Pepper Plants Leaf Spectral Reflectance Changes as a Result of Root Rot Damage
Abstract
1. Introduction
2. Materials and Methods
Spectroscopic Measurements and Spectral Processing
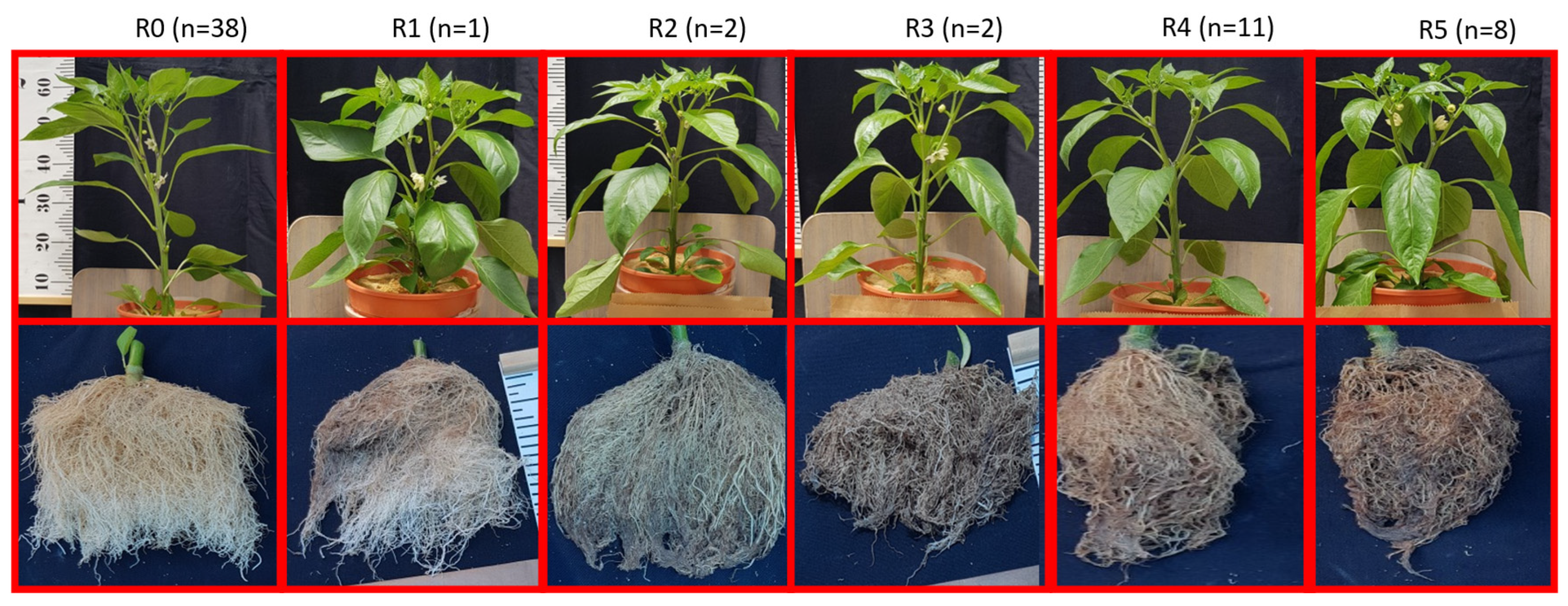
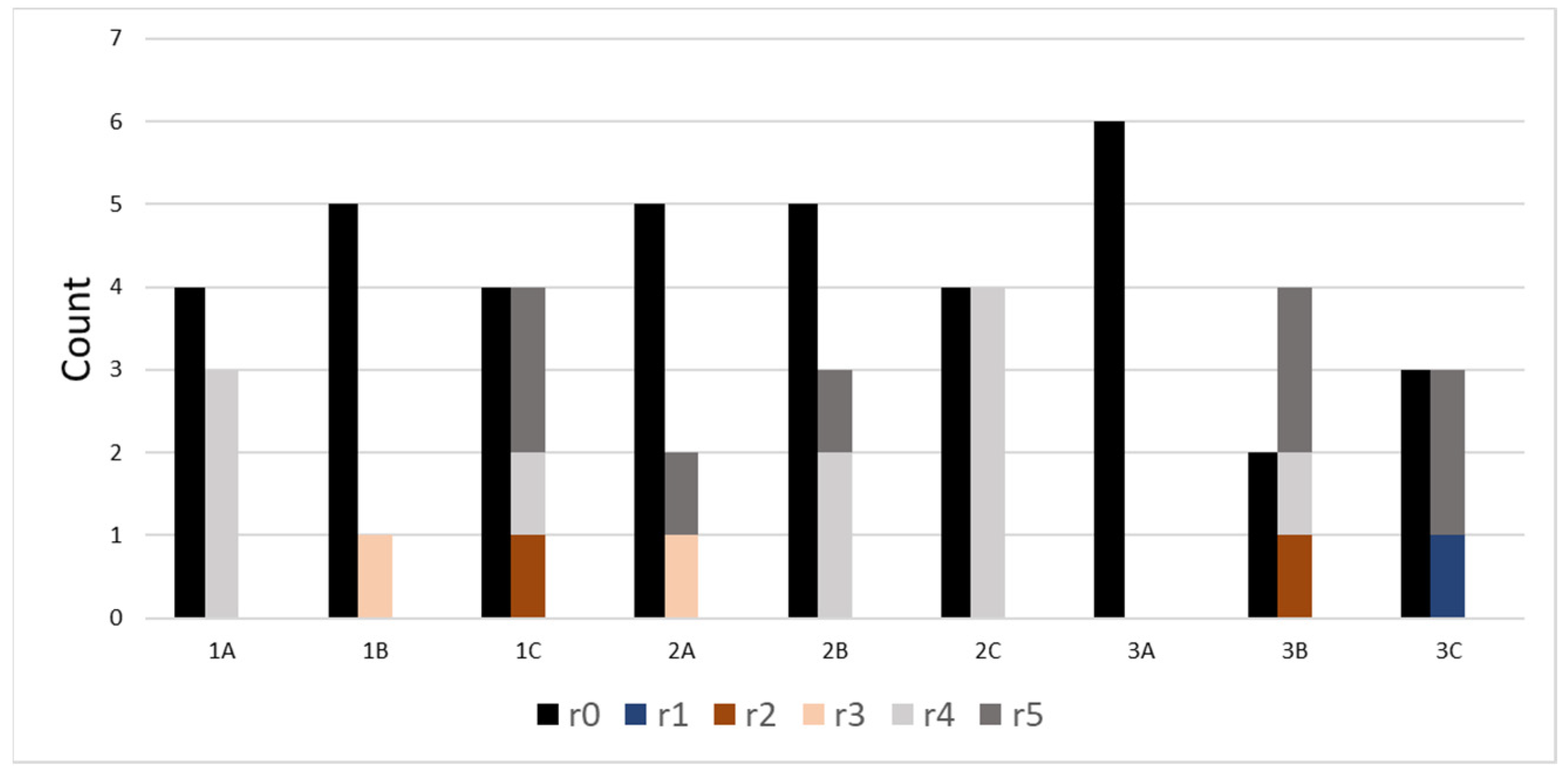
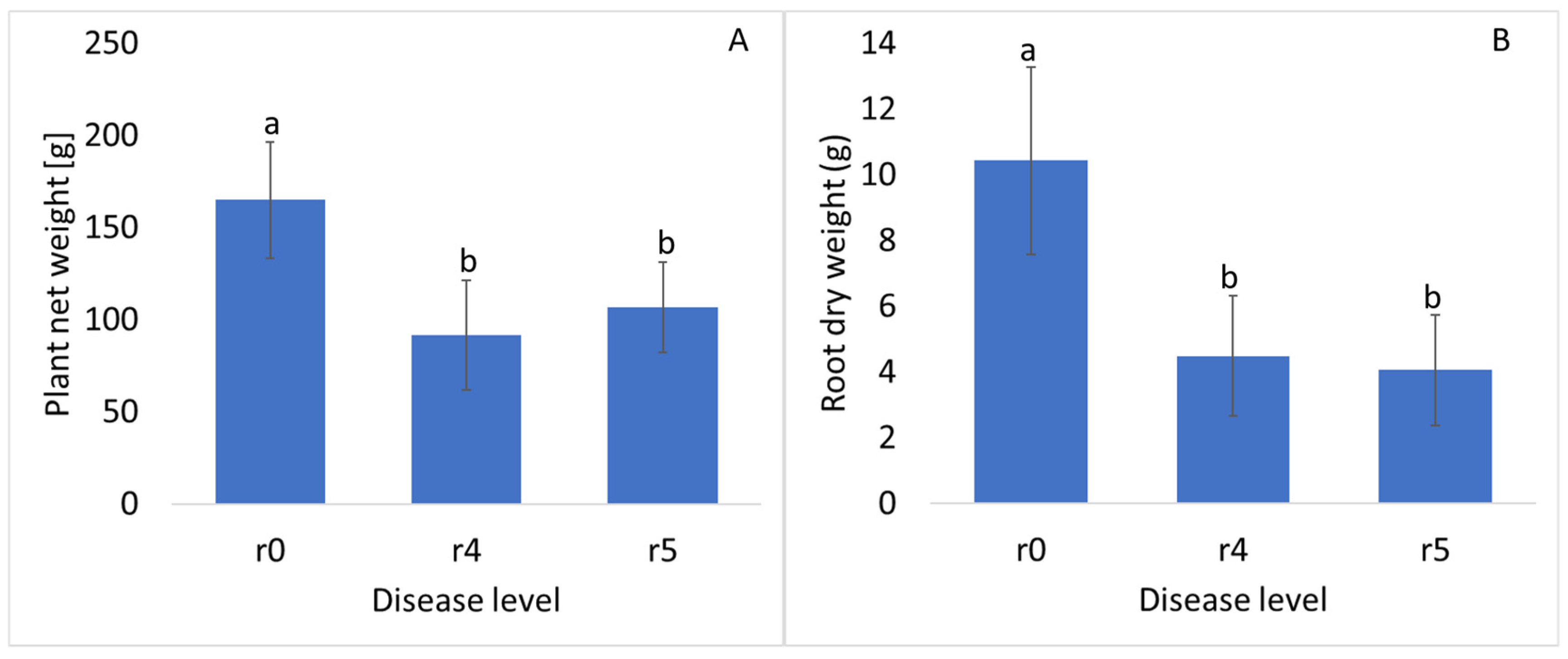
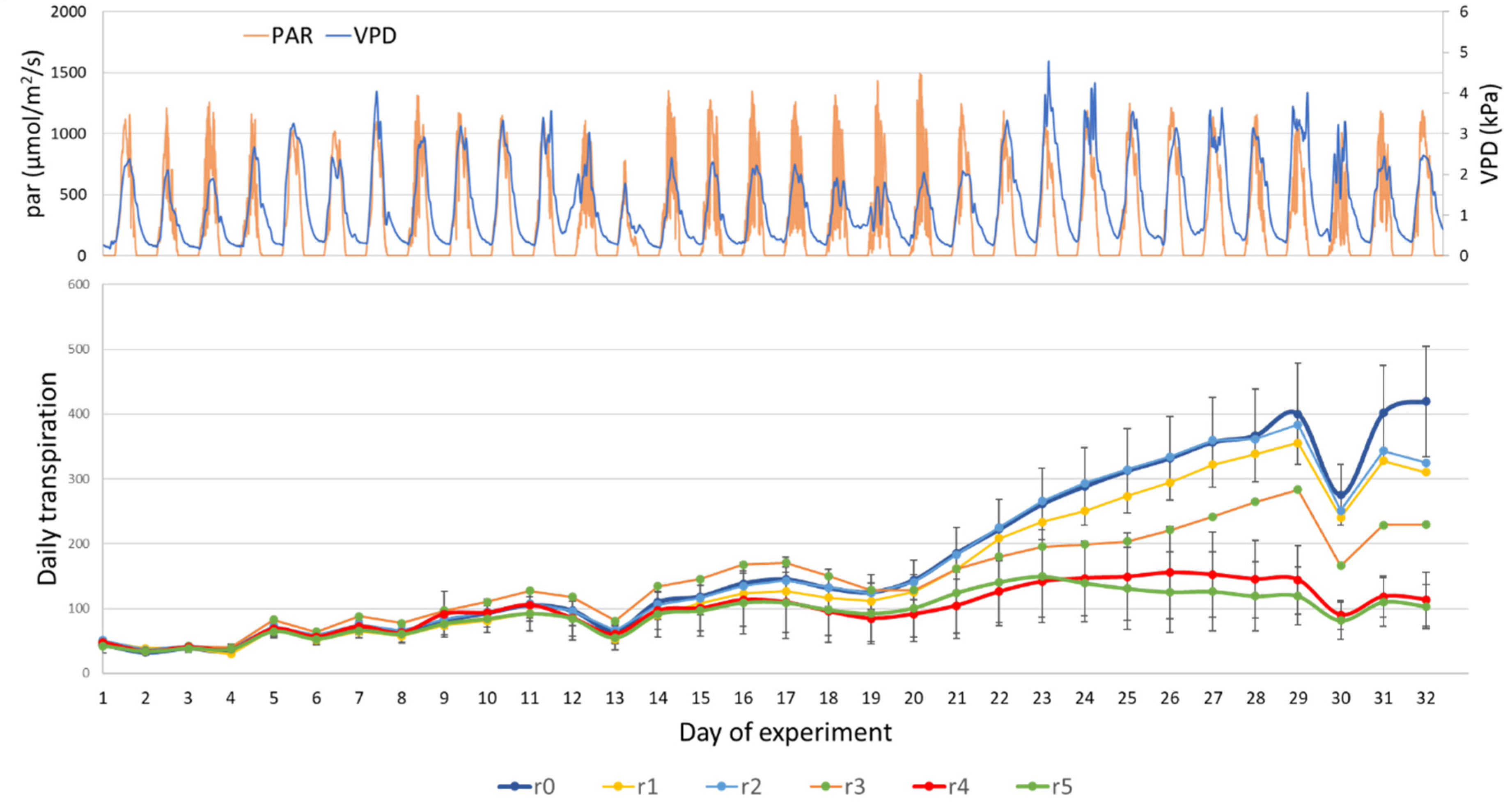
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- MacDonald, J.D. Temperature and Water Stress Effects on Sporangium Viability and Zoospore Discharge in Phytophthora cryptogea and P. megasperma. Phytopathology 1978, 68, 1449. [Google Scholar] [CrossRef]
- Sade, N.; Gebretsadik, M.; Seligmann, R.; Schwartz, A.; Wallach, R.; Moshelion, M. The role of tobacco Aquaporin1 in improving water use efficiency, hydraulic conductivity, and yield production under salt stress. Plant Physiol. 2010, 152, 245–254. [Google Scholar] [CrossRef]
- Robinson, J.B.D. Nutrient Deficiencies and Toxicities in Crop Plants. Exp. Agric. 1995, 31, 391. [Google Scholar] [CrossRef]
- Lloyd, J. Plant Health Care for Woody Ornamentals: A Professional’s Guide to Preventing and Managing Environmental Stresses and Pests; Cooperative Extension Service, College of Agricultural, Consumer and Environmental Sciences, University of Illinois at Urbana-Champaign: Urbana, IL, USA, 1997; ISBN 1883097177. [Google Scholar]
- Al-Sohaibani, S.A.; Mahmoud, M.A.; Al-Othman, M.R.; Ragab, M.M.M.; Saber, M.M.; Abd El-Aziz, A.R.M. Influence of some biotic and abiotic inducers on root rot disease incidence of sweet basil. Afr. J. Microbiol. Res. 2011, 5, 3628–3639. [Google Scholar] [CrossRef]
- Kühn, J.; Rippel, R.; Schmidhalter, U. Abiotic soil properties and the occurrence of Rhizoctonia crown and root rot in sugar beet. J. Plant. Nutr. Soil Sci. 2009, 172, 661–668. [Google Scholar] [CrossRef]
