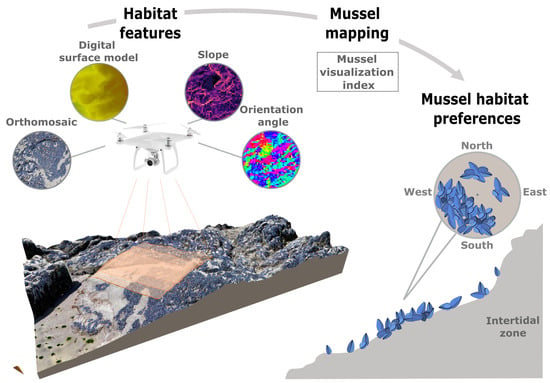
High-Resolution Drone Images Show That the Distribution of Mussels Depends on Microhabitat Features of Intertidal Rocky Shores
Abstract
1. Introduction
2. Methods
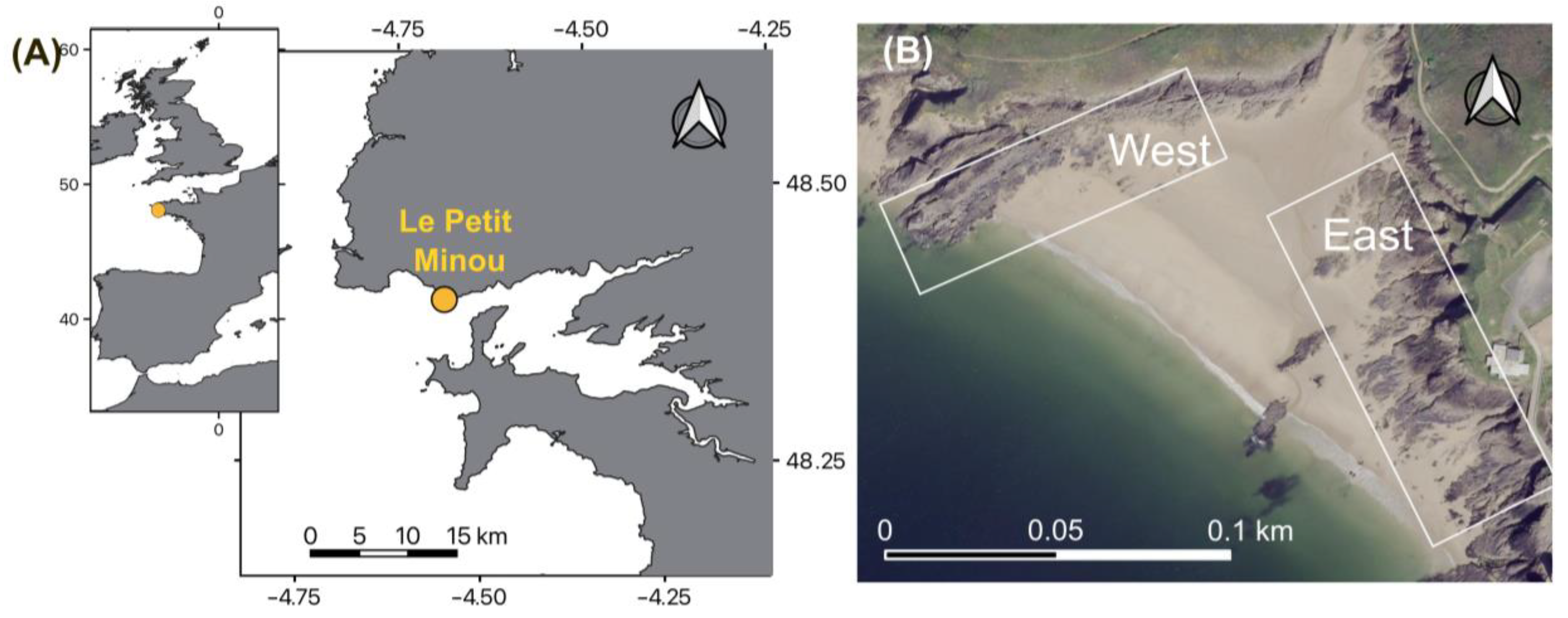
2.1. Study Site
2.2. Drone Survey and SfM Photogrammetry Processing
- Detection of distinct features (key points, limited to 40,000) on the images and detection and matching of tie points (homologous key points on overlapping photographs, limited to 4000) to perform image alignment by bundle adjustment and to generate a sparse point cloud. The “High” accuracy parameter was selected so that the software used original size photos to obtain a more accurate estimation of camera exterior orientation.
- Manual pointing of GCP position in the images (with their GPS position downloaded beforehand) to georeference the scene and to refine the camera calibration to exterior parameters (position, orientation).
- Generation of a dense point cloud by dense image matching, based on the previously estimated camera external and internal parameters. The quality is set to “High” to obtain a more detailed and accurate reconstruction.
- Construction of a 3D polygonal mesh.
- DSM computation by interpolation of the irregular polygonal mesh into a regular XY grid. This DSM is a “2.5D” reconstruction.
- Generation of the orthomosaic (a mosaic of geometrically corrected aerial photographs).
