Leaf Age Matters in Remote Sensing: Taking Ground Truth for Spectroscopic Studies in Hemiboreal Deciduous Trees with Continuous Leaf Formation
Abstract
1. Introduction
2. Materials and Methods
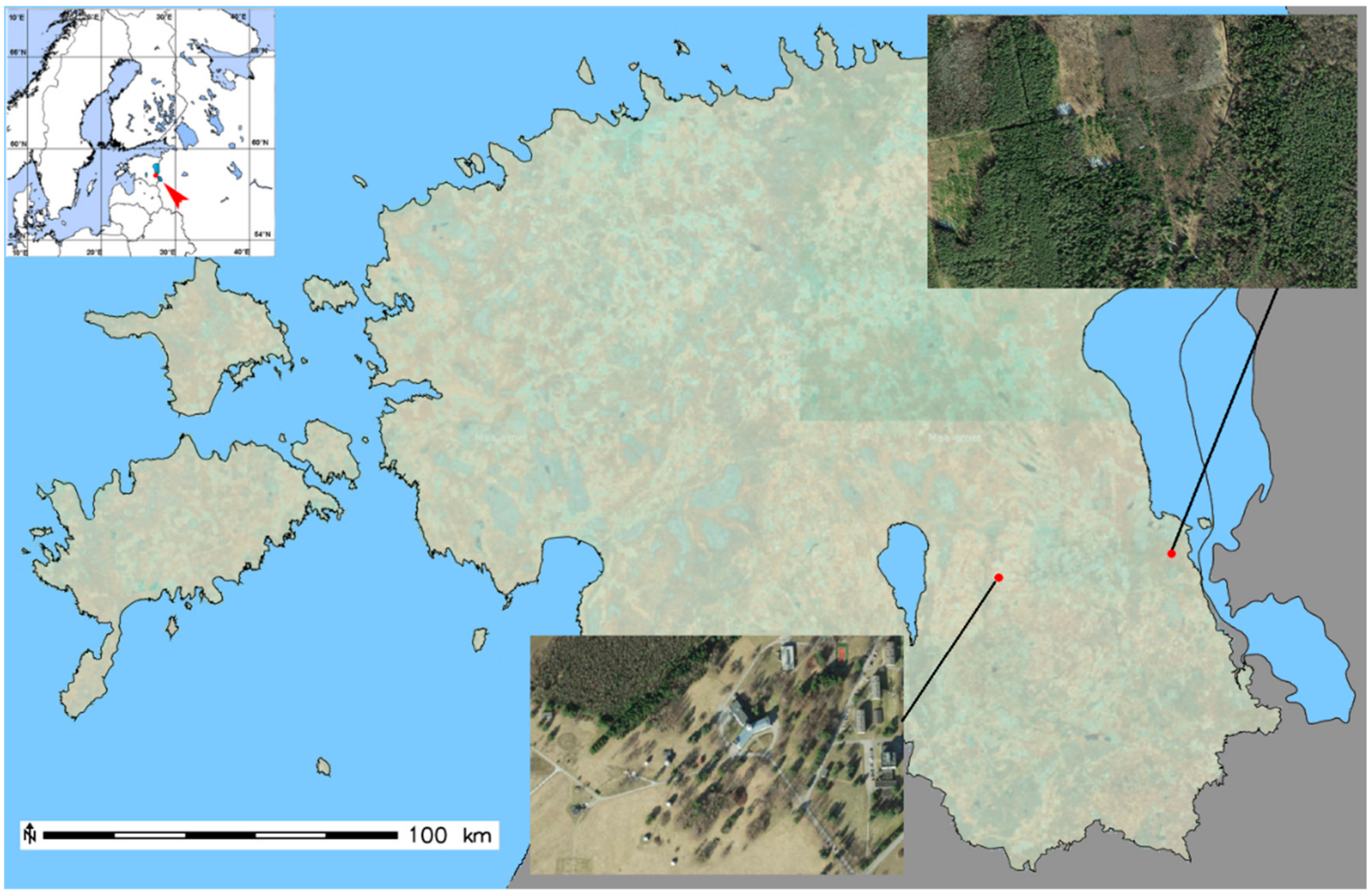
2.1. Plant Sampling
2.2. Measurement of Leaf Optical Properties
2.3. Biophysical and Anatomical Analysis
2.4. Statistical Processing of Optical, Biophysical, and Anatomical Leaf Traits
3. Results
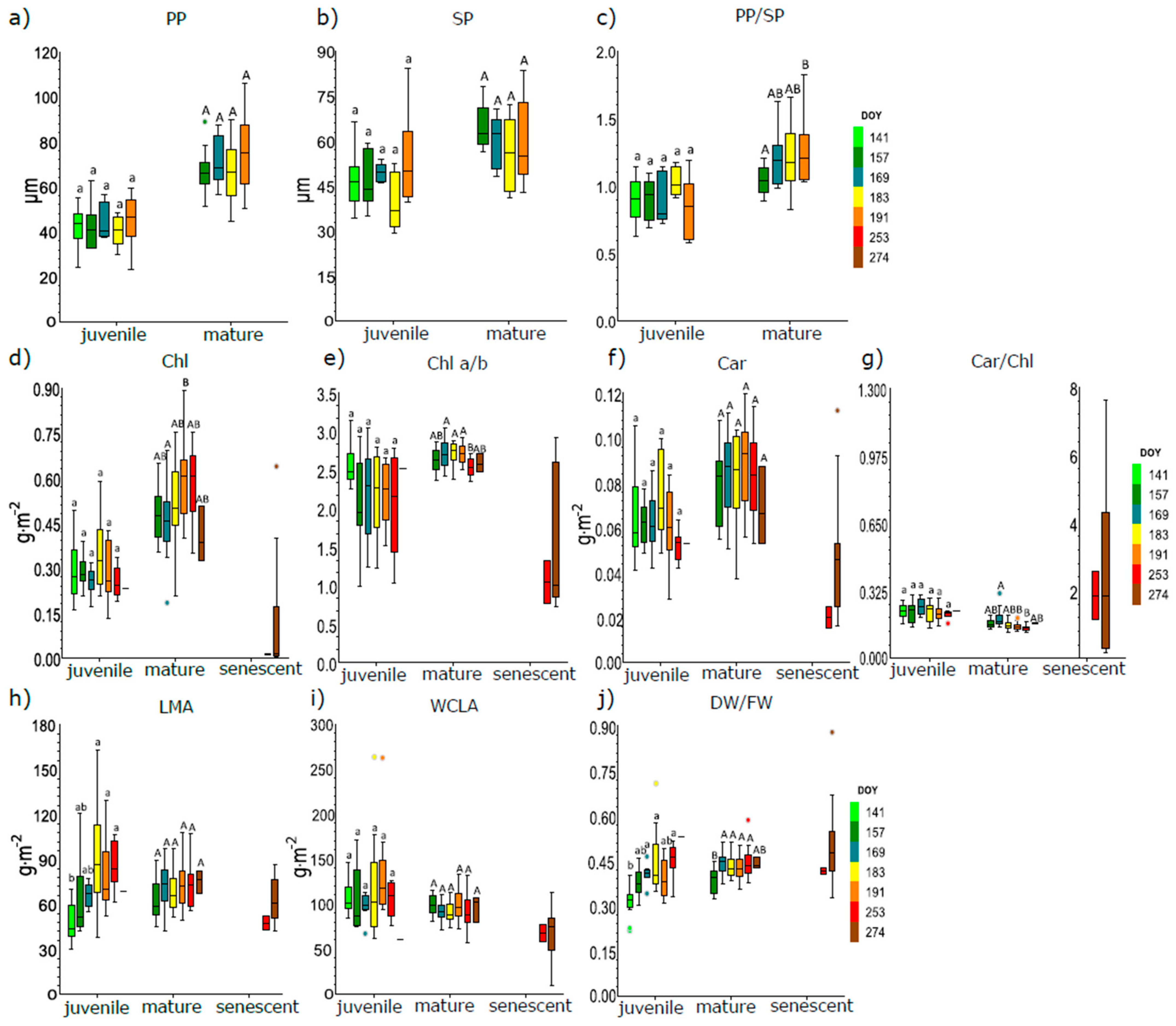
3.1. Seasonal Course of Anatomical and Biophysical Traits of Juvenile, Mature, and Senescent Leaves
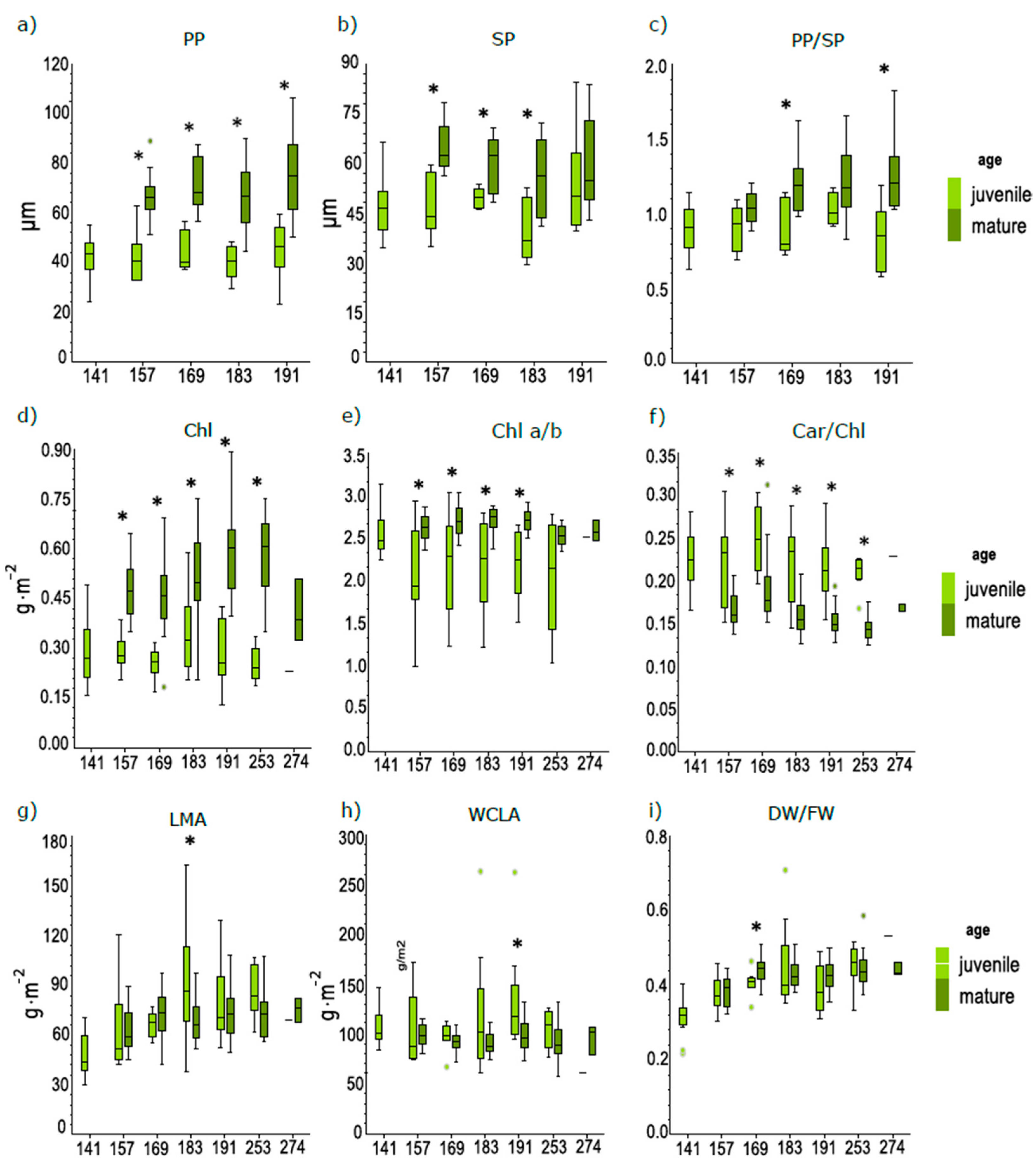
3.2. Differences in Anatomical and Biophysical Traits between Juvenile and Mature Leaves during the Season
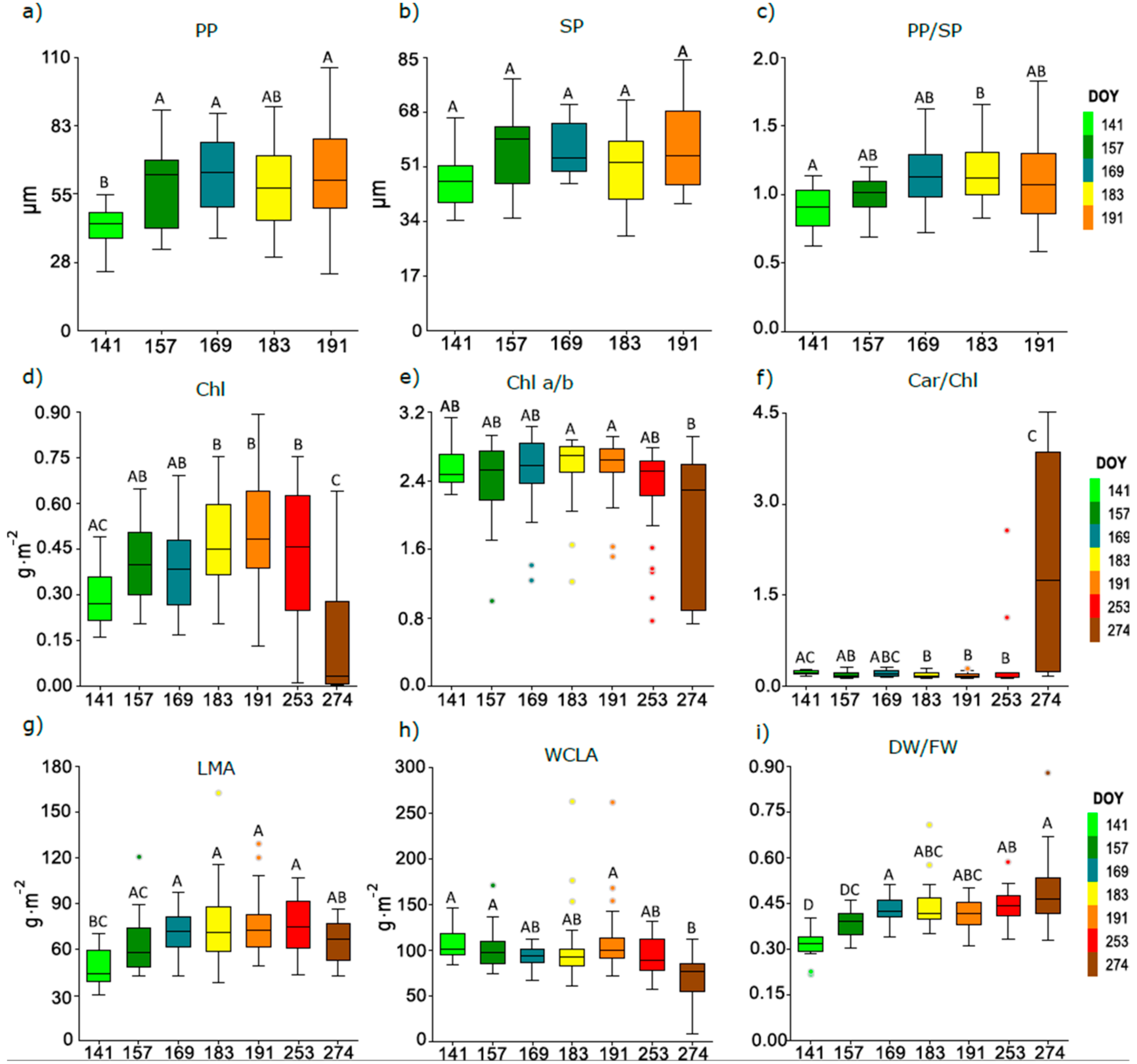
3.3. Seasonal Course of Anatomical and Biophysical Traits of Pooled Leaves
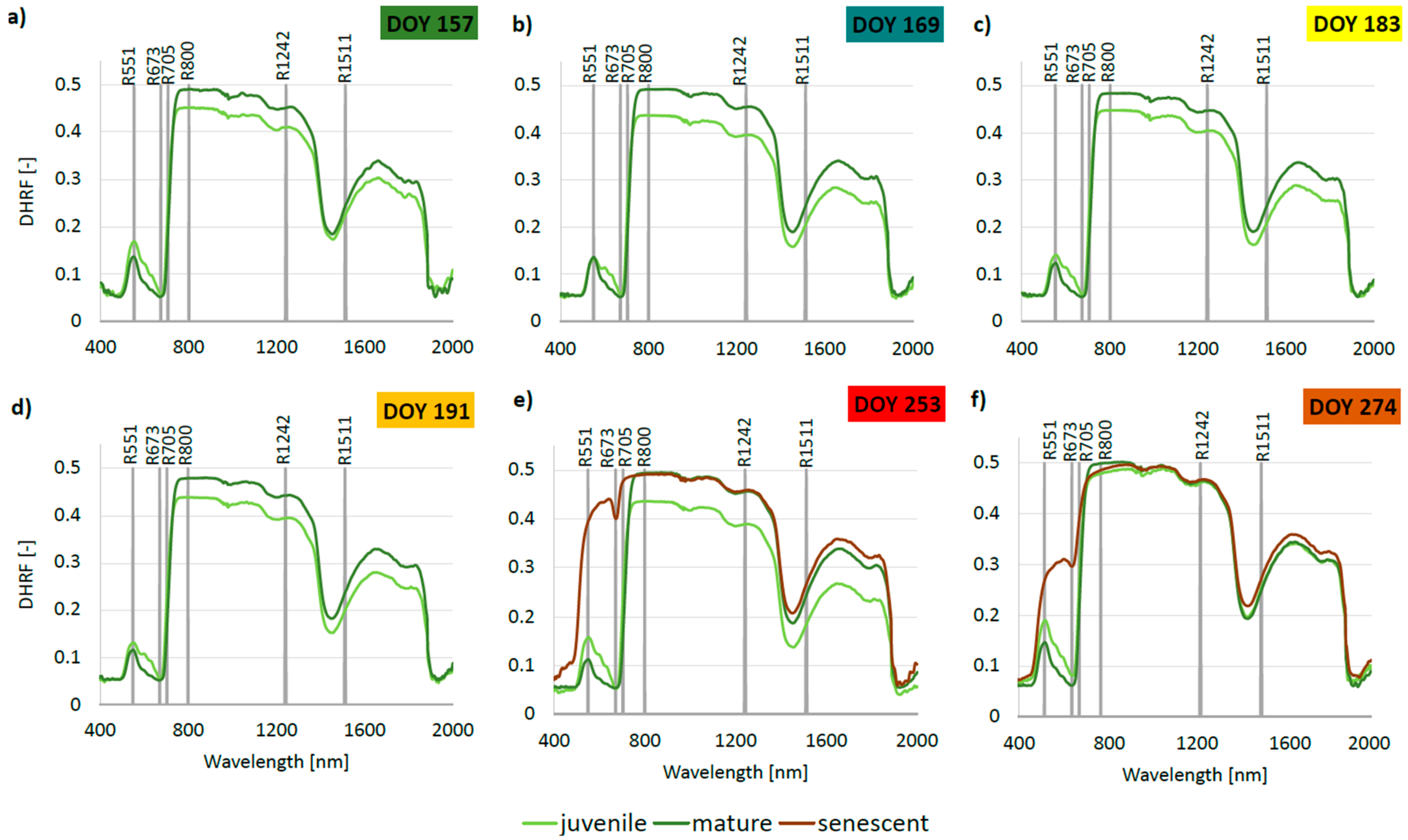
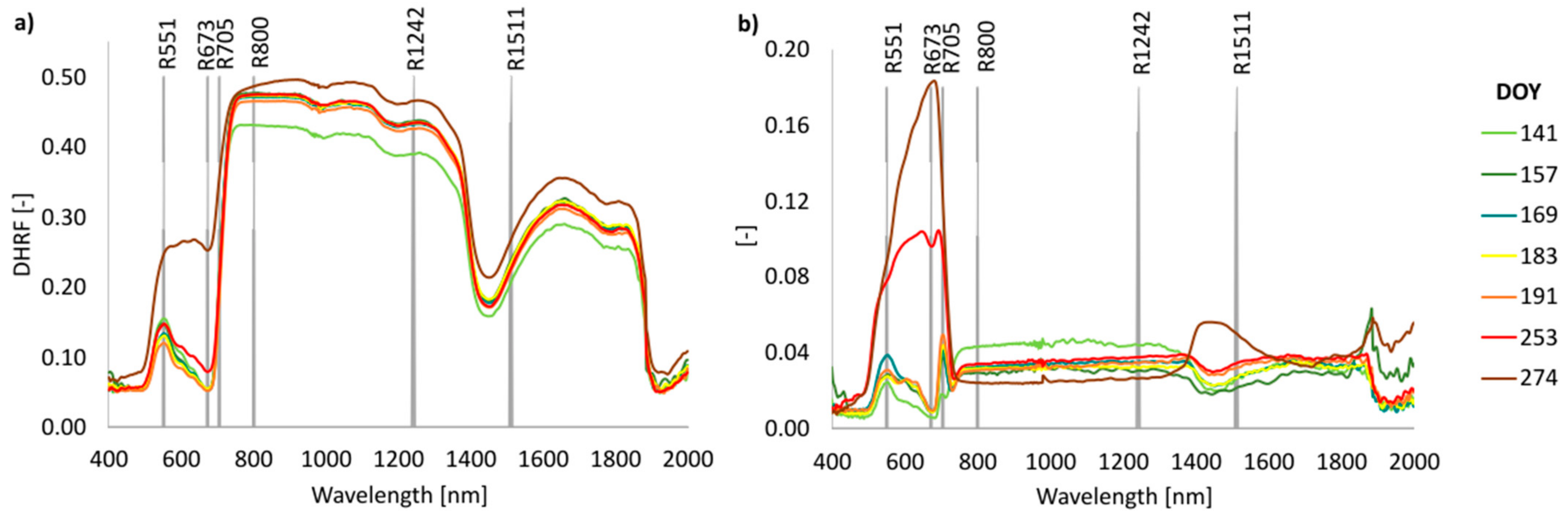
3.4. Phenology of Leaf Reflectance Related to Leaf Developmental Category
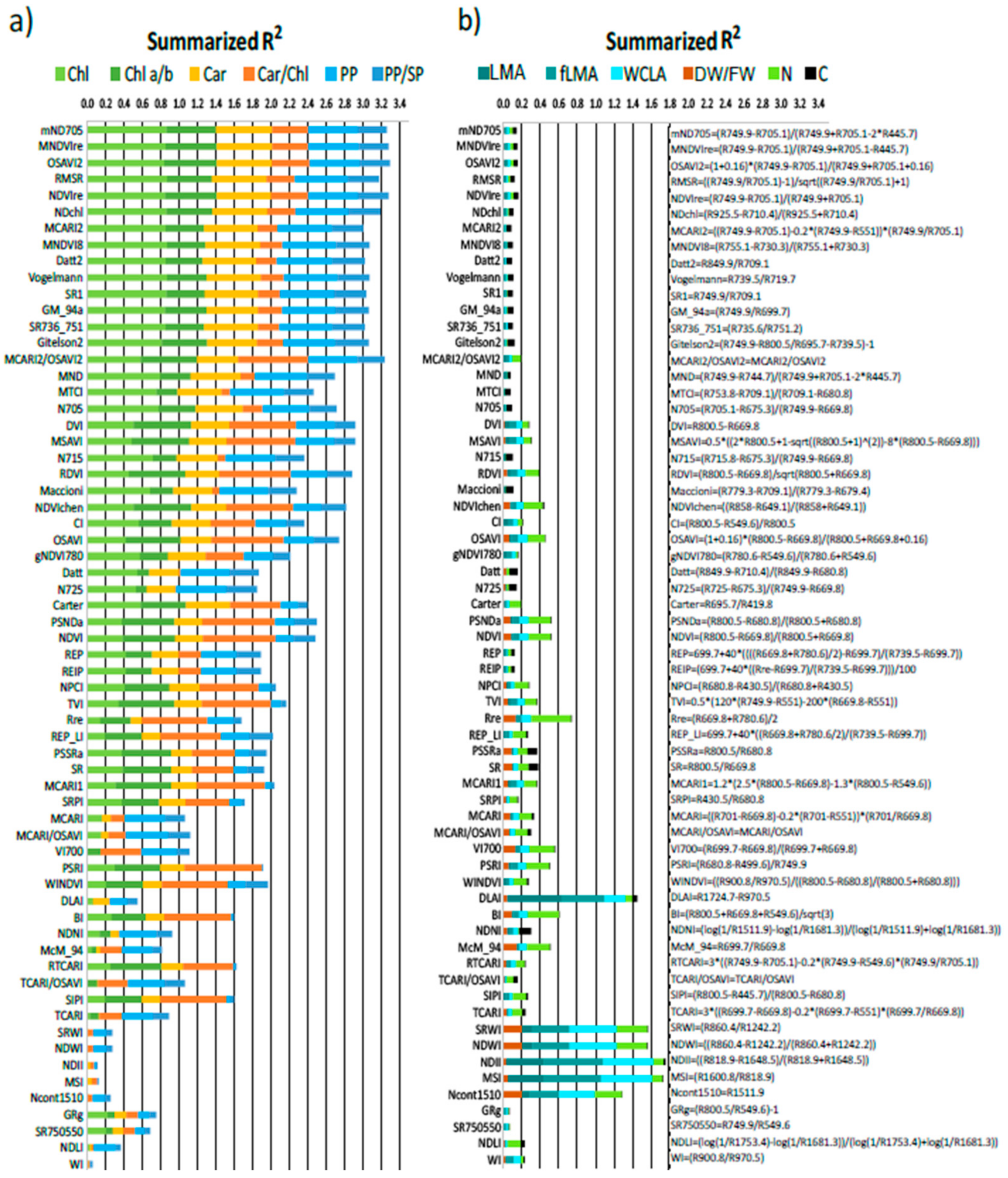
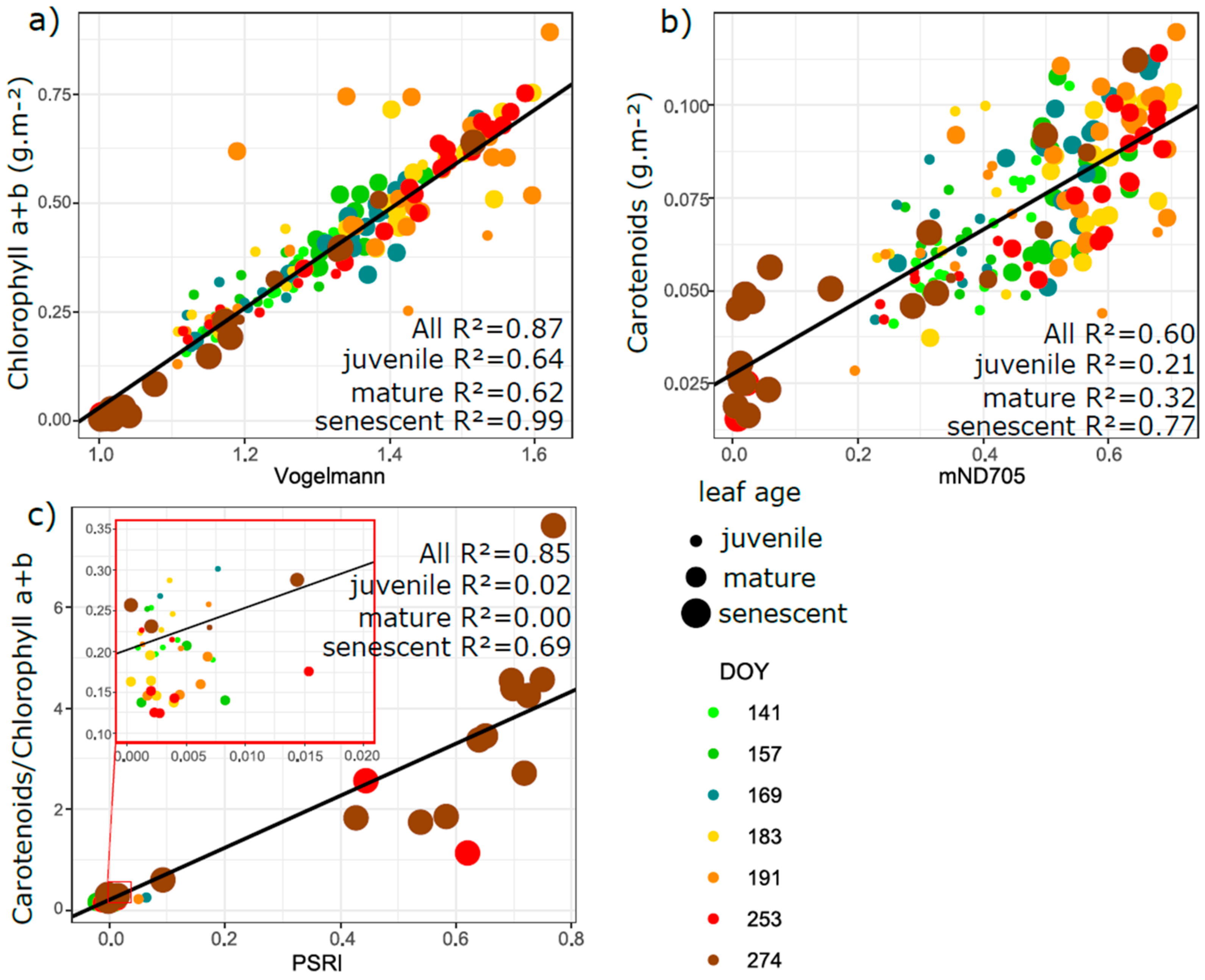
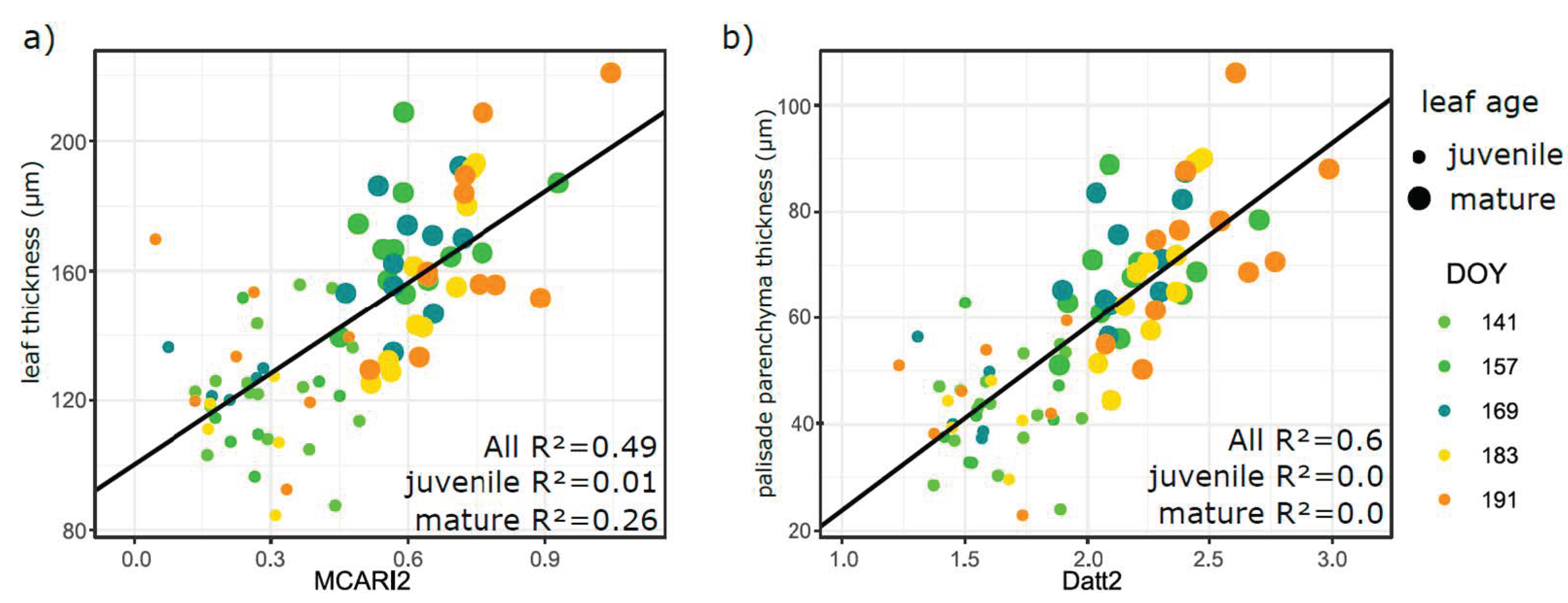
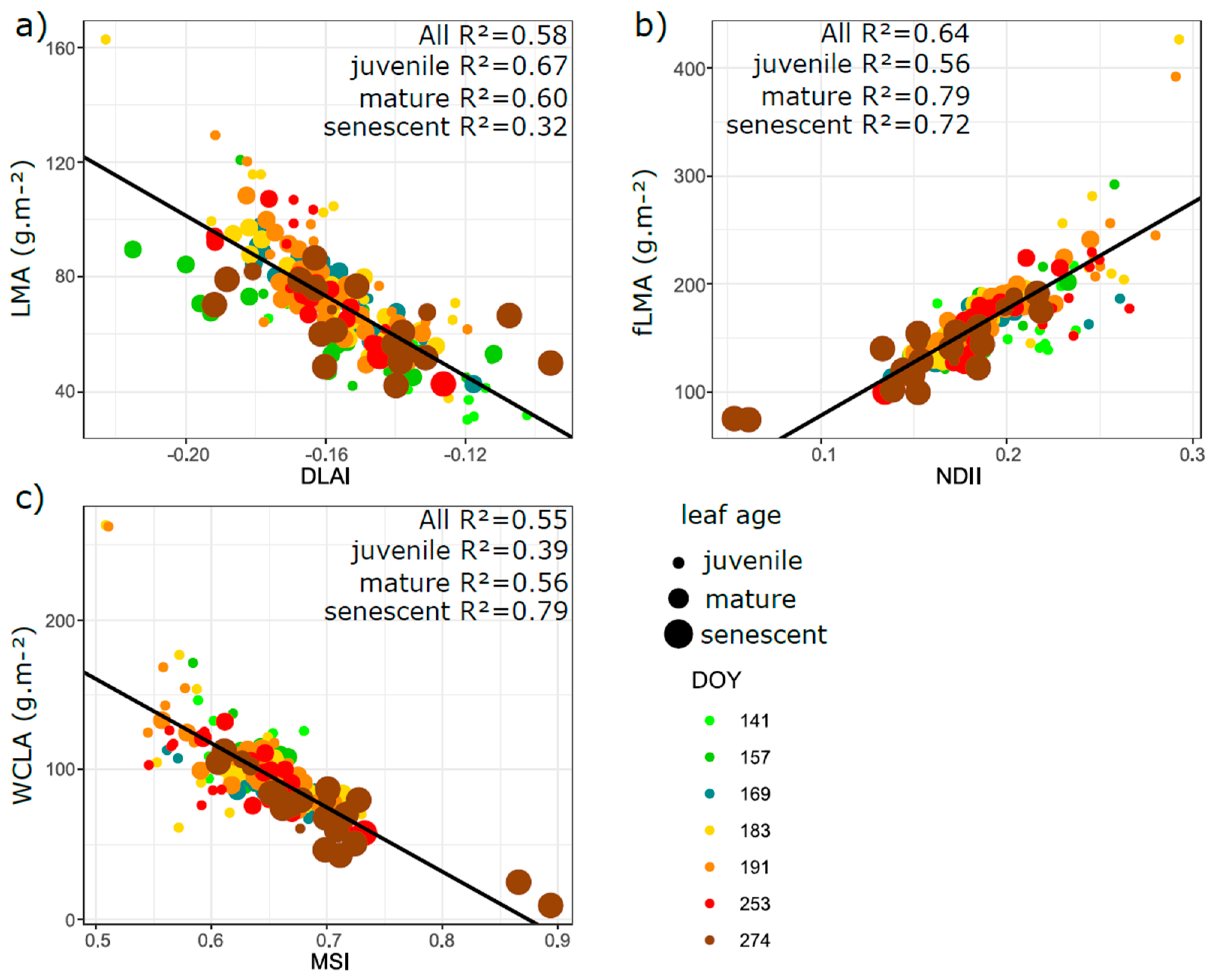
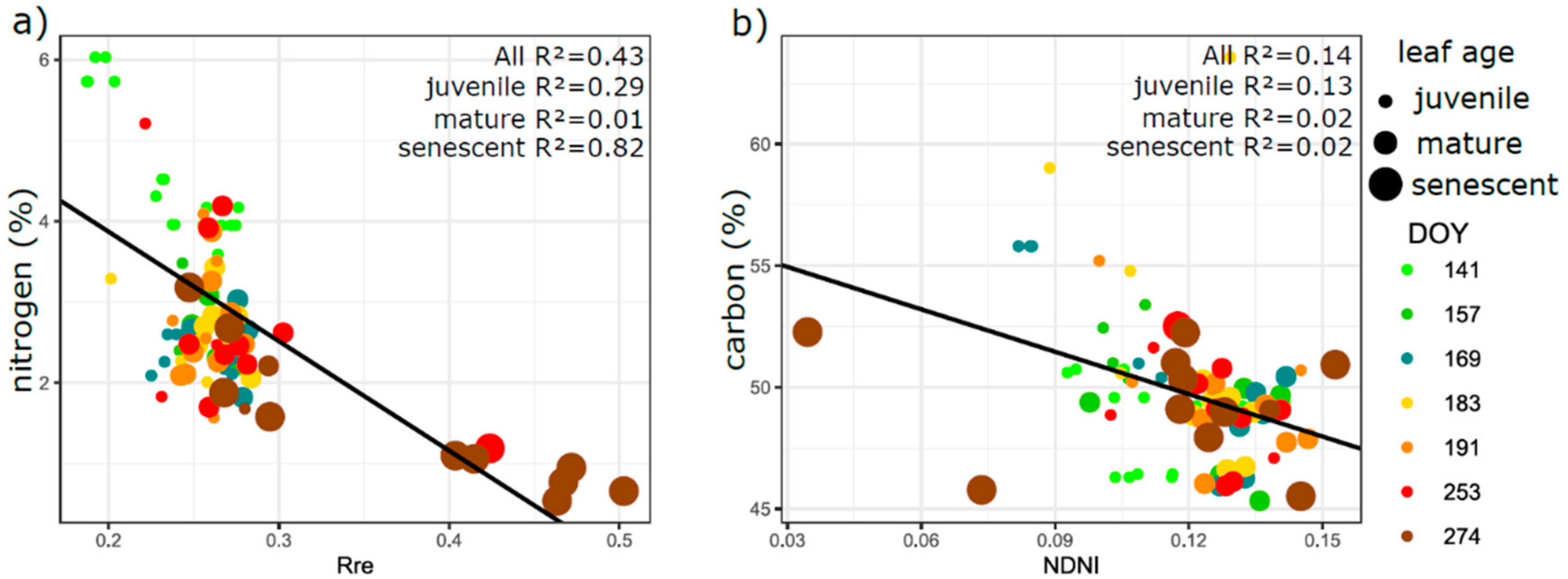
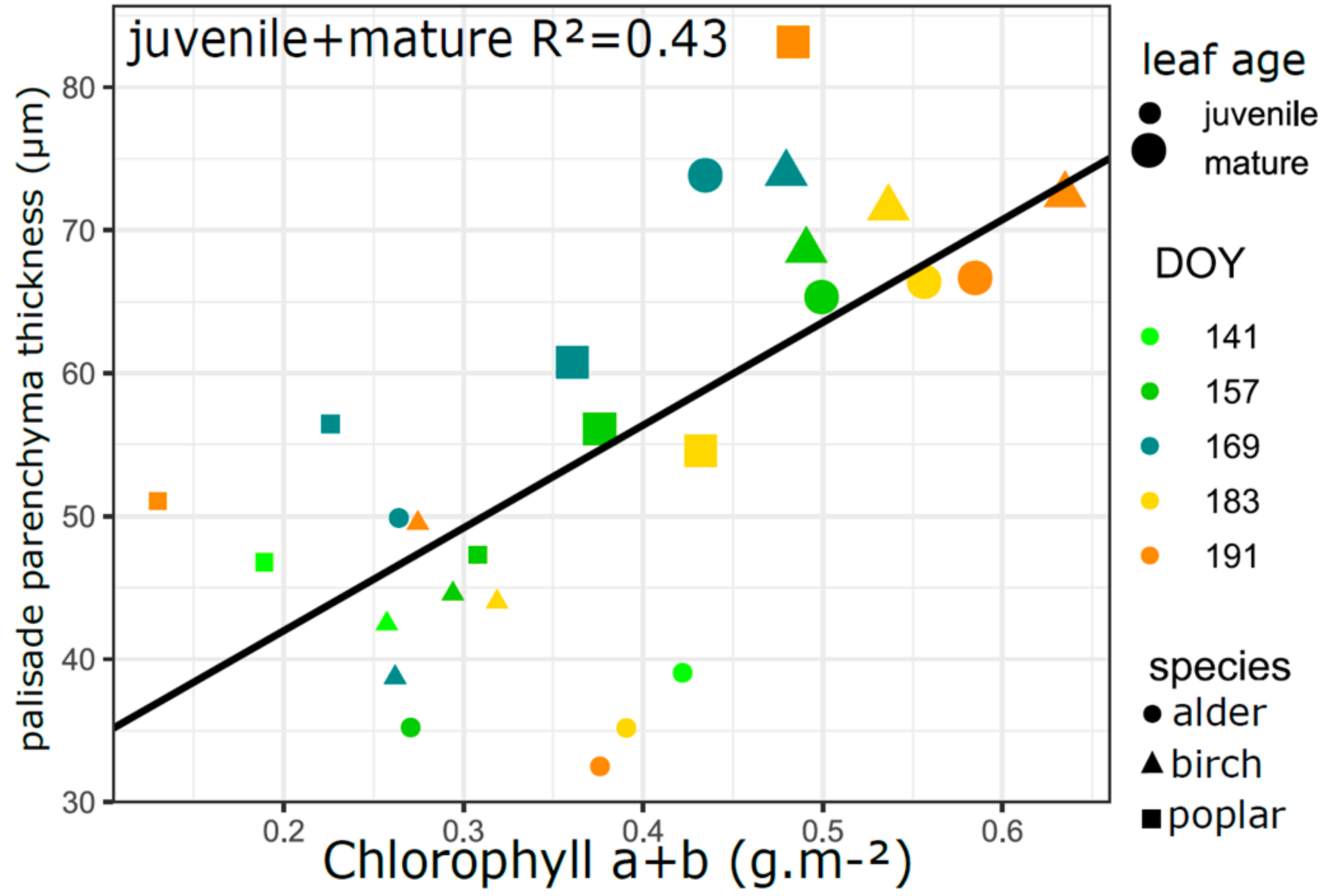
3.5. Relation of VIs with Biophysical and Anatomical Traits
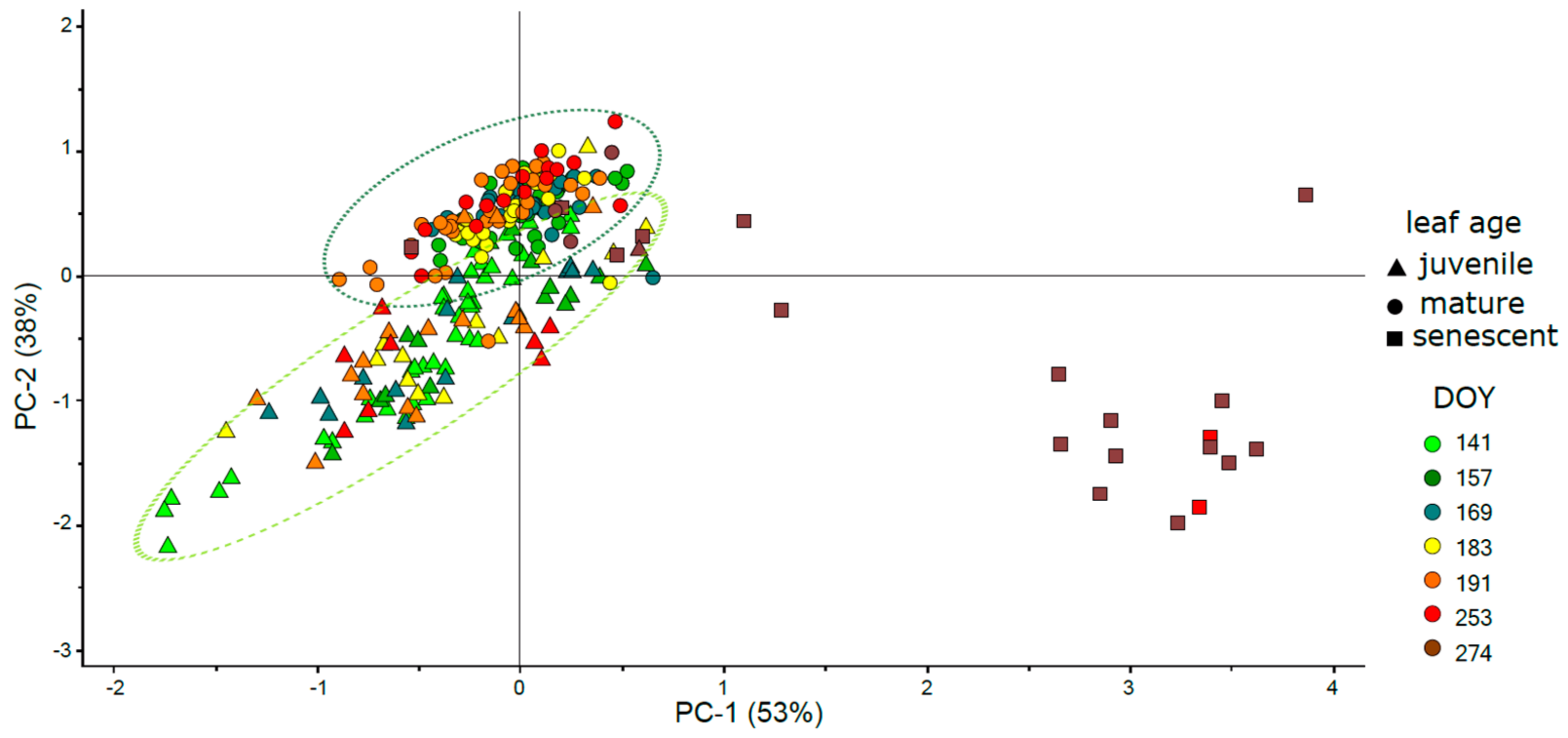
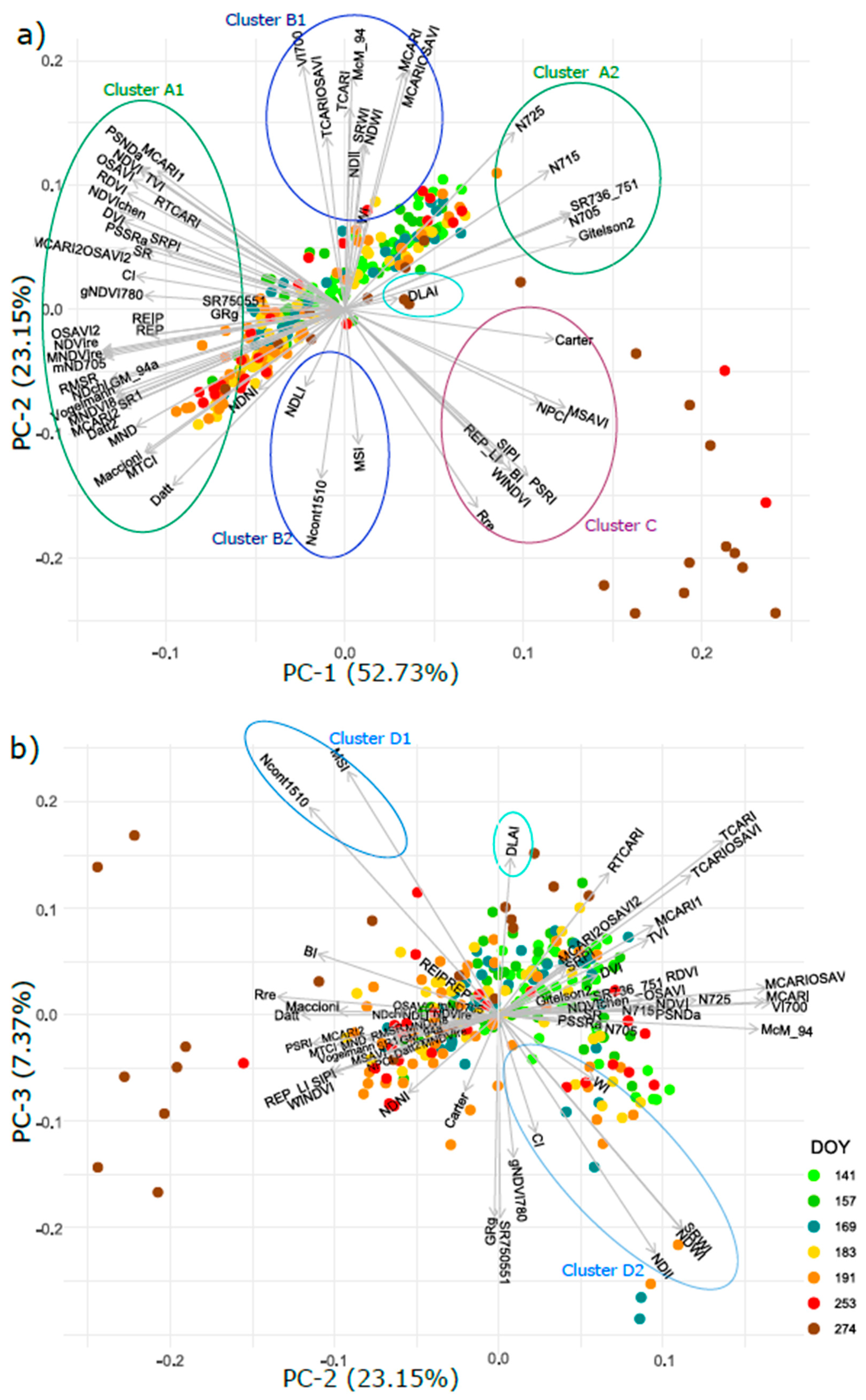
3.6. Intercorrelation of Vegetation Indices and Their Clustering
4. Discussion
4.1. Seasonal Course and Variability in Biophysical and Anatomical Traits Related to Leaf Developmental Category
4.2. Seasonal Course of Leaf Optical Properties Related to Leaf Developmental Category
4.3. Performance of Vegetation Indices in Leaf Traits Prediction—Implications for Interpretation of Canopy Level Remote Sensing Signal
4.3.1. Effect of Phenology and Leaf Developmental Category
4.3.2. Effect of Vegetation Indices Intercorrelation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeLucia, E.H.; Hamilton, J.G.; Naidu, S.L.; Thomas, R.B.; Andrews, J.A.; Finzi, A.C.; Lavine, M.; Matamala, R.; Mohan, J.E.; Hendrey, G.R.; et al. Net Primary Production of a Forest Ecosystem with Experimental CO2 Enrichment. Science 1999, 284, 1177–1179. [Google Scholar] [CrossRef]
- Zhao, M.; Running, S.W. Drought-Induced Reduction in Global Terrestrial Net Primary Production from 2000 through 2009. Science 2010, 329, 940–943. [Google Scholar] [CrossRef]
- Walker, A.P. Integrating the Evidence for a Terrestrial Carbon Sink Caused by Increasing Atmospheric CO2. New Phytol. 2020, 229, 2413–2445. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Reichstein, M.; Bahn, M.; Thonicke, K.; Frank, D.; Mahecha, M.D.; Smith, P.; van der Velde, M.; Vicca, S.; Babst, F.; et al. Effects of Climate Extremes on the Terrestrial Carbon Cycle: Concepts, Processes and Potential Future Impacts. Glob. Chang. Biol. 2015, 21, 2861–2880. [Google Scholar] [CrossRef] [PubMed]
- Ahti, T.; Hämet-Ahti, L.; Jalas, J. Vegetation Zones and Their Sections in Northwestern Europe. Ann. Bot. Fenn. 1968, 5, 169–211. [Google Scholar]
