Laboratory Evaluation of the (355, 532) nm Particle Depolarization Ratio of Pure Pollen at 180.0° Lidar Backscattering Angle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lidar Particle Depolarization Ratio as Derived from Scattering Matrix Formalism
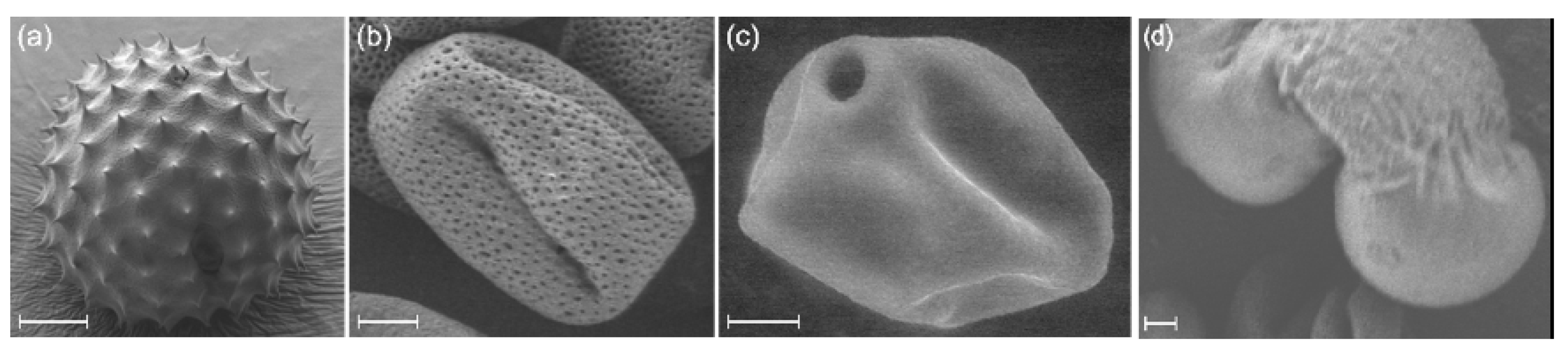
2.2. Pollen Samples
- Ragweed (Ambrosia artemisiifolia)
- Ash (Fraxinus americana)
- Birch (Betula pendula)
- Pine (Pinus strobus)
- Cypress (Cupressus sempervirens)
- Spruce (Picea abies)
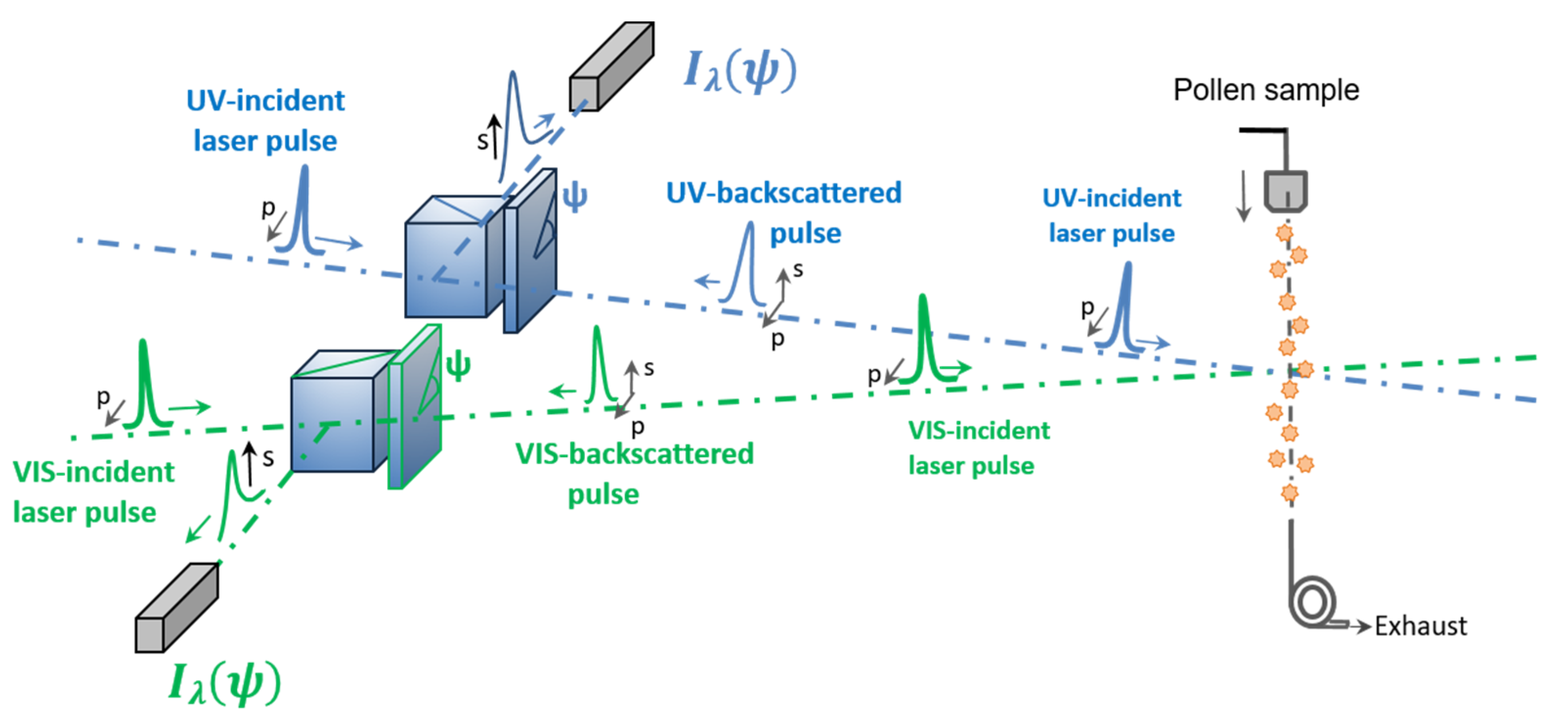
2.3. Lidar PDR Retrieval with a Laboratory π-Polarimeter at Lidar Backscattering Angle of 180.0°
