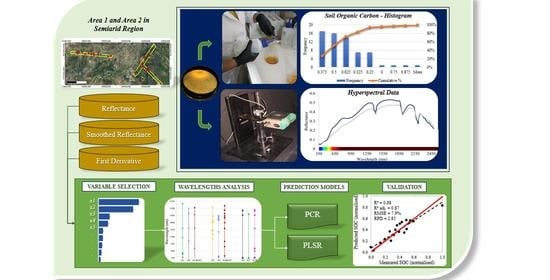
Soil Organic Carbon Content Prediction Using Soil-Reflected Spectra: A Comparison of Two Regression Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Study Areas
2.2. Collection of the Samples
2.3. Acquisition of the Hyperspectral Data
2.4. Quantification of the Soil Organic Carbon
2.5. Exploratory Data Analysis
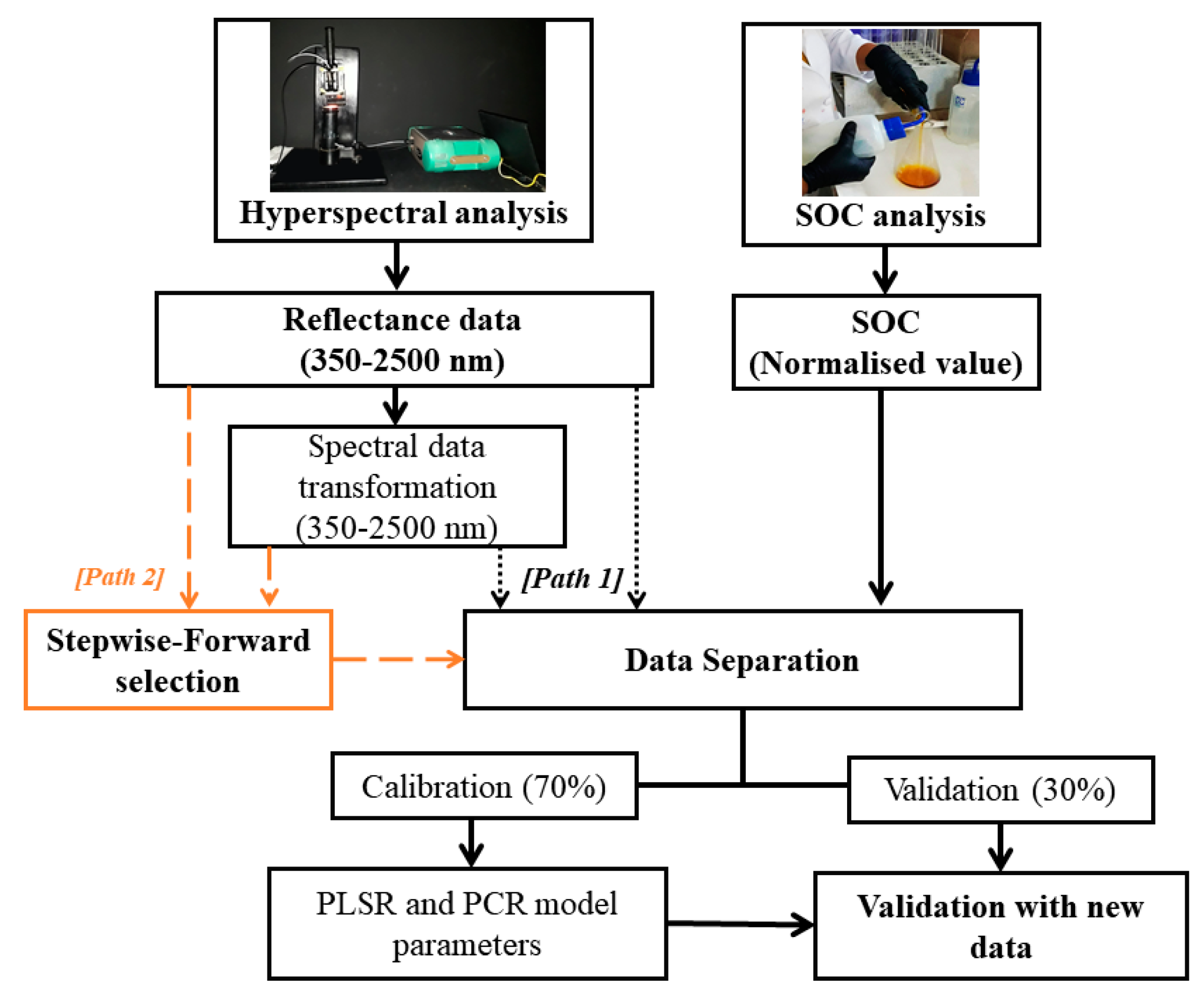
2.6. Selection of the Significant Variables
2.7. Treatment of the Hyperspectral Data
2.7.1. First Derivative
2.7.2. Savitzky–Golay Smoothing
2.8. Calibration and Validation of the Predictive Models
3. Results
3.1. Descriptive Statistics
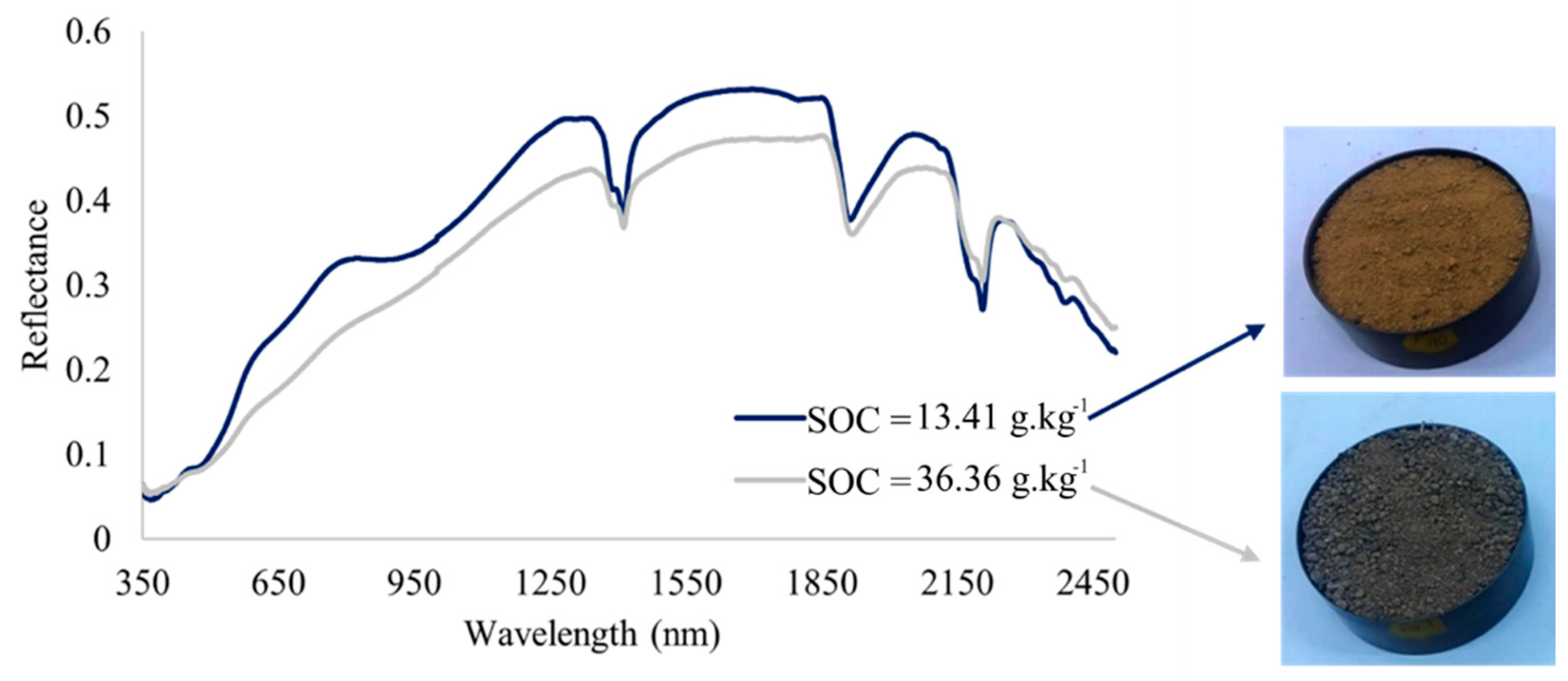
