Remote Sensing Detection of Algal Blooms in a Lake Impacted by Petroleum Hydrocarbons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Satellite Monitoring
2.3. Phytoplankton Detection
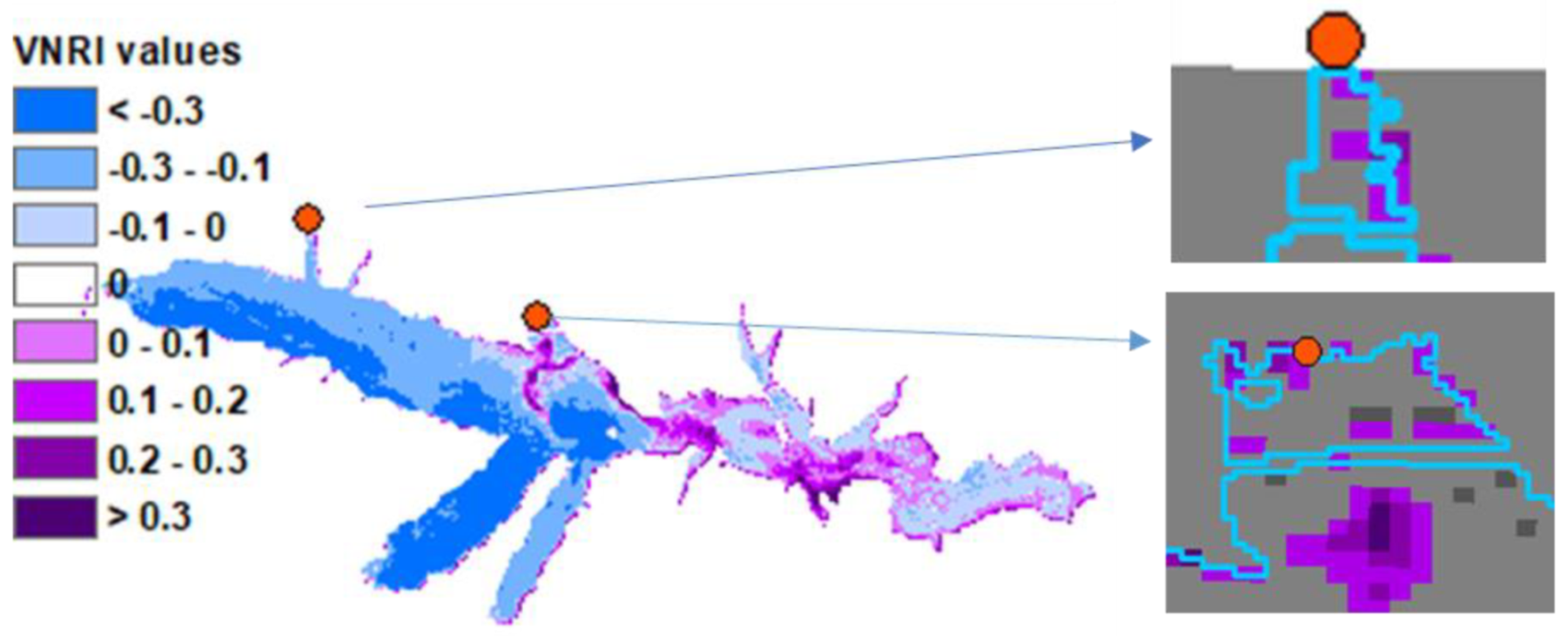
3. Results
3.1. Data Collection
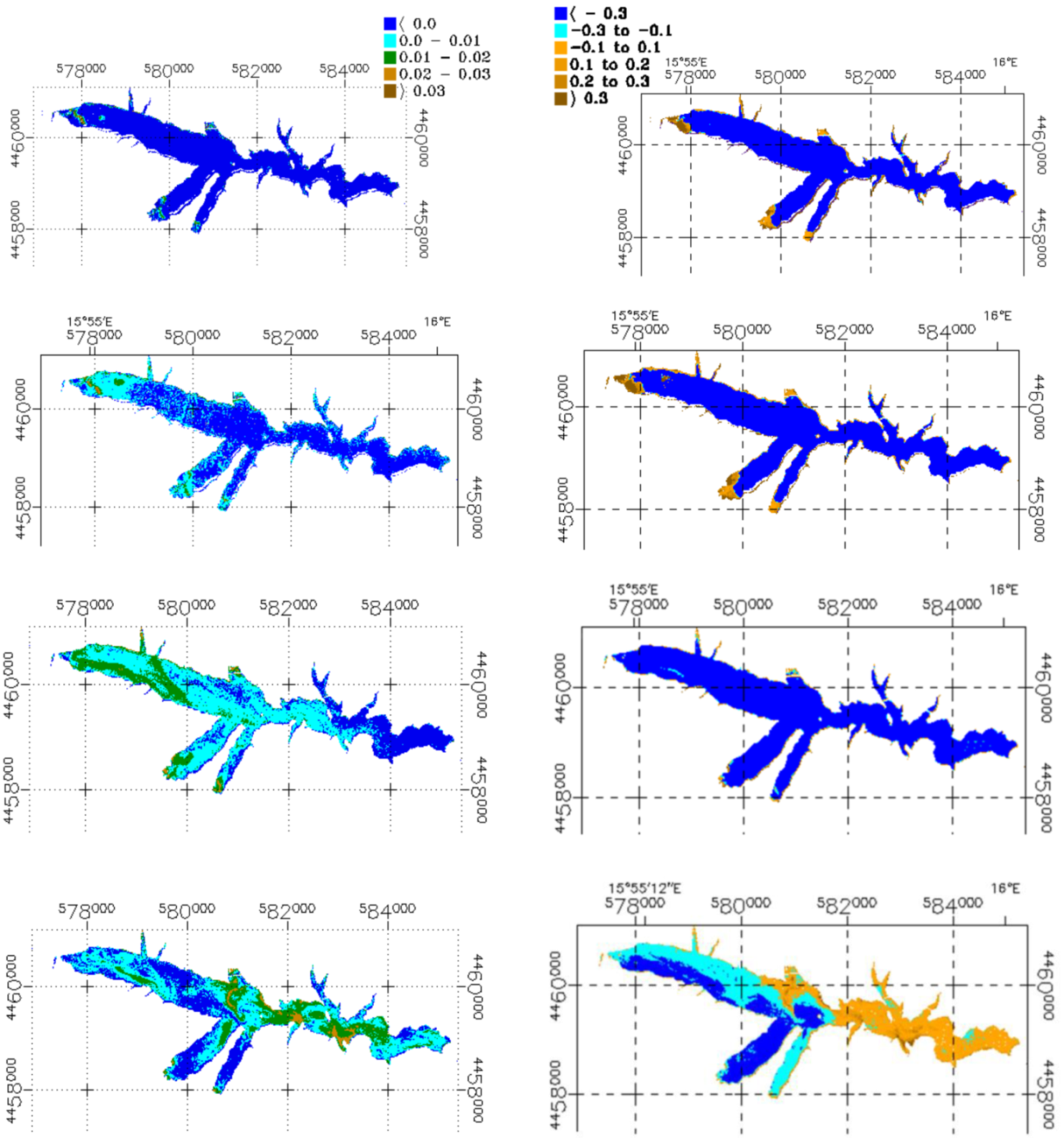
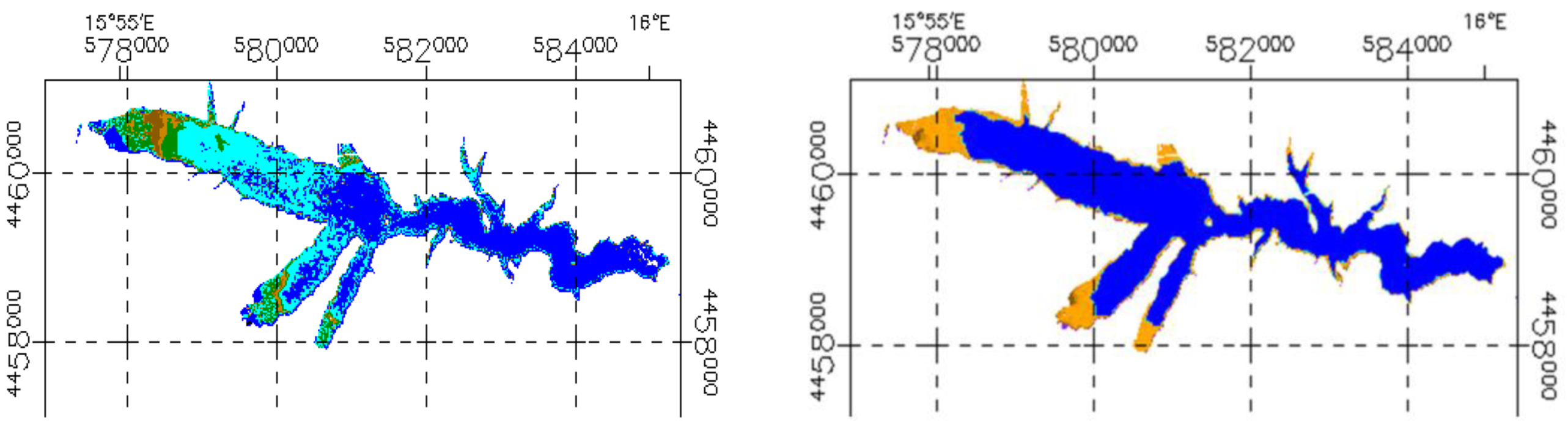
3.2. Sentinel-2 Imagery
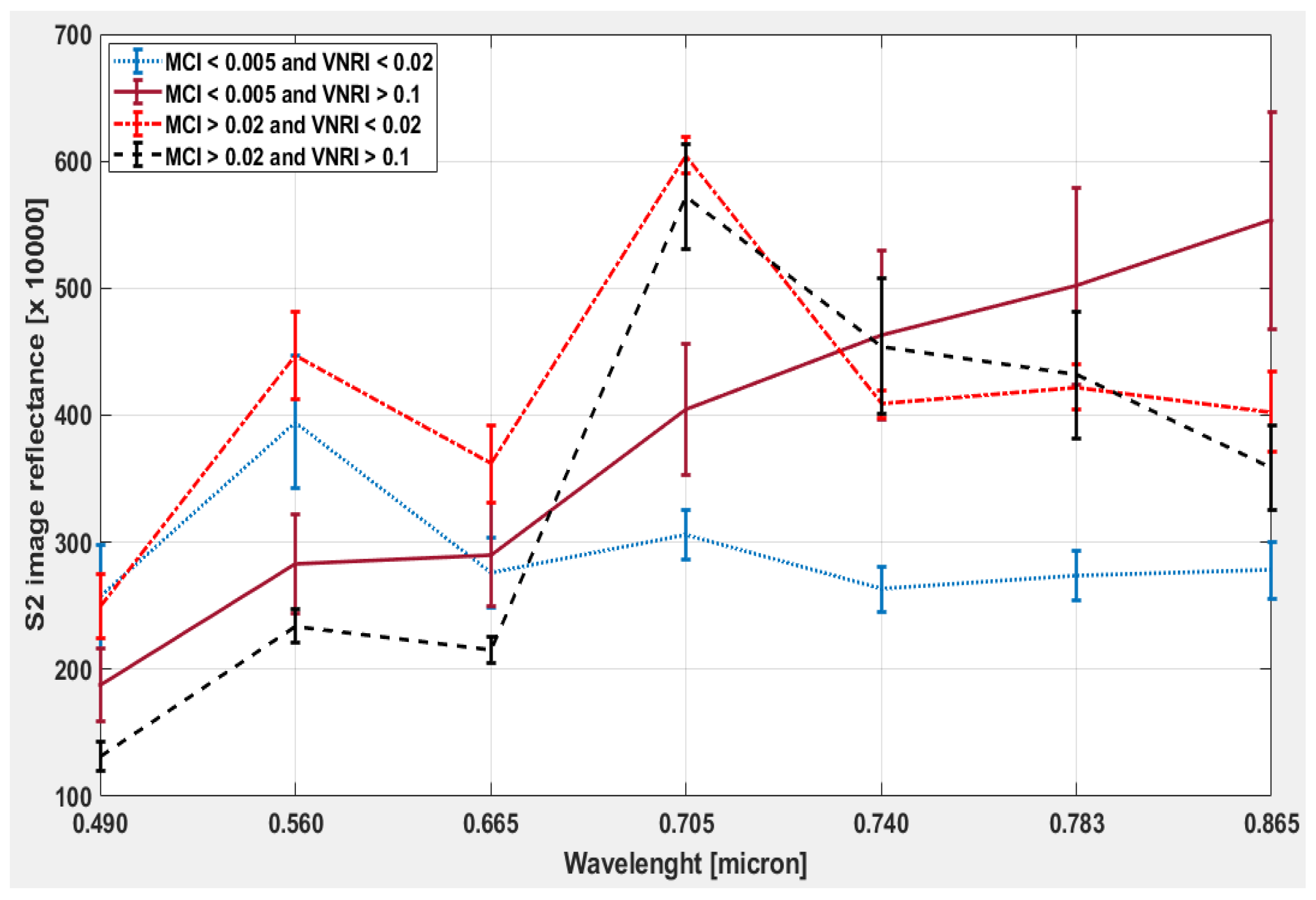
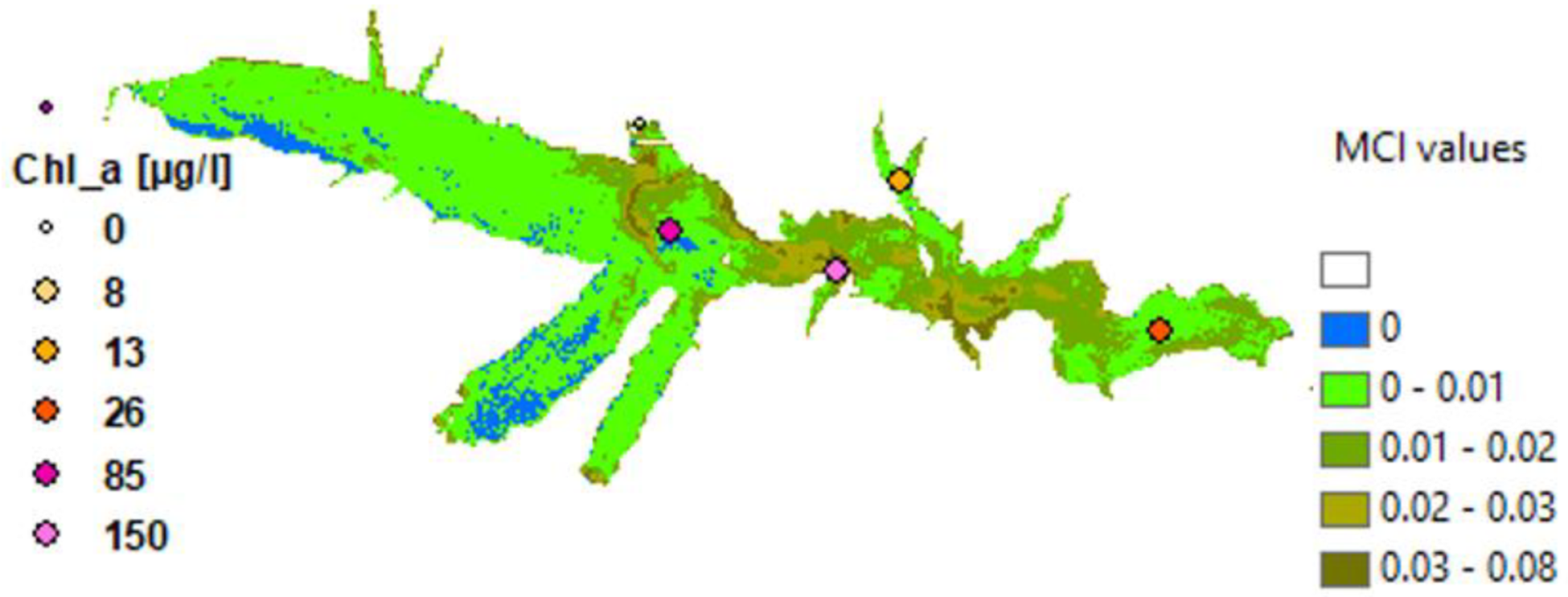
3.3. Phytoplankton Detection
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, D.Q.; Yu, Y.-X.; Zhang, X.Y.; Zhang, S.H.; Pang, Y.P.; Zhang, X.L.; Yu, Z.Q.; Wu, M.H.; Fu, J.M. Polycyclic aromatic hydrocarbons and organochlorine pesticides in fish from Taihu Lake: Their levels, sources, and biomagnification. Ecotoxicol. Environ. Saf. 2012, 82, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Akporido, S.O.; Onianwa, P.C. Heavy metals and total petroleum hydrocarbon concentrations in surface water of Esi River, western Niger Delta. Res. J. Environ. Sci. 2015, 9, 88–100. [Google Scholar] [CrossRef]
- Cova Contro. 2017. Available online: http://analizebasilicata.altervista.org/blog/idrocarburi-nel-pertusillo-presenti-in-tre-campioni-su-tre-oltrea-fosfati-e-manganese/?doing_wp_cronm1517936436.7830340862274169921875 (accessed on 15 November 2021).
- Ostgaard, K. The oil, the water and the phytoplankton. In Algae and Water Pollution; Advances in Limnology; Rai, L.C., Gaur, J.P., Soeder, C.J., Eds.; Springer: Berlin, Germany, 1994; Volume 42, pp. 167–193. [Google Scholar]
- Almeda, R.; Cosgrove, S.; Buskey, E.J. Oil spills and dispersants can cause the initiation of potentially harmful dinoflagellate blooms (“red tides”). Environ. Sci. Technol. 2018, 52, 5718–5724. [Google Scholar] [CrossRef] [PubMed]
- Feron, V.J.; Groten, J.P.; van Bladeren, P.J. Exposure of Humans to Complex Chemical Mixtures: Hazard Identification and Risk Assessment. In Diversification in Toxicology—Man and Environment; Archives of Toxicology; Seiler, J.P., Autrup, J.L., Autrup, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; Volume 20, pp. 363–373. [Google Scholar]
- Krewski, D.; Thorslund, T.; Withey, J. Carcinogenic Risk Assessment of Complex Mixtures. Toxicol. Ind. Health 1989, 5, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Heys, K.A.; Shore, R.F.; Pereira, M.G.; Jones, K.C.; Martin, F.L. Risk assessment of environmental mixture effects. RSC Adv. 2016, 6, 47844–47857. [Google Scholar] [CrossRef] [Green Version]
