Spectral Estimation of In Vivo Wheat Chlorophyll a/b Ratio under Contrasting Water Availabilities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Data Collection
2.3. Data Preprocessing and Analyses
3. Results and Discussion
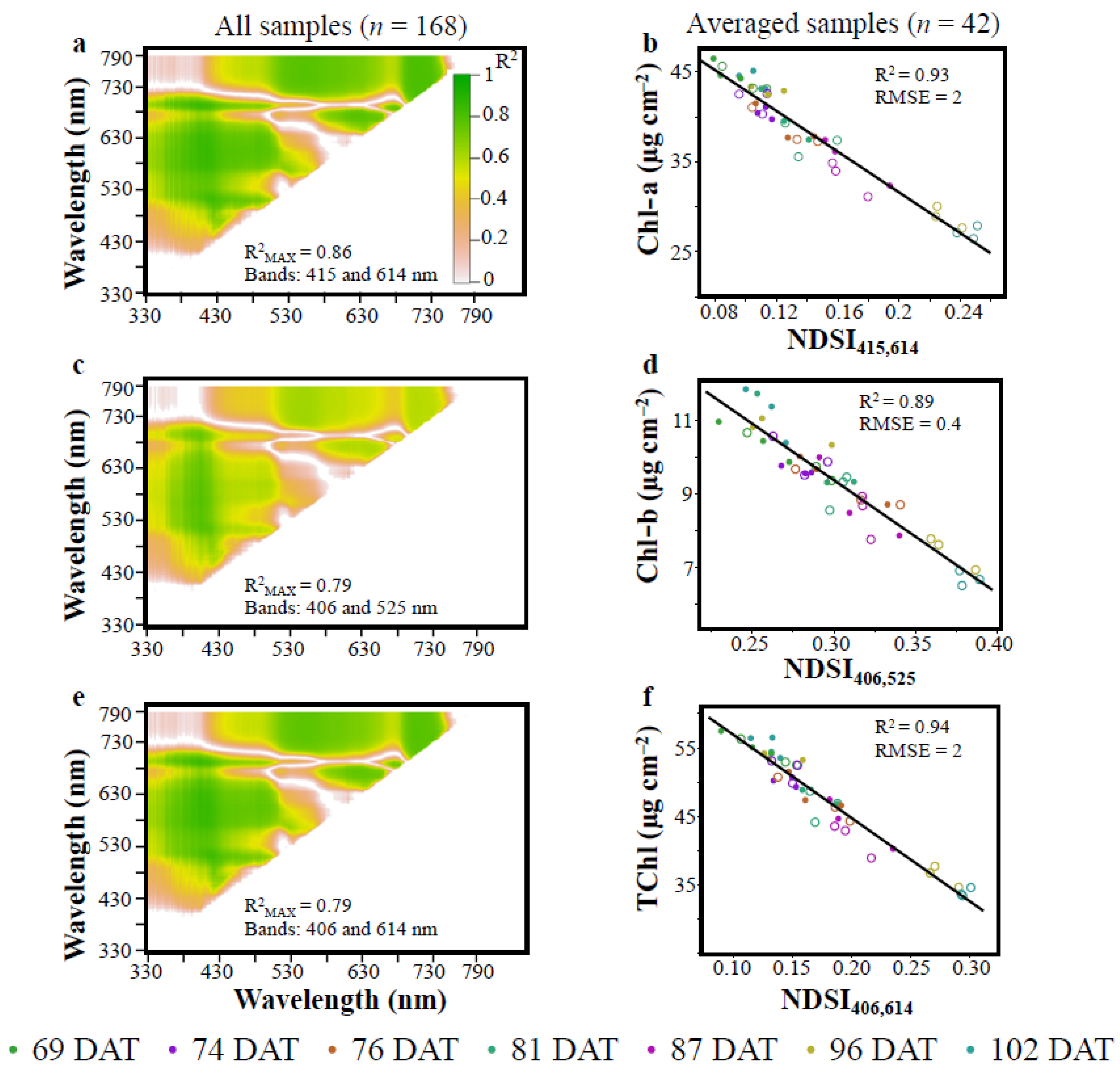