- Hickler, T.; Vohland, K.; Feehan, J.; Miller, P.A.; Smith, B.; Costa, L.; Giesecke, T.; Fronzek, S.; Carter, T.R.; Cramer, W.; et al. Projecting the Future Distribution of European Potential Natural Vegetation Zones with a Generalized, Tree Species-Based Dynamic Vegetation Model. Glob. Ecol. Biogeogr. 2012, 21, 50–63. [Google Scholar] [CrossRef]
- Wu, R.; Hinckley, T.M. Phenotypic Plasticity of Sylleptic Branching: Genetic Design of Tree Architecture. Crit. Rev. Plant Sci. 2001, 20, 467–485. [Google Scholar] [CrossRef]
- Halle, F.; Oldeman, R.A.A.; Tomlinson, P.B. Tropical Trees and Forests: An Architectural Analysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-81190-6. [Google Scholar]
- Critchfield, W.B. Leaf Dimorphism in Populus Trichocarpa. Am. J. Bot. 1960, 47, 699–711. [Google Scholar] [CrossRef]
- Cox, S.E. Elevational Gradient of Neoformed Shoot Growth in Populus tremuloides. Can. J. Bot. 2005, 83, 1340–1344. [Google Scholar] [CrossRef]
- Ceulemans, R.; Stettler, R.F.; Hinckley, T.M.; Isebrands, J.G.; Heilman, P.E. Crown Architecture of Populus Clones as Determined by Branch Orientation and Branch Characteristics. Tree Physiol. 1990, 7, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Deepak, M.; Keski-Saari, S.; Fauch, L.; Granlund, L.; Oksanen, E.; Keinänen, M. Leaf Canopy Layers Affect Spectral Reflectance in Silver Birch. Remote Sens. 2019, 11, 2884. [Google Scholar] [CrossRef]
- Broeckx, L.S.; Verlinden, M.S.; Vangronsveld, J.; Ceulemans, R. Importance of Crown Architecture for Leaf Area Index of Different Populus Genotypes in a High-Density Plantation. Tree Physiol. 2012, 32, 1214–1226. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.D.; Lersten, N.R. Heterophylly in Populus Grandidentata (Salicaceae) with Emphasis on Resin Glands and Extrafloral Nectaries. Am. J. Bot. 1978, 65, 1003–1010. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Chen, G.; Li, M.; Liu, M.; Liu, D. Epidermal Micromorphology and Mesophyll Structure of Populus Euphratica Heteromorphic Leaves at Different Development Stages. PLoS ONE 2015, 10, e0137701. [Google Scholar] [CrossRef]
- Evert, R. Esau’s Plant Anatomy, Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development, 3rd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2006; Volume 99, pp. 785–786. [Google Scholar] [CrossRef]
- Kenzo, T.; Ichie, T.; Yoneda, R.; Kitahashi, Y.; Watanabe, Y.; Ninomiya, I.; Koike, T. Interspecific Variation of Photosynthesis and Leaf Characteristics in Canopy Trees of Five Species of Dipterocarpaceae in a Tropical Rain Forest. Tree Physiol. 2004, 24, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Caemmerer, S.; Setchell, B.; Hudson, G. The Relationship Between CO2 Transfer Conductance and Leaf Anatomy in Transgenic Tobacco With a Reduced Content of Rubisco. Funct. Plant Biol. 1994, 21, 475. [Google Scholar] [CrossRef]
- Lhotáková, Z.; Kopačková-Strnadová, V.; Oulehle, F.; Homolová, L.; Neuwirthová, E.; Švik, M.; Janoutová, R.; Albrechtová, J. Foliage Biophysical Trait Prediction from Laboratory Spectra in Norway Spruce Is More Affected by Needle Age Than by Site Soil Conditions. Remote Sens. 2021, 13, 391. [Google Scholar] [CrossRef]
