Feasibility of Using Small UAVs to Derive Morphometric Measurements of Australian Snubfin (Orcaella heinsohni) and Humpback (Sousa sahulensis) Dolphins
Abstract
:1. Introduction
2. Methods
2.1. Assessing the Accuracy and Precision of Morphometric Measurements Using a Dolphin Replica
2.2. Collection and Processing of Morphometric Data from Live Dolphins
2.3. Reliability of Morphometric Measurements on Live Dolphins
3. Results
3.1. Assessing the Accuracy of Estimating the Size of the Replica
3.2. Accuracy of UAV Derived Length Measurements of Australian Snubfin and Humpback Dolphins
3.2.1. Data Collection
3.2.2. Morphometric Measurements of Australian Snubfin Dolphins
3.2.3. Morphometric Measurements of Australian Humpback Dolphins
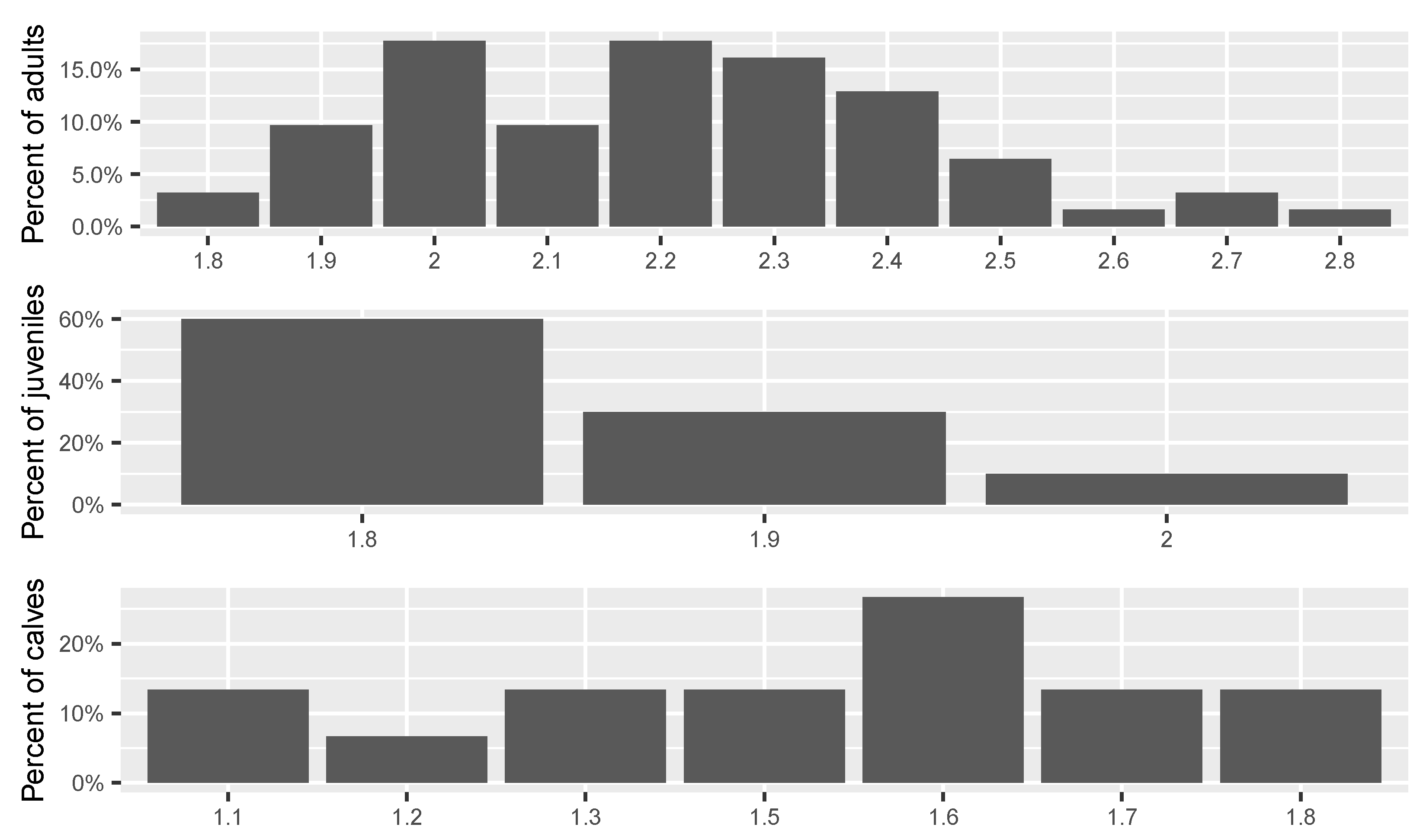
3.3. Morphometric Characteristics
4. Discussion
4.1. The Effect of the Covariates on the Reliability of Total Body Length Estimates
4.2. Comparison of Total Body Length Estimates with Previous Studies Available Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodgson, J.C.; Holman, D.; Terauds, A.; Koh, L.P.; Goldsworthy, S.D. Rapid condition monitoring of an endangered marine vertebrate using precise, non-invasive morphometrics. Biol. Conserv. 2020, 242, 108402. [Google Scholar] [CrossRef]
- Castrillon, J.; Bengtson Nash, S. Evaluating cetacean body condition; a review of traditional approaches and new developments. Ecol. Evol. 2020, 10, 6144–6162. [Google Scholar] [CrossRef]
- Berger, J. Estimation of body-size traits by photogrammetry in large mammals to inform conservation. Conserv. Biol. 2012, 26, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Vindenes, Y.; Edeline, E.; Ohlberger, J.; Langangen, Ø.; Winfield, I.J.; Stenseth, N.C.; Vøllestad, L.A. Effects of climate change on trait-based dynamics of a top predator in freshwater ecosystems. Am. Nat. 2014, 183, 243–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulanger, J.; Cattet, M.; Nielsen, S.E.; Stenhouse, G.; Cranston, J. Use of multi-state models to explore relationships between changes in body condition, habitat and survival of grizzly bearsUrsus arctos horribilis. Wildl. Biol. 2013, 19, 274–288. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, F.; Dawson, S.M.; Durban, J.W.; Fearnbach, H.; Miller, C.A.; Bejder, L.; Uhart, M.; Sironi, M.; Corkeron, P.; Rayment, W. Population comparison of right whale body condition reveals poor state of the North Atlantic right whale. Mar. Ecol. Prog. Ser. 2020, 640, 1–16. [Google Scholar] [CrossRef]
- Pastene, L.A.; Acevedo, J.; Branch, T.A. Morphometric analysis of Chilean blue whales and implications for their taxonomy. Mar. Mammal Sci. 2020, 36, 116–135. [Google Scholar] [CrossRef]
- Woodward, B.L.; Winn, J.P.; Fish, F.E. Morphological specializations of baleen whales associated with hydrodynamic performance and ecological niche. J. Morphol. 2006, 267, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Joblon, M.J.; Pokras, M.A.; Morse, B.; Harry, C.T.; Rose, K.S.; Sharp, S.M.; Niemeyer, M.E.; Patchett, K.M.; Sharp, W.B.; Moore, M.J. Body condition scoring system for delphinids based on short-beaked common dolphins (Delphinus delphis). J. Mar. Anim. Ecol. 2014, 7, 5–13. [Google Scholar]
- Cardona-Maldonado, M.A.; Mignucci-Giannoni, A.A. Pygmy and dwarf sperm whales in Puerto Rico and the Virgin Islands, with a review of Kogia in the Caribbean. Caribb. J. Sci. 1999, 35, 29–37. [Google Scholar]
- Turner, J.P.; Worthy, G.A. Skull morphometry of bottlenose dolphins (Tursiops truncatus) from the Gulf of Mexico. J. Mammal. 2003, 84, 665–672. [Google Scholar] [CrossRef] [Green Version]