2.3.1. Laboratory π-Polarimeter at Lidar Exact Backscattering Angle of 180.0°
2.3.2. Detected Backscattered Light Intensity and Lidar PDR Retrieval Methodology
3. Results and Discussion
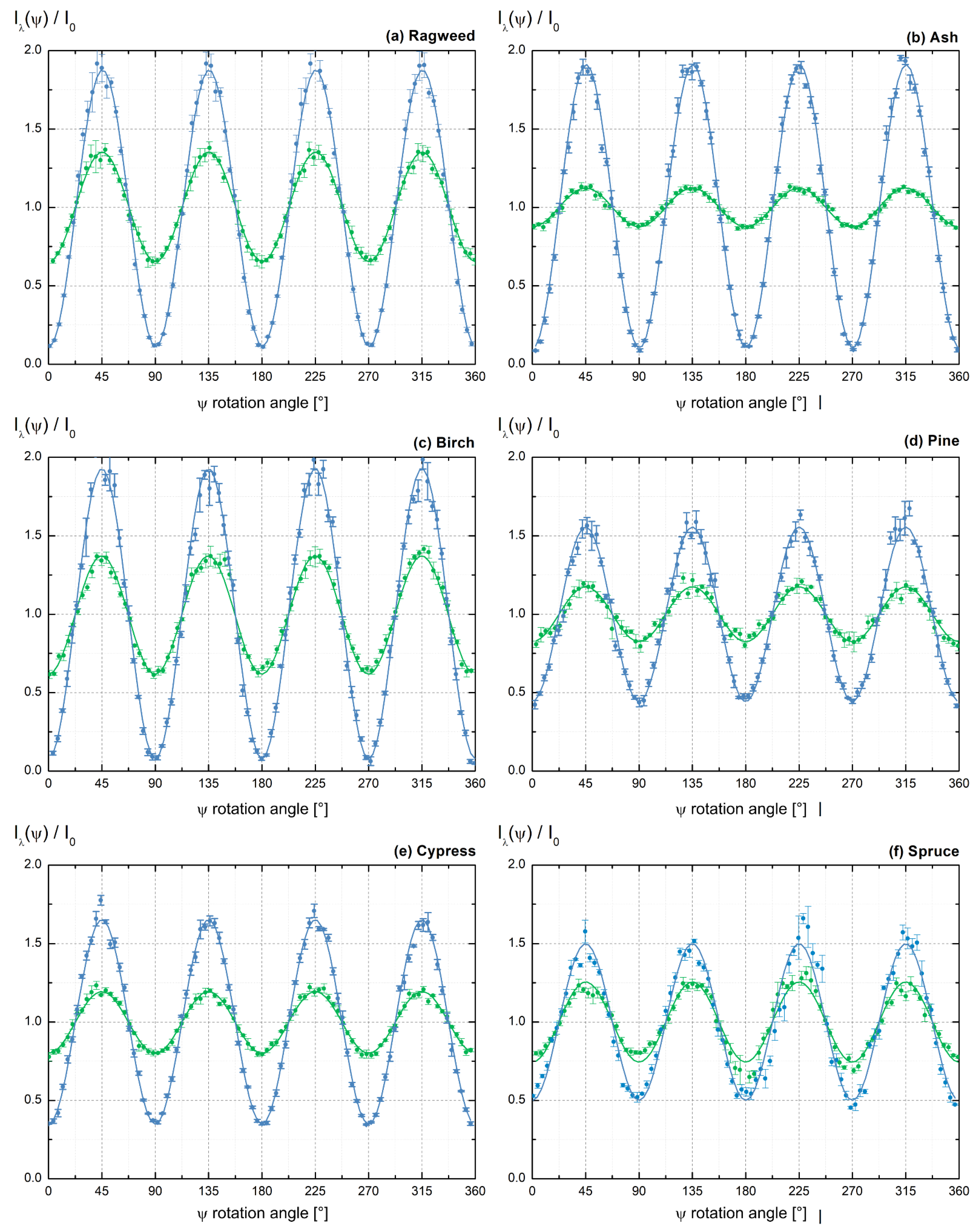
3.1. Light Backscattering Acquisition Curves
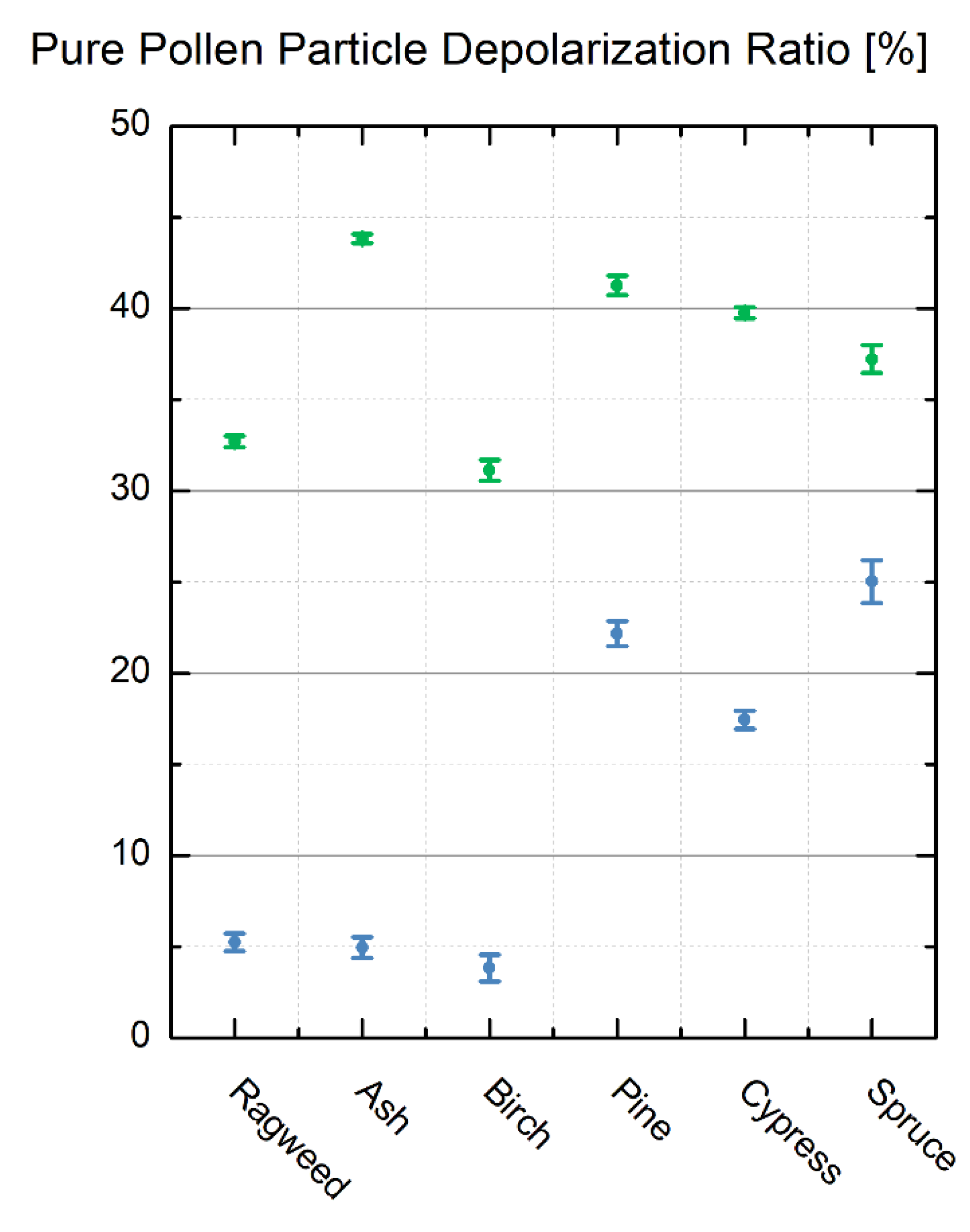
3.2. Lidar PDR-Retrieval for Pure Pollen at 355 and 532 nm Wavelength
3.3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Ragweed Pollen
Appendix A.2. Ash Pollen
Appendix A.3. Birch Pollen
Appendix A.4. Pine Pollen
Appendix A.5. Cypress Pollen
Appendix A.6. Spruce Pollen
References
