Virtual Laser Scanning Approach to Assessing Impact of Geometric Inaccuracy on 3D Plant Traits
Abstract
1. Introduction
- To what extent progressive inaccuracy of 3D plant reconstruction simulated by different types of geometric noise affects the resulting 3D plant traits?
- Can partially inaccurate measurements of 3D plant traits provide consistent quantitative description of plant morphology and physiology by combining them with the results of computational simulations of synthetic plant models?
2. Methods
2.1. Modelling Platform
2.2. Synthetic Plant Models
2.3. Simulation Scenarios
2.3.1. Virtual Laser Scanner
2.3.2. Light Simulation
2.3.3. Geometrical Perturbation Scenarios and Simulations
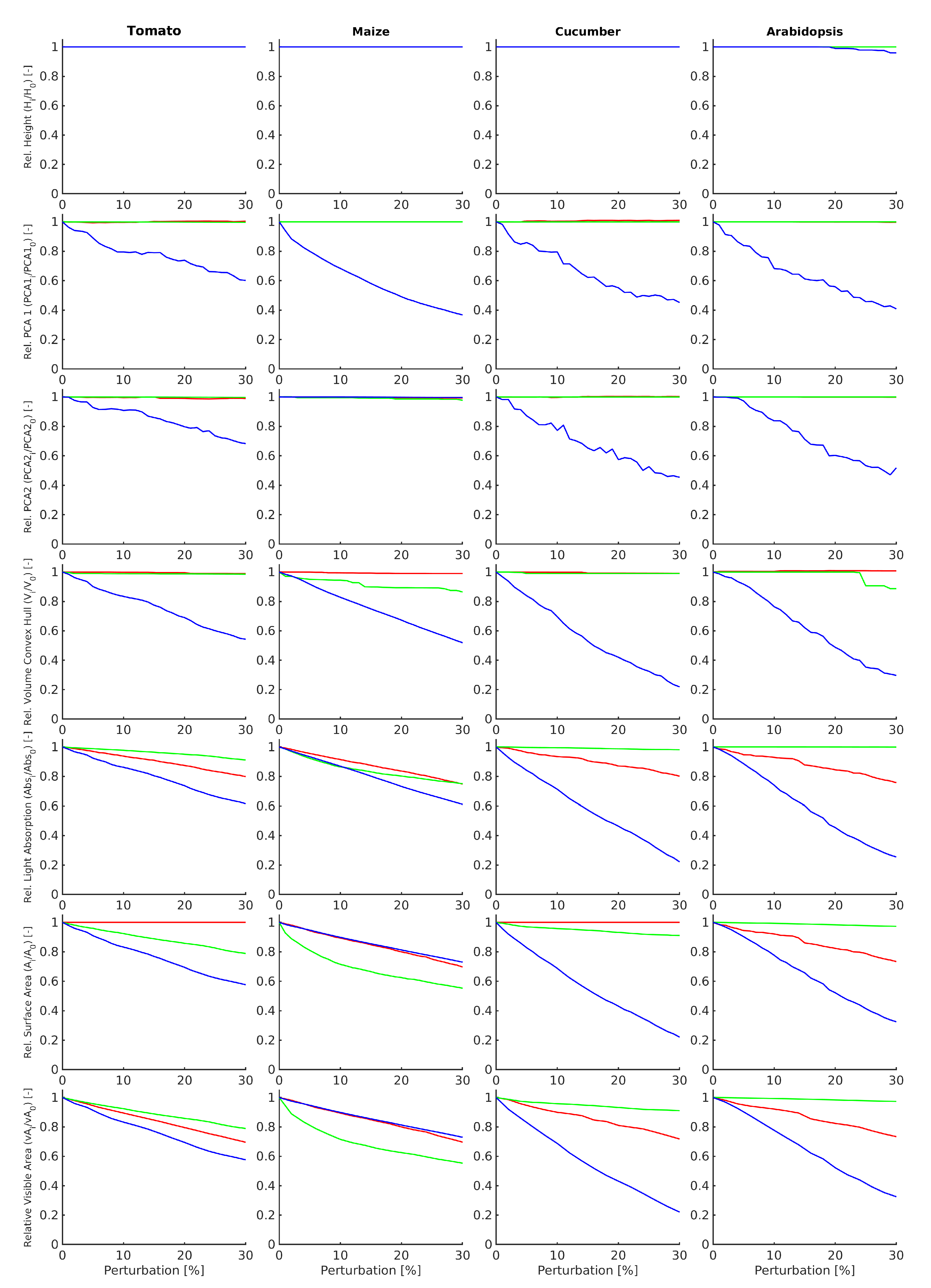
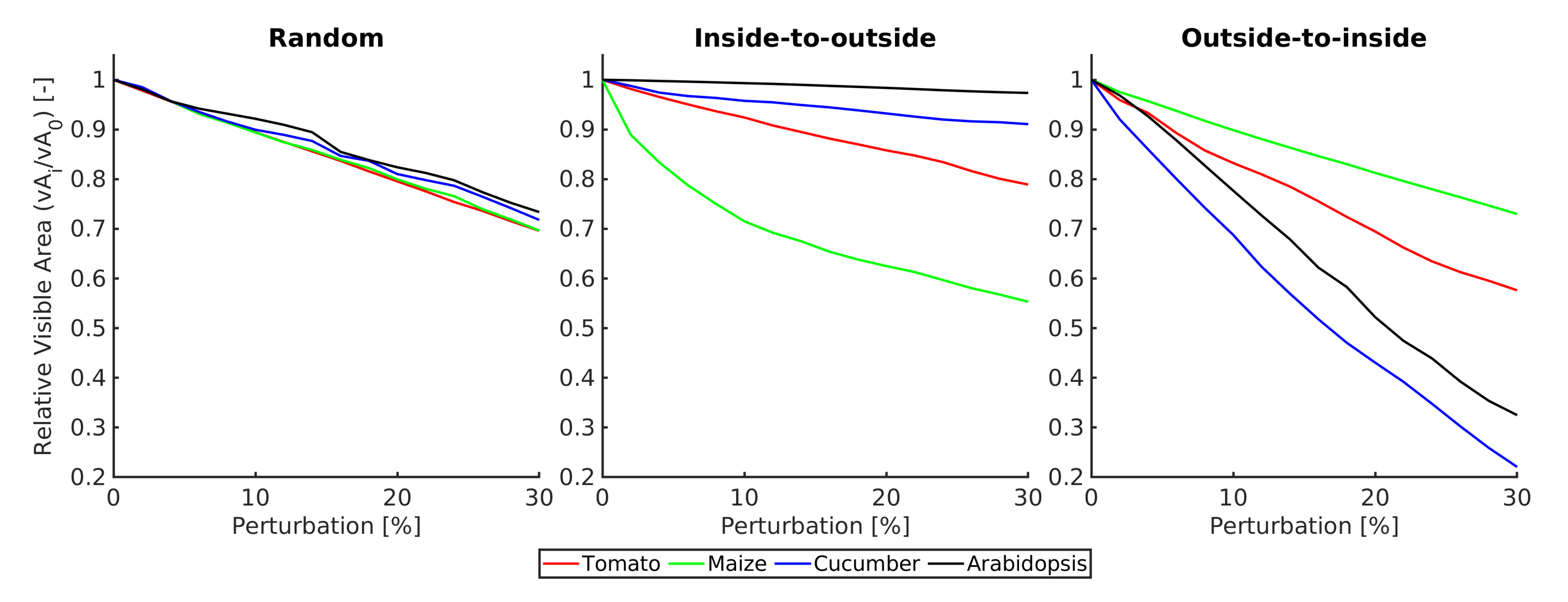
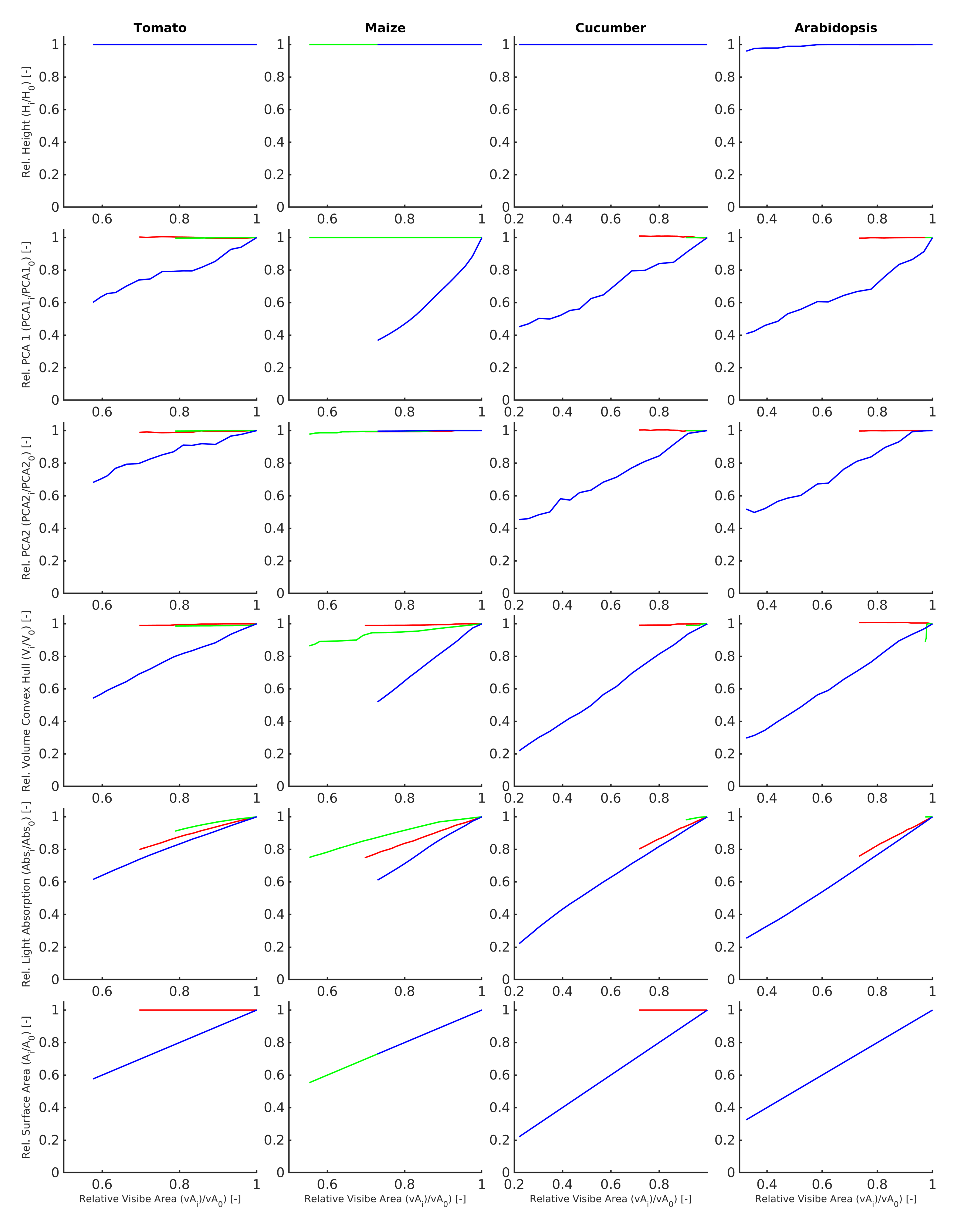
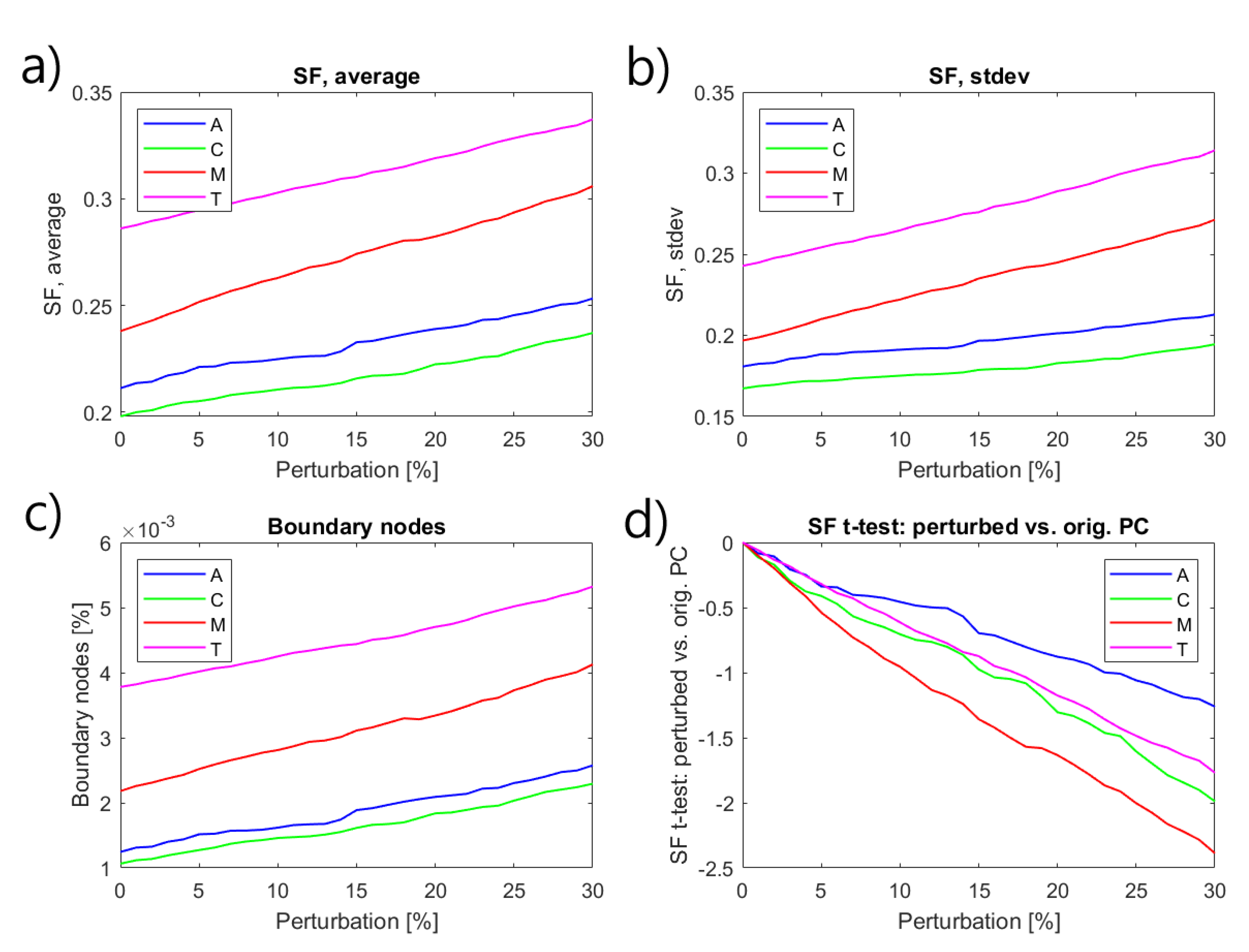
2.3.4. Data Analysis
- Height. Total plant height in metre defined as highest Z-coordinate of the point cloud above the ground.