- Castro, K.L.; Sanchez-Azofeifa, G.A. Changes in Spectral Properties, Chlorophyll Content and Internal Mesophyll Structure of Senescing Populus Balsamifera and Populus Tremuloides Leaves. Sensors 2008, 8, 51–69. [Google Scholar] [CrossRef]
- Gara, T.W.; Darvishzadeh, R.; Skidmore, A.K.; Wang, T. Impact of Vertical Canopy Position on Leaf Spectral Properties and Traits across Multiple Species. Remote Sens. 2018, 10, 346. [Google Scholar] [CrossRef]
- Mähler, N.; Schiffthaler, B.; Robinson, K.M.; Terebieniec, B.K.; Vučak, M.; Mannapperuma, C.; Bailey, M.E.S.; Jansson, S.; Hvidsten, T.R.; Street, N.R. Leaf Shape in Populus tremula Is a Complex, Omnigenic Trait. Ecol. Evol. 2020, 10, 11922–11940. [Google Scholar] [CrossRef] [PubMed]
- Lukeš, P.; Neuwirthová, E.; Lhotáková, Z.; Janoutová, R.; Albrechtová, J. Upscaling Seasonal Phenological Course of Leaf Dorsiventral Reflectance in Radiative Transfer Model. Remote Sens. Environ. 2020, 246, 111862. [Google Scholar] [CrossRef]
- Nezval, O.; Krejza, J.; Světlík, J.; Šigut, L.; Horáček, P. Comparison of Traditional Ground-Based Observations and Digital Remote Sensing of Phenological Transitions in a Floodplain Forest. Agric. For. Meteorol. 2020, 291, 108079. [Google Scholar] [CrossRef]
- Dengler, N.G.; Mackay, L.B.; Gregory, L.M. Cell Enlargement and Tissue Differentiation during Leaf Expansion in Beech, Fagus grandifolia. Can. J. Bot. 1975, 53, 2846–2865. [Google Scholar] [CrossRef]
- Maillard, A.; Diquélou, S.; Billard, V.; Laîné, P.; Garnica, M.; Prudent, M.; Garcia-Mina, J.-M.; Yvin, J.-C.; Ourry, A. Leaf Mineral Nutrient Remobilization during Leaf Senescence and Modulation by Nutrient Deficiency. Front. Plant. Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Keskitalo, J.; Bergquist, G.; Gardeström, P.; Jansson, S. A Cellular Timetable of Autumn Senescence. Plant Physiol. 2005, 139, 1635–1648. [Google Scholar] [CrossRef] [PubMed]
- Junker, L.V.; Ensminger, I. Relationship between Leaf Optical Properties, Chlorophyll Fluorescence and Pigment Changes in Senescing Acer saccharum Leaves. Tree Physiol. 2016, 36, 694–711. [Google Scholar] [CrossRef]
- Neuwirthová, E.; Lhotáková, Z.; Albrechtová, J. The Effect of Leaf Stacking on Leaf Reflectance and Vegetation Indices Measured by Contact Probe during the Season. Sensors 2017, 17, 1202. [Google Scholar] [CrossRef]
- Yang, X.; Tang, J.; Mustard, J.F.; Wu, J.; Zhao, K.; Serbin, S.; Lee, J.-E. Seasonal Variability of Multiple Leaf Traits Captured by Leaf Spectroscopy at Two Temperate Deciduous Forests. Remote Sens. Environ. 2016, 179, 1–12. [Google Scholar] [CrossRef]
- Dillen, S.Y.; de Beeck, M.O.; Hufkens, K.; Buonanduci, M.; Phillips, N.G. Seasonal Patterns of Foliar Reflectance in Relation to Photosynthetic Capacity and Color Index in Two Co-Occurring Tree Species, Quercus Rubra and Betula Papyrifera. Agric. For. Meteorol. 2012, 160, 60–68. [Google Scholar] [CrossRef]
- White, K.; Pontius, J.; Schaberg, P. Remote Sensing of Spring Phenology in Northeastern Forests: A Comparison of Methods, Field Metrics and Sources of Uncertainty. Remote Sens. Environ. 2014, 148, 97–107. [Google Scholar] [CrossRef]