- Whipker, B.E.; Evans, M.R. Regulation of plant growth. In Greenhouse Operation and Management; Nelson, P.V., Ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2012; pp. 373–389. [Google Scholar]
- Duniway, J.M. Movement of Zoospores of Phytophthora cryptogea in Soils of Various Textures and Matric Potentials. Phytopathology 1976, 66, 877. [Google Scholar] [CrossRef]
- le Maire, G.; François, C.; Soudani, K.; Berveiller, D.; Pontailler, J.Y.; Bréda, N.; Genet, H.; Davi, H.; Dufrêne, E. Calibration and validation of hyperspectral indices for the estimation of broadleaved forest leaf chlorophyll content, leaf mass per area, leaf area index and leaf canopy biomass. Remote Sens. Environ. 2008, 112, 3846–3864. [Google Scholar] [CrossRef]
- Bartholomeus, H.; Kooistra, L.; Stevens, A.; van Leeuwen, M.; van Wesemael, B.; Ben-Dor, E.; Tychon, B. Soil Organic Carbon mapping of partially vegetated agricultural fields with imaging spectroscopy. Int. J. Appl. Earth Obs. Geoinf. 2011, 13, 81–88. [Google Scholar] [CrossRef]
- Horler, D.N.H.; Dockray, M.; Barber, J. The red edge of plant leaf reflectance. Int. J. Remote Sens. 1983, 4, 273–288. [Google Scholar] [CrossRef]
- Curran, P.J.; Windham, W.R.; Gholz, H.L. Exploring the relationship between reflectance red edge and chlorophyll concentration in slash pine leaves. Tree Physiol. 1995, 15, 203–206. [Google Scholar] [CrossRef]
- Peñuelas, J.; Gamon, J.A.; Fredeen, A.L.; Merino, J.; Field, C.B. Reflectance indices associated with physiological changes in nitrogen- and water-limited sunflower leaves. Remote Sens. Environ. 1994, 48, 135–146. [Google Scholar] [CrossRef]