- PCA1. The length of the largest PCA axis of the scanned point cloud in [m].
- PCA2. The length of the smallest PCA axis of the scanned point cloud in [m]. ize Convex_Hull_Volume. The 3D volume [m] of the convex hull (Figure 5) enclosing all points of the scanned point cloud.
- Plant_AbsorbedRadiation. Total amount of radiation absorbed by the plant structure in Watt [W].
- Plant_SurfaceArea. Total surface area [m] of the plant structure, computed as a sum of areas of all single-side faces.
- Visible_Plant_SurfaceArea. The visible surface area [m] is defined as the sum of areas all single-side faces that are “visible” to the virtual laser scanner and obtained an intersection with at least one virtual light ray emitted by the scanner.
- Number_ScanPoints. Total number of scanner points, i.e., number of points within the point cloud, generated by the virtual laser scanner.
3. Experimental Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pieruschka, R.; Schurr, U. Plant Phenotyping: Past, Present, and Future. Plant Phenomics 2019, 2019, 7507131. [Google Scholar] [CrossRef] [PubMed]
- Minervini, M.; Scharr, H.; Tsaftaris, S.A. Image Analysis: The New Bottleneck in Plant Phenotyping. IEEE Signal Proc. Mag. 2015, 32, 126–131. [Google Scholar] [CrossRef]
- Klukas, C.; Chen, D.; Pape, J.M. Integrated Analysis Platform: An Open-Source Information System for High-Throughput Plant Phenotyping. Plant Physiol. 2014, 165, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Paulus, S. Measuring crops in 3D: Using geometry for plant phenotyping. Plant Methods 2019, 15, 103. [Google Scholar] [CrossRef]
- Paturkar, A.; Sen Gupta, G.; Bailey, D. Making Use of 3D Models for Plant Physiognomic Analysis: A Review. Remote Sens. 2021, 13, 2232. [Google Scholar] [CrossRef]
- Dornbusch, T.; Lorrain, S.; Kuznetsov, D.; Fortier, A.; Liechti, R.; Xenarios, I.; Fankhauser, C. Measuring the diurnal pattern of leaf hyponasty and growth in Arabidopsis–a novel phenotyping approach using laser scanning. Funct. Plant Biol. 2012, 39, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Paulus, S.; Schumann, H.; Kuhlmann, H.; Léon, J. High-precision laser scanning system for capturing 3D plant architecture and analysing growth of cereal plants. Biosyst. Eng. 2014, 121, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, W.; Wu, S.; Wang, C.; Yu, Z.; Guo, X.; Zhao, C. Maize Plant Phenotyping: Comparing 3D Laser Scanning, Multi-View Stereo Reconstruction, and 3D Digitizing Estimates. Remote Sens. 2019, 11, 63. [Google Scholar] [CrossRef]
