Forest Biodiversity Monitoring Based on Remotely Sensed Spectral Diversity—A Review
Abstract
1. Introduction
1.1. Relevance of Biodiversity Monitoring
1.2. Forest Biodiversity Monitoring Based on Remote Sensing Data
1.3. The Objectives and Structure of This Review
- The introduction in Section 1 presents the relevance of forest biodiversity monitoring and highlights the possibilities of concepts from remote sensing.
- The literature selection process is explained in Section 2 by giving an overview on the literature databases and keywords used for identifying relevant articles for this review.
- The results section (Section 3) is structured into a general introduction on the number of publications by year and main publishers and authors, followed by a spatial analysis of the author affiliations and study areas. In addition, the sensors used and temporal periods of remote sensing data are covered. The results chapter ends with a thematic analysis by classifying the studies into the three concepts of spectral diversity and by providing information on spectral diversity metrics and biodiversity scales.
- The concepts of spectral diversity, contrary findings and challenges are discussed in Section 4.
- A conclusive statement on forest biodiversity monitoring from remotely sensed spectral diversity based on airborne and spaceborne sensors is given in Section 5.
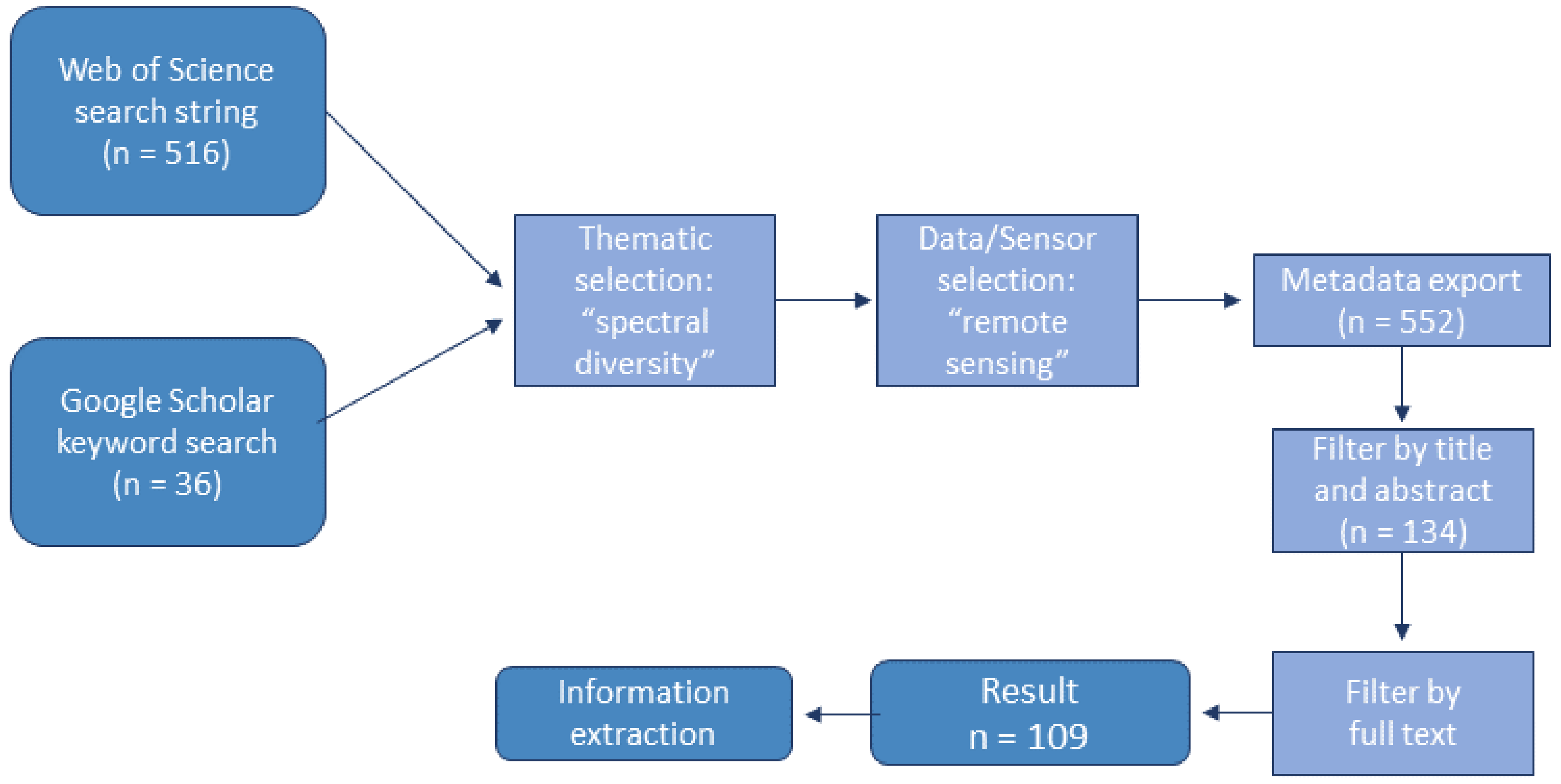
2. Materials and Methods
3. Results of the Review
- In a first step, general information about the number of publications based on reclassified Web of Science categories, and main publishers, journals and authors are presented (Section 3.1).
- The following chapter (Section 3.2) on spatial analysis, displays on the one hand the countries of the first authors affiliations, and on the other hand, the spatial distribution of study areas grouped by country as maps.
- To investigate sensors used in the studies and compare different spatial scales and spatial resolutions, the third chapter serves as an overview on different sensor characteristics (Section 3.3).
- The varying temporal periods of remote sensing data grouped by sensors, and the proportions of mono-temporal, multi-temporal and time-series approaches in field and remote sensing data are the focus of Section 3.4.
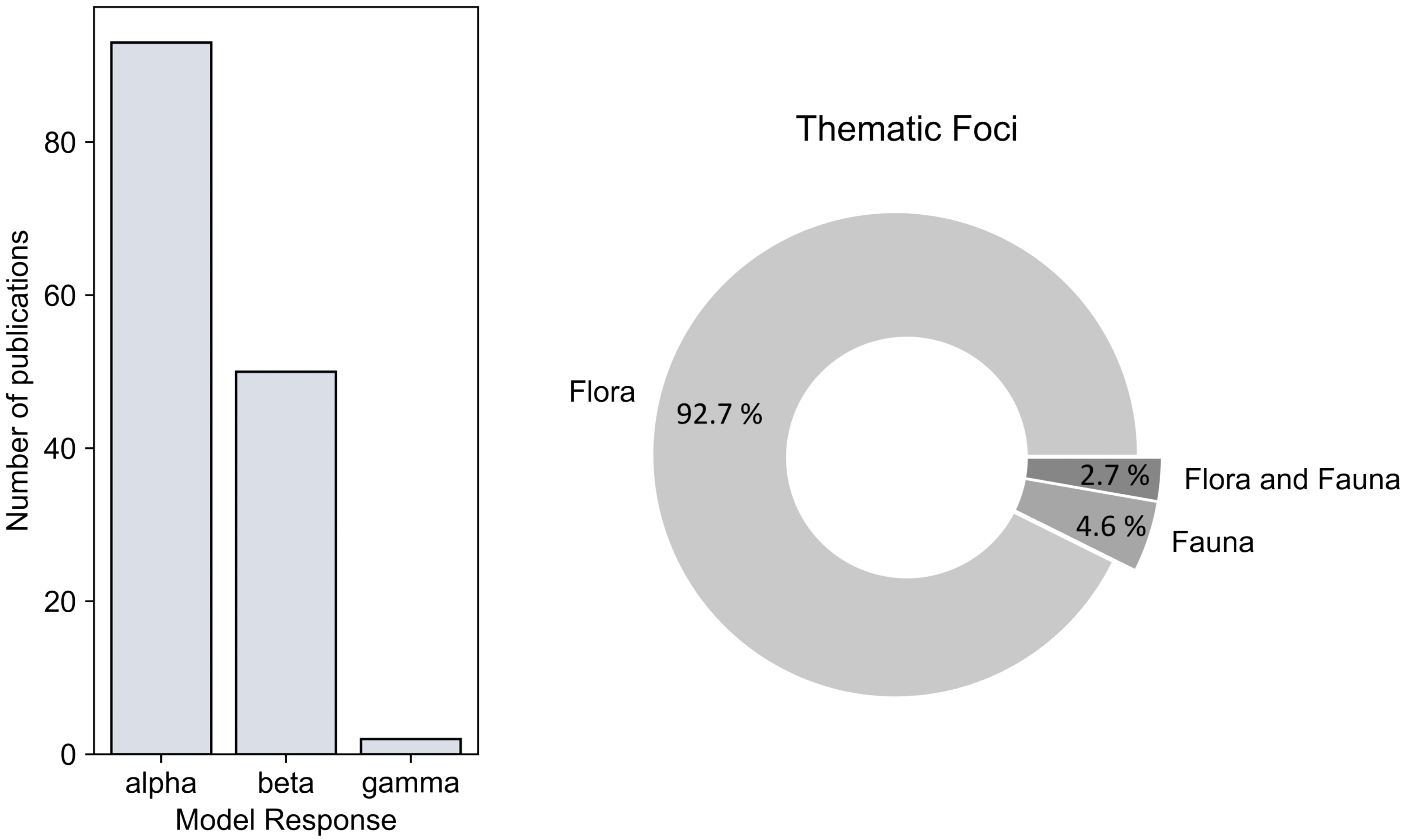
- As a final result, the thematic analysis (Section 3.5) covers the temporal distribution of spectral diversity concepts and presents the most frequently used spectral indices. In addition, the share of analyzed biodiversity scales (, , and diversity), and focus on flora/fauna is presented.
