Spatial Characterisation of Vegetation Diversity in Groundwater-Dependent Ecosystems Using In-Situ and Sentinel-2 MSI Satellite Data
Abstract
1. Introduction
2. Materials and Methods
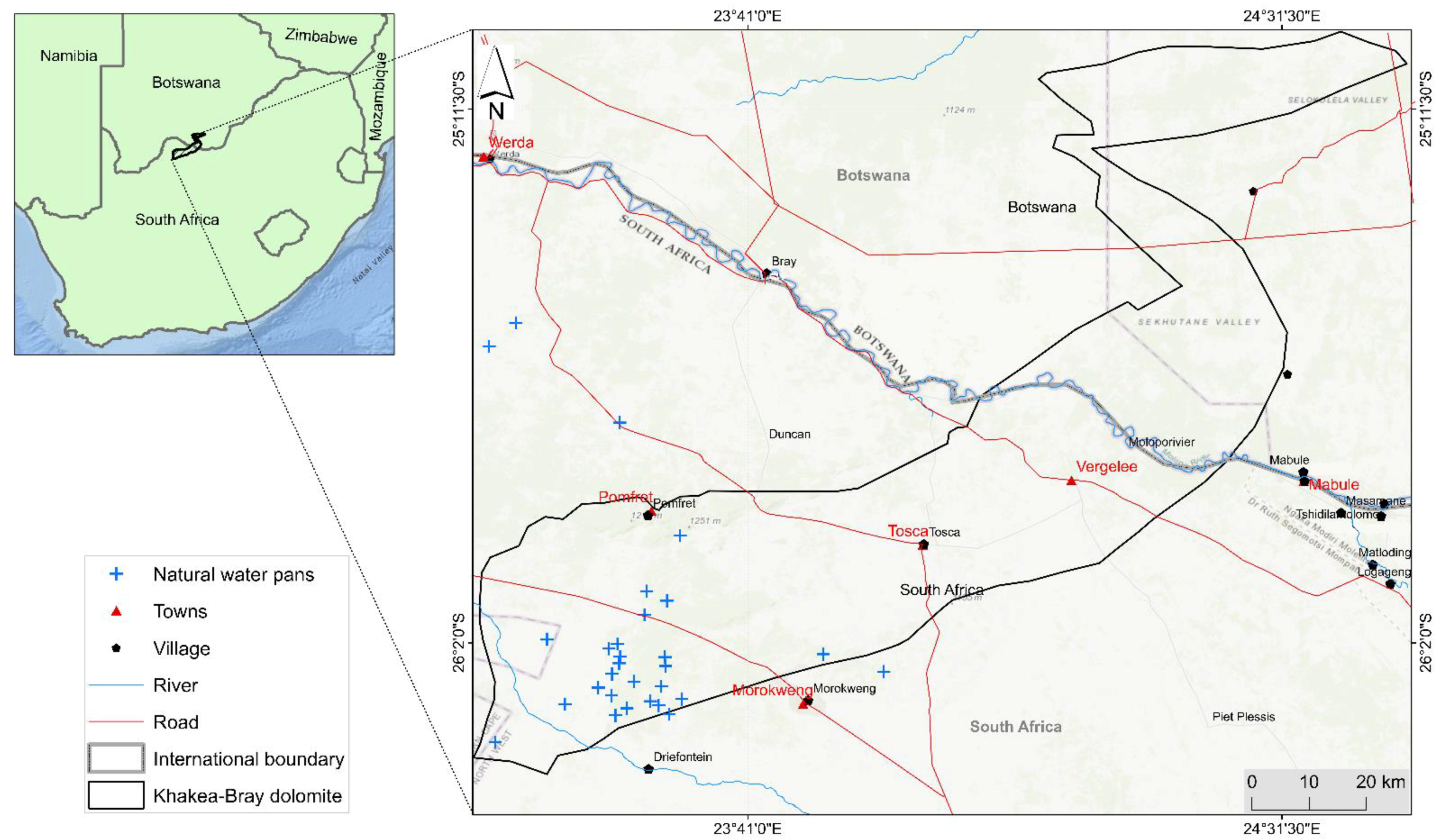
2.1. Study Area
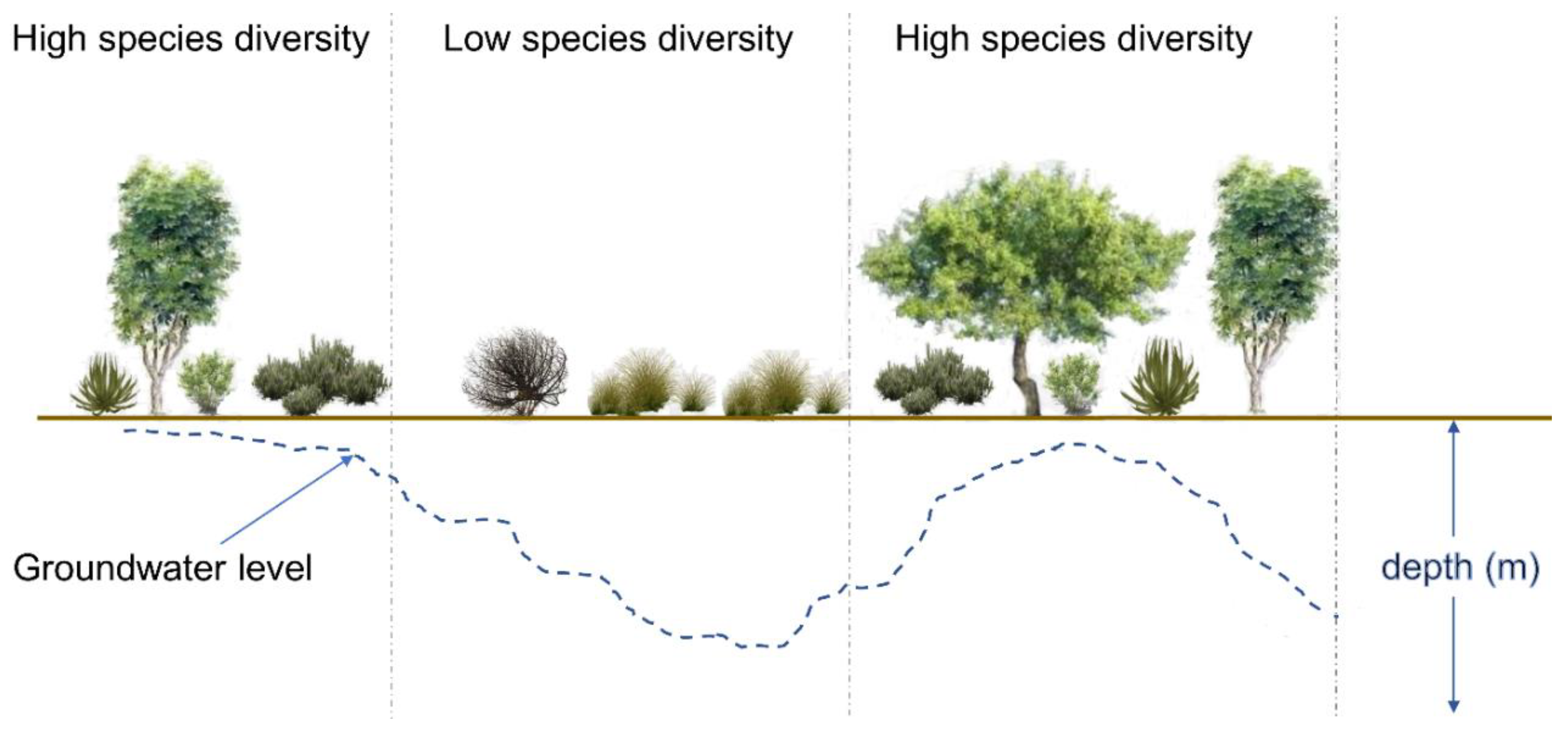
2.2. Field Campaign and Measuring Vegetation Diversity
Community Composition and Species Dominance
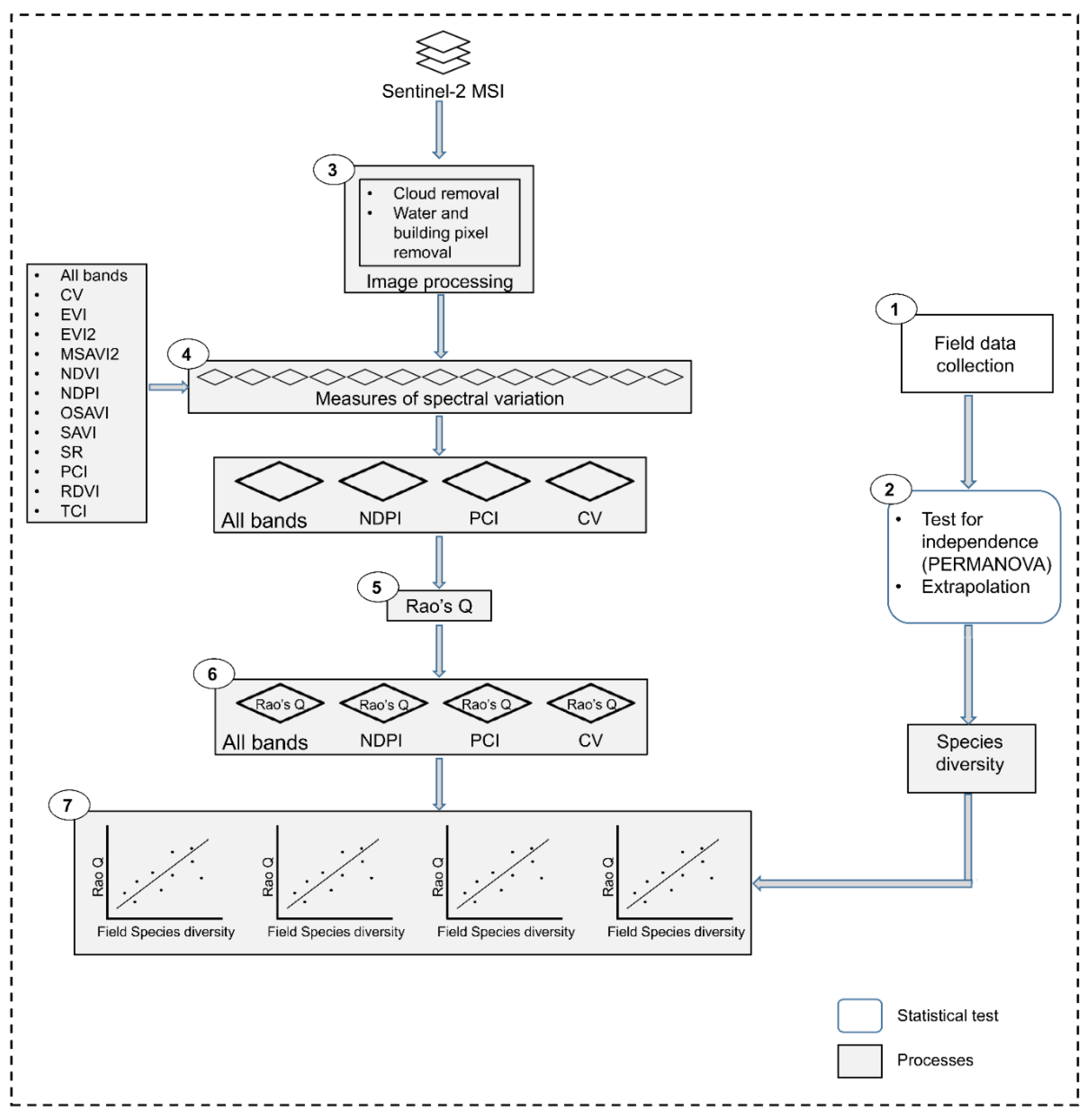
2.3. Image Acquisition and Processing
2.3.1. Calculating Measures of Spectral Variation
2.3.2. Calculating Vegetation Diversity with the Rao’s Q Using Remote Sensing Data
2.3.3. Evaluating Remote Sensing-Derived Diversity
2.3.4. Effect of Distance from the Natural Water Pan on Vegetation Diversity
3. Results
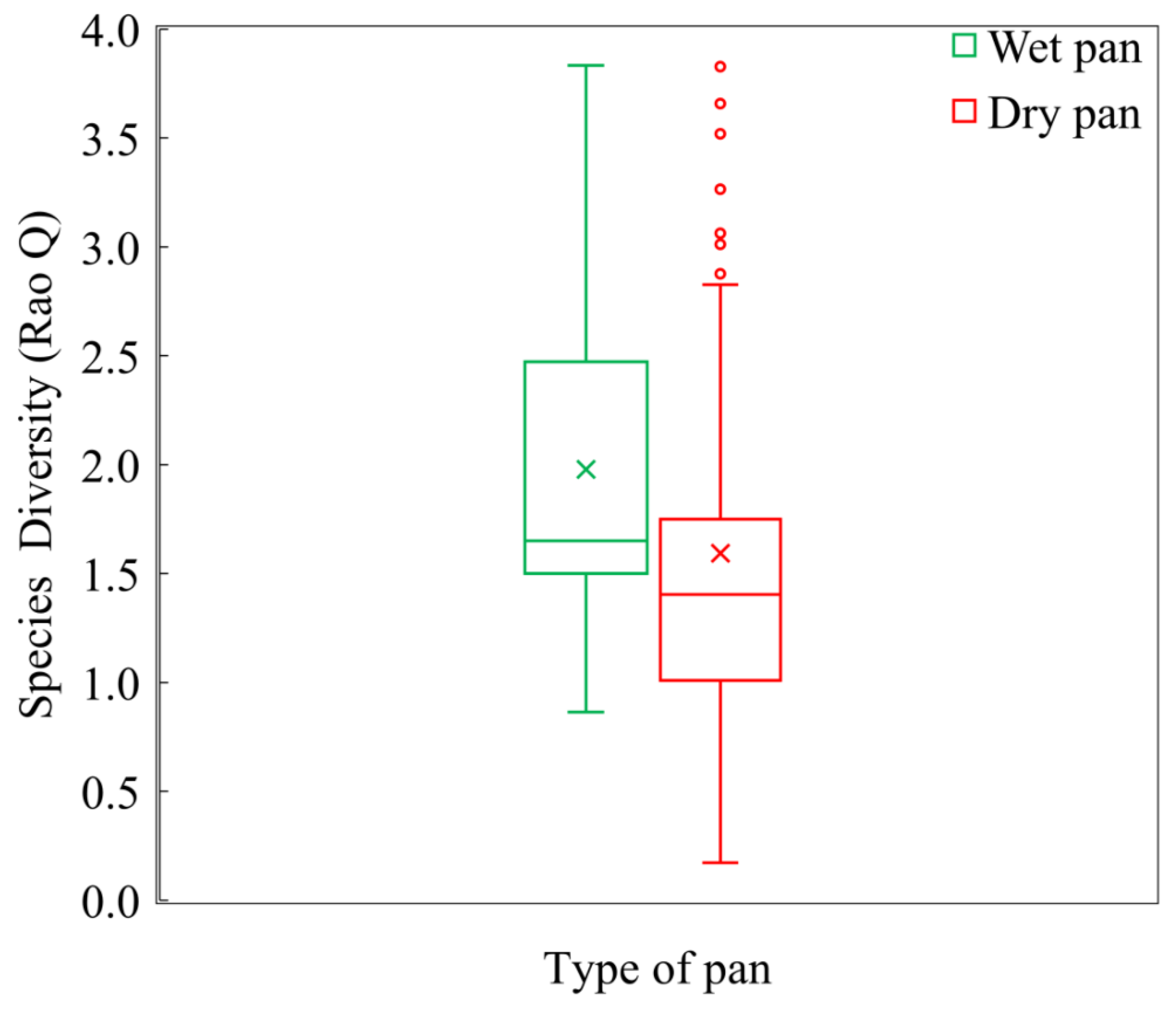
3.1. Species Composition, Diversity, and Dominance
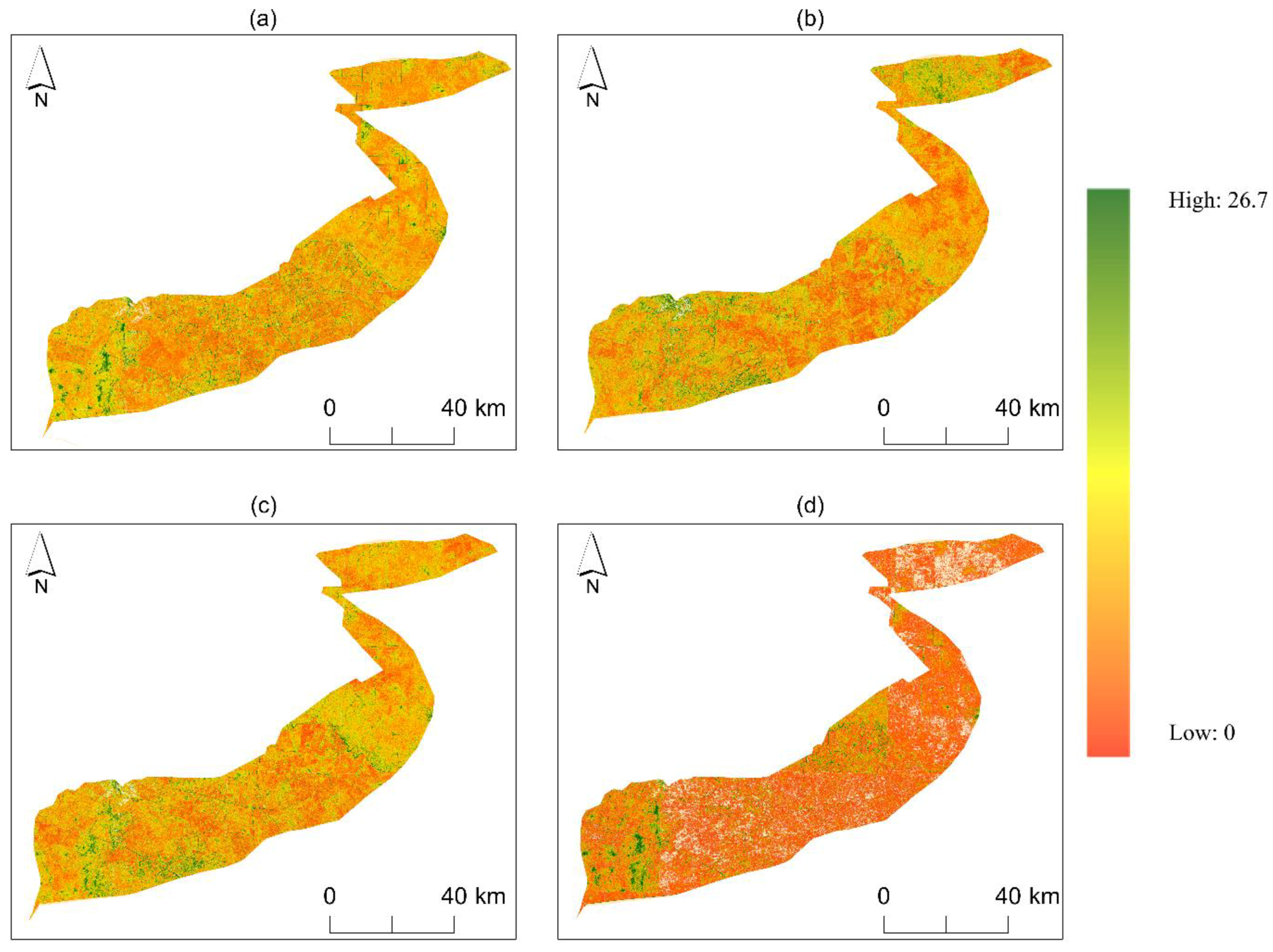
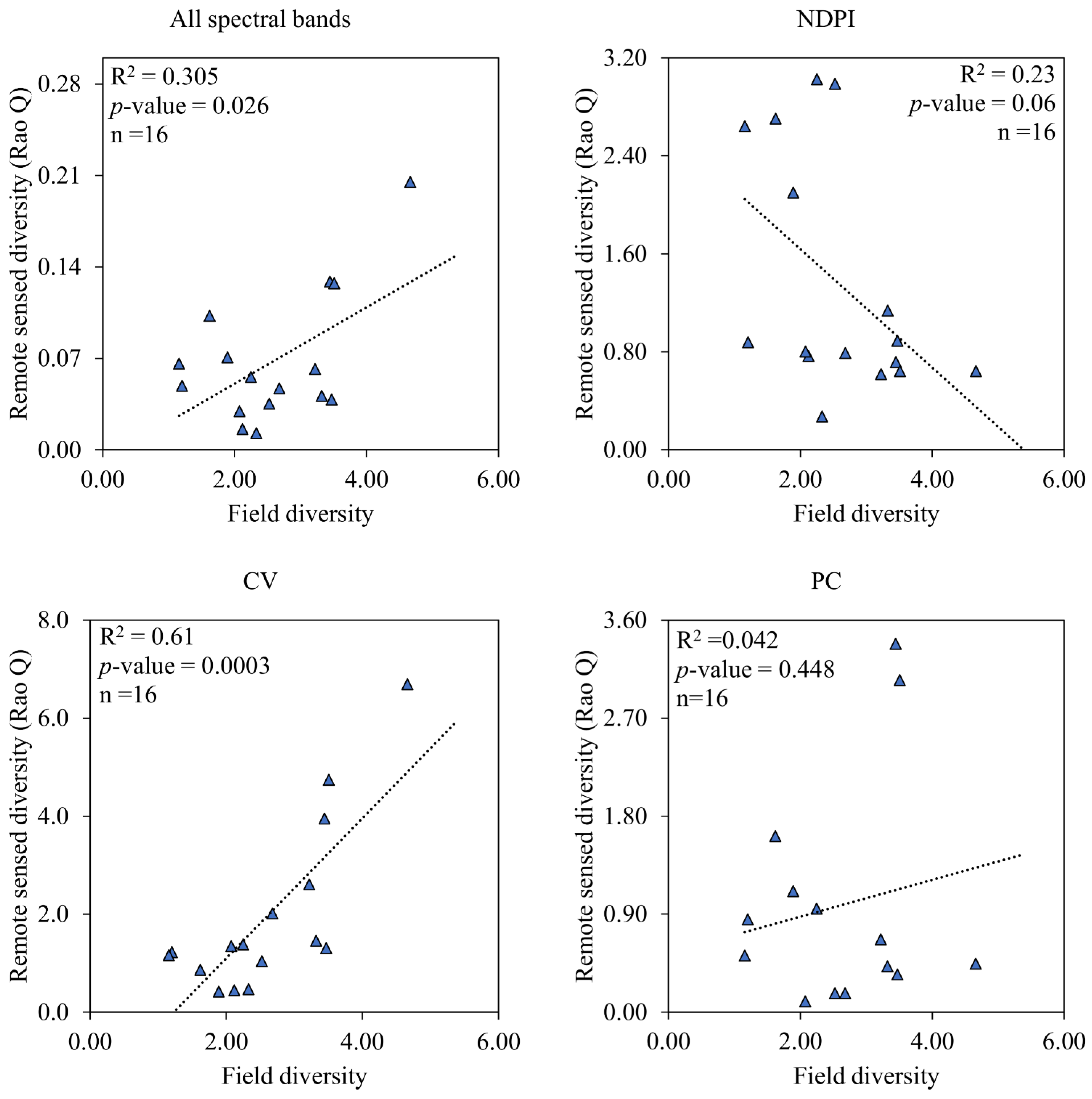
3.2. Distribution and Performance of Spectral Diversity from Remote Sensing Data
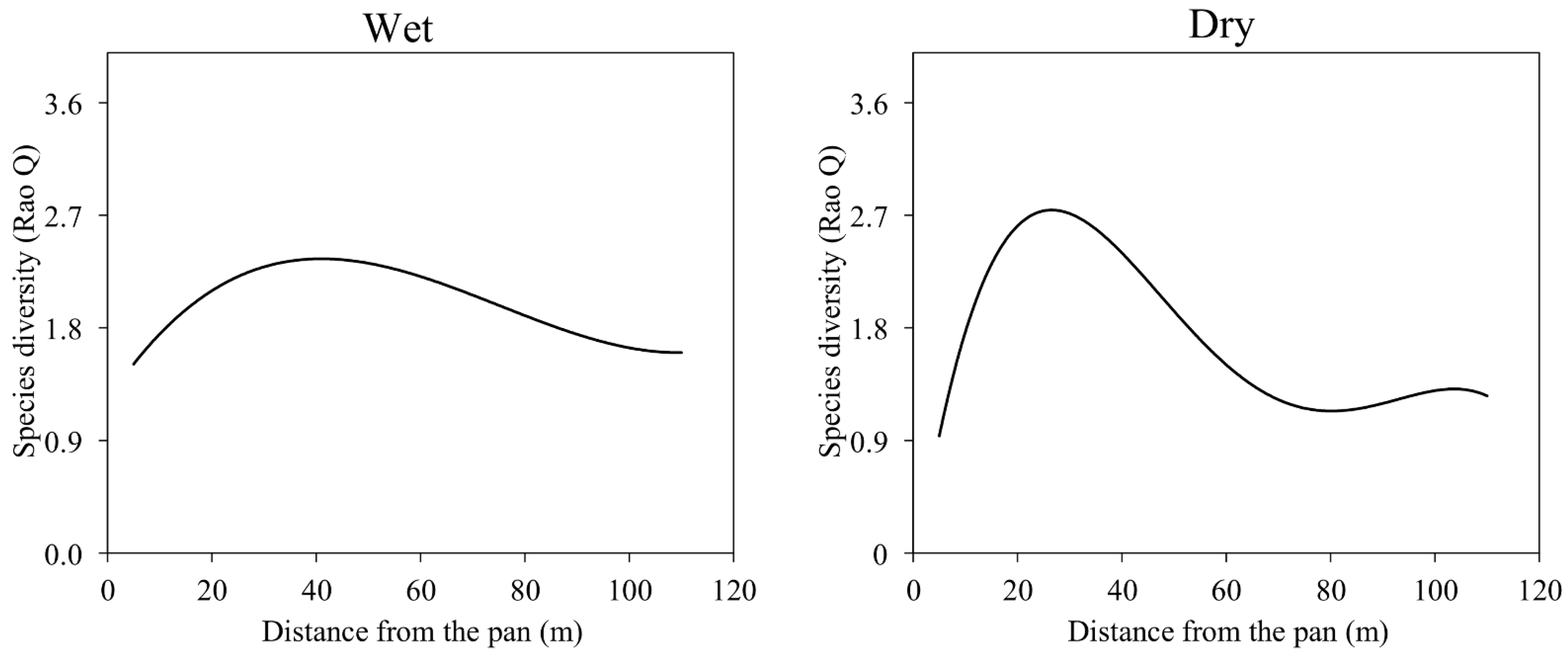
3.3. Vegetation Diversity and Distance from the Natural Pan
4. Discussion
4.1. Species Composition and the Performance of Measures of Spectral Variation in Estimating Vegetation Diversity
4.2. Distribution of Vegetation Diversity in the Khakea-Bray TBA
4.3. Implications of Using Vegetation Diversity on Monitoring and Conserving GDE
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seward, P.; van Dyk, G.S.d.T. Turning the tide–curbing groundwater over-abstraction in the Tosca-Molopo area, South Africa. In Advances in Groundwater Governance; Villholth, K.G., López-Gunn, E., Conti, K., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 511–525. [Google Scholar]
- Turton, A.; Godfrey, L.; Julien, F.; Hattingh, H. Unpacking Groundwater Governance through the Lens of a Trialogue: A Southern African Case Study. In Proceedings of the International Symposium on Groundwater Sustainability (ISGWAS), Alicante, Spain, 23–27 January 2006; pp. 24–27. [Google Scholar]
