Abstract
Environmental factors often limit plant establishment and survival through increased seedling mortality. Understanding plant growth and the causes of mortality can be helpful in developing solutions that enhance seeding success and improve restoration monitoring. The purpose of our research was to assess the efficacy of time lapse and motion sensing cameras for monitoring seedling height, density, and fate. We conducted this study in a salt desert shrub community in northwest Utah, USA. In spring 2017, we placed 28 cameras in fenced and unfenced plots seeded with bottlebrush squirreltail and collected hourly images of the seedlings’ development for the initial four months post-seeding. The seedling attributes were recorded in-field and compared with camera images to determine accuracy and reliability. We found that the optimal period for capturing imagery occurred near the sun’s zenith when shadows were minimized. We were able to detect both the timing of the plant emergence, plant height, density, growth rate, and seedling death. The average seedling height and density were underestimated by 14% and 30% between the camera and field estimates, respectively. We recognize that it could be beneficial to adjust for the effect of the date. The reduced seedling density improved the measurement accuracy through a lower visual obscurity. Managers can utilize remote cameras to effectively measure vegetation that can provide an insight into environmental influences.
1. Introduction
Rangelands account for more than 50% of the terrestrial land surface area and include areas which support biodiversity and provide important ecological services [1]. The human uses of rangelands are varied, extensive, and can lead to degraded conditions and shifts in vegetation structure, such as a reduction in the abundance, diversity, and vigor of native species [2,3]. Degraded rangelands often promote the colonization and dominance of invasive annual species [4,5,6,7]. In contrast to plant communities dominated by perennial vegetation, annual invasive weeds can impair ecosystems by altering fire regimes, exposing surface soils that promote wind and water erosion, and alter plant community composition and successional pathways [8,9,10]. Additionally, degraded plant communities support a poorer forage quality and cover for wildlife and reduced animal diversity [11]. These influences have been associated with diminished biosphere integrity which is currently at risk worldwide [12].
In an effort to increase biotic integrity, improve plant community function, and reduce fire risk, land managers regularly reseed degraded rangelands using perennial grasses, forbs, and shrubs that protect soils, provide forage for wildlife and livestock, and increase ecological resilience [9]. Annually, more than 100 million USD are spent in an effort to restore degraded rangelands worldwide [13,14,15], however, these efforts have historically experienced low rates of success [9,13,16]. Different methods are needed to better monitor and assess reseeding efforts that can lead to a better restoration success, improved ecosystem function, and extensive cost savings [9,13,14].
Technological advances are integral for improving restoration success through an increased understanding of seed ecology and a recognition of limitations in plant establishment. Developing improved monitoring techniques for characterizing demographic stages, including seed germination, seedling emergence, seedling establishment, and survival to an adult plant, will increase our understanding of limitations to revegetation success [17,18,19,20]. An effective restoration is typically correlated with plant establishment in response to environmental variability (i.e., climate, herbivory, soils, and invasive species). Monitoring these characteristics requires frequent site visits to quantify the numbers of seedlings (density), the rate of plant growth, and seedling survival. Restoration monitoring is often challenging because of field–travel logistics, a limited amount of time, and a lack of resource availability decreases the frequency that samples can be collected. This may be especially difficult in remote study areas or sites with a poor access. Current monitoring methods can provide valuable information but are generally on a limited temporal scale. Methods that can record a greater detail of what transpires in reseeded areas will increase our ability to monitor restoration efforts. Remote sensing technologies are widely accepted and applied across scientific and professional disciplines, where they reduce the time and effort required to collect vegetation data with a greater data resolution [21,22,23].
Remote sensing technology has been widely developed for natural resource management applied at broad spatial scales, such as classifying landscape vegetation types or creating digital elevation models [24,25,26]. There are increasing opportunities to expand the use of remotely sensed data at multiple spatial and temporal scales, including agricultural and wildland seedling monitoring [23,27]. A recent development in remote sensing is the use of remote camera technology, also referred to as trail cameras, game cameras, or camera traps. These devices were developed to be a non-invasive sensor that acquires imagery using timed, motion detection, and infrared-sensing capabilities [28,29]. Resource managers use these cameras to monitor disturbance and vegetation change that often affect rangeland resource availability and ecological services [30,31]. The use of remote cameras for research has varied widely and includes applications such as the estimation of tiger densities in India [32], monitoring wildlife interactions with feral horses at water sources in the Great Basin [33], determining watering sites selected by chukar [34], and monitoring invasive rodents and rodent granivory [35,36]. Compared to wildlife monitoring, literature describing the use of remote cameras for monitoring vegetation is limited, however, this technology is currently being applied to improve vegetation detection, monitoring, and habitat assessment [30,37,38].
There have been significant advancements in camera technology since their first use to quantify vegetation characteristics in the 1920s, when an apparatus for photographing vegetation quadrats was developed [39,40,41,42]. A prominent development in vegetation monitoring has been the incorporation of high-resolution image acquisition acquired from a variety of sensors and platforms, such as unmanned aerial systems (UAS) [43,44,45,46,47]. These methods have been demonstrated to be accurate for measuring mature plants in both rangeland and agricultural settings [23,46,47,48,49]. The photographic monitoring of vegetation is currently used for measuring plant canopy covers, species composition, plant health, and changes in the plant community or individual plants over time for mature individuals [50,51,52,53,54]. The use of photogrammetry in seedling research is much more limited and focuses mainly on large seedlings in precision agriculture and forestry or measuring seedling characteristics in a lab setting with specialized equipment [27,55,56,57,58,59].
The purpose of this study was to determine the efficacy of using commercially available camera traps to detect small seedling characteristics including vegetation height, seedling density and the variability in density over time which is associated with plant growth and herbivory, and the timing and cause of seedling mortality at a fine scale. We evaluated the use of remote cameras to measure seedling physical characteristics and specific causes of mortality while simultaneously obtaining frequent data that minimizes the amount of time required to collect these data in the field. To accomplish this, we compared the data collected from high-resolution cameras with field-based measurements and evaluated the efficacy and limitations of remote cameras to accurately quantify vegetation growth and detect the causes of mortality. We also assessed the appropriate sensor types (camera), optional camera settings, and the appropriate positioning of the sensor for optimal data capture.
2. Materials and Methods
2.1. Study Site Description
This study was conducted at two locations, Murray’s Mesa (41.036394°N, −112.979465°W) and Arctic Road (41.078425°N, −112.927195°W), on the Utah Test and Training Range (UTTR) located in the West Desert of Utah, United States. This military-managed land is in a relatively low precipitation area of the semi-arid Great Basin Region, receiving approximately 258 mm of precipitation annually [60]. Murray’s Mesa is located at a 1399 m elevation with a <4% slope. We determined through Brigham Young University’s Environmental Analytical Lab (Provo, UT, USA) that the top 15 cm of soil contained 37.4% of silt, 22.4% of clay, and 40.2% of sand with a pH of 7.8 and 1.3% of organic matter. The Arctic Road site is located at a 1338 m elevation with a <4% slope and soil containing 47.4% of silt, 26.4% of clay, and 26.2% of sand with a pH of 7.6 and 2.7% of organic matter. Both sites consist of a degraded salt desert shrub community. Remnant native perennial plants include Sarcobatus vermiculatus (Hook.) Torr. (greasewood), Atriplex confertifolia (Torr. & Frem.) S. Watson (shadscale), Artemisia spinescens D.C. Eaton (bud sagebrush), Elymus elymoides (Raf.) Swezey (bottlebrush squirreltail), and Achnatherum hymenoides (Roemer & J.A. Schultes) Barkworth. Within these communities, military activity has contributed to an increased fire frequency and the invasion of annual grasses and forbs including Bromus tectorum L. (cheatgrass), Halogeton glomeratus (Bieb.) C.A. Mey (halogeton), Salsola iberica (Sennen & Pau) Botsch. (Russian thistle), and Sisymbrium altissimum L. (tumble mustard). A revegetation from seed with a mix of native and introduced species was attempted at the Arctic Road site in 2016 with very little success, and at the Murray’s Mesa site in 2017 with a limited plant establishment.
2.2. Study Design
We installed 28 Reconyx PC900 (Reconyx, Holmen, WI, USA) remotely triggered cameras at each study plot for a total of 28 study plots. This model of camera was selected because of its factory-installed weatherproof protection, ability to be programmed for both time-lapse and motion-triggered image acquisition, and common use in wildlife research. We modified the focal range of these cameras to capture images at a close range (≤60 cm). We placed 14 cameras at each site, arranged in a randomized split-plot design for a total of seven replications. Half of the plots were fenced to exclude herbivores. Each plot was hand-seeded on 27 May with E. elymoides in four 75 cm rows placed perpendicular to the camera position. The first row was oriented 35 cm from the camera, and the rows were spaced 20 cm apart to ensure the visibility of individual rows on each image (Figure 1). The rows were marked on each end with a wood dowel to help in locating and counting the seedlings both in the field and on images. Each row was seeded at a 0.5 cm depth with 50 pure live seeds, totaling 200 seeds per plot. Due to the dry climate at the study sites (~258 mm precipitation annually) [47], we watered the plots daily to ensure a sufficient soil moisture for seed germination and seedling emergence. One plot was selected at each site to monitor the soil moisture from 0 to 10 cm depths using Decagon MPS-6 dielectric water potential sensors (Meter Group Inc., Pullman, WA, USA). The plots were brought to the field capacity (−33 kPa, 0.301 g of water 1 g soil−1) three days after planting and were maintained at ≥50% of field capacity until all the plots reached a 50% emergence. Upon reaching a 50% emergence, watering was reduced to only twice per week and completely discontinued five weeks after planting.

Figure 1.
Photo of the plot setup with camera on left and seedlings in four rows marked by wooden dowels in the center. An unfenced plot is observed in the foreground, and a fenced plot in the background. This photograph was taken at the Murray’s Mesa site located on the Utah Test and Training Range (UTTR), Utah.
We placed cameras in each plot 10 cm above the soil surface and angled forward 15°. Keeping the cameras at this slightly elevated height reduced the amount of dust that collected on the lens and allowed for multiple rows to be visible in the camera’s field of view. If cameras were kept level with the soil surface, the first row of seedlings would have blocked the seedlings in the rows behind it. Additionally, our preliminary work indicated that it was difficult to detect seedlings if the cameras were placed at nadir (directly above seedlings) because the plant surface area that was visible was much less than if viewed at an oblique perspective. The camera focal length was factory adjusted to 61 cm. The cameras were programmed to capture one photo every hour from 8 AM to 7 PM (approximate light hours) daily. The cameras were also set to trigger with changes in infrared heat (caused by motion), taking three photos per trigger, with a wait period of 15 s between the triggers (Figure 2). The plots were cleared of weeds and the camera lenses were cleaned to maintain the visibility of the seedlings throughout the sample period.