3.1. General Information on the Research Interest over Time
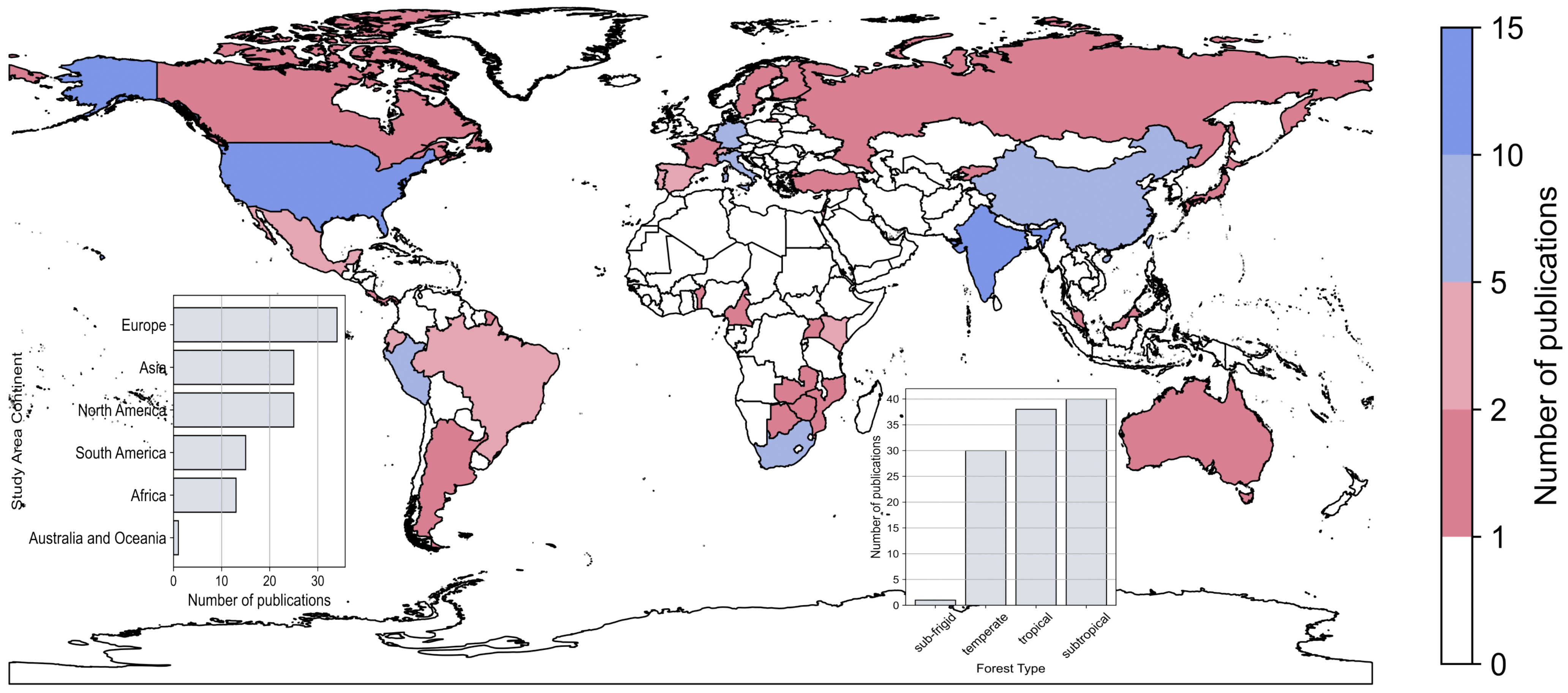
3.2. Spatial Analysis on Affiliations and Study Areas
3.3. Analysis on Remote Sensing Sensors
3.4. Temporal Analysis on Remote Sensing and Field Data
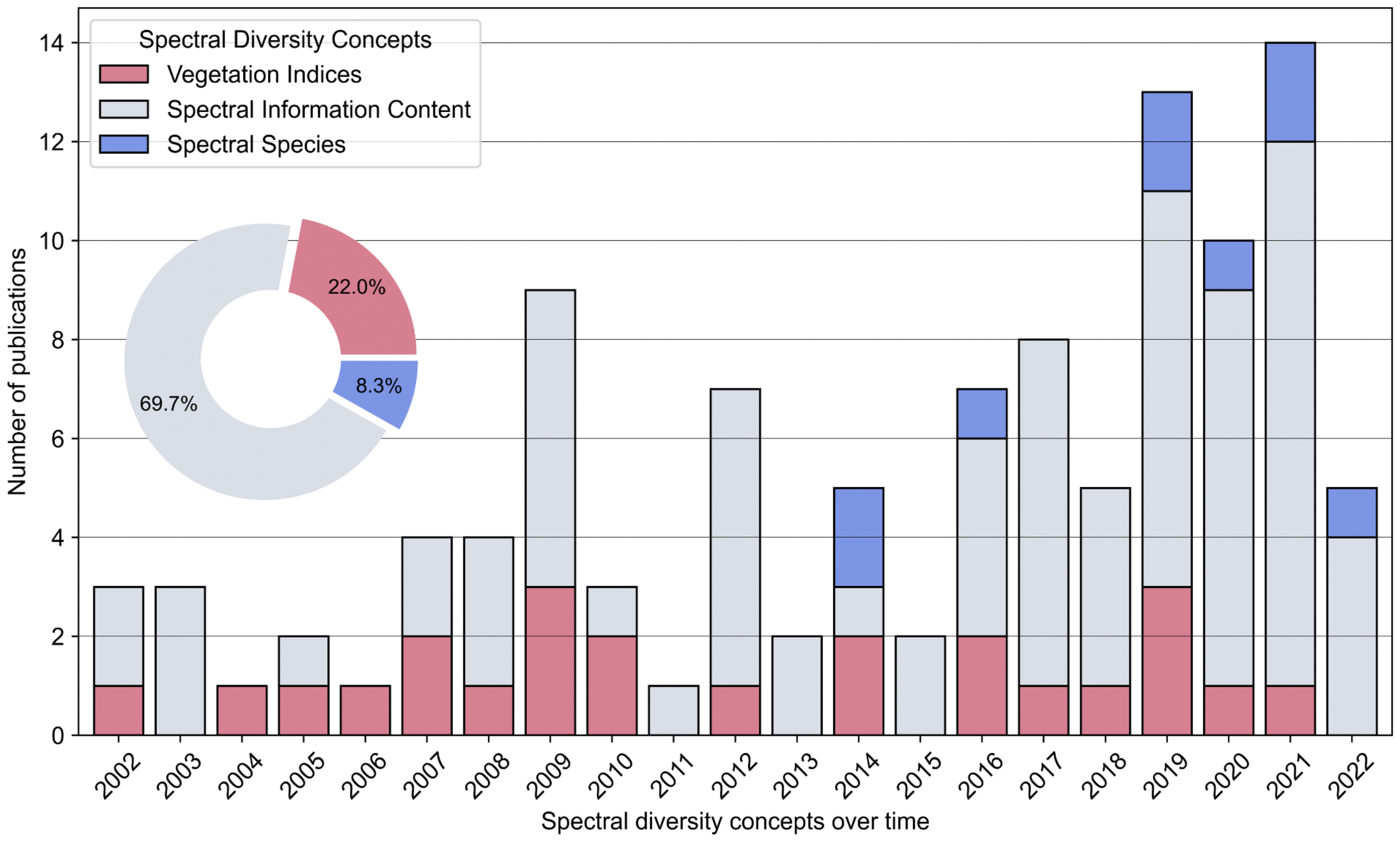
3.5. Review of Thematic Foci
3.5.1. Comparison of the Different Spectral Diversity Concepts: Vegetation Indices, Spectral Information Content, and Spectral Species
3.5.2. Model Responses and Environmental Foci
3.5.3. Spectral Indices for the Analysis of Optical Diversity
4. Discussion
4.1. Overall Discussion on the Validity of the Spectral Variation Hypothesis
4.2. Benefits and Limitations of the Three Spectral Diversity Concepts
4.3. Future Research Directions
5. Conclusions
- In recent years there was an increasing number of studies on forest biodiversity monitoring from remotely sensed spectral diversity. Since 2016, more than 56% of all studies were published which underlines the increasing relevance of forest-related research in the context of climate change.
- Several research hotspots were identified with most studies investigating forest biodiversity in the United States and India. Grouped by continent, about one third is focusing on European forests, followed by Asia and North America (each continent holds about one fourth). Overall, there is a strong focus on temperate, sub-tropical and tropical forests, while other forest types (e.g., sub-frigid) are only investigated in a single study. Strong discrepancies between the country of the first author affiliation and the country or continent under study were identified: at continental scale, the strongest discrepancy is found for South America which holds a share of about 6% of all first authors and about 14% of all study sites. At country level, about 19% of the affiliations of first authors are in Italy, while only about 8% of all studies are investigating forest biodiversity in Italy.
- Research on forest biodiversity based on remotely sensed spectral diversity derived from vegetation indices, spectral information content and spectral species has a strong focus on optical sensors. About 70% of all reviewed articles are integrating multispectral imagery, and about 10% are based on hyperspectral data. Most commonly used multispectral sensors are Landsat 7 (24 applications), Sentinel-2 (15 applications), Landsat 8 (13 publications), MODIS (13 publications), and Landsat 5 (11 publications).
- Most studies are integrating data from field work as estimate of in situ biodiversity (94 articles). Remotely sensed spectral diversity is dominantly assessed using spaceborne sensors (85 applications), while data from airborne sensors are applied in 33 reviewed articles. Furthermore, there is a tendency towards the integration of very high (≤5 m, 46 applications) on the one hand, and medium spatial resolution imagery (30 m, 58 publications) on the other hand.
- The analysis of temporal scales of remote sensing and field data present a strong focus on mono-temporal resolution. About 66% of all remote sensing data are from one time step, while multi-temporal (about 12%) and time series approaches (about 22%) hold much lower shares. Overall, all time series approaches are either based on multispectral imagery (about 13%) or data from multiple sensors (about 9%). Mono-temporal data from field work amount to 84%, 15% of all reviewed articles did not use field data, and only a minor proportion of about 1% collected bi-temporal in situ measurements of forest biodiversity.
- The comparative statistics of spectral diversity concepts show that most reviewed articles are based on spectral information content (about 70%), followed by vegetation indices (about 22%), and spectral species (about 8%). It is important to note that the spectral species concept was introduced in 2014, whereas articles based on vegetation indices or spectral information content were published since 2002. The promising findings on forest biodiversity using spectral species are highlighted by the adaption of the original concept using airborne hyperspectral data towards Sentinel-2 and MODIS data.
- Forest biodiversity was assessed at multiple scales: , , and diversity. Most of the articles (n = 93) analyzed diversity, followed by 50 articles on diversity, and a combined analysis of and diversity in 34 articles. An explicit estimate of diversity was only calculated in two studies. The analysis on floristic characteristics as in situ biodiversity measure amounts to more than 92%, while analysis solely on fauna (about 5%), and combined analysis on flora and fauna (less than 3%) hold much lower shares.