- Eamus, D.; Fu, B.; Springer, A.E.; Stevens, L.E. Groundwater dependent ecosystems: Classification, identification techniques and threats. In Integrated Groundwater Management: Concepts, Approaches and Challenges; Jakeman, A.J., Barreteau, O., Hunt, R.J., Rinaudo, J.-D., Ross, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 313–346. [Google Scholar]
- Murray, B.R.; Hose, G.C.; Eamus, D.; Licari, D. Valuation of groundwater-dependent ecosystems: A functional methodology incorporating ecosystem services. Aust. J. Bot. 2006, 54, 221–229. [Google Scholar] [CrossRef]
- Sousa, M.R.; Rudolph, D.L.; Frind, E.O. Threats to groundwater resources in urbanizing watersheds: The waterloo moraine and beyond. Can. Water Resour. J. Rev. Can. Des Ressour. Hydr. 2014, 39, 193–208. [Google Scholar] [CrossRef]
- Wu, W.-Y.; Lo, M.-H.; Wada, Y.; Famiglietti, J.S.; Reager, J.T.; Yeh, P.J.F.; Ducharne, A.; Yang, Z.-L. Divergent effects of climate change on future groundwater availability in key mid-latitude aquifers. Nat. Commun. 2020, 11, 3710. [Google Scholar] [CrossRef]
- Clifton, C.; Evans, R.; Hayes, S.; Hirji, R.; Puz, G.; Pizarro, C. Water and Climate Change: Impacts on Groundwater Resources and Adaptation Options; World Bank: Washington, DC, USA, 2010. [Google Scholar]
- Kreamer, D.K.; Stevens, L.E.; Ledbetter, J.D. Groundwater dependent ecosystems—Science, challenges, and policy directions. Groundwater 2015, 205, 230. [Google Scholar]
- Kløve, B.; Ala-Aho, P.; Bertrand, G.; Gurdak, J.J.; Kupfersberger, H.; Kværner, J.; Muotka, T.; Mykrä, H.; Preda, E.; Rossi, P. Climate change impacts on groundwater and dependent ecosystems. J. Hydrol. 2014, 518, 250–266. [Google Scholar] [CrossRef]