2.3. Microhabitat Features
2.4. Mussel Mapping
2.5. Mussel Habitat Preferences
3. Results
3.1. Microhabitat Features
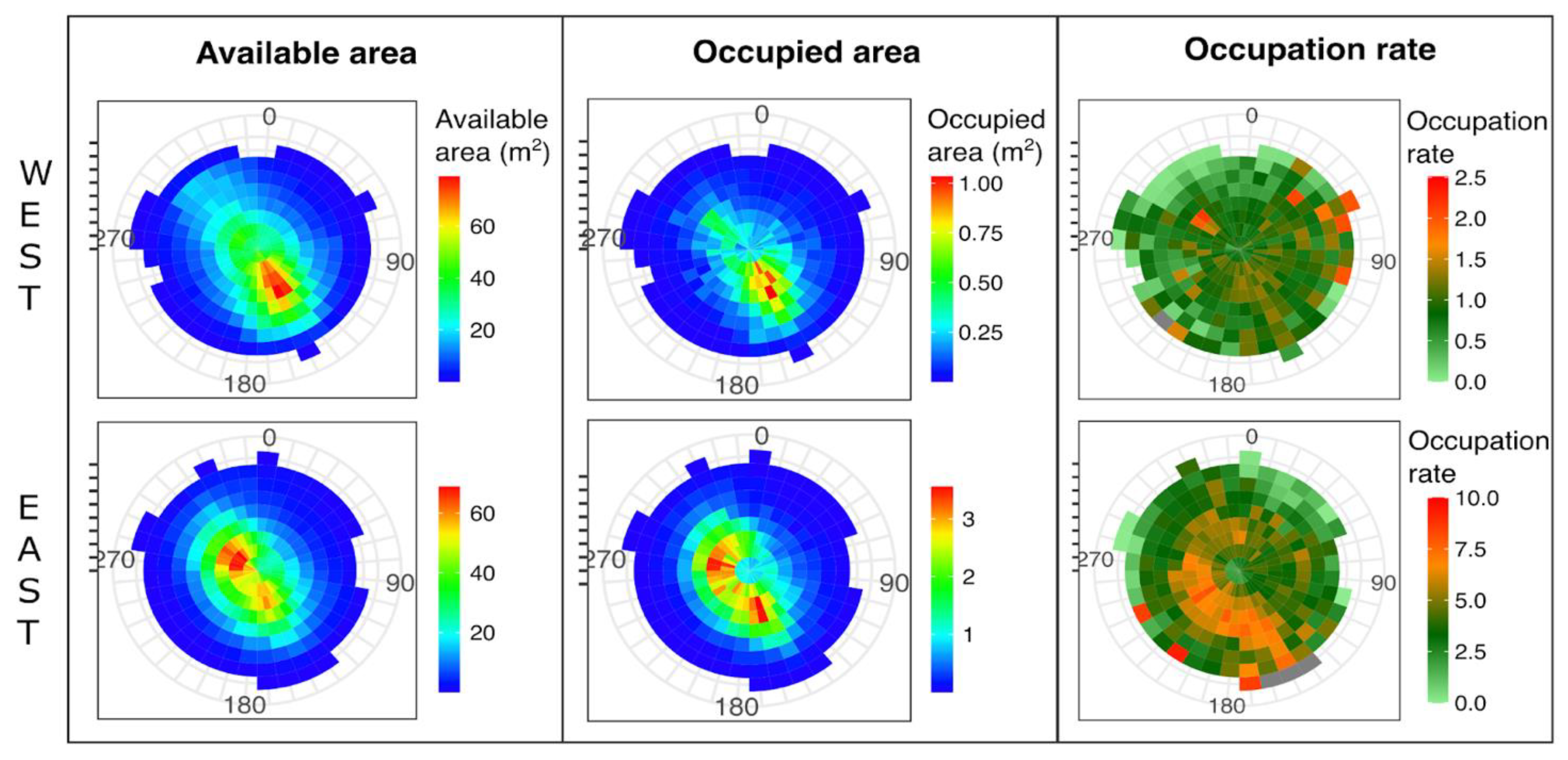
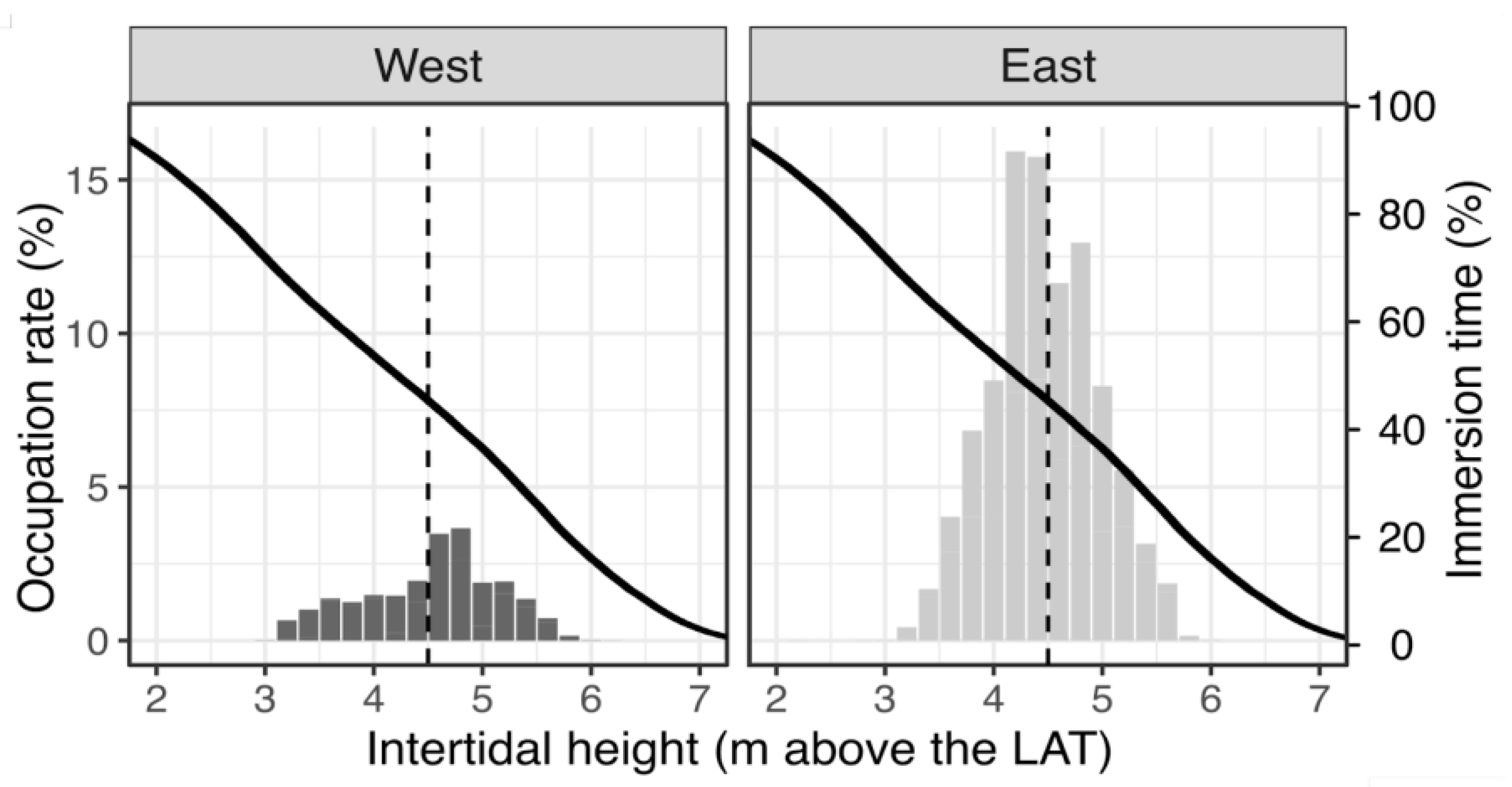
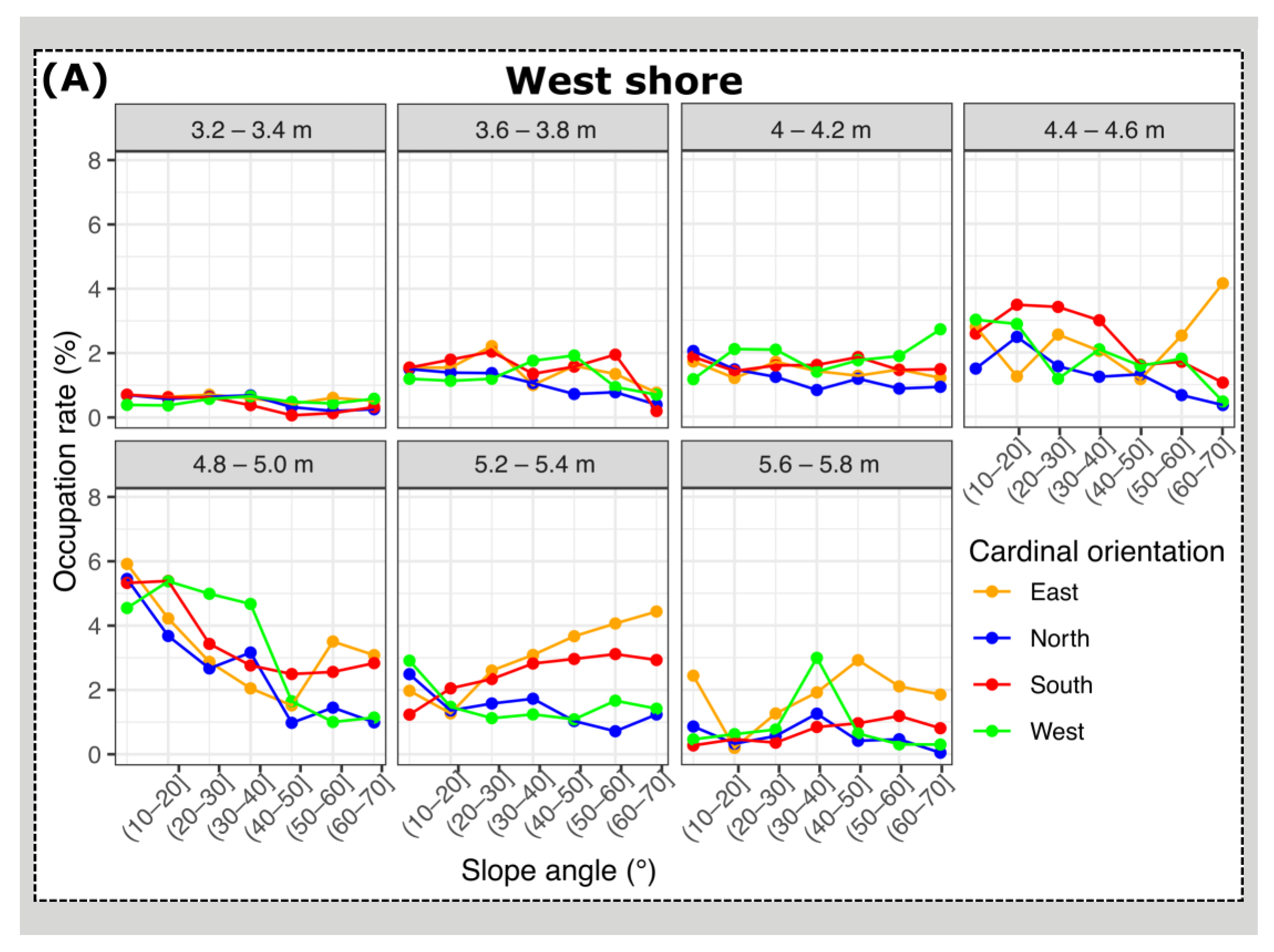
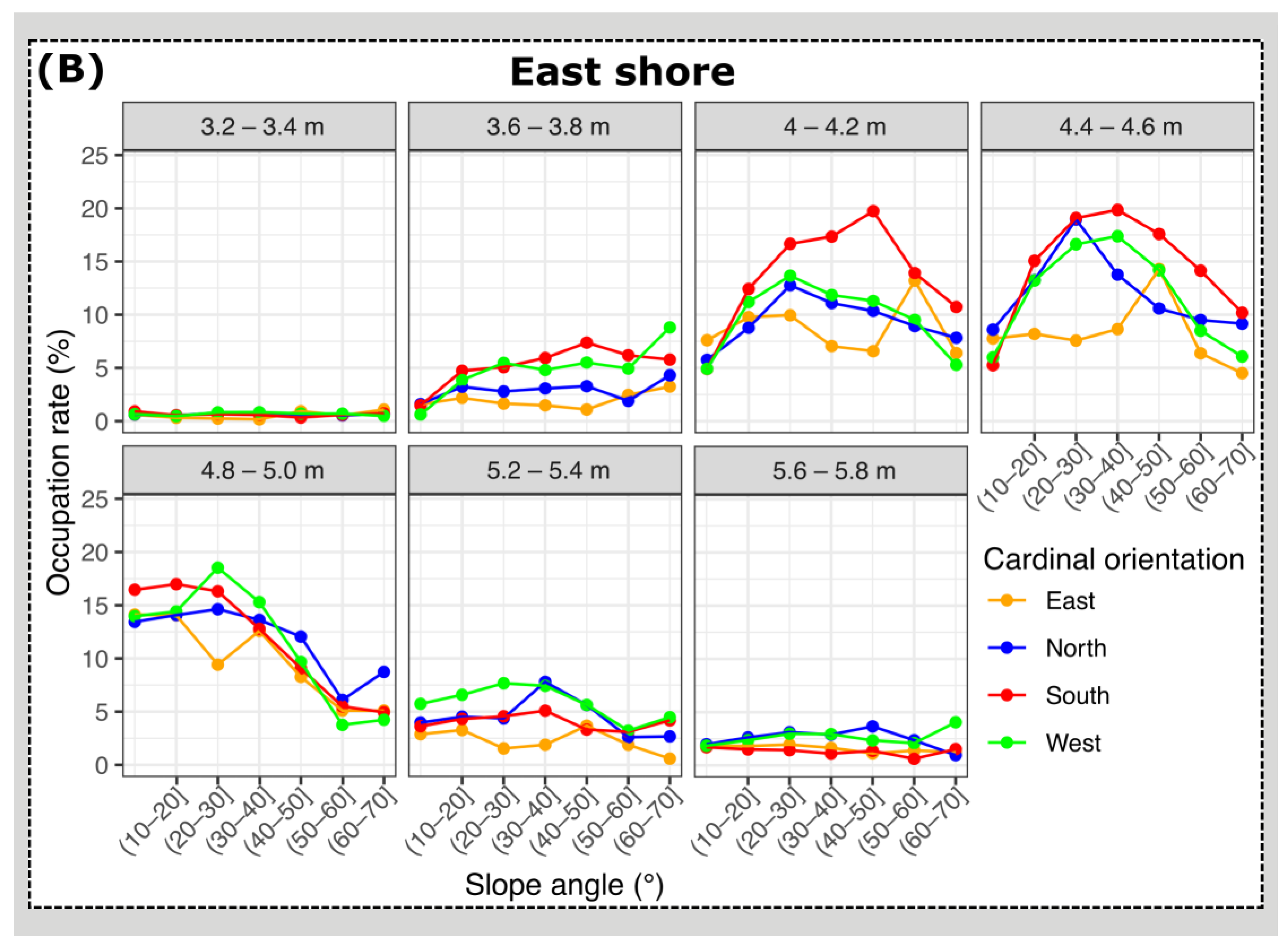
3.2. Mussel Habitat Preferences
4. Discussion
4.1. Mussel Distribution along the Variable Intertidal Height, Slope, and Orientation Features
4.2. Approach Advantages and Limitations
4.3. Insights into Intertidal Population Monitoring
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Donnell, M.J.; Denny, M.W. Hydrodynamic Forces and Surface Topography: Centimeter-Scale Spatial Variation in Wave Forces. Limnol. Oceanogr. 2008, 53, 579–588. [Google Scholar] [CrossRef]
- Burel, T.; Schaal, G.; Grall, J.; Le Duff, M.; Chapalain, G.; Schmitt, B.; Gemin, M.; Boucher, O.; Ar Gall, E. Small-Scale Effects of Hydrodynamics on the Structure of Intertidal Macroalgal Communities: A Novel Approach. Estuar. Coast. Shelf Sci. 2019, 226, 106290. [Google Scholar] [CrossRef]
- Helmuth, B.; Broitman, B.R.; Blanchette, C.A.; Gilman, S.; Halpin, P.; Harley, C.D.G.; O’Donnell, M.J.; Hofmann, G.E.; Menge, B.; Strickland, D. Mosaic Patterns of Thermal Stress in the Rocky Intertidal Zone: Implications for Climate Change. Ecol. Monogr. 2006, 76, 461–479. [Google Scholar] [CrossRef]
- Meager, J.; Schlacher, T.; Green, M. Topographic Complexity and Landscape temperature Patterns Create a Dynamic Habitat Structure on a Rocky Intertidal Shore. Mar. Ecol. Prog. Ser. 2011, 428, 1–12. [Google Scholar] [CrossRef]
- Bouchet, P.J.; Meeuwig, J.J.; Kent, C.P.S.; Letessier, T.B.; Jenner, C.K. Topographic Determinants of Mobile Vertebrate Predator Hotspots: Current Knowledge and Future Directions. Biol. Rev. 2015, 90, 699–728. [Google Scholar] [CrossRef] [PubMed]
- Reichwaldt, E.S.; Ghadouani, A. Can Mussels Be Used as Sentinel Organisms for Characterization of Pollution in Urban Water Systems? Hydrol. Earth Syst. Sci. 2016, 20, 2679–2689. [Google Scholar] [CrossRef]
- Beyer, J.; Green, N.W.; Brooks, S.; Allan, I.J.; Ruus, A.; Gomes, T.; Bråte, I.L.N.; Schøyen, M. Blue Mussels (Mytilus edulis Spp.) as Sentinel Organisms in Coastal Pollution Monitoring: A Review. Mar. Environ. Res. 2017, 130, 338–365. [Google Scholar] [CrossRef] [PubMed]
