Mapping Forest Stability within Major Biomes Using Canopy Indices Derived from MODIS Time Series
Abstract
:1. Introduction
2. Materials and Methods
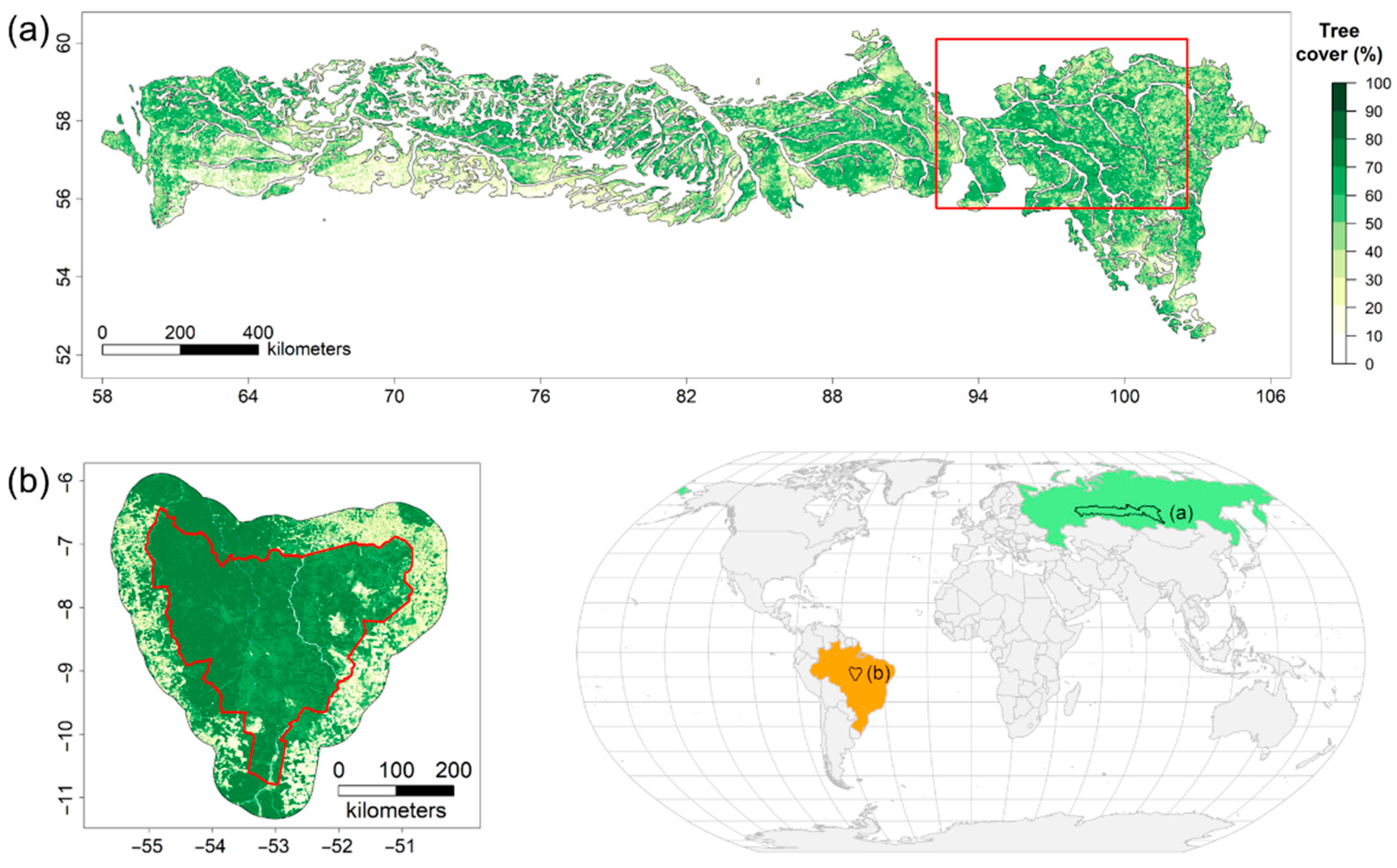
2.1. Study Regions
2.2. Data Analysis
2.2.1. Input Parameters
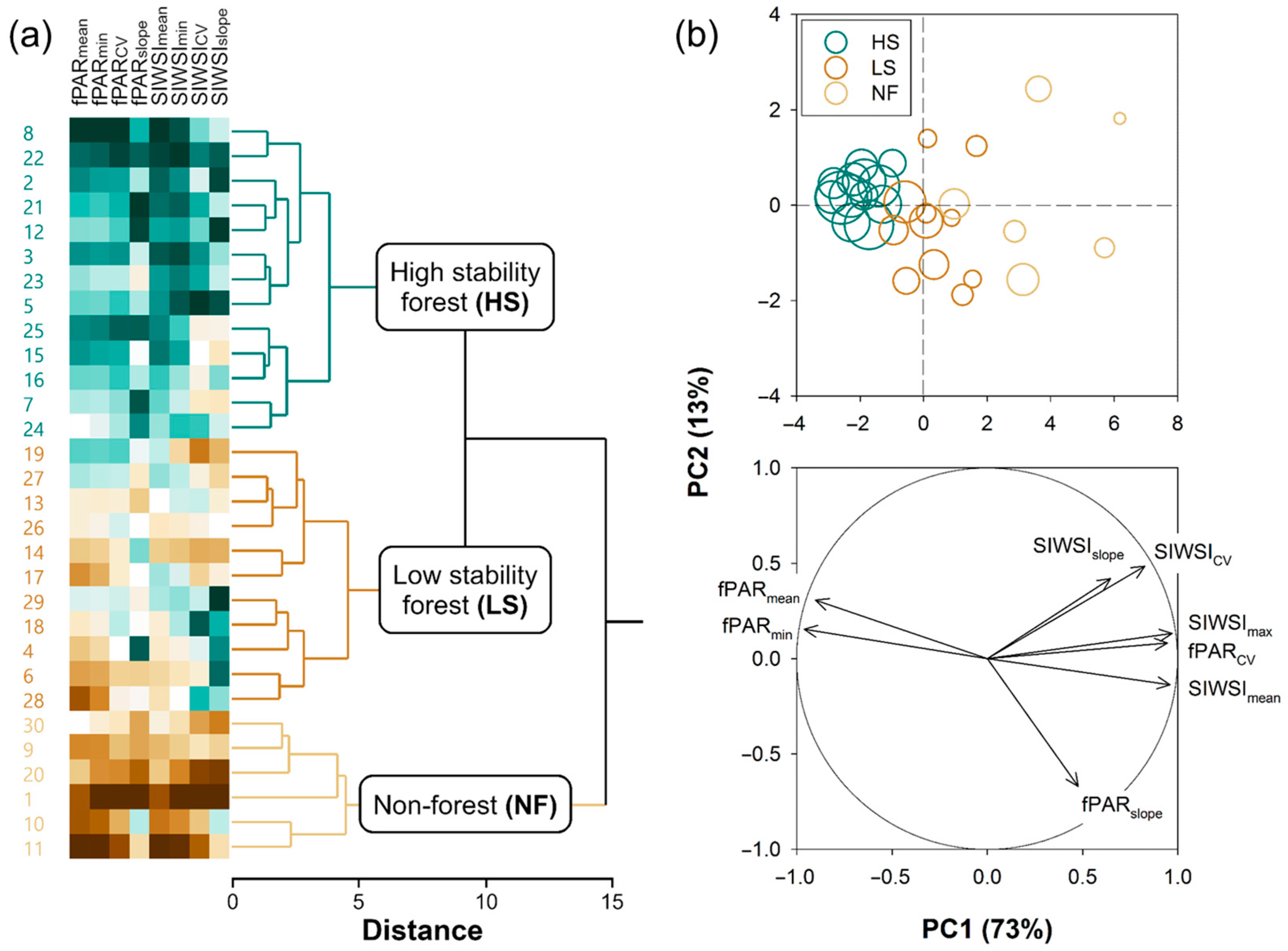
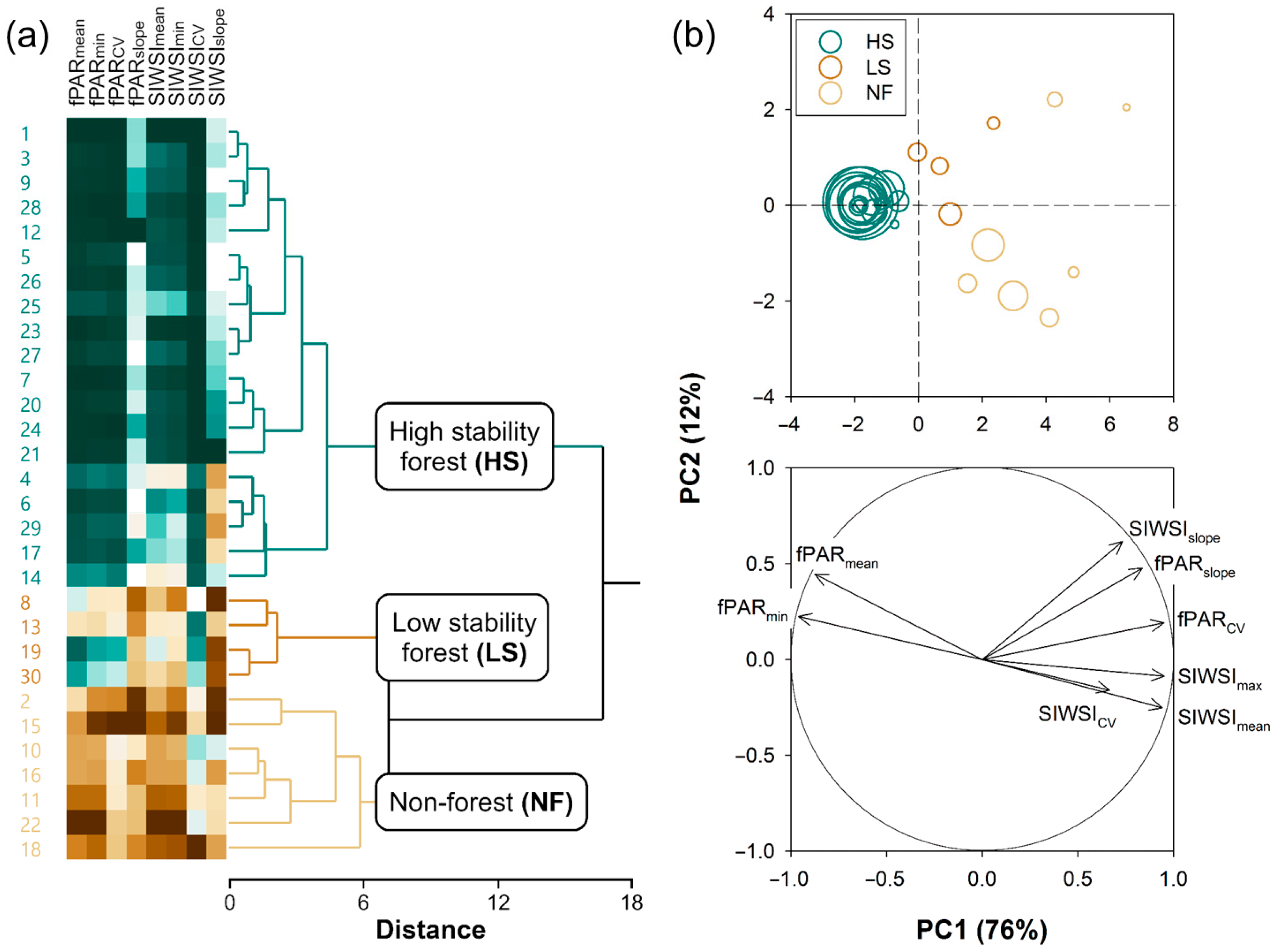
2.2.2. k-Means Clustering of Pixels
- HS forests to be characterized by high fPAR and low SIWSI mean values over the time series and fPAR minimum and SIWSI maximum values close to the means, with high temporal stability of these metrics, i.e., low CV and slope;
- LS forests to be characterized by lower fPAR and higher SIWSI mean values, more variable fPAR minimum and SIWSI maximum values, and less temporal stability, i.e., relatively high inter-annual fluctuation, high slope, or both; and
- NF areas to display the lowest fPAR and highest SIWSI mean values, and also highly variable fPAR minimum and SIWSI maximum values, together with a large temporal instability, i.e., high CV and/or slope.
