic matter components. Thus, we conclude that pH value can be successfully predicted for Oh horizons using vis-NIR spectral data. It is remarkable that predictions are more accurate for pH than for C content, despite the high C content values. One reason might be the differences in wavelengths important for the predictions. In another study investigating vis-NIRS for SOM and pH prediction, Yang et al. also found different relevant wavelengths [70]. The results underline that predictions through indirect spectral assignments can be useful as well.
The RMSE values for CEC prediction in the Oh horizon were high, with values around 100 µeq/g. As a result, RPIQ values do not reach the threshold of 1.89. One reason for the high values were the few sample points with high CEC values, which are not predicted precisely. Another explanation could be the absence of clay in the organic horizon, as CEC correlation to vis-NIR spectra is suggested to be indirect and due to the correlation of CEC and clay [33]. This is also likely as the model relies mostly on the visual range. Even though BS has no direct spectral assignment, the models achieved meaningful results with RPIQ between 2.51 and 2.61 and R2 between 0.69 and 0.71. The important features around 2200 and 1930 nm suggest indirect assignments via hydroxy and carbonyl groups [25]. The scatter plot reveals a cluster of points at highest possible measured value. BS can not be greater than 100 per definition. However, some points were predicted to have values exceeding this threshold. The cluster therefore indicates that the defined valid values can be a problem for predictive modeling purposes. For the potential usage of predicted values in practical application, it is necessary to find a way on how to deal with such occurrences.
4.4. Predicting Ah Properties
Models calibrated for mineral Ah horizon achieved higher prediction accuracy for C and N content. The estimates of all algorithms resulted in RPIQ values ranging from 2.22 to 2.54 and R2 values between 0.59 and 0.72. According to the generally lower C and N percentages in mineral soil, RMSE was also lower (C: 1.56–1.77%, N: 0.07–0.08%). Therefore, vis-NIRS is suitable for C and N prediction in Ah horizons of forest soils. The better results compared to Oh samples could lie in the more homogeneous soil material producing a clearer spectral response. Gholizadeh et al. [22] reported R2 values of 0.53 when estimating C content of mineral horizons from Czech forests using SVM, which is less precise than our findings. However, they were using soil material from depths up to 40 cm. For A horizons, they reached an R2 of 0.72. On the other hand, e.g., Coûteaux et al. [71] reached R2 values of 0.8 for mineral soils. Therefore, our findings are in-between the results of other similar investigations.
Regarding N content, other studies reported similar prediction accuracy. Shi et al. [53] reached R2 values of 0.68 for PLSR and 0.76 for SVM on soil samples from different land use forms. A study investigating N content on grasslands in Norway reported models with R2 between 0.68 and 0.8 [72]. Thus, our results are within the same prediction accuracy as similar studies. For C and N content, the visual range as well as features around 1900 and 2200 nm were found to be important. These ranges were also found relevant in other studies [69].
Despite the results for C and N content prediction, model accuracy was weaker for C/N ratio for the mineral samples, with RMSE values being higher compared to models for Oh horizon. This is surprising, as more accurate values for single C and N values should also result in precise estimations for C/N ratio.
Other research reported likewise results for estimating C/N ratio. Ludwig et al. [73] had comparable PLSR results on forest soils, with R2 values C and N content being higher (>0.9) than the ones for C/N ratio (0.57). On agricultural sites, Mutuo et al. [74] observed similar findings. In their predictions, R2 for C (0.84) and N (0.87) was much more accurate than their attempt to estimate C/N ratio (0.37). Contrariwise, other studies indicate more precise predictions of C/N ratio. Chang et al. [51] obtained R2 of 0.88 when predicting using PLSR. Their samples were originating from grassland and agricultural sites. This contradictions raise the question if N is measured by direct response to vis-NIR spectra or as a result of correlation with C content within the samples. Chang et al. [51] interpret their results in favour of direct measurement. Ludwig et al. [18] name possible error propagation within the single measurement as possible source for the poorer performance, whereas Mutuo et al. [74] did not suggest any reasons for the different outcomes.