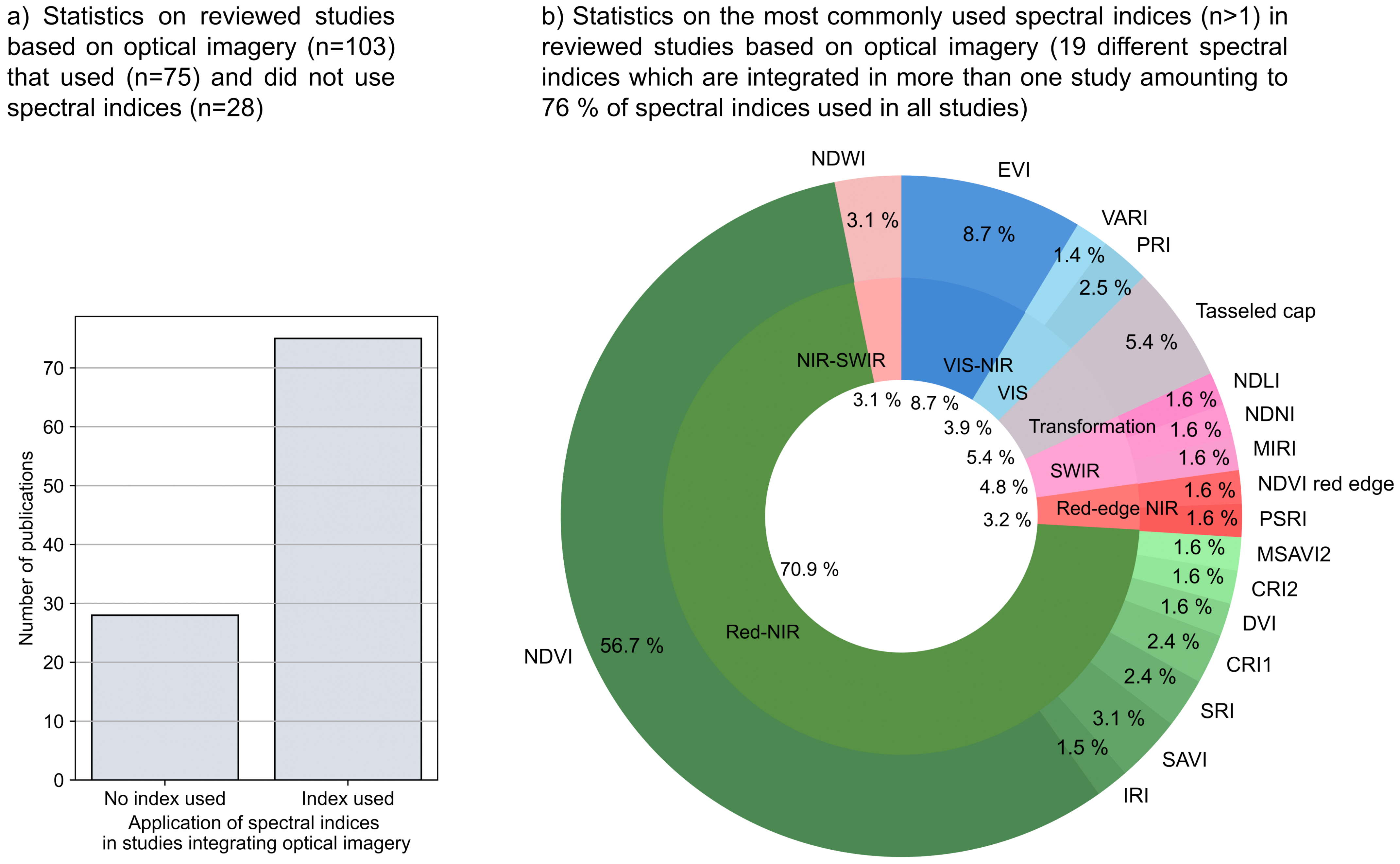
- Many studies integrating optical imagery (n = 103) calculated spectral indices (n = 75). About 71% of those studies calculated spectral indices based on red to near-infrared bands. The most often used spectral index is the NDVI (about 57%), followed by the EVI (about 9%).
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| diversity | Alpha diversity (local community diversity) |
| AIRSAR | Airborne SAR |
| ALS | Airborne Laser Scanning |
| AVHRR | Advanced Very High Resolution Radiometer |
| AVIRIS | Airborne Visible/Infrared Imaging Spectrometer |
| diversity | Beta diversity (turnover in species composition) |
| CAO | Carnegie Airborne Observatory |
| CEOS | Committee on Earth Observation Systems |
| CRI1 | Carotenoid Reflectance Index 1 |
| CRI2 | Carotenoid Reflectance Index 2 |
| DT | Document Type |
| DVI | Difference Vegetation Index |
| EBV | Essential Biophysical Variables |
| ESA | European Space Agency |
| EVI | Enhanced Vegetation Index |
| G-LiHT | Goddard’s LiDAR, Hyperspectral and Thermal Imager |
| diversity | Gamma diversity (landscape diversity) |
| GEDI | Global Ecosystem Dynamics Investigation |
| GEO BON | Group on Earth Observations Biodiversity Observation Network |
| HVH | Height Variation Hypothesis |
| IGBP | International Geosphere Biosphere Programme |
| IRI | Infrared Index |
| LA | Language |
| LiDAR | Light Detection And Ranging |
| MIRI | Mid-Infrared Index |
| MODIS | Moderate-resolution Imaging Spectroradiometer |
| MSAVI2 | Modified Soil Adjusted Vegetation Index 2 |
| NASA | National Aeronautics and Space Administration |
| NDLI | Normalized Difference Lignin Index |
| NDNI | Normalized Difference Nitrogen Index |
| NDVI | Normalized Difference Vegetation Index |
| NDWI | Normalized Difference Water Index |
| NIR | Near-Infrared |
| NMDS | Nonmetric Mulit-Dimensional Scaling |
| PCA | Principle Component Analysis |
| PRI | Photochemical Reflectance Index |
| PSRI | Plant Senescence Reflectance Index |
| SAR | Synthetic Aperture Radar |
| SAVI | Soil Adjusted Vegetation Index |
| SRI | Simple Ratio Index |
| SRTM | Shuttle Radar Topography Mission |
| SWIR | Short wave infrared |
| SVH | Spectral Variation Hypothesis |
| TS | Topic |
| USGS | United States Geological Survey |
| WDRVI | Wide Dynamic Range Vegetation Index |
| VV | Vertical transmit, Vertical receive |
| VARI | Visible Atmospherically Resistant Index |
References
- Wilson, E.O. Biodiversity; National Academies Press: Washington, DC, USA, 1988. [Google Scholar]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Maclaurin, J.; Sterelny, K. What is biodiversity? In What Is Biodiversity? University of Chicago Press: Chicago, IL, USA, 2008. [Google Scholar]
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global Biodiversity Conservation: The Critical Role of Hotspots. In Biodiversity Hotspots; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–22. [Google Scholar] [CrossRef]
- Reid, W.V. Biodiversity hotspots. Trends Ecol. Evol. 1998, 13, 275–280. [Google Scholar] [CrossRef]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef] [PubMed]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, G.; Ehrlich, P.R.; Dirzo, R. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. USA 2017, 114, E6089–E6096. [Google Scholar] [CrossRef] [PubMed]
- Dirzo, R.; Young, H.S.; Galetti, M.; Ceballos, G.; Isaac, N.J.; Collen, B. Defaunation in the Anthropocene. Science 2014, 345, 401–406. [Google Scholar] [CrossRef]
- Almond, R.E.; Grooten, M.; Peterson, T. Living Planet Report 2020-Bending the Curve of Biodiversity Loss; World Wildlife Fund: Washington, DC, USA, 2020. [Google Scholar]
- Betts, M.G.; Wolf, C.; Ripple, W.J.; Phalan, B.; Millers, K.A.; Duarte, A.; Butchart, S.H.M.; Levi, T. Global forest loss disproportionately erodes biodiversity in intact landscapes. Nature 2017, 547, 441–444. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2022-1. 2022. Available online: https://www.iucnredlist.org (accessed on 9 May 2022).
- Jones, J.P.; Collen, B.; Atkinson, G.; Baxter, P.W.; Bubb, P.; Illian, J.B.; Katzner, T.E.; Keane, A.; Loh, J.; Mcdonald-Madden, E.; et al. The why, what, and how of global biodiversity indicators beyond the 2010 target. Conserv. Biol. 2011, 25, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Keenan, R.J.; Reams, G.A.; Achard, F.; de Freitas, J.V.; Grainger, A.; Lindquist, E. Dynamics of global forest area: Results from the FAO Global Forest Resources Assessment 2015. For. Ecol. Manag. 2015, 352, 9–20. [Google Scholar] [CrossRef]
- Butchart, S.H.; Walpole, M.; Collen, B.; Van Strien, A.; Scharlemann, J.P.; Almond, R.E.; Baillie, J.E.; Bomhard, B.; Brown, C.; Bruno, J.; et al. Global biodiversity: Indicators of recent declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef]
- FAO. Agriculture Organization: Global Forest Resources Assessment; FAO: Rome, Italy, 2010. [Google Scholar]
- Sayer, J.; Sheil, D.; Galloway, G.; Riggs, R.A.; Mewett, G.; MacDicken, K.G.; Arts, B.; Boedhihartono, A.K.; Langston, J.; Edwards, D.P. SDG 15 Life on land–the central role of forests in sustainable development. In Sustainable Development Goals: Their Impacts on Forest and People; Cambridge University Press: Cambridge, MA, USA, 2019; pp. 482–509. [Google Scholar]
- Brockerhoff, E.G.; Barbaro, L.; Castagneyrol, B.; Forrester, D.I.; Gardiner, B.; González-Olabarria, J.R.; Lyver, P.O.; Meurisse, N.; Oxbrough, A.; Taki, H.; et al. Forest biodiversity, ecosystem functioning and the provision of ecosystem services. Biodivers. Conserv. 2017, 26, 3005–3035. [Google Scholar] [CrossRef]
- Potapov, P.; Hansen, M.C.; Laestadius, L.; Turubanova, S.; Yaroshenko, A.; Thies, C.; Smith, W.; Zhuravleva, I.; Komarova, A.; Minnemeyer, S.; et al. The last frontiers of wilderness: Tracking loss of intact forest landscapes from 2000 to 2013. Sci. Adv. 2017, 3, e1600821. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 1960, 30, 279–338. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and measurement of species diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Colwell, R.K. Biodiversity: Concepts, patterns, and measurement. Princet. Guide Ecol. 2009, 663, 257–263. [Google Scholar]
- Shannon, C.E.; Weaver, W. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 623–656. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Jaccard, P. The distribution of the flora in the alpine zone. 1. New Phytol. 1912, 11, 37–50. [Google Scholar] [CrossRef]
- Sørensen, T.J. A Method of Establishing Groups of Equal Amplitude in Plant Sociology Based on Similarity of Species Content and Its Application to Analyses of the Vegetation on Danish Commons; I kommission hos E. Munksgaard: Copenhagen, Denmark, 1948. [Google Scholar]
- Clarke, K.R.; Somerfield, P.J.; Chapman, M.G. On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray–Curtis coefficient for denuded assemblages. J. Exp. Mar. Biol. Ecol. 2006, 330, 55–80. [Google Scholar] [CrossRef]
- Tuomisto, H. A diversity of beta diversities: Straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography 2010, 33, 2–22. [Google Scholar] [CrossRef]
- Tuomisto, H. A diversity of beta diversities: Straightening up a concept gone awry. Part 2. Quantifying beta diversity and related phenomena. Ecography 2010, 33, 23–45. [Google Scholar] [CrossRef]
- Jost, L. Independence of alpha and beta diversities. Ecology 2010, 91, 1969–1974. [Google Scholar] [CrossRef] [PubMed]
- Veech, J.A.; Crist, T.O. Toward a unified view of diversity partitioning. Ecology 2010, 91, 1988–1992. [Google Scholar] [CrossRef] [PubMed]
- Veech, J.A.; Crist, T.O. Diversity partitioning without statistical independence of alpha and beta. Ecology 2010, 91, 1964–1969. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Palmer, M.W. How should one count species? Nat. Area J. 1995, 15, 124–135. [Google Scholar]