- Gazala, I.F.S.; Sahoo, R.N.; Pandey, R.; Mandal, B.; Gupta, V.K.; Singh, R.; Sinha, P. Spectral reflectance pattern in soybean for assessing yellow mosaic disease. Indian J. Virol. 2013, 24, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhou, G. Estimation of vegetation water content using hyperspectral vegetation indices: A comparison of crop water indicators in response to water stress treatments for summer maize. BMC Ecol. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Ballester, C.; Brinkhoff, J.; Quayle, W.C.; Hornbuckle, J. Monitoring the effects of water stress in cotton using the green red vegetation index and red edge ratio. Remote Sens. 2019, 11. [Google Scholar] [CrossRef]
- Zhao, D.; Reddy, K.R.; Kakani, V.G.; Reddy, V.R. Nitrogen deficiency effects on plant growth, leaf photosynthesis, and hyperspectral reflectance properties of sorghum. Eur. J. Agron. 2005, 22, 391–403. [Google Scholar] [CrossRef]
- Houborg, R.; Boegh, E. Mapping leaf chlorophyll and leaf area index using inverse and forward canopy reflectance modeling and SPOT reflectance data. Remote Sens. Environ. 2008, 112, 186–202. [Google Scholar] [CrossRef]
- El-Hendawy, S.; Al-Suhaibani, N.; Hassan, W.; Tahir, M.; Schmidhalter, U. Hyperspectral reflectance sensing to assess the growth and photosynthetic properties of wheat cultivars exposed to different irrigation rates in an irrigated arid region. PLoS ONE 2017, 1–22. [Google Scholar] [CrossRef]
- El-Hendawy, S.E.; Al-Suhaibani, N.A.; Hassan, W.M.; Dewir, Y.H. Evaluation of wavelengths and spectral re fl ectance indices for high- throughput assessment of growth, water relations and ion contents of wheat irrigated with saline water. Agric. Water Manag. 2019, 212, 358–377. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Mišurec, J.; Kopačková, V.; Mielke, C.; Rogass, C. Assessment of red-edge position extraction techniques: A case study for norway spruce forests using hymap and simulated sentinel-2 data. Forests 2016, 7. [Google Scholar] [CrossRef]