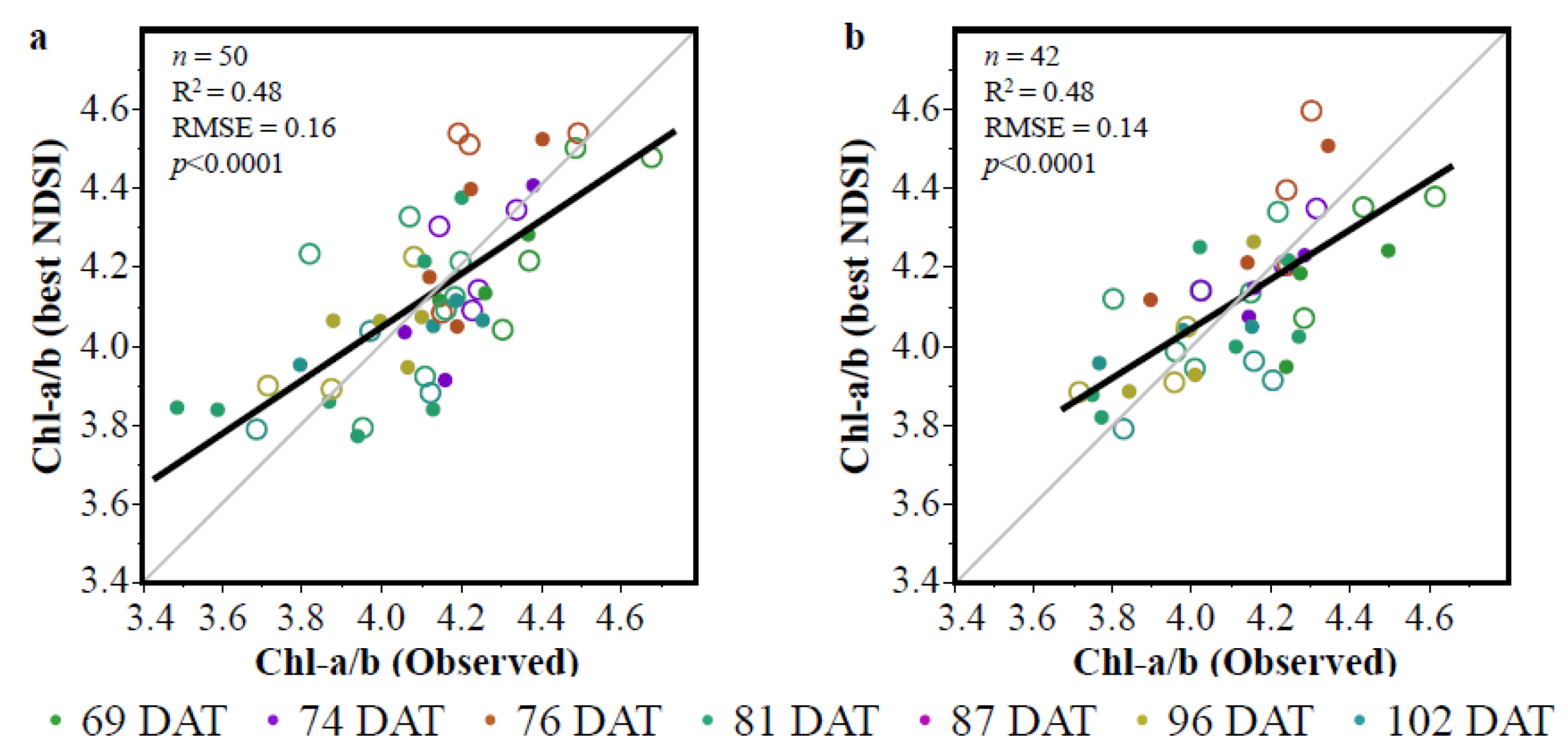
3.1. Vegetation Indices (VIs)
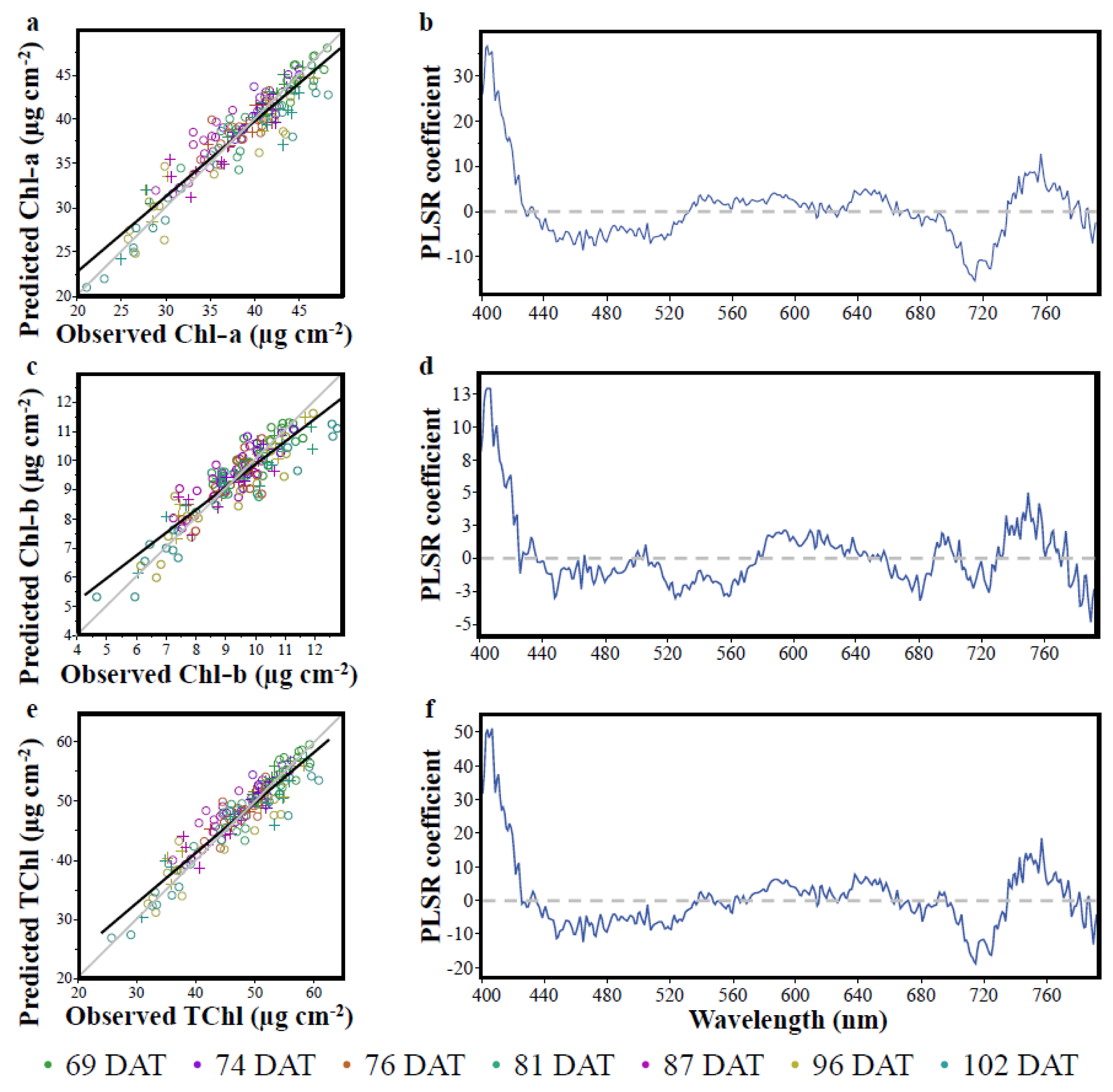
3.2. The Partial Least Squares Regression (PLSR)
4. Conclusions
- (i)
- The new VIs that were developed in the current study resulted in highly accurate Chl-a and Chl-b estimation.