- Bell, C.H.; Kemper, C.M.; Conran, J.G. Common dolphins (Delphinus delphis) in Southern Australia: A morphometric study. Aust. Mammal. 2002, 24, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Robeck, T.R.; Monfort, S.L.; Calle, P.P.; Dunn, J.L.; Jensen, E.; Boehm, J.R.; Young, S.; Clark, S.T. Reproduction, growth and development in captive beluga (Delphinapterus leucas). Zoo Biol. 2005, 24, 29–49. [Google Scholar] [CrossRef]
- Clark, S.T.; Odell, D.K. Allometric relationships and sexual dimorphism in captive killer whales (Orcinus orca). J. Mammal. 1999, 80, 777–785. [Google Scholar] [CrossRef] [Green Version]
- Tolley, K.; Read, A.; Wells, R.; Urian, K.; Scott, M.; Irvine, A.; Hohn, A. Sexual dimorphism in wild bottlenose dolphins (Tursiops truncatus) from Sarasota, Florida. J. Mammal. 1995, 76, 1190–1198. [Google Scholar] [CrossRef]
- Đuras, M.; Brnić, D.D.; Gomerčić, T.; Galov, A. Craniometry of bottlenose dolphins (Tursiops truncatus) from the Adriatic Sea. Vet. Arh. 2014, 84, 649–666. [Google Scholar]
- Booth, C.G.; Sinclair, R.R.; Harwood, J. Methods for monitoring for the population consequences of disturbance in marine mammals: A review. Front. Mar. Sci. 2020, 7, 115. [Google Scholar] [CrossRef] [Green Version]
- Beltran, R.S.; Ruscher-Hill, B.; Kirkham, A.L.; Burns, J.M. An evaluation of three-dimensional photogrammetric and morphometric techniques for estimating volume and mass in Weddell seals Leptonychotes weddellii. PLoS ONE 2018, 13, e0189865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnett, J.D.; Lemos, L.; Barlow, D.; Wing, M.G.; Chandler, T.; Torres, L.G. Estimating morphometric attributes of baleen whales with photogrammetry from small UASs: A case study with blue and gray whales. Mar. Mammal Sci. 2019, 35, 108–139. [Google Scholar] [CrossRef] [Green Version]
- Webster, T.; Dawson, S.; Slooten, E. A simple laser photogrammetry technique for measuring Hector’s dolphins (Cephalorhynchus hectori) in the field. Mar. Mammal Sci. 2010, 26, 296–308. [Google Scholar] [CrossRef]
- Wong, J.B.; Auger-Méthé, M. Using laser photogrammetry to measure long-finned pilot whales (Globicephala melas). Proc. Nova Scotian Inst. Sci. 2018, 49, 269. [Google Scholar] [CrossRef]
- Durban, J.; Parsons, K. Laser-metrics of free-ranging killer whales. Mar. Mammal Sci. 2006, 22, 735–743. [Google Scholar] [CrossRef]
- Van Aswegen, M.; Christiansen, F.; Symons, J.; Mann, J.; Nicholson, K.; Sprogis, K.; Bejder, L. Morphological differences between coastal bottlenose dolphin (Tursiops aduncus) populations identified using non-invasive stereo-laser photogrammetry. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fearnbach, H.; Durban, J.W.; Ellifrit, D.K.; Balcomb, K.C. Using aerial photogrammetry to detect changes in body condition of endangered southern resident killer whales. Endanger. Species Res. 2018, 35, 175–180. [Google Scholar] [CrossRef]
- Perryman, W.L.; Lynn, M.S. Evaluation of nutritive condition and reproductive status of migrating gray whales (Eschrichtius robustus) based on analysis of photogrammetric data. J. Cetacean Res. Manag. 2002, 4, 155–164. [Google Scholar]
- Miller, C.A.; Best, P.B.; Perryman, W.L.; Baumgartner, M.F.; Moore, M.J. Body shape changes associated with reproductive status, nutritive condition and growth in right whales Eubalaena glacialis and E. australis. Mar. Ecol. Prog. Ser. 2012, 459, 135–156. [Google Scholar] [CrossRef] [Green Version]
- Ratnaswamy, M.J.; Winn, H.E. Photogrammetric estimates of allometry and calf production in fin whales, Balaenoptera physalus. J. Mammal. 1993, 74, 323–330. [Google Scholar] [CrossRef]