- Palikova, M.; Papezikova, I.; Kopp, R.; Mares, J.; Markova, Z.; Navratil, S.; Adamovsky, O.; Kohoutek, J.; Navratil, L.; Blaha, L. Effect of arsenic and cyanobacterial co-exposure on pathological, haematological and immunological parameters of rainbow trout (Oncorhynchus mykiss). Neuroendocrinol. Lett. 2014, 35, 101–107. [Google Scholar]
- Kopp, R.; Mares, J.; Soukupova, Z.; Navratil, S.; Palikova, M. Influence of arsenic and cyanobacteria coexposure on plasmatic parameters of rainbow trout (Oncorhynchus mykiss W.). Neuroendocrinol. Lett. 2014, 35, 57–63. [Google Scholar]
- Harris, T.D.; Smith, V.H. Do persistent organic pollutants stimulate cyanobacterial blooms? Inland Waters 2016, 6, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.C.; Dai, J.Y.; Ma, B.X.; Luo, X.J.; Cao, H.; Wang, J.S.; Li, F.C.; Xu, M.Q. Concentrations and accumulation features of organochlorine pesticides on the Baiyangdian Lake freshwater food web of North China. Arch. Environ. Contam. Toxicol. 2010, 58, 700–710. [Google Scholar] [CrossRef]
- Takeuchi, I.; Miyoshi, N.; Mizukawa, K.; Takada, H.; Ikemoto, T.; Omori, K.; Tsuchiya, K. Biomagnification profiles of polycyclic aromatic hydrocarbons, alkylphenols and polychlorinated biphenyls in Tokyo Bay elucidated by 13C and 15N isotope ratios as guides to trophic web structure. Mar. Pollut. Bull. 2009, 58, 663–671. [Google Scholar] [CrossRef]
- Santodonato, J. Review of the estrogenic and antiestrogenic activity of polycyclic aromatic hydrocarbons: Relationship to carcinogenicity. Chemosphere 1997, 34, 835–848. [Google Scholar] [CrossRef]
- Mnif, W.; Hassine, A.I.H.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of endocrine disruptor pesticides: A review. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collier, T.; Varanasi, U. Hepatic activities of xenobiotic metabolizing enzymes and biliary levels of xenobiotics in English sole (Parophrys vetulus) exposed to environmental contaminants. Arch. Environ. 1991, 20, 462–473. [Google Scholar] [CrossRef]
- Livingstone, D.R. The fate of organic xenobiotics in aquatic ecosystems: Quantitative and qualitative differences in bio-transformation by invertebrates and fish. Comp. Biochem. Physiol. 1998, 120, 43–49. [Google Scholar] [CrossRef]