- Herrmann, I.; Berenstein, M.; Sade, A.; Karnieli, A.; Bonfil, D.J.; Weintraub, P.G. Spectral monitoring of two-spotted spider mite damage to pepper leaves. Remote Sens. Lett. 2012, 3, 277–283. [Google Scholar] [CrossRef]
- Marshall, M.; Thenkabail, P.; Biggs, T.; Post, K. Hyperspectral narrowband and multispectral broadband indices for remote sensing of crop evapotranspiration and its components (transpiration and soil evaporation). Agric. For. Meteorol. 2016, 218–219, 122–134. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Smith, R.B.; De Pauw, E. Hyperspectral vegetation indices and their relationships with agricultural crop characteristics. Remote Sens. Environ. 2000, 71, 158–182. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Pacumbaba, R.O.; Beyl, C.A. Changes in hyperspectral reflectance signatures of lettuce leaves in response to macronutrient deficiencies. Adv. Sp. Res. 2011, 48, 32–42. [Google Scholar] [CrossRef]
- Pandey, P.; Ge, Y.; Stoerger, V.; Schnable, J.C. High Throughput In vivo Analysis of Plant Leaf Chemical Properties Using Hyperspectral Imaging. Front. Plant Sci. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Leckie, D.G.; Jay, C.; Gougeon, F.A.; Sturrock, R.N.; Paradine, D. Detection and assessment of trees with Phellinus weirii (laminated root rot) using high resolution multi-spectral imagery. Int. J. Remote Sens. 2004, 25, 793–818. [Google Scholar] [CrossRef]
- Reynolds, G.J.; Windels, C.E.; MacRae, I.V.; Laguette, S. Remote sensing for assessing rhizoctonia crown and root rot severity in sugar beet. Plant Dis. 2012, 96, 497–505. [Google Scholar] [CrossRef]