Figure 2.
An example of timed (left) and motion triggered (right) images obtained from a camera trap that were analyzed for seedling height and herbivory.
In the field, we physically measured the seedling height and density every 2–3 days between 10 June–5 July 2017, for a total of 11 measurements. We then collected a final measurement for both characteristics on 19 September 2017. The seedling density and average height were measured in each plot by counting the number of seedlings per row and measuring the height of each seedling in each row. These measurements were taken in the field at the same time that an image was collected from a remotely placed camera, ensuring that the two measurements could reliably be compared.
After all images were collected, the seedling height and density were manually extracted from the images on a computer monitor by counting the number of seedlings per row in the images and using a ruler to measure the seedling height on the screen. Since the photographs put the seedlings on a one-dimensional image, the seedlings farther back in the image appear smaller than the seedlings closer to the camera and calibration samples were necessary to extract the seedling height. To account for the seedling size distortion in the images and the fact that we had to calibrate the image height measurements, we randomly selected 3 image collection dates to compare the extracted height measurements of the seedlings in the images to the physical measurements that were taken in the field at the exact same time as the image was collected. The average seedling height of the in-field measurements was divided by the on-screen extracted average seedling height from the matching image to create a ratio (calibration sample field height ÷ calibration sample image height = calibration ratio) that could then be used to calibrate the image seedling height measurements using the following calculations: image height x calibration ratio = corrected image height. The corrected image height was used to compare to the field measured height for our analysis.
Motion-triggered images were collected continuously from 27 May to 20 September 2017. Animals were identified as accurately as possible from the images, usually to the level of the genus, and when possible, to the species. Images of the seedlings from before, during, and after the animals were detected and were used to determine whether the animal was grazing or otherwise damaging the seedlings and how many seedlings had been damaged. The causes of damage to the plants were quantified by tracking individual plants and documenting when entire plants or parts of plants failed to occur in subsequent images. If images showed a herbivore consuming part or all of the plant, or if the plant was missing all or part of its vegetation directly after a herbivore was foraging at the plant, it was classified as a herbivory event for that animal. If no herbivore was detected when a plant was partially or wholly removed, it was classified as an unknown herbivory. If a seedling was otherwise damaged by being buried or trampled, it was labeled accordingly.
Our ability to accurately use data collected in the field and using remote cameras was dependent on several conditions which included (1) the proper camera settings, (2) the correct positioning of the camera, (3) establishing unobstructed conditions, and (4) developing strategies for working through the challenges that we encountered, including balancing the need for detail with managing large quantities of data collected, collecting different datasets with the same sensor (e.g., motion triggered images and time-lapse images from the same camera), and determining the correct interval for checking and cleaning sensors. The time required for data collection was also an important consideration when comparing the field data to the remote camera data, and we compared the time spent in the field collecting data to the time required for a remote camera setup, maintenance, and a post-collection analysis of images. Based on these assessments, we created a decision flow chart to inform users on the optimal camera settings for an improved reliability and an assessment of the nuances of the data collection and image analysis for acquiring accurate plant growth and survival data. We divided this decision-making approach into 6 main categories: (1) the primary focus of the study, (2) size/scale of the study subject, (3) primary features of the subjects to be studied/measured, (4) plot setup, (5) camera settings, and (6) camera maintenance.
2.3. Analysis
We compared the seedling density and average seedling height between our images and in-field measurements. We analyzed the overall accuracy of the seedling density and average seedling height from the images and identified the factors that affected the measurement accuracy using a mixed model analysis of variance in SAS® (SAS Institute Inc., Cary, NC, USA), with α = 0.05. The factors included in the model for the seedling height included the date, row order from the camera, fencing, and the interaction of the fencing and the date. We performed the same analysis for the seedling density including the date, row order from the camera, fencing, and the interaction of the date with the fencing and row. After adjusting for these factors, we used a mixed model analysis of variance to determine whether the time of day affected the measurement accuracy from the images. To determine the accuracy of the cameras in detecting herbivory, we calculated the frequency of herbivory events for each herbivore, and included a category for an unknown cause of herbivory.
3. Results
3.1. Average Height
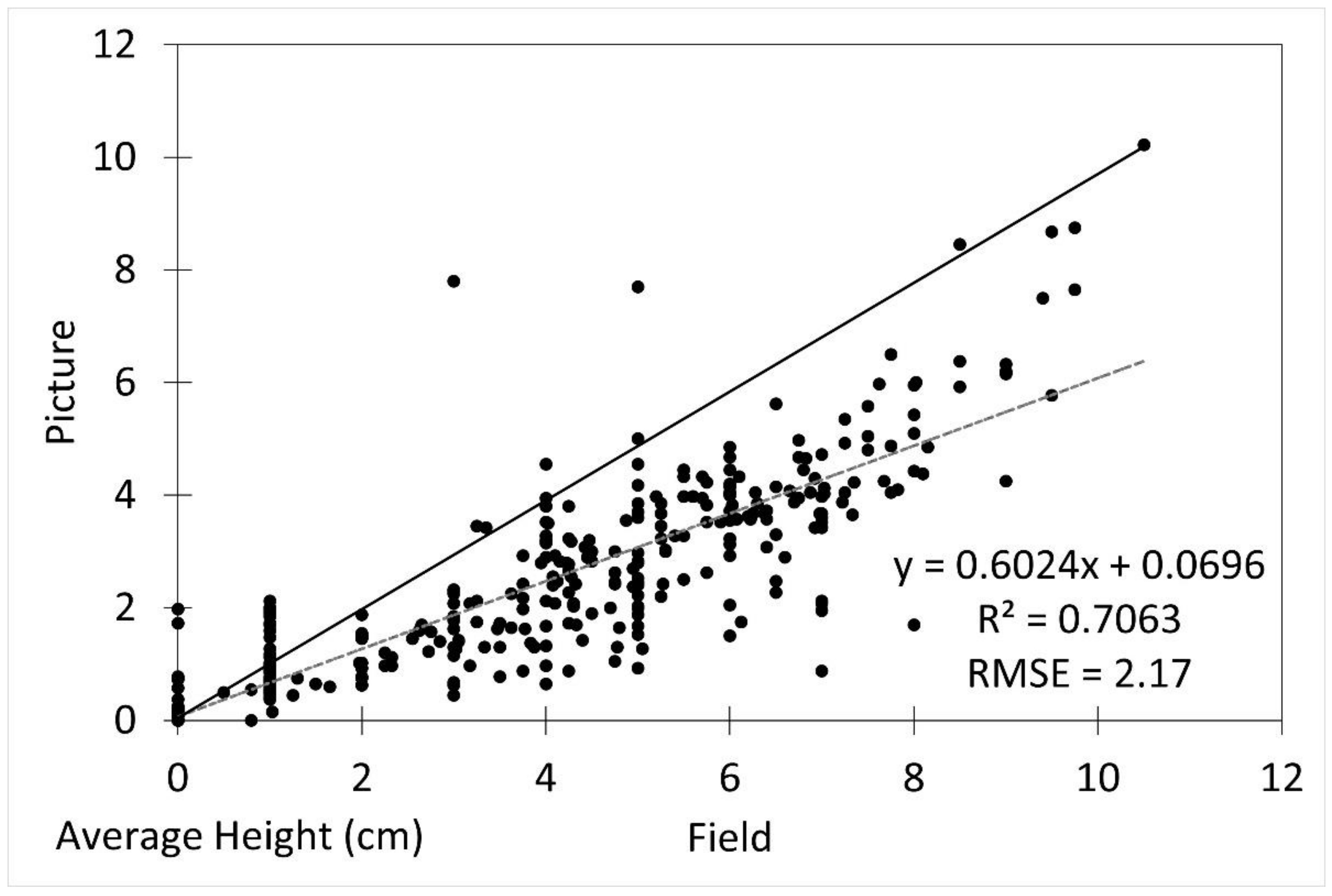
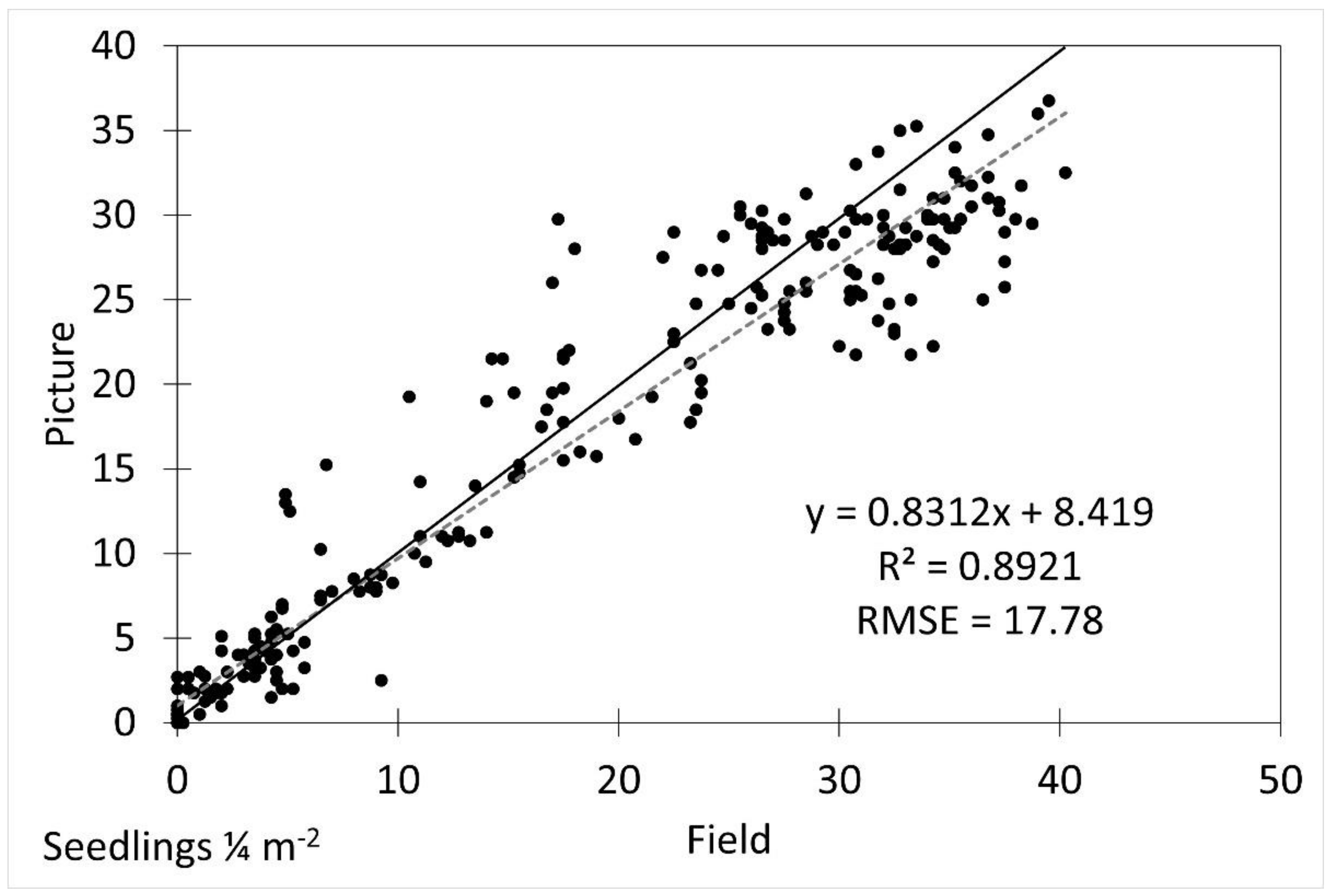
The average seedling height measured from the imagery was underestimated by approximately 14% compared to the field measurements (p = 0.03). The image height measurements were reliably generally accurate (R2 = 0.7063, RMSE 2.17) after underestimation was accounted for (Figure 3).