- (ii)
- The developed VIs were able to indirectly estimate Chl-a/b.
- (iii)
- The VIs developed in the current study performed similarly to the PLSR.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Myers, S.S.; Smith, M.R.; Guth, S.; Golden, C.D.; Vaitla, B.; Mueller, N.D.; Dangour, A.D.; Huybers, P. Climate change and global food systems: Potential impacts on food security and undernutrition. Annu. Rev. Public Health 2017, 38, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Sade, N.; Peleg, Z. Future challenges for global food security under climate change. Plant Sci. 2020, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Croft, H.; Chen, J.M.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Chang. Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef] [PubMed]
- Croce, R.; Van Amerongen, H. Natural strategies for photosynthetic light harvesting. Nat. Chem. Biol. 2014, 10, 492–501. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M. Leaf Pigment Content. Compr. Remote Sens. 2018, 117–142. [Google Scholar] [CrossRef]
- Lenaerts, B.; Collard, B.C.Y.; Demont, M. Review: Improving global food security through accelerated plant breeding. Plant Sci. 2019, 287, 110207. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Yu, H.Y.; Kong, D.S.; Yan, F.; Zhang, Y.J. Effects of drought stress on growth and chlorophyll fluorescence of Lycium ruthenicum Murr. seedlings. Photosynthetica 2016, 54, 524–531. [Google Scholar] [CrossRef]
- Moran, R.; Porath, D. Chlorophyll determination in intact tissues using N,N -Dimethylformamide. Plant Physiol. 1980, 65, 478–479. [Google Scholar] [CrossRef] [Green Version]
- Wellburn, A.R. The Spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-band model for noninvasive estimation of chlorophyll, carotenoids, and anthocyanin contents in higher plant leaves. Geophys. Res. Lett. 2006, 33, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Lopes, D.d.C.; Moura, L.d.O.; Steidle Neto, A.J.; Ferraz, L.d.C.L.; Carlos, L.d.A.; Martins, L.M. Spectral indices for non-destructive determination of lettuce pigments. Food Anal. Methods 2017, 10, 2807–2814. [Google Scholar] [CrossRef]
- Yamamoto, A.; Nakamura, T.; Adu-Gyamfi, J.J.; Saigusa, M. Relationship between chlorophyll content in leaves of sorghum and pigeonpea determined by extraction method and by chlorophyll meter (SPAD-502). J. Plant Nutr. 2002, 25, 2295–2301. [Google Scholar] [CrossRef]
- Joynson, R.; Molero, G.; Coombes, B.; Gardiner, L.J.; Rivera-Amado, C.; Piñera-Chávez, F.J.; Evans, J.R.; Furbank, R.T.; Reynolds, M.P.; Hall, A. Uncovering candidate genes involved in photosynthetic capacity using unexplored genetic variation in Spring Wheat. Plant Biotechnol. J. 2021, 19, 1537–1552. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Mariotto, I.; Gumma, M.K.; Middleton, E.M.; Landis, D.R.; Huemmrich, K.F. Selection of hyperspectral narrowbands (HNBs) and composition of hyperspectral two band vegetation indices (HVIs) for biophysical characterization and discrimination of crop types using field reflectance and Hyperion/EO-1 data. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2013, 6, 427–439. [Google Scholar] [CrossRef] [Green Version]