- Palmer, M.W.; Earls, P.G.; Hoagland, B.W.; White, P.S.; Wohlgemuth, T. Quantitative tools for perfecting species lists. Environ. Off. J. Int. Environ. Soc. 2002, 13, 121–137. [Google Scholar] [CrossRef]
- Nagendra, H. Using remote sensing to assess biodiversity. Int. J. Remote Sens. 2001, 22, 2377–2400. [Google Scholar] [CrossRef]
- Gillespie, T.W.; Foody, G.M.; Rocchini, D.; Giorgi, A.P.; Saatchi, S. Measuring and modelling biodiversity from space. Prog. Phys. Geogr. 2008, 32, 203–221. [Google Scholar] [CrossRef]
- Rouse, J., Jr.; Haas, R.; Schell, J.; Deering, D. Paper a 20. In Proceedings of the Third Earth Resources Technology Satellite-1 Symposium: The Proceedings of a Symposium, Washington, DC, USA, 10–14 December 1973; Volume 351, p. 309. [Google Scholar]
- Kuenzer, C.; Ottinger, M.; Wegmann, M.; Guo, H.; Wang, C.; Zhang, J.; Dech, S.; Wikelski, M. Earth observation satellite sensors for biodiversity monitoring: Potentials and bottlenecks. Int. J. Remote Sens. 2014, 35, 6599–6647. [Google Scholar] [CrossRef]
- Zeng, Y.; Hao, D.; Huete, A.; Dechant, B.; Berry, J.; Chen, J.M.; Joiner, J.; Frankenberg, C.; Bond-Lamberty, B.; Ryu, Y.; et al. Optical vegetation indices for monitoring terrestrial ecosystems globally. Nat. Rev. Earth Environ. 2022, 3, 477–493. [Google Scholar] [CrossRef]
- Claverie, M.; Ju, J.; Masek, J.G.; Dungan, J.L.; Vermote, E.F.; Roger, J.C.; Skakun, S.V.; Justice, C. The Harmonized Landsat and Sentinel-2 surface reflectance data set. Remote Sens. Environ. 2018, 219, 145–161. [Google Scholar] [CrossRef]
- Kuenzer, C.; Dech, S.; Wagner, W. Remote sensing time series. Remote Sens. Digit. Image Process. 2015, 22, 225–245. [Google Scholar]
- Asner, G.P.; Knapp, D.E.; Boardman, J.; Green, R.O.; Kennedy-Bowdoin, T.; Eastwood, M.; Martin, R.E.; Anderson, C.; Field, C.B. Carnegie Airborne Observatory-2: Increasing science data dimensionality via high-fidelity multi-sensor fusion. Remote Sens. Environ. 2012, 124, 454–465. [Google Scholar]
- Gillespie, T.W.; Saatchi, S.; Pau, S.; Bohlman, S.; Giorgi, A.P.; Lewis, S. Towards quantifying tropical tree species richness in tropical forests. Int. J. Remote Sens. 2009, 30, 1629–1634. [Google Scholar] [CrossRef]
- Kamoske, A.G.; Dahlin, K.M.; Read, Q.D.; Record, S.; Stark, S.C.; Serbin, S.P.; Zarnetske, P.L.; Dornelas, M. Towards mapping biodiversity from above: Can fusing lidar and hyperspectral remote sensing predict taxonomic, functional, and phylogenetic tree diversity in temperate forests? Glob. Ecol. Biogeogr. 2022, 31, 1440–1460. [Google Scholar] [CrossRef]
- Ribeiro, I.; Proença, V.; Serra, P.; Palma, J.; Domingo-Marimon, C.; Pons, X.; Domingos, T. Remotely sensed indicators and open-access biodiversity data to assess bird diversity patterns in Mediterranean rural landscapes. Sci. Rep. 2019, 9, 6826. [Google Scholar] [CrossRef]
- Guerra-Hernández, J.; Pascual, A. Using GEDI lidar data and airborne laser scanning to assess height growth dynamics in fast-growing species: A showcase in Spain. For. Ecosyst. 2021, 8, 1–17. [Google Scholar] [CrossRef]
- Kacic, P.; Hirner, A.; Da Ponte, E. Fusing Sentinel-1 and-2 to Model GEDI-Derived Vegetation Structure Characteristics in GEE for the Paraguayan Chaco. Remote Sens. 2021, 13, 5105. [Google Scholar] [CrossRef]
- Lang, N.; Kalischek, N.; Armston, J.; Schindler, K.; Dubayah, R.; Wegner, J.D. Global canopy height regression and uncertainty estimation from GEDI LIDAR waveforms with deep ensembles. Remote Sens. Environ. 2022, 268, 112760. [Google Scholar] [CrossRef]
- Potapov, P.; Li, X.; Hernandez-Serna, A.; Tyukavina, A.; Hansen, M.C.; Kommareddy, A.; Pickens, A.; Turubanova, S.; Tang, H.; Silva, C.E.; et al. Mapping global forest canopy height through integration of GEDI and Landsat data. Remote Sens. Environ. 2021, 253, 112165. [Google Scholar] [CrossRef]
- Wang, R.; Gamon, J.A. Remote sensing of terrestrial plant biodiversity. Remote Sens. Environ. 2019, 231, 111218. [Google Scholar] [CrossRef]
- Lausch, A.; Bannehr, L.; Beckmann, M.; Boehm, C.; Feilhauer, H.; Hacker, J.; Heurich, M.; Jung, A.; Klenke, R.; Neumann, C.; et al. Linking Earth Observation and taxonomic, structural and functional biodiversity: Local to ecosystem perspectives. Ecol. Indic. 2016, 70, 317–339. [Google Scholar] [CrossRef]
- Carlson, K.M.; Asner, G.P.; Hughes, R.F.; Ostertag, R.; Martin, R.E. Hyperspectral Remote Sensing of Canopy Biodiversity in Hawaiian Lowland Rainforests. Ecosystems 2007, 10, 536–549. [Google Scholar] [CrossRef]
- Farwell, L.S.; Gudex-Cross, D.; Anise, I.E.; Bosch, M.J.; Olah, A.M.; Radeloff, V.C.; Razenkova, E.; Rogova, N.; Silveira, E.M.; Smith, M.M.; et al. Satellite image texture captures vegetation heterogeneity and explains patterns of bird richness. Remote Sens. Environ. 2021, 253, 112175. [Google Scholar] [CrossRef]
- Féret, J.B.; Asner, G.P. Mapping tropical forest canopy diversity using high-fidelity imaging spectroscopy. Ecol. Appl. 2014, 24, 1289–1296. [Google Scholar] [CrossRef]
- Laliberté, E.; Schweiger, A.K.; Legendre, P. Partitioning plant spectral diversity into alpha and beta components. Ecol. Lett. 2019, 23, 370–380. [Google Scholar] [CrossRef]
- Rocchini, D.; Balkenhol, N.; Carter, G.A.; Foody, G.M.; Gillespie, T.W.; He, K.S.; Kark, S.; Levin, N.; Lucas, K.; Luoto, M.; et al. Remotely sensed spectral heterogeneity as a proxy of species diversity: Recent advances and open challenges. Ecol. Inform. 2010, 5, 318–329. [Google Scholar] [CrossRef]
- Tuomisto, H.; Ruokolainen, K.; Aguilar, M.; Sarmiento, A. Floristic patterns along a 43-km long transect in an Amazonian rain forest. J. Ecol. 2003, 91, 743–756. [Google Scholar] [CrossRef]
- Tuomisto, H.; Poulsen, A.D.; Ruokolainen, K.; Moran, R.C.; Quintana, C.; Celi, J.; Cañas, G. Linking floristic patterns with soil heterogeneity and satellite imagery in ecuadorian amazonia. Ecol. Appl. 2003, 13, 352–371. [Google Scholar] [CrossRef]
- Schmidtlein, S.; Fassnacht, F.E. The spectral variability hypothesis does not hold across landscapes. Remote Sens. Environ. 2017, 192, 114–125. [Google Scholar] [CrossRef]
- Fassnacht, F.E.; Müllerová, J.; Conti, L.; Malavasi, M.; Schmidtlein, S. About the link between biodiversity and spectral variation. Appl. Veg. Sci. 2022, 25, e12643. [Google Scholar] [CrossRef]
- Rocchini, D.; Bacaro, G.; Chirici, G.; Re, D.D.; Feilhauer, H.; Foody, G.M.; Galluzzi, M.; Garzon-Lopez, C.X.; Gillespie, T.W.; He, K.S.; et al. Remotely sensed spatial heterogeneity as an exploratory tool for taxonomic and functional diversity study. Ecol. Indic. 2018, 85, 983–990. [Google Scholar] [CrossRef]
- Stoms, D.M.; Estes, J.E. A remote sensing research agenda for mapping and monitoring biodiversity. Int. J. Remote Sens. 1993, 14, 1839–1860. [Google Scholar] [CrossRef]
- Jennings, M.D. Gap analysis: Concepts, methods, and recent results. Landsc. Ecol. 2000, 15, 5–20. [Google Scholar] [CrossRef]
- Kerr, J.T.; Southwood, T.R.E.; Cihlar, J. Remotely sensed habitat diversity predicts butterfly species richness and community similarity in Canada. Proc. Natl. Acad. Sci. USA 2001, 98, 11365–11370. [Google Scholar] [CrossRef]
- Roberts, D.; Gardner, M.; Church, R.; Ustin, S.; Scheer, G.; Green, R. Mapping Chaparral in the Santa Monica Mountains Using Multiple Endmember Spectral Mixture Models. Remote Sens. Environ. 1998, 65, 267–279. [Google Scholar] [CrossRef]
- Ustin, S.L.; Roberts, D.A.; Gamon, J.A.; Asner, G.P.; Green, R.O. Using imaging spectroscopy to study ecosystem processes and properties. BioScience 2004, 54, 523–534. [Google Scholar] [CrossRef]
- Asner, G.P.; Hughes, R.F.; Vitousek, P.M.; Knapp, D.E.; Kennedy-Bowdoin, T.; Boardman, J.; Martin, R.E.; Eastwood, M.; Green, R.O. Invasive plants transform the three-dimensional structure of rain forests. Proc. Natl. Acad. Sci. USA 2008, 105, 4519–4523. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Gamon, J.A.; Hobbie, S.E.; Madritch, M.D.; Meireles, J.E.; Schweiger, A.K.; Townsend, P.A. Harnessing plant spectra to integrate the biodiversity sciences across biological and spatial scales. Am. J. Bot. 2017, 104, 966–969. [Google Scholar] [CrossRef]