- Alaibakhsh, M.; Emelyanova, I.; Barron, O.; Khiadani, M.; Warren, G. Large-scale regional delineation of riparian vegetation in the arid and semi-arid Pilbara region, WA. Hydrol. Process. 2017, 31, 4269–4281. [Google Scholar] [CrossRef]
- Pengra, B.W.; Johnston, C.A.; Loveland, T.R. Mapping an invasive plant, Phragmites australis, in coastal wetlands using the eo-1 hyperion hyperspectral sensor. Remote Sens. Environ. 2007, 108, 74–81. [Google Scholar] [CrossRef]
- Xu, C.; Holmgren, M.; Van Nes, E.H.; Maestre, F.T.; Soliveres, S.; Berdugo, M.; Kéfi, S.; Marquet, P.A.; Abades, S.; Scheffer, M. Can we infer plant facilitation from remote sensing? A test across global drylands. Ecol. Appl. 2015, 25, 1456–1462. [Google Scholar] [CrossRef]
- Lv, J.; Wang, X.S.; Zhou, Y.; Qian, K.; Wan, L.; Eamus, D.; Tao, Z. Groundwater-dependent distribution of vegetation in Hailiutu river catchment, a semi-arid region in China. Ecohydrology 2013, 6, 142–149. [Google Scholar] [CrossRef]
- Barron, O.V.; Emelyanova, I.; Van Niel, T.G.; Pollock, D.; Hodgson, G. Mapping groundwater-dependent ecosystems using remote sensing measures of vegetation and moisture dynamics. Hydrol. Process. 2014, 28, 372–385. [Google Scholar] [CrossRef]
- De Klerk, A.; De Klerk, L.; Oberholster, P.; Ashton, P.; Dini, J.; Holness, S. A Review of Depressional Wetlands (Pans) in South Africa, Including a Water Quality Classification System; WRC Report No 2230/1/16; Water Research Commission: Gezina, South Africa, 2016. [Google Scholar]
- Eamus, D.; Froend, R.; Loomes, R.; Hose, G.; Murray, B. A functional methodology for determining the groundwater regime needed to maintain the health of groundwater-dependent vegetation. Aust. J. Bot. 2006, 54, 97–114. [Google Scholar] [CrossRef]
- Orellana, F.; Verma, P.; Loheide, S.P.; Daly, E. Monitoring and modeling water-vegetation interactions in groundwater-dependent ecosystems. Rev. Geophys. 2012, 50, RG3003. [Google Scholar] [CrossRef]