- Yang, C.; Everitt, J.H.; Fernandez, C.J. Comparison of airborne multispectral and hyperspectral imagery for mapping cotton root rot. Biosyst. Eng. 2010, 107, 131–139. [Google Scholar] [CrossRef]
- Weksler, S.; Rozenstein, O.; Haish, N.; Moshelion, M.; Walach, R.; Ben-Dor, E. A hyperspectral-physiological phenomics system: Measuring diurnal transpiration rates and diurnal reflectance. Remote Sens. 2020, 12, 1493. [Google Scholar] [CrossRef]
- Halperin, O.; Gebremedhin, A.; Wallach, R.; Moshelion, M. High-throughput physiological phenotyping and screening system for the characterization of plant—Environment interactions. Plant J. 2017, 89, 839–850. [Google Scholar] [CrossRef]
- Mahajan, G.R.; Sahoo, R.N.; Pandey, R.N.; Gupta, V.K.; Kumar, D. Using hyperspectral remote sensing techniques to monitor nitrogen, phosphorus, sulphur and potassium in wheat (Triticum aestivum L.). Precis. Agric. 2014, 15, 499–522. [Google Scholar] [CrossRef]
- Serbin, S.P.; Dillaway, D.N.; Kruger, E.L.; Townsend, P.A. Leaf optical properties reflect variation in photosynthetic metabolism and its sensitivity to temperature. J. Exp. Bot. 2012, 63, 489–502. [Google Scholar] [CrossRef]
- Ullah, S.; Schlerf, M.; Skidmore, A.K.; Hecker, C. Identifying plant species using mid-wave infrared (2.5–6 μm) and thermal infrared (8–14 μm) emissivity spectra. Remote Sens. Environ. 2012, 118, 95–102. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS- MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Quantitative estimation of chlorophyll-a using reflectance spectra: Experiments with autumn chestnut and maple leaves. J. Photochem. Photobiol. B Biol. 1994, 22, 247–252. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; De Colstoun, E.B.; McMurtrey, J.E. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Gitelsona, A.A.; Kaufmanb, Y.J.; Starkc, R.; Rundquist, D. Novel Algorithms for Remote Estimation of Vegetation Fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef]