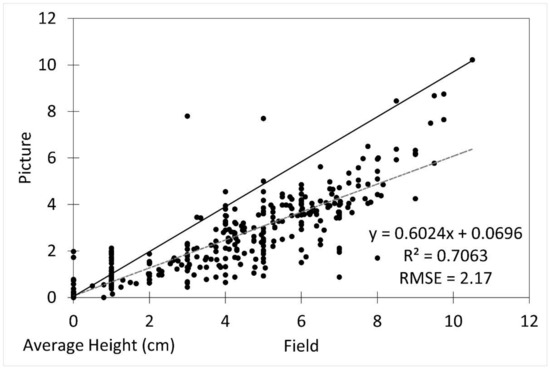
Figure 3.
Scatterplot of field vs. picture average seedling height with fitted linear regression line (dotted line) and 1:1 line (solid line). R2 was 0.7063 and RMSE was 2.17.
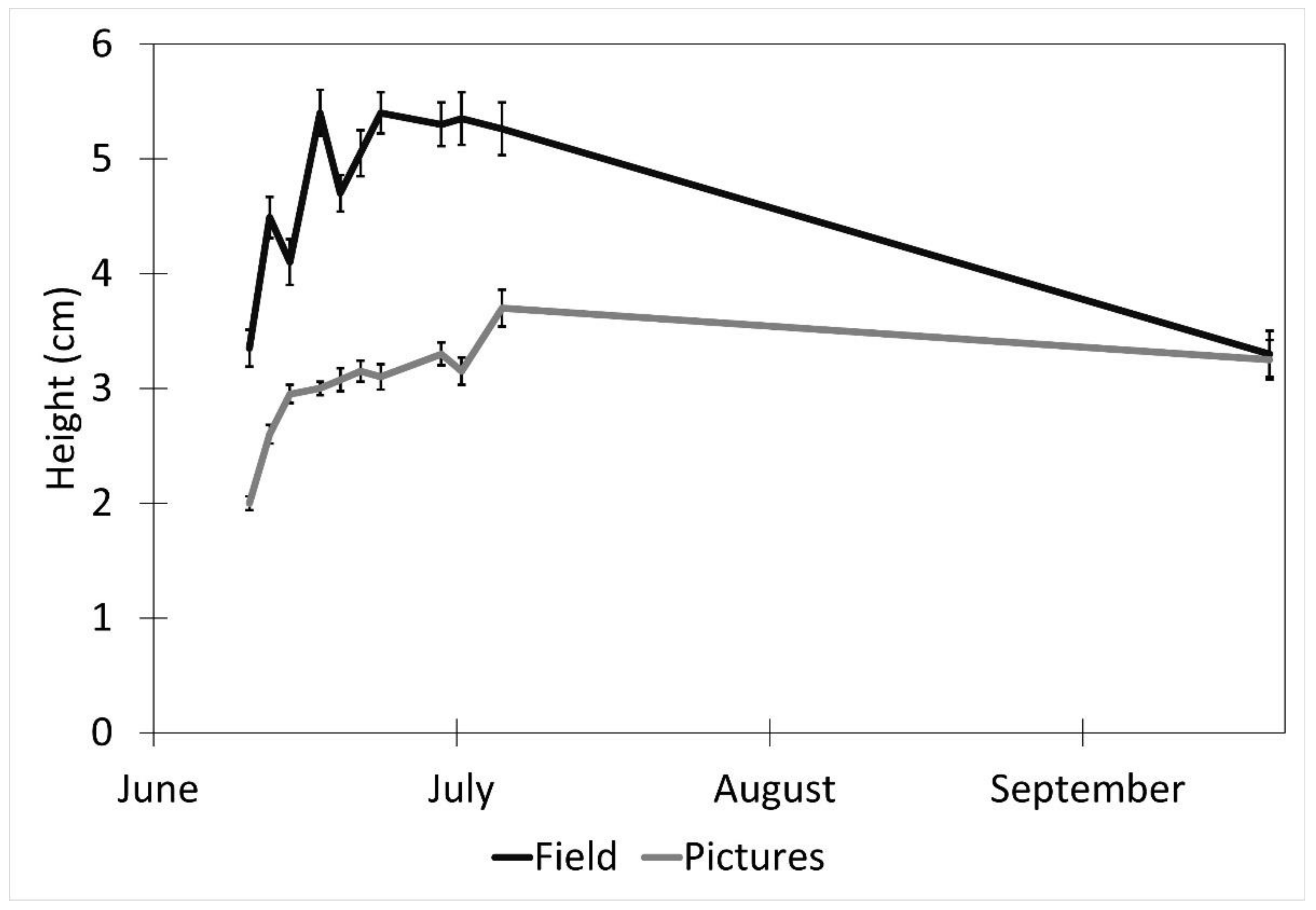
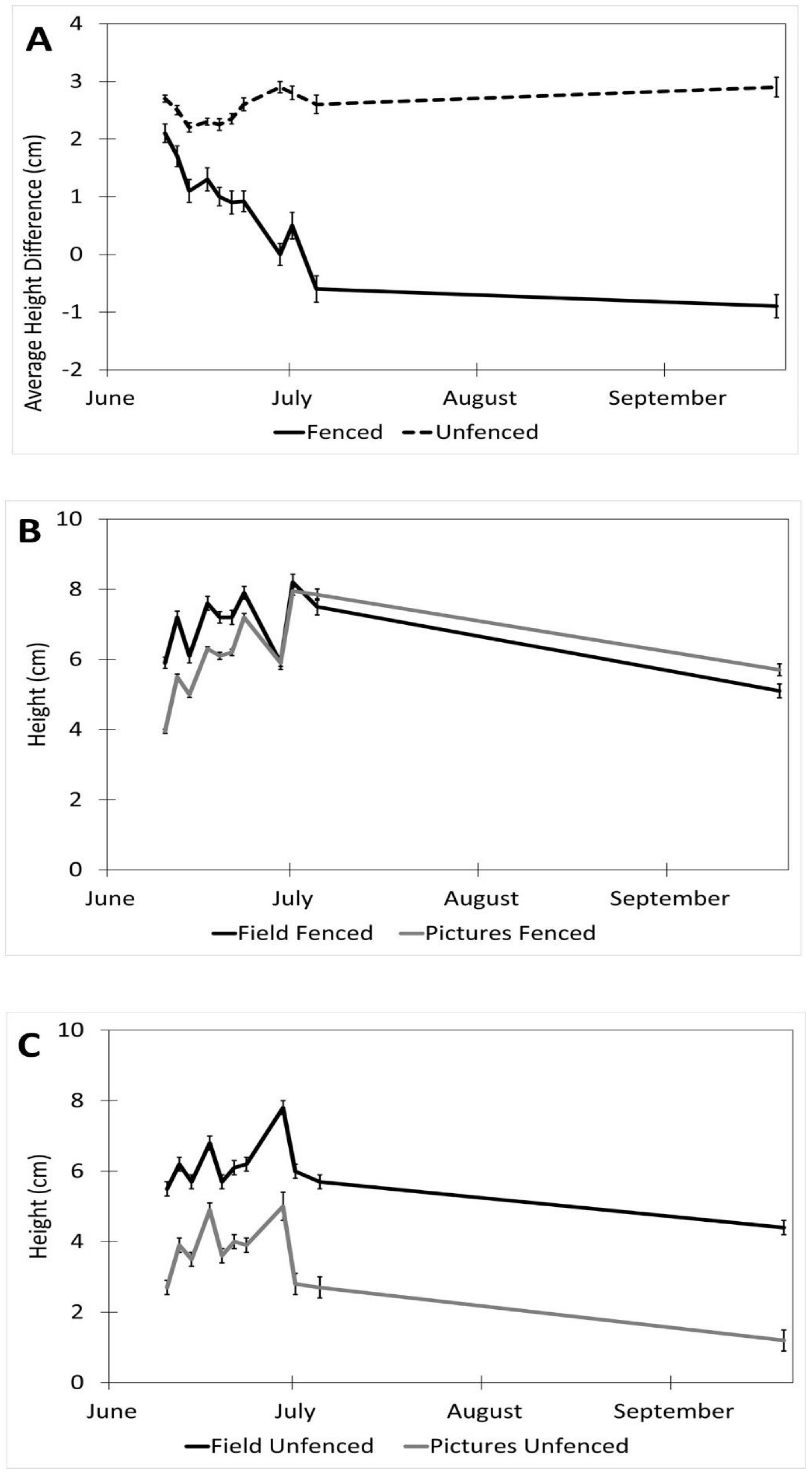
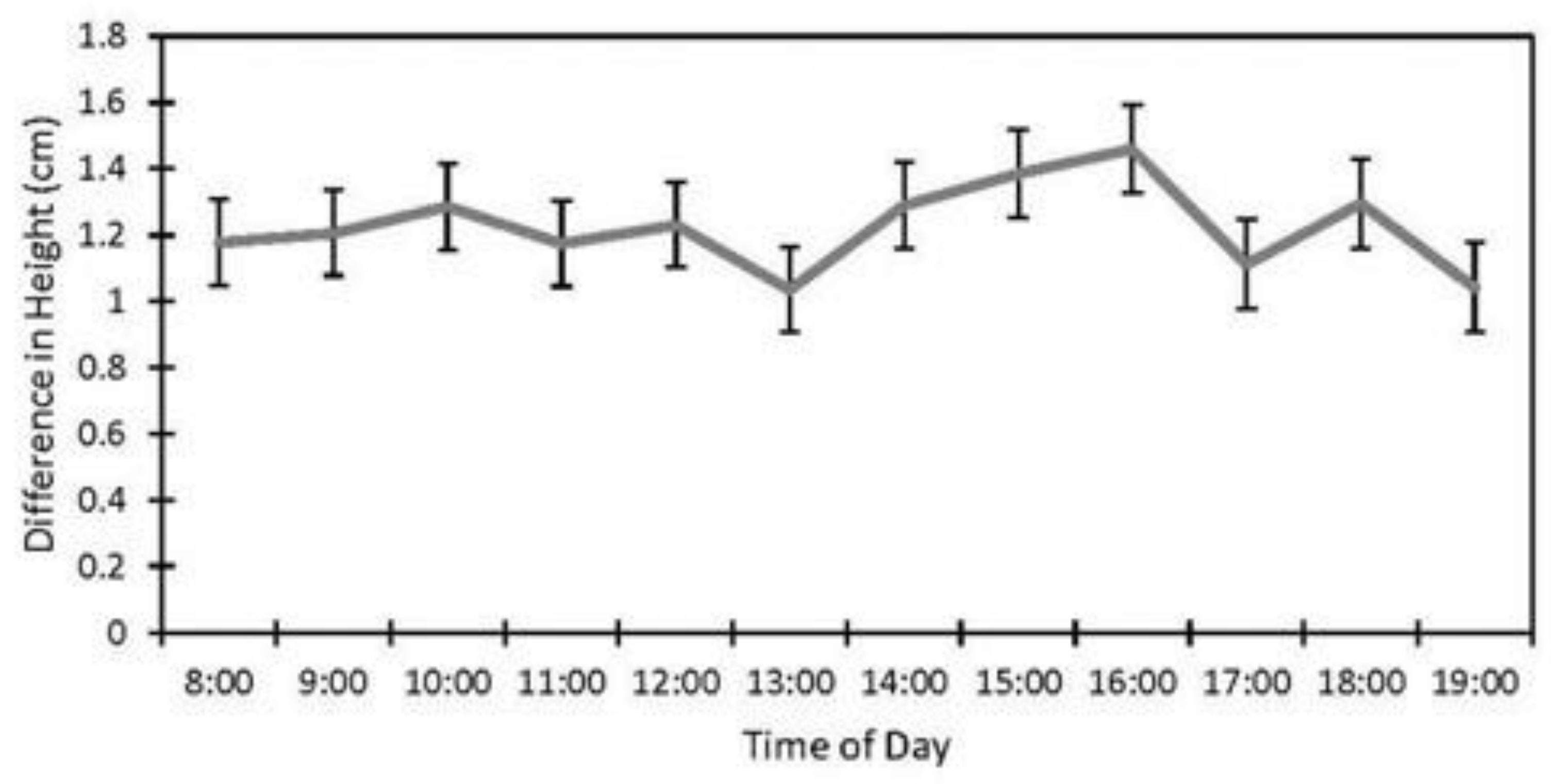
Factors affecting the accuracy of the image estimates for the height included the date (p < 0.001), fencing (p < 0.001), and the interaction of the date and the fencing (p < 0.001). The row order from the camera was the only factor that did not affect the accuracy of the seedling height estimates (p = 0.4). (Table 1). The measurements were underestimated at earlier dates by as much as 27% (14 June) and became more accurate over time with a difference of 8% on 5 July and no difference between the field and image estimates at the last measurement (19 September, Figure 4). The fenced image estimates were more accurate than the unfenced ones, but they varied by up to 2.9 cm, while the unfenced image estimates were very precise, varying a maximum of 0.75 cm. The fenced plot estimates of the average seedling height were underestimated by approximately 2 cm in June and became more accurate over time (Figure 5B), while the unfenced plot estimates were consistently underestimated by about 2.5 cm (Figure 5C). The accuracy of the plant height from the images did not differ between the dates in the unfenced plots (Figure 5A). After adjusting for the effects of the date, row, and fencing, the time of day had an effect on the accuracy of the height estimates from the images (p = 0.026). No major patterns were observed, but 3:00 and 4:00 PM were less accurate than 1:00, 5:00, and 7:00 PM, but not different from the other hours (Figure 6).

Table 1.
Results of mixed model analysis for average seedling height.
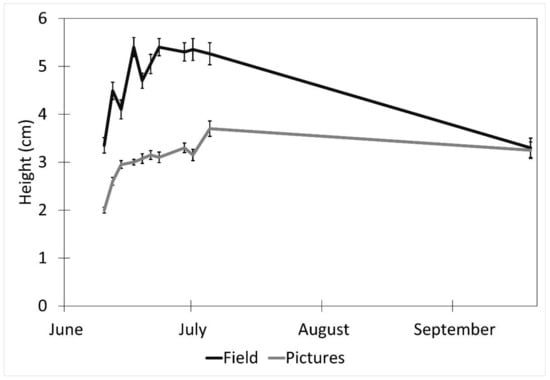
Figure 4.