- Herlyn, M. Quantitative Assessment of Intertidal Blue Mussel (Mytilus edulis L.) Stocks: Combined Methods of Remote Sensing, Field Investigation and Sampling. J. Sea Res. 2005, 53, 243–253. [Google Scholar] [CrossRef]
- Folmer, E.O.; Drent, J.; Troost, K.; Büttger, H.; Dankers, N.; Jansen, J.; van Stralen, M.; Millat, G.; Herlyn, M.; Philippart, C.J.M. Large-Scale Spatial Dynamics of Intertidal Mussel (Mytilus edulis L.) Bed Coverage in the German and Dutch Wadden Sea. Ecosystems 2014, 17, 550–566. [Google Scholar] [CrossRef]
- D’Urban Jackson, T.; Williams, G.J.; Walker-Springett, G.; Davies, A.J. Three-Dimensional Digital Mapping of Ecosystems: A New Era in Spatial Ecology. Proc. R. Soc. B Biol. Sci. 2020, 287, 20192383. [Google Scholar] [CrossRef] [PubMed]
- Murfitt, S.L.; Allan, B.M.; Bellgrove, A.; Rattray, A.; Young, M.A.; Ierodiaconou, D. Applications of Unmanned Aerial Vehicles in Intertidal Reef Monitoring. Sci. Rep. 2017, 7, 10259. [Google Scholar] [CrossRef] [PubMed]
- Bajjouk, T.; Jauzein, C.; Drumetz, L.; Dalla Mura, M.; Duval, A.; Dubois, S.F. Hyperspectral and Lidar: Complementary Tools to Identify Benthic Features and Assess the Ecological Status of Sabellaria alveolata Reefs. Front. Mar. Sci. 2020, 7, 804. [Google Scholar] [CrossRef]
- Sejr, M.K.; Mouritsen, K.N.; Krause-Jensen, D.; Olesen, B.; Blicher, M.E.; Thyrring, J. Small Scale Factors Modify Impacts of Temperature, Ice Scour and Waves and Drive Rocky Intertidal Community Structure in a Greenland Fjord. Front. Mar. Sci. 2021, 7, 607135. [Google Scholar] [CrossRef]
- Collin, A.; Dubois, S.; James, D.; Houet, T. Improving Intertidal Reef Mapping Using UAV Surface, Red Edge, and Near-Infrared Data. Drones 2019, 3, 67. [Google Scholar] [CrossRef]
- Jackson-Bué, T.; Williams, G.J.; Walker-Springett, G.; Rowlands, S.J.; Davies, A.J. Three-Dimensional Mapping Reveals Scale-Dependent Dynamics in Biogenic Reef Habitat Structure. Remote Sens. Ecol. Conserv. 2021, 7, 621–637. [Google Scholar] [CrossRef]