- Durban, J.; Fearnbach, H.; Ellifrit, D.; Balcomb, K. Size and body condition of southern resident killer whales. In Contract Report to National Marine Fisheries Service; Northwest Regional Office: Seattle, WA, USA, 2009. [Google Scholar]
- Suydam, R.S. Age, Growth, Reproduction, and Movements of Beluga Whales (Delphinapterus leucas) from the Eastern Chukchi Sea. Ph.D. Thesis, University of Washington, Seattle, WA, USA, 2009. [Google Scholar]
- Christie, K.S.; Gilbert, S.L.; Brown, C.L.; Hatfield, M.; Hanson, L. Unmanned aircraft systems in wildlife research: Current and future applications of a transformative technology. Front. Ecol. Environ. 2016, 14, 241–251. [Google Scholar] [CrossRef]
- Watts, A.C.; Perry, J.H.; Smith, S.E.; Burgess, M.A.; Wilkinson, B.E.; Szantoi, Z.; Ifju, P.G.; Percival, H.F. Small unmanned aircraft systems for low-altitude aerial surveys. J. Wildl. Manag. 2010, 74, 1614–1619. [Google Scholar] [CrossRef]
- Linchant, J.; Lisein, J.; Semeki, J.; Lejeune, P.; Vermeulen, C. Are unmanned aircraft systems (UAS s) the future of wildlife monitoring? A review of accomplishments and challenges. Mammal. Rev. 2015, 45, 239–252. [Google Scholar] [CrossRef]
- Gray, P.C.; Bierlich, K.C.; Mantell, S.A.; Friedlaender, A.S.; Goldbogen, J.A.; Johnston, D.W. Drones and convolutional neural networks facilitate automated and accurate cetacean species identification and photogrammetry. Methods Ecol. Evol. 2019, 10, 1490–1500. [Google Scholar] [CrossRef]
- Durban, J.W.; Moore, M.J.; Chiang, G.; Hickmott, L.S.; Bocconcelli, A.; Howes, G.; Bahamonde, P.A.; Perryman, W.L.; LeRoi, D.J. Photogrammetry of blue whales with an unmanned hexacopter. Mar. Mammal Sci. 2016, 32, 1510–1515. [Google Scholar] [CrossRef]
- Christiansen, F.; Dujon, A.M.; Sprogis, K.R.; Arnould, J.P.; Bejder, L. Noninvasive unmanned aerial vehicle provides estimates of the energetic cost of reproduction in humpback whales. Ecosphere 2016, 7, e01468. [Google Scholar] [CrossRef]
- Dawson, S.M.; Bowman, M.H.; Leunissen, E.; Sirguey, P. Inexpensive aerial photogrammetry for studies of whales and large marine animals. Front. Mar. Sci. 2017, 4, 366. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, F.; Vivier, F.; Charlton, C.; Ward, R.; Amerson, A.; Burnell, S.; Bejder, L. Maternal body size and condition determine calf growth rates in southern right whales. Mar. Ecol. Prog. Ser. 2018, 592, 267–281. [Google Scholar] [CrossRef] [Green Version]
- Hughes, S.; Diggins, D.; Cerchio, S.; Bennett, A. Morphometric measurements of Omura’s whales using consumer grade sUASs: A methodological study. In Proceedings of the OCEANS 2019-Marseille, Marseille, France, 17–20 June 2019; pp. 1–8. [Google Scholar] [CrossRef]
- Durban, J.W.; Fearnbach, H.; Barrett-Lennard, L.; Perryman, W.; Leroi, D. Photogrammetry of killer whales using a small hexacopter launched at sea. J. Unmanned Veh. Syst. 2015, 3, 131–135. [Google Scholar] [CrossRef]
- Noren, S.; Schwarz, L.; Chase, K.; Aldrich, K.; McMahon-Van Oss, K.; Leger, J.S. Validation of the photogrammetric method to assess body condition of an odontocete, the shortfinned pilot whale Globicephala macrorhynchus. Mar. Ecol. Prog. Ser. 2019, 620, 185–200. [Google Scholar] [CrossRef]
- Adamczak, S.K.; Pabst, A.; McLellan, W.; Thorne, L. Using 3D models to improve estimates of marine mammal size and external morphology. Front. Mar. Sci. 2019, 6, 334. [Google Scholar] [CrossRef]
- Claridge, D.; Dunn, C.; Durban, J.; Fearnbach, H.; Perryman, W. Photogrammetry with an Unmanned Aerial System to Assess. Body Condition and Growth of Blainville’s Beaked Whales; National Oceanic and Atmospheric Administration: La Jolla, CA, USA, 2015.