- Biskup, B.; Scharr, H.; Schurr, U.; Rascher, U. A stereo imaging system for measuring structural parameters of plant canopies. Plant Cell Environ. 2007, 30, 1299–1308. [Google Scholar] [CrossRef]
- Mizuno, S.; Noda, K.; Ezaki, N.; Takizawa, H.; Yamamoto, S. Detection of Wilt by Analyzing Color and Stereo Vision Data of Plant. In International Conference on Computer Vision/Computer Graphics Collaboration Techniques and Applications; Springer: Berlin/Heidelberg, Germany, 2007; Volume 4418, pp. 400–411. [Google Scholar] [CrossRef]
- Jin, J.; Tang, L. Corn plant sensing using real-time stereo vision. Field Robot. 2009, 26, 591–608. [Google Scholar] [CrossRef]
- Nguyen, C.V.; Fripp, J.; Lovell, D.R.; Furbank, R.; Kuffner, P.; Daily, H.; Sirault, X. 3D Scanning System for Automatic High-Resolution Plant Phenotyping. In Proceedings of the 2016 International Conference on Digital Image Computing: Techniques and Applications (DICTA), Gold Coast, QLD, Australia, 30 November–2 December 2016; pp. 1–8. [Google Scholar] [CrossRef]
- Das Choudhury, S.; Maturu, S.; Samal, A.; Stoerger, V.; Awada, T. Leveraging Image Analysis to Compute 3D Plant Phenotypes Based on Voxel-Grid Plant Reconstruction. Front. Plant Sci. 2020, 11, 1963. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.; Wang, H.; Fukano, Y.; Kato, Y.; Ninomiya, S.; Guo, W. EasyDCP: An affordable, high-throughput tool to measure plant phenotypic traits in 3D. Methods Ecol Evol. 2021, 12, 1679–1686. [Google Scholar] [CrossRef]
- Chéné, Y.; Rousseau, D.; Lucidarme, P.; Bertheloot, J.; Caffier, V.; Morel, P.; Belin, É.; Chapeau-Blondeau, F. On the use of depth camera for 3d phenotyping of entire plants. Comput. Electr. Agric. 2012, 82, 122–127. [Google Scholar] [CrossRef]
- Azzari, G.; Goulden, M.; Rusu, R. Rapid characterization of vegetation structure with a microsoft kinect sensor. Sensors 2013, 13, 2384–2398. [Google Scholar] [CrossRef] [PubMed]
- Paulus, S.; Behmann, J.; Mahlein, A.K.; Plümer, L.; Kuhlmann, H. Low-cost 3D systems: Suitable tools for plant phenotyping. Sensors 2014, 14, 3001–3018. [Google Scholar] [CrossRef] [PubMed]