- Gomes, I.; Peteiro, L.; Bueno-Pardo, J.; Albuquerque, R.; Pérez-Jorge, S.; Oliveira, E.R.; Alves, F.L.; Queiroga, H. What’s a Picture Really Worth? On the Use of Drone Aerial Imagery to Estimate Intertidal Rocky Shore Mussel Demographic Parameters. Estuar. Coast. Shelf Sci. 2018, 213, 185–198. [Google Scholar] [CrossRef]
- Kaplanis, N.J.; Edwards, C.B.; Eynaud, Y.; Smith, J.E. Future Sea-Level Rise Drives Rocky Intertidal Habitat Loss and Benthic Community Change. PeerJ 2020, 8, e9186. [Google Scholar] [CrossRef]
- Anderson, K.; Gaston, K.J. Lightweight Unmanned Aerial Vehicles Will Revolutionize Spatial Ecology. Front. Ecol. Environ. 2013, 11, 138–146. [Google Scholar] [CrossRef]
- Yang, J.; El-Kassaby, Y.A.; Guan, W. The Effect of Slope Aspect on Vegetation Attributes in a Mountainous Dry Valley, Southwest China. Sci. Rep. 2020, 10, 16465. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Romero, E.; Petrlic, K.; Verachtert, E.; Bochet, E.; Poesen, J. Effects of Slope Angle and Aspect on Plant Cover and Species Richness in a Humid Mediterranean Badland. Earth Surf. Process. Landf. 2014, 39, 1705–1716. [Google Scholar] [CrossRef]
- Bochet, E.; García-Fayos, P.; Poesen, J. Topographic Thresholds for Plant Colonization on Semi-Arid Eroded Slopes. Earth Surf. Process. Landf. 2009, 34, 1758–1771. [Google Scholar] [CrossRef]
- Lozano-García, B.; Parras-Alcántara, L.; Brevik, E.C. Impact of Topographic Aspect and Vegetation (Native and Reforested Areas) on Soil Organic Carbon and Nitrogen Budgets in Mediterranean Natural Areas. Sci. Total Environ. 2016, 544, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Seabra, R.; Wethey, D.S.; Santos, A.M.; Lima, F.P. Side Matters: Microhabitat Influence on Intertidal Heat Stress over a Large Geographical Scale. J. Exp. Mar. Biol. Ecol. 2011, 400, 200–208. [Google Scholar] [CrossRef]
- Guichard, F.; Halpin, P.M.; Allison, G.W.; Lubchenco, J.; Menge, B.A. Mussel Disturbance Dynamics: Signatures of Oceanographic Forcing from Local Interactions. Am. Nat. 2003, 161, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, C.A.; Helmuth, B.; Gaines, S.D. Spatial Patterns of Growth in the Mussel, Mytilus californianus, across a Major Oceanographic and Biogeographic Boundary at Point Conception, California, USA. J. Exp. Mar. Biol. Ecol. 2007, 340, 126–148. [Google Scholar] [CrossRef]