- Krause, D.J.; Hinke, J.T.; Perryman, W.L.; Goebel, M.E.; LeRoi, D.J. An accurate and adaptable photogrammetric approach for estimating the mass and body condition of pinnipeds using an unmanned aerial system. PLoS ONE 2017, 12, e0187465. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, O.; Braun, C.; Esefeld, J.; Knetsch, S.; Maercker, J.; Pfeifer, C.; Rümmler, M.-C. Detecting antarctic seals and flying seabirds by uav. ISPRS Ann. Photogramm. Remote Sens. Spat. Inf. Sci. 2019, 4, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Goebel, M.E.; Perryman, W.L.; Hinke, J.T.; Krause, D.J.; Hann, N.A.; Gardner, S.; LeRoi, D.J. A small unmanned aerial system for estimating abundance and size of Antarctic predators. Polar Biol. 2015, 38, 619–630. [Google Scholar] [CrossRef] [Green Version]
- Allan, B.M.; Ierodiaconou, D.; Hoskins, A.J.; Arnould, J.P. A rapid UAV method for assessing body condition in fur seals. Drones 2019, 3, 24. [Google Scholar] [CrossRef] [Green Version]
- Fudala, K.; Bialik, R.J. Breeding colony dynamics of southern elephant seals at patelnia point, King George Island, Antarctica. Remote Sens. 2020, 12, 2964. [Google Scholar]
- Alvarado, D.C.; Robinson, P.W.; Frasson, N.C.; Costa, D.P.; Beltran, R.S. Calibration of aerial photogrammetry to estimate elephant seal mass. Mar. Mammal Sci. 2020, 36, 1347–1355. [Google Scholar]
- Stepien, E.N. Using UAVs for morphometric measurements of harbour porpoises (Phocoena phocoena). In Proceedings of the Nordic Remote Sensing, Aarhus, Denmark, 17–19 September 2019. [Google Scholar]
- Cagnazzi, D. Conservation Status of Australian Snubfin Dolphin, Orcaella heinsohni, and Indo-Pacific Humpback Dolphin, Sousa Chinensis, in the Capricorn Coast, Central Queensland Australia. Ph.D. Thesis, Southern Cross University, Lismore, Australia, 2010. [Google Scholar]
- Beasley, I.; Robertson, K.M.; Arnold, P. Description of a new dolphin, the Australian snubfin dolphin Orcaella heinsohni sp. n. (Cetacea, Delphinidae). Mar. Mammal Sci. 2005, 21, 365–400. [Google Scholar] [CrossRef]
- Arnold, P.; Heinsohn, G. Phylogenetic status of the Irrawaddy dolphin Orcaella brevirostris (Owen in Gray): A cladistic analysis. Mem. Qld. Mus. 1996, 39, 141–204. [Google Scholar]
- Jefferson, T.A.; Rosenbaum, H.C. Taxonomic revision of the humpback dolphins (Sousa spp.), and description of a new species from Australia. Mar. Mammal Sci. 2014, 30, 1494–1541. [Google Scholar] [CrossRef]
- Ross, G.J.; Heinsohn, G.; Cockcroft, V. Humpback dolphins Sousa chinensis (Osbeck, 1765), Sousa plumbea (G. Cuvier, 1829) and Sousa teuszii (Kukenthal, 1892). Handb. Mar. Mamm. 1994, 5, 23–42. [Google Scholar]