- Eamus, D.; Froend, R. Groundwater-dependent ecosystems: The where, what and why of gdes. Aust. J. Bot. 2006, 54, 91–96. [Google Scholar] [CrossRef]
- Chiloane, C.; Dube, T.; Shoko, C. Impacts of groundwater and climate variability on terrestrial groundwater dependent ecosystems: A review of geospatial assessment approaches and challenges and possible future research directions. Geocarto Int. 2021. [Google Scholar] [CrossRef]
- John, R.; Chen, J.; Lu, N.; Guo, K.; Liang, C.; Wei, Y.; Noormets, A.; Ma, K.; Han, X. Predicting plant diversity based on remote sensing products in the semi-arid region of inner mongolia. Remote Sens. Environ. 2008, 112, 2018–2032. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Cheng, X.; Liao, Y.; Chen, G. Comparison and integration of feature reduction methods for land cover classification with rapideye imagery. Multimed. Tools Appl. 2017, 76, 23041–23057. [Google Scholar] [CrossRef]
- Woods, J.; Sekhwela, M.B.M. The vegetation resources of botswana’s savannas: An overview. S. Afr. Geogr. J. 2003, 85, 69–79. [Google Scholar] [CrossRef]
- Rocchini, D.; Ricotta, C.; Chiarucci, A. Using satellite imagery to assess plant species richness: The role of multispectral systems. Appl. Veg. Sci. 2007, 10, 325–331. [Google Scholar] [CrossRef]
- Nagendra, H.; Gadgil, M. Satellite imagery as a tool for monitoring species diversity: An assessment. J. Appl. Ecol. 1999, 36, 388–397. [Google Scholar] [CrossRef]
- Rocchini, D.; Chiarucci, A.; Loiselle, S.A. Testing the spectral variation hypothesis by using satellite multispectral images. Acta Oecologica 2004, 26, 117–120. [Google Scholar] [CrossRef]
- Rocchini, D.; Marcantonio, M.; Ricotta, C. Measuring Rao’s Q diversity index from remote sensing: An open source solution. Ecol. Indic. 2017, 72, 234–238. [Google Scholar] [CrossRef]