- Oróstica, M.H.; Wyness, A.J.; Monsinjon, J.R.; Nicastro, K.R.; Zardi, G.I.; Barker, C.; McQuaid, C.D. Effects of Habitat Quality on Abundance, Size and Growth of Mussel Recruits. Hydrobiologia 2022, 849, 4341–4356. [Google Scholar] [CrossRef] [PubMed]
- Harley, C. Tidal Dynamics, Topographic Orientation, and Temperature-Mediated Mass Mortalities on Rocky Shores. Mar. Ecol. Prog. Ser. 2008, 371, 37–46. [Google Scholar] [CrossRef]
- Carrington, E.; Moeser, G.M.; Dimond, J.; Mello, J.J.; Boller, M.L. Seasonal Disturbance to Mussel Beds: Field Test of a Mechanistic Model Predicting Wave Dislodgment. Limnol. Oceanogr. 2009, 54, 978–986. [Google Scholar] [CrossRef]
- Bierne, N.; Borsa, P.; Daguin, C.; Jollivet, D.; Viard, F.; Bonhomme, F.; David, P. Introgression Patterns in the Mosaic Hybrid Zone between Mytilus edulis and M. galloprovincialis. Mol. Ecol. 2003, 12, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Arbiol, C.; Nielsen, E.E.; Couteau, J.; Sussarellu, R.; Burgeot, T.; Bernard, I.; Coolen, J.W.P.; Lamy, J.-B.; Robert, S.; et al. Replicated Anthropogenic Hybridisations Reveal Parallel Patterns of Admixture in Marine Mussels. Evol. Appl. 2020, 13, 575–599. [Google Scholar] [CrossRef]
- Barbosa, R.V.; Bacher, C.; Jean, F.; Thomas, Y. Linking Individual and Population Patterns of Rocky-Shore Mussels. PeerJ 2021, 9, e12550. [Google Scholar] [CrossRef] [PubMed]
- Jaud, M.; Passot, S.; Allemand, P.; Le Dantec, N.; Grandjean, P.; Delacourt, C. Suggestions to Limit Geometric Distortions in the Reconstruction of Linear Coastal Landforms by SfM Photogrammetry with PhotoScan® and MicMac® for UAV Surveys with Restricted GCPs Pattern. Drones 2019, 3, 2. [Google Scholar] [CrossRef]
- Fonstad, M.A.; Dietrich, J.T.; Courville, B.C.; Jensen, J.L.; Carbonneau, P.E. Topographic Structure from Motion: A New Development in Photogrammetric Measurement. Earth Surf. Process. Landf. 2013, 38, 421–430. [Google Scholar] [CrossRef]
- Hijmans, R.J.; van Etten, J. Raster: Geographic Analysis and Modeling with Raster Data. R Package Version2.0-12. 2012. Available online: http://CRAN.R-Project.or/Package=raster (accessed on 30 March 2021).