Regarding pH value, models were as well less precise for the Ah samples. None of the used algorithms were able to reach the proposed RPIQ threshold. The relevant wavelengths in the visual range and around 1900 nm suggest a indirect assignment to humic acids and hydroxy groups. However, the small range in the data set and the majority of samples having low pH values also results in low RPIQ values for pH. Further, the underestimation of few high values increases the RMSE value. Thus, we conclude that the PLSR results with R2 = 0.62 and RMSE = 0.29 could nevertheless be useful for forest soil mapping.
Investigating samples from 0 to 20 cm depth, Conforti et al. [75] calibrated models that reached higher prediction accuracy with R2 = 0.7 and RMSE = 0.15. In another study using only 0–5 cm depth from wetlands, Cohen et al. [76] reported RMSE values of 0.36, which is less precise than our PLSR results. Again, our predictions are in between the results of other investigations.
CEC prediction was successful for mineral samples as all algorithms achieved RPIQ values > 1.89. RMSE values were much lower than for organic samples, best R2 was 0.67. The better results and the importance of spectral features around 1900 nm underline the indirect prediction through correlation with clay, as mineral horizon contain more clay than organic layers. In comparison, Chang et al. [67] reported models with R2 = 0.81. Leone et al. [50] presented R2 values between 0.59 and 0.85, depending on the study site. Reasons for the occurring differences in some cases could be the usage of deeper depths up to 30 and 60 cm.
It was not possible to create meaningful models for BS in Ah horizons. Even though RMSE values were not much higher than for the Oh samples, predictions were unreliable as scatter was high and occurred through the whole range. One reason could be the different chemical analysis, which was carried out using ammonium chloride whilst Oh samples were analyzed using barium chloride. Another explanation could lie in the divergent percentages of organic compounds.
4.5. Classification Based on pH Values
To assess the prediction accuracy with regards to practical application for forest soil mapping, the Oh samples were classified based on predicted and observed pH values. Balanced accuracy for the different classes lay between 0.5 and 0.87. We conclude that the values obtained from vis-NIRS can therefore be used to classify the samples based on pH values to provide easy to interpret information for assessing humus conditions. This can be used to support silvicultural decision making regarding, e.g., selection of tree species or soil liming measures.
It is important to notice that the less acidic soils are highly underrepresented in the data. This impacts the classification result, as the majority of the samples ended up in the strongly and very acidic classes. Only a few samples have pH values high enough to be classified in the other classes. Especially the accuracy for the classes weakly acidic and neutral are therefore not very meaningful. However, we show how a classification could be implemented in future forest monitoring and the acidic conditions emphasize the need for management strategies. The approach should be repeated with samples that are more evenly distributed and not biased towards one class. One problem of this approach is that the location of the values within classification influences the precision. Unfortunately, there is no solution for this when a classification of the samples is desired.
4.6. Further Implications
We expected generally better prediction results for Ah horizons due to numerous examples for successful application on forest areas as well as other land use forms. Despite the more complex and heterogeneous structure of the humic material, the prediction accuracy was higher for samples from Oh horizons regarding the properties C/N ratio, pH-value and BS. As the latter are likely to be predicted through indirect spectral assignments, the better results can be explained by the different composition of the horizons regarding organic compounds.
As already mentioned, 18 samples collected as Oh horizon do not contain enough C to be classified as organic samples. On the other hand, it is also not possible to assume that these samples originate from the Ah horizon, as the separation during the sample collection was erroneous.
One way to overcome this problem when applying vis-NIRS to assess forest soil humus could lie in a combined investigation of every sample point. This way, an unclear separation of the horizons during the sample collection could not interfere with the model result. This approach has already been applied, e.g., for mixed samples of whole soil profiles [22] as well as modelling samples from both Oh and A horizon with the same model [71]. On the other hand, this could imply further difficulties as the wider data range for C and N contents could result in overoptimistic error measures [19]. Another reason against combined modeling is the different spectral behaviour of the investigated horizons.