- Hovi, A.; Raitio, P.; Rautiainen, M. A Spectral Analysis of 25 Boreal Tree Species. Silva Fenn. 2017, 51. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Verhoef, W.; Baret, F.; Bacour, C.; Zarco-Tejada, P.J.; Asner, G.P.; François, C.; Ustin, S.L. PROSPECT + SAIL Models: A Review of Use for Vegetation Characterization. Remote Sens. Environ. 2009, 113 (Suppl. 1), S56–S66. [Google Scholar] [CrossRef]
- Wu, Q.; Song, C.; Song, J.; Wang, J.; Chen, S.; Yu, B. Impacts of Leaf Age on Canopy Spectral Signature Variation in Evergreen Chinese Fir Forests. Remote Sens. 2018, 10, 262. [Google Scholar] [CrossRef]
- Demarez, V. Seasonal Variation of Leaf Chlorophyll Content of a Temperate Forest. Inversion of the PROSPECT Model. Int. J. Remote Sens. 1999, 20, 879–894. [Google Scholar] [CrossRef]
- Gausman, H.W.; Allen, W.A. Optical Parameters of Leaves of 30 Plant Species. Plant Physiol. 1973, 52, 57–62. [Google Scholar] [CrossRef]
- Allen, W.A.; Gausman, H.W.; Richardson, A.J. Willstätter-Stoll Theory of Leaf Reflectance Evaluated by Ray Tracing. Appl. Opt. 1973, 12, 2448–2453. [Google Scholar] [CrossRef] [PubMed]
- Slaton, M.R.; Hunt, E.R.; Smith, W.K. Estimating Near-Infrared Leaf Reflectance from Leaf Structural Characteristics. Am. J. Bot. 2001, 88, 278–284. [Google Scholar] [CrossRef]
- Buschmann, C.; Lenk, S.; Lichtenthaler, H.K. Reflectance Spectra and Images of Green Leaves with Different Tissue Structure and Chlorophyll Content. Isr. J. Plant Sci. 2012, 60, 49–64. [Google Scholar] [CrossRef]
- Rapaport, T.; Hochberg, U.; Rachmilevitch, S.; Karnieli, A. The Effect of Differential Growth Rates across Plants on Spectral Predictions of Physiological Parameters. PLoS ONE 2014, 9, e88930. [Google Scholar] [CrossRef]
- Van Wittenberghe, S.; Alonso, L.; Verrelst, J.; Moreno, J.; Samson, R. Bidirectional Sun-Induced Chlorophyll Fluorescence Emission Is Influenced by Leaf Structure and Light Scattering Properties—A Bottom-up Approach. Remote Sens. Environ. 2015, 158, 169–179. [Google Scholar] [CrossRef]
- Lu, S.; Lu, X.; Zhao, W.; Liu, Y.; Wang, Z.; Omasa, K. Comparing Vegetation Indices for Remote Chlorophyll Measurement of White Poplar and Chinese Elm Leaves with Different Adaxial and Abaxial Surfaces. J. Exp. Bot. 2015, 66, 5625–5637. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between Leaf Pigment Content and Spectral Reflectance across a Wide Range of Species, Leaf Structures and Developmental Stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Verrelst, J.; Malenovský, Z.; Van der Tol, C.; Camps-Valls, G.; Gastellu-Etchegorry, J.-P.; Lewis, P.; North, P.; Moreno, J. Quantifying Vegetation Biophysical Variables from Imaging Spectroscopy Data: A Review on Retrieval Methods. Surv. Geophys. 2019, 40, 589–629. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Signature Analysis of Leaf Reflectance Spectra: Algorithm Development for Remote Sensing of Chlorophyll. J. Plant Physiol. 1996, 148, 494–500. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote Sensing of Chlorophyll Concentration in Higher Plant Leaves. Adv. Space Rese. 1998, 22, 689–692. [Google Scholar] [CrossRef]
- Hallik, L.; Kuusk, A.; Lang, M.; Kuusk, J. Reflectance Properties of Hemiboreal Mixed Forest Canopies with Focus on Red Edge and Near Infrared Spectral Regions. Remote Sens. 2019, 11, 1717. [Google Scholar] [CrossRef]