- Casa, R.; Castaldi, F.; Pascucci, S.; Pignatti, S. Chlorophyll estimation in field crops: An assessment of handheld leaf meters and spectral reflectance measurements. J. Agric. Sci. 2015, 153, 876–890. [Google Scholar] [CrossRef]
- Pérez-Patricio, M.; Camas-Anzueto, J.L.; Sanchez-Alegría, A.; Aguilar-González, A.; Gutiérrez-Miceli, F.; Escobar-Gómez, E.; Voisin, Y.; Rios-Rojas, C.; Grajales-Coutiño, R. Optical method for estimating the chlorophyll contents in plant leaves. Sensors 2018, 18, 650. [Google Scholar] [CrossRef] [Green Version]
- Chappelle, E.W.; Kim, M.S.; McMurtrey, J.E. Ratio analysis of reflectance spectra (RARS): An algorithm for the remote estimation of the concentrations of chlorophyll A, chlorophyll B, and carotenoids in soybean leaves. Remote Sens. Environ. 1992, 39, 239–247. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote estimation of canopy chlorophyll content in crops. Geophys. Res. Lett. 2005, 32, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Gnyp, M.L.; Gao, L.; Miao, Y.; Chen, X.; Bareth, G. Estimate leaf chlorophyll of rice using reflectance indices and partial least squares. Photogramm. Fernerkund. Geoinf. 2015, 2015, 45–54. [Google Scholar] [CrossRef]
- Gausman, H.W.; Allen, W.A. Optical parameters of leaves of 30 plant species. Plant Physiol. 1973, 52, 57–62. [Google Scholar] [CrossRef]
- Bannari, A.; Morin, D.; Bonn, F.; Huete, A.R. A review of vegetation indices. Remote Sens. Rev. 1995, 13, 95–120. [Google Scholar] [CrossRef]
- Giovos, R.; Tassopoulos, D.; Kalivas, D.; Lougkos, N.; Priovolou, A. Remote sensing vegetation indices in viticulture: A critical review. Agriculture 2021, 11, 457. [Google Scholar] [CrossRef]
- Banerjee, B.P.; Joshi, S.; Thoday-Kennedy, E.; Pasam, R.K.; Tibbits, J.; Hayden, M.; Spangenberg, G.; Kant, S. High-throughput phenotyping using digital and hyperspectral imaging-derived biomarkers for genotypic nitrogen response. J. Exp. Bot. 2020, 71, 4604–4615. [Google Scholar] [CrossRef] [Green Version]
- Bannari, A.; Khurshid, K.S.; Staenz, K.; Schwarz, J.W. A comparison of hyperspectral chlorophyll indices for wheat crop chlorophyll content estimation using laboratory reflectance measurements. IEEE Trans. Geosci. Remote Sens. 2007, 45, 3063–3074. [Google Scholar] [CrossRef]
- Chen, X.; Dong, Z.; Liu, J.; Wang, H.; Zhang, Y.; Chen, T.; Du, Y.; Shao, L.; Xie, J. Hyperspectral characteristics and quantitative analysis of leaf chlorophyll by reflectance spectroscopy based on a genetic algorithm in combination with partial least squares regression. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 243, 118786. [Google Scholar] [CrossRef]
- Gitelson, A.A. Nondestructive estimation of foliar pigment (chlorophylls, carotenoids, and anthocyanins) contents: Evaluating a semianalytical three-band model. In Hyperspectral Remote Sensing of Vegetation; Thenkabail, P.S., Lyon, J.G., Eds.; CRC: Boca Raton, FL, USA, 2011; pp. 141–165. [Google Scholar]