- Ustin, S.L.; Gitelson, A.; Jacquemoud, S.; Schaepman, M.; Asner, G.P.; Gamon, J.A.; Zarco-Tejada, P. Retrieval of foliar information about plant pigment systems from high resolution spectroscopy. Remote Sens. Environ. 2009, 113, S67–S77. [Google Scholar] [CrossRef]
- Ustin, S.L.; Gamon, J.A. Remote sensing of plant functional types. New Phytol. 2010, 186, 795–816. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, T.W. Predicting woody-plant species richness in tropical dry forests: A case study from south florida, USA. Ecol. Appl. 2005, 15, 27–37. [Google Scholar] [CrossRef]
- Dahlin, K.M. Spectral diversity area relationships for assessing biodiversity in a wildland–agriculture matrix. Ecol. Appl. 2016, 26, 2758–2768. [Google Scholar] [CrossRef]
- Hakkenberg, C.R.; Zhu, K.; Peet, R.K.; Song, C. Mapping multi-scale vascular plant richness in a forest landscape with integrated LiDAR and hyperspectral remote-sensing. Ecology 2018, 99, 474–487. [Google Scholar] [CrossRef]
- Schäfer, E.; Heiskanen, J.; Heikinheimo, V.; Pellikka, P. Mapping tree species diversity of a tropical montane forest by unsupervised clustering of airborne imaging spectroscopy data. Ecol. Indic. 2016, 64, 49–58. [Google Scholar] [CrossRef]
- Levin, N.; Shmida, A.; Levanoni, O.; Tamari, H.; Kark, S. Predicting mountain plant richness and rarity from space using satellite-derived vegetation indices. Divers. Distrib. 2007, 13, 692–703. [Google Scholar] [CrossRef]
- Oindo, B.O.; Skidmore, A.K. Interannual variability of NDVI and species richness in Kenya. Int. J. Remote Sens. 2002, 23, 285–298. [Google Scholar] [CrossRef]
- Rocchini, D.; Chiarucci, A.; Loiselle, S.A. Testing the spectral variation hypothesis by using satellite multispectral images. Acta Oecol. 2004, 26, 117–120. [Google Scholar] [CrossRef]
- Torresani, M.; Rocchini, D.; Sonnenschein, R.; Zebisch, M.; Hauffe, H.C.; Heym, M.; Pretzsch, H.; Tonon, G. Height variation hypothesis: A new approach for estimating forest species diversity with CHM LiDAR data. Ecol. Indic. 2020, 117, 106520. [Google Scholar] [CrossRef]
- Foody, G.M.; Cutler, M.E.J. Tree biodiversity in protected and logged Bornean tropical rain forests and its measurement by satellite remote sensing. J. Biogeogr. 2003, 30, 1053–1066. [Google Scholar] [CrossRef]
- Rocchini, D.; Santos, M.J.; Ustin, S.L.; Féret, J.B.; Asner, G.P.; Beierkuhnlein, C.; Dalponte, M.; Feilhauer, H.; Foody, G.M.; Geller, G.N.; et al. The spectral species concept in living color. J. Geophys. Res. Biogeosci. 2022, 127, e2022JG007026. [Google Scholar] [CrossRef] [PubMed]
- Da Re, D.; De Clercq, E.; Tordoni, E.; Madder, M.; Rousseau, R.; Vanwambeke, S. Looking for Ticks from Space: Using Remotely Sensed Spectral Diversity to Assess Amblyomma and Hyalomma Tick Abundance. Remote Sens. 2019, 11, 770. [Google Scholar] [CrossRef]
- Rocchini, D.; Dadalt, L.; Delucchi, L.; Neteler, M.; Palmer, M. Disentangling the role of remotely sensed spectral heterogeneity as a proxy for North American plant species richness. Community Ecol. 2014, 15, 37–43. [Google Scholar] [CrossRef]
- Rocchini, D.; Marcantonio, M.; Ricotta, C. Measuring Rao’s Q diversity index from remote sensing: An open source solution. Ecol. Indic. 2017, 72, 234–238. [Google Scholar] [CrossRef]
- Rocchini, D.; Salvatori, N.; Beierkuhnlein, C.; Chiarucci, A.; de Boissieu, F.; Förster, M.; Garzon-Lopez, C.X.; Gillespie, T.W.; Hauffe, H.C.; He, K.S.; et al. From local spectral species to global spectral communities: A benchmark for ecosystem diversity estimate by remote sensing. Ecol. Inform. 2021, 61, 101195. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, X.; Hernandez-Clemente, R.; Lu, W.; Manzanedo, R.D. Global Forest Types Based on Climatic and Vegetation Data. Sustainability 2022, 14, 634. [Google Scholar] [CrossRef]
- Zhirin, V.M.; Knyazeva, S.V.; Eydlina, S.P. Long-term dynamics of vegetation indices in dark coniferous forest after Siberian moth disturbance. Contemp. Probl. Ecol. 2016, 9, 834–843. [Google Scholar] [CrossRef]
- Sinha, S.; Jeganathan, C.; Sharma, L.K.; Nathawat, M.S. A review of radar remote sensing for biomass estimation. Int. J. Environ. Sci. Technol. 2015, 12, 1779–1792. [Google Scholar] [CrossRef]
- Toth, C.; Jóźków, G. Remote sensing platforms and sensors: A survey. ISPRS J. Photogramm. Remote Sens. 2016, 115, 22–36. [Google Scholar] [CrossRef]
- Turner, W.; Spector, S.; Gardiner, N.; Fladeland, M.; Sterling, E.; Steininger, M. Remote sensing for biodiversity science and conservation. Trends Ecol. Evol. 2003, 18, 306–314. [Google Scholar] [CrossRef]
- Scaramuzza, P.; Barsi, J. Landsat 7 scan line corrector-off gap-filled product development. In Proceedings of the Pecora 16 “Global Priorities in Land Remote Sensing”, Sioux Falls, SD, USA, 23–27 October 2005; Volume 16, pp. 23–27. [Google Scholar]
- Wulder, M.A.; Ortlepp, S.M.; White, J.C.; Maxwell, S. Evaluation of Landsat-7 SLC-off image products for forest change detection. Can. J. Remote Sens. 2008, 34, 93–99. [Google Scholar] [CrossRef]
- Chi, Y.; Sun, J.; Fu, Z.; Xie, Z. Spatial pattern of plant diversity in a group of uninhabited islands from the perspectives of island and site scales. Sci. Total Environ. 2019, 664, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Wulder, M.A.; Masek, J.G.; Cohen, W.B.; Loveland, T.R.; Woodcock, C.E. Opening the archive: How free data has enabled the science and monitoring promise of Landsat. Remote Sens. Environ. 2012, 122, 2–10. [Google Scholar] [CrossRef]
- Berger, M.; Moreno, J.; Johannessen, J.A.; Levelt, P.F.; Hanssen, R.F. ESA’s sentinel missions in support of Earth system science. Remote Sens. Environ. 2012, 120, 84–90. [Google Scholar] [CrossRef]
- Malenovskỳ, Z.; Rott, H.; Cihlar, J.; Schaepman, M.E.; García-Santos, G.; Fernandes, R.; Berger, M. Sentinels for science: Potential of Sentinel-1,-2, and-3 missions for scientific observations of ocean, cryosphere, and land. Remote Sens. Environ. 2012, 120, 91–101. [Google Scholar] [CrossRef]
- Oindo, B.O. Patterns of herbivore species richness in Kenya and current ecoclimatic stability. Biodivers. Conserv. 2002, 11, 1205–1221. [Google Scholar] [CrossRef]
- He, K.S.; Zhang, J.; Zhang, Q. Linking variability in species composition and MODIS NDVI based on beta diversity measurements. Acta Oecol. 2009, 35, 14–21. [Google Scholar] [CrossRef]
- Rocchini, D.; He, K.S.; Zhang, J. Is spectral distance a proxy of beta diversity at different taxonomic ranks? A test using quantile regression. Ecol. Inform. 2009, 4, 254–259. [Google Scholar] [CrossRef]
- Viña, A.; Tuanmu, M.N.; Xu, W.; Li, Y.; Qi, J.; Ouyang, Z.; Liu, J. Relationship between floristic similarity and vegetated land surface phenology: Implications for the synoptic monitoring of species diversity at broad geographic regions. Remote Sens. Environ. 2012, 121, 488–496. [Google Scholar] [CrossRef]
- Pau, S.; Gillespie, T.W.; Wolkovich, E.M. Dissecting NDVI-species richness relationships in Hawaiian dry forests. J. Biogeogr. 2012, 39, 1678–1686. [Google Scholar] [CrossRef]
- Mackey, B.; Berry, S.; Hugh, S.; Ferrier, S.; Harwood, T.D.; Williams, K.J. Ecosystem greenspots: Identifying potential drought, fire, and climate-change micro-refuges. Ecol. Appl. 2012, 22, 1852–1864. [Google Scholar] [CrossRef]
- Maeda, E.E.; Heiskanen, J.; Thijs, K.W.; Pellikka, P.K. Season-dependence of remote sensing indicators of tree species diversity. Remote Sens. Lett. 2014, 5, 404–412. [Google Scholar] [CrossRef]
- Muro, J.; doninck, J.V.; Tuomisto, H.; Higgins, M.A.; Moulatlet, G.M.; Ruokolainen, K. Floristic composition and across-track reflectance gradient in Landsat images over Amazonian forests. ISPRS J. Photogramm. Remote Sens. 2016, 119, 361–372. [Google Scholar] [CrossRef]
