Evaluation of Near-Infrared Reflectance and Transflectance Sensing System for Predicting Manure Nutrients
Abstract
1. Introduction
2. Material and Methods
2.1. Manure Sampling and Chemical Analysis
2.2. Manure Spiking
2.3. Near-Infrared (NIR) Spectroscopic Device and Measurement
2.4. Statistical Analysis and PLS Calibration Assessment
3. Results
3.1. NIR Spectra
3.2. Chemical Composition of Manure Samples
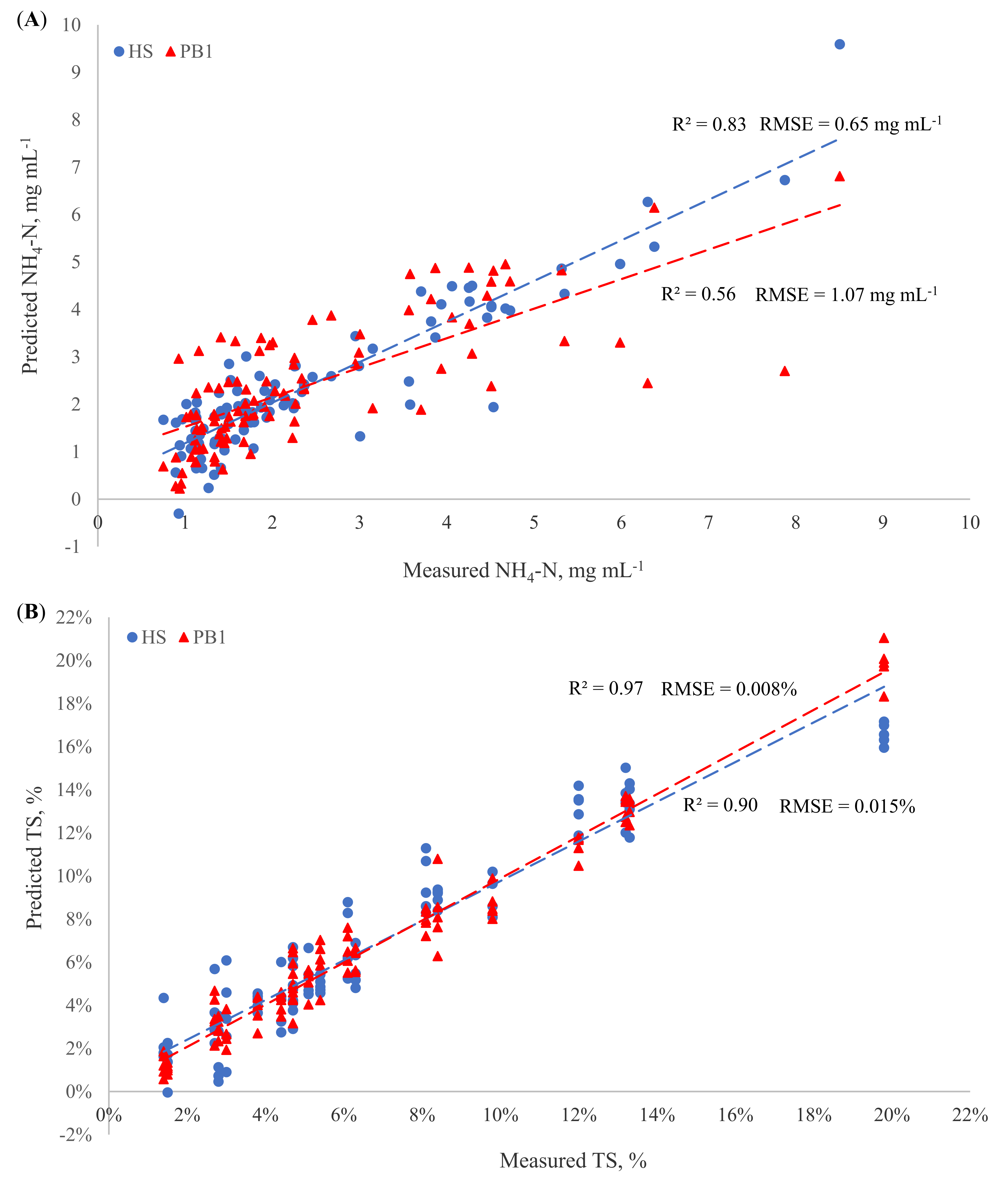
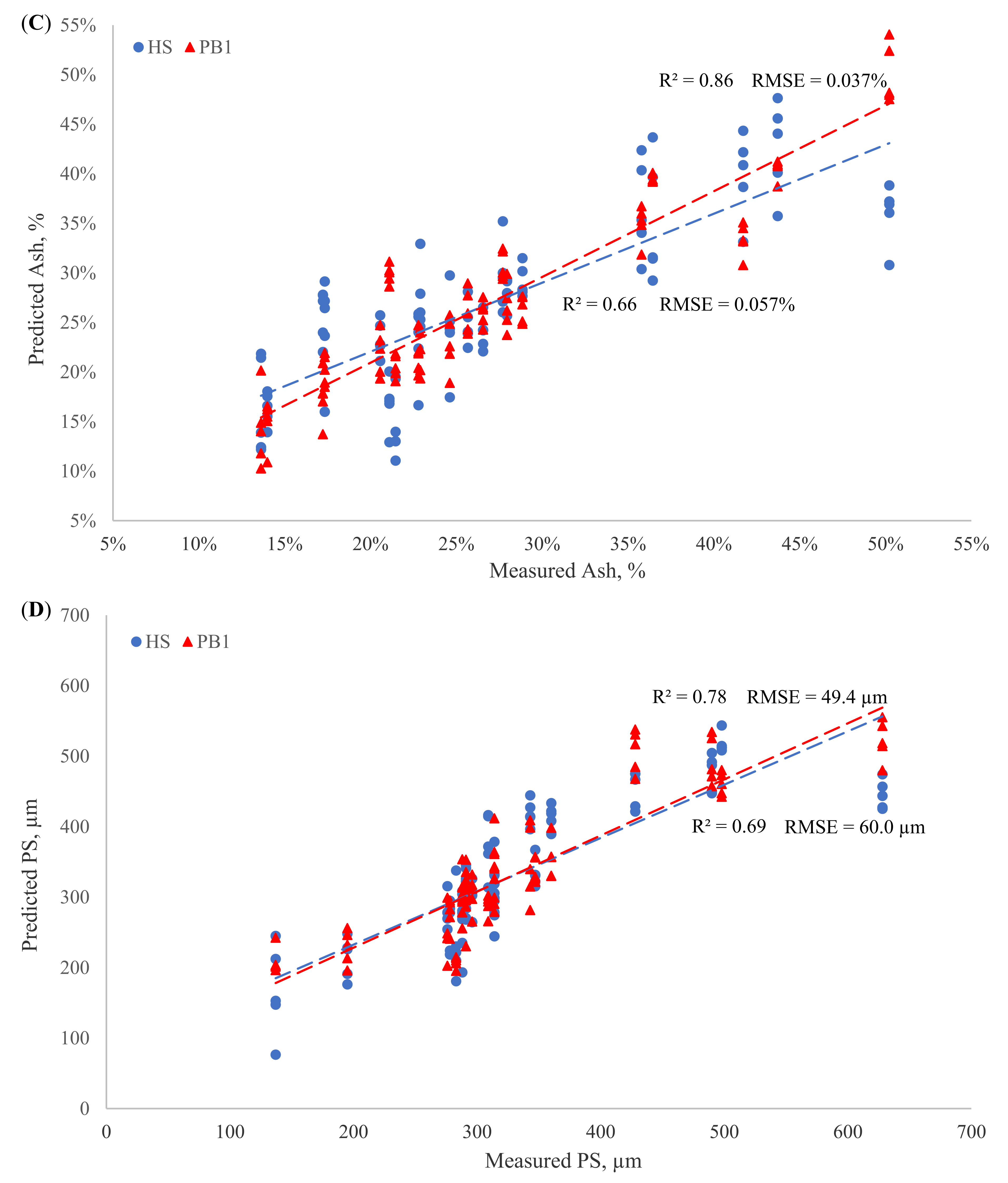
3.3. Constituent PLS Calibrations of Reflectance and Transflectance Sensors
3.3.1. Path Lengths
3.3.2. Reflectance and Transflectance PLS Calibration
3.4. Relationship between Nitrogen and Other Manure Parameters
4. Discussion
4.1. Effect of Path Lengths of Transflectance Mode
4.2. NIR Prediction for Nitrogen Concentrations
4.3. Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aguirre-Villegas, H.A.; Sharara, M.A.; Larson, R.A. Nutrient variability following dairy manure storage agitation. Appl. Eng. Agric. 2018, 34, 908–917. [Google Scholar] [CrossRef]
- Sanford, J.R.; Larson, R.A.; Digman, M.F. Assessing certified manure analysis laboratory accuracy and variability. Appl. Eng. Agric. 2020, 36, 905–912. [Google Scholar] [CrossRef]
- Dou, Z.; Galligan, D.T.; Allshouse, R.D.; Toth, J.D.; Ramberg, C.F., Jr.; Ferguson, J.D. Manure sampling for nutrient analysis: Variability and sampling efficacy. J. Environ. Qual. 2001, 30, 1432–1437. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pasquini, C. Near infrared spectroscopy: A mature analytical technique with new perspectives—A review. Anal. Chim. Acta 2018, 1026, 8–36. [Google Scholar] [CrossRef]
- Huang, G.; Han, L.; Liu, X. Rapid estimation of the composition of animal manure compost by near infrared reflectance spectroscopy. J. Near Infrared Spectrosc. 2007, 15, 387–394. [Google Scholar] [CrossRef]