- Wang, R.; Gamon, J.A.; Emmerton, C.A.; Li, H.; Nestola, E.; Pastorello, G.Z.; Menzer, O. Integrated analysis of productivity and biodiversity in a southern alberta prairie. Remote Sens. 2016, 8, 214. [Google Scholar] [CrossRef]
- Wang, R.; Gamon, J.A. Remote sensing of terrestrial plant biodiversity. Remote Sens. Environ. 2019, 231, 111218. [Google Scholar] [CrossRef]
- Torresani, M.; Feilhauer, H.; Rocchini, D.; Féret, J.-B.; Zebisch, M.; Tonon, G. Which optical traits enable an estimation of tree species diversity based on the spectral variation hypothesis? Appl. Veg. Sci. 2021, 24, e12586. [Google Scholar] [CrossRef]
- Nagendra, H.; Rocchini, D.; Ghate, R.; Sharma, B.; Pareeth, S. Assessing plant diversity in a dry tropical forest: Comparing the utility of landsat and ikonos satellite images. Remote Sens. 2010, 2, 478–496. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Schweiger, A.K.; Pinto-Ledezma, J.N.; Meireles, J.E. Applying remote sensing to biodiversity science. In Remote Sensing of Plant Biodiversity; Springer: Cham, Switzerland, 2020; pp. 13–42. [Google Scholar]
- Schmidtlein, S.; Fassnacht, F.E. The spectral variability hypothesis does not hold across landscapes. Remote Sens. Environ. 2017, 192, 114–125. [Google Scholar] [CrossRef]
- Mandanici, E.; Bitelli, G. Preliminary comparison of sentinel-2 and landsat 8 imagery for a combined use. Remote Sens. 2016, 8, 1014. [Google Scholar] [CrossRef]
- Rocchini, D. Effects of spatial and spectral resolution in estimating ecosystem α-diversity by satellite imagery. Remote Sens. Environ. 2007, 111, 423–434. [Google Scholar] [CrossRef]
- Gould, W. Remote sensing of vegetation, plant species richness, and regional biodiversity hotspots. Ecol. Appl. 2000, 10, 1861–1870. [Google Scholar] [CrossRef]