- Bae, S.; Levick, S.R.; Heidrich, L.; Magdon, P.; Leutner, B.F.; Wöllauer, S.; Serebryanyk, A.; Nauss, T.; Krzystek, P.; Gossner, M.M.; et al. Radar vision in the mapping of forest biodiversity from space. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Torresani, M.; Rocchini, D.; Sonnenschein, R.; Zebisch, M.; Marcantonio, M.; Ricotta, C.; Tonon, G. Estimating tree species diversity from space in an alpine conifer forest: The Rao’s Q diversity index meets the spectral variation hypothesis. Ecol. Inform. 2019, 52, 26–34. [Google Scholar] [CrossRef]
- Chitale, V.S.; Behera, M.D.; Roy, P.S. Deciphering plant richness using satellite remote sensing: A study from three biodiversity hotspots. Biodivers. Conserv. 2019, 28, 2183–2196. [Google Scholar] [CrossRef]
- Rocchini, D.; Marcantonio, M.; Re, D.D.; Chirici, G.; Galluzzi, M.; Lenoir, J.; Ricotta, C.; Torresani, M.; Ziv, G. Time-lapsing biodiversity: An open source method for measuring diversity changes by remote sensing. Remote Sens. Environ. 2019, 231, 111192. [Google Scholar] [CrossRef]
- Hoffmann, S.; Schmitt, T.M.; Chiarucci, A.; Irl, S.D.; Rocchini, D.; Vetaas, O.R.; Tanase, M.A.; Mermoz, S.; Bouvet, A.; Beierkuhnlein, C. Remote sensing of β-diversity: Evidence from plant communities in a semi-natural system. Appl. Veg. Sci. 2019, 22, 13–26. [Google Scholar] [CrossRef]
- Mensah, A.A.; Petersson, H.; Saarela, S.; Goude, M.; Holmström, E. Using heterogeneity indices to adjust basal area – Leaf area index relationship in managed coniferous stands. For. Ecol. Manag. 2020, 458, 117699. [Google Scholar] [CrossRef]
- Chaves, P.; Zuquim, G.; Ruokolainen, K.; doninck, J.V.; Kalliola, R.; Rivero, E.G.; Tuomisto, H. Mapping Floristic Patterns of Trees in Peruvian Amazonia Using Remote Sensing and Machine Learning. Remote Sens. 2020, 12, 1523. [Google Scholar] [CrossRef]
- Torresani, M.; Feilhauer, H.; Rocchini, D.; Féret, J.B.; Zebisch, M.; Tonon, G. Which optical traits enable an estimation of tree species diversity based on the Spectral Variation Hypothesis? Appl. Veg. Sci. 2021, 24, e12586. [Google Scholar] [CrossRef]
- Chaves, P.P.; Echeverri, N.R.; Ruokolainen, K.; Kalliola, R.; doninck, J.V.; Rivero, E.G.; Zuquim, G.; Tuomisto, H. Using forestry inventories and satellite imagery to assess floristic variation in bamboo-dominated forests in Peruvian Amazonia. J. Veg. Sci. 2021, 32, e12938. [Google Scholar] [CrossRef]
- Silveira, E.M.; Radeloff, V.C.; Martinuzzi, S.; Pastur, G.J.M.; Rivera, L.O.; Politi, N.; Lizarraga, L.; Farwell, L.S.; Elsen, P.R.; Pidgeon, A.M. Spatio-temporal remotely sensed indices identify hotspots of biodiversity conservation concern. Remote Sens. Environ. 2021, 258, 112368. [Google Scholar] [CrossRef]
- Rocchini, D.; Marcantonio, M.; Re, D.D.; Bacaro, G.; Feoli, E.; Foody, G.M.; Furrer, R.; Harrigan, R.J.; Kleijn, D.; Iannacito, M.; et al. From zero to infinity: Minimum to maximum diversity of the planet by spatio-parametric Rao’s quadratic entropy. Glob. Ecol. Biogeogr. 2021, 30, 1153–1162. [Google Scholar] [CrossRef]
- Khare, S.; Latifi, H.; Rossi, S. A 15-year spatio-temporal analysis of plant β-diversity using Landsat time series derived Rao’s Q index. Ecol. Indic. 2021, 121, 107105. [Google Scholar] [CrossRef]
- Senf, C.; Mori, A.S.; Müller, J.; Seidl, R. The response of canopy height diversity to natural disturbances in two temperate forest landscapes. Landsc. Ecol. 2020, 35, 2101–2112. [Google Scholar] [CrossRef]
- Schneider, F.D.; Ferraz, A.; Hancock, S.; Duncanson, L.I.; Dubayah, R.O.; Pavlick, R.P.; Schimel, D.S. Towards mapping the diversity of canopy structure from space with GEDI. Environ. Res. Lett. 2020, 15, 115006. [Google Scholar] [CrossRef]
- Heidrich, L.; Bae, S.; Levick, S.; Seibold, S.; Weisser, W.; Krzystek, P.; Magdon, P.; Nauss, T.; Schall, P.; Serebryanyk, A.; et al. Heterogeneity–diversity relationships differ between and within trophic levels in temperate forests. Nat. Ecol. Evol. 2020, 4, 1204–1212. [Google Scholar] [CrossRef]
- Tamburlin, D.; Torresani, M.; Tomelleri, E.; Tonon, G.; Rocchini, D. Testing the Height Variation Hypothesis with the R rasterdiv Package for Tree Species Diversity Estimation. Remote Sens. 2021, 13, 3569. [Google Scholar] [CrossRef]
- Khare, S.; Latifi, H.; Ghosh, S.K. Multi-scale assessment of invasive plant species diversity using Pléiades 1A, RapidEye and Landsat-8 data. Geocarto Int. 2018, 33, 681–698. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, Y.; Zheng, Z.; Dong, W.; Zhao, D.; Wu, B.; Zhao, Q. Forest species diversity mapping using airborne LiDAR and hyperspectral data in a subtropical forest in China. Remote Sens. Environ. 2018, 213, 104–114. [Google Scholar] [CrossRef]
- Mohapatra, J.; Singh, C.P.; Hamid, M.; Khuroo, A.A.; Malik, A.H.; Pandya, H.A. Assessment of the alpine plant species biodiversity in the western Himalaya using Resourcesat-2 imagery and field survey. J. Earth Syst. Sci. 2019, 128, 1–16. [Google Scholar] [CrossRef]
- Khare, S.; Latifi, H.; Rossi, S. Forest beta-diversity analysis by remote sensing: How scale and sensors affect the Rao’s Q index. Ecol. Indic. 2019, 106, 105520. [Google Scholar] [CrossRef]
- George-Chacon, S.P.; Dupuy, J.M.; Peduzzi, A.; Hernandez-Stefanoni, J.L. Combining high resolution satellite imagery and lidar data to model woody species diversity of tropical dry forests. Ecol. Indic. 2019, 101, 975–984. [Google Scholar] [CrossRef]
- Hauser, L.T.; Timmermans, J.; van der Windt, N.; Sil, Â.F.; de Sá, N.C.; Soudzilovskaia, N.A.; van Bodegom, P.M. Explaining discrepancies between spectral and in-situ plant diversity in multispectral satellite earth observation. Remote Sens. Environ. 2021, 265, 112684. [Google Scholar] [CrossRef]
- Wang, D.; Qiu, P.; Wan, B.; Cao, Z.; Zhang, Q. Mapping α- and β-diversity of mangrove forests with multispectral and hyperspectral images. Remote Sens. Environ. 2022, 275, 113021. [Google Scholar] [CrossRef]
- Agarwal, S.; Rocchini, D.; Marathe, A.; Nagendra, H. Exploring the Relationship between Remotely-Sensed Spectral Variables and Attributes of Tropical Forest Vegetation under the Influence of Local Forest Institutions. ISPRS Int. J. -Geo-Inf. 2016, 5, 117. [Google Scholar] [CrossRef]
- Arekhi, M.; Yılmaz, O.Y.; Yılmaz, H.; Akyüz, Y.F. Can tree species diversity be assessed with Landsat data in a temperate forest? Environ. Monit. Assess. 2017, 189, 1–14. [Google Scholar] [CrossRef]
- Bawa, K.; Rose, J.; Ganeshaiah, K.; Barve, N.; Kiran, M.; Umashaanker, R. Assessing biodiversity from space: An example from the Western Ghats, India. Conserv. Ecol. 2002, 6. [Google Scholar] [CrossRef]
- Fairbanks, D.H.K.; McGwire, K.C. Patterns of floristic richness in vegetation communities of California: Regional scale analysis with multi-temporal NDVI. Glob. Ecol. Biogeogr. 2004, 13, 221–235. [Google Scholar] [CrossRef]
- Gillespie, T.W.; Zutta, B.R.; Early, M.K.; Saatchi, S. Predicting and quantifying the structure of tropical dry forests in South Florida and the Neotropics using spaceborne imagery. Glob. Ecol. Biogeogr. 2006, 15, 225–236. [Google Scholar] [CrossRef]
- Hernández-Stefanoni, J.L.; Dupuy, J.M.; Castillo-Santiago, M.A. Assessing species density and abundance of tropical trees from remotely sensed data and geostatistics. Appl. Veg. Sci. 2009, 12, 398–414. [Google Scholar] [CrossRef]
- Nagendra, H.; Rocchini, D.; Ghate, R.; Sharma, B.; Pareeth, S. Assessing Plant Diversity in a Dry Tropical Forest: Comparing the Utility of Landsat and Ikonos Satellite Images. Remote Sens. 2010, 2, 478–496. [Google Scholar] [CrossRef]
- Parviainen, M.; Luoto, M.; Heikkinen, R.K. The role of local and landscape level measures of greenness in modelling boreal plant species richness. Ecol. Model. 2009, 220, 2690–2701. [Google Scholar] [CrossRef]