Further, our samples originate from various forest types and from soils with different parent material. However, deciduous stands are highly underrepresented. This, e.g., impacts the distribution of pH values, for which it leads to an over-representation of acidic conditions. In addition, forest management strategies vary through the stands. The heterogeneity of the data set can affect the model accuracy, and local modelling approaches may have higher prediction power [44].
It would therefore be of great interest to expand the investigations to other study areas covering different forest types, soil forms and parent materials. Our results indicate that vis-NIRS could also be applied for humus assessment in other regions.
5. Conclusions
In this study, we investigated the applicability of vis-NIRS to predict soil properties relevant for humus assessment for samples from Oh and Ah horizon in Saxony.
Applications of vis-NIRS for the prediction of properties of organic soil layers of forests soils were not investigated so far, and our findings enrich the knowledge about the feasibility of this scenario.
For the Oh samples, we found useful predictions for N content, C/N ratio, pH value and base saturation based on the proposed classification scheme. Model results for C content and CEC did not reach the required threshold (RPIQ < 1.89).
Oh and Ah horizons showed distinct absorbance features in wavelength ranges that are known to be sensitive for organic matter. Thus, we assume that the much higher percentage of organic matter in organic layers leads to the observed differences. Therefore our results point to the relevance of the different composition of the humus layer and organic horizon for vis-NIRS. This is also reflected in the results.
In the Ah we achieved satisfying accuracy for C and N content, pH value and CEC. However, our results for C/N ratio were poor, and the predictions for BS turned out to be unreliable. We used calibrated regression models based on different algorithms. While SVM turned out to be most accurate for C and N content prediction, differences were minor for the other properties. None of the used algorithms clearly outperformed the others regarding the prediction of the investigated properties. With respect to its usefulness for forest soil mapping based on pH values, we compared classification into classes based on measured and predicted pH values and provided classification accuracy for the different classes, with satisfying results. We would like to emphasize the potential of retained soil samples for building spectral libraries. A great benefit for future studies can be achieved by adding samples from deciduous sites with less acidic conditions as well. We can conclude that our results indicate useful possibilities for the usage of vis-NIR spectroscopy for forest soil mapping and could help identifying areas in need for mitigation strategies to face soil degradation. Based on the feasibility of predictions and the comparably low effort and costs, we recommend the further development of the method to supplement the periodic forest soil mapping procedure.
Author Contributions
Conceptualization, F.T., U.W. and R.P.; methodology, F.T. and U.W.; software, F.T.; validation, F.T., U.W., C.B. and R.P.; formal analysis, F.T.; investigation, F.T., S.L. and C.B.; resources, R.P., U.W. and H.M.; data curation, F.T., S.L. and C.B.; writing—original draft preparation, F.T.; writing—review and editing, R.P., H.M., C.B. and U.W.; visualization, F.T.; supervision, U.W. and R.P.; project administration, U.W. and R.P.; funding acquisition, U.W. and R.P. All authors have read and agreed to the published version of the manuscript.
Funding
The study is part of the joint research project DIGI-Humus: “Verbundvorhaben: Erfassung und Regionalisierung von Humuseigenschaften mittels VIS-NIR und Digitaler Bodenkartierung” and was funded by the Federal Ministry of Food and Agriculure (project no. 22014417 and 22001316).
Data Availability Statement
The data set from this study is available through the EUDAT online archive under https://10.23728/b2share.20727e01b826450ebe59600c315925cb (accessed on 25 January 2022).