- Grohmann, C.H. Effects of Spatial Resolution on Slope and Aspect Derivation for Regional-Scale Analysis. Comput. Geosci. 2015, 77, 111–117. [Google Scholar] [CrossRef]
- Chang, K.; Tsai, B. The Effect of DEM Resolution on Slope and Aspect Mapping. Cartogr. Geogr. Inf. Syst. 1991, 18, 69–77. [Google Scholar] [CrossRef]
- Benaglia, T.; Chauveau, D.; Hunter, D.R.; Young, D.S. Mixtools: An R Package for Analyzing Mixture Models. J. Stat. Softw. 2009, 32, 1–29. [Google Scholar] [CrossRef]
- Thrun, M.; Hansen-Goos, O.; Ultsch, A. AdaptGauss: Gaussian Mixture Models (GMM). R Package. 2020. Available online: https://CRAN.R-project.org/package=AdaptGauss (accessed on 20 October 2020).
- Congalton, R.G. A Review of Assessing the Accuracy of Classifications of Remotely Sensed Data. Remote Sens. Environ. 1991, 37, 35–46. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 1 March 2021).
- Thyrring, J.; Wegeberg, S.; Blicher, M.E.; Krause-Jensen, D.; Høgslund, S.; Olesen, B.; Jozef Jr., W.; Mouritsen, K.N.; Peck, L.S.; Sejr, M.K. Latitudinal Patterns in Intertidal Ecosystem Structure in West Greenland Suggest Resilience to Climate Change. Ecography 2021, 44, 1156–1168. [Google Scholar] [CrossRef]
- Blicher, M.E.; Sejr, M.K.; Høgslund, S. Population Structure of Mytilus edulis in the Intertidal Zone in a Sub-Arctic Fjord, SW Greenland. Mar. Ecol. Prog. Ser. 2013, 487, 89–100. [Google Scholar] [CrossRef]
- Blanchette, C.A.; Gaines, S.D. Distribution, Abundance, Size and Recruitment of the Mussel, Mytilus californianus, across a Major Oceanographic and Biogeographic Boundary at Point Conception, California, USA. J. Exp. Mar. Biol. Ecol. 2007, 340, 268–279. [Google Scholar] [CrossRef]
- Gascoigne, J.C.; Beadman, H.A.; Saurel, C.; Kaiser, M.J. Density Dependence, Spatial Scale and Patterning in Sessile Biota. Oecologia 2005, 145, 371–381. [Google Scholar] [CrossRef]
- Alunno-Bruscia, M.; Petraitis, P.S.; Bourget, E.; Fréchette, M. Body Size-Density Relationship for Mytilus edulis in an Experimental Food-Regulated Situation. Oikos 2000, 90, 28–42. [Google Scholar] [CrossRef]
- Beukema, J.; Flach, E. Factors Controlling the Upper and Lower Limits of the Intertidal Distribution of Two Corophium Species in the Wadden Sea. Mar. Ecol. Prog. Ser. 1995, 125, 117–126. [Google Scholar] [CrossRef]
- Connell, J.H. The Influence of Interspecific Competition and Other Factors on the Distribution of the Barnacle Chthamalus Stellatus. Ecology 1961, 42, 710–723. [Google Scholar] [CrossRef]
- Somero, G.N. Thermal Physiology and Vertical Zonation of Intertidal Animals: Optima, Limits, and Costs of Living. Integr. Comp. Biol. 2002, 42, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Suchanek, T.H. The Ecology of Mytilus Edulis L. in Exposed Rocky Intertidal Communities. J. Exp. Mar. Biol. Ecol. 1978, 31, 105–120. [Google Scholar] [CrossRef]
- Foster, B.A. Tolerance of High Temperatures by Some Intertidal Barnacles. Mar. Biol. 1969, 4, 326–332. [Google Scholar] [CrossRef]
- Hopkins, G.A.; Prince, M.; Cahill, P.L.; Fletcher, L.M.; Atalah, J. Desiccation as a Mitigation Tool to Manage Biofouling Risks: Trials on Temperate Taxa to Elucidate Factors Influencing Mortality Rates. Biofouling 2016, 32, 1–11. [Google Scholar] [CrossRef]
- Foster, B.A. On the Determinants of the Upper Limit of Intertidal Distribution of Barnacles (Crustacea: Cirripedia). J. Anim. Ecol. 1971, 40, 33–48. [Google Scholar] [CrossRef]