Effect of date on picture height measurements (mean ± SE) estimated from images obtained with remote cameras and actual heights measured in the field every 2–3 days between 10 June and 5 July 2017, and a final measurement on 19 September 2017. Data were collected from study plots located at the Utah Testing Range, Utah.
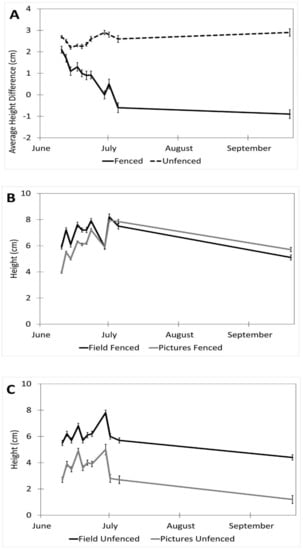
Figure 5.
Interaction of date and fencing for height estimates in images vs. field (estimates ± SE). (A) Shows the difference in height for fenced vs. unfenced plots (field measurement–image measurement). (B) A comparison of image and field measurements for height in fenced plots, over time. (C) A comparison of image and field measurements for height in unfenced plots, over time. Data were recorded in the field every 2–3 days between 10 June and 5 July 2017, with a final sample collected on 19 September 2017 at the Utah Testing Range, Utah.
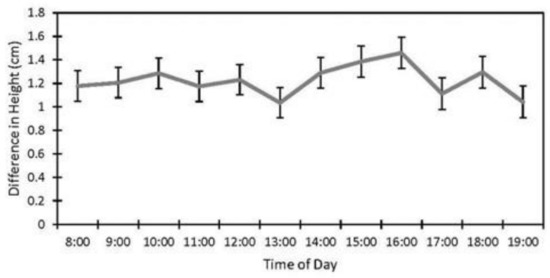
Figure 6.
Effect of time of day on accuracy of image height estimates (estimate ± SE) from data collected at study sites located at the Utah Test and Training Range (UTTR) in northwest Utah.
3.2. Seedling Density
The density estimates in images were different from the field measurements and were underestimated by approximately 30% (5.3 seedlings ¼ m−2, p = 0.019). The image extracted densities were generally accurate (R2 = 0.8921) after accounting for the underestimation, but had a higher error (RMSE = 17.78) than the height estimates.
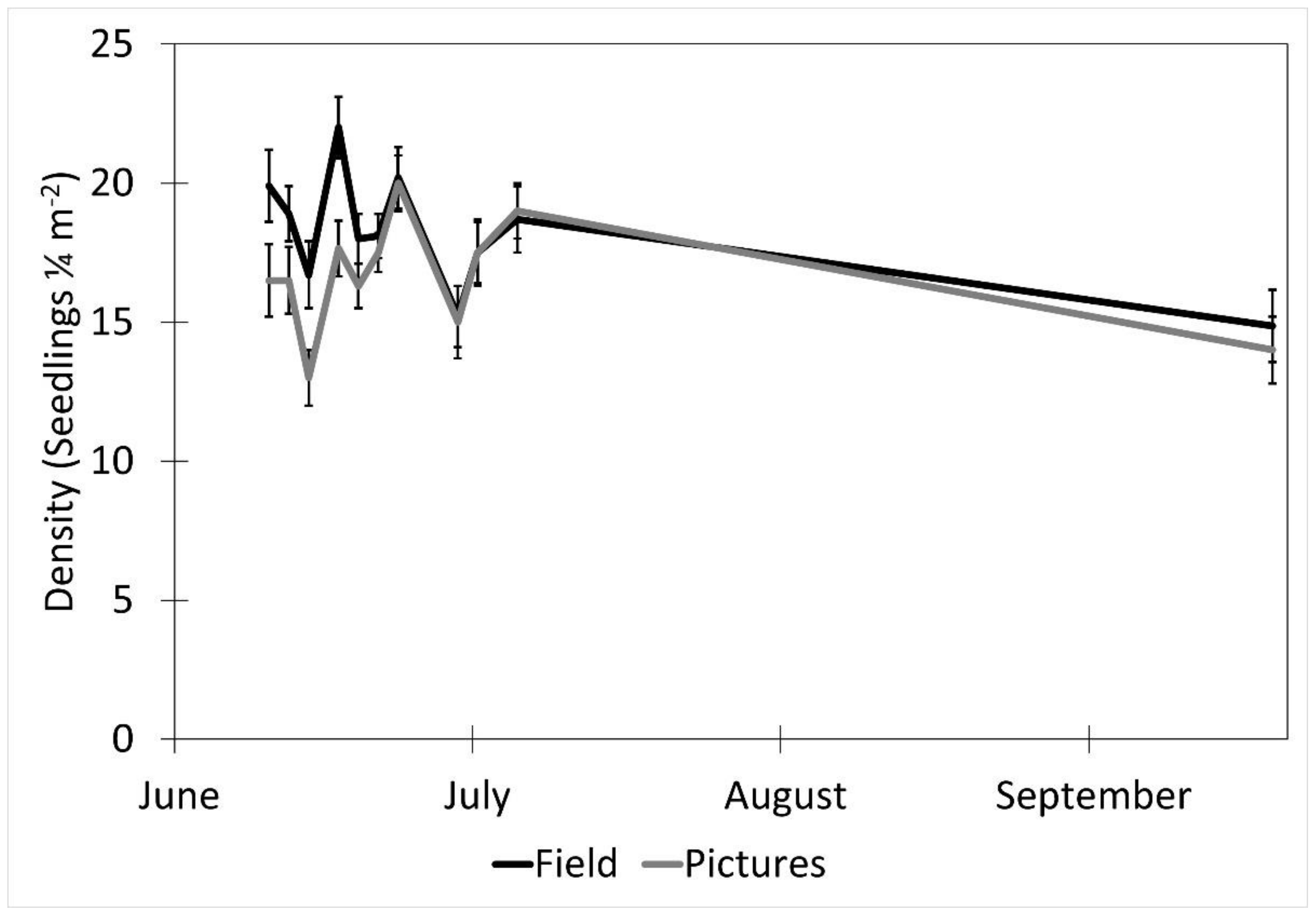
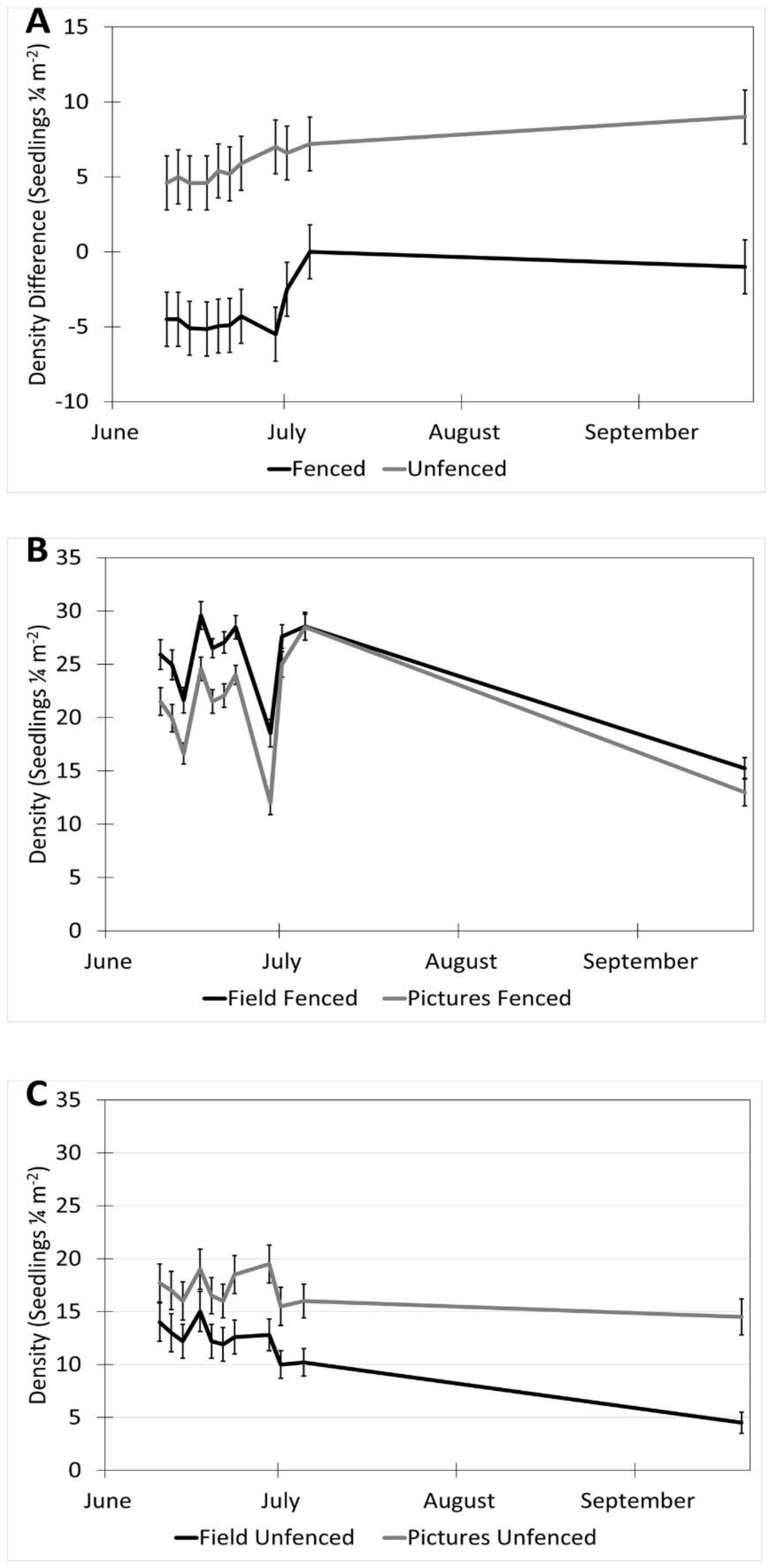
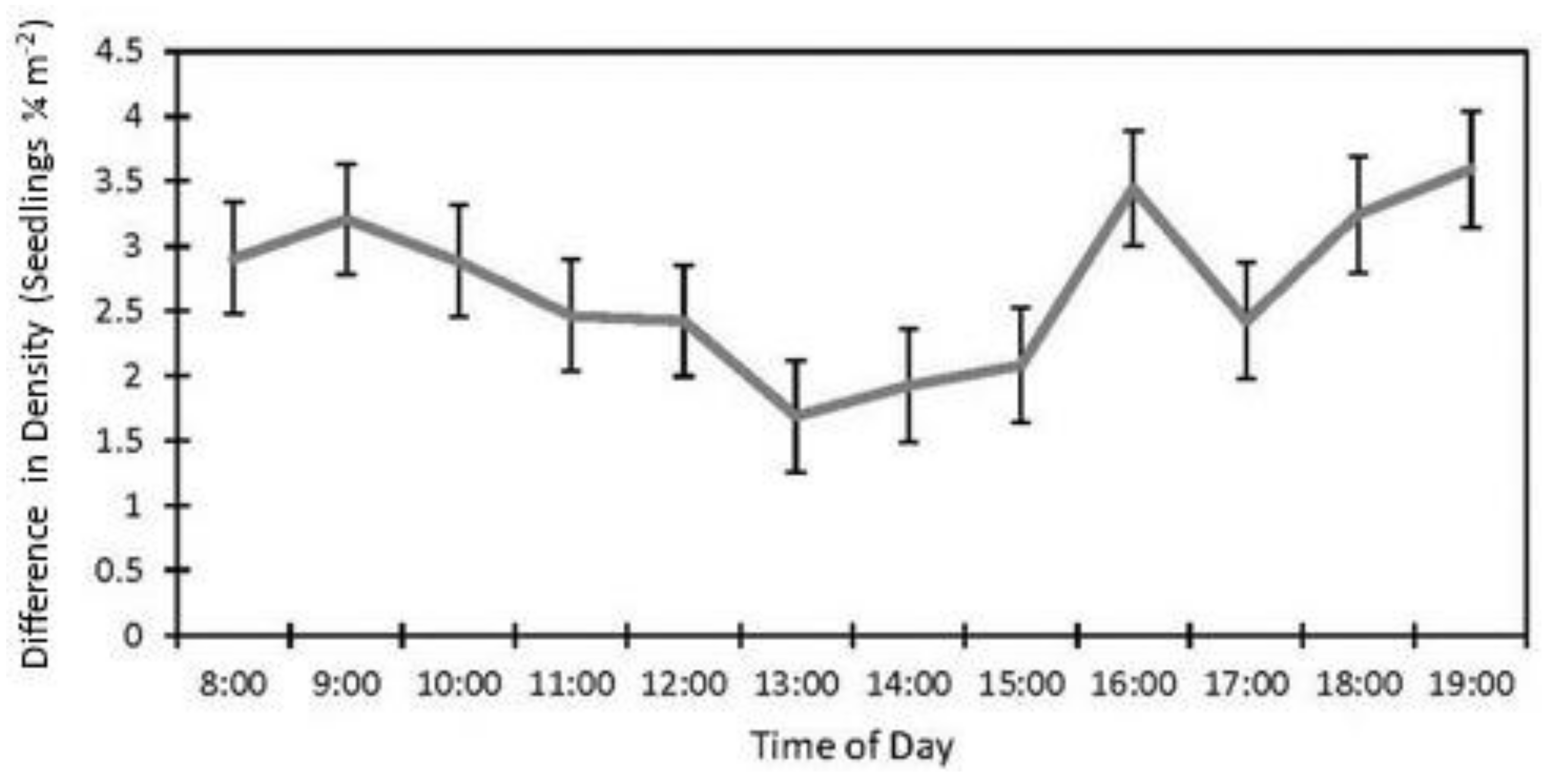
The date (p < 0.001), fencing (p < 0.001), the interactions of the date with the row order from the camera (p < 0.001) and with the fencing (p = 0.016), and the time of day affected the accuracy of the image estimates for the seedling density. The row order alone did not significantly affect the accuracy of image estimates for the seedling density (p = 0.069, Table 