Acknowledgments
We want to thank the chemical laboratory of Public Enterprise Sachsenforst for analyzing the soil samples used in this study, and Marco Pohle and Helko Kotas for their technical support conducting the spectral measurements. We thank the Federal Ministry of Food and Agriculture (BMEL) and the Fachagentur Nachwachsende Rohstoffe (FNR) for permanent support of the project DIGI-Humus (Erfassung und Regionalisierung von Humuseigenschaften mittels VIS-NIR und Digitaler Bodenkartierung). This study received funding for F.T. (#22014417) and C.B. (#22001316) within the project DIGI-Humus.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| vis | visual |
| NIR | near infrared |
| C | carbon |
| N | nitrogen |
| CEC | cation exchange capacity |
| BS | base saturation |
| NFSI | national forest soil inventory |
| PSS | proximal soil sensing |
| vis-NIRS | visual and near-infrared reflectance spectroscopy |
| SOC | soil organic carbon |
| PLSR | partial least squares regression |
| SVM | support vector machine |
| RMSE | root mean square error |
| RPIQ | ratio of performance to interquartile |
| RPD | ratio of performance to deviation |
References
- Schuldt, B.; Buras, A.; Arend, M.; Vitasse, Y.; Beierkuhnlein, C.; Damm, A.; Gharun, M.; Grams, T.E.; Hauck, M.; Hajek, P.; et al. A first assessment of the impact of the extreme 2018 summer drought on Central European forests. Basic Appl. Ecol. 2020, 45, 86–103. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.T.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Nussbaum, M.; Spiess, K.; Baltensweiler, A.; Grob, U.; Keller, A.; Greiner, L.; Schaepman, M.E.; Papritz, A.J. Evaluation of digital soil mapping approaches with large sets of environmental covariates. Soil 2018, 4, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Conen, F.; Zerva, A.; Arrouays, D.; Jolivet, C.; Jarvis, P.G.; Grace, J.; Mencuccini, M. The carbon balance of forest soils: Detectability of changes in soil carbon stocks in temperate and boreal forests. Carbon Balance For. Biomes 2004, 9, 233–247. [Google Scholar]
- Petzold, R.; Benning, R.; Gauer, J. Bodeninformationen in den verschiedenen Standortserkundungssystemen Deutschlands: Gegenwärtiger Stand und Perspektiven. Wald. Landsch. Nat. 2016, 16, 7–17. [Google Scholar]
- Prescott, C.E.; Maynard, D.G.; Laiho, R. Humus in northern forests: Friend or foe? For. Ecol. Manag. 2000, 133, 23–36. [Google Scholar] [CrossRef]
- Binkley, D.; Fisher, R.F. Ecology and Management of Forest Soils, 5th ed.; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar]
- Schulze, G.; Kopp, D. Anleitung für die forstliche Standortserkundung im nordostdeutschen Tiefland. Standortserkundungsanleitung 2013, 95, 1–178. [Google Scholar]
- Wellbrock, N.; Ahrends, B.; Bögelein, R.; Bolte, A.; Eickenscheidt, N.; Grüneberg, E.; König, N.; Schmitz, A.; Fleck, S.; Ziche, D. Concept and methodology of the national forest soil inventory. In Status and Dynamics of Forests in Germany; Springer: Cham, Switzerland, 2019; pp. 1–28. [Google Scholar]
- Rossel, R.V.; Adamchuk, V.; Sudduth, K.; McKenzie, N.; Lobsey, C. Proximal soil sensing: An effective approach for soil measurements in space and time. In Advances in Agronomy; Elsevier: Amsterdam, The Netherland, 2011; Volume 113, pp. 243–291. [Google Scholar]
- Viscarra Rossel, R.; Lark, R. Improved analysis and modelling of soil diffuse reflectance spectra using wavelets. Eur. J. Soil Sci. 2009, 60, 453–464. [Google Scholar] [CrossRef]