- Main, R.; Cho, M.A.; Mathieu, R.; O’Kennedy, M.M.; Ramoelo, A.; Koch, S. An Investigation into Robust Spectral Indices for Leaf Chlorophyll Estimation. ISPRS J. Photogramm. Remote Sens. 2011, 66, 751–761. [Google Scholar] [CrossRef]
- Hallik, L.; Kazantsev, T.; Kuusk, A.; Galmés, J.; Tomás, M.; Niinemets, Ü. Generality of Relationships between Leaf Pigment Contents and Spectral Vegetation Indices in Mallorca (Spain). Reg. Environ. Chang. 2017, 17, 2097–2109. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation; NASA/GSFC Type III Final Report; NASA: Washington, DC, USA, 1974; 371p. [Google Scholar]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the Radiometric and Biophysical Performance of the MODIS Vegetation Indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Campbell, P.K.E.; Rock, B.N.; Martin, M.E.; Neefus, C.D.; Irons, J.R.; Middleton, E.M.; Albrechtova, J. Detection of Initial Damage in Norway Spruce Canopies Using Hyperspectral Airborne Data. Int. J. Remote Sens. 2004, 25, 5557–5583. [Google Scholar] [CrossRef]
- Kuusk, A.; Lang, M.; Nilson, T. Forest Test Site at Järvselja, Estonia. In Proceedings of the Third Workshop CHRIS/Proba, Frascati, Italy, 21–23 March 2005; ESA Publication SP-593. ISBN 92-9092-904-9. ISSN 1609-042X. [Google Scholar]
- R Core Team R: The R Project for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.r-project.org/ (accessed on 24 November 2020).
- Wendlandt, W.W.; Hecht, H.G. Reflectance Spectroscopy; John Wiley and Sons, Inc.: New York, NY, USA, 1966; Volume 21, vii+298p. [Google Scholar]
- Rabiner, L.R.; Gold, B. Theory and Application of Digital Signal Processing; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 1975; 777p. [Google Scholar]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Esbensen, K.H.; Swarbrick, B.; Westad, F.; Whitcomb, P.; Anderson, M. Multivariate Data Analysis: An Introduction to Multivariate Analysis. In Process Analytical Technology and Quality by Design, 6th ed.; CAMO Software AS: Oslo Magnolia, TX, USA, 2018; ISBN 978-82-691104-0-1. [Google Scholar]
- Tang, Y.; Horikoshi, M.; Li, W. Ggfortify: Unified Interface to Visualize Statistical Results of Popular R Packages. R J. 2016, 8, 474. [Google Scholar] [CrossRef]
- Gao, B. NDWI—A Normalized Difference Water Index for Remote Sensing of Vegetation Liquid Water from Space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Curran, P.J. Remote Sensing of Foliar Chemistry. Remote Sens. Environ. 1989, 30, 271–278. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, G.; Liu, Z. Contribution of Leaf Anatomical Traits to Leaf Mass per Area among Canopy Layers for Five Coexisting Broadleaf Species across Shade Tolerances at a Regional Scale. For. Ecol. Manag. 2019, 452, 117569. [Google Scholar] [CrossRef]
- Cline, M.G.; Harrington, C.A. Apical Dominance and Apical Control in Multiple Flushing of Temperate Woody Species. J. For. Res. 2007, 37, 74–83. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, F.; Li, F.; Jin, G. Coordination of Intra and Inter-Species Leaf Traits According to Leaf Phenology and Plant Age for Three Temperate Broadleaf Species with Different Shade Tolerances. For. Ecol. Manag. 2019, 434, 63–75. [Google Scholar] [CrossRef]