2.3. Validation Framework
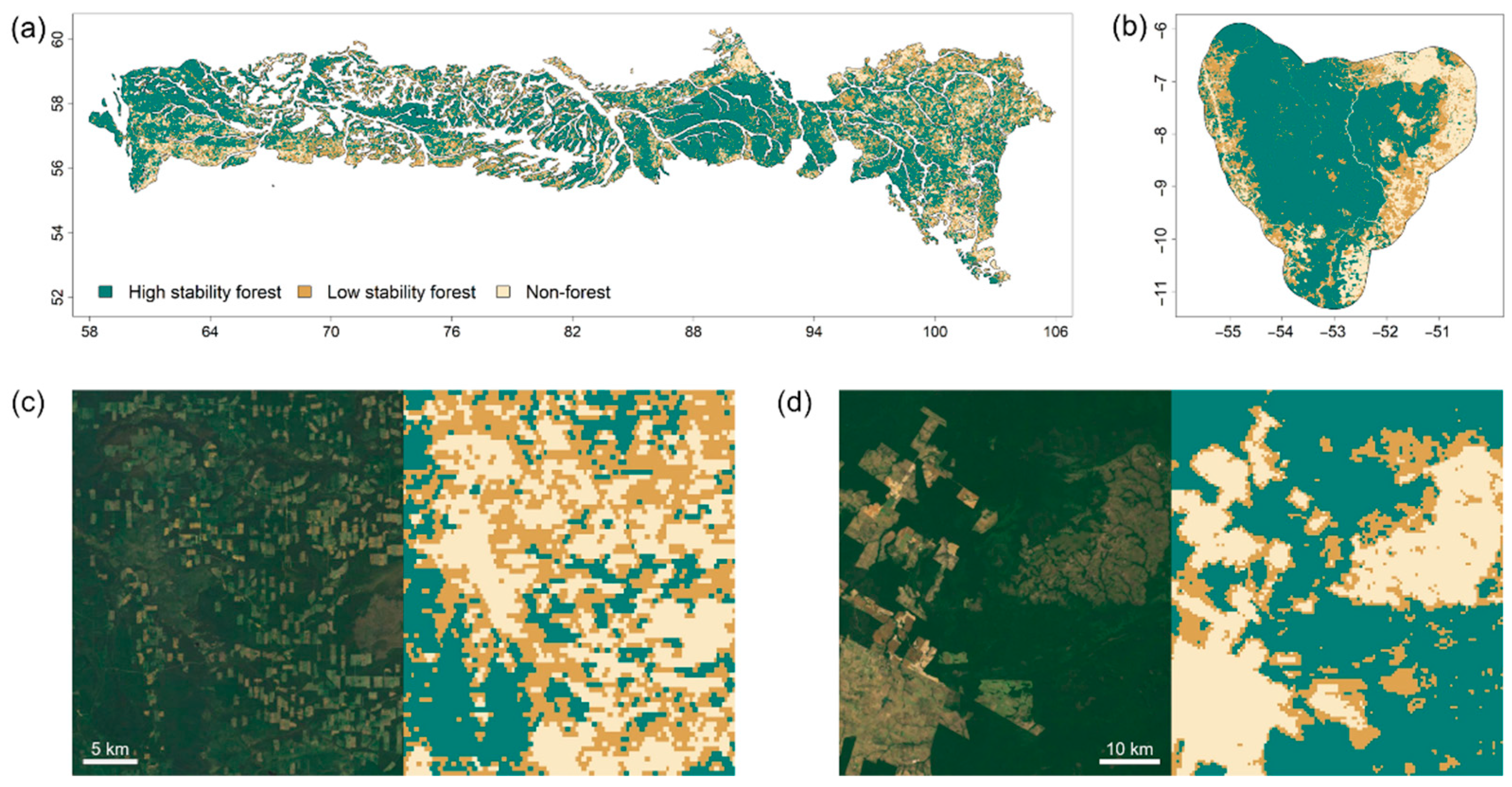
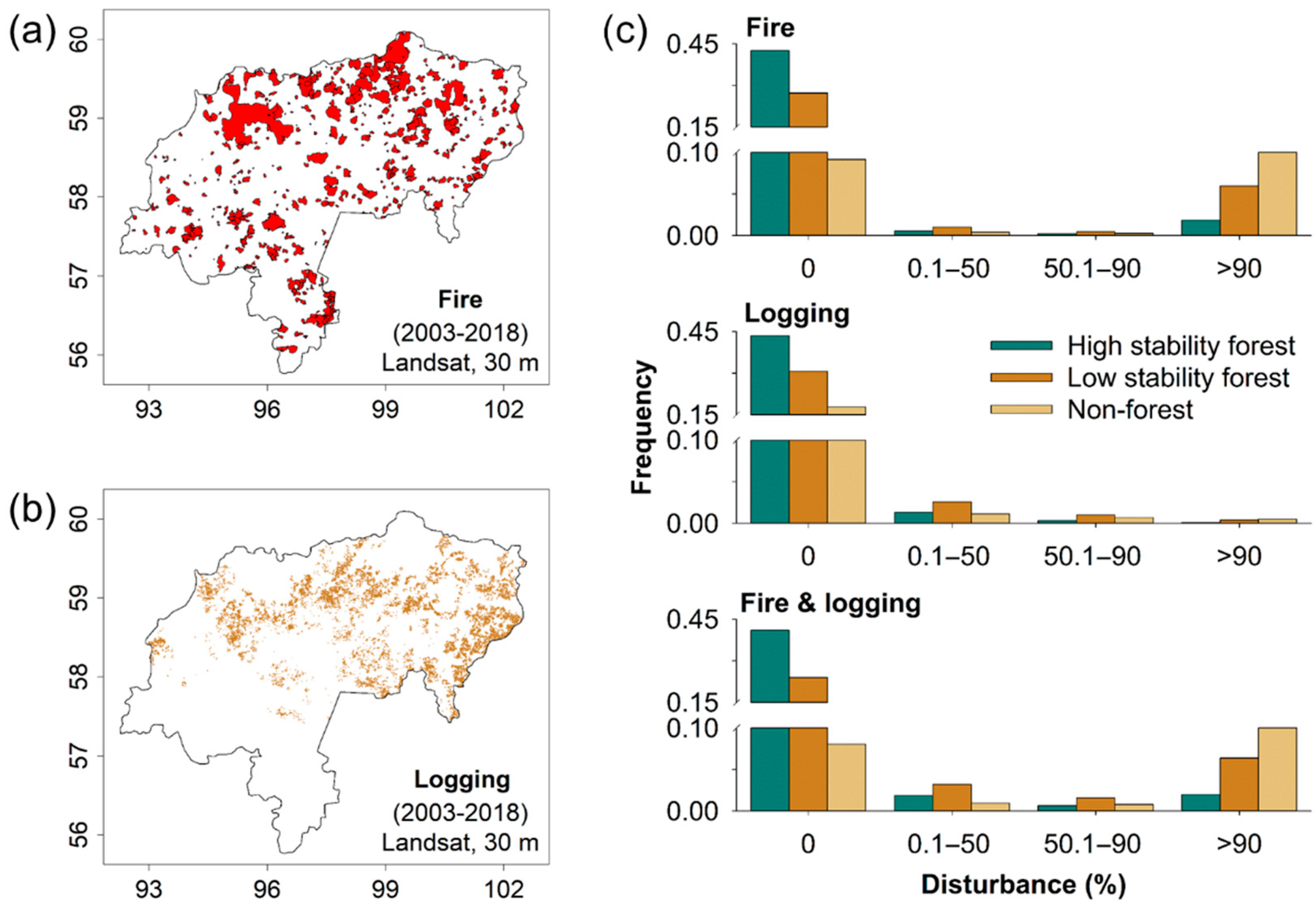
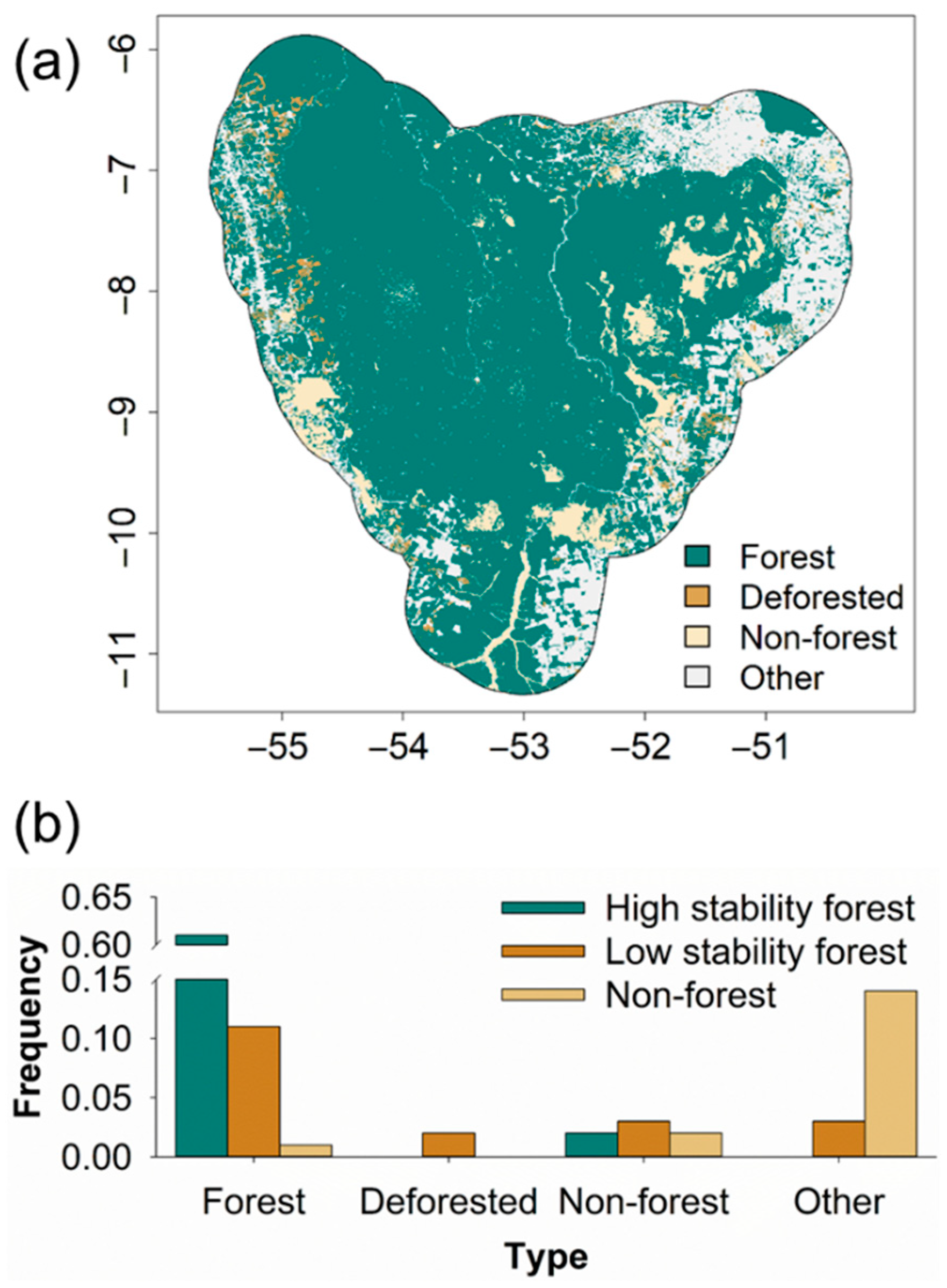
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO (Food and Agriculture Organization). The State of the World’s Forests 2020: Forests, Biodiversity and People; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- IUCN (International Union for Conservation of Nature). IUCN Policy Statement on Primary Forests Including Intact Forest Landscapes. In International Union for Conservation of Nature; IUCN: Glan, Switzerland, 2020; Available online: http://forestsandclimate.org.au/cms/wp-content/uploads/iucn-pf-ifl-policy-2020-approved-version-2-1.pdf (accessed on 13 July 2022).
- FAO (Food and Agriculture Organization). Global Forest Resources Assessment 2020: Terms and Definitions. In Forest Resources Assessment Working Paper 188; FAO: Rome, Italy, 2020; Available online: http://www.fao.org/3/I8661EN/i8661en.pdf (accessed on 13 July 2022).
- Thompson, I.D.; Guariguata, M.R.; Okabe, K.; Bahamondez, C.; Nasi, R.; Heymell, V.; Sabogal, C. An operational framework for defining and monitoring forest degradation. Ecol. Soc. 2013, 18, 20. [Google Scholar] [CrossRef]
- Mackey, B.; DellaSala, D.A.; Kormos, C.; Lindenmayer, D.; Kumpel, N.; Zimmerman, B.; Hugh, S.; Young, V.; Foley, S.; Arsenis, K.; et al. Policy options for the world’s primary forests in multilateral environmental agreements. Conserv. Lett. 2015, 8, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Roche, P.K.; Campagne, C.S. From ecosystem integrity to ecosystem condition: A continuity of concepts supporting different aspects of ecosystem sustainability. Curr. Opin. Environ. Sustain. 2017, 29, 63–68. [Google Scholar] [CrossRef]
- Kormos, C.F.; Mackey, B.; Della Sala, D.A.; Kumpe, N.; Jaeger, T.; Mittermeier, R.A.; Filardi, C. Primary forests: Definition, status and future prospects for global conservation. In Encyclopedia of the Anthropocene; DellaSala, D.A., Goldstein, M.I., Eds.; Elsevier: Oxford, UK, 2018; Volume 2, pp. 31–41. [Google Scholar] [CrossRef]
- Watson, J.E.M.; Evans, T.; Venter, O.; Williams, B.; Tulloch, A.; Stewart, C.; Thompson, I.; Ray, J.C.; Murray, K.; Salazar, A.; et al. The exceptional value of intact forest ecosystems. Nat. Ecol. Evol. 2018, 2, 599–610. [Google Scholar] [CrossRef]
- Funk, J.M.; Aguilar-Amuchastegui, N.; Baldwin-Cantello, W.; Busch, J.; Chuvasov, E.; Evans, T.; Griffin, B.; Harris, N.; Ferreira, M.N.; Petersen, K.; et al. Securing the climate benefits of stable forests. Clim. Policy 2019, 19, 845–860. [Google Scholar] [CrossRef] [Green Version]
- Thom, D.; Golivets, M.; Edling, L.; Meigs, G.W.; Gourevitch, J.D.; Sonter, L.J.; Galford, G.L.; Keeton, W.S. The climate sensitivity of carbon, timber, and species richness covaries with forest age in boreal–temperate North America. Glob. Chang. Biol. 2019, 25, 2446–2458. [Google Scholar] [CrossRef]
- Mackey, B.; Kormos, C.F.; Keith, H.; Moomaw, W.R.; Houghton, R.A.; Mittermeier, R.A.; Hole, D.; Hugh, S. Understanding the importance of primary tropical forest protection as a mitigation strategy. Mitig. Adapt. Strateg. Glob. Chang. 2020, 25, 763–787. [Google Scholar] [CrossRef] [Green Version]
- Asner, G.; Knapp, D.E.; Broadbent, E.N.; Oliveira, J.C.; Keller, M.; Silva, J.N. Selective logging in the Brazilian Amazon. Science 2005, 310, 480–482. [Google Scholar] [CrossRef]
- Broadbent, E.N.; Asner, G.P.; Keller, M.; Knapp, D.E.; Oliveira, P.J.C.; Silva, J.N. Forest fragmentation and edge effects from deforestation and selective logging in the Brazilian Amazon. Biol. Conserv. 2008, 141, 1745–1757. [Google Scholar] [CrossRef]
- Tyukavina, A.; Hansen, M.C.; Potapov, P.V.; Stehman, S.V.; Smith-Rodriguez, K.; Okpa, C.; Aguilar, R. Types and rates of forest disturbance in Brazilian Legal Amazon, 2000–2013. Sci. Adv. 2017, 3, e1601047. [Google Scholar] [CrossRef] [Green Version]