- Dahlin, K.M. Spectral diversity area relationships for assessing biodiversity in a wildland–agriculture matrix. Ecol. Appl. 2016, 26, 2758–2768. [Google Scholar] [CrossRef] [PubMed]
- Torresani, M.; Rocchini, D.; Sonnenschein, R.; Zebisch, M.; Marcantonio, M.; Ricotta, C.; Tonon, G. Estimating tree species diversity from space in an alpine conifer forest: The Rao’s Q diversity index meets the spectral variation hypothesis. Ecol. Inform. 2019, 52, 26–34. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
- Ayyad, M.A. Case studies in the conservation of biodiversity: Degradation and threats. J. Arid. Environ. 2003, 54, 165–182. [Google Scholar] [CrossRef]
- Bongaarts, J. IPBES, 2019. Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the intergovernmental science-policy platform on biodiversity and ecosystem services. Popul. Dev. Rev. 2019, 45, 680–681. [Google Scholar] [CrossRef]
- Brown, J.; Wyers, A.; Aldous, A.; Bach, L. Groundwater and Biodiversity Conservation: A Methods Guide for Integrating Groundwater Needs of Ecosystems and Species into Conservation Plans in the Pacific Northwest; The Nature Conservancy: Portland, OR, USA, 2007. [Google Scholar]
- Eales, K. Equity and Efficiency in Water Services: The Climate Change Dimension; Water Institute of Southern Africa: Midrand, South Africa, 2010. [Google Scholar]
- Davies, J.; Robins, N.S.; Farr, J.; Sorensen, J.; Beetlestone, P.; Cobbing, J.E. Identifying transboundary aquifers in need of international resource management in the southern african development community region. Hydrogeol. J. 2013, 21, 321–330. [Google Scholar] [CrossRef]
- Ngobe, T. Investigation of Groundwater Discharge Processes in GDEs in the Khakea Bray Transboundary Aquifer. 2021. Available online: https://sadc-gmi.org/wp-content/uploads/2021/08/SADC-JRS-project-presentation-Thandeka.pdf (accessed on 15 November 2021).