- Parra, G.J.; Cagnazzi, D. Conservation status of the Australian humpback dolphin (Sousa sahulensis) using the IUCN Red List Criteria. In Advances in Marine Biology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 73, pp. 157–192. [Google Scholar] [CrossRef]
- Parra, G.; Cagnazzi, D.; Beasley, I. Orcaella Heinsohni. The IUCN Red List of Threatened Species. 2017. Available online: https://www.iucnredlist.org/species/136315/123793740 (accessed on 16 December 2021).
- Hodgson, A.; Kelly, N.; Peel, D. Unmanned aerial vehicles (UAVs) for surveying marine fauna: A dugong case study. PLoS ONE 2013, 8, e79556. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, N.M.; Panebianco, A.; Gonzalez Musso, R.; Carmanchahi, P. An experimental approach to evaluate the potential of drones in terrestrial mammal research: A gregarious ungulate as a study model. R. Soc. Open Sci. 2020, 7, 191482. [Google Scholar] [CrossRef] [Green Version]
- Cagnazzi, D.; Parra, G.J.; Harrison, P.L.; Brooks, L.; Rankin, R. Vulnerability of threatened Australian humpback dolphins to flooding and port development within the southern Great Barrier Reef coastal region. Glob. Ecol. Conserv. 2020, 24, e01203. [Google Scholar] [CrossRef]
- Cagnazzi, D.; Parra, G.J.; Westley, S.; Harrison, P.L. At the heart of the industrial boom: Australian snubfin dolphins in the Capricorn Coast, Queensland, need urgent conservation action. PLoS ONE 2013, 8, e56729. [Google Scholar] [CrossRef] [Green Version]
- Colefax, A.P.; Butcher, P.A.; Pagendam, D.E.; Kelaher, B.P. Reliability of marine faunal detections in drone-based monitoring. Ocean Coast. Manag. 2019, 174, 108–115. [Google Scholar] [CrossRef]
- Raoult, V.; Colefax, A.P.; Allan, B.M.; Cagnazzi, D.; Castelblanco-Martínez, N.; Ierodiaconou, D.; Johnston, D.W.; Landeo-Yauri, S.; Lyons, M.; Pirotta, V. Operational protocols for the use of drones in marine animal research. Drones 2020, 4, 64. [Google Scholar] [CrossRef]
- Aleixo, F.; O’Callaghan, S.A.; Ducla Soares, L.; Nunes, P.; Prieto, R. AragoJ: A free, open-source software to aid single camera photogrammetry studies. Methods Ecol. Evol. 2020, 11, 670–677. [Google Scholar] [CrossRef] [Green Version]
- Colefax, A.P.; Kelaher, B.P.; Pagendam, D.E.; Butcher, P.A. Assessing white shark (Carcharodon carcharias) behavior along coastal beaches for conservation-focused shark mitigation. Front. Mar. Sci. 2020, 7, 268. [Google Scholar] [CrossRef]
- Tucker, J.P.; Colefax, A.P.; Santos, I.R.; Kelaher, B.P.; Pagendam, D.E.; Butcher, P.A. White shark behaviour altered by stranded whale carcasses: Insights from drones and implications for beach management. Ocean Coast. Manag. 2021, 200, 105477. [Google Scholar] [CrossRef]