- Scharr, H.; Briese, C.; Embgenbroich, P.; Fischbach, A.; Fiorani, F.; Müller-Linow, M. Fast High Resolution Volume Carving for 3D Plant Shoot Reconstruction. Front. Plant Sci. 2017, 8, 1680. [Google Scholar] [CrossRef]
- Klose, R.; Penlington, J.; Ruckelshausen, A. Usability study of 3D time-of-flight cameras for automatic plant phenotyping. Bornimer Agrartech. Ber. 2009, 69, 93–105. [Google Scholar]
- Kraft, M.; Salomão de Freitas, N.; Munack, A. Test of a 3d time of flight camera for shape measurements of plants. In Proceedings of the CIGR Workshop on Image Analysis in Agriculture, Budapest, Hungary, 26–27 August 2010; pp. 108–115. [Google Scholar]
- Bishop, T.; Favaro, P. The light field camera: Extended depth of field, aliasing, and superresolution. Pattern Anal. Mach. Intell. IEEE Trans. 2012, 34, 972–986. [Google Scholar] [CrossRef]
- Omasa, K.; Hosoi, F.; Konishi, A. 3D lidar imaging for detecting and understanding plant responses and canopy structure. J. Exp. Bot. 2007, 58, 881–898. [Google Scholar] [CrossRef]
- Lichti, D. Error modelling, calibration and analysis of an AM–CW terrestrial laser scanner system. ISPRS J. Photogramm. Remote Sens. 2007, 61, 307–324. [Google Scholar] [CrossRef]
- Hosoi, F.; Omasa, K. Estimating vertical plant area density profile and growth parameters of a wheat canopy at different growth stages using three-dimensional portable lidar imaging. ISPRS J. Photogramm. Remote Sens. 2009, 64, 151–158. [Google Scholar] [CrossRef]
- Panjvani, K.; Dinh, A.V.; Wahid, K.A. LiDARPheno—A Low-Cost LiDAR-Based 3D Scanning System for Leaf Morphological Trait Extraction. Front. Plant Sci. 2019, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Gélard, W.; Herbulot, A.; Devy, M.; Debaeke, P.; Mccormick, R.F.; Truong, S.K.; Mullet, J. Leaves Segmentation in 3D Point Cloud. In Proceedings of the Advanced Concepts for Intelligent Vision Systems 18th International Conference, ACIVS 2017, Antwerp, Belgium, 18–21 September 2017; pp. 664–674. [Google Scholar]
- Chaudhury, A.; Boudon, F.; Godin, C. 3D Plant Phenotyping: All You Need is Labelled Point Cloud Data. In Proceedings of the ECCV 2020 Workshop on Computer Vision Problems in Plant Phenotyping, Glasgow, UK, 28 August 2020; pp. 244–260. [Google Scholar] [CrossRef]
- Miao, T.; Wen, W.; Li, Y.; Wu, S.; Zhu, C.; Guo, X. Label3DMaize: Toolkit for 3D point cloud data annotation of maize shoots. GigaScience 2021, 10, giab031. [Google Scholar] [CrossRef] [PubMed]