- Guo, P.; Li, T.; Gao, H.; Chen, X.; Cui, Y.; Huang, Y. Evaluating Calibration and Spectral Variable Selection Methods for Predicting Three Soil Nutrients Using Vis-NIR Spectroscopy. Remote Sens. 2021, 13, 4000. [Google Scholar] [CrossRef]
- Kawamura, K.; Tsujimoto, Y.; Nishigaki, T.; Andriamananjara, A.; Rabenarivo, M.; Asai, H.; Rakotoson, T.; Razafimbelo, T. Laboratory visible and near-infrared spectroscopy with genetic algorithm-based partial least squares regression for assessing the soil phosphorus content of upland and lowland rice fields in Madagascar. Remote Sens. 2019, 11, 506. [Google Scholar] [CrossRef]
- Millmier, A.; Lorimor, J.; Hurburgh Jr, C.; Fulhage, C.; Hattey, J.; Zhang, H. Near-infrared sensing of manure nutrients. Trans. ASAE 2000, 43, 903. [Google Scholar] [CrossRef]
- Nam, J.J.; Lee, S.H. Non-destructive analysis of compost by near infrared spectroscopy. J. Korean Chem. Soc. 2000, 445, 410–414. [Google Scholar]
- Kemsley, E.K.; Tapp, H.S.; Scarlett, A.J.; Miles, S.J.; Hammond, R.; Wilson, R.H. Comparison of spectroscopic techniques for the determination of Kjeldahl and ammoniacal nitrogen content of farmyard manure. J. Agric. Food Chem. 2001, 49, 603–609. [Google Scholar] [CrossRef]
- Reeves, J.B. Near-infrared diffuse reflectance spectroscopy for the analysis of poultry manures. J. Agric. Food Chem. 2001, 49, 2193–2197. [Google Scholar] [CrossRef]
- Saeys, W.; Darius, P.; Ramon, H. Potential for on-site analysis of hog manure using a visual and near infrared diode array reflectance spectrometer. J. Near Infrared Spectrosc. 2004, 12, 299–309. [Google Scholar] [CrossRef]