- Main, R.; Cho, M.A.; Mathieu, R.; O’Kennedy, M.M.; Ramoelo, A.; Koch, S. An investigation into robust spectral indices for leaf chlorophyll estimation. ISPRS J. Photogramm. Remote Sens. 2011, 66, 751–761. [Google Scholar] [CrossRef]
- Sonobe, R.; Yamashita, H.; Mihara, H.; Morita, A.; Ikka, T. Estimation of leaf chlorophyll a, b and carotenoid contents and their ratios using hyperspectral reflectance. Remote Sens. 2020, 12, 3265. [Google Scholar] [CrossRef]
- Ray, D.; Mueller, N.; West, P.; Foley, J. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [Green Version]
- Abbo, S.; Pinhasi van-Oss, R.; Gopher, A.; Saranga, Y.; Ofner, I.; Peleg, Z. Plant domestication versus crop evolution: A conceptual framework for cereals and grain legumes. Trends Plant Sci. 2014, 19, 351–360. [Google Scholar] [CrossRef]
- Golan, G.; Hendel, E.; Méndez Espitia, G.E.; Schwartz, N.; Peleg, Z. Activation of seminal root primordia during wheat domestication reveals underlying mechanisms of plant resilience. Plant Cell Environ. 2018, 41, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Bacher, H.; Zhu, F.; Gao, T.; Liu, K.; Dhatt, B.K.; Awada, T.; Zhang, C.; Distelfeld, A.; Yu, H.; Peleg, Z.; et al. Wild emmer introgression alters root-to-shoot growth dynamics in durum wheat in response to water stress. Plant Physiol. 2021, 187, 1149–1162. [Google Scholar] [CrossRef] [PubMed]
- Bacher, H.; Sharaby, Y.; Walia, H.; Peleg, Z. Modifying root-to-shoot ratio improves root water influxes in wheat under drought stress. J. Exp. Bot. 2022, 73, 1643–1654. [Google Scholar] [CrossRef] [PubMed]
- Peleg, Z.; Fahima, T.; Abbo, S.; Krugman, T.; Nevo, E.; Yakir, D.; Saranga, Y. Genetic diversity for drought resistance in wild emmer wheat and its ecogeographical associations. Plant Cell Environ. 2005, 28, 176–191. [Google Scholar] [CrossRef]
- Herrmann, I.; Karnieli, A.; Bonfil, D.J.; Cohen, Y.; Alchanatis, V. SWIR-based spectral indices for assessing nitrogen content in potato fields. Int. J. Remote Sens. 2010, 31, 5127–5143. [Google Scholar] [CrossRef]
- Inoue, Y.; Peñuelas, J.; Miyata, A.; Mano, M. Normalized difference spectral indices for estimating photosynthetic efficiency and capacity at a canopy scale derived from hyperspectral and CO2 flux measurements in rice. Remote Sens. Environ. 2008, 112, 156–172. [Google Scholar] [CrossRef]
- Singh, A.; Serbin, S.P.; McNeil, B.E.; Kingdon, C.C.; Townsend, P.A. Imaging spectroscopy algorithms for mapping canopy foliar chemical and morphological traits and their uncertainties. Ecol. Appl. 2015, 25, 2180–2197. [Google Scholar] [CrossRef] [Green Version]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Yang, G.; Liu, J.; Zhao, C.; Li, Z.; Huang, Y.; Yu, H.; Xu, B.; Yang, X.; Zhu, D.; Zhang, X.; et al. Unmanned aerial vehicle remote sensing for field-based crop phenotyping: Current status and perspectives. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- McKinney, W. Data structures for statistical computing in python. Proc. 9th Python Sci. Conf. 2010, 1, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Pedregosa, F.; Grisel, O.; Weiss, R.; Passos, A.; Brucher, M.; Varoquax, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; et al. Scikit-learn: Machine learning in python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Pyke, K.A.; Jellings, A.J.; Leech, R.M. Variation in mesophyll cell number and size in wheat leaves. Ann. Bot. 1990, 65, 679–683. [Google Scholar] [CrossRef]