- Blackburn, G.A. Relationships between Spectral Reflectance and Pigment Concentrations in Stacks of Deciduous Broadleaves. Remote Sens. Environ. 1999, 70, 224–237. [Google Scholar] [CrossRef]
- Marchi, S.; Tognetti, R.; Minnocci, A.; Borghi, M.; Sebastiani, L. Variation in Mesophyll Anatomy and Photosynthetic Capacity during Leaf Development in a Deciduous Mesophyte Fruit Tree (Prunus persica) and an Evergreen Sclerophyllous Mediterranean Shrub (Olea europaea). Trees 2008, 22, 559–571. [Google Scholar] [CrossRef]
- Uliassi, D.D.; Ruess, R.W. Limitations to Symbiotic Nitrogen Fixation in Primary Succession on the Tanana River Floodplain. Ecology 2002, 83, 88–103. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.-P.; Niyogi, K.K. Non-Photochemical Quenching. A Response to Excess Light Energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Photoprotection in an Ecological Context: The Remarkable Complexity of Thermal Energy Dissipation. New Phytol. 2006, 172, 11–21. [Google Scholar] [CrossRef]
- Matsubara, S.; Krause, G.H.; Aranda, J.; Virgo, A.; Beisel, K.G.; Jahns, P.; Winter, K. Sun-Shade Patterns of Leaf Carotenoid Composition in 86 Species of Neotropical Forest Plants. Funct. Plant Biol. 2009, 36, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Marin, A.; Passarini, F.; van Stokkum, I.H.M.; van Grondelle, R.; Croce, R. Minor Complexes at Work: Light-Harvesting by Carotenoids in the Photosystem II Antenna Complexes CP24 and CP26. Biophys. J. 2011, 100, 2829–2838. [Google Scholar] [CrossRef]
- Niinemets, Ü. Responses of Forest Trees to Single and Multiple Environmental Stresses from Seedlings to Mature Plants: Past Stress History, Stress Interactions, Tolerance and Acclimation. For. Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Féret, J.-B.; François, C.; Gitelson, A.; Asner, G.P.; Barry, K.M.; Panigada, C.; Richardson, A.D.; Jacquemoud, S. Optimizing Spectral Indices and Chemometric Analysis of Leaf Chemical Properties Using Radiative Transfer Modeling. Remote Sens. Environ. 2011, 115, 2742–2750. [Google Scholar] [CrossRef]
- Mänd, P.; Hallik, L.; Peñuelas, J.; Kull, O. Electron Transport Efficiency at Opposite Leaf Sides: Effect of Vertical Distribution of Leaf Angle, Structure, Chlorophyll Content and Species in a Forest Canopy. Tree Physiol. 2013, 33, 202–210. [Google Scholar] [CrossRef]
- Kull, O. Acclimation of Photosynthesis in Canopies: Models and Limitations. Oecologia 2002, 133, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Hallik, L.; Kull, O.; Niinemets, Ü.; Aan, A. Contrasting Correlation Networks between Leaf Structure, Nitrogen and Chlorophyll in Herbaceous and Woody Canopies. Basic Appl. Ecol. 2009, 10, 309–318. [Google Scholar] [CrossRef]
- Mottus, M.; Sulev, M.; Hallik, L. Seasonal Course of the Spectral Properties of Alder and Birch Leaves. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 2496–2505. [Google Scholar] [CrossRef]