- Golbach, F.; Kootstra, G.; Damjanovic, S.; Otten, G.; van de Zedde, R. Validation of plant part measurements using a 3D reconstruction method suitable for high-throughput seedling phenotyping. Mach. Vis. Appl. 2016, 27, 663–680. [Google Scholar] [CrossRef]
- Zhou, J.; Fu, X.; Schumacher, L.; Zhou, J. Evaluating Geometric Measurement Accuracy Based on 3D Reconstruction of Automated Imagery in a Greenhouse. Sensors 2018, 18, 2270. [Google Scholar] [CrossRef]
- Amador, P.; Müller-Linow, M.; Scharr, H. Measuring Ground Truth for 3D Reconstruction of Plants. In Proceedings of the BMVC Workshop on Computer Vision Problems in Plant Phenotyping, Newcastle upon Tyne, UK, 6 September 2018; p. 2. [Google Scholar]
- Kniemeyer, O. Design and Implementation of a Graph Grammar Based Language for Functional-Structural Plant Modelling. Ph.D. Thesis, Brandenburg University of Technology Cottbus, Cottbus, Germany, 2008. [Google Scholar]
- Hemmerling, R.; Kniemeyer, O.; Lanwert, D.; Kurth, W.; Buck-Sorlin, G.H. The rule-based language XL and the modelling environment GroIMP illustrated with simulated tree competition. Funct. Plant Biol. 2008, 35, 739–750. [Google Scholar] [CrossRef]
- Henke, M.; Buck-Sorlin, G.H. Using a Full Spectral Raytracer for Calculating Light Microclimate in Functional-Structural Plant Modelling. Comput. Inform. 2018, 36, 1492–1522. [Google Scholar] [CrossRef]
- Zhang, Y.; Henke, M.; Li, Y.; Yue, X.; Xu, D.; Liu, X.; Li, T. High resolution 3D simulation of light climate and thermal performance of a solar greenhouse model under tomato canopy structure. Renew. Energy 2020, 160, 730–745. [Google Scholar] [CrossRef]
- Evers, J.B.; Vos, J.; Yin, X.; Romero, P.; van der Putten, P.; Struik, P. Simulation of wheat growth and development based on organ-level photosynthesis and assimilate allocation. J. Exp. Bot. 2010, 61, 2203–2216. [Google Scholar] [CrossRef]
- Zhu, J.; van der Werf, W.; Anten, N.P.R.; Vos, J.; Evers, J.B. The contribution of phenotypic plasticity to complementary light capture in plant mixtures. New Phytol. 2015, 207, 1213–1222. [Google Scholar] [CrossRef]
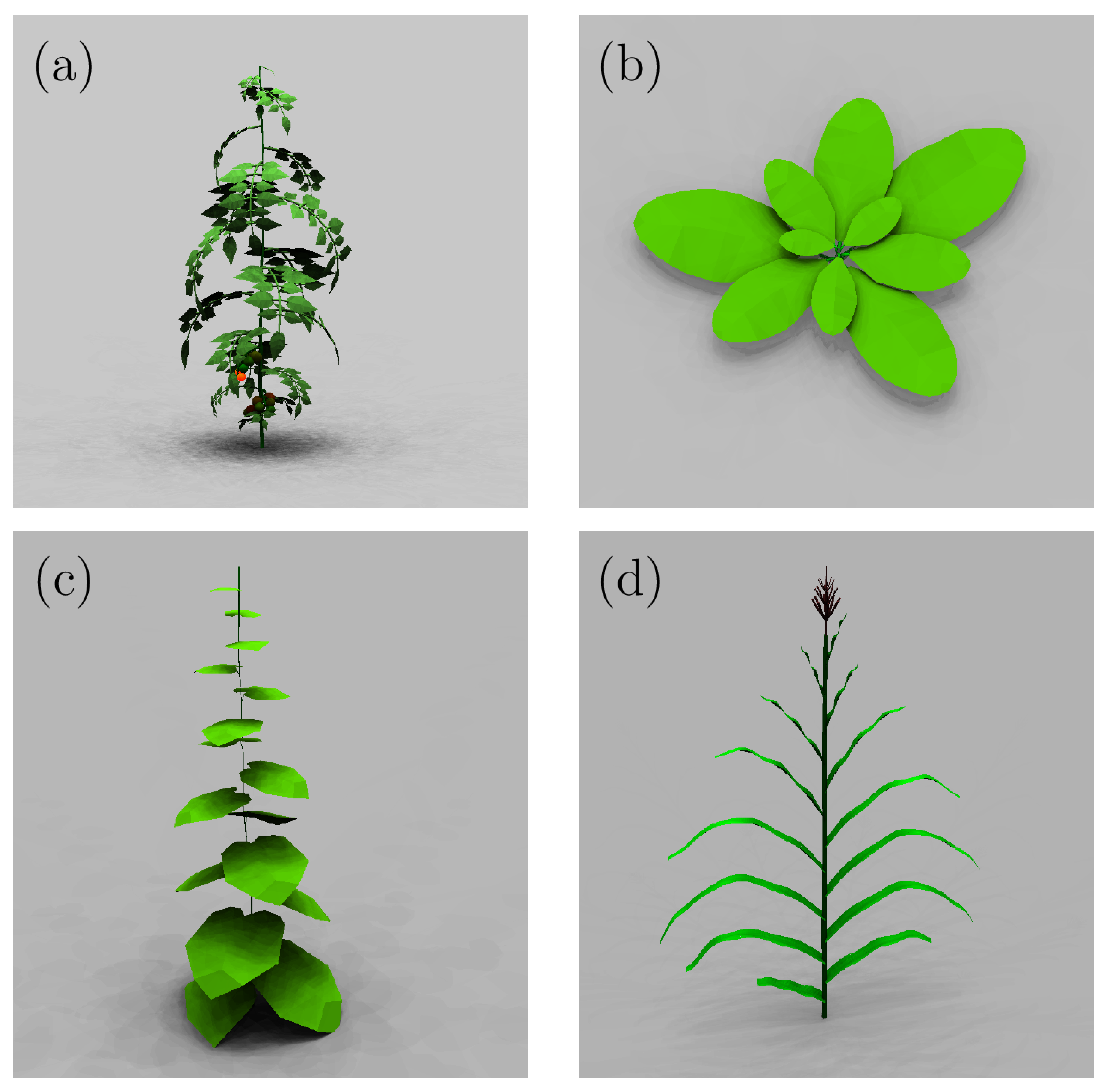


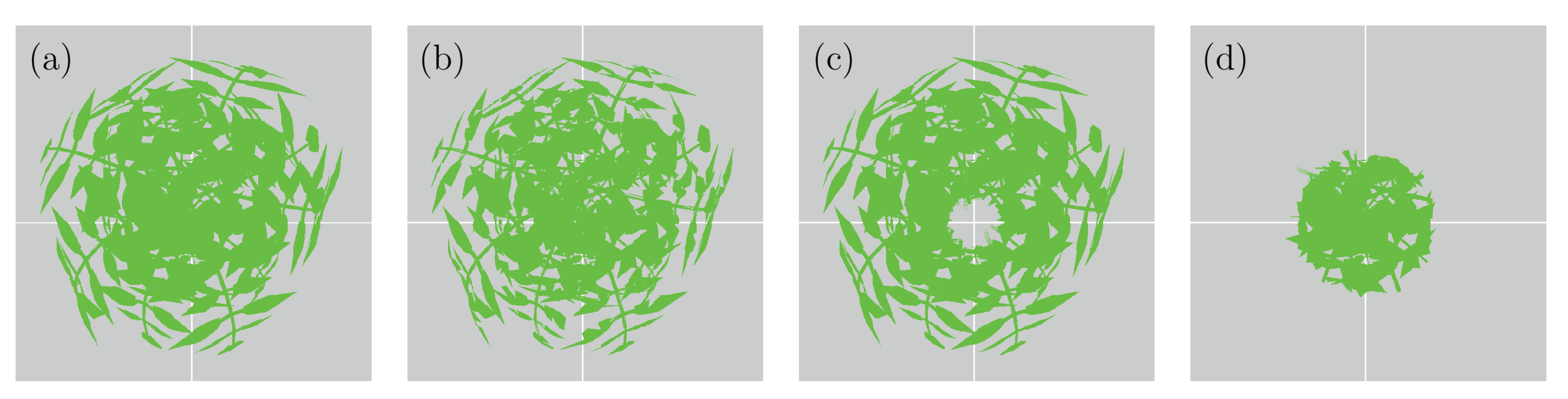

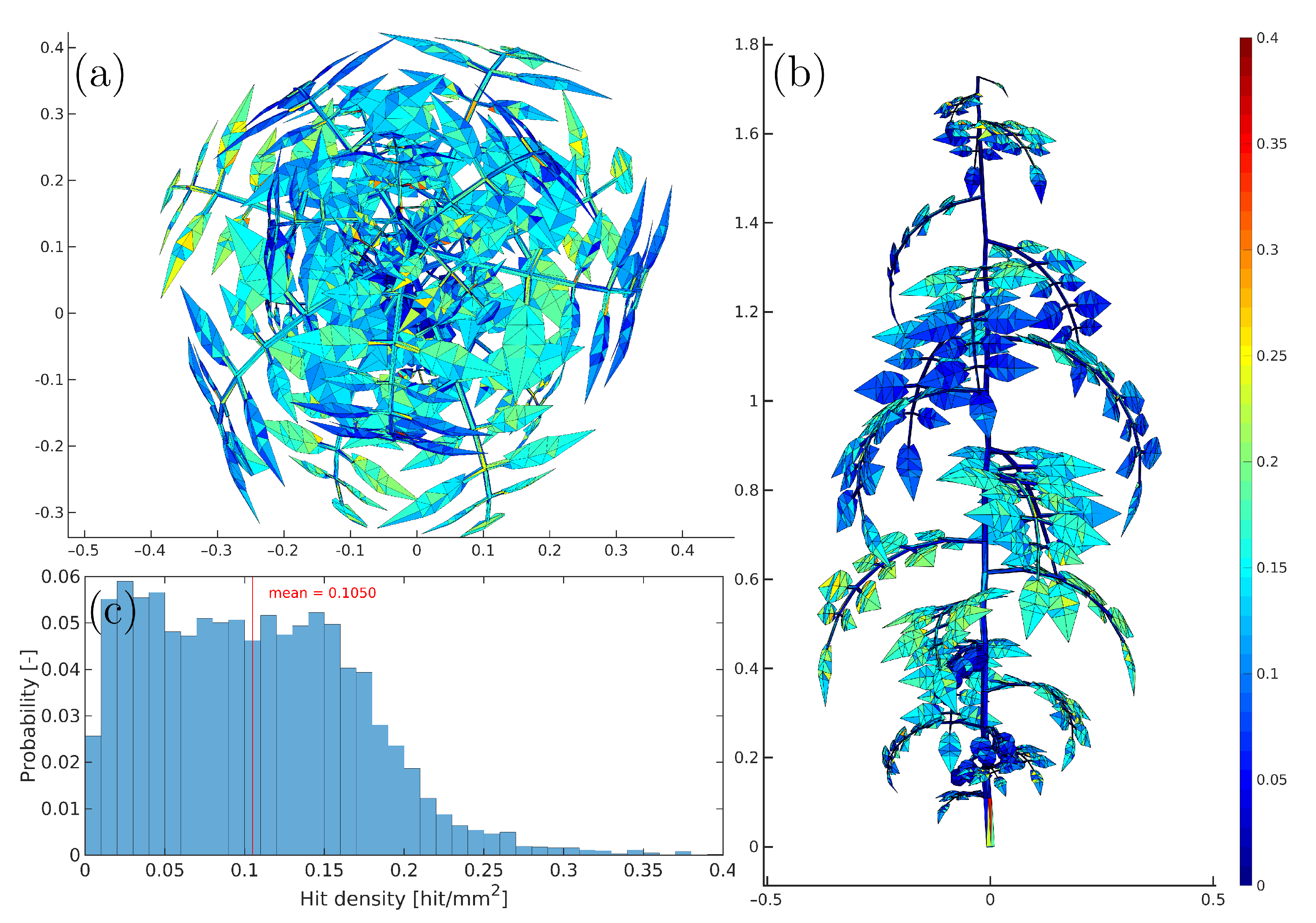



| Plant | Number | Number of | Max | Max | Number | Number | Mean Face |