- Roberts, D.A.; Hofmann, G.E.; Somero, G.N. Heat-Shock Protein Expression in Mytilus californianus: Acclimatization (Seasonal and Tidal-Height Comparisons) and Acclimation Effects. Biol. Bull. 1997, 192, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Buckley, B.A.; Owen, M.-E.; Hofmann, G.E. Adjusting the Thermostat: The Threshold Induction Temperature for the Heat-Shock Response in Intertidal Mussels (Genus Mytilus) Changes as a Function of Thermal History. J. Exp. Biol. 2001, 204, 3571–3579. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, B.L.; Sanders, J.G.; Somero, G.N. Transcriptomic Responses to Heat Stress in Invasive and Native Blue Mussels (Genus Mytilus): Molecular Correlates of Invasive Success. J. Exp. Biol. 2010, 213, 3548–3558. [Google Scholar] [CrossRef] [PubMed]
- De Vooys, C.G.N.; De Zwaan, A. The Rate of Oxygen Consumption and Ammonia Excretion by Mytilus edulis after Various Periods of Exposure to Air. Comp. Biochem. Physiol. A Physiol. 1978, 60, 343–347. [Google Scholar] [CrossRef]
- Thomas, Y.; Bacher, C. Assessing the Sensitivity of Bivalve Populations to Global Warming Using an Individual-Based Modelling Approach. Glob. Change Biol. 2018, 24, 4581–4597. [Google Scholar] [CrossRef]
- Monaco, C.J.; McQuaid, C.D. Applicability of Dynamic Energy Budget (DEB) Models across Steep Environmental Gradients. Sci. Rep. 2018, 8, 16384. [Google Scholar] [CrossRef]
- Thomas, Y.; Mazurié, J.; Alunno-Bruscia, M.; Bacher, C.; Bouget, J.-F.; Gohin, F.; Pouvreau, S.; Struski, C. Modelling Spatio-Temporal Variability of Mytilus edulis (L.) Growth by Forcing a Dynamic Energy Budget Model with Satellite-Derived Environmental Data. J. Sea Res. 2011, 66, 308–317. [Google Scholar] [CrossRef]
- Rius, M.; McQuaid, C.D. Wave Action and Competitive Interaction between the Invasive Mussel Mytilus Galloprovincialis and the Indigenous Perna Perna in South Africa. Mar. Biol. 2006, 150, 69–78. [Google Scholar] [CrossRef]
- Moritsch, M.M. Expansion of Intertidal Mussel Beds Following Disease-Driven Reduction of a Keystone Predator. Mar. Environ. Res. 2021, 169, 105363. [Google Scholar] [CrossRef]
- Zardi, G.I.; Nicastro, K.R.; McQuaid, C.D.; Erlandsson, J. Sand and Wave Induced Mortality in Invasive (Mytilus galloprovincialis) and Indigenous (Perna perna) Mussels. Mar. Biol. 2008, 153, 853–858. [Google Scholar] [CrossRef]
- Bagur, M.; Gutiérrez, J.L.; González, J.A.; Arribas, L.P.; Palomo, M.G. Physical Rather than Biotic Factors Set the Lower Limit of Mussel Beds in a Horizontal Rocky Intertidal Platform. J. Exp. Mar. Biol. Ecol. 2022, 548, 151680. [Google Scholar] [CrossRef]
- Denny, M.W.; Dowd, W.W.; Bilir, L.; Mach, K.J. Spreading the Risk: Small-Scale Body Temperature Variation among Intertidal Organisms and Its Implications for Species Persistence. J. Exp. Mar. Biol. Ecol. 2011, 400, 175–190. [Google Scholar] [CrossRef]
- McQuaid, C.; Lindsay, T. Effect of Wave Exposure on Growth and Mortality Rates of the Mussel Perna Perna: Bottom-up Regulation of Intertidal Populations. Mar. Ecol. Prog. Ser. 2000, 206, 147–154. [Google Scholar] [CrossRef]
- McQuaid, C.; Branch, G. Trophic Structure of Rocky Intertidal Communities Response to Wave Action and Implications for Energy Flow. Mar. Ecol. Prog. Ser. 1985, 22, 153–161. [Google Scholar] [CrossRef]
- Erlandsson, J.; McQuaid, C.D.; Kostylev, V.E. Contrasting Spatial Heterogeneity of Sessile Organisms within Mussel (Perna Perna L.) Beds in Relation to Topographic Variability. J. Exp. Mar. Biol. Ecol. 2005, 314, 79–97. [Google Scholar] [CrossRef]
- Focht, R.C.; Shima, J.S. Acceleration Loggers Reveal Fine-Scale Heterogeneity in Wave Exposure along an Open Coast. Estuar. Coast. Shelf Sci. 2020, 233, 106507. [Google Scholar] [CrossRef]
- McQuaid, C.D.; Mostert, B.P. The Effects of Within-Shore Water Movement on Growth of the Intertidal Mussel Perna perna: An Experimental Field Test of Bottom-up Control at Centimetre Scales. J. Exp. Mar. Biol. Ecol. 2010, 384, 119–123. [Google Scholar] [CrossRef]
- Guiñez, R. A Review on Self-Thinning in Mussels. Rev. Biol. Mar. Oceanogr. 2005, 40, 1–6. [Google Scholar] [CrossRef]