- Odermatt, D.; Stelzer, K.; Koponen, S.; Philipson, P.; Brockmann, C.; Saile, P.; Koetz, B. Water Quality Remote Sensing in Support of the UN Sustainable Development Goals. In Proc. ESA Living Planet Symposium; ESA/ESRIN: Prague, Czech Republic, 2016; p. 5. [Google Scholar]
- Schaeffer, B.A.; Schaeffer, K.G.; Keith, D.; Lunetta, R.S.; Conmy, R.; Gould, R.W. Barriers to adopting satellite remote sensing for water quality management. Int. J. Remote Sens. 2013, 34, 7534–7544. [Google Scholar] [CrossRef]
- Richardson, L.L.; Bachoon, D.; lngram-Willey, V.; Chee Chow, C.; Weinstock, K. Remote Sensing of the biological dynamics of large-scale salt evaporation ponds. In Proceedings of the International Symposium on Remote Sensing of Environment, Rio de Janeiro, Brazil, 27–31 May 1991; pp. 511–623. [Google Scholar]
- Richardson, L.L.; Buisson, D.; Ambrosia, V. Use of Remote Sensing coupled with algal accessory pigment data to study phytoplankton bloom dynamics in Florida Bay. In Proceedings of the Third Thematic Conference on Remote Sensing for Marine and Coastal Environments, Seattle, WA, USA, 18–20 September 1995; Volume 1, pp. 183–192. [Google Scholar]
- Blondeau-Patissier, D.; Gower, J.F.R.; Dekker, A.G.; Phinn, S.R.; Brando, V.E. A review of ocean color remote sensing methods and statistical techniques for the detection, mapping and analysis of phytoplankton blooms in coastal and open oceans. Prog. Oceanogr. 2014, 123, 123–144. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Mishra, D.R. A novel remote sensing algorithm to quantify phycocyanin in cyanobacterial algal blooms. Environ. Res. Lett. 2014, 9, 114003. [Google Scholar] [CrossRef]
- Gorham, T.; Jia, Y.; Shumb, C.K.; Lee, J. Ten-year survey of cyanobacterial blooms in Ohio’s waterbodies using satellite remote sensing. Harmful Algae 2017, 66, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lee, J.; Liang, S.; Shum, C.K. Cyanobacteria blooms and non-alcoholic liver disease: Evidence from a county level ecological study in the United States. Environ. Health 2015, 14, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Li, G.; Chen, J.; Lin, J.; Zeng, C.; Chen, J.; Deng, J.; Xie, P. Prolonged exposure to low-dose microcystin induces nonalcoholic steatohepatitis in mice: A system toxicology study. Arch. Toxicol. 2017, 91, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Drusch, M.; Del Bello, U.; Carlier, S.; Colin, O.; Fernandez, V.; Gascon, F.; Hoersch, B.; Isola, C.; Laberinti, P.; Martimort, P.; et al. Sentinel-2: ESA’s Optical High-Resolution Mission for GMES Operational Services. Remote Sens. Environ. 2012, 120, 25–36. [Google Scholar] [CrossRef]