- Herold, M.; Román-Cuesta, R.M.; Mollicone, D.; Hirata, Y.; van Laake, P.; Asner, G.P.; Souza, C.; Skutsch, M.; Avitabile, V.; MacDicken, K. Options for monitoring and estimating historical carbon emissions from forest degradation in the context of REDD+. Carbon Balance Manag. 2011, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Skutsch, M.; Paneque-Gálvez, J.; Ghilardi, A. Remote sensing of forest degradation: A review. Environ. Res. Lett. 2020, 15, 103001. [Google Scholar] [CrossRef]
- Lambin, E.F. Monitoring forest degradation in tropical regions by remote sensing: Some methodological issues. Glob. Ecol. Biogeogr. 1999, 8, 191–198. [Google Scholar] [CrossRef]
- Berenguer, E.; Ferreira, J.; Gardner, T.A.; Aragao, L.E.O.C.; de Camargo, P.B.; Cerri, C.E.; Durigan, M.; Cosme De Oliveira, R.J.; Guimarães Vieira, I.C.; Barlow, J. A large-scale field assessment of carbon stocks in human-modified tropical forests. Glob. Chang. Biol. 2014, 20, 3713–3726. [Google Scholar] [CrossRef] [Green Version]
- Baccini, A.; Walker, W.; Carvalho, L.; Farina, M.; Sulla-Menashe, D.; Houghton, R.A. Tropical forests are a net carbon source based on aboveground measurements of gain and loss. Science 2017, 358, 230–233. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, A.L.; Rosenqvist, A.; Mora, B. Current remote sensing approaches to monitoring forest degradation in support of countries measurement, reporting and verification (MRV) systems for REDD+. Carbon Balance Manag. 2017, 12, 9. [Google Scholar] [CrossRef] [Green Version]
- Potapov, P.; Li, X.; Hernandez-Serna, A.; Tyukavina, A.; Hansen, M.C.; Kommareddy, A.; Pickens, A.; Turubanova, S.; Tang, H.; Silva, C.E.; et al. Mapping and monitoring global forest canopy height through integration of GEDI and Landsat data. Remote Sens. Environ. 2020, 253, 112165. [Google Scholar] [CrossRef]
- Turubanova, S.; Potapov, P.V.; Tyukavina, A.; Hansen, M.C. Ongoing primary forest loss in Brazil, Democratic Republic of the Congo, and Indonesia. Environ. Res. Lett. 2018, 13, 074028. [Google Scholar] [CrossRef] [Green Version]
- Bergeron, Y.; Fenton, N.J. Boreal forests of eastern Canada revisited: Old growth, nonfire disturbances, forest succession, and biodiversity. Botany 2012, 90, 509–523. [Google Scholar] [CrossRef]
- Martin, M.; Morin, H.; Fenton, N.J. Secondary disturbances of low and moderate severity drive the dynamics of eastern Canadian boreal old-growth forests. Ann. For. Sci. 2019, 76, 108. [Google Scholar] [CrossRef]
- Senf, C.; Pflugmacher, D.; Hostert, P.; Seidl, R. Using Landsat time series for characterizing forest disturbance dynamics in the coupled human and natural systems of Central Europe. ISPRS J. Photogramm. Remote Sens. 2017, 130, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Levin, S.A.; Grenfell, B.; Hastings, A.; Perelson, A.S. Mathematical and computational challenges in population biology and ecosystems science. Science 1997, 275, 334–343. [Google Scholar] [CrossRef] [Green Version]
- Carnahan, J.A. Mapping Australia’s vegetation—200 years of change. Cartography 1990, 19, 1–6. [Google Scholar] [CrossRef]
- Gauthier, S.; De Grandpré, L.; Bergeron, Y. Differences in forest composition in two boreal forest ecoregions of Quebec. J. Veg. Sci. 2000, 11, 781–790. [Google Scholar] [CrossRef]
- Chávez, V.; Macdonald, S.E. The influence of canopy patch mosaics on understory plant community composition in boreal mixedwood forest. For. Ecol. Manag. 2010, 259, 1067–1075. [Google Scholar] [CrossRef]
- Grimm, V.; Wissel, C. Babel, or the ecological stability discussions: An inventory and analysis of terminology and a guide for avoiding confusion. Oecologia 1997, 109, 323–334. [Google Scholar] [CrossRef]
- Hughes, J.B.; Ives, A.R.; Norberg, J. Do species interactions buffer environmental variation (in theory)? In Biodiversity and Ecosystem Functioning. Synthesis and Perspectives; Loreau, M., Naeem, S., Inchausti, P., Eds.; Oxford University Press: Oxford, UK, 2002; pp. 92–101. [Google Scholar]