- Kopačková-Strnadová, V.; Koucká, L.; Jelének, J.; Lhotáková, Z.; Oulehle, F. Canopy Top, Height and Photosynthetic Pigment Estimation Using Parrot Sequoia Multispectral Imagery and the Unmanned Aerial Vehicle (UAV). Remote Sens. 2021, 13, 705. [Google Scholar] [CrossRef]
- Chavana-Bryant, C.; Malhi, Y.; Anastasiou, A.; Enquist, B.J.; Cosio, E.G.; Keenan, T.F.; Gerard, F.F. Leaf Age Effects on the Spectral Predictability of Leaf Traits in Amazonian Canopy Trees. Sci. Total Environ. 2019, 666, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Rogers, A.; Albert, L.P.; Ely, K.; Prohaska, N.; Wolfe, B.T.; Oliveira, R.C.; Saleska, S.R.; Serbin, S.P. Leaf Reflectance Spectroscopy Captures Variation in Carboxylation Capacity across Species, Canopy Environment and Leaf Age in Lowland Moist Tropical Forests. New Phytol. 2019, 224, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chavana-Bryant, C.; Prohaska, N.; Serbin, S.P.; Guan, K.; Albert, L.P.; Yang, X. Convergence in Relationships between Leaf Traits, Spectra and Age across Diverse Canopy Environments and Two Contrasting Tropical Forests. New Phytol. 2017, 214, 1033–1048. [Google Scholar] [CrossRef] [PubMed]
- Croft, H.; Chen, J.M.; Zhang, Y. The Applicability of Empirical Vegetation Indices for Determining Leaf Chlorophyll Content over Different Leaf and Canopy Structures. Ecol. Complex. 2014, 17, 119–130. [Google Scholar] [CrossRef]
- He, N.; Liu, C.; Tian, M.; Li, M.; Yang, H.; Yu, G.; Guo, D.; Smith, M.D.; Yu, Q.; Hou, J. Variation in Leaf Anatomical Traits from Tropical to Cold-temperate Forests and Linkage to Ecosystem Functions. Funct. Ecol. 2018, 32, 10–19. [Google Scholar] [CrossRef]
- Le Maire, G.; Francois, C.; Soudani, K.; Berveiller, D.; Pontailler, J.; Breda, N.; Genet, H.; Davi, H.; Dufrene, E. Calibration and Validation of Hyperspectral Indices for the Estimation of Broadleaved Forest Leaf Chlorophyll Content, Leaf Mass per Area, Leaf Area Index and Leaf Canopy Biomass. Remote Sens. Environ. 2008, 112, 3846–3864. [Google Scholar] [CrossRef]
- Homolová, L.; Lukeš, P.; Malenovský, Z.; Lhotáková, Z.; Kaplan, V.; Hanuš, J. Measurement Methods and Variability Assessment of the Norway Spruce Total Leaf Area: Implications for Remote Sensing. Trees 2013, 27, 111–121. [Google Scholar] [CrossRef]
- Dawson, T.P.; Curran, P.J.; Plummer, S.E. LIBERTY—Modeling the Effects of Leaf Biochemical Concentration on Reflectance Spectra. Remote Sens. Environ. 1998, 65, 50–60. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Baret, F. PROSPECT: A Model of Leaf Optical Properties Spectra. Remote Sens. Environ. 1990, 34, 75–91. [Google Scholar] [CrossRef]
- Du, L.; Shi, S.; Yang, J.; Sun, J.; Gong, W. Using Different Regression Methods to Estimate Leaf Nitrogen Content in Rice by Fusing Hyperspectral LiDAR Data and Laser-Induced Chlorophyll Fluorescence Data. Remote Sens. 2016, 8, 526. [Google Scholar] [CrossRef]
- Schlemmer, M.R.; Francis, D.D.; Shanahan, J.F.; Schepers, J.S. Remotely Measuring Chlorophyll Content in Corn Leaves with Differing Nitrogen Levels and Relative Water Content. Agron. J. 2005, 97, 106–112. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y.U. Non-Destructive Optical Detection of Pigment Changes during Leaf Senescence and Fruit Ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef]