- Geraeds, M.; van Emmerik, T.; de Vries, R.; bin Ab Razak, M.S. Riverine plastic litter monitoring using unmanned aerial vehicles (UAVs). Remote Sens. 2019, 11, 2045. [Google Scholar] [CrossRef] [Green Version]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Aho, K.; Derryberry, D.; Peterson, T. Model selection for ecologists: The worldviews of AIC and BIC. Ecology 2014, 95, 631–636. [Google Scholar] [CrossRef]
- Ripley, B.D. Modern Applied Statistics with S; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Nieuwenhuis, R.; Te Grotenhuis, H.; Pelzer, B. Influence. ME: Tools for Detecting Influential Data in Mixed Effects Models; Radboud University: Nijmegen, The Netherlands, 2012. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: Thousand Oaks, CA, USA, 2018. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. J. Math. Methods Biosci. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- Parra, G.J.; Corkeron, P.J.; Marsh, H. Population sizes, site fidelity and residence patterns of Australian snubfin and Indo-Pacific humpback dolphins: Implications for conservation. Biol. Conserv. 2006, 129, 167–180. [Google Scholar] [CrossRef]
- Parra, G.J.; Corkeron, P.J.; Arnold, P. Grouping and fission–fusion dynamics in Australian snubfin and Indo-Pacific humpback dolphins. Anim. Behav. 2011, 82, 1423–1433. [Google Scholar] [CrossRef]
- Mariani, M.; Miragliuolo, A.; Mussi, B.; Russo, G.F.; Ardizzone, G.; Pace, D.S. Analysis of the natural markings of Risso’s dolphins (Grampus griseus) in the central Mediterranean Sea. J. Mammal. 2016, 97, 1512–1524. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, F.; Sprogis, K.R.; Gross, J.; Castrillon, J.; Warick, H.A.; Leunissen, E.; Nash, S.B. Variation in outer blubber lipid concentration does not reflect morphological body condition in humpback whales. J. Exp. Biol. 2020, 223. [Google Scholar] [CrossRef]
- Smith, C.E.; Sykora-Bodie, S.T.; Bloodworth, B.; Pack, S.M.; Spradlin, T.R.; LeBoeuf, N.R. Assessment of known impacts of unmanned aerial systems (UAS) on marine mammals: Data gaps and recommendations for researchers in the United States. J. Unmanned Veh. Syst. 2016, 4, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Ramos, E.A.; Maloney, B.; Magnasco, M.O.; Reiss, D. Bottlenose dolphins and Antillean manatees respond to small multi-rotor unmanned aerial systems. Front. Mar. Sci. 2018, 5, 316. [Google Scholar] [CrossRef]
- Fettermann de Oliveira, T. Unmanned Aerial Vehicle (UAV) Remote Sensing of Behaviour and Habitat Use of the Nationally Endangered Bottlenose Dolphin (Tursiops truncatus) off Great Barrier Island. Ph.D. Thesis, Auckland University of Technology, Auckland, New Zealand, 2018. [Google Scholar]