- Schaffner, U.; Steinbach, S.; Sun, Y.; Skjøth, C.A.; de Weger, L.A.; Lommen, S.T.; Augustinus, B.A.; Bonini, M.; Karrer, G.; Šikoparija, B.; et al. Biological weed control to relieve millions from Ambrosia allergies in Europe. Nat. Commun. 2020, 11, 1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lake, I.R.; Jones, N.R.; Agnew, M.; Goodess, C.M.; Giorgi, F.; Hamaoui-Laguel, L.; Semenov, M.A.; Solomon, F.; Storkey, J.; Vautard, R.; et al. Climate Change and Future Pollen Allergy in Europe. Environ. Health Perspect. 2017, 125, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Steiner, A.; Solmon, F. Investigating the direct climatic forcing of pollen and subpollen particles. NASA/ADS 2013, 2013, A11B-0020. [Google Scholar]
- Spänkuch, D.; Döhler, W.; Güldner, J. Effect of coarse biogenic aerosol on downwelling infrared flux at the surface. J. Geophys. Res. Atmos. 2000, 105, 17341–17350. [Google Scholar] [CrossRef]
- Pope, F.D. Pollen grains are efficient cloud condensation nuclei. Environ. Res. Lett. 2010, 5, 044015. [Google Scholar] [CrossRef]
- Sam, S.; Halbritter, H.; Heigl, H. PalDat-A Palynological Database. Available online: https://www.paldat.org/pub/Ambrosia_artemisiifolia/304617 (accessed on 21 July 2021).