- Ye, W.; Lorimor, J.C.; Hurburgh, C.; Zhang, H.; Hattey, J. Application of near-infrared reflectance spectroscopy for determination of nutrient contents in liquid and solid manures. Trans. ASAE 2005, 485, 1911–1918. [Google Scholar] [CrossRef]
- Malley, D.F.; Yesmin, L.; Eilers, R.G. Rapid analysis of hog manure and manure-amended soils using near-infrared spectroscopy. Soil Sci. Soc. Am. J. 2002, 66, 1677–1686. [Google Scholar] [CrossRef]
- Raju, C.S.; Løkke, M.M.; Sutaryo, S.; Ward, A.J.; Møller, H.B. NIR monitoring of ammonia in anaerobic digesters using a diffuse reflectance probe. Sensors 2012, 12, 2340–2350. [Google Scholar] [CrossRef]
- Saeys, W.; Xing, J.; De Baerdemaeker, J.; Ramon, H. Comparison of transflectance and reflectance to analyse hog manures. J. Near Infrared Spectrosc. 2005, 13, 99–107. [Google Scholar] [CrossRef]
- Kuang, B.; Mouazen, A.M. Effect of spiking strategy and ratio on calibration of on-line visible and near infrared soil sensor for measurement in European farms. Soil Tillage Res. 2013, 128, 125–136. [Google Scholar] [CrossRef]
- Waiser, T.H.; Morgan, C.L.; Brown, D.J.; Hallmark, C.T. In situ characterization of soil clay content with visible near-infrared diffuse reflectance spectroscopy. Soil Sci. Soc. Am. J. 2007, 71, 389–396. [Google Scholar] [CrossRef]
- Sankey, J.B.; Brown, D.J.; Bernard, M.L.; Lawrence, R.L. Comparing local vs. global visible and near-infrared (VisNIR) diffuse reflectance spectroscopy (DRS) calibrations for the prediction of soil clay, organic C and inorganic C. Geoderma 2008, 148, 149–158. [Google Scholar] [CrossRef]
- Minasny, B.; McBratney, A.B.; Pichon, L.; Sun, W.; Short, M.G. Evaluating near infrared spectroscopy for field prediction of soil properties. Soil Res. 2009, 47, 664–673. [Google Scholar] [CrossRef]