|---|---|---|---|---|---|---|---|
| Model | of Leaves | Internodes | Height [m] | Radius [m] | of Vertices | of Faces | Area [mm] |
| Tomato | 21 | 21 | 1.7 | 0.43 | 25,674 | 8558 | 142.53 |
| Arabidopsis | 10 | 11 | 0.03 | 0.06 | 3600 | 1200 | 189.92 |
| Maize | 14 | 15 | 2.5 | 0.97 | 15,450 | 5150 | 699.83 |
| Cucumber | 17 | 18 | 2.0 | 0.43 | 5793 | 1931 | 12.74 |
| Species | Visible Faces [%] | Visible Area [%] |
|---|---|---|
| Tomato | 0.59 | 0.78 |
| Arabidopsis | 0.79 | 0.81 |
| Maize | 0.67 | 0.94 |
| Cucumber | 0.48 | 0.94 |
| Height | PCA1 | PCA2 | Volume | Absorption | Area | |
|---|---|---|---|---|---|---|
| , p-Value | , p-Value | , p-Value | , p-Value | , p-Value | , p-Value | |
| Tomato | ||||||
| Random | NaN, NaN | −0.75, | 0.87, | 0.94, | 1.00, | NaN, NaN |
| i2o | NaN, NaN | 0.98, | 0.95, | 0.78, | 0.99, | 1.00, |
| o2i | NaN, NaN | 0.98, | 0.98, | 1.00, | 1.00, | 1.00, |
| Maize | ||||||
| Random | NaN, NaN | 0.80, | 0.87, | 0.94, | 1.00, | 1.00, |
| i2o | NaN, NaN | 0.95, | 0.87, | 0.97, | 0.99, | 1.00, |
| o2i | NaN, NaN | 0.99, | 0.92, | 1.00, | 1.00, | 1.00, 0.00 |
| Cucumber | ||||||
| Random | NaN, NaN | −0.85, | −0.73, | 0.91, | 1.00, | NaN, NaN |
| i2o | NaN, NaN | 0.43, | 0.42, | 0.75, | 0.98, | 1.00, |
| o2i | NaN, NaN | 0.99, | 1.00, | 1.00, | 1.00, | 1.00, 0.00 |
| Arabidopsis | ||||||
| Random | 0.71, | 0.89, | 0.83, | −0.77, | 1.00, | 1.00, 0.00 |
| i2o | NaN, NaN | −0.88, | 0.87, | 0.73, | 0.95, | 1.00, |
| o2i | 0.82, | 0.98, | 0.99, | 1.00, | 1.00, | 1.00, 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henke, M.; Gladilin, E. Virtual Laser Scanning Approach to Assessing Impact of Geometric Inaccuracy on 3D Plant Traits. Remote Sens. 2022, 14, 4727. https://doi.org/10.3390/rs14194727
Henke M, Gladilin E. Virtual Laser Scanning Approach to Assessing Impact of Geometric Inaccuracy on 3D Plant Traits. Remote Sensing. 2022; 14(19):4727. https://doi.org/10.3390/rs14194727
Chicago/Turabian StyleHenke, Michael, and Evgeny Gladilin. 2022. "Virtual Laser Scanning Approach to Assessing Impact of Geometric Inaccuracy on 3D Plant Traits" Remote Sensing 14, no. 19: 4727. https://doi.org/10.3390/rs14194727
APA StyleHenke, M., & Gladilin, E. (2022). Virtual Laser Scanning Approach to Assessing Impact of Geometric Inaccuracy on 3D Plant Traits. Remote Sensing, 14(19), 4727. https://doi.org/10.3390/rs14194727