- Halbritter, H.; Ulrich, S.; Grímsson, F.; Weber, M.; Zetter, R.; Hesse, M.; Buchner, R.; Svojtka, M.; Frosch-Radivo, A. Illustrated Pollen Terminology; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-71364-9. [Google Scholar]
- Liu, C.; Yin, Y. Inherent optical properties of pollen particles: A case study for the morning glory pollen. Opt. Express 2016, 24, A104. [Google Scholar] [CrossRef]
- Thibaudon, M.; Šikoparija, B.; Oliver, G.; Smith, M.; Skjøth, C.A. Ragweed pollen source inventory for France–The second largest centre of Ambrosia in Europe. Atmos. Environ. 2014, 83, 62–71. [Google Scholar] [CrossRef]
- Szczepanek, K.; Myszkowska, D.; Worobiec, E.; Piotrowicz, K.; Ziemianin, M.; Bielec-Bąkowska, Z. The long-range transport of Pinaceae pollen: An example in Kraków (Southern Poland). Aerobiologia 2017, 33, 109–125. [Google Scholar] [CrossRef] [Green Version]
- Sofiev, M.; Belmonte, J.; Gehrig, R.; Izquierdo, R.; Smith, M.; Dahl, Å.; Siljamo, P. Airborne Pollen Transport. In Allergenic Pollen; Sofiev, M., Bergmann, K.-C., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 127–159. ISBN 978-94-007-4880-4. [Google Scholar]
- Makra, L.; Matyasovszky, I.; Thibaudon, M.; Bonini, M. Forecasting ragweed pollen characteristics with nonparametric regression methods over the most polluted areas in Europe. Int. J. Biometeorol. 2011, 55, 361–371. [Google Scholar] [CrossRef]
- Zink, K.; Kaufmann, P.; Petitpierre, B.; Broennimann, O.; Guisan, A.; Gentilini, E.; Rotach, M.W. Numerical ragweed pollen forecasts using different source maps: A comparison for France. Int. J. Biometeorol. 2017, 61, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Sassen, K. Boreal tree pollen sensed by polarization lidar: Depolarizing biogenic chaff. Geophys. Res. Lett. 2008, 35, 18. [Google Scholar] [CrossRef]
- Noh, Y.M.; Müller, D.; Lee, H.; Choi, T.J. Influence of biogenic pollen on optical properties of atmospheric aerosols observed by lidar over Gwangju, South Korea. Atmos. Environ. 2013, 69, 139–147. [Google Scholar] [CrossRef]
- Veselovskii, I.; Hu, Q.; Goloub, P.; Podvin, T.; Choël, M.; Visez, N.; Korenskiy, M. Mie–Raman–fluorescence lidar observations of aerosols during pollen season in the north of France. Atmos. Meas. Tech. 2021, 14, 4773–4786. [Google Scholar] [CrossRef]
- Kiselev, D.; Bonacina, L.; Wolf, J.-P. A flash-lamp based device for fluorescence detection and identification of individual pollen grains. Rev. Sci. Instrum. 2013, 84, 033302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miffre, A.; David, G.; Thomas, B.; Rairoux, P. Atmospheric non-spherical particles optical properties from UV-polarization lidar and scattering matrix. Geophys. Res. Lett. 2011, 38, L16804. [Google Scholar] [CrossRef]
- Sicard, M.; Izquierdo, R.; Alarcón, M.; Belmonte, J.; Comerón, A.; Baldasano, J.M. Near-surface and columnar measurements with a micro pulse lidar of atmospheric pollen in Barcelona, Spain. Atmos. Chem. Phys. 2016, 16, 6805–6821. [Google Scholar] [CrossRef] [Green Version]