- Guru, T.; Padma, V.; Reddy, D.V.V.; Rao, P.R.; Sanjeeva Rao, D.; Ramesh, T.; Radhakrishna, K.V. Natural variation of top three leaf traits and their association with grain yield in rice hybrids. Indian J. Plant Physiol. 2017, 22, 141–146. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Carter, G.A. Ratios of leaf reflectances in narrow wavebands as indicators of plant stress. Int. J. Remote Sens. 1994, 15, 517–520. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Noland, T.L.; Mohammed, G.H.; Sampson, P.H. Scaling-up and model inversion methods with narrowband optical indices for chlorophyll content estimation in closed forest canopies with hyperspectral data. IEEE Trans. Geosci. Remote Sens. 2001, 39, 1491–1507. [Google Scholar] [CrossRef] [Green Version]
- Barnes, E.M.; Clarke, T.R.; Richards, S.E. Coincident detection of crop water stress, nitrogen status and canopy density using ground-based multispectral data. In Proceedings of the Fifth International Conference on Precision Agriculture, Bloomington, MN, USA, 16–19 July 2000; Robert, P.C., Rust, R.H., Larson, W.E., Eds.; American Society of Agronomy: Madison, WI, USA, 2000. [Google Scholar]
- Guyot, G.; Baret, F.; Major, D.J. High spectral resolution: Determination of spectral shifts between the red and infrared. Int. Arch. Photogramm. Remote Sens. 1988, 11, 750–760. [Google Scholar] [CrossRef]
- Raymond Hunt, E.; Daughtry, C.S.T.; Eitel, J.U.H.; Long, D.S. Remote sensing leaf chlorophyll content using a visible band index. Agron. J. 2011, 103, 1090–1099. [Google Scholar] [CrossRef] [Green Version]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Datt, B. Remote sensing of chlorophyll a, chlorophyll b, chlorophyll a+b, and total carotenoid content in eucalyptus leaves. Remote Sens. Environ. 1998, 66, 111–121. [Google Scholar] [CrossRef]
- Ustin, S.L.; Jacquemoud, S. How the optical properties of leaves modify the absorption and scattering of energy and enhance leaf functionality. In Remote Sensing of Plant Biodiversity; Cavender-Bares, J., Gamon, J.A., Townsend, P.A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 349–384. ISBN 9783030331573. [Google Scholar]
- Hallik, L.; Kazantsev, T.; Kuusk, A.; Galmés, J.; Tomás, M.; Niinemets, Ü. Generality of relationships between leaf pigment contents and spectral vegetation indices in Mallorca (Spain). Reg. Environ. Chang. 2017, 17, 2097–2109. [Google Scholar] [CrossRef]
- Herrmann, I.; Pimstein, A.; Karnieli, A.; Cohen, Y.; Alchanatis, V.; Bonfil, D.J. LAI assessment of wheat and potato crops by VENμS and Sentinel-2 bands. Remote Sens. Environ. 2011, 115, 2141–2151. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation; Texas A&M University Remote Sensing Center: College Station, TX, USA, 1974. [Google Scholar]