- Liu, H.; Li, Q.; Shi, T.; Hu, S.; Wu, G.; Zhou, Q. Application of Sentinel 2 MSI images to retrieve suspended particulate matter concentrations in Poyang Lake. Remote Sens. 2017, 9, 761. [Google Scholar] [CrossRef] [Green Version]
- Caballero, I.; Fernández, R.; Escalante, O.M. New capabilities of Sentinel-2A/B satellites combined with in situ data for monitoring small harmful algal blooms in complex coastal waters. Sci. Rep. 2020, 10, 8743. [Google Scholar] [CrossRef]
- Rodríguez-Benito, C.V.; Navarro, G.; Caballero, I. Using Copernicus Sentinel-2 and Sentinel-3 data to monitor harmful algal blooms in Southern Chile during the COVID-19 lockdown. Marine Poll. Bull. 2020, 161, 111722. [Google Scholar] [CrossRef]
- Bramich, J.; Bolch, C.J.S.; Fischer, A. Improved red-edge chlorophyll a detection for Sentinel 2. Ecol. Ind. 2021, 120, 106876. [Google Scholar] [CrossRef]
- Pahlevan, N.; Sarkar, S.; Franz, B.A.; Balasubramanian, S.V.; He, J. Sentinel-2 MultiSpectral Instrument (MSI) data processing for aquatic science applications: Demonstrations and validations. Remote Sens. Environ. 2017, 201, 47–56. [Google Scholar] [CrossRef]
- Toming, K.; Kutser, T.; Laas, A.; Sepp, M.; Paavel, B.; Nõges, T. First experiences in mapping lake water quality parameters with Sentinel-2 MSI Imagery. Remote Sens. 2016, 8, 640. [Google Scholar] [CrossRef] [Green Version]
- Gitelson, A. The peak near 700 nm on radiance spectra of algae and water: Relationships of its magnitude and position with chlorophyll concentration. Int. J. Remote Sens. 1992, 13, 3367–3373. [Google Scholar] [CrossRef]
- Kolokoussis, P.; Karathanassi, V. Oil spill detection and mapping using Sentinel 2 Imagery. J. Mar. Sci. Eng. 2018, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Bulgarelli, B.; Djavidnia, S. On MODIS retrieval of oil spill spectral properties in the marine environment. IEEE Geosci. Remote Sens. Lett. 2012, 9, 398–402. [Google Scholar] [CrossRef]