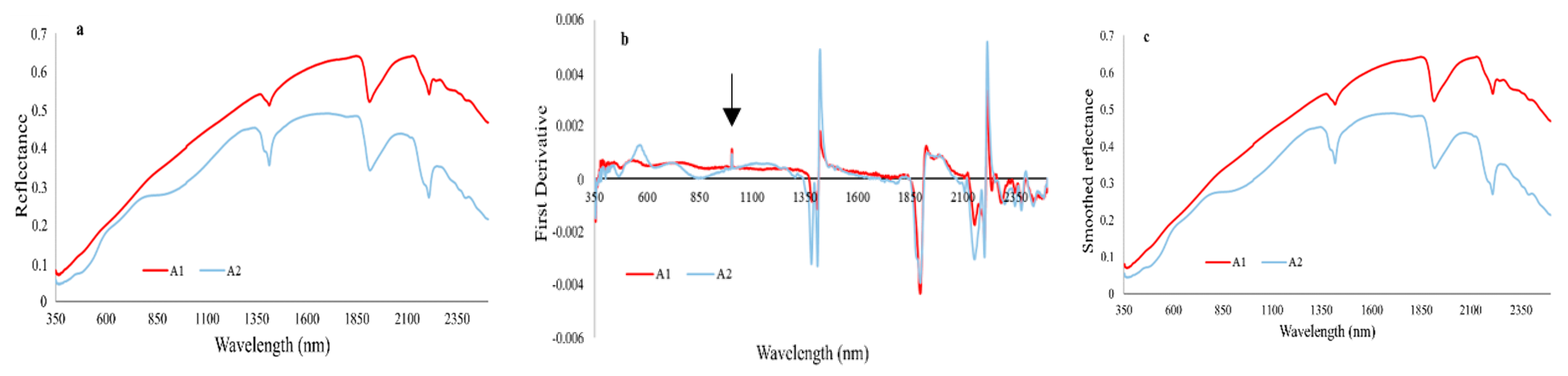
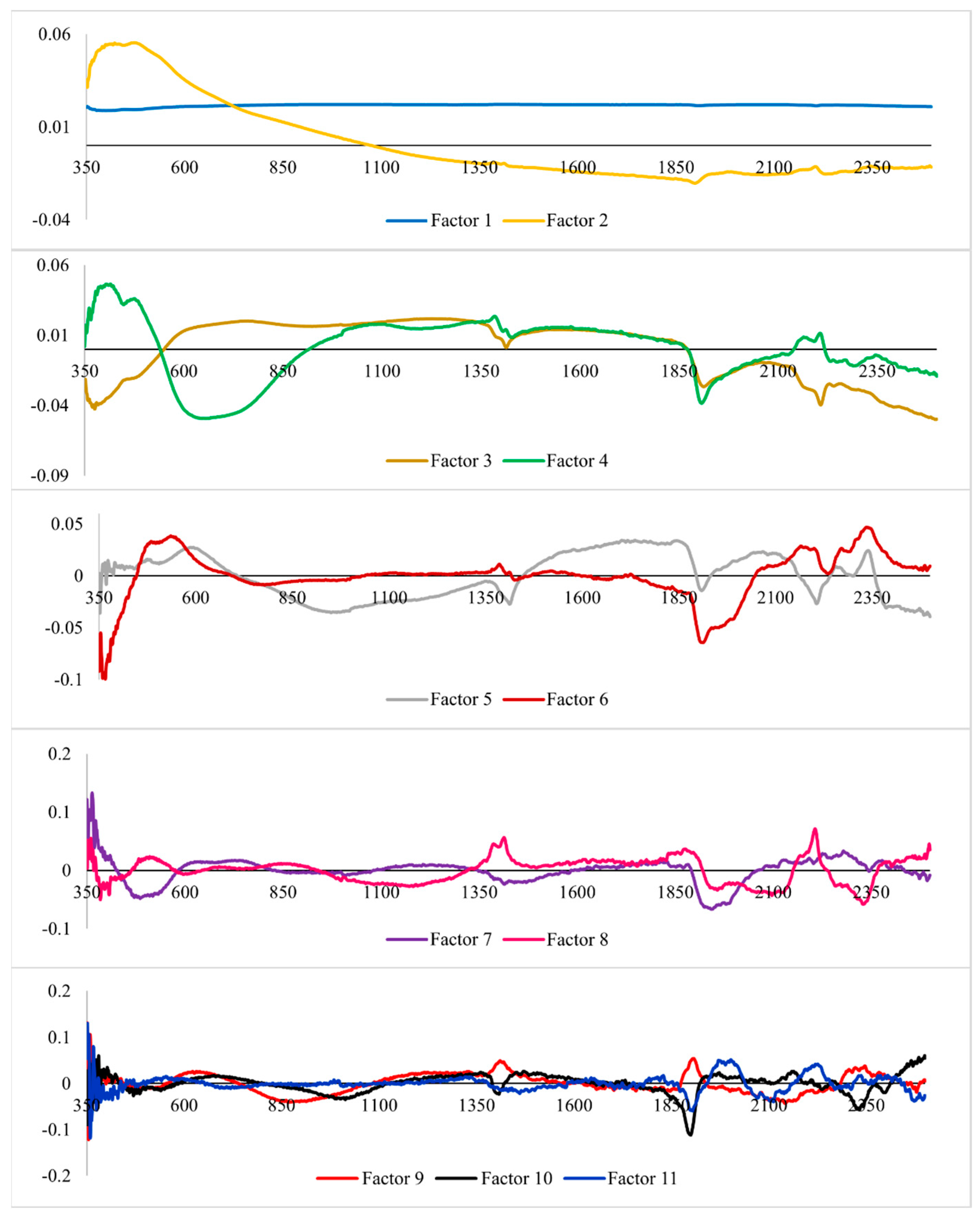
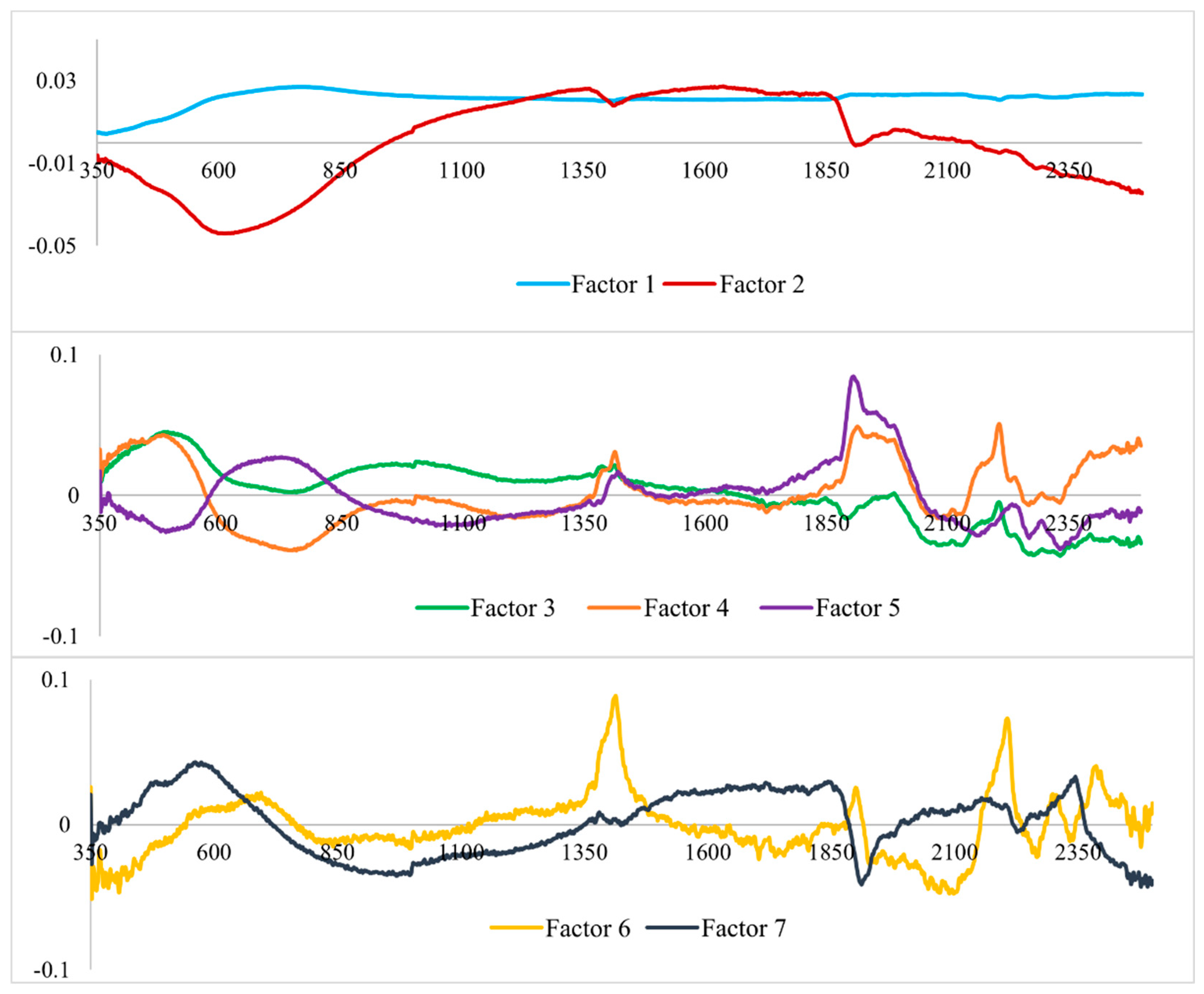
3.2. Analysis of the Hyperspectral Data
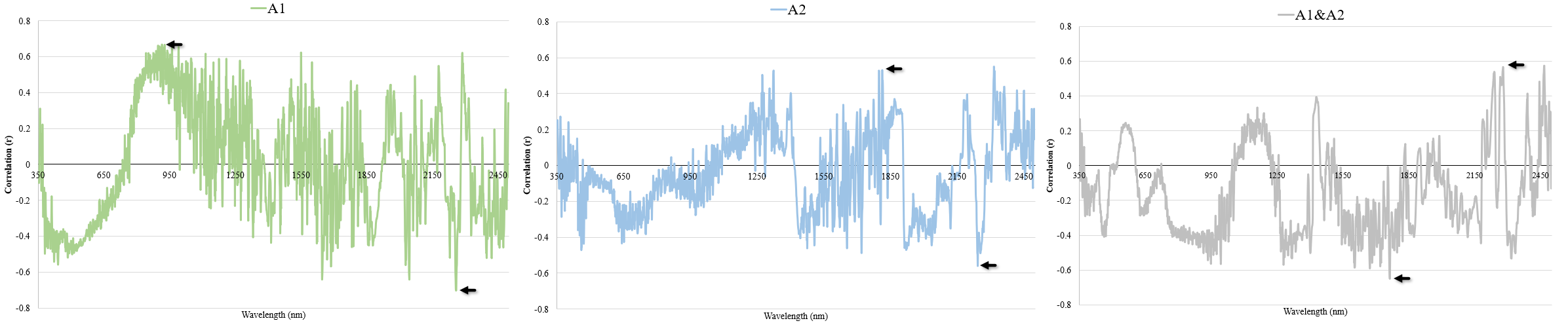
3.3. Pearson Correlation Coefficients
3.4. Full-Spectrum Estimation of SOC Content
3.4.1. Principal Component Regression (PCR)