- Shang, X.; Giannakaki, E.; Bohlmann, S.; Filioglou, M.; Saarto, A.; Ruuskanen, A.; Leskinen, A.; Romakkaniemi, S.; Komppula, M. Optical characterization of pure pollen types using a multi-wavelength Raman polarization lidar. Atmos. Chem. Phys. 2020, 20, 15323–15339. [Google Scholar] [CrossRef]
- Bohlmann, S.; Shang, X.; Giannakaki, E.; Filioglou, M.; Saarto, A.; Romakkaniemi, S.; Komppula, M. Detection and characterization of birch pollen in the atmosphere using multi-wavelength Raman lidar in Finland. Atmos. Chem. Phys. Discuss. 2019, 19, 14559–14569. [Google Scholar] [CrossRef] [Green Version]
- Tesche, M.; Ansmann, A.; Müller, D.; Althausen, D.; Engelmann, R.; Freudenthaler, V.; Groß, S. Vertically resolved separation of dust and smoke over Cape Verde using multiwavelength Raman and polarization lidars during Saharan Mineral Dust Experiment 2008. J. Geophys. Res. 2009, 114, 13. [Google Scholar] [CrossRef]
- David, G.; Thomas, B.; Nousiainen, T.; Miffre, A.; Rairoux, P. Retrieving simulated volcanic, desert dust and sea-salt particle properties from two/three-component particle mixtures using UV-VIS polarization lidar and T matrix. Atmos. Chem. Phys. 2013, 13, 6757–6776. [Google Scholar] [CrossRef] [Green Version]
- Haarig, M.; Ansmann, A.; Engelmann, R.; Baars, H.; Toledano, C.; Torres, B.; Althausen, D.; Radenz, M.; Wandinger, U. First triple-wavelength lidar observations of depolarization and extinction-to-backscatter ratios of Saharan dust. Atmos. Chem. Phys. 2022, 22, 355–369. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; Wiley-VCH: Weinheim, German, 1983; ISBN 978-0-471-29340-8. [Google Scholar]
- Mishchenko, M.I.; Travis, L.D.; Lacis, A.A. Scattering, Absorption, and Emission of Light by Small Particles; Cambridge University Press: Cambridge, UK, 2002; ISBN 978-0-521-78252-4. [Google Scholar]
- Cholleton, D.; Bialic, É.; Dumas, A.; Kaluzny, P.; Rairoux, P.; Miffre, A. Laboratory evaluation of the scattering matrix of ragweed, ash, birch and pine pollen towards pollen classification. Atmos. Meas. Tech. 2022, 15, 1021–1032. [Google Scholar] [CrossRef]
- David, G.; Thomas, B.; Coillet, E.; Miffre, A.; Rairoux, P. Polarization-resolved exact light backscattering by an ensemble of particles in air. Opt. Express 2013, 21, 18624. [Google Scholar] [CrossRef] [PubMed]
- Miffre, A.; Cholleton, D.; Mehri, T.; Rairoux, P. Remote Sensing Observation of New Particle Formation Events with a (UV, VIS) Polarization Lidar. Remote Sens. 2019, 11, 1761. [Google Scholar] [CrossRef] [Green Version]
- Cholleton, D.; Bialic, E.; Dumas, A.; Kaluzny, P.; Rairoux, P.; Miffre, A. Laboratory evaluation of the (VIS, IR) scattering matrix of complex-shaped ragweed pollen particles. J. Quant. Spectrosc. Radiat. Transf. 2020, 254, 107223. [Google Scholar] [CrossRef]
- Heslop-Harrison, J. An Interpretation of the Hydrodynamics of Pollen. Am. J. Bot. 1979, 66, 737–743. [Google Scholar] [CrossRef]
- Katifori, E.; Alben, S.; Cerda, E.; Nelson, D.R.; Dumais, J. Foldable structures and the natural design of pollen grains. Proc. Natl. Acad. Sci. USA 2010, 107, 7635–7639. [Google Scholar] [CrossRef] [Green Version]