- Zhao, J.; Temimi, M.; Ghedira, H.; Hu, C. Exploring the potential of optical remote sensing for oil spill detection in shallow coastal waters-a case study in the Arabian Gulf. Opt. Express 2014, 22, 13755–13772. [Google Scholar] [CrossRef]
- Luciani, R.; Laneve, G. Oil Spill detection using optical sensors: A multi-temporal approach. In Satellite Oceanography and Meteorology; Whioce Publishing Pte. Ltd.: Singapore, 2018. [Google Scholar] [CrossRef] [Green Version]
- Fingas, M.; Brown, C.E. A review of oil spill remote sensing. Sensors 2018, 18, 91. [Google Scholar] [CrossRef] [Green Version]
- Haule, K.; Freda, W.; Darecki, M.; Toczek, H. Possibilities of optical remote sensing of dispersed oil in coastal waters. Estuar. Coast. Shelf Sci. 2017, 195, 76–87. [Google Scholar] [CrossRef]
- USEPA. 2011. Available online: https://www.epa.gov/sites/production/files/documents/comparisonofhffluidscompositionwithproducedformationwater.pdf (accessed on 15 November 2021).
- ARPAB. 2017. Available online: http://www.arpab.it/risorse_idriche/rapporti2017.asp (accessed on 15 November 2021).
- Zohary, T.; Sukenik, A.; Berman, T.; Nishri, A. Lake Kinneret: Ecology and Management; Springer: Dordrecht, The Netherlands, 2014; p. 683. [Google Scholar]
- De Pace, R.; Storelli, M.M.; Barone, G.; Messineo, V.; Bruno, M. Co-occurrence of polychlorinated biphenyls, cyanotoxins and trace elements in commercial fish species from a freshwater protected area (Pertusillo Lake, Southern Italy). J. Geogr. Environ. Earth Sci. Int. 2019, 22, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Cova Contro. 2017. Available online: http://analizebasilicata.altervista.org/blog/ancora-idrocarburi-ed-azoto-nel-pertusillo-anche-il-quartocampione-e-contaminato (accessed on 15 November 2021).
- ARPAB. 2017. Available online: http://www.arpab.it/risorse_idriche/public/RELAZIONE%20PERTUSILLO_19102017_pubblicato_rev_031117.pdf (accessed on 15 November 2021).
- D’Ugo, E.; Bruno, M.; Mukherjee, A.; Chattopadhyay, D.; Giuseppetti, R.; De Pace, R.; Magurano, F. Characterization of microbial response to petroleum hydrocarbon contamination in a lacustrine ecosystem. Environ. Sci. Poll. Res. 2021, 28, 26187–26196. [Google Scholar] [CrossRef] [PubMed]
- Calderoni, A.; Mosello, R. Caratteristiche termiche e chimiche. In Il Lago Di Pietra Del Pertusillo: Definizione Delle Sue Caratteristiche Limnoecologiche, Ed. Ist. Ital. Idrobiol; Istituto Italiano di Idrobiologia: Pallanza, Italy, 1978; pp. 3–66. [Google Scholar]