- Jordan, C.F. Derivation of Leaf-Area Index from Quality of Light on the Forest Floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of Soil-Adjusted Vegetation Indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Smith, R.; Adams, J.; Stephens, D.; Hick, P. Forecasting Wheat Yield in a Mediterranean-Type Environment from the NOAA Satellite. Crop Pasture Sci. 1995, 46, 113–125. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; Brown de Colostoun, E.; McMurtrey, J.E. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing Prediction Power and Stability of Broadband and Hyperspectral Vegetation Indices for Estimation of Green Leaf Area Index and Canopy Chlorophyll Density. Remote Sens. Environ. 2001, 76, 156–172. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I. Reflectance Assessment of Mite Effects on Apple Trees. Int. J. Remote Sens. 1995, 16, 2727–2733. [Google Scholar] [CrossRef]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A Reappraisal of the Use of DMSO for the Extraction and Determination of Chlorophylls a and b in Lichens and Higher Plants. Environ. Exp. Bot. 1992, 32, 85–100. [Google Scholar] [CrossRef]
- Gamon, J.A.; Penuelas, J.; Field, C.B. A Narrow-Waveband Spectral Index That Tracks Diurnal Changes in Photosynthetic Efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Peñuelas, J.; Gamon, J.A.; Fredeen, A.L.; Merino, J.; Field, C.B. Reflectance Indices Associated with Physiological Changes in Nitrogen- and Water-Limited Sunflower Leaves. Remote Sens. Environ. 1994, 48, 135–146. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. The Stress Concept in Plants: An Introduction. Plant Physiol. Biochem. 1996, 148, 4–14. [Google Scholar] [CrossRef]
- Penuelas, J.; Baret, F.; Filella, I. Semi-Empirical Indices to Assess Carotenoids/Chlorophyll a Ratio from Leaf Spectral Reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical Properties and Nondestructive Estimation of Anthocyanin Content in Plant Leaves. Photochem. Photobiol. 2001, 74, 38. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing Carotenoid Content in Plant Leaves with Reflectance Spectroscopy. Photochem. Photobiol. 2002, 75, 272. [Google Scholar] [CrossRef]
- Roujean, J.L.; Breon, F.M. Estimating PAR Absorbed by Vegetation from Bidirectional Reflectance Measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-Destructive Optical Detection of Pigment Changes during Leaf Senescence and Fruit Ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef] [Green Version]


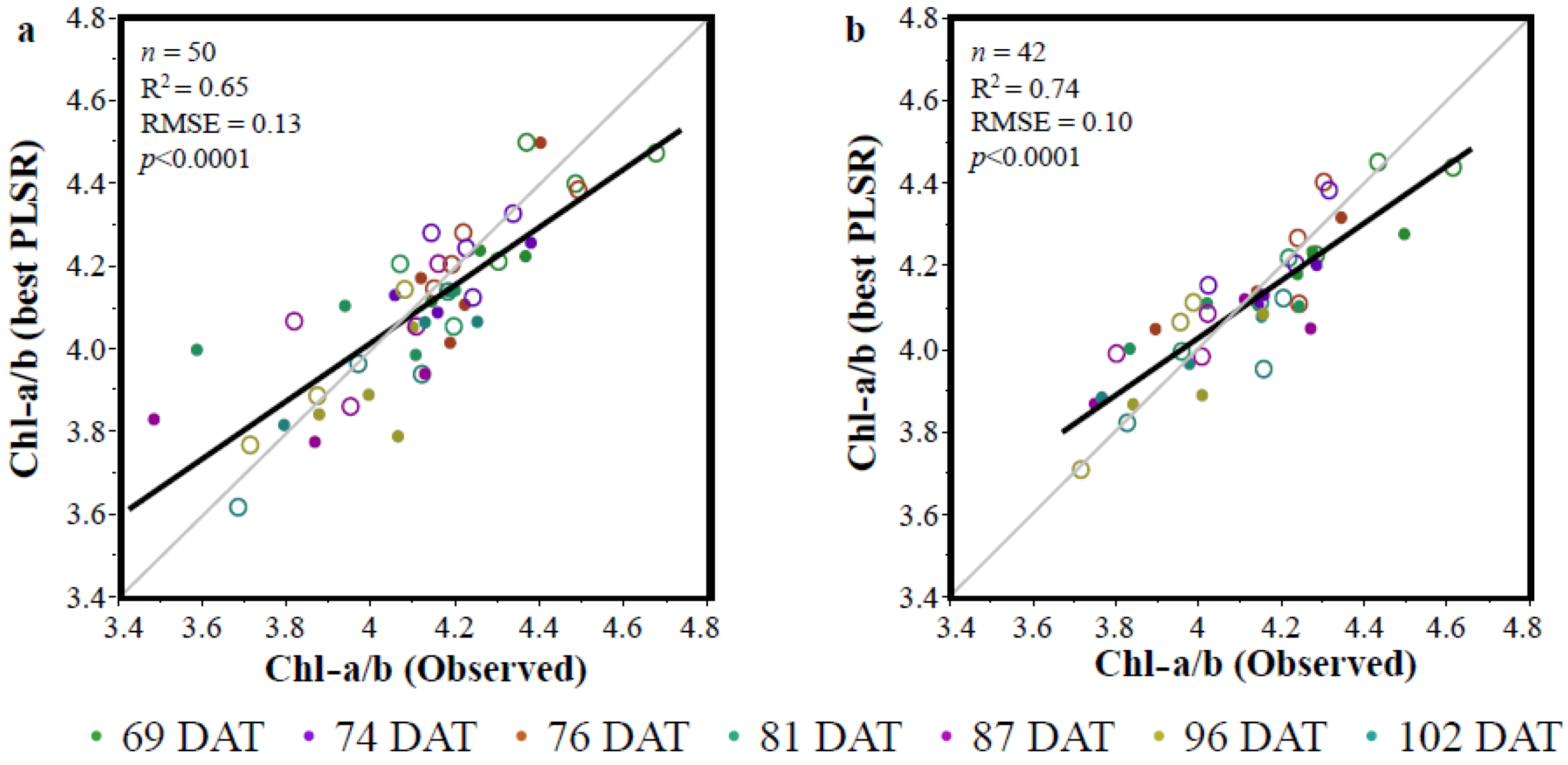
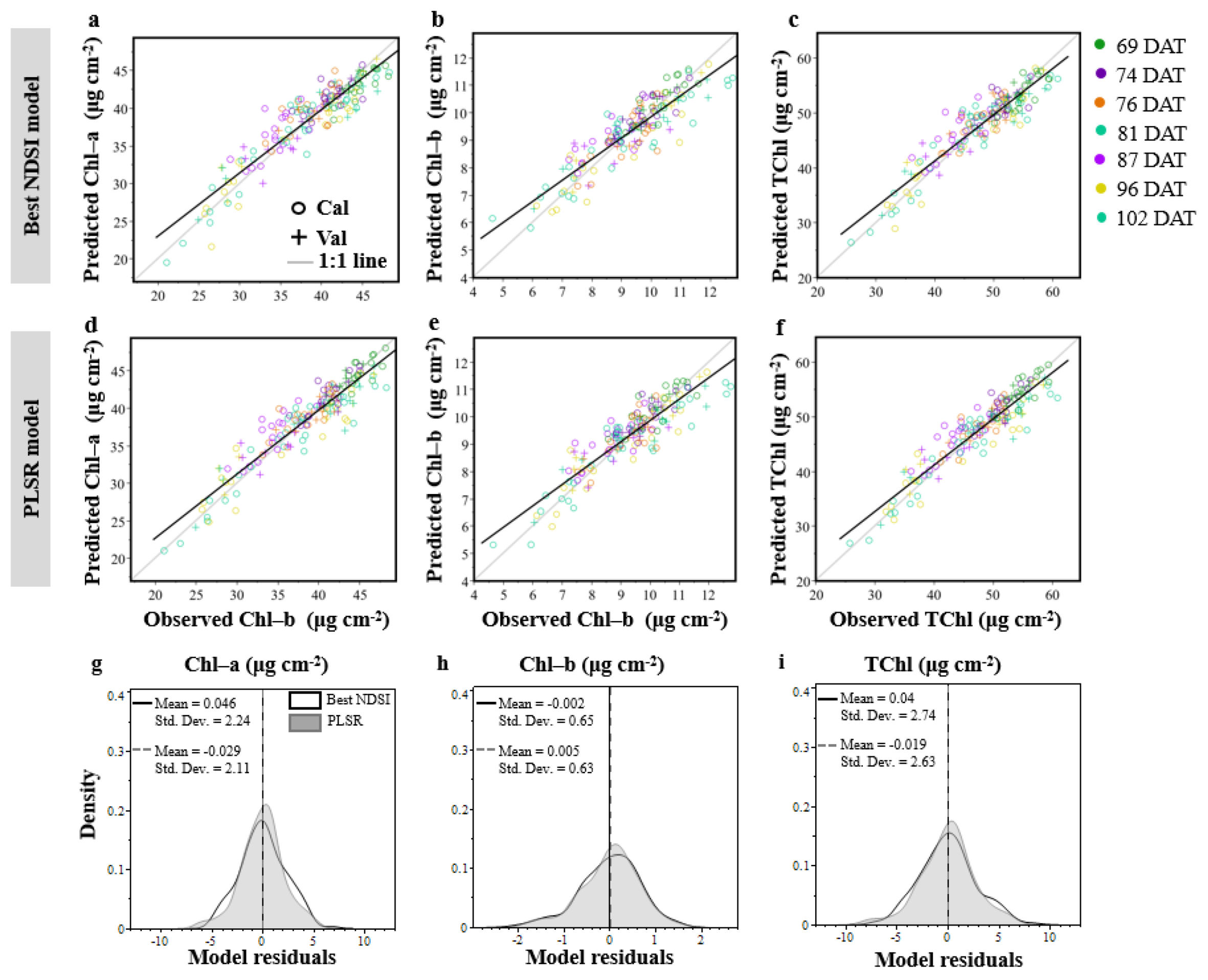
| Ranking 1 | VI | Cal R2 | RMSEPC (μg cm−2) | % RMSEPC | Val R2 | RMSEPV (μg cm−2) | % RMSEPV | References |
|---|---|---|---|---|---|---|---|---|
| Chl-a | (n = 118) | (n = 50) | ||||||
| 1 | NDSI415,614 | 0.85 | 2.34 | 8.54 | 0.87 | 2.02 | 9.24 | Current study |
| 2 | ZMI | 0.82 | 2.52 | 9.30 | 0.80 | 2.47 | 11.31 | [49] |
| 3 | CIred-edge | 0.82 | 2.54 | 9.37 | 0.81 | 2.43 | 11.10 | [19] |
| 4 | Carter 1 | 0.82 | 2.57 | 9.45 | 0.82 | 2.38 | 10.89 | [48] |