2). At earlier dates, the seedling density was underestimated in images by approximately 16% and increased with time until the density estimates were accurate, then overestimated at the latest dates by 3% (5 July) and 31% (19 September, Figure 6). In the fenced plots, the seedling density was underestimated in images by 5.7%, whereas the seedling density estimates were not affected in the unfenced plots. The fenced plot densities were underestimated at earlier dates by up to 25% (14 June) and were not different from the field measurements on 5 July and 19 September (Figure 7). At earlier dates, the row order from the camera did not affect the accuracy of the image density estimates compared to the field measurements, but on 19 September, rows one and four (the first and last rows) were overestimated compared to the field measurements by 47% and 56%, respectively. After adjusting for the date, row, and treatment, the time of day had an effect on the accuracy of the density estimates (p < 0.001). Afternoon hours from 1:00 PM to 3:00 PM had more accurate seedling density estimates than the morning hours (8:00 AM to 10:00 AM) and some evening hours (4:00 AM, 6:00 PM, and 7:00 PM, Figure 8). The interaction of the date and the fencing for the density estimates suggest that there is a difference in density when comparing the fenced and unfenced plots (Figure 9). Additionally, when comparing the images and field measurements, we found a greater density in the fenced plots over time. We also noted that the time of day that the photos were taken in influenced the accuracy of the results (Figure 10).

Table 2.
Results of mixed model analysis for seedling density.
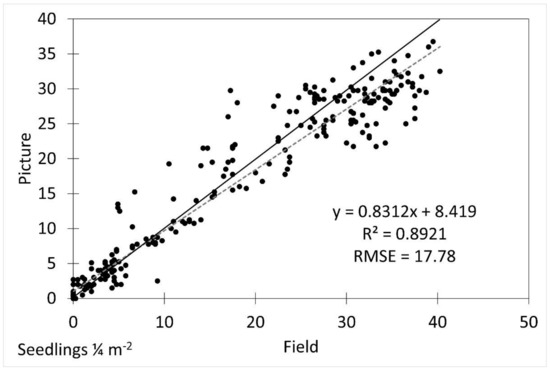
Figure 7.
Scatterplot of field vs. picture average seedling density with fitted linear regression line (dotted line) and 1:1 line (solid line). R2 was 0.8921 and RMSE was 17.78.
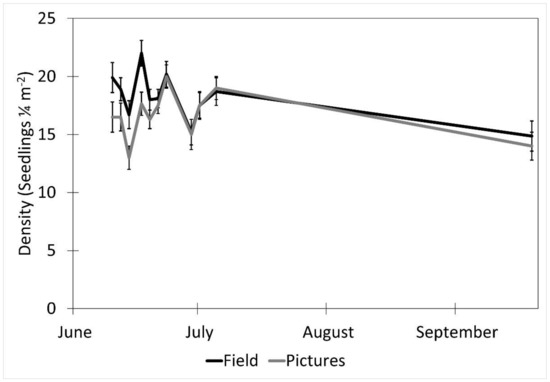
Figure 8.