- Wetterlind, J.; Stenberg, B. Near-infrared spectroscopy for within-field soil characterization: Small local calibrations compared with national libraries spiked with local samples. Eur. J. Soil Sci. 2010, 61, 823–843. [Google Scholar] [CrossRef]
- Guerrero, C.; Zornoza, R.; Gómez, I.; Mataix-Beneyto, J. Spiking of NIR regional models using samples from target sites: Effect of model size on prediction accuracy. Geoderma 2010, 158, 66–77. [Google Scholar] [CrossRef]
- Cezar, E.; Nanni, M.R.; Crusiol, L.G.T.; Sun, L.; Chicati, M.S.; Furlanetto, R.H.; Rodrigues, M.; Sibaldelli, R.N.R.; Silva, G.F.C.; Oliveira, K.M.D.; et al. Strategies for the development of spectral models for soil organic matter estimation. Remote Sens. 2021, 13, 1376. [Google Scholar] [CrossRef]
- ASTM Committee D-18 on Soil and Rock. Standard Test Methods for Particle-Size Distribution (Gradation) of Soils Using Sieve Analysis; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- Peters, J.; Combs, S.; Hoskins, B.; Jarman, J.; Kovar, J.; Watson, M.; Wolf, A.; Wolf, N. Recommended Methods of Manure Analysis; University of Wisconsin Cooperative Extension Publishing: Madison, WI, USA, 2003. [Google Scholar]
- Wise, B.M.; Gallagher, N.B. PLS Toolbox for Use with MATLAB; Eigenvector Technologies: West Richland, WA, USA, 1998. [Google Scholar]
- Williams, P.; Dardenne, P.; Flinn, P. Tutorial: Items to be included in a report on a near infrared spectroscopy project. J. Near Infrared Spectrosc. 2017, 25, 85–90. [Google Scholar] [CrossRef]
- Dardenne, P. Some considerations about NIR spectroscopy: Closing speech at NIR-2009. NIR News 2010, 21, 8–14. [Google Scholar] [CrossRef]
- Williams, P. Implementation of Near-Infrared Technology. In Near-Infrared Technology in the Agricultural and Food Industries, 2nd ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2001. [Google Scholar]