- Bussotti, F.; Gerosa, G.; Digrado, A.; Pollastrini, M. Selection of Chlorophyll Fluorescence Parameters as Indicators of Photosynthetic Efficiency in Large Scale Plant Ecological Studies. Ecol. Indic. 2020, 108, 105686. [Google Scholar] [CrossRef]
- Jay, S.; Bendoula, R.; Hadoux, X.; Féret, J.-B.; Gorretta, N. A Physically-Based Model for Retrieving Foliar Biochemistry and Leaf Orientation Using Close-Range Imaging Spectroscopy. Remote Sens. Environ. 2016, 177, 220–236. [Google Scholar] [CrossRef]
- Vanbrabant, Y.; Tits, L.; Delalieux, S.; Pauly, K.; Verjans, W.; Somers, B. Multitemporal Chlorophyll Mapping in Pome Fruit Orchards from Remotely Piloted Aircraft Systems. Remote Sens. 2019, 11, 1468. [Google Scholar] [CrossRef]
- Singhal, G.; Bansod, B.; Mathew, L.; Goswami, J.; Choudhury, B.U.; Raju, P.L.N. Chlorophyll Estimation Using Multi-Spectral Unmanned Aerial System Based on Machine Learning Techniques. Remote Sens. Appl. Soc. Environ. 2019, 15, 100235. [Google Scholar] [CrossRef]
- Švik, M.; Oulehle, F.; Krám, P.; Janoutová, R.; Tajovská, K.; Homolová, L. Landsat-Based Indices Reveal Consistent Recovery of Forested Stream Catchments from Acid Deposition. Remote Sens. 2020, 12, 1944. [Google Scholar] [CrossRef]
- Claverie, M.; Ju, J.; Masek, J.G.; Dungan, J.L.; Vermote, E.F.; Roger, J.-C.; Skakun, S.V.; Justice, C. The Harmonized Landsat and Sentinel-2 Surface Reflectance Data Set. Remote Sens. Environ. 2018, 219, 145–161. [Google Scholar] [CrossRef]
- Shang, R.; Zhu, Z. Harmonizing Landsat 8 and Sentinel-2: A Time-Series-Based Reflectance Adjustment Approach. Remote Sens. Environ. 2019, 235, 111439. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Henebry, G.M.; Gao, F. Development and Evaluation of a New Algorithm for Detecting 30 m Land Surface Phenology from VIIRS and HLS Time Series. ISPRS J. Photogramm. Remote Sens. 2020, 161, 37–51. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neuwirthová, E.; Kuusk, A.; Lhotáková, Z.; Kuusk, J.; Albrechtová, J.; Hallik, L. Leaf Age Matters in Remote Sensing: Taking Ground Truth for Spectroscopic Studies in Hemiboreal Deciduous Trees with Continuous Leaf Formation. Remote Sens. 2021, 13, 1353. https://doi.org/10.3390/rs13071353
Neuwirthová E, Kuusk A, Lhotáková Z, Kuusk J, Albrechtová J, Hallik L. Leaf Age Matters in Remote Sensing: Taking Ground Truth for Spectroscopic Studies in Hemiboreal Deciduous Trees with Continuous Leaf Formation. Remote Sensing. 2021; 13(7):1353. https://doi.org/10.3390/rs13071353
Chicago/Turabian StyleNeuwirthová, Eva, Andres Kuusk, Zuzana Lhotáková, Joel Kuusk, Jana Albrechtová, and Lea Hallik. 2021. "Leaf Age Matters in Remote Sensing: Taking Ground Truth for Spectroscopic Studies in Hemiboreal Deciduous Trees with Continuous Leaf Formation" Remote Sensing 13, no. 7: 1353. https://doi.org/10.3390/rs13071353
APA StyleNeuwirthová, E., Kuusk, A., Lhotáková, Z., Kuusk, J., Albrechtová, J., & Hallik, L. (2021). Leaf Age Matters in Remote Sensing: Taking Ground Truth for Spectroscopic Studies in Hemiboreal Deciduous Trees with Continuous Leaf Formation. Remote Sensing, 13(7), 1353. https://doi.org/10.3390/rs13071353