The effect of date on picture density estimates (mean ± SE) from an image and measured in the field over the period of the study. Data were recorded in the field every 2–3 days between 10 June and 5 July 2017, with a final sample collected on 19 September 2017 at the Utah Testing Range, Utah.
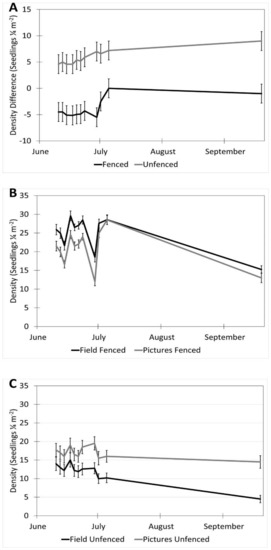
Figure 9.
Interaction of date and fencing for density estimates in images vs. field (estimates ± SE). (A) Shows the difference in density for fenced vs. unfenced plots. (B) A comparison of image and field measurements for density in fenced plots, over time. (C) A comparison of image and field measurements for density in unfenced plots, over time. Data were recorded in the field every 2–3 days between 10 June and 5 July 2017, with a final sample collected on 19 September 2017 at the Utah Testing Range, Utah.
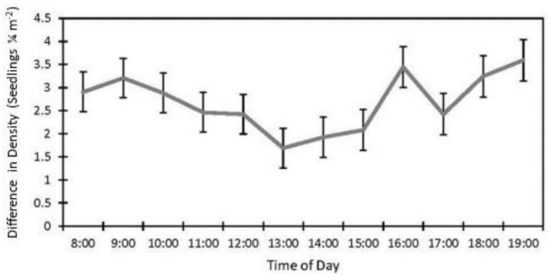
Figure 10.
Effect of time of day on seedling density estimates in images vs. field (estimate ±SE) from data collected at study sites located at the Utah Test and Training Range (UTTR) in northwest Utah.
3.3. Herbivory Detection
A large suite of herbivores was detected consuming seedlings in herbivory events (Table 3). Herbivory was detected and assigned to specific herbivores for 69.1% of the damaged seedlings. Seedling herbivory often decreased the density and/or height of the seedlings in the unfenced plots. Larger herbivores like L. californicus and T. bottae generally consumed larger amounts of seedlings and also consumed more of the tissue on the individual seedlings than smaller herbivores. The cause of the seedling herbivory was unknown for 22.6% of the seedlings, and 8.3% of the seedlings were trampled or buried (Table 4). The smallest herbivore detected was the Acrididae family (grasshoppers), which accounted for 5.6% of known herbivory to the seedlings. The majority (91.66%) of seedling damage and death was caused by herbivory.

Table 3.
Frequency and percent of herbivory events on seedlings separated by herbivore event types.

Table 4.
Percent damage caused to seedlings separated by category. Herbivores damaged the largest proportion of seedlings (69%).
The fenced plots did account for 23.5% of the herbivory events. T. bottae caused 73.18% of the herbivory events in the fenced plots. This was in two plots where T. bottae were able to burrow under the fence to access the seedlings late in the summer (August). Other herbivory in fenced plots was mostly undetected (82%).
4. Discussion
Research that evaluates vegetation characteristics using airborne or satellite imagery more than 2 m distant from the plant surface is common in the published literature [25,50,61,62]. The small size of E. elymoides seedlings relative to more mature plants creates a unique challenge in accurately assessing the plant height in images. In our study, we were able to measure even the slightest differences in plant growth because of the high-frequency image availability and the high resolution. While the total height and growth patterns over time were detected with both the field and remote measurement techniques, our limited calibration between these two approaches resulted in an underestimation of the seedling height by 14%. One method to improve the accuracy of the image height estimates would be to place a ruler or a small Robel pole-style instrument [50] attached to a small dowel vertically next to the seedlings at each row as a reference scale. This would eliminate the need for a calibration of the picture estimates because the visual marker would allow for a direct extraction of the height from the images.
The fencing appeared to decrease the precision of the height measurements from the images. At later dates, the height estimates from the images were more accurate than at the beginning of the summer. The unfenced plots were underestimated but very precise, which allowed for an easier adjustment of the measurements. A reasonable explanation for the patterns with the date, fencing, and their interaction, is that a higher seedling density made it more difficult to obtain consistently accurate seedling measurements in the images due to more visual obstruction from the seedlings in the front rows. In the unfenced plots, the seedlings were often grazed by herbivores, maintaining or reducing the average height and density, and thus indirectly increasing the precision of the estimates. The fenced plots were subject to some herbivory, likely due to small invertebrate herbivores, and in two plots where T. bottae were able to burrow under the fence. While this may have reduced some density in the fenced plots, 73% of the herbivory happened between the last two measurements and did not affect the majority of the density estimates in the fenced plots. The densities were much lower in the unfenced plots due to much higher herbivory rates by large herbivores across all plots. Since less dense plants could lead to more precise image estimates, this should be a consideration when measuring the height of very small seedlings. When the time-of-day analysis for the height is considered, 1:00 PM, 5:00 PM, and 7:00 PM were the only hours that had more accurate image estimates than the others. This is most likely due to the effects of shadows and the angle of the sun. To sample at the best time of day, researchers should consider the angle of the sun and visual obstructions that may cause shadows.
The number of plants present tended to reduce the accuracy of the plant density estimates from the images. For example, the seedling densities were highest at the earlier dates before the seedlings had been grazed, which was also when the seedlings densities were underestimated the most. The density estimates from the images were underestimated more in the fenced plots, which had higher plant densities than the unfenced plots. The high-density averages were approximately 22 seedlings ¼ m−2, and the low values ranged between 10 and 15 seedlings ¼ m−2. Overall, the density estimates became more accurate over time, which correlated with a reduction in the seedling density. We felt that our ability to detect seedlings from images decreased with an increasing seedling density because individual seedlings in rows closer to the camera would obstruct the view of other seedlings in the rows farther from the camera. Herbivory in the unfenced plots could indirectly affect the density estimates by reducing the actual density of the seedlings, making it easier to estimate the densities from the images. Additionally, seedlings growing close together made it difficult to determine from the images whether they were individual plants or tillers from the same plant. It could be possible that the influence of the date on the accuracy is partially attributed to larger seedlings being easier to see and measure than the smaller plants.
Similar to the aerial wildlife surveys reported in the literature it appears that the seedling density and height estimates could be influenced by different sightability factors (factors affecting the probability of seeing an individual), like the number of seedlings in the image (analogous to group size in wildlife), visual obstruction (seedlings themselves, analogous to cover for wildlife), and the size of the individual [63]. Though this study did not calculate sightability adjustments for the seedlings, models similar to wildlife sightability could be developed to adjust the estimates based on the probability of seeing individual seedlings [64].
Afternoon hours from 1:00 PM to 3:00 PM had the most accurate seedling density estimates. At the extremes of the day, the sun casts long shadows, affecting the visibility of the seedlings in the images. The sun is overhead at noon, but with no shadow, the seedlings may be washed out in the image and thus be hard to see. As the sun passes its zenith, shadows may be cast from the seedlings, increasing the visibility, but not being overcast from larger shadows as they are in the morning and evening. The best time of day for reducing the effect of shadows will also depend on the season and location [65].
The cameras were effective at capturing most herbivory events (69%). It is likely that much of the unknown herbivory was caused by the Formicidae family (ants), Acrididae family (grasshoppers), or other small invertebrate herbivores that are too small, with temperatures near the ambient temperature, to trigger the camera’s infrared sensor. All but two (99.1%) unknown herbivory events occurred during the day, which coincides with the activity of diurnal species such as small invertebrate herbivores. A large suite of invertebrate herbivores such as the Formicidae family, Coleoptera order (beetles), and Acrididae family can be encountered in the Great Basin, which feed primarily on grasses like E. elymoides [66]. Additionally, 23.5% of herbivory events occurred in the fenced plots. In these fenced plots, the herbivory events were from animals that were able to get past the boundary fence by flying over, burrowing under, or fitting through the spaces in the wire, such as Eremophila alpestris (horned lark), the Acrididae family, and Thomomys bottae (Botta’s pocket gopher). Unknown herbivores that were small enough to enter the fenced plots probably were in the same proportions as in the unfenced plots (22.7%), adding to evidence that undetected herbivory events were by small invertebrate herbivores such as the Formicidae family (Table 5).

Table 5.
Frequency of herbivory events on seedlings in fenced plots.
One of the advantages of using motion-sensitive cameras to track the seedlings is the flexibility which is provided. In order to effectively utilize cameras to address the unique research objectives, many decisions must be made based on the specific research needs of individual studies. The time required for data collection is an important consideration when looking at using remote cameras for studies. We assume that the study setup time will be roughly the same for each method, but the camera setup for us required an additional 4 h. In our study, we wanted to collect data a minimum of two times per week. Our study site was approximately 2 hours’ drive from our regular workstation. To collect data in the field, this meant a minimum of 8 h travel time just for the basic amount of data needed. We collected data in the field 12 times during the course of the summer, which is 48 h of driving time alone. The actual collection of the field data took approximately 1 h, bringing the total to 60 h required to collect the data. The extraction of data from the images took approximately the same amount of time as the actual field collection, totaling approximately 12 h. We determined that 2-week intervals were sufficient for maintaining the cameras (checking the batteries, cleaning the lenses, and replacing the memory cards). The travel to maintain the cameras over the course of the summer required seven site visits, totaling 28 h of travel time and 1 h of maintenance time. This brought the total for the camera data collection and maintenance to 45 h. For this frequency of data collection and distance to the study site, we saved 15 h of work time. We collected additional data on our cameras as frequently as 12 times per day, including motion data. These data would be practically impossible to obtain if requiring a person to be present at all times. While we did not estimate the time cost, we believe that cameras are required to collect this type and frequency of data.
This study design was developed specifically using the Reconyx PC900 camera, but other cameras could be used. Some considerations for selecting a camera include the availability of the cameras, price/cost of using the cameras, durability, weather resistance, focal length and the ability to adjust the focal length, field of view size, and type of trigger available (timed, motion, or manual) [28]. While these cameras can be relatively expensive, their utility for collecting novel data should be taken into consideration. Additionally, the density of the cameras required will depend on the type and scale of monitoring desired, which can influence the total overall costs.
Once a camera is selected, the user should determine the size of the plants to be studied. Studies of large plants can have a longer focal length and a larger field of view (FOV), because the plants are more easily visible and a larger field of view may be necessary to capture the images of larger plants. Conversely, small plants are more difficult to detect in images and the camera must be closer to the plants, creating a narrower FOV (Figure 1).