- Loreau, M.; Downing, A.; Emmerson, M.; Gonzalez, A.; Hughes, J.; Inchausti, P.; Joshi, J.; Norberg, J.; Sala, O. A new look at the relationship between diversity and stability. In Biodiversity and Ecosystem Functioning. Synthesis and Perspectives; Loreau, M., Naeem, S., Inchausti, P., Eds.; Oxford University Press: Oxford, UK, 2002; pp. 79–91. [Google Scholar]
- Roderick, M.L.; Berry, S.L.; Noble, I.R. A framework for understanding the linkage between environment and vegetation based on the surface area to volume ratio of leaves. Funct. Ecol. 2000, 14, 423–437. [Google Scholar] [CrossRef]
- Migliavacca, M.; Musavi, T.; Mahecha, M.D.; Nelson, J.A.; Knauer, J.; Baldocchi, D.D.; Perez-Priego, O.; Christiansen, R.; Peters, J.; Anderson, K.; et al. The three major axes of terrestrial ecosystem function. Nature 2021, 598, 468–472. [Google Scholar] [CrossRef]
- Sellers, P.J. Canopy reflectance, photosynthesis and transpiration. Int. J. Remote Sens. 1985, 6, 1335–1372. [Google Scholar] [CrossRef]
- Berry, S.; Mackey, B.; Brown, T. Potential applications of remotely sensed vegetation greenness to habitat analysis and the conservation of dispersive fauna. Pac. Conserv. Biol. 2007, 13, 120–127. [Google Scholar] [CrossRef]
- Tucker, C.J.; Sellers, P.J. Satellite remote sensing of primary production. Int. J. Remote Sens. 1986, 7, 1395–1416. [Google Scholar] [CrossRef]
- Prince, S.D. A model of regional primary production for use with course resolution satellite data. Int. J. Remote Sens. 1991, 12, 1313–1330. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Estimation of vegetation water content and photosynthetic tissue area from spectral reflectance: A comparison of indices based on liquid water and chlorophyll absorption features. Remote Sens. Environ. 2003, 84, 526–537. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Save, R. The reflectance at the 950–970 nm region as an indicator of plant water status. Int. J. Remote Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Potapov, P.; Hansen, M.C.; Laestadius, L.; Turubanova, S.; Yaroshenko, A.; Thies, C.; Smith, W.; Zhuravleva, I.; Komarova, A.; Minnemeyer, S.; et al. The last frontiers of wilderness: Tracking loss of intact forest landscapes from 2000 to 2013. Sci. Adv. 2017, 3, e1600821. [Google Scholar] [CrossRef] [Green Version]
- Achard, F.; Mollicone, D.; Stibig, H.J.; Aksenov, D.; Laestadius, L.; Li, Z.; Potapov, P.; Yaroshenko, A. Areas of rapid forest-cover change in boreal Eurasia. For. Ecol. Manag. 2006, 237, 322–334. [Google Scholar] [CrossRef]
- Cochrane, M.A.; Laurance, W.F. Synergisms among fire, land use, and climate change in the Amazon. AMBIO J. Hum. Environ. 2008, 37, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, M.A.; Laurance, W.F. Fire as a large-scale edge effect in Amazonian forests. J. Trop. Ecol. 2002, 18, 311–325. [Google Scholar] [CrossRef] [Green Version]
- Cochrane, M.A. Fire science for rainforests. Nature 2003, 421, 913–919. [Google Scholar] [CrossRef]
- Achard, F.; Eva, H.D.; Mollicone, D.; Beuchle, R. The effect of climate anomalies and human ignition factor on wildfires in Russian boreal forests. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 2331–2339. [Google Scholar] [CrossRef] [Green Version]
- Kukavskaya, E.A.; Buryak, L.V.; Ivanova, G.; Conard, S.; Kalenskaya, O.; Zhila, S.V.; McRae, D.J. Influence of logging on the effects of wildfire in Siberia. Environ. Res. Lett. 2013, 8, 045034. [Google Scholar] [CrossRef]
- Matricardi, E.A.T.; Skole, D.L.; Costa, O.B.; Pedlowski, M.A.; Samek, J.H.; Miguel, E.P. Long-term forest degradation surpasses deforestation in the Brazilian Amazon. Science 2020, 369, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Garrett, R.D.; Cammelli, F.; Ferreira, J.; Levy, S.A.; Valentim, J.; Vieira, I. Forests and Sustainable Development in the Brazilian Amazon: History, Trends, and Future Prospects. Annu. Rev. Environ. Resour. 2021, 46, 625–652. [Google Scholar] [CrossRef]