- Williams, P. The RPD statistic: A tutorial note. NIR News 2014, 25, 22–26. [Google Scholar] [CrossRef]
- Chen, L.; Xing, L.; Han, L. Review of the application of near-infrared spectroscopy technology to determine the chemical composition of animal manure. J. Environ. Qual. 2013, 42, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, E.; Castaldelli, G.; Ferrari, G.; Marchetti, M.G.; Pedrini, P.; Aschonitis, V.G. Onsite and online FT-NIR spectroscopy for the estimation of total nitrogen and moisture content in poultry manure. Environ. Technol. 2015, 36, 2285–2294. [Google Scholar] [CrossRef] [PubMed]
- Saeys, W.; Mouazen, A.M.; Ramon, H. Potential for onsite and online analysis of pig manure using visible and near infrared reflectance spectroscopy. Biosyst. Eng. 2005, 91, 393–402. [Google Scholar] [CrossRef]
- Finzi, A.; Oberti, R.; Negri, A.S.; Perazzolo, F.; Cocolo, G.; Tambone, F.; Cabassi, G.; Provolo, G. Effects of measurement technique and sample preparation on NIR spectroscopy analysis of livestock slurry and digestates. Biosyst. Eng. 2015, 134, 42–54. [Google Scholar] [CrossRef]
- Lorimor, J.; Powers, W.; Sutton, A. Manure Characteristics: Manure Management Systems Series; MWPS 18, Section 1; Midwest Plan Service: Ames, IA, USA, 2004. [Google Scholar]
- Cabassi, G.; Cavalli, D.; Fuccella, R.; Gallina, P.M. Evaluation of four NIR spectrometers in the analysis of cattle slurry. Biosyst. Eng. 2015, 133, 1–13. [Google Scholar] [CrossRef]
- Reeves, J.B.; Van Kessel, J.S. Near-infrared spectroscopic determination of carbon, total nitrogen, and ammonium-N in dairy manures. J. Dairy Sci. 2000, 83, 1829–1836. [Google Scholar] [CrossRef]
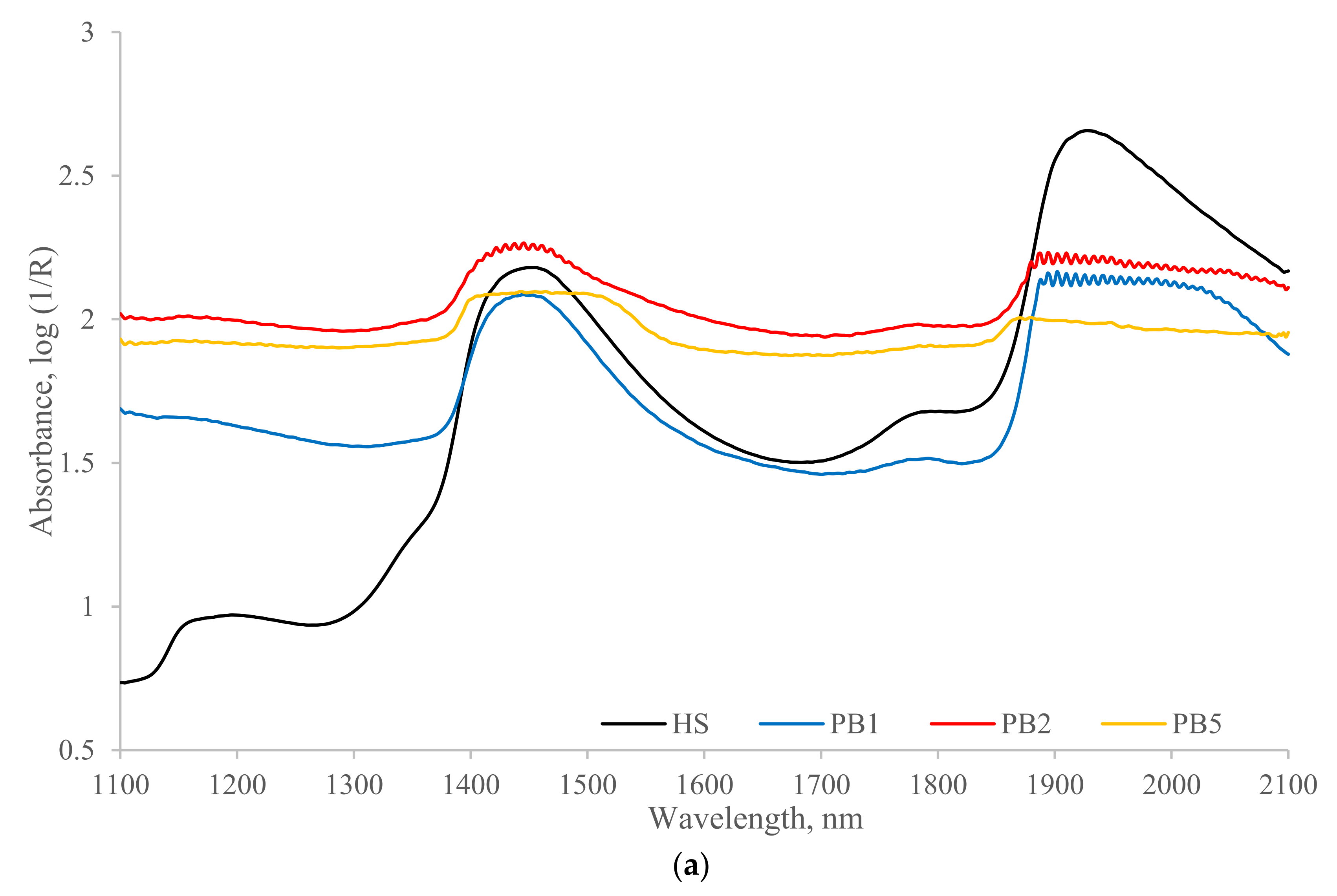
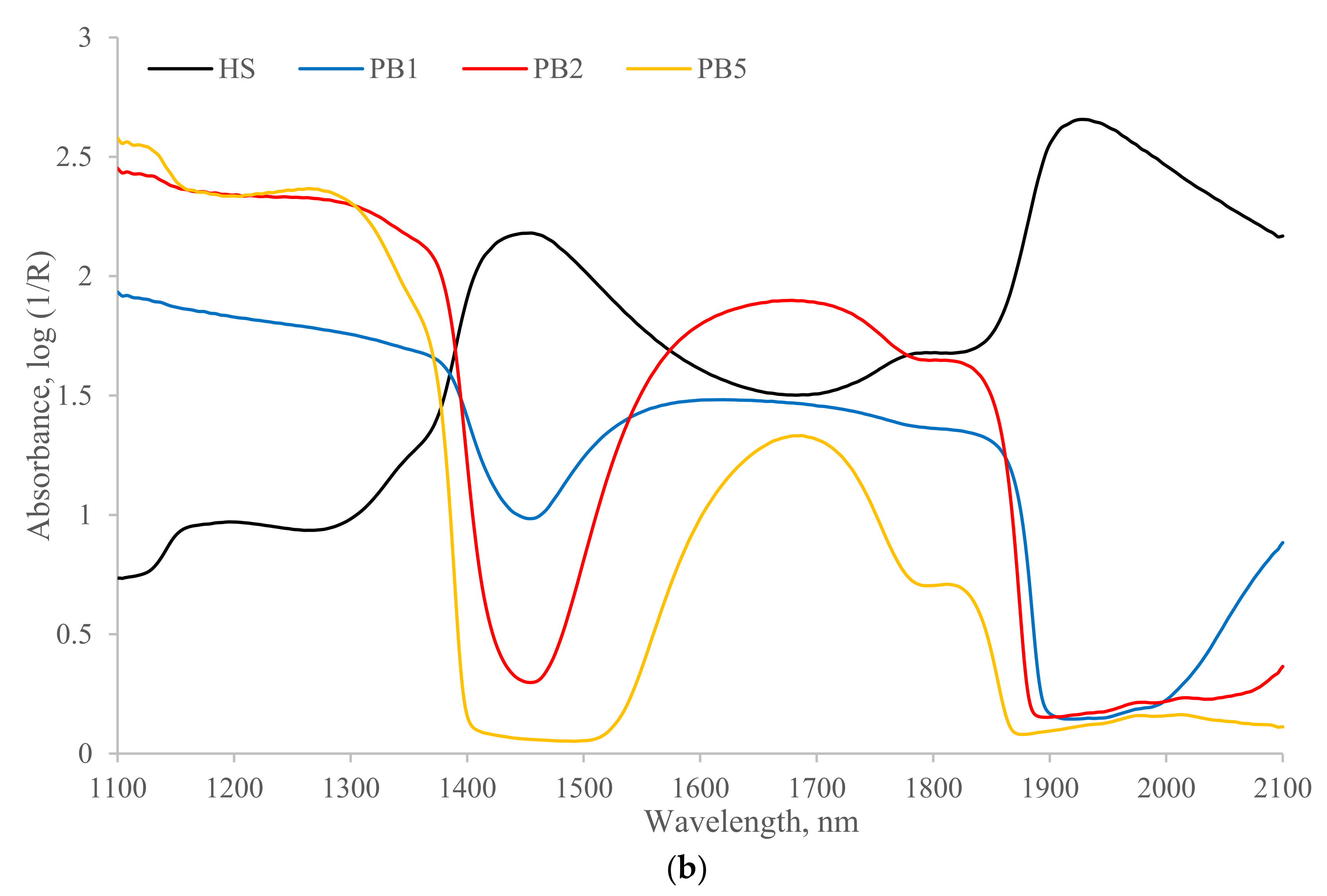




| Sample ID | TS (%) | Ash (%) | PS (µM) | NH4-N (mg mL−1) | Org-N (mg mL−1) | P (mg mL−1) | K (mg mL−1) |