We recommend that primarily two factors should be used to determine the position that cameras should be placed in: the data to be collected and physical characteristics of the plant. If a study emphasizes the vertical plant characteristics such as the height or changes in height, we recommend positioning the camera parallel to the ground, allowing the camera focus to be perpendicular to the plant for ab adequate feature capture. Horizontal plant characteristics such as the plant width, increases in foliage, and even biomass [63] can often also be determined with the camera being positioned parallel to the ground. If cameras are placed parallel to the ground, we recommend that they be elevated a minimum of approximately 10 cm off the ground and angled forward approximately 15 degrees. This helps prevent dust and debris buildup on the lens of the camera. If larger plants are being studied, the camera may be placed higher without an angle, since dust and debris will be less of a concern. One advantage of placing the camera parallel to the ground is that cameras will cast less of a shadow than a camera which is placed above the plants. If a study does not require height estimates but requires a cover or other similar estimates, we recommend placing the camera above the plants, perpendicular to the ground. Most plant cover estimates and measurements are taken from above the plant looking down [67]. Placing the camera in this position facilitates an image collection that allows the estimation of cover. Again, depending on the size of the plants, the camera height should be adjusted based on the size of the plants being studied. We also recommend that plants’ physical characteristics should be considered when determining the camera position. E. elymoides seedlings are slender with a much higher surface area visible from the side than from above, especially directly after seedling emergence. These characteristics of the plant make it important to place the camera parallel to the ground so that the images capture the largest amount of surface area for an easier identification. If the plant has more surface area visible from above and height measurements are not required, it may be better to place the camera above the plants.
5. Conclusions and Implications
The ability to acquire imagery using remote camera technology is a powerful tool for acquiring plant morphological and growth pattern data. The use of these cameras in a near-ground setting offers the opportunity to collect details unavailable with higher-altitude sensors [49]. The ability to adjust the camera position and settings allows for a flexibility in creating the study design. Remote cameras can be set up and checked by one person in a few hours and can collect data even when researchers are not present, which reduces the need to make frequent visits to the site. While cameras may require less fieldwork, the amount of time required pre- and post-collection can be substantial, and this trade-off should be considered before using cameras in such research. A careful and thorough planning and decision-making process is imperative for an effective data collection and for post-collection processing.
This study focused on tracking one species (E. elymoides) in small, watered plots within the area of rangeland revegetation efforts. Future research should be conducted with cameras to determine if they can be successfully used to monitor multiple species in rangeland reseeding efforts. Using cameras to monitor reseeding efforts may also require research on how the camera measurements of height, density, and herbivory are affected in very low densities of seedlings, and the number of cameras required to achieve an acceptable statistical power with low densities of seedlings. By applying these methods, we were able to assess the efficacy of detecting seedling dynamics at a fine scale, optimizing the frequency of sampling and reducing travel costs to improve the seedling research. This information can be used to inform managers on the application of technology as a sampling strategy for monitoring seeding success with a greater accuracy and efficiency.
Though there are potential drawbacks to using remote cameras for research, creativity and thoughtfulness will allow cameras to be a powerful tool for researchers and land managers to study plants, especially seedlings. Potential areas of research or monitoring using remote cameras could include tracking seedling emergence, densities, demographics, and survival, among others. The ability to track specific causes of seedling death using direct photographic evidence could be useful for identifying the causes of seedling death in restoration efforts. Using cameras for seedling monitoring and research during restoration will inform the post-seeding management of rangeland restoration projects and possibly lead to more effective restoration efforts.
Author Contributions
Conceptualization, J.R.M., S.L.P., C.R.L., B.R.M. and M.D.M.; methodology, J.R.M., S.L.P., B.R.M., M.D.M. and D.L.E.; software, J.R.M. and D.L.E.; validation, J.R.M., S.L.P. and D.L.E.; formal analysis, J.R.M., S.L.P. and D.L.E.; investigation, J.R.M. and S.L.P.; resources, S.L.P. and C.R.L.; data curation, J.R.M.; writing—original draft preparation, J.R.M. and S.L.P.; writing—review and editing, B.R.M. and M.D.M.; visualization, J.R.M., S.L.P. and C.R.L.; supervision, J.R.M., S.L.P., B.R.M. and M.D.M.; project administration, S.L.P. and C.R.L.; funding acquisition, S.L.P. and C.R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Funding was provided by the Hill Air Force Base Natural Resource Management Program and Brigham Young University. We appreciate Jace Taylor, Jacob Hall, Tyler Glazier, and BYU student field technicians who participated in the fieldwork and data collection. We also wish to thank Nick Brown and Mike Shane for helping sure access to the study sites and facilitating our data collection efforts at the UTTR.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Institute, W.R. Forests and Rangelands. In World Resources 1986; Basic Books, Inc.: New York, NY, USA, 1986; pp. 273–280. [Google Scholar]
- Bestelmeyer, B.T.; Brown, J.R.; Havstad, K.M.; Alexander, R.; Chavez, G.; Herrick, J.E. Development and use of state-and-transition models for rangelands. Rangel. Ecol. Manag. J. Range Manag. Arch. 2003, 56, 114–126. [Google Scholar]
- Bedunah, D.J.; Angerer, J.P. Rangeland degradation, poverty, and conflict: How can rangeland scientists contribute to effective responses and solutions? Rangel. Ecol. Manag. 2012, 65, 606–612. [Google Scholar] [CrossRef]
- Burke, M.J.; Grime, J. An experimental study of plant community invasibility. Ecology 1996, 77, 776–790. [Google Scholar] [CrossRef]
- Brooks, M.L.; D’antonio, C.M.; Richardson, D.M.; Grace, J.B.; Keeley, J.E.; DiTomaso, J.M.; Hobbs, R.J.; Pellant, M.; Pyke, D. Effects of invasive alien plants on fire regimes. BioScience 2004, 54, 677–688. [Google Scholar] [CrossRef]
- Holle, B.V.; Simberloff, D. Ecological resistance to biological invasion overwhelmed by propagule pressure. Ecology 2005, 86, 3212–3218. [Google Scholar] [CrossRef]
- Scasta, J.D.; Weir, J.R.; Stambaugh, M.C. Droughts and wildfires in Western U.S. rangelands. Rangelands 2016, 38, 197–203. [Google Scholar] [CrossRef]
- Mack, M.C.; D’Antonio, C.M. Impacts of biological invasions on disturbance regimes. Trends Ecol. Evol. 1998, 13, 195–198. [Google Scholar] [CrossRef]
- Svejcar, T.; Boyd, C.; Davies, K.; Hamerlynck, E.; Svejcar, L. Challenges and limitations to native species restoration in the Great Basin, USA. Plant Ecol. 2017, 218, 81–94. [Google Scholar] [CrossRef]
- Stringham, T.K.; Krueger, W.C.; Shaver, P.L. State and transition modeling: An ecological process approach. Rangel. Ecol. Manag. J. Range Manag. Arch. 2003, 56, 106–113. [Google Scholar]
- Charles, H.; Dukes, J.S. Impacts of invasive species on ecosystem services. In Biological Invasions; Springer: Berlin/Heidelberg, Germany, 2008; pp. 217–237. [Google Scholar]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; De Vries, W.; De Wit, C.A. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef]
- Hardegree, S.P.; Jones, T.A.; Roundy, B.A.; Shaw, N.L.; Monaco, T.A. Assessment of range planting as a conservation practice [chapter 4]. In Conservation Benefits of Rangeland Practices: Assessment, Recommendations, and Knowledge Gaps; Briske, D.D., Ed.; Allen Press: Lawrence, KS, USA, 2011; pp. 171–212. [Google Scholar]
- Merritt, D.J.; Dixon, K.W. Restoration seed banks—A matter of scale. Science 2011, 332, 424–425. [Google Scholar] [CrossRef]
- Office, U.S.A. Wildland Fires; Office, G.A., Ed.; Washington, DC, USA, 2003.
- Sheley, R.; James, J.; Rinella, M.; Blumenthal, D.; DiTomasso, J. A scientific assessment of invasive plant management on anticipated conservation benefits. In Conservation Benefits of Rangeland Practices: Assessment, Recommendations, and Knowledge Gaps; Allen Press: Lawrence, KS, USA, 2011; pp. 291–335. [Google Scholar]
- Bosco, T.; Bertiller, M.B.; Carrera, A.L. Micro-environmental conditions affect grass and shrub seedling emergence in denuded areas of the arid Patagonian Monte, Argentina. Flora-Morphol. Distrib. Funct. Ecol. Plants 2015, 210, 66–71. [Google Scholar] [CrossRef]
- Boyd, C.S.; James, J.J. Variation in timing of planting influences bluebunch wheatgrass demography in an arid system. Rangel. Ecol. Manag. 2013, 66, 117–126. [Google Scholar] [CrossRef]
- Hardegree, S.P.; Moffet, C.A.; Flerchinger, G.N.; Cho, J.; Roundy, B.A.; Jones, T.A.; James, J.J.; Clark, P.E.; Pierson, F.B. Hydrothermal assessment of temporal variability in seedbed microclimate. Rangel. Ecol. Manag. 2013, 66, 127–135. [Google Scholar] [CrossRef]
- James, J.J.; Svejcar, T. Limitations to postfire seedling establishment: The role of seeding technology, water availability, and invasive plant abundance. Rangel. Ecol. Manag. 2010, 63, 491–495. [Google Scholar] [CrossRef]
- Kerr, J.T.; Ostrovsky, M. From space to species: Ecological applications for remote sensing. Trends Ecol. Evol. 2003, 18, 299–305. [Google Scholar] [CrossRef]
- Jones, M.O.; Naugle, D.E.; Twidwill, D.; Uden, D.R.; Maetas, J.D.; Allred, B.W. Beyond inventories: Emergence of a new era in rangeland monitoring. Rangel. Ecol. Manag. 2020, 73, 577–583. [Google Scholar] [CrossRef]
- Lawley, V.; Lewis, M.; Clarke, M.; Ostendorf, B. Site-based and remote sensing methods for monitoring indicators of vegetation condition: An Australian review. Ecol. Indic. 2016, 60, 1273–1283. [Google Scholar] [CrossRef]
- What Is Remote Sensing? Available online: https://oceanservice.noaa.gov/facts/remotesensing.html (accessed on 25 June 2018).