- Shvetsov, E.G.; Kukavskaya, E.A.; Shestakova, T.A.; Laflamme, J.; Rogers, B.M. Increasing fire and logging disturbances in Siberian boreal forests: A case study of the Angara region. Environ. Res. Lett. 2021, 16, 115007. [Google Scholar] [CrossRef]
- Alexeyev, V.A.; Birdsey, R.A. Carbon Storage in Forests and Peatlands of Russia; General Technical Report NE-244; USDA Forest Service: Radnor, PA, USA, 1998. [Google Scholar] [CrossRef]
- Soja, A.J.; Cofer, W.R.; Shugart, H.H.; Sukhinin, A.I.; Stackhouse, P.W., Jr.; McRae, D.J.; Conard, S.G. Estimating fire emissions and disparities in boreal Siberia (1998–2002). J. Geophys. Res. 2004, 109, D14S06. [Google Scholar] [CrossRef]
- Myneni, R.; Knyazikhin, Y.; Park, T. MCD15A3H MODIS/Terra + Aqua Leaf Area Index/FPAR 4-Day L4 Global 500 m SIN Grid V006 [Data Set]; NASA EOSDIS LP DAAC: Sioux Falls, SD, USA, 2015. [Google Scholar] [CrossRef]
- Fensholt, R.; Sandholt, I. Derivation of a shortwave infrared water stress index from MODIS near- and shortwave infrared data in a semiarid environment. Remote Sens. Environ. 2003, 87, 111–121. [Google Scholar] [CrossRef]
- Briant, G.; Gond, V.; Laurance, S.G.W. Habitat fragmentation and the desiccation of forest canopies: A case study from eastern Amazonia. Biol. Conserv. 2010, 143, 2763–2769. [Google Scholar] [CrossRef]
- Friedl, M.; Sulla-Menashe, D. MCD12Q1 MODIS/Terra + Aqua Land Cover Type Yearly L3 Global 500 m SIN Grid V006 [Data Set]; NASA EOSDIS LP DAAC: Sioux Falls, SD, USA, 2019. [Google Scholar] [CrossRef]
- Enderle, D.I.M.; Weih, R.C., Jr. Integrating supervised and unsupervised classification methods to develop a more accurate land cover classification. J. Ark. Acad. Sci. 2005, 59, 10. [Google Scholar]
- Ward, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Mackey, B.; Berry, S.; Hugh, S.; Ferrier, S.; Harwood, T.D.; Williams, K.J. Ecosystem greenspots: Identifying potential drought, fire, and climate-change micro-refuges. Ecol. Appl. 2012, 22, 1852–1864. [Google Scholar] [CrossRef] [Green Version]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.r-project.org (accessed on 13 July 2022).
- Stehman, S.V.; Czaplewski, R.L. Design and analysis for thematic map accuracy assessment: Fundamental principles. Remote Sens. Environ. 1998, 64, 331–344. [Google Scholar] [CrossRef]
- Hislop, S.; Haywood, A.; Alaibakhsh, M.; Nguyen, T.H.; Soto-Berelov, M.; Jones, S.; Stone, C. A reference data framework for the application of satellite time series to monitor forest disturbance. Int. J. Appl. Earth Obs. Geoinf. 2021, 105, 102636. [Google Scholar] [CrossRef]
- INPE (Instituto Nacional de Pesquisas Espaciais). Projeto PRODES: Monitoramento da Floresta Amazônica Brasileira por Satélite [Data Set]; INPE: São José dos Campos, Brasil, 2019; Available online: http://www.obt.inpe.br/prodes (accessed on 19 November 2021).
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-resolution global maps of 21st-century forest cover change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef] [Green Version]
- Packham, J.R.; Harding, D.J.L.; Hilton, G.M.; Stuttard, R.A. Functional Ecology of Woodlands and Forests; Chapman and Hall: London, UK, 1992. [Google Scholar]
- Larsen, J.B. Ecological stability of forests and sustainable silviculture. For. Ecol. Manag. 1994, 73, 85–96. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization). Global Forest Resources Assessment 2020: Main Report; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Mackey, B.; Skinner, E.; Norman, P. A Review of Current Reporting and Available Data on Primary Forest and Potential Methods for Country-Level Assessment; FAO: Rome, Italy, 2020; Available online: http://assets.fsnforum.fao.org.s3-eu-west-1.amazonaws.com/public/files/163_Primary_Forests/DRAFT_Primary_Forest_Report_12Feb20.pdf (accessed on 13 July 2022).