- Stickler, C.M.; Southworth, J. Application of multi-scale spatial and spectral analysis for predicting primate occurrence and habitat associations in Kibale National Park, Uganda. Remote Sens. Environ. 2008, 112, 2170–2186. [Google Scholar] [CrossRef]
- Viedma, O.; Torres, I.; Pérez, B.; Moreno, J.M. Modeling plant species richness using reflectance and texture data derived from QuickBird in a recently burned area of Central Spain. Remote Sens. Environ. 2012, 119, 208–221. [Google Scholar] [CrossRef]
- Féret, J.B.; Asner, G.P. Microtopographic controls on lowland Amazonian canopy diversity from imaging spectroscopy. Ecol. Appl. 2014, 24, 1297–1310. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E. Airborne spectranomics: Mapping canopy chemical and taxonomic diversity in tropical forests. Front. Ecol. Environ. 2009, 7, 269–276. [Google Scholar] [CrossRef]
- Chraibi, E.; Arnold, H.; Luque, S.; Deacon, A.; Magurran, A.; Féret, J.B. A Remote Sensing Approach to Understanding Patterns of Secondary Succession in Tropical Forest. Remote Sens. 2021, 13, 2148. [Google Scholar] [CrossRef]
- Féret, J.B.; De Boissieu, F. biodivMapR: An r package for α-and β-diversity mapping using remotely sensed images. Methods Ecol. Evol. 2020, 11, 64–70. [Google Scholar] [CrossRef]
- Gastauer, M.; Nascimento, W.R.; Caldeira, C.F.; Ramos, S.J.; Souza-Filho, P.W.M.; Féret, J.B. Spectral diversity allows remote detection of the rehabilitation status in an Amazonian iron mining complex. Int. J. Appl. Earth Obs. Geoinf. 2022, 106, 102653. [Google Scholar] [CrossRef]
- Kalacska, M.; Sanchez-Azofeifa, G.; Rivard, B.; Caelli, T.; White, H.P.; Calvo-Alvarado, J. Ecological fingerprinting of ecosystem succession: Estimating secondary tropical dry forest structure and diversity using imaging spectroscopy. Remote Sens. Environ. 2007, 108, 82–96. [Google Scholar] [CrossRef]
- White, J.C.; Gómez, C.; Wulder, M.A.; Coops, N.C. Characterizing temperate forest structural and spectral diversity with Hyperion EO-1 data. Remote Sens. Environ. 2010, 114, 1576–1589. [Google Scholar] [CrossRef]
- Baldeck, C.; Asner, G. Estimating Vegetation Beta Diversity from Airborne Imaging Spectroscopy and Unsupervised Clustering. Remote Sens. 2013, 5, 2057–2071. [Google Scholar] [CrossRef]
- Chaurasia, A.N.; Dave, M.G.; Parmar, R.M.; Bhattacharya, B.; Marpu, P.R.; Singh, A.; Krishnayya, N.S.R. Inferring Species Diversity and Variability over Climatic Gradient with Spectral Diversity Metrics. Remote Sens. 2020, 12, 2130. [Google Scholar] [CrossRef]
- Feilhauer, H.; Schmidtlein, S. Mapping continuous fields of forest alpha and beta diversity. Appl. Veg. Sci. 2009, 12, 429–439. [Google Scholar] [CrossRef]
- Ferreira, M.P.; Zortea, M.; Zanotta, D.C.; Shimabukuro, Y.E.; de Souza Filho, C.R. Mapping tree species in tropical seasonal semi-deciduous forests with hyperspectral and multispectral data. Remote Sens. Environ. 2016, 179, 66–78. [Google Scholar] [CrossRef]
- Fricker, G.A.; Wolf, J.A.; Saatchi, S.S.; Gillespie, T.W. Predicting spatial variations of tree species richness in tropical forests from high-resolution remote sensing. Ecol. Appl. 2015, 25, 1776–1789. [Google Scholar] [CrossRef]
- Hauser, L.T.; Féret, J.B.; Binh, N.A.; van der Windt, N.; Sil, Â.F.; Timmermans, J.; Soudzilovskaia, N.A.; van Bodegom, P.M. Towards scalable estimation of plant functional diversity from Sentinel-2: In-situ validation in a heterogeneous (semi-)natural landscape. Remote Sens. Environ. 2021, 262, 112505. [Google Scholar] [CrossRef]
- Hernández-Stefanoni, J.L.; Gallardo-Cruz, J.A.; Meave, J.A.; Rocchini, D.; Bello-Pineda, J.; López-Martínez, J.O. Modeling α- and β-diversity in a tropical forest from remotely sensed and spatial data. Int. J. Appl. Earth Obs. Geoinf. 2012, 19, 359–368. [Google Scholar] [CrossRef]
- Higgins, M.A.; Asner, G.P.; Perez, E.; Elespuru, N.; Tuomisto, H.; Ruokolainen, K.; Alonso, A. Use of Landsat and SRTM Data to Detect Broad-Scale Biodiversity Patterns in Northwestern Amazonia. Remote Sens. 2012, 4, 2401–2418. [Google Scholar] [CrossRef]
- Krishnaswamy, J.; Bawa, K.S.; Ganeshaiah, K.; Kiran, M. Quantifying and mapping biodiversity and ecosystem services: Utility of a multi-season NDVI based Mahalanobis distance surrogate. Remote Sens. Environ. 2009, 113, 857–867. [Google Scholar] [CrossRef]
- Lucas, K.; Cater, G.A. The use of hyperspectral remote sensing to assess vascular plant species richness on Horn Island, Mississippi. Remote Sens. Environ. 2008, 112, 3908–3915. [Google Scholar] [CrossRef]
- Madonsela, S.; Cho, M.A.; Mathieu, R.; Mutanga, O.; Ramoelo, A.; Kaszta, Ż.; Kerchove, R.V.D.; Wolff, E. Multi-phenology WorldView-2 imagery improves remote sensing of savannah tree species. Int. J. Appl. Earth Obs. Geoinf. 2017, 58, 65–73. [Google Scholar] [CrossRef]
- Madonsela, S.; Cho, M.A.; Ramoelo, A.; Mutanga, O. Remote sensing of species diversity using Landsat 8 spectral variables. ISPRS J. Photogramm. Remote Sens. 2017, 133, 116–127. [Google Scholar] [CrossRef]
- Madonsela, S.; Cho, M.; Ramoelo, A.; Mutanga, O. Investigating the Relationship between Tree Species Diversity and Landsat-8 Spectral Heterogeneity across Multiple Phenological Stages. Remote Sens. 2021, 13, 2467. [Google Scholar] [CrossRef]
- Mapfumo, R.B.; Murwira, A.; Masocha, M.; Andriani, R. The relationship between satellite-derived indices and species diversity across African savanna ecosystems. Int. J. Appl. Earth Obs. Geoinf. 2016, 52, 306–317. [Google Scholar] [CrossRef]
- Mpakairi, K.S.; Dube, T.; Dondofema, F.; Dalu, T. Spatio–temporal variation of vegetation heterogeneity in groundwater dependent ecosystems within arid environments. Ecol. Inform. 2022, 69, 101667. [Google Scholar] [CrossRef]
- Paz-Kagan, T.; Caras, T.; Herrmann, I.; Shachak, M.; Karnieli, A. Multiscale mapping of species diversity under changed land use using imaging spectroscopy. Ecol. Appl. 2017, 27, 1466–1484. [Google Scholar] [CrossRef]
- Paz-Kagan, T.; Chang, J.G.; Shoshany, M.; Sternberg, M.; Karnieli, A. Assessment of plant species distribution and diversity along a climatic gradient from Mediterranean woodlands to semi-arid shrublands. GISci. Remote Sens. 2021, 58, 929–953. [Google Scholar] [CrossRef]
- Rocchini, D. Distance decay in spectral space in analysing ecosystem β-diversity. Int. J. Remote Sens. 2007, 28, 2635–2644. [Google Scholar] [CrossRef]
- Rocchini, D.; Cade, B.S. Quantile Regression Applied to Spectral Distance Decay. IEEE Geosci. Remote Sens. Lett. 2008, 5, 640–643. [Google Scholar] [CrossRef]
- Rocchini, D.; Wohlgemuth, T.; Ghisleni, S.; Chiarucci, A. Spectral rarefaction: Linking ecological variability and plant species diversity. Community Ecol. 2008, 9, 169–176. [Google Scholar] [CrossRef]
- Rocchini, D.; Nagendra, H.; Ghate, R.; Cade, B.S. Spectral distance decay. Photogramm. Eng. Remote Sens. 2009, 75, 1225–1230. [Google Scholar] [CrossRef]
- Rocchini, D.; Wohlgemuth, T.; Ricotta, C.; Ghisleni, S.; Stefanini, A.; Chiarucci, A. Rarefaction theory applied to satellite imagery for relating spectral and species diversity. Riv. Ital. Telerilevamento 2009, 41, 109–123. [Google Scholar] [CrossRef]
- Rocchini, D.; Vannini, A. What is up? Testing spectral heterogeneity versus NDVI relationship using quantile regression. Int. J. Remote Sens. 2010, 31, 2745–2756. [Google Scholar] [CrossRef]
- Rocchini, D.; McGlinn, D.; Ricotta, C.; Neteler, M.; Wohlgemuth, T. Landscape complexity and spatial scale influence the relationship between remotely sensed spectral diversity and survey-based plant species richness. J. Veg. Sci. 2011, 22, 688–698. [Google Scholar] [CrossRef]
- Rocchini, D.; Neteler, M. Spectral rank–abundance for measuring landscape diversity. Int. J. Remote Sens. 2012, 33, 4458–4470. [Google Scholar] [CrossRef]