- Nocita, M.; Stevens, A.; van Wesemael, B.; Aitkenhead, M.; Bachmann, M.; Barthès, B.; Dor, E.B.; Brown, D.J.; Clairotte, M.; Csorba, A.; et al. Soil spectroscopy: An alternative to wet chemistry for soil monitoring. Adv. Agron. 2015, 132, 139–159. [Google Scholar]
- Reeves, J.; McCarty, G.; Meisinger, J. Near infrared reflectance spectroscopy for the analysis of agricultural soils. J. Near Infrared Spectrosc. 1999, 7, 179–193. [Google Scholar] [CrossRef]
- Kuang, B.; Mouazen, A. Calibration of visible and near infrared spectroscopy for soil analysis at the field scale on three European farms. Eur. J. Soil Sci. 2011, 62, 629–636. [Google Scholar] [CrossRef]
- Rossel, R.V.; Walvoort, D.; McBratney, A.; Janik, L.J.; Skjemstad, J. Visible, near infrared, mid infrared or combined diffuse reflectance spectroscopy for simultaneous assessment of various soil properties. Geoderma 2006, 131, 59–75. [Google Scholar] [CrossRef]
- Ribeiro, S.G.; Teixeira, A.d.S.; de Oliveira, M.R.R.; Costa, M.C.G.; Araújo, I.C.d.S.; Moreira, L.C.J.; Lopes, F.B. Soil Organic Carbon Content Prediction Using Soil-Reflected Spectra: A Comparison of Two Regression Methods. Remote Sens. 2021, 13, 4752. [Google Scholar] [CrossRef]
- Pietrzykowski, M.; Chodak, M. Near infrared spectroscopy—A tool for chemical properties and organic matter assessment of afforested mine soils. Ecol. Eng. 2014, 62, 115–122. [Google Scholar] [CrossRef]
- Ludwig, B.; Vormstein, S.; Niebuhr, J.; Heinze, S.; Marschner, B.; Vohland, M. Estimation accuracies of near infrared spectroscopy for general soil properties and enzyme activities for two forest sites along three transects. Geoderma 2017, 288, 37–46. [Google Scholar] [CrossRef]
- Thomas, F.; Petzold, R.; Becker, C.; Werban, U. Application of Low-Cost MEMS Spectrometers for Forest Topsoil Properties Prediction. Sensors 2021, 21, 3927. [Google Scholar] [CrossRef]
- Liu, S.; Shen, H.; Chen, S.; Zhao, X.; Biswas, A.; Jia, X.; Shi, Z.; Fang, J. Estimating forest soil organic carbon content using vis-NIR spectroscopy: Implications for large-scale soil carbon spectroscopic assessment. Geoderma 2019, 348, 37–44. [Google Scholar] [CrossRef]
- Pinheiro, É.F.; Ceddia, M.B.; Clingensmith, C.M.; Grunwald, S.; Vasques, G.M. Prediction of soil physical and chemical properties by visible and near-infrared diffuse reflectance spectroscopy in the central Amazon. Remote Sens. 2017, 9, 293. [Google Scholar] [CrossRef] [Green Version]
- Gholizadeh, A.; Viscarra Rossel, R.A.; Saberioon, M.; Borůvka, L.; Kratina, J.; Pavlů, L. National-scale spectroscopic assessment of soil organic carbon in forests of the Czech Republic. Geoderma 2021, 385, 114832. [Google Scholar] [CrossRef]
- Ertlen, D.; Schwartz, D.; Trautmann, M.; Webster, R.; Brunet, D. Discriminating between organic matter in soil from grass and forest by near-infrared spectroscopy. Eur. J. Soil Sci. 2010, 61, 207–216. [Google Scholar] [CrossRef]
- Wellbrock, N.; Grüneberg, E.; Ziche, D.; Eickenscheidt, N.; Holzhausen, M.; Höhle, J.; Gemballa, R.; Andreae, H. Entwicklung einer Methodik zur stichprobengestützten Erfassung und Regionalisierung von Zustandseigenschaften der Waldstandorte; Thünen Report 36; Johann Heinrich von Thünen-Institut: Braunschweig, Germany, 2015. [Google Scholar] [CrossRef]