- Venter, O.; Sanderson, E.W.; Magrach, A.; Allan, J.R.; Beher, J.; Jones, K.R.; Possingham, H.P.; Laurance, W.F.; Wood, P.; Fekete, B.M.; et al. Global terrestrial Human Footprint maps for 1993 and 2009. Sci. Data 2016, 3, 160067. [Google Scholar] [CrossRef] [Green Version]
- Ibisch, P.L.; Hoffmann, M.T.; Kreft, S.; Pe′Er, G.; Kati, V.; Biber-Freudenberger, L.; DellaSala, D.A.; Vale, M.M.; Hobson, P.R.; Selva, N. A global map of roadless areas and their conservation status. Science 2016, 354, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Beyer, H.L.; Venter, O.; Grantham, H.S.; Watson, J.E.M. Substantial losses in ecoregion intactness highlight urgency of globally coordinated action. Conserv. Lett. 2020, 13, e12692. [Google Scholar] [CrossRef]
- Grantham, H.S.; Duncan, A.; Evans, T.D.; Jones, K.; Beyer, H.; Schuster, R.; Walston, J.; Ray, J.; Robinson, J.; Callow, M.; et al. Modification of forests by people means only 40% of remaining forests have high ecosystem integrity. bioRxiv 2020. [Google Scholar] [CrossRef]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef] [Green Version]
- Tyukavina, A.; Hansen, M.C.; Potapov, P.V.; Krylov, A.M.; Goetz, S.J. Pan-tropical hinterland forests: Mapping minimally disturbed forests. Glob. Ecol. Biogeogr. 2016, 25, 151–163. [Google Scholar] [CrossRef]
- Hansen, A.; Barnett, K.; Jantz, P.; Phillips, L.; Goetz, S.J.; Hansen, M.C.; Venter, O.; Watson, J.E.M.; Burns, P.; Atkinson, S.; et al. Global humid tropics forest structural condition and forest structural integrity maps. Sci. Data 2019, 6, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, A.J.; Burns, P.; Ervin, J.; Goetz, S.J.; Hansen, M.; Venter, O.; Watson, J.E.M.; Jantz, P.A.; Virnig, A.L.S.; Barnett, K.; et al. A policy-driven framework for conserving the best of Earth’s remaining moist tropical forests. Nat. Ecol. Evol. 2020, 4, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Tropek, R.; Sedláček, O.; Beck, J.; Keil, P.; Musilová, Z.; Šímová, I.; Storch, D. Comment on “High-resolution global maps of 21st-century forest cover change”. Science 2014, 344, 981. [Google Scholar] [CrossRef] [Green Version]
- Laurance, W.F.; Laurance, S.G.; Ferreira, L.V.; RankindeMerona, J.M.; Gascon, C.; Lovejoy, T.E. Biomass collapse in Amazonian forest fragments. Science 1997, 278, 1117–1118. [Google Scholar] [CrossRef]
- Chaplin-Kramer, R.; Ramler, I.; Sharp, R.; Haddad, N.M.; Gerber, J.S.; West, P.C.; Mandle, L.; Engstrom, P.; Baccini, A.; Sim, S.; et al. Degradation in carbon stocks near tropical forest edges. Nat. Commun. 2015, 6, 10158. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Brancalion, P.H.S.; Laestadius, L.; Bennett-Curry, A.; Buckingham, K.; Kumar, C.; Moll-Rocek, J.; Vieira, I.C.G.; Wilson, S.J. When is a forest a forest? Forest concepts and definitions in the era of forest and landscape restoration. Ambio 2016, 45, 538–550. [Google Scholar] [CrossRef]
- Hernández-Ruedas, M.A.; Arroyo-Rodríguez, V.; Meave, J.A.; Martínez-Ramos, M.; Ibarra-Manríquez, G.; Martínez, E.; Jamangapé, G.; Melo, F.P.L.; Santos, B.A. Conserving tropical tree diversity and forest structure: The value of small rainforest patches in moderately-managed landscapes. PLoS ONE 2014, 9, e98931. [Google Scholar] [CrossRef] [Green Version]
- Le Roux, D.S.; Ikin, K.; Lindenmayer, D.B.; Manning, A.D.; Gibbons, P. Single large or several small? Applying biogeographic principles to tree-level conservation and biodiversity offsets. Biol. Conserv. 2015, 191, 558–566. [Google Scholar] [CrossRef]
- Tulloch, A.I.T.; Barnes, M.D.; Ringma, J.; Fuller, R.A.; Watson, J.E.M. Understanding the importance of small patches of habitat for conservation. J. Appl. Ecol. 2016, 53, 418–429. [Google Scholar] [CrossRef] [Green Version]
- Häkkilä, M.; Savilaakso, S.; Johansson, A.; Sandgren, T.; Uusitalo, A.; Mönkkönen, M.; Puttonen, P. Do small protected habitat patches within boreal production forests provide value for biodiversity conservation? A systematic review protocol. Environ. Evid. 2019, 8, 30. [Google Scholar] [CrossRef]
- Wintle, B.A.; Kujala, H.; Whitehead, A.; Cameron, A.; Veloz, S.; Kukkala, A.; Moilanen, A.; Gordon, A.; Lentini, P.E.; Cadenhead, N.C.R.; et al. Global synthesis of conservation studies reveals the importance of small habitat patches for biodiversity. Proc. Natl. Acad. Sci. USA 2019, 116, 909–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boucher, Y.; St-Laurent, M.H.; Grondin, P. Logging-induced edge and configuration of old-growth forest remnants in the eastern North American boreal forests. Nat. Areas J. 2011, 31, 300–306. [Google Scholar] [CrossRef]
- Dantas De Paula, M.; Groeneveld, J.; Huth, A. The extent of edge effects in fragmented landscapes: Insights from satellite measurements of tree cover. Ecol. Indic. 2016, 69, 196–204. [Google Scholar] [CrossRef]
- De Frenne, P.; Lenoir, J.; Luoto, M.; Scheffers, B.R.; Zellweger, F.; Aalto, J.; Ashcroft, M.B.; Christiansen, D.M.; Decocq, G.; De Pauw, K.; et al. Forest microclimates and climate change: Importance, drivers and future research agenda. Glob. Chang. Biol. 2021, 27, 2279–2297. [Google Scholar] [CrossRef]
- Sims, R.A.; Mackey, B.G.; Baldwin, K.A. Stand and landscape level applications of a forest ecosystem classification for Northwestern Ontario, Canada. Ann. For. Sci. 1995, 52, 573–588. [Google Scholar] [CrossRef] [Green Version]
- Ibáñez, I.; Katz, D.S.; Peltier, D.; Wolf, S.M.; Connor Barrie, B.T. Assessing the integrated effects of landscape fragmentation on plants and plant communities: The challenge of multiprocess-multiresponse dynamics. J. Ecol. 2014, 102, 882–895. [Google Scholar] [CrossRef] [Green Version]
- Kay, J.J.; Regier, H.A. Uncertainty, complexity, and ecological integrity: Insights from an ecosystem approach. In Implementing Ecological Integrity; Crabbé, P., Holland, A., Ryszkowski, L., Westra, L., Eds.; Springer: Dordrecht, the Netherlands, 2000; pp. 121–156. [Google Scholar] [CrossRef]
- Specht, R.L. Vegetation. In The Australian Environment, 4th ed.; Leeper, G.W., Ed.; CSIRO and Melbourne University Press: Melbourne, Australia, 1970; pp. 44–67. [Google Scholar]
- Nelson, B.W.; Kapos, V.; Adams, J.B.; Oliveira, W.J.; Braun, O.P.G. Forest disturbance by large blowdowns in the Brazilian Amazon. Ecology 1994, 75, 853–858. [Google Scholar] [CrossRef]
- Ibanez, T.; Keppel, G.; Menkes, C.; Gillespie, T.W.; Lengaigne, M.; Mangeas, M.; Rivas-Torres, G.; Birnbaum, P. Globally consistent impact of tropical cyclones on the structure of tropical and subtropical forests. J. Ecol. 2019, 107, 279–292. [Google Scholar] [CrossRef]
- Robbins, K.R.; Saxton, A.M.; Southern, L.L. Estimation of nutrient requirements using broken-line regression analysis. J. Anim. Sci. 2006, 84, E155–E165. [Google Scholar] [CrossRef]
- Ma, L.; Li, M.; Ma, X.; Cheng, L.; Du, P.; Liu, Y. A review of supervised object-based land-cover image classification. ISPRS J. Photogramm. Remote Sens. 2017, 130, 277–293. [Google Scholar] [CrossRef]
- Boucher, D.; De Grandpré, L.; Kneeshaw, D.; St-Onge, B.; Ruel, J.C.; Waldron, K.; Lussier, J.M. Effects of 80 years of forest management on landscape structure and pattern in the eastern Canadian boreal forest. Landsc. Ecol. 2015, 30, 1913–1929. [Google Scholar] [CrossRef]
- Jentsch, A.; Beierkuhnlein, C.; White, P.S. Scale, the dynamic stability of forest ecosystems, and the persistence of biodiversity. Silva. Fenn. 2002, 36, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Wulder, M.A.; Hall, R.J.; Coops, N.C.; Franklin, S.E. High spatial resolution remotely sensed data for ecosystem characterization. Bioscience 2004, 54, 511–521. [Google Scholar] [CrossRef] [Green Version]
- Mackey, B.; Watson, J.; Worboys, G.L. Connectivity Conservation and the Great Eastern Ranges Corridor: An Independent Report to the Interstate Agency Working Group (Alps to Atherton Connectivity Conservation Working Group) Convened under the Environment Heritage and Protection Council/Natural Resource Management Ministerial Council; Department of Environment, Climate Change and Water NSW: Sydney, Australia, 2010; Available online: https://conservationcorridor.org/cpb/Mackey_et_al_2010.pdf (accessed on 13 July 2022).