- Rocchini, D.; Luque, S.; Pettorelli, N.; Bastin, L.; Doktor, D.; Faedi, N.; Feilhauer, H.; Féret, J.B.; Foody, G.M.; Gavish, Y.; et al. Measuring β-diversity by remote sensing: A challenge for biodiversity monitoring. Methods Ecol. Evol. 2018, 9, 1787–1798. [Google Scholar] [CrossRef]
- Schneider, F.D.; Morsdorf, F.; Schmid, B.; Petchey, O.L.; Hueni, A.; Schimel, D.S.; Schaepman, M.E. Mapping functional diversity from remotely sensed morphological and physiological forest traits. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shahtahmassebi, A.R.; Lin, Y.; Lin, L.; Atkinson, P.M.; Moore, N.; Wang, K.; He, S.; Huang, L.; Wu, J.; Shen, Z.; et al. Reconstructing Historical Land Cover Type and Complexity by Synergistic Use of Landsat Multispectral Scanner and CORONA. Remote Sens. 2017, 9, 682. [Google Scholar] [CrossRef]
- Sirén, A.; Tuomisto, H.; Navarrete, H. Mapping environmental variation in lowland Amazonian rainforests using remote sensing and floristic data. Int. J. Remote Sens. 2012, 34, 1561–1575. [Google Scholar] [CrossRef]
- Somers, B.; Asner, G.P.; Martin, R.E.; Anderson, C.B.; Knapp, D.E.; Wright, S.J.; Kerchove, R.V.D. Mesoscale assessment of changes in tropical tree species richness across a bioclimatic gradient in Panama using airborne imaging spectroscopy. Remote Sens. Environ. 2015, 167, 111–120. [Google Scholar] [CrossRef]
- Tagliabue, G.; Panigada, C.; Celesti, M.; Cogliati, S.; Colombo, R.; Migliavacca, M.; Rascher, U.; Rocchini, D.; Schüttemeyer, D.; Rossini, M. Sun–induced fluorescence heterogeneity as a measure of functional diversity. Remote Sens. Environ. 2020, 247, 111934. [Google Scholar] [CrossRef]
- Thessler, S.; Ruokolainen, K.; Tuomisto, H.; Tomppo, E. Mapping gradual landscape-scale floristic changes in Amazonian primary rain forests by combining ordination and remote sensing. Glob. Ecol. Biogeogr. 2005, 14, 315–325. [Google Scholar] [CrossRef]
- Végh, L.; Tsuyuzaki, S. Remote sensing of forest diversities: The effect of image resolution and spectral plot extent. Int. J. Remote Sens. 2021, 42, 5985–6002. [Google Scholar] [CrossRef]
- Warren, S.D.; Alt, M.; Olson, K.D.; Irl, S.D.; Steinbauer, M.J.; Jentsch, A. The relationship between the spectral diversity of satellite imagery, habitat heterogeneity, and plant species richness. Ecol. Inform. 2014, 24, 160–168. [Google Scholar] [CrossRef]
- Draper, F.C.; Baraloto, C.; Brodrick, P.G.; Phillips, O.L.; Martinez, R.V.; Coronado, E.N.H.; Baker, T.R.; Gómez, R.Z.; Guerra, C.A.A.; Flores, M.; et al. Imaging spectroscopy predicts variable distance decay across contrasting Amazonian tree communities. J. Ecol. 2018, 107, 696–710. [Google Scholar] [CrossRef]
- Jha, C.S.; Singhal, J.; Reddy, C.S.; Rajashekar, G.; Maity, S.; Patnaik, C.; Das, A.; Misra, A.; Singh, C.P.; Mohapatra, J.; et al. Characterization of Species Diversity and Forest Health using AVIRIS-NG Hyperspectral Remote Sensing Data. Curr. Sci. 2019, 116, 1124. [Google Scholar] [CrossRef]
- Rao, C.R. Diversity and dissimilarity coefficients: A unified approach. Theor. Popul. Biol. 1982, 21, 24–43. [Google Scholar] [CrossRef]
- Botta-Dukát, Z. Rao’s quadratic entropy as a measure of functional diversity based on multiple traits. J. Veg. Sci. 2005, 16, 533–540. [Google Scholar] [CrossRef]
- Rocchini, D.; Boyd, D.S.; Féret, J.B.; Foody, G.M.; He, K.S.; Lausch, A.; Nagendra, H.; Wegmann, M.; Pettorelli, N. Satellite remote sensing to monitor species diversity: Potential and pitfalls. Remote Sens. Ecol. Conserv. 2016, 2, 25–36. [Google Scholar] [CrossRef]
- Skidmore, A.K.; Pettorelli, N.; Coops, N.C.; Geller, G.N.; Hansen, M.; Lucas, R.; Mücher, C.A.; O’Connor, B.; Paganini, M.; Pereira, H.M.; et al. Environmental science: Agree on biodiversity metrics to track from space. Nature 2015, 523, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, A.K.; Coops, N.C.; Neinavaz, E.; Ali, A.; Schaepman, M.E.; Paganini, M.; Kissling, W.D.; Vihervaara, P.; Darvishzadeh, R.; Feilhauer, H.; et al. Priority list of biodiversity metrics to observe from space. Nat. Ecol. Evol. 2021, 5, 896–906. [Google Scholar] [CrossRef]
- Rocchini, D.; Thouverai, E.; Marcantonio, M.; Iannacito, M.; Da Re, D.; Torresani, M.; Bacaro, G.; Bazzichetto, M.; Bernardi, A.; Foody, G.M.; et al. rasterdiv—An Information Theory tailored R package for measuring ecosystem heterogeneity from space: To the origin and back. Methods Ecol. Evol. 2021, 12, 1093–1102. [Google Scholar] [CrossRef]
- Guanter, L.; Kaufmann, H.; Segl, K.; Foerster, S.; Rogass, C.; Chabrillat, S.; Kuester, T.; Hollstein, A.; Rossner, G.; Chlebek, C.; et al. The EnMAP spaceborne imaging spectroscopy mission for earth observation. Remote Sens. 2015, 7, 8830–8857. [Google Scholar] [CrossRef]
- Lopinto, E.; Ananasso, C. The Prisma hyperspectral mission. In Proceedings of the 33rd EARSeL Symposium, towards Horizon, Matera, Italy, 3–6 June 2020. [Google Scholar]
- Dubayah, R.; Blair, J.B.; Goetz, S.; Fatoyinbo, L.; Hansen, M.; Healey, S.; Hofton, M.; Hurtt, G.; Kellner, J.; Luthcke, S.; et al. The Global Ecosystem Dynamics Investigation: High-resolution laser ranging of the Earth’s forests and topography. Sci. Remote Sens. 2020, 1, 100002. [Google Scholar] [CrossRef]
- Dubayah, R.; Armston, J.; Healey, S.P.; Bruening, J.M.; Patterson, P.L.; Kellner, J.R.; Duncanson, L.; Saarela, S.; Ståhl, G.; Yang, Z.; et al. GEDI launches a new era of biomass inference from space. Environ. Res. Lett. 2022, 17, 095001. [Google Scholar] [CrossRef]
- Le Toan, T.; Quegan, S.; Davidson, M.; Balzter, H.; Paillou, P.; Papathanassiou, K.; Plummer, S.; Rocca, F.; Saatchi, S.; Shugart, H.; et al. The BIOMASS mission: Mapping global forest biomass to better understand the terrestrial carbon cycle. Remote Sens. Environ. 2011, 115, 2850–2860. [Google Scholar] [CrossRef]
- Kellogg, K.; Hoffman, P.; Standley, S.; Shaffer, S.; Rosen, P.; Edelstein, W.; Dunn, C.; Baker, C.; Barela, P.; Shen, Y.; et al. NASA-ISRO synthetic aperture radar (NISAR) mission. In Proceedings of the 2020 IEEE Aerospace Conference, Big Sky, MT, USA, 7–14 March 2020; pp. 1–21. [Google Scholar]
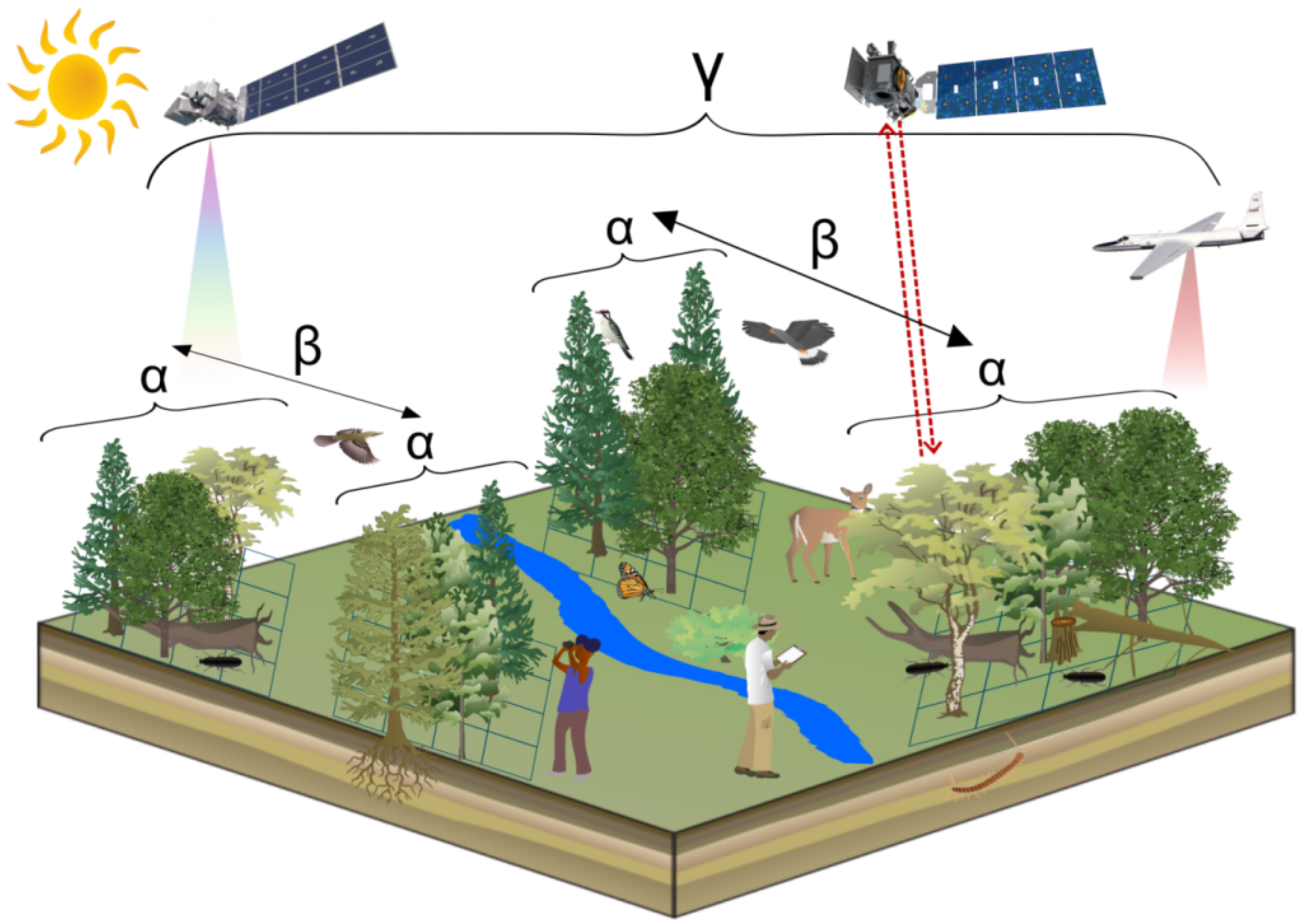







| Biodiversity Scale | Explanation | Exemplary Field Measurement Metrics | Examples of Publications |
|---|---|---|---|
| diversity | within community diversity; local scale; habitat preferences | species richness, Shannon–Wiener index, Simpson index | [22,23,24] |
| diversity | between community diversity; turn-over in species composition; connection between local and regional scales | Jaccard index, Sørensen index, Bray–Curtis dissimilarity | [25,26,27] |
| diversity | landscape diversity; subdivided into and diversity | total species richness (true diversity) | [33] |
| Categories | Concepts | Exemplary Publications |
|---|---|---|
| Habitat mapping | Species area curve | [63] |
| Habitat heterogeneity | [64,65] | |
| Species mapping | Species distribution | [66,67,68] |
| Functional diversity | Plant functional traits | [69,70,71] |
| Vegetation indices | [53,72] | |
| Spectral diversity | Spectral information content | [73,74] |
| Spectral species | [55,75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kacic, P.; Kuenzer, C. Forest Biodiversity Monitoring Based on Remotely Sensed Spectral Diversity—A Review. Remote Sens. 2022, 14, 5363. https://doi.org/10.3390/rs14215363
Kacic P, Kuenzer C. Forest Biodiversity Monitoring Based on Remotely Sensed Spectral Diversity—A Review. Remote Sensing. 2022; 14(21):5363. https://doi.org/10.3390/rs14215363
Chicago/Turabian StyleKacic, Patrick, and Claudia Kuenzer. 2022. "Forest Biodiversity Monitoring Based on Remotely Sensed Spectral Diversity—A Review" Remote Sensing 14, no. 21: 5363. https://doi.org/10.3390/rs14215363
APA StyleKacic, P., & Kuenzer, C. (2022). Forest Biodiversity Monitoring Based on Remotely Sensed Spectral Diversity—A Review. Remote Sensing, 14(21), 5363. https://doi.org/10.3390/rs14215363