- Frampton, W.J.; Dash, J.; Watmough, G.; Milton, E.J. Evaluating the capabilities of Sentinel-2 for quantitative estimation of biophysical variables in vegetation. ISPRS J. Photogramm. Remote Sens. 2013, 82, 83–92. [Google Scholar] [CrossRef]
- Milton, N.M.; Ager, C.M.; Eiswerth, B.A.; Power, M.S. Arsenic- and selenium-induced changes in spectral reflectance and morphology of soybean plants. Remote Sens. Environ. 1989, 30, 263–269. [Google Scholar] [CrossRef]
- Curran, P.J. Remote sensing of foliar chemistry. Remote Sens. Environ. 1989, 30, 271–278. [Google Scholar] [CrossRef]
- Fletcher, R.S.; Skaria, M.; Escobar, D.E.; Everitt, J.H. Field spectra and airborne digital imagery for detecting phytophthora foot rot infections in citrus trees. HortScience 2001, 36, 94–97. [Google Scholar] [CrossRef]
- Zhang, T.T.; Zeng, S.L.; Gao, Y.; Ouyang, Z.T.; Li, B.; Fang, C.M.; Zhao, B. Using hyperspectral vegetation indices as a proxy to monitor soil salinity. Ecol. Indic. 2011, 11, 1552–1562. [Google Scholar] [CrossRef]


| Sentinel 2 Band Index | Center Wavelength (nm) | Bandwidth (nm) |
|---|---|---|
| Ultra-blue B1 | 442.7 | 432.2–453.2 |
| Blue B2 | 492.4 | 459.4–525.4 |
| Green B3 | 559.8 | 541.8–577.8 |
| Red B4 | 664.6 | 649.1–680.1 |
| Re1 B5 | 704.1 | 696.6–711.6 |
| Re2 B6 | 740.5 | 733–748 |
| Re3 B7 | 782.8 | 772.8–792.8 |
| Nir B8 | 832.8 | 779.8–885.8 |
| Nir_n B9 | 864.7 | 854.2–875.2 |
| SWIR1 | 1613.7 | 1567.2–1659.2 |
| SWIR2 | 2202.4 | 2114.9–2289.9 |
| Vegetation Index | Abbreviation | Formula | References |
|---|---|---|---|
| Normalized difference vegetation index | NDVI | Tucker (1979) [36] | |
| Green normalized difference vegetation index | GNDVI | Gitelson et al. (1996) [37] | |