- Griscom, B.W.; Adams, J.; Ellis, P.W.; Houghton, R.A.; Lomax, G.; Miteva, D.A.; Schlesinger, W.H.; Shoch, D.; Siikamäki, J.V.; Smith, P.; et al. Natural climate solutions. Proc. Natl. Acad. Sci. USA 2017, 114, 11645–11650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IPCC (Intergovernmental Panel on Climate Change). Summary for Policymakers. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Moomaw, W.R.; Masino, S.A.; Faison, E.K. Intact forests in the United States: Proforestation mitigates climate change and serves the greatest good. Front. For. Glob. Chang. 2019, 2, 27. [Google Scholar] [CrossRef] [Green Version]
- Roe, S.; Streck, C.; Obersteiner, M.; Frank, S.; Griscom, B.; Drouet, L.; Fricko, O.; Gusti, M.; Harris, N.; Hasegawa, T.; et al. Contribution of the land sector to a 1.5 °C world. Nat. Clim. Chang. 2019, 9, 817–828. [Google Scholar] [CrossRef]
- Cook-Patton, S.C.; Leavitt, S.M.; Gibbs, D.; Harris, N.L.; Lister, K.; Anderson-Teixeira, K.J.; Briggs, R.D.; Chazdon, R.L.; Crowther, T.W.; Ellis, P.W.; et al. Mapping carbon accumulation potential from global natural forest regrowth. Nature 2020, 585, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Dorren, L.K.A.; Berger, F.; Imeson, A.C.; Maier, B.; Rey, F. Integrity, stability and management of protection forests in the European Alps. For. Ecol. Manag. 2004, 195, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Lindenmayer, D.B.; Westgate, M.J.; Scheele, B.C.; Foster, C.N.; Blair, D.P. Key perspectives on early successional forests subject to stand-replacing disturbances. For. Ecol. Manag. 2019, 454, 117656. [Google Scholar] [CrossRef]
| fPAR * | SIWSI | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Min | CV | Slope (abs) | Mean | Max | CV | Slope (abs) | |
| Southern taiga ecoregion (Russia) | ||||||||
| HS | 86.77 ± 2.40 | 81.41 ± 3.12 | 0.03 ± 0.01 | 0.11 ± 0.09 | −0.35 ± 0.03 | −0.28 ± 0.05 | 0.05 ± 0.02 | 0.002 ± 0.002 |
| LS | 79.42 ± 6.01 | 72.67 ± 6.33 | 0.04 ± 0.01 | 0.24 ± 0.16 | −0.29 ± 0.05 | −0.18 ± 0.06 | 0.07 ± 0.02 | 0.003 ± 0.003 |
| NF | 72.65 ± 8.78 | 60.53 ± 10.73 | 0.08 ± 0.03 | 0.61 ± 0.42 | −0.21 ± 0.07 | −0.05 ± 0.10 | 0.10 ± 0.03 | 0.008 ± 0.006 |
| Kayapó territory (Brazil) | ||||||||
| HS | 89.83 ± 0.26 | 89.03 ± 0.75 | 0.004 ± 0.002 | 0.56 ± 0.51 | −0.35 ± 0.02 | −0.33 ± 0.02 | −0.03 ± 0.01 | 0.014 ± 0.012 |
| LS | 87.82 ± 2.01 | 84.30 ± 5.27 | 0.02 ± 0.02 | 3.48 ± 6.53 | −0.26 ± 0.07 | −0.17 ± 0.10 | −0.22 ± 0.23 | 0.077 ± 0.084 |
| NF | 65.15 ± 8.14 | 56.62 ± 8.72 | 0.08 ± 0.03 | 17.36 ± 20.43 | −0.09 ± 0.09 | −0.01 ± 0.10 | −0.64 ± 1.35 | 0.178 ± 0.206 |
| Classified Data | Reference Data | User’s Accuracy (%) | Producer’s Accuracy (%) | Overall Accuracy (%) | ||
|---|---|---|---|---|---|---|
| HS | NF | Total | ||||
| Southern taiga ecoregion (Russia) | ||||||
| HS | 381 | 3 | 384 | 99 | 91 | 92 |
| NF | 38 | 123 | 161 | 76 | 98 | |
| Total | 419 | 126 | 545 | |||
| Kayapó territory (Brazil) | ||||||
| HS | 251 | 2 | 253 | 99 | 100 | 99 |
| NF | 1 | 66 | 67 | 99 | 97 | |
| Total | 252 | 68 | 320 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shestakova, T.A.; Mackey, B.; Hugh, S.; Dean, J.; Kukavskaya, E.A.; Laflamme, J.; Shvetsov, E.G.; Rogers, B.M. Mapping Forest Stability within Major Biomes Using Canopy Indices Derived from MODIS Time Series. Remote Sens. 2022, 14, 3813. https://doi.org/10.3390/rs14153813
Shestakova TA, Mackey B, Hugh S, Dean J, Kukavskaya EA, Laflamme J, Shvetsov EG, Rogers BM. Mapping Forest Stability within Major Biomes Using Canopy Indices Derived from MODIS Time Series. Remote Sensing. 2022; 14(15):3813. https://doi.org/10.3390/rs14153813
Chicago/Turabian StyleShestakova, Tatiana A., Brendan Mackey, Sonia Hugh, Jackie Dean, Elena A. Kukavskaya, Jocelyne Laflamme, Evgeny G. Shvetsov, and Brendan M. Rogers. 2022. "Mapping Forest Stability within Major Biomes Using Canopy Indices Derived from MODIS Time Series" Remote Sensing 14, no. 15: 3813. https://doi.org/10.3390/rs14153813
APA StyleShestakova, T. A., Mackey, B., Hugh, S., Dean, J., Kukavskaya, E. A., Laflamme, J., Shvetsov, E. G., & Rogers, B. M. (2022). Mapping Forest Stability within Major Biomes Using Canopy Indices Derived from MODIS Time Series. Remote Sensing, 14(15), 3813. https://doi.org/10.3390/rs14153813