- Godfrey, L.; van Dyk, G. Reserve Determination for the Pomfret-Vergelegen Dolomitic Aquifer, North West Province, Part of Catchments D41C, D, E and F; CSIR Report Env-P-C; CSIR: Pretoria, South Africa, 2002. [Google Scholar]
- Altchenko, Y.; Villholth, K.G. Transboundary aquifer mapping and management in africa: A harmonised approach. Hydrogeol. J. 2013, 21, 1497–1517. [Google Scholar] [CrossRef]
- Nijsten, G.-J.; Christelis, G.; Villholth, K.G.; Braune, E.; Gaye, C.B. Transboundary aquifers of africa: Review of the current state of knowledge and progress towards sustainable development and management. J. Hydrol. Reg. Stud. 2018, 20, 21–34. [Google Scholar] [CrossRef]
- Van Dyk, G.S.D.T. Managing the Impact of Irrigation on the Tosca-Molopo Groundwater Resource. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2005. [Google Scholar]
- Environmental Systems Research Institute. Arcgis 10.8; Environmental Systems Research Institute: Redlands, CA, USA, 2020. [Google Scholar]
- Arendt, R.; Reinhardt-Imjela, C.; Schulte, A.; Faulstich, L.; Ullmann, T.; Beck, L.; Martinis, S.; Johannes, P.; Lengricht, J. Natural pans as an important surface water resource in the Cuvelai basin—metrics for storage volume calculations and identification of potential augmentation sites. Water 2021, 13, 177. [Google Scholar] [CrossRef]
- Van Wyk, B. Field Guide to Trees of Southern Africa; Penguin Random House South Africa: Cape Town, South Africa, 2013. [Google Scholar]
- Jost, L. The relation between evenness and diversity. Diversity 2010, 2, 207–232. [Google Scholar] [CrossRef]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.; Sander, E.L.; Ma, K.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Chao, A.; Chiu, C.-H.; Jost, L. Unifying species diversity, phylogenetic diversity, functional diversity, and related similarity and differentiation measures through hill numbers. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 297–324. [Google Scholar] [CrossRef]
- Smith, E.P.; van Belle, G. Nonparametric estimation of species richness. Biometrics 1984, 40, 119–129. [Google Scholar] [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M. The vegan package. Community Ecol. Package 2007, 10, 719. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA); John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–15. [Google Scholar]
- Guo, C.; Zhu, G.; Qin, B.; Zhang, Y.; Zhu, M.; Xu, H.; Chen, Y.; Paerl, H.W. Climate exerts a greater modulating effect on the phytoplankton community after 2007 in eutrophic Lake Taihu, China: Evidence from 25 years of recordings. Ecol. Indic. 2019, 105, 82–91. [Google Scholar] [CrossRef]
- Lin, D.; Li, X.; Fang, H.; Dong, Y.; Huang, Z.; Chen, J. Calanoid copepods assemblages in Pearl river estuary of China in summer: Relationships between species distribution and environmental variables. Estuar. Coast. Shelf Sci. 2011, 93, 259–267. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, Y. Aggregated intensity of dominant species of zooplankton in autumn in the east China Sea and Yellow Sea. J. Ecol. 1989, 8, 13–15. [Google Scholar]
- Bhatti, S.S.; Tripathi, N.K. Built-up area extraction using landsat 8 oli imagery. GIScience Remote Sens. 2014, 51, 445–467. [Google Scholar] [CrossRef]
- Jiang, Z.; Qi, J.; Su, S.; Zhang, Z.; Wu, J. Water body delineation using index composition and his transformation. Int. J. Remote Sens. 2012, 33, 3402–3421. [Google Scholar] [CrossRef]
- Madonsela, S.; Cho, M.A.; Ramoelo, A.; Mutanga, O. Investigating the relationship between tree species diversity and landsat-8 spectral heterogeneity across multiple phenological stages. Remote Sens. 2021, 13, 2467. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the modis vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Jiang, Z.; Huete, A.R.; Didan, K.; Miura, T. Development of a two-band enhanced vegetation index without a blue band. Remote Sens. Environ. 2008, 112, 3833–3845. [Google Scholar] [CrossRef]
- Jiang, Z.; Huete, A.R.; Li, J.; Qi, J. Interpretation of the modified soil-adjusted vegetation index isolines in red-nir reflectance space. J. Appl. Remote Sens. 2007, 1, 013503. [Google Scholar] [CrossRef]
- Jiang, Z.; Huete, A.R.; Chen, J.; Chen, Y.; Li, J.; Yan, G.; Zhang, X. Analysis of NDVI and scaled difference vegetation index retrievals of vegetation fraction. Remote Sens. Environ. 2006, 101, 366–378. [Google Scholar] [CrossRef]
- Xu, D.; Wang, C.; Chen, J.; Shen, M.; Shen, B.; Yan, R.; Li, Z.; Karnieli, A.; Chen, J.; Yan, Y. The superiority of the normalized difference phenology index (NDPI) for estimating grassland aboveground fresh biomass. Remote Sens. Environ. 2021, 264, 112578. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Hayashi, M.; Van der Kamp, G. Simple equations to represent the volume–area–depth relations of shallow wetlands in small topographic depressions. J. Hydrol. 2000, 237, 74–85. [Google Scholar] [CrossRef]
- Huete, A.R. A soil-adjusted vegetation index (savi). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green lai of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Crist, E.P.; Kauth, R. The tasseled cap de-mystified. [transformations of mss and tm data]. Photogramm. Eng. Remote Sens. 1986, 52, 81–86. [Google Scholar]
- Laliberté, E.; Schweiger, A.K.; Legendre, P. Partitioning plant spectral diversity into alpha and beta components. Ecol. Lett. 2020, 23, 370–380. [Google Scholar] [CrossRef]
- R Core Team. R: The R Project for Statistical Computing. 2019. Available online: https://www.r-project.org/ (accessed on 28 February 2021).
- Kletting, P.; Glatting, G. Model selection for time-activity curves: The corrected akaike information criterion and the f-test. Z. Für Med. Phys. 2009, 19, 200–206. [Google Scholar] [CrossRef]
- Ruxton, G.D. The unequal variance t-test is an underused alternative to Student’s t-test and the Mann–Whitney U test. Behav. Ecol. 2006, 17, 688–690. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Zhou, M.; Ai, D.; Wang, G.; Wang, Y.; Chu, C.; Lundholm, J.T. The effect of environmental heterogeneity on species richness depends on community position along the environmental gradient. Sci. Rep. 2015, 5, 15723. [Google Scholar] [CrossRef]
- van Mazijk, R.; Cramer, M.D.; Verboom, G.A. Environmental heterogeneity explains contrasting plant species richness between the South African Cape and Southwestern Australia. J. Biogeogr. 2021, 48, 1875–1888. [Google Scholar] [CrossRef]
- Oldeland, J.; Wesuls, D.; Rocchini, D.; Schmidt, M.; Jürgens, N. Does using species abundance data improve estimates of species diversity from remotely sensed spectral heterogeneity? Ecol. Indic. 2010, 10, 390–396. [Google Scholar] [CrossRef]