- Raudino, H.C.; Tyne, J.A.; Smith, A.; Ottewell, K.; McArthur, S.; Kopps, A.M.; Chabanne, D.; Harcourt, R.G.; Pirotta, V.; Waples, K. Challenges of collecting blow from small cetaceans. Ecosphere 2019, 10, e02901. [Google Scholar] [CrossRef] [Green Version]
- Centelleghe, C.; Carraro, L.; Gonzalvo, J.; Rosso, M.; Esposti, E.; Gili, C.; Bonato, M.; Pedrotti, D.; Cardazzo, B.; Povinelli, M. The use of Unmanned Aerial Vehicles (UAVs) to sample the blow microbiome of small cetaceans. PLoS ONE 2020, 15, e0235537. [Google Scholar] [CrossRef]
- Meager, J. Marine wildlife stranding and mortality database annual report 2013–2015. Cetacean and Pinniped. Conserv. Tech. Data Rep. 2016, 1, 1–33. [Google Scholar]
- Meager, J. Marine wildlife stranding and mortality database annual report 2012. II. Cetacean Pinniped. Conserv. Tech. Data Rep. 2013, 2, 1–38. [Google Scholar]
- Meager, J.J.; Sumpton, W.D. Bycatch and strandings programs as ecological indicators for data-limited cetaceans. Ecol. Indic. 2016, 60, 987–995. [Google Scholar] [CrossRef] [Green Version]
- Parra, G.J. Behavioural Ecology of Irrawaddy, Orcaella brevirostris (Owen in Gray, 1866), and Indo-Pacific Humpback Dolphins, Sousa chinensis (Osbeck, 1765), in Northeast Queensland, Australia: A Comparative Study. Ph.D. Thesis, James Cook University, Townsville, Australia, 2005. [Google Scholar]
- Parra, G.J.; Jedensjö, M. Stomach contents of Australian snubfin (Orcaella heinsohni) and Indo-Pacific humpback dolphins (Sousa chinensis). Mar. Mammal Sci. 2014, 30, 1184–1198. [Google Scholar] [CrossRef]
- Weijs, L.; Vijayasarathy, S.; Villa, C.A.; Neugebauer, F.; Meager, J.J.; Gaus, C. Screening of organic and metal contaminants in Australian humpback dolphins (Sousa sahulensis) inhabiting an urbanised embayment. Chemosphere 2016, 151, 253–262. [Google Scholar] [CrossRef]

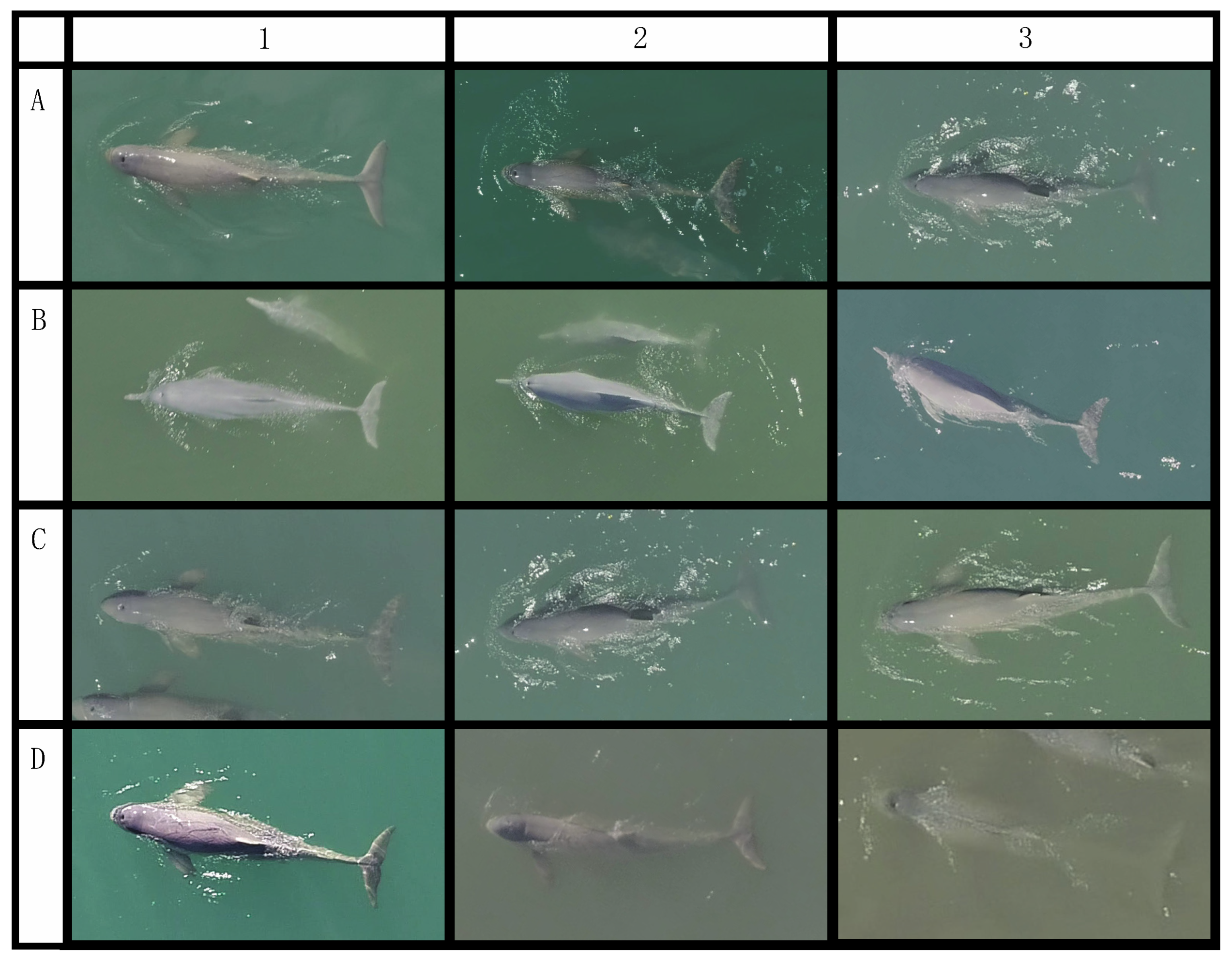
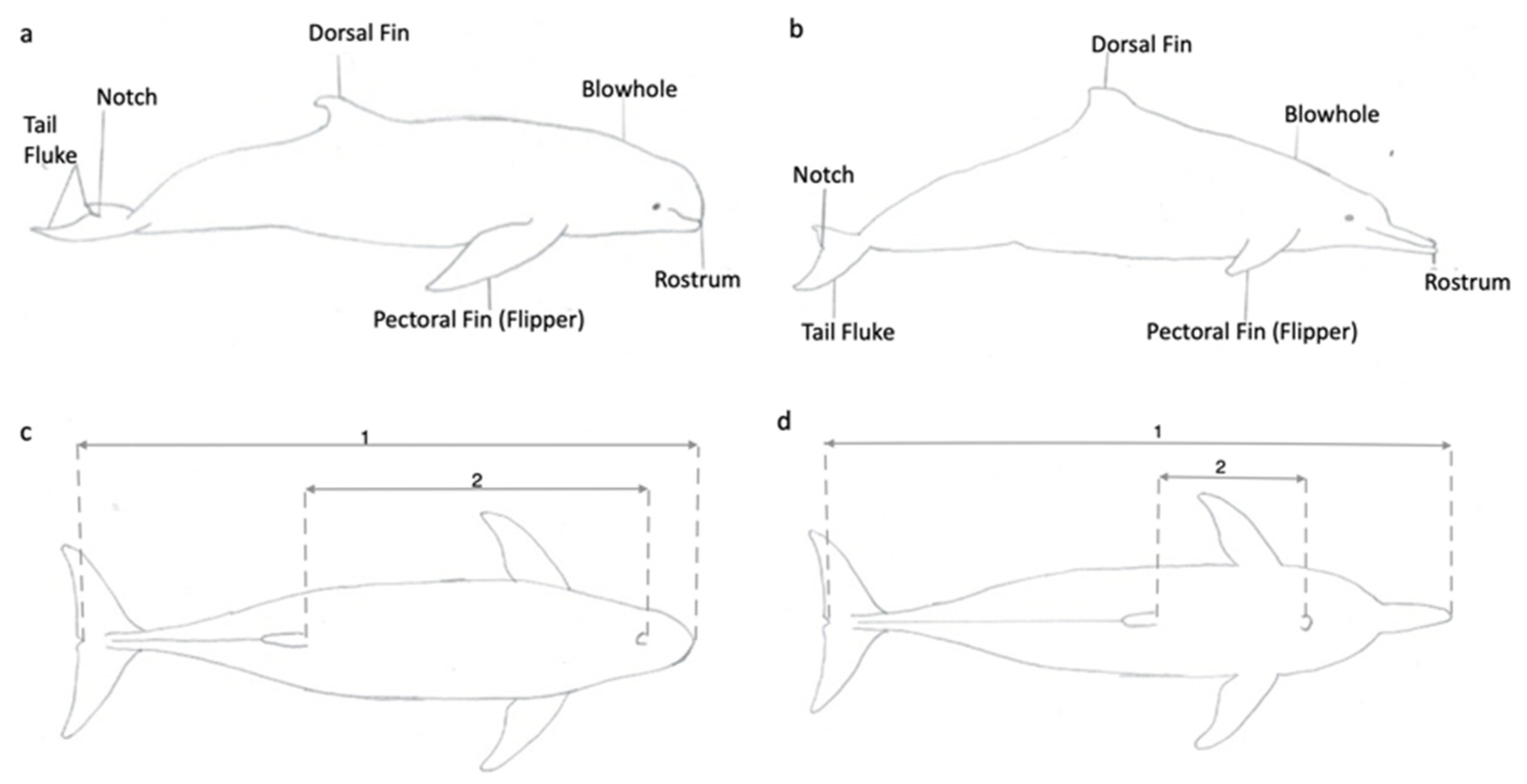

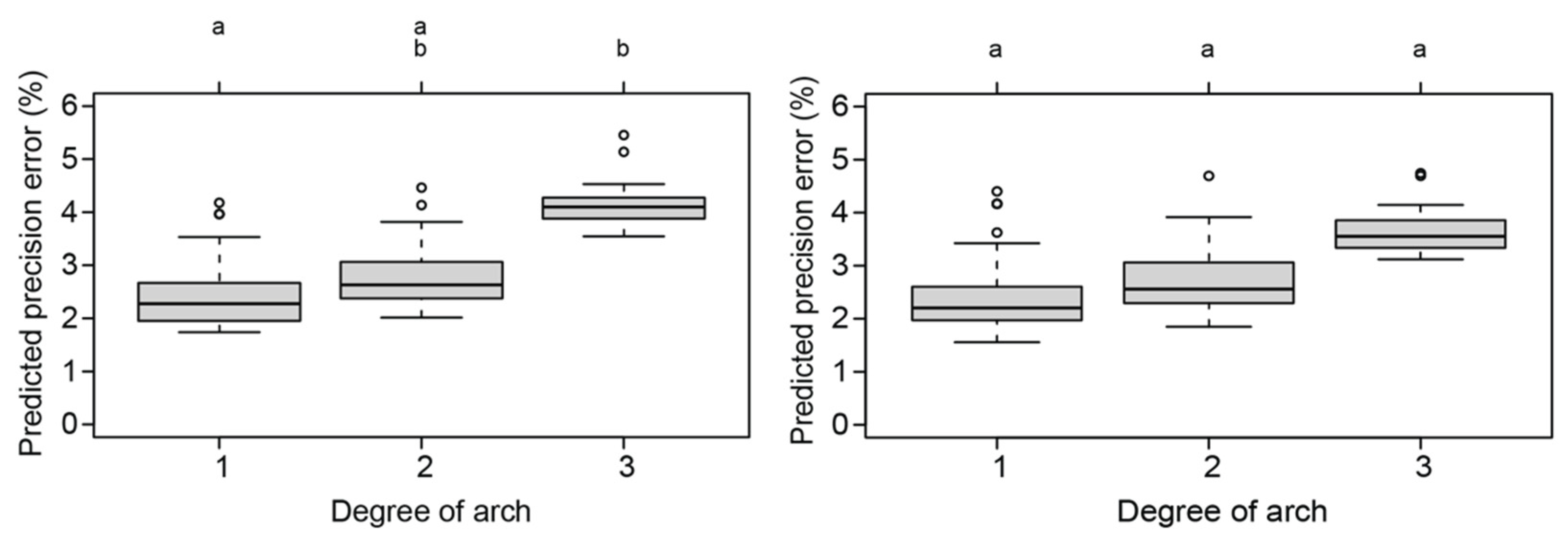
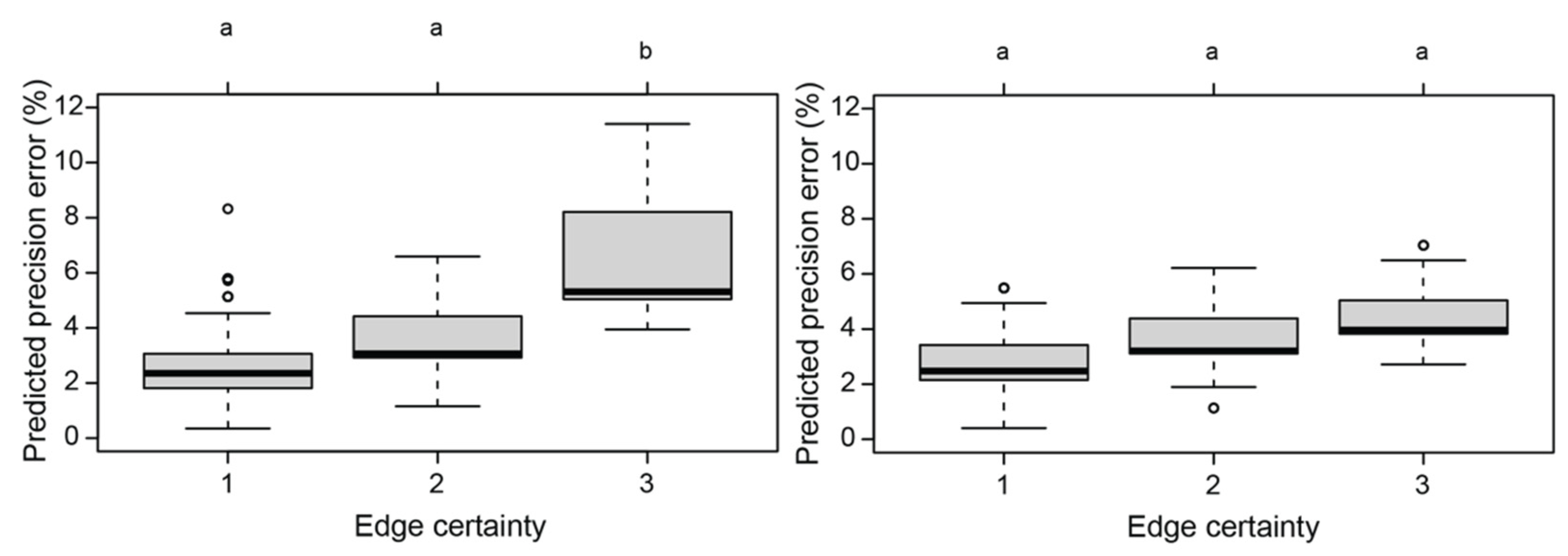

| Covariable | Good (1) | Medium (2) | Poor (3) | Unmeasurable (4) |
|---|---|---|---|---|
| Degree of arch | No visible bending or arching of the body. Animal is lying vertically at the surface. | The head and/or tail of the animal is slightly bent downwards. | The head and/or tail of the animal is significantly bent downward. | The head and/or tail of the animals is bent downward to the point where it is not visible. |
| Degree of straightness | The straight line from the rostrum to the fluke notch runs down the midline of the body axis along the dorsal fin and middle of the peduncle. | The straight line from the rostrum to the fluke notch runs closer to the edge of the animal, not along the dorsal fin and middle of the peduncle. | The straight line from the rostrum to the fluke notch runs outside of the edge of the animal, not along the dorsal fin and middle of the peduncle. | The straight line from the rostrum to the fluke notch run completely off the animals, not along the dorsal fin and middle of the peduncle. |