3.4.2. Partial Least Squares Regression (PLSR)
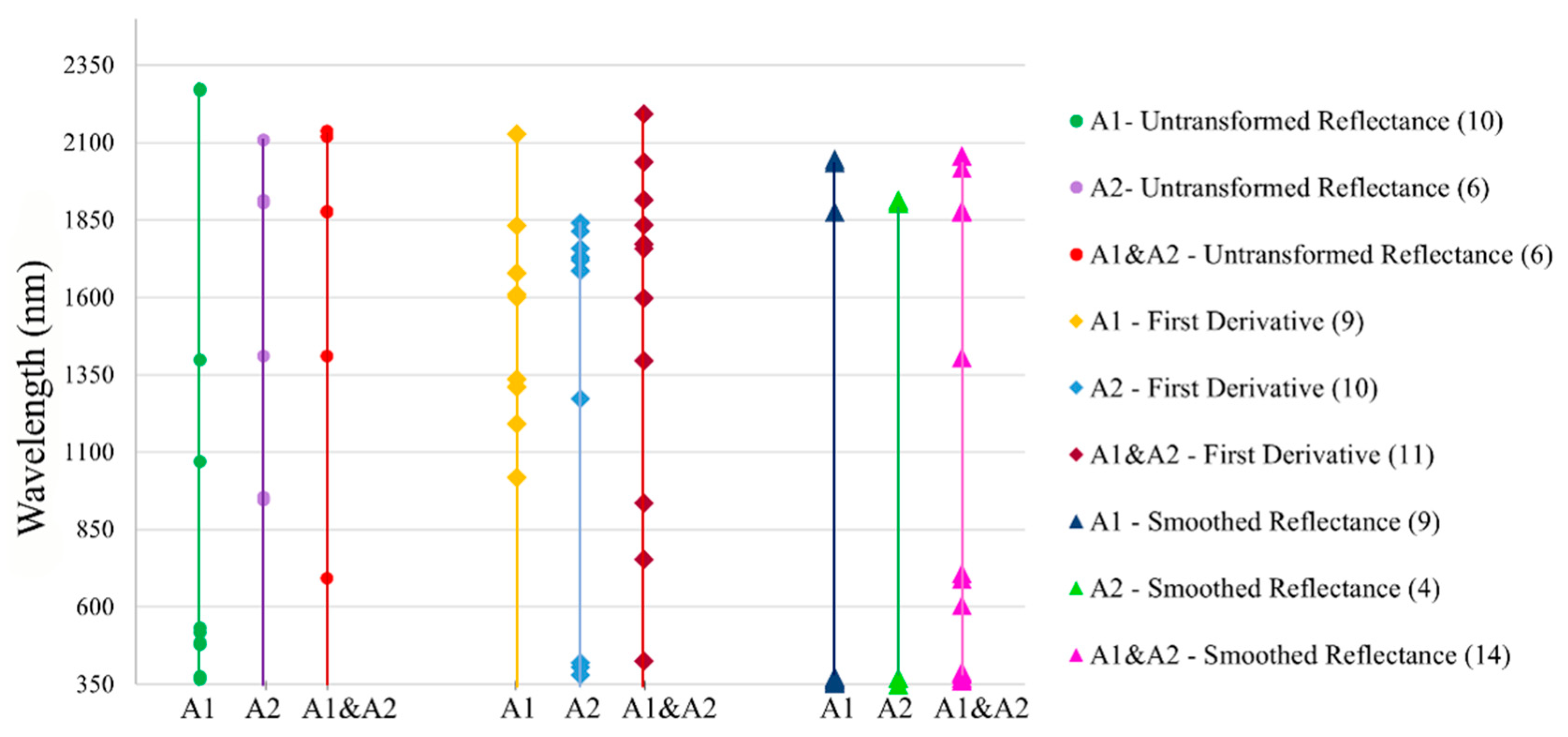
3.5. Selection of Significant Bands
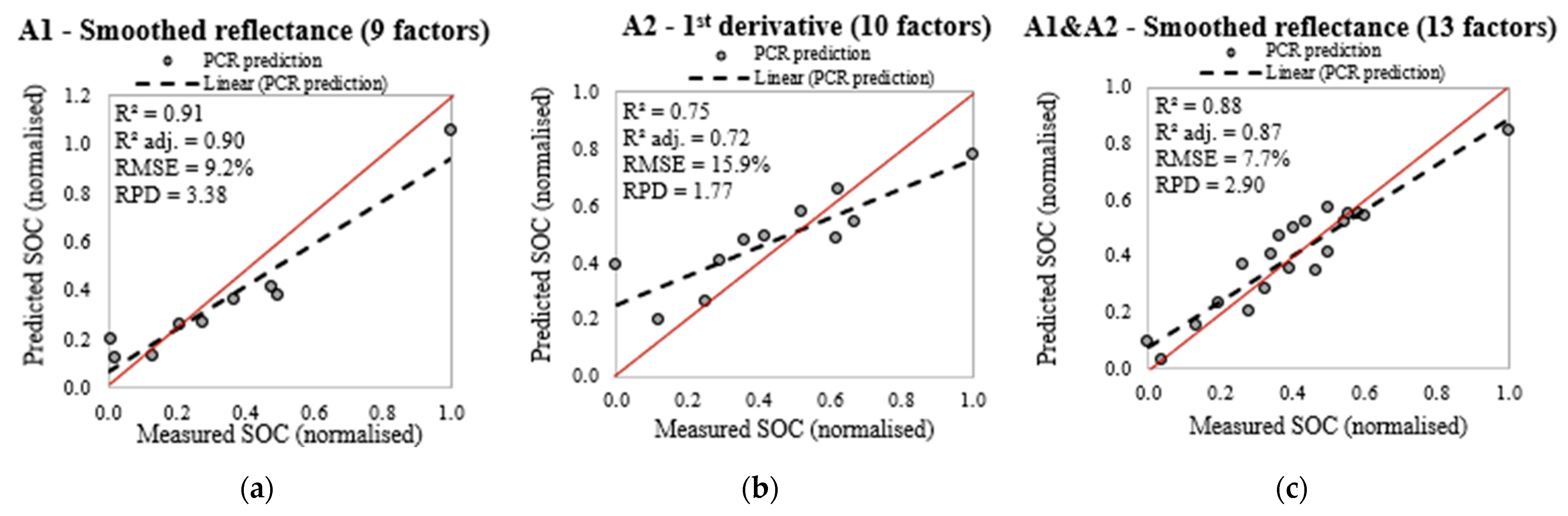
3.5.1. Principal Component Regression after Band Selection
3.5.2. Partial Least Squares Regression after Band Selection
4. Discussion
4.1. Descriptive Statistics
4.2. Hyperspectral Data Analysis
4.3. Correlation between the Variation in Soc and the First Derivative of Reflectance
4.4. Estimating the SOC Content before and after Selecting the Spectral Bands
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrews, S.S.; Karlen, D.L.; Cambardella, C.A. The Soil Management Assessment Framework: A Quantitative Soil Quality Evaluation Method. Soil Sci. Soc. Am. J. 2004, 68, 1945–1962. [Google Scholar] [CrossRef]
- Allory, V.; Cambou, A.; Moulin, P.; Schwartz, C.; Cannavo, P.; Vidal-Beaudet, L.; Barthès, B.G. Quantification of soil organic carbon stock in urban soils using visible and near infrared reflectance spectroscopy (VNIRS) in situ or in laboratory conditions. Sci. Total Environ. 2019, 686, 764–773. [Google Scholar] [CrossRef]
- Gomez, C.; Chevallier, T.; Moulin, P.; Bouferra, I.; Hmaidi, K.; Arrouays, D.; Jolivet, C.; Barthès, B.G. Prediction of soil organic and inorganic carbon concentrations in tunisian samples by mid-infrared reflectance spectroscopy using a french national library. Geoderma 2020, 375, 114