- Rossel, R.V.; Behrens, T. Using data mining to model and interpret soil diffuse reflectance spectra. Geoderma 2010, 158, 46–54. [Google Scholar] [CrossRef]
- Rossel, R.V.; Behrens, T.; Ben-Dor, E.; Brown, D.; Demattê, J.; Shepherd, K.D.; Shi, Z.; Stenberg, B.; Stevens, A.; Adamchuk, V.; et al. A global spectral library to characterize the world’s soil. Earth-Sci. Rev. 2016, 155, 198–230. [Google Scholar] [CrossRef] [Green Version]
- Christy, C.D. Real-time measurement of soil attributes using on-the-go near infrared reflectance spectroscopy. Comput. Electron. Agric. 2008, 61, 10–19. [Google Scholar] [CrossRef]
- Gubler, A. Quantitative Estimations of Soil Properties by Visible and Near Infrared Spectroscopy: Applications for Laboratory and Field Measurements. Ph.D. Thesis, University of Bern, Bern, Switzerland, 2011. [Google Scholar]
- Knadel, M.; Masís-Meléndez, F.; de Jonge, L.W.; Moldrup, P.; Arthur, E.; Greve, M.H. Assessing soil water repellency of a sandy field with visible near infrared spectroscopy. J. Near Infrared Spectrosc. 2016, 24, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Baumgardner, M.F.; Silva, L.F.; Biehl, L.L.; Stoner, E.R. Reflectance properties of soils. Adv. Agron. 1986, 38, 1–44. [Google Scholar]
- Hunt, G.R. Spectral signatures of particulate minerals in the visible and near infrared. Geophysics 1977, 42, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Fourty, T.; Baret, F.; Jacquemoud, S.; Schmuck, G.; Verdebout, J. Leaf optical properties with explicit description of its biochemical composition: Direct and inverse problems. Remote Sens. Environ. 1996, 56, 104–117. [Google Scholar] [CrossRef]
- Ben-Dor, E.; Banin, A. Near-infrared analysis as a rapid method to simultaneously evaluate several soil properties. Soil Sci. Soc. Am. J. 1995, 59, 364–372. [Google Scholar] [CrossRef]
- Stenberg, B.; Rossel, R.A.V.; Mouazen, A.M.; Wetterlind, J. Visible and near infrared spectroscopy in soil science. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2010; Volume 107, pp. 163–215. [Google Scholar]
- Xu, D.; Ma, W.; Chen, S.; Jiang, Q.; He, K.; Shi, Z. Assessment of important soil properties related to Chinese Soil Taxonomy based on vis–NIR reflectance spectroscopy. Comput. Electron. Agric. 2018, 144, 1–8. [Google Scholar] [CrossRef]
- Gutachterausschuss Forstliche Analytik. Handbuch Forstliche Analytik. Eine Loseblatt-Sammlung der Analysemethoden im Forstbereich. 2014. Available online: https://www.nw-fva.de/fileadmin/nwfva/publikationen/pdf/konig_handbuch_forstliche.pdf (accessed on 28 September 2021).
- DIN ISO 10390: 2005; Bodenbeschaffenheit—Bestimmung des pH-Wertes. Deutsches Insitut für Normung: Berlin, Germany, 2005.
- Höhle, J.; Bielefeldt, J.; Dühnelt, P.; König, N.; Ziche, D.; Eickenscheidt, N.; Grüneberg, E.; Hilbrig, L.; Wellbrock, N. Bodenzustandserhebung im Wald-Dokumentation und Harmonisierung der Methoden; Technical Report, Thünen Working Paper; Johann Heinrich von Thünen-Institut: Braunschweig, Germany, 2018. [Google Scholar]
- DIN 10694; Bestimmung des Organischen Kohlenstoffgehaltes und des Gesamtkohlenstoffgehaltes Nach Trockener Verbrennung (Elementaranalyse); Deutsche Normen (ed. Fachnormenausschuß Wasserwesen, FNW, im DIN Deutsches Institut für Normung e.V.). Beuth Verlag: Berlin, Germany, 1994.
- Avian Technologies. Fluorilon Gray Scale Standards & Targets. 2019. Available online: https://aviantechnologies.com/product/gray-scale-standards/ (accessed on 13 June 2019).