|---|---|---|---|---|---|---|---|
| S1 | 9.8 | 13.6 | 490 | 1.07 | 1.46 | 0.41 | 1.70 |
| S2 | 8.1 | 14.0 | 498 | 1.13 | 1.37 | 0.42 | 1.56 |
| S3 | 5.4 | 20.6 | 291 | 1.13 | 1.29 | 0.45 | 1.52 |
| S4 | 5.1 | 22.9 | 291 | 1.12 | 1.27 | 0.44 | 1.52 |
| S5 | 3.8 | 28.9 | 347 | 1.13 | 1.25 | 0.43 | 1.40 |
| S6 | 4.7 | 24.6 | 296 | 1.17 | 1.26 | 0.44 | 1.38 |
| S7 | 4.4 | 25.7 | 288 | 1.18 | 1.23 | 0.47 | 1.36 |
| S8 | 3.0 | 28.0 | 278 | 0.96 | 0.98 | 0.36 | 1.30 |
| S9 | 2.8 | 26.6 | 276 | 0.89 | 0.95 | 0.34 | 1.14 |
| S10 | 2.7 | 27.7 | 195 | 0.97 | 0.94 | 0.33 | 1.18 |
| S11 | 19.8 | 50.2 | 343 | 1.50 | 2.84 | 0.81 | 2.00 |
| S12 | 6.1 | 35.8 | 288 | 4.25 | 1.57 | 0.53 | 5.05 |
| S13 | 12.0 | 17.2 | 360 | 1.58 | 2.84 | 0.92 | 3.67 |
| S14 | 4.7 | 21.5 | 314 | 1.01 | 1.09 | 0.47 | 1.85 |
| S15 | 1.4 | 36.4 | 283 | 0.75 | 0.36 | 0.16 | 1.08 |
| S16 | 1.5 | 43.7 | 137 | 0.90 | 0.37 | 0.16 | 1.40 |
| S17 | 8.4 | 41.7 | 314 | 1.34 | 1.42 | 0.61 | 2.20 |
| S18 | 6.3 | 21.1 | 309 | 1.07 | 1.44 | 0.46 | 1.67 |
| S19 | 13.2 | 22.8 | 428 | 1.97 | 2.76 | 0.83 | 2.92 |
| S20 | 13.3 | 17.3 | 628 | 0.93 | 3.57 | 1.10 | 1.49 |
| Parameter | TS (%) | Ash (%) | PS (µM) | NH4-N (mg mL−1) | Org-N (mg mL−1) | ||
|---|---|---|---|---|---|---|---|
| Spiking | N/A | N/A | N/A | Before | After | Before | After |
| N | 100 | 100 | 100 | 20 | 100 | 20 | 100 |
| Minimum | 1.4 | 13.6 | 137 | 0.75 | 0.75 | 0.36 | 0.36 |
| Mean | 6.8 | 27.0 | 333 | 1.30 | 2.54 | 1.51 | 2.95 |
| Maximum | 19.8 | 50.2 | 628 | 4.25 | 17.01 | 3.57 | 14.28 |
| SD | 4.6 | 9.8 | 107 | 0.75 | 2.16 | 0.84 | 2.46 |
| Parameter | TS | Ash | PS | NH4-N | Org-N | TN | P | K |
|---|---|---|---|---|---|---|---|---|
| TS | 1.00 | |||||||
| Ash | −0.32 | 1.00 | ||||||
| PS | 0.17 | −0.62 | 1.00 | |||||
| NH4-N | 0.18 | 0.16 | 0.00 | 1.00 | ||||