| 5 | CIgreen | 0.81 | 2.61 | 9.60 | 0.83 | 2.28 | 10.42 | [19] |
| 6 | GM1 | 0.81 | 2.62 | 9.64 | 0.83 | 2.28 | 10.43 | [47] |
| 7 | GM2 | 0.82 | 2.57 | 9.46 | 0.80 | 2.48 | 11.37 | [47] |
| 8 | NDRE | 0.81 | 2.62 | 9.66 | 0.80 | 2.47 | 11.31 | [50] |
| 9 | REIP | 0.80 | 2.71 | 9.97 | 0.81 | 2.44 | 11.15 | [51] |
| 10 | TGI | 0.78 | 2.81 | 10.35 | 0.80 | 2.51 | 11.49 | [52] |
| Chl-b | (n = 117) | (n = 50) | ||||||
| 1 | NDSI406,525 | 0.78 | 0.73 | 8.47 | 0.82 | 0.56 | 9.59 | Current study |
| 2 | Carter 1 | 0.67 | 0.88 | 10.86 | 0.56 | 0.79 | 13.49 | [48] |
| 3 | TGI | 0.64 | 0.90 | 11.15 | 0.63 | 0.76 | 12.90 | [52] |
| 4 | GM1 | 0.64 | 0.90 | 11.10 | 0.63 | 0.77 | 13.16 | [47] |
| 5 | CIgreen | 0.63 | 0.91 | 11.25 | 0.62 | 0.78 | 13.22 | [19] |
| 6 | TCARI | 0.62 | 0.92 | 11.37 | 0.61 | 0.78 | 13.26 | [53] |
| 7 | Datt1 | 0.60 | 0.95 | 11.71 | 0.56 | 0.85 | 14.42 | [54] |
| 8 | REIP | 0.59 | 0.96 | 11.84 | 0.54 | 0.84 | 14.30 | [51] |
| 9 | MCARI | 0.59 | 0.96 | 11.84 | 0.54 | 0.84 | 14.34 | [53] |
| 10 | NDRE | 0.58 | 0.97 | 11.84 | 0.55 | 0.85 | 14.45 | [50] |
| TChl | (n = 116) | (n = 50) | ||||||
| 1 | NDSI406,614 | 0.86 | 2.82 | 8.03 | 0.87 | 2.51 | 9.08 | Current study |
| 2 | GM1/Carter 1 | 0.85 | 2.91 | 8.30 | 0.85 | 2.61 | 9.42 | Current study |
| 3 | GM1-Carter 1 | 0.83 | 3.05 | 8.70 | 0.84 | 2.68 | 9.69 | Current study |
| 4 | Carter 1 | 0.80 | 3.30 | 9.42 | 0.79 | 3.07 | 11.09 | [48] |
| 5 | GM1 | 0.79 | 3.38 | 9.63 | 0.81 | 2.94 | 10.63 | [47] |
| 6 | CIgreen | 0.79 | 3.39 | 9.66 | 0.81 | 2.95 | 10.65 | [19] |
| 7 | ZMI | 0.79 | 3.40 | 9.68 | 0.77 | 3.28 | 11.84 | [49] |
| 8 | CIred-edge | 0.79 | 3.42 | 9.76 | 0.77 | 3.22 | 11.65 | [19] |
| 9 | GM2 | 0.79 | 3.44 | 9.81 | 0.76 | 3.31 | 11.95 | [47] |
| 10 | TGI | 0.77 | 3.55 | 10.12 | 0.78 | 3.14 | 11.34 | [52] |
| Chlorophyll | Cal R2 | RMSEPC (μg cm−2) | % RMSEPC | Val R2 | RMSEPV (μg cm−2) | % RMSEPV |
|---|---|---|---|---|---|---|
| Chl-a | 0.88 | 2.11 | 7.76 | 0.86 | 2.08 | 9.52 |
| Chl-b | 0.80 | 0.66 | 8.10 | 0.81 | 0.57 | 9.63 |
| TChl | 0.87 | 2.68 | 7.63 | 0.86 | 2.51 | 9.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulero, G.; Bacher, H.; Kleiner, U.; Peleg, Z.; Herrmann, I. Spectral Estimation of In Vivo Wheat Chlorophyll a/b Ratio under Contrasting Water Availabilities. Remote Sens. 2022, 14, 2585. https://doi.org/10.3390/rs14112585
Mulero G, Bacher H, Kleiner U, Peleg Z, Herrmann I. Spectral Estimation of In Vivo Wheat Chlorophyll a/b Ratio under Contrasting Water Availabilities. Remote Sensing. 2022; 14(11):2585. https://doi.org/10.3390/rs14112585
Chicago/Turabian StyleMulero, Gabriel, Harel Bacher, Uri Kleiner, Zvi Peleg, and Ittai Herrmann. 2022. "Spectral Estimation of In Vivo Wheat Chlorophyll a/b Ratio under Contrasting Water Availabilities" Remote Sensing 14, no. 11: 2585. https://doi.org/10.3390/rs14112585
APA StyleMulero, G., Bacher, H., Kleiner, U., Peleg, Z., & Herrmann, I. (2022). Spectral Estimation of In Vivo Wheat Chlorophyll a/b Ratio under Contrasting Water Availabilities. Remote Sensing, 14(11), 2585. https://doi.org/10.3390/rs14112585