- Miffre, A.; Mehri, T.; Francis, M.; Rairoux, P. UV–VIS depolarization from Arizona Test Dust particles at exact backscattering angle. J. Quant. Spectrosc. Radiat. Transf. 2016, 169, 79–90. [Google Scholar] [CrossRef]
- Dubois, C.; Cholleton, D.; Gemayel, R.; Chen, Y.; Surratt, J.D.; George, C.; Rairoux, P.; Miffre, A.; Riva, M. Decrease in sulfate aerosol light backscattering by reactive uptake of isoprene epoxydiols. Phys. Chem. Chem. Phys. 2021, 23, 5927–5935. [Google Scholar] [CrossRef]
- Cao, X.; Roy, G.A.; Bernier, R. Lidar polarization discrimination of bioaerosols. Opt. Eng. 2010, 49, 116201. [Google Scholar] [CrossRef]
- Bohlmann, S.; Shang, X.; Vakkari, V.; Giannakaki, E.; Leskinen, A.; Lehtinen, K.E.J.; Pätsi, S.; Komppula, M. Lidar depolarization ratio of atmospheric pollen at multiple wavelengths. Atmos. Chem. Phys. 2021, 21, 7083–7097. [Google Scholar] [CrossRef]
- Veselovskii, I.; Goloub, P.; Podvin, T.; Bovchaliuk, V.; Derimian, Y.; Augustin, P.; Fourmentin, M.; Tanre, D.; Korenskiy, M.; Whiteman, D.N.; et al. Retrieval of optical and physical properties of African dust from multiwavelength Raman lidar measurements during the SHADOW campaign in Senegal. Atmos. Chem. Phys. 2016, 16, 7013–7028. [Google Scholar] [CrossRef] [Green Version]
- Miffre, A.; Cholleton, D.; Noël, C.; Rairoux, P. Investigating the dependence of mineral dust depolarization on complex refractive index and size with a laboratory polarimeter at 180.0° lidar backscattering angle. Atmos. Meas. Tech. 2022. submitted. [Google Scholar]
- Smith, M.; Cecchi, L.; Skjøth, C.A.; Karrer, G.; Šikoparija, B. Common ragweed: A threat to environmental health in Europe. Environ. Int. 2013, 61, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Dahl, Å.; Strandhede, S.-O.; Wihl, J.-Å. Ragweed–An allergy risk in Sweden? Aerobiologia 1999, 15, 293–297. [Google Scholar] [CrossRef]
- Hamaoui-Laguel, L.; Vautard, R.; Liu, L.; Solmon, F.; Viovy, N.; Khvorostyanov, D.; Essl, F.; Chuine, I.; Colette, A.; Semenov, M.A.; et al. Effects of climate change and seed dispersal on airborne ragweed pollen loads in Europe. Nat. Clim. Chang. 2015, 5, 766–771. [Google Scholar] [CrossRef]
- Prank, M.; Chapman, D.S.; Bullock, J.M.; Belmonte, J.; Berger, U.; Dahl, A.; Jäger, S.; Kovtunenko, I.; Magyar, D.; Niemelä, S.; et al. An operational model for forecasting ragweed pollen release and dispersion in Europe. Agric. For. Meteorol. 2013, 182–183, 43–53. [Google Scholar] [CrossRef]
- Imhof, K.; Probst, E.; Seifert, B.; Regenass, S.; Schmid-Grendelmeier, P. Ash pollen allergy: Reliable detection of sensitization on the basis of IgE to Ole e 1. Allergo J. Int. 2014, 23, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Biedermann, T.; Winther, L.; Till, S.J.; Panzner, P.; Knulst, A.; Valovirta, E. Birch pollen allergy in Europe. Allergy 2019, 74, 1237–1248. [Google Scholar] [CrossRef] [Green Version]