| Org-N | −0.17 | −0.34 | 0.70 | 0.25 | 1.00 | |||
| TN | −0.01 | −0.13 | 0.46 | 0.76 | 0.82 | 1.00 | ||
| P | −0.14 | −0.31 | 0.65 | 0.25 | 0.98 | 0.80 | 1.00 | |
| K | 0.07 | −0.01 | 0.09 | 0.91 | 0.41 | 0.82 | 0.44 | 1.00 |
| Configuration | Parameter | Pretreatment | Outliers | LVs | R2 (CV) | RMSE (CV) | RPD (CV) |
|---|---|---|---|---|---|---|---|
| HS | NH4-N | MC; D-1,1,15 | 1 | 7 | 0.83 | 0.65 | 2.45 |
| TS | MSC; D-1,1,15 | 0 | 7 | 0.90 | 0.015 | 3.16 | |
| Ash | MC; D-1,2,15 | 0 | 7 | 0.66 | 0.057 | 1.71 | |
| PS | D-2,2,15 | 0 | 5 | 0.69 | 60.0 | 1.79 | |
| PB1 | NH4-N | D-1,2,15 | 1 | 9 | 0.56 | 1.07 | 1.50 |
| TS | D-1,1,15 | 0 | 8 | 0.97 | 0.008 | 5.50 | |
| Ash | MC; D-2,2,15 | 0 | 7 | 0.86 | 0.037 | 2.64 | |
| PS | MC; D-1,2,15 | 0 | 8 | 0.78 | 49.4 | 2.15 | |
| PB2 | NH4-N | MSC; D-1,2,15 | 1 | 1 | 0.30 | 1.64 | 1.20 |
| TS | MC | 0 | 8 | 0.88 | 0.016 | 2.86 | |
| Ash | MSC | 0 | 8 | 0.90 | 0.031 | 3.10 | |
| PS | MSC | 0 | 5 | 0.70 | 58.5 | 1.81 | |
| PB5 | NH4-N | MSC; D-2,2,15 | 1 | 1 | 0.37 | 1.65 | 1.26 |
| TS | MSC | 0 | 8 | 0.89 | 0.015 | 3.03 | |
| Ash | MSC | 0 | 9 | 0.89 | 0.032 | 3.00 | |
| PS | MSC; D-1,2,15 | 0 | 8 | 0.74 | 53.9 | 1.97 |
| Configuration | Parameter | Pretreatment | Outliers | LVs | R2 (CV) | RMSE (CV) | RPD (CV) |
|---|---|---|---|---|---|---|---|
| HS | Org-N | D-1,2,15 | 2 | 12 | 0.66 | 1.18 | 1.73 |
| TS | MC; D-1,2,15 | 0 | 9 | 0.90 | 0.015 | 3.16 | |
| Ash | MC; D-1,1,15 | 0 | 9 | 0.72 | 0.053 | 1.88 | |
| PS | MC; D-2,2,15 | 0 | 5 | 0.67 | 61.9 | 1.73 | |
| PB1 | Org-N | MC; D-1,1,15 | 2 | 6 | 0.34 | 1.67 | 1.23 |
| TS | MC; D-1,1,15 | 0 | 6 | 0.97 | 0.009 | 5.42 | |
| Ash | MC; D-1,1,15 | 0 | 6 | 0.87 | 0.035 | 2.77 | |
| PS | MSC; D-1,1,15 | 0 | 7 | 0.77 | 50.7 | 2.09 | |