| Degree of body roll | Dorsal fin aligned within the midline of the body axis, with equal amount of the body visible on both sides. | Dorsal fin deviates slightly from midline, with larger amount of the body visible on one side. | Dorsal fin deviates significantly from the midline, with only one side of the body visible. | Dorsal fin is not visible, with only a section of one side and stomach visible. |
| Edge certainty | Points being measured are both clearly visible. | One of the points being measured is unclear. | Both of the points being measured is not visible. | One of the points being measured is not visible. |
| Altitude (m) | Number of Photos | |
|---|---|---|
| Snubfin Dolphins | Humpback Dolphins | |
| 15 | 285 | 85 |
| 20 | 97 | 27 |
| 30 | 70 | 50 |
| 40 | 28 | 46 |
| 50 | 31 | 20 |
| Fixed Effect | χ2 | Df | p | |
|---|---|---|---|---|
| Snubfin dolphins | Arch | 7.830 | 2 | 0.020 |
| Roll | 5.010 | 2 | 0.082 | |
| Snubfin dolphins: after removal of outliers | Arch | 7.830 | 2 | 0.020 |
| Roll | 5.010 | 2 | 0.082 | |
| Humpback dolphins | Edge cert. | 11.435 | 2 | 0.003 |
| Straightness | 2.949 | 1 | 0.086 | |
| Position | 5.120 | 2 | 0.078 | |
| Humpback dolphins: after removal of outliers | Edge cert. | 4.125 | 2 | 0.127 |
| Straightness | 3.835 | 1 | 0.050 | |
| Position | 4.728 | 2 | 0.094 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christie, A.I.; Colefax, A.P.; Cagnazzi, D. Feasibility of Using Small UAVs to Derive Morphometric Measurements of Australian Snubfin (Orcaella heinsohni) and Humpback (Sousa sahulensis) Dolphins. Remote Sens. 2022, 14, 21. https://doi.org/10.3390/rs14010021
Christie AI, Colefax AP, Cagnazzi D. Feasibility of Using Small UAVs to Derive Morphometric Measurements of Australian Snubfin (Orcaella heinsohni) and Humpback (Sousa sahulensis) Dolphins. Remote Sensing. 2022; 14(1):21. https://doi.org/10.3390/rs14010021
Chicago/Turabian StyleChristie, Anna I., Andrew P. Colefax, and Daniele Cagnazzi. 2022. "Feasibility of Using Small UAVs to Derive Morphometric Measurements of Australian Snubfin (Orcaella heinsohni) and Humpback (Sousa sahulensis) Dolphins" Remote Sensing 14, no. 1: 21. https://doi.org/10.3390/rs14010021
APA StyleChristie, A. I., Colefax, A. P., & Cagnazzi, D. (2022). Feasibility of Using Small UAVs to Derive Morphometric Measurements of Australian Snubfin (Orcaella heinsohni) and Humpback (Sousa sahulensis) Dolphins. Remote Sensing, 14(1), 21. https://doi.org/10.3390/rs14010021