- Colella, A. Hydrocarbons and metals in waters and sediments of the Pertusillo Lake, Italy. Fresenius Environ. Bull. 2012, 21, 3003–3011. [Google Scholar]
- Colella, A.; D’ Orsogna, M.R. Hydrocarbon contamination in waters and sediments of the Pertusillo freshwater reservoir, Val D’agri, Southern Italy. Fresenius Environ. Bull. 2014, 23, 3286–3295. [Google Scholar]
- Improta, L.; Valoroso, L.; Piccinini, D.; Chiarabba, C. A detailed analysis of wastewater-induced seismicity in the Val d’Agri oil field (Italy). Geophys. Res. Lett. 2015, 42, 2682–2690. [Google Scholar] [CrossRef]
- Buttinelli, M.; Improta, L.; Bagh, S.; Chiarabba, C. Inversion of inherited thrusts by wastewater injection induced seismicity at the Val d’Agri oilfield (Italy). Scient. Rep. 2016, 6, 37165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soriano-González, J.; Angelats, E.; Fernández-Tejedor, M.; Diogene, J.; Alcaraz, C. First results of phytoplankton spatial dynamics in two NW-Mediterranean bays from chlorophyll a estimates using Sentinel 2: Potential implications for aquaculture. Remote Sens. 2019, 11, 1756. [Google Scholar] [CrossRef] [Green Version]
- Gower, J.; King, S.; Borstad, G.; Brown, L. Detection of intense plankton blooms using the 709 nm band of the MERIS imaging spectrometer. Int. J. Remote Sens. 2005, 26, 2005–2012. [Google Scholar] [CrossRef]
- Salem, S.I.; Strand, M.H.; Higa, H.; Kim, H.; Kazuhiro, K.; Oki, K.; Oki, T. Evaluation of MERIS chlorophyll a retrieval processors in a complex turbid Lake Kasumigaura over a 10-year mission. Remote Sens. 2017, 9, 1022. [Google Scholar] [CrossRef] [Green Version]
- Lund, J.W.G.; Kipling, C.; Le Cren, E. The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 1958, 11, 143–170. [Google Scholar] [CrossRef]
- Utermőhl, H. Neue Wege in der quantitativen Earfassung des Planktons (mit besonderer Berücksichtigung des Ultraplanktons). Verh. Int. Ver. Theor. Angew. Limnol. 1931, 5, 567–596. [Google Scholar]
- Kutser, T.; Paavel, B.; Verpoorter, C.; Ligi, M.; Soomets, T.; Toming, K.; Casal, G. Remote Sensing of Black Lakes and using 810 nm reflectance peak for retrieving water quality parameters of optically complex waters. Remote Sens. 2016, 8, 497. [Google Scholar] [CrossRef]