- Davies, K.W.; Petersen, S.L.; Johnson, D.D.; Davis, D.B.; Madsen, M.D.; Zvirzdin, D.L.; Bates, J.D. Estimating juniper cover from National Agriculture Imagery Program (NAIP) imagery and evaluating relationships between potential cover and environmental variables. Rangel. Ecol. Manag. 2010, 63, 630–637. [Google Scholar] [CrossRef]
- Madsen, M.D.; Zvirzdin, D.L.; Davis, B.D.; Petersen, S.L.; Roundy, B.A. Feature extraction techniques for measuring pinon and juniper tree cover and density, and comparison with field-based management surveys. Environ. Manag. 2011, 47, 766–776. [Google Scholar] [CrossRef]
- Yu, Z.; Cao, Z.; Wu, X.; Bai, X.; Qin, Y.; Zhuo, W.; Xiao, Y.; Zhang, X.; Xue, H. Automatic image-based detection technology for two critical growth stages of maize: Emergence and three-leaf stage. Agric. For. Meteorol. 2013, 174, 65–84. [Google Scholar] [CrossRef]
- Rovero, F.; Zimmermann, F.; Berzi, D.; Meek, P. “Which camera trap type and how many do I need?” A review of camera features and study designs for a range of wildlife research applications. Hystrix 2013, 24, 148–156. [Google Scholar]
- Kays, R.; Kranstauber, B.; Jansen, P.; Carbone, C.; Rowcliffe, M.; Fountain, T.; Tilak, S. Camera traps as sensor networks for monitoring animal communities. In Proceedings of the 2009 IEEE 34th Conference on Local Computer Networks, Zurich, Switzerland, 20–23 October 2009; pp. 811–818. [Google Scholar]
- Sun, C.; Beirne, C.; Burgar, J.M.; Howey, T.; Fisher, J.T.; Burton, A.C. Simultaneous monitoring of vegetation dynamics and wildlife activity with camera traps to assess habitat change. Remote Sens. Ecol. Conserv. 2021, 7, 666–684. [Google Scholar] [CrossRef]
- Hofmeester, T.R.; Young, S.; Juthberg, S.; Singh, N.J.; Widemo, F.; Andren, H.; Linnell, J.D.C.; Cromsigt, J.P.G.M. Using by-catch data from wildlife surveys to quantify climatic parameters and timing of phenology for plants and animals using camera traps. Remote Sens. Ecol. Conserv. 2020, 6, 129–140. [Google Scholar] [CrossRef]
- Karanth, K.U.; Nichols, J.D. Estimation of tiger densities in India using photographic captures and recaptures. Ecology 1998, 79, 2852–2862. [Google Scholar] [CrossRef]
- Hall, L.K.; Larsen, R.T.; Knight, R.N.; McMillan, B.R. Feral horses influence both spatial and temporal patterns of water use by native ungulates in a semi-arid environment. Ecosphere 2018, 9, e02096. [Google Scholar] [CrossRef]
- Larsen, R.T.; Flinders, J.T.; Mitchell, D.L.; Perkins, E.R.; Whiting, D.G. Chukar watering patterns and water site selection. Rangel. Ecol. Manag. 2007, 60, 559–565. [Google Scholar] [CrossRef]
- Rendall, A.R.; Sutherland, D.R.; Cooke, R.; White, J. Camera trapping: A contemporary approach to monitoring invasive rodents in high conservation priority ecosystems. PLoS ONE 2014, 9, e86592. [Google Scholar]
- White, J.D.; Bronner, G.N.; Midgley, J.J. Camera-trapping and seed-labelling reveals widespread granivory and scatter-hoarding of nuts by rodents in the Fynbos Biome. Afr. Zool. 2017, 52, 31–41. [Google Scholar] [CrossRef]
- Burton, A.C.; Neilson, E.; Moreira, D.; Ladle, A.; Steenweg, R.; Fisher, J.T.; Bayne, E.; Boutin, S. Wildlife camera trapping: A review and recommendations for linking surveys to ecological processes. J. Appl. Ecol. 2015, 52, 675–685. [Google Scholar] [CrossRef]
- Questad, E.J.; Antill, M.; Liu, N.; Stavros, E.N.; Townsend, P.A.; Bonfield, S.; Schimel, D. A camera-based method for collecting rapid vegetation data to support remote-sensing studies of shrubland biodiversity. Remote Sens. 2022, 14, 1933. [Google Scholar] [CrossRef]
- Cooper, W.S. An apparatus for photographic recording of quadrats. J. Ecol. 1924, 12, 317–321. [Google Scholar] [CrossRef]
- Booth, D.T.; Cox, S.E.; Louhaichi, M.; Johnson, D.E. Lightweight camera stand for close-to-earth remote sensing. Rangel. Ecol. Manag. 2004, 57, 675–679. [Google Scholar] [CrossRef]
- Claveran, R.A. Two modifications to the vegetation photographic charting method. J. Range Manag. 1966, 19, 371–373. [Google Scholar]
- Owens, M.; Gardiner, H.; Norton, B. A photographic technique for repeated mapping of rangeland plant populations in permanent plots. Rangel. Ecol. Manag. J. Range Manag. Arch. 1985, 38, 231–232. [Google Scholar] [CrossRef]
- Laliberte, A.S.; Winters, C.; Rango, A. UAS remote sensing missions for rangeland applications. Geocarto Int. 2011, 26, 141–156. [Google Scholar] [CrossRef]
- Louhaichi, M.; Borman, M.M.; Johnson, D.E. Spatially Located Platform and Aerial Photography for Documentation of Grazing Impacts on Wheat. Geocarto Int. 2001, 16, 65–70. [Google Scholar] [CrossRef]
- Tarhouni, M.; Ben Salem, F.; Tlili, A.; Ouled Belgacem, A.; Neffati, M.; Louhaichi, M. Measurement of the aboveground biomass of some rangeland species using a digital non-destructive technique. Bot. Lett. 2016, 163, 281–287. [Google Scholar] [CrossRef]
- Chianucci, F.; Lucibelli, A.; Dell’Abate, M.T. Estimation of ground canopy cover in agricultural crops using downward-looking photography. Biosyst. Eng. 2018, 169, 209–216. [Google Scholar] [CrossRef]
- Sankey, J.B.; Sankey, T.T.; Li, J.; Ravi, S.; Wang, G.; Caster, J.; Kasprak, A. Quantifying plant-soil-nutrient dynamics in rangelands: Fusion of UAV hyperspectral LiDAR, UAV multispectral-photogrammetry, and ground-based LiDAR-digital photography in a shrub-encroached desert grassland. Remote Sens. Environ. 2021, 253, 112223. [Google Scholar] [CrossRef]
- Terrance Booth, D.; Cox, S.E.; Fifield, C.; Phillips, M.; Williamson, N. Image analysis compared with other methods for measuring ground cover. Arid. Land Res. Manag. 2005, 19, 91–100. [Google Scholar] [CrossRef]
- Booth, D.T.; Tueller, P.T. Rangeland monitoring using remote sensing. Arid. Land Res. Manag. 2003, 17, 455–467. [Google Scholar] [CrossRef]
- Booth, D.T.; Cox, S.E. Image-based monitoring to measure ecological change in rangeland. Front. Ecol. Environ. 2008, 6, 185–190. [Google Scholar] [CrossRef]
- Bennett, L.T.; Judd, T.S.; Adams, M.A. Close-range vertical photography for measuring cover changes in perennial grasslands. Rangel. Ecol. Manag. J. Range Manag. Arch. 2000, 53, 634–641. [Google Scholar]
- Shafran-Nathan, R.; Svoray, T.; Perevolotsky, A. Continuous droughts’ effect on herbaceous vegetation cover and productivity in rangelands: Results from close-range photography and spatial analysis. Int. J. Remote Sens. 2013, 34, 6263–6281. [Google Scholar] [CrossRef]
- Tobler, M.W.; Hartley, A.Z.; Carrillo-Percastegui, S.E.; Powell, G.V.N. Spatiotemporal hierarchical modelling of species richness and occupancy using camera trap data. J. Appl. Ecol. 2015, 52, 413–421. [Google Scholar] [CrossRef]
- Iglhaut, J.; Cabo, C.; Stefano, P.; Piermattei, L.; O’Connor, J.; Rosette, J. Structure from motion photogrammetry in forestry: A review. Curr. For. Rep. 2019, 5, 155–168. [Google Scholar] [CrossRef]
- Wilhoit, J.; Kutz, L.; Fly, D.; South, D. PC-based multiple camera machine vision systems for pine seedling measurements. Appl. Eng. Agric. 1994, 10, 841–847. [Google Scholar] [CrossRef]
- Kaizu, Y.; Imou, K. A dual-spectral camera system for paddy rice seedling row detection. Comput. Electron. Agric. 2008, 63, 49–56. [Google Scholar] [CrossRef]
- Niwa, H.; Imai, Y.; Kamada, M. The effectiveness of a method that uses stabilized cameras and photogrammetry to survey the size and distribution of individual trees in a mangrove forest. J. For. Res. 2021, 26, 314–320. [Google Scholar] [CrossRef]
- Putro, A.W.; Nugroho, A.P.; Sutiarso, L.; Okayasu, T. Application of 3D reconstruction system based on close-range photogrammetry method for plant growth estimation. IOP Conf. Ser. Earth Environ. Sci. 2022, 1038, 012051. [Google Scholar] [CrossRef]
- Lu, H.; Tang, L.; Whitham, S.A.; Mei, Y. A robotic platform for corn seedling morphological traits characterization. Sensors 2017, 17, 2082. [Google Scholar] [CrossRef]
- PRISM Climate Group, Oregon State University PRISM Climate Data. 2016. Available online: https://prism.oregonstate.edu (accessed on 8 September 2022).
- Nijland, W.; Coops, N.; Coogan, S.; Bater, C.; Wulder, M.; Nielsen, S.; McDermid, G.; Stenhouse, G. Vegetation phenology can be captured with digital repeat photography and linked to variability of root nutrition in Hedysarum alpinum. Appl. Veg. Sci. 2013, 16, 317–324. [Google Scholar] [CrossRef]
- Hardin, P.J.; Jackson, M.W. An unmanned aerial vehicle for rangeland photography. Rangel. Ecol. Manag. 2005, 58, 439–442. [Google Scholar] [CrossRef]
- Robel, R.; Briggs, J.; Dayton, A.; Hulbert, L. Relationships between visual obstruction measurements and weight of grassland vegetation. J. Range Manag. 1970, 23, 295–297. [Google Scholar] [CrossRef]
- Fieberg, J. Estimating population abundance using sightability models: R SightabilityModel package. J. Stat. Softw. 2012, 51, 1–20. [Google Scholar] [CrossRef]
- Yamazaki, F.; Liu, W.; Takasaki, M. Characteristics of shadow and removal of its effects for remote sensing imagery. In Proceedings of the 2009 IEEE International Geoscience and Remote Sensing Symposium, Cape Town, South Africa, 12–17 July 2009; pp. IV-426–IV-429. [Google Scholar]
- Youtie, B.A.; Stafford, M.; Johnson, J.B. Herbivorous and parasitic insect guilds associated with Great Basin wildrye (Elymus cinereus) in southern Idaho. Great Basin Nat. 1987, 47, 644–651. [Google Scholar]
- Damgaard, C. Estimating mean plant cover from different types of cover data: A coherent tatistical framework. Ecosphere 2014, 5, 20. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).