- Mac Arthur, A.; MacLellan, C.J.; Malthus, T. The fields of view and directional response functions of two field spectroradiometers. IEEE Trans. Geosci. Remote Sens. 2012, 50, 3892–3907. [Google Scholar] [CrossRef]
- Rinnan, Å.; Van Den Berg, F.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Stevens, A.; Nocita, M.; Tóth, G.; Montanarella, L.; van Wesemael, B. Prediction of soil organic carbon at the European scale by visible and near infrared reflectance spectroscopy. PLoS ONE 2013, 8, e66409. [Google Scholar] [CrossRef]
- Barnes, R.; Dhanoa, M.S.; Lister, S.J. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Stevens, A.; Ramirez-Lopez, L. An Introduction to the Prospectr Package, Version 0.1.3; R Core Team: Vienna, Austria, 2013.
- Filzmoser, P.; Gschwandtner, M. Mvoutlier: Multivariate Outlier Detection Based on Robust Methods; Version 2.0.9; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Filzmoser, P.; Garrett, R.G.; Reimann, C. Multivariate outlier detection in exploration geochemistry. Comput. Geosci. 2005, 31, 579–587. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Leone, A.; Viscarra-Rossel, R.; Amenta, P.; Buondonno, A. Prediction of soil properties with PLSR and vis-NIR spectroscopy: Application to mediterranean soils from Southern Italy. Curr. Anal. Chem. 2012, 8, 283–299. [Google Scholar] [CrossRef]
- Chang, C.W.; Laird, D.A. Near-infrared reflectance spectroscopic analysis of soil C and N. Soil Sci. 2002, 167, 110–116. [Google Scholar] [CrossRef]
- Reeves, J.B., III. Near-versus mid-infrared diffuse reflectance spectroscopy for soil analysis emphasizing carbon and laboratory versus on-site analysis: Where are we and what needs to be done? Geoderma 2010, 158, 3–14. [Google Scholar] [CrossRef]
- Shi, T.; Cui, L.; Wang, J.; Fei, T.; Chen, Y.; Wu, G. Comparison of multivariate methods for estimating soil total nitrogen with visible/near-infrared spectroscopy. Plant Soil 2013, 366, 363–375. [Google Scholar] [CrossRef]
- Sorenson, P.T.; Small, C.; Tappert, M.; Quideau, S.A.; Drozdowski, B.; Underwood, A.; Janz, A. Monitoring organic carbon, total nitrogen, and pH for reclaimed soils using field reflectance spectroscopy. Can. J. Soil Sci. 2017, 97, 241–248. [Google Scholar] [CrossRef]
- Wold, S.; Martens, H.; Wold, H. The multivariate calibration problem in chemistry solved by the PLS method. In Matrix Pencils; Springer: Cham, Switzerland, 1983; pp. 286–293. [Google Scholar]
- Ramirez-Lopez, L.; Behrens, T.; Schmidt, K.; Stevens, A.; Demattê, J.A.M.; Scholten, T. The spectrum-based learner: A new local approach for modeling soil vis–NIR spectra of complex datasets. Geoderma 2013, 195, 268–279. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer Science & Business Media: Cham, Switzerland, 2009. [Google Scholar]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Müller, A.C.; Guido, S. Introduction to Machine Learning with Python: A Guide For Data Scientists, 1st ed.; O’Reilly Media, Inc.: Newton, MA, USA, 2016. [Google Scholar]
- Quinlan, J.R. Learning with continuous classes. In Proceedings of the 5th Australian Joint Conference on Artificial Intelligence, World Scientific, Canberra, ACT, Australia, 2–5 December 1992; Volume 92, pp. 343–348. [Google Scholar]
- Appelhans, T.; Mwangomo, E.; Hardy, D.R.; Hemp, A.; Nauss, T. Evaluating machine learning approaches for the interpolation of monthly air temperature at Mt. Kilimanjaro, Tanzania. Spat. Stat. 2015, 14, 91–113. [Google Scholar] [CrossRef] [Green Version]