| PB2 | Org-N | MC; D-1,2,15 | 2 | 8 | 0.27 | 1.83 | 1.17 |
| TS | MC | 0 | 8 | 0.92 | 0.013 | 3.58 | |
| Ash | MSC | 0 | 8 | 0.88 | 0.034 | 2.86 | |
| PS | MSC | 0 | 8 | 0.78 | 50.4 | 2.11 | |
| PB5 | Org-N | MSC; D-1,2,15 | 2 | 3 | 0.15 | 1.89 | 1.08 |
| TS | MSC | 0 | 7 | 0.80 | 0.021 | 2.26 | |
| Ash | MSC; D-1,2,15 | 0 | 8 | 0.85 | 0.038 | 2.56 | |
| PS | MC | 0 | 7 | 0.77 | 51.6 | 2.07 |
| Parameter | NH4-N | Org-N | ||||
|---|---|---|---|---|---|---|
| Sensor | Equation | Rr2 | Sensor | Equation | Rr2 | |
| TS | HS | 1.89x + 0.69 | 0.14 | HS | 2.71x + 0.55 | 0.27 |
| PB1 | −3.33x + 1.03 | 0.30 | PB1 | −1.81x + 0.77 | 0.10 | |
| Ash | HS | −0.28x + 0.90 | 0.01 | HS | −1.20x + 1.05 | 0.24 |
| PB1 | 0.42x + 0.69 | 0.02 | PB1 | −1.30x + 1.00 | 0.22 | |
| PS | HS | 0.00x + 0.80 | 0.00 | HS | 0.00x + 0.22 | 0.47 |
| PB1 | −0.00x + 1.10 | 0.12 | PB1 | 0.00x + 0.56 | 0.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Larson, R.A.; Digman, M.F. Evaluation of Near-Infrared Reflectance and Transflectance Sensing System for Predicting Manure Nutrients. Remote Sens. 2022, 14, 963. https://doi.org/10.3390/rs14040963
Feng X, Larson RA, Digman MF. Evaluation of Near-Infrared Reflectance and Transflectance Sensing System for Predicting Manure Nutrients. Remote Sensing. 2022; 14(4):963. https://doi.org/10.3390/rs14040963
Chicago/Turabian StyleFeng, Xiaoyu, Rebecca A. Larson, and Matthew F. Digman. 2022. "Evaluation of Near-Infrared Reflectance and Transflectance Sensing System for Predicting Manure Nutrients" Remote Sensing 14, no. 4: 963. https://doi.org/10.3390/rs14040963
APA StyleFeng, X., Larson, R. A., & Digman, M. F. (2022). Evaluation of Near-Infrared Reflectance and Transflectance Sensing System for Predicting Manure Nutrients. Remote Sensing, 14(4), 963. https://doi.org/10.3390/rs14040963