- Ruggiu, D.; Saraceni, C. Struttura dei popolamenti algali e produzione primaria. In II Lago Di Pietra Del Pertusillo: Definizione Delle Sue Caratteristiche Limnoecologiche, Ed. Ist. Ital. Idrobiol; Istituto Italiano di Idrobiologia: Pallanza, Italiy, 1978; pp. 67–98. [Google Scholar]
- Dagenais-Bellefeuille, S.; Morse, D. Putting the N in dinoflagellates. Front. Microbiol. 2013, 4, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smalley, G.W.; Coats, D.W.; Stoecker, D.K. Feeding in the mixotrophic dinoflagellate Ceratium furca is influenced by intracellular nutrient concentrations. Mar. Ecol. Prog. Ser. 2013, 262, 137–151. [Google Scholar] [CrossRef]
- Das, N.; Chandran, P. Microbial Degradation of Petroleum Hydrocarbon Contaminants: An Overview. Biotech. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-R, L.M.; Overholt, W.A.; Hagan, C.; Huettel, M.; Kostka, J.E.; Konstantinidis, K.T. Microbial community successional patterns in beach sands impacted by the Deepwater Horizon oil spill. ISME J. 2015, 9, 1928–1940. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Weisberg, R.H.; Liu, Y.; Zheng, L.; Daly, K.L.; English, D.C.; Zhao, J.; Vargo, G.A. Did the northeastern Gulf of Mexico become greener after the Deepwater Horizon oil spill? Geophys. Res. Lett. 2011, 38, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Hisakawa, N.; Quistad, S.D.; Hester, E.R. Metagenomic and satellite analyses of red snow in the Russian Arctic. PeerJ 2015, 3, e1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francini-Filho, R.B.; Cordeiro, M.C.; Omachi, C.Y.; Rocha, A.M.; Bahiense, L.; Garcia, G.D.; Tschoeke, D.; de Almeida, M.G.; Rangel, T.P.; De Oliveira, B.C.V.; et al. Remote sensing, isotopic composition and metagenomics analyses revealed Doce River ore plume reached the southern Abrolhos Bank Reefs. Sci. Total Environ. 2019, 697, 134038. [Google Scholar] [CrossRef] [PubMed]
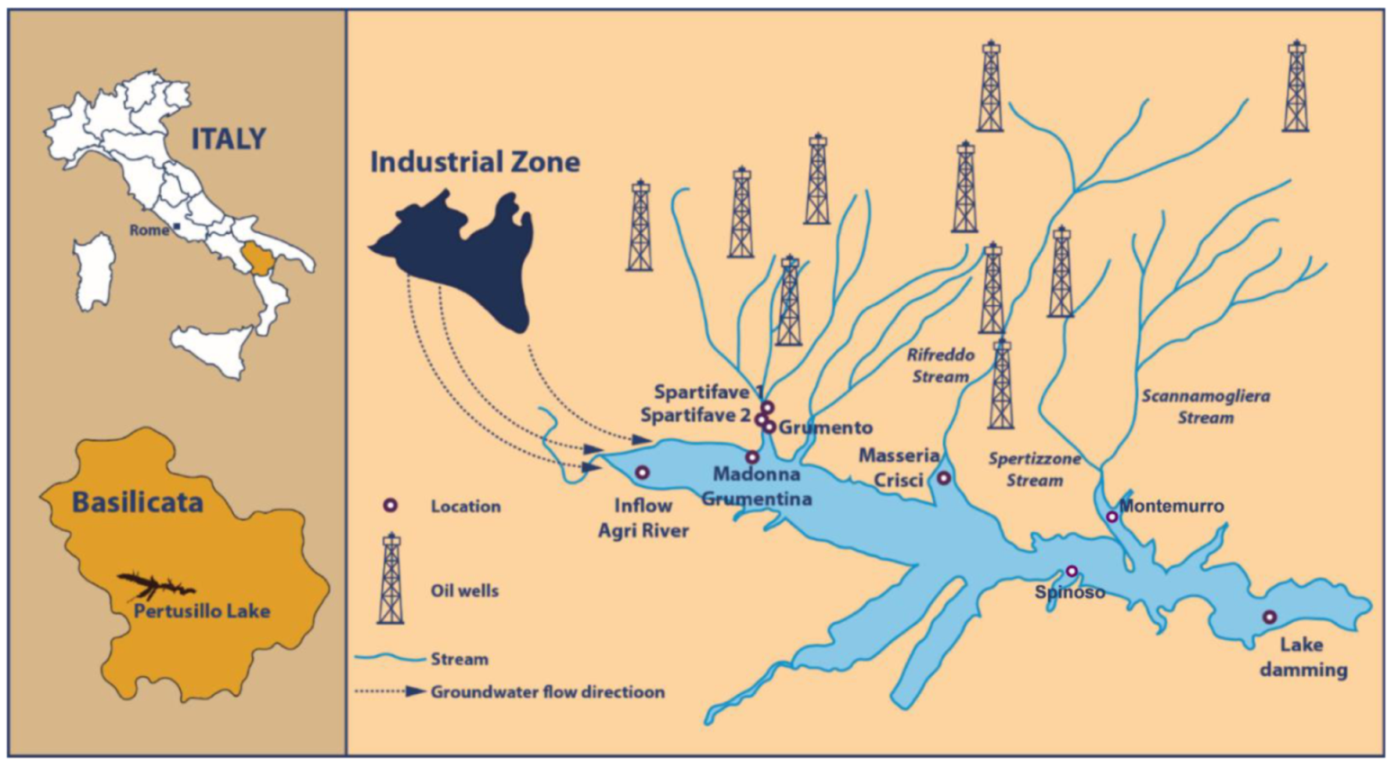
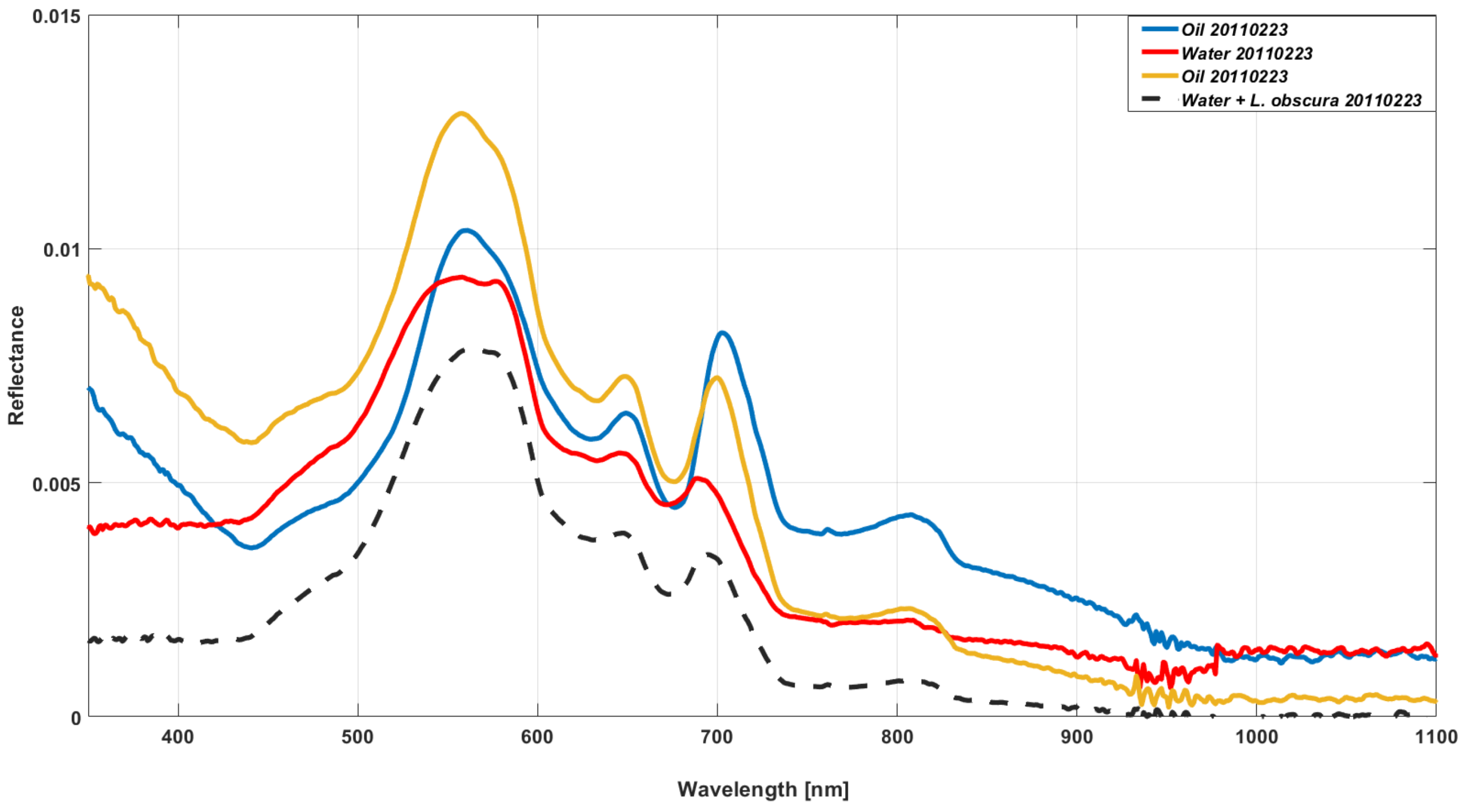
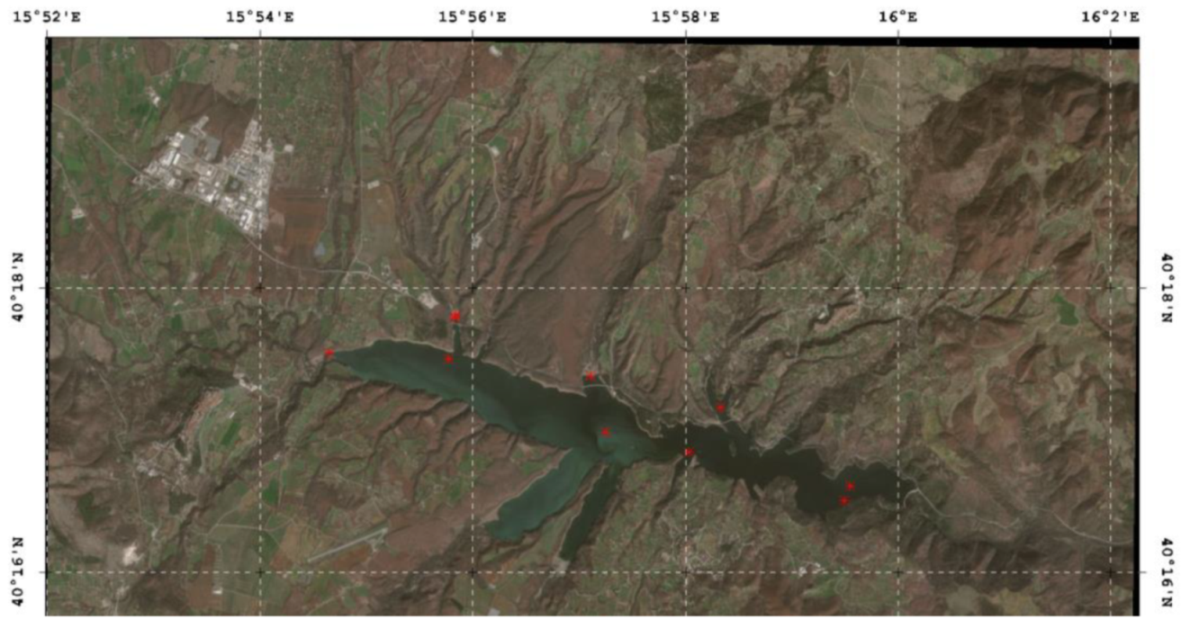

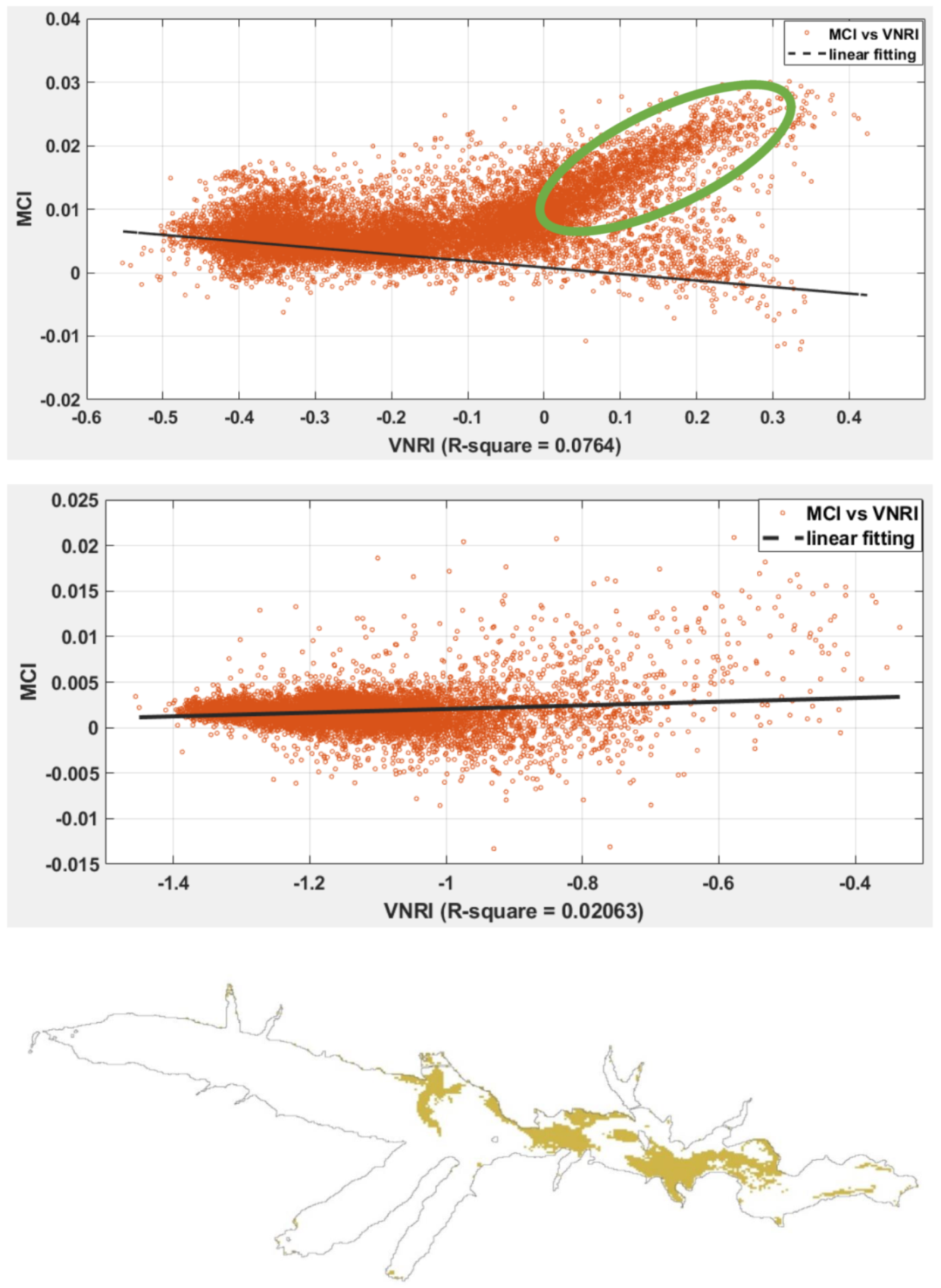

| Collection Site | Coordinates | Total Petroleum Hydrocarbons µg/L (at Time of Collection) | Sampling Date | Peridinium sp. Cells/L | Chlorophyll a µg/L | |
|---|---|---|---|---|---|---|
| Sampling and certified analyses by local civic associations | Spartifave 1 | N 40.29687 E 15.93075 | 38 | 27 February 2017 | N.A. | N.A. |