- Hughes, R.N.; Griffiths, C.L. Self-Thinning in Barnacles and Mussels: The Geometry of Packing. Am. Nat. 1988, 132, 484–491. [Google Scholar] [CrossRef]
- Cubillo, A.M.; Peteiro, L.G.; Fernández-Reiriz, M.J.; Labarta, U. Influence of Stocking Density on Growth of Mussels (Mytilus galloprovincialis) in Suspended Culture. Aquaculture 2012, 342–343, 103–111. [Google Scholar] [CrossRef]
- Blaschke, T. Object Based Image Analysis for Remote Sensing. ISPRS J. Photogramm. Remote Sens. 2010, 65, 2–16. [Google Scholar] [CrossRef]
- Lupo, C.; Bougeard, S.; Le Bihan, V.; Blin, J.L.; Allain, G.; Azéma, P.; Benoit, F.; Béchemin, C.; Bernard, I.; Blachier, P.; et al. Mortality of Marine Mussels Mytilus edulis and M. galloprovincialis: Systematic Literature Review of Risk Factors and Recommendations for Future Research. Rev. Aquac. 2021, 13, 504–536. [Google Scholar] [CrossRef]
- Jaud, M.; Bertin, S.; Beauverger, M.; Augereau, E.; Delacourt, C. RTK GNSS-Assisted Terrestrial SfM Photogrammetry without GCP: Application to Coastal Morphodynamics Monitoring. Remote Sens. 2020, 12, 1889. [Google Scholar] [CrossRef]
- Charles, M.; Bernard, I.; Villalba, A.; Oden, E.; Burioli, E.A.V.; Allain, G.; Trancart, S.; Bouchart, V.; Houssin, M. High Mortality of Mussels in Northern Brittany—Evaluation of the Involvement of Pathogens, Pathological Conditions and Pollutants. J. Invertebr. Pathol. 2020, 170, 107308. [Google Scholar] [CrossRef] [PubMed]
- Baden, S.; Hernroth, B.; Lindahl, O. Declining Populations of Mytilus Spp. in North Atlantic Coastal Waters—A Swedish Perspective. J. Shellfish Res. 2021, 40, 269–296. [Google Scholar] [CrossRef]
- Caza, F.; Bernet, E.; Veyrier, F.J.; Betoulle, S.; St-Pierre, Y. Hemocytes Released in Seawater Act as Trojan Horses for Spreading of Bacterial Infections in Mussels. Sci. Rep. 2020, 10, 19696. [Google Scholar] [CrossRef]
- Thomas, Y.; Razafimahefa, N.R.; Ménesguen, A.; Bacher, C. Multi-Scale Interaction Processes Modulate the Population Response of a Benthic Species to Global Warming. Ecol. Model. 2020, 436, 109295. [Google Scholar] [CrossRef]
- Thomas, Y.; Cassou, C.; Gernez, P.; Pouvreau, S. Oysters as Sentinels of Climate Variability and Climate Change in Coastal Ecosystems. Environ. Res. Lett. 2018, 13, 104009. [Google Scholar] [CrossRef]
- Choi, F.; Gouhier, T.; Lima, F.; Rilov, G.; Seabra, R.; Helmuth, B. Mapping Physiology: Biophysical Mechanisms Define Scales of Climate Change Impacts. Conserv. Physiol. 2019, 7, coz028. [Google Scholar] [CrossRef]


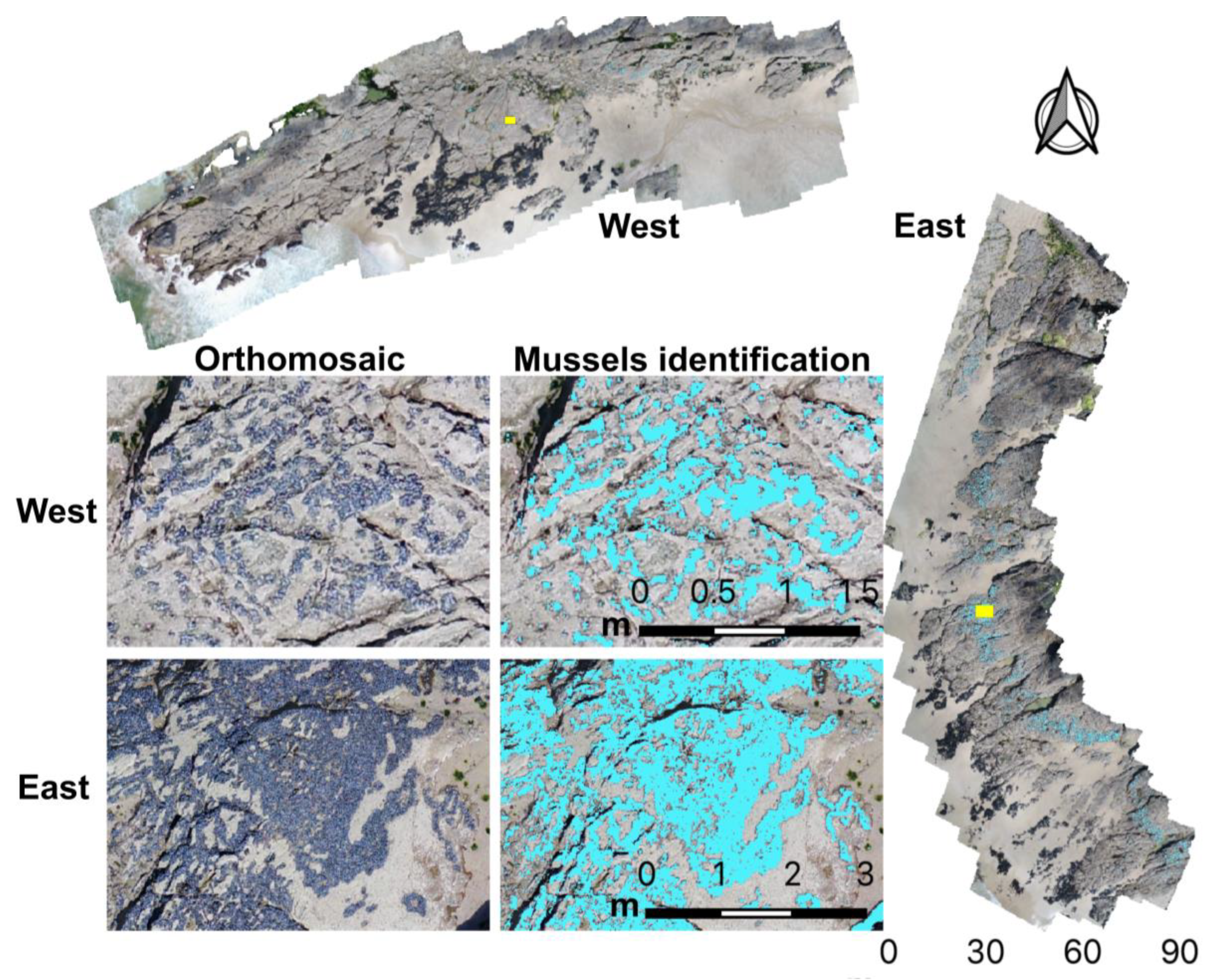
| Rocky Shore | Overall Accuracy (%) | Kappa Index |
|---|---|---|
| West | 0.98 | 0.93 |
| East | 0.93 | 0.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, R.V.; Jaud, M.; Bacher, C.; Kerjean, Y.; Jean, F.; Ammann, J.; Thomas, Y. High-Resolution Drone Images Show That the Distribution of Mussels Depends on Microhabitat Features of Intertidal Rocky Shores. Remote Sens. 2022, 14, 5441. https://doi.org/10.3390/rs14215441
Barbosa RV, Jaud M, Bacher C, Kerjean Y, Jean F, Ammann J, Thomas Y. High-Resolution Drone Images Show That the Distribution of Mussels Depends on Microhabitat Features of Intertidal Rocky Shores. Remote Sensing. 2022; 14(21):5441. https://doi.org/10.3390/rs14215441
Chicago/Turabian StyleBarbosa, Romina Vanessa, Marion Jaud, Cédric Bacher, Yann Kerjean, Fred Jean, Jérôme Ammann, and Yoann Thomas. 2022. "High-Resolution Drone Images Show That the Distribution of Mussels Depends on Microhabitat Features of Intertidal Rocky Shores" Remote Sensing 14, no. 21: 5441. https://doi.org/10.3390/rs14215441
APA StyleBarbosa, R. V., Jaud, M., Bacher, C., Kerjean, Y., Jean, F., Ammann, J., & Thomas, Y. (2022). High-Resolution Drone Images Show That the Distribution of Mussels Depends on Microhabitat Features of Intertidal Rocky Shores. Remote Sensing, 14(21), 5441. https://doi.org/10.3390/rs14215441