- Morellos, A.; Pantazi, X.E.; Moshou, D.; Alexandridis, T.; Whetton, R.; Tziotzios, G.; Wiebensohn, J.; Bill, R.; Mouazen, A.M. Machine learning based prediction of soil total nitrogen, organic carbon and moisture content by using VIS-NIR spectroscopy. Biosyst. Eng. 2016, 152, 104–116. [Google Scholar] [CrossRef] [Green Version]
- Walton, J.T. Subpixel urban land cover estimation. Photogramm. Eng. Remote Sens. 2008, 74, 1213–1222. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, M. caret: Classification and Regression Training; Version 6.0-81; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Nash, J.E.; Sutcliffe, J.V. River flow forecasting through conceptual models part I—A discussion of principles. J. Hydrol. 1970, 10, 282–290. [Google Scholar] [CrossRef]
- Bellon-Maurel, V.; Fernandez-Ahumada, E.; Palagos, B.; Roger, J.M.; McBratney, A. Critical review of chemometric indicators commonly used for assessing the quality of the prediction of soil attributes by NIR spectroscopy. TrAC Trends Anal. Chem. 2010, 29, 1073–1081. [Google Scholar] [CrossRef]
- Chang, C.W.; Laird, D.A.; Mausbach, M.J.; Hurburgh, C.R. Near-infrared reflectance spectroscopy–principal components regression analyses of soil properties. Soil Sci. Soc. Am. J. 2001, 65, 480–490. [Google Scholar] [CrossRef] [Green Version]
- Nocita, M.; Stevens, A.; Toth, G.; Panagos, P.; van Wesemael, B.; Montanarella, L. Prediction of soil organic carbon content by diffuse reflectance spectroscopy using a local partial least square regression approach. Soil Biol. Biochem. 2014, 68, 337–347. [Google Scholar] [CrossRef]
- Vohland, M.; Ludwig, M.; Thiele-Bruhn, S.; Ludwig, B. Determination of soil properties with visible to near-and mid-infrared spectroscopy: Effects of spectral variable selection. Geoderma 2014, 223, 88–96. [Google Scholar] [CrossRef]
- Yang, M.; Xu, D.; Chen, S.; Li, H.; Shi, Z. Evaluation of machine learning approaches to predict soil organic matter and pH using Vis-NIR spectra. Sensors 2019, 19, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coûteaux, M.M.; Berg, B.; Rovira, P. Near infrared reflectance spectroscopy for determination of organic matter fractions including microbial biomass in coniferous forest soils. Soil Biol. Biochem. 2003, 35, 1587–1600. [Google Scholar] [CrossRef]
- Fystro, G. The prediction of C and N content and their potential mineralisation in heterogeneous soil samples using Vis–NIR spectroscopy and comparative methods. Plant Soil 2002, 246, 139–149. [Google Scholar] [CrossRef]
- Ludwig, B.; Khanna, P.; Bauhus, J.; Hopmans, P. Near infrared spectroscopy of forest soils to determine chemical and biological properties related to soil sustainability. For. Ecol. Manag. 2002, 171, 121–132. [Google Scholar] [CrossRef]
- Mutuo, P.K.; Shepherd, K.D.; Albrecht, A.; Cadisch, G. Prediction of carbon mineralization rates from different soil physical fractions using diffuse reflectance spectroscopy. Soil Biol. Biochem. 2006, 38, 1658–1664. [Google Scholar] [CrossRef]
- Conforti, M.; Matteucci, G.; Buttafuoco, G. Using laboratory Vis-NIR spectroscopy for monitoring some forest soil properties. J. Soils Sediments 2018, 18, 1009–1019. [Google Scholar] [CrossRef]
- Cohen, M.J.; Prenger, J.P.; DeBusk, W.F. Visible-near infrared reflectance spectroscopy for rapid, nondestructive assessment of wetland soil quality. J. Environ. Qual. 2005, 34, 1422–1434. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).