| Spartifave 2 | N 40.29687 E 15.93075 | 213 | 27 February 2017 | N.A. | N.A. | |
| Masseria Crisci | N 40.28977 E 15.95180 | 192 | 27 February 2017 | N.A *1 | N.A. | |
| Grumento | N 40.29665 E 15.93056 | 286 | 1 March 2017 | N.A. | N.A. | |
| Madonna Grumentina | N 40.29172 E 15.92957 | 900 | 22 May 2017 | N.A. | N.A. | |
| Lake damming | N 40.27522 E 15.99157 | 87 | 3 August 2017 | N.A. | N.A. | |
| Sampling and analyses by the Regional Agency for Environment Protection of Basilicata (ARPAB) and ISS | Station 1 Lake damming Surface | N 40.276913 E 15.992453 | N.D. *2 | 24 February 2017 | 7800.000 | 120 |
| Station 1 Lake damming Surface | N 40.276913 E 15.992453 | N.D. | 27 February 2017 | 1822.311 | 25–28 | |
| Station 2 Montemurro Surface | N 40.286077 E 15.972118 | N.D. | 27 February 2017 | 439.544 | 12–15 | |
| Station 3 Spinoso Superficiale | N 40.280857 E 15.967185 | N.D. | 27 February 2017 | 6684.275 | ≥150 | |
| Station 4 Masseria Crisci Surface | N 40.283217 E 15.954102 | N.D. | 27 February 2017 | 402.733 | 85 | |
| Masseria Crisci | N 40.28977 E 15.95180 | N.A. | 27 February 2017 | 10,000.000 *3 | N.A. | |
| Station 5 Grumento Surface | N 40.29665 E 15.93056 | N.D. | 27 February 2017 | 255.125 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laneve, G.; Bruno, M.; Mukherjee, A.; Messineo, V.; Giuseppetti, R.; De Pace, R.; Magurano, F.; D'Ugo, E. Remote Sensing Detection of Algal Blooms in a Lake Impacted by Petroleum Hydrocarbons. Remote Sens. 2022, 14, 121. https://doi.org/10.3390/rs14010121
Laneve G, Bruno M, Mukherjee A, Messineo V, Giuseppetti R, De Pace R, Magurano F, D'Ugo E. Remote Sensing Detection of Algal Blooms in a Lake Impacted by Petroleum Hydrocarbons. Remote Sensing. 2022; 14(1):121. https://doi.org/10.3390/rs14010121
Chicago/Turabian StyleLaneve, Giovanni, Milena Bruno, Arghya Mukherjee, Valentina Messineo, Roberto Giuseppetti, Rita De Pace, Fabio Magurano, and Emilio D'Ugo. 2022. "Remote Sensing Detection of Algal Blooms in a Lake Impacted by Petroleum Hydrocarbons" Remote Sensing 14, no. 1: 121. https://doi.org/10.3390/rs14010121
APA StyleLaneve, G., Bruno, M., Mukherjee, A., Messineo, V., Giuseppetti, R., De Pace, R., Magurano, F., & D'Ugo, E. (2022). Remote Sensing Detection of Algal Blooms in a Lake Impacted by Petroleum Hydrocarbons. Remote Sensing, 14(1), 121. https://doi.org/10.3390/rs14010121