- Siljamo, P.; Sofiev, M.; Filatova, E.; Grewling, Ł.; Jäger, S.; Khoreva, E.; Linkosalo, T.; Ortega Jimenez, S.; Ranta, H.; Rantio-Lehtimäki, A.; et al. A numerical model of birch pollen emission and dispersion in the atmosphere. Model evaluation and sensitivity analysis. Int. J. Biometeorol. 2013, 57, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Schwendemann, A.B.; Wang, G.; Mertz, M.L.; McWilliams, R.T.; Thatcher, S.L.; Osborn, J.M. Aerodynamics of saccate pollen and its implications for wind pollination. Am. J. Bot. 2007, 94, 1371–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.; Lee, S.; Shin, S.; Park, Y. Three-dimensional label-free imaging and analysis of Pinus pollen grains using optical diffraction tomography. Sci. Rep. 2018, 8, 1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sicard, M.; Jorba, O.; Ho, J.J.; Izquierdo, R.; De Linares, C.; Alarcón, M.; Comerón, A.; Belmonte, J. Measurement report: Characterization of the vertical distribution of airborne Pinus pollen in the atmosphere with lidar-derived profiles: A modelling case study in the region of Barcelona, NE Spain. Atmos. Chem. Phys. 2021, 21, 17807–17832. [Google Scholar] [CrossRef]
- Charpin, D.; Calleja, M.; Lahoz, C.; Pichot, C.; Waisel, Y. Allergy to cypress pollen. Allergy 2005, 60, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Charpin, D.; Pichot, C.; Belmonte, J.; Sutra, J.-P.; Zidkova, J.; Chanez, P.; Shahali, Y.; Sénéchal, H.; Poncet, P. Cypress pollinosis: From tree to clinic. Clin. Rev. Allergy Immunol. 2019, 56, 174–195. [Google Scholar] [CrossRef] [PubMed]
- Hesse, M. (Ed.) Pollen Terminology: An Illustrated Handbook; Springer: Wien, NY, USA, 2009; ISBN 978-3-211-79893-5. [Google Scholar]
| Pollen | λ (nm) | ||
|---|---|---|---|
| Ragweed | 355 | 0.90 ± 0.01 | 5.3 ± 0.5 |
| 532 | 0.51 ± 0.01 | 32.7 ± 0.3 | |
| Ash | 355 | 0.91 ± 0.01 | 5.0 ± 0.6 |
| 532 | 0.39 ± 0.01 | 43.8 ± 0.3 | |
| Birch | 355 | 0.93 ± 0.02 | 3.8 ± 0.8 |
| 532 | 0.39 ± 0.01 | 31.1 ± 0.6 | |
| Pine | 355 | 0.64 ± 0.01 | 22.2 ± 0.7 |
| 532 | 0.42 ± 0.01 | 41.3 ± 0.5 | |
| Cypress | 355 | 0.70 ± 0.01 | 17.5 ± 0.6 |
| 532 | 0.43 ± 0.01 | 39.8 ± 0.3 | |
| Spruce | 355 | 0.60 ± 0.02 | 25.0 ± 1.2 |
| 532 | 0.46 ± 0.01 | 37.2 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cholleton, D.; Rairoux, P.; Miffre, A. Laboratory Evaluation of the (355, 532) nm Particle Depolarization Ratio of Pure Pollen at 180.0° Lidar Backscattering Angle. Remote Sens. 2022, 14, 3767. https://doi.org/10.3390/rs14153767
Cholleton D, Rairoux P, Miffre A. Laboratory Evaluation of the (355, 532) nm Particle Depolarization Ratio of Pure Pollen at 180.0° Lidar Backscattering Angle. Remote Sensing. 2022; 14(15):3767. https://doi.org/10.3390/rs14153767
Chicago/Turabian StyleCholleton, Danaël, Patrick Rairoux, and Alain Miffre. 2022. "Laboratory Evaluation of the (355, 532) nm Particle Depolarization Ratio of Pure Pollen at 180.0° Lidar Backscattering Angle" Remote Sensing 14, no. 15: 3767. https://doi.org/10.3390/rs14153767
APA StyleCholleton, D., Rairoux, P., & Miffre, A. (2022). Laboratory Evaluation of the (355, 532) nm Particle Depolarization Ratio of Pure Pollen at 180.0° Lidar Backscattering Angle. Remote Sensing, 14(15), 3767. https://doi.org/10.3390/rs14153767