- Madonsela, S.; Cho, M.A.; Ramoelo, A.; Mutanga, O. Remote sensing of species diversity using landsat 8 spectral variables. ISPRS J. Photogramm. Remote Sens. 2017, 133, 116–127. [Google Scholar] [CrossRef]
- Wang, R.; Gamon, J.A.; Cavender-Bares, J.; Townsend, P.A.; Zygielbaum, A.I. The spatial sensitivity of the spectral diversity–biodiversity relationship: An experimental test in a prairie grassland. Ecol. Appl. 2018, 28, 541–556. [Google Scholar] [CrossRef]
- Mapfumo, R.B.; Murwira, A.; Masocha, M.; Andriani, R. The relationship between satellite-derived indices and species diversity across African savanna ecosystems. Int. J. Appl. Earth Obs. Geoinf. 2016, 52, 306–317. [Google Scholar] [CrossRef]
- Arnall, D.; Raun, W.; Solie, J.; Stone, M.; Johnson, G.; Girma, K.; Freeman, K.; Teal, R.; Martin, K. Relationship between coefficient of variation measured by spectral reflectance and plant density at early growth stages in winter wheat. J. Plant Nutr. 2006, 29, 1983–1997. [Google Scholar] [CrossRef]
- Jansson, R.; Laudon, H.; Johansson, E.; Augspurger, C. The importance of groundwater discharge for plant species number in riparian zones. Ecology 2007, 88, 131–139. [Google Scholar] [CrossRef]
- Egeru, A.; Wasonga, O.; MacOpiyo, L.; Mburu, J.; Tabuti, J.R.S.; Majaliwa, M.G.J. Piospheric influence on forage species composition and abundance in semi-arid karamoja sub-region, uganda. Pastoralism 2015, 5, 12. [Google Scholar] [CrossRef]
- Mpakairi, K.S. Waterhole distribution and the piosphere effect in heterogeneous landscapes: Evidence from North-Western Zimbabwe. Trans. R. Soc. S. Afr. 2019, 74, 219–222. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, J.; Wang, P.; Yu, Q.; Eamus, D. Distribution patterns of groundwater-dependent vegetation species diversity and their relationship to groundwater attributes in Northwestern China. Ecohydrology 2013, 6, 191–200. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Y.; Zhu, C.; Li, W. The variation in soil moisture and the appropriate groundwater table for desert riparian forest along the lower Tarim river. J. Geogr. Sci. 2011, 21, 150–162. [Google Scholar] [CrossRef]
- Spellerberg, I. Ecological effects of roads and traffic: A literature review. Glob. Ecol. Biogeogr. Lett. 1998, 7, 317–333. [Google Scholar] [CrossRef]
- Fowler, J.F.; Sieg, C.H.; Dickson, B.G.; Saab, V. Exotic plant species diversity: Influence of roads and prescribed fire in Arizona Ponderosa pine forests. Rangel. Ecol. Manag. 2008, 61, 284–293. [Google Scholar] [CrossRef]
- Fallahchai, M.M.; Haghverdi, K.; Mojaddam, M.S. Ecological effects of forest roads on plant species diversity in Caspian forests of iran. Acta Ecol. Sin. 2018, 38, 255–261. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J.; Ning, K.; Du, S.; Han, G.; Qu, F.; Wang, G.; Fu, Y.; Zhan, C. Ecological effects of roads on the plant diversity of coastal wetland in the Yellow River delta. Sci. World J. 2014, 2014, 952051. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, K. The influence of roads on the species diversity of forest vascular flora in central Poland. Biodivers. Res. Conserv. 2007, 5–8, 71–80. [Google Scholar]
- Roberts, J.; Florentine, S.; van Etten, E.; Turville, C. Germination biology, distribution and control of the invasive species Eragrostis curvula [Schard. Nees] (African lovegrass): A global synthesis of current and future management challenges. Weed Res. 2021, 61, 154–163. [Google Scholar] [CrossRef]
- Roberts, J.; Florentine, S.; van Etten, E.; Turville, C. Germination biology of four climatically varied populations of the invasive species African lovegrass (Eragrostis curvula). Weed Sci. 2021, 69, 210–218. [Google Scholar] [CrossRef]
- Harvey, C.A.; Villanueva, C.; Villacís, J.; Chacón, M.; Muñoz, D.; López, M.; Ibrahim, M.; Gómez, R.; Taylor, R.; Martinez, J. Contribution of live fences to the ecological integrity of agricultural landscapes. Agric. Ecosyst. Environ. 2005, 111, 200–230. [Google Scholar] [CrossRef]
- Pulido-Santacruz, P.; Renjifo, L.M. Live fences as tools for biodiversity conservation: A study case with birds and plants. Agrofor. Syst. 2011, 81, 15–30. [Google Scholar] [CrossRef]
- Lopes, M.; Fauvel, M.; Ouin, A.; Girard, S. Spectro-temporal heterogeneity measures from dense high spatial resolution satellite image time series: Application to grassland species diversity estimation. Remote Sens. 2017, 9, 993. [Google Scholar] [CrossRef]
- Sætersdal, M.; Gjerde, I. Prioritising conservation areas using species surrogate measures: Consistent with ecological theory? J. Appl. Ecol. 2011, 48, 1236–1240. [Google Scholar] [CrossRef]
- Regos, A.; Arenas-Castro, S.; Tapia, L.; Domínguez, J.; Honrado, J.P. Using remotely sensed indicators of primary productivity to improve prioritization of conservation areas for top predators. Ecol. Indic. 2021, 125, 107503. [Google Scholar] [CrossRef]
- Knight, A.T.; Cowling, R.M.; Rouget, M.; Balmford, A.; Lombard, A.T.; Campbell, B.M. Knowing but not doing: Selecting priority conservation areas and the research-implementation gap. Conserv. Biol. 2008, 22, 610–617. [Google Scholar] [CrossRef]

| Formula | Type | Wet Pan | Dry Pan |
|---|---|---|---|
| AICc | AICc | ||
| Y = b1X + C | Linear | −69.47 | −66.61 |
| Y = C + b1log(X) | Logarithmic | −353.54 | −348.76 |
| Y = C + b1/X | Inverse | −280.18 | −123.34 |
| Y = C + b1X + b2X2 | Quadratic | −78.07 | −64.51 |
| Y = C + b1X + b2X2 + b3X3 | Cubic | −78.02 | −77.09 |
| Y = C + b1X + b2X2 + b3X3+ b4X4 | Polynomial | −74.40 | −93.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mpakairi, K.S.; Dube, T.; Dondofema, F.; Dalu, T. Spatial Characterisation of Vegetation Diversity in Groundwater-Dependent Ecosystems Using In-Situ and Sentinel-2 MSI Satellite Data. Remote Sens. 2022, 14, 2995. https://doi.org/10.3390/rs14132995
Mpakairi KS, Dube T, Dondofema F, Dalu T. Spatial Characterisation of Vegetation Diversity in Groundwater-Dependent Ecosystems Using In-Situ and Sentinel-2 MSI Satellite Data. Remote Sensing. 2022; 14(13):2995. https://doi.org/10.3390/rs14132995
Chicago/Turabian StyleMpakairi, Kudzai Shaun, Timothy Dube, Farai Dondofema, and Tatenda Dalu. 2022. "Spatial Characterisation of Vegetation Diversity in Groundwater-Dependent Ecosystems Using In-Situ and Sentinel-2 MSI Satellite Data" Remote Sensing 14, no. 13: 2995. https://doi.org/10.3390/rs14132995
APA StyleMpakairi, K. S., Dube, T., Dondofema, F., & Dalu, T. (2022). Spatial Characterisation of Vegetation Diversity in Groundwater-Dependent Ecosystems Using In-Situ and Sentinel-2 MSI Satellite Data. Remote Sensing, 14(13), 2995. https://doi.org/10.3390/rs14132995