| Red-edge normalized vegetation index | RENDVI | Gitelson et al. (1994) [38] | |
| Modified chlorophyll absorption in reflectance | MCARI | Daughtry et al. (2000) [39] | |
| Visible Atmospherically Resistant Index | VARI | Gitelson et al. (2002) [40] | |
| Sentinel 2 red-edge position | S2REP | 705 + 35((((B7 + B4)/2) − B5)/(B6 − B5)) | Frampton et al. 2013 [41] |
| ID | Treatment |
|---|---|
| 1A | Low potassium + H2O |
| 1B | Medium potassium + H2O |
| 1C | High potassium + H2O |
| 2A | Low potassium + medium salinity |
| 2B | Medium potassium + medium salinity |
| 2C | High potassium + medium salinity |
| 3A | Low potassium + high salinity |
| 3B | Medium potassium + high salinity |
| 3C | High potassium + high salinity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weksler, S.; Rozenstein, O.; Haish, N.; Moshelion, M.; Wallach, R.; Ben-Dor, E. Pepper Plants Leaf Spectral Reflectance Changes as a Result of Root Rot Damage. Remote Sens. 2021, 13, 980. https://doi.org/10.3390/rs13050980
Weksler S, Rozenstein O, Haish N, Moshelion M, Wallach R, Ben-Dor E. Pepper Plants Leaf Spectral Reflectance Changes as a Result of Root Rot Damage. Remote Sensing. 2021; 13(5):980. https://doi.org/10.3390/rs13050980
Chicago/Turabian StyleWeksler, Shahar, Offer Rozenstein, Nadav Haish, Menachem Moshelion, Rony Wallach, and Eyal Ben-Dor. 2021. "Pepper Plants Leaf Spectral Reflectance Changes as a Result of Root Rot Damage" Remote Sensing 13, no. 5: 980. https://doi.org/10.3390/rs13050980
APA StyleWeksler, S., Rozenstein, O., Haish, N., Moshelion, M., Wallach, R., & Ben-Dor, E. (2021). Pepper Plants Leaf Spectral Reflectance Changes as a Result of Root Rot Damage. Remote Sensing, 13(5), 980. https://doi.org/10.3390/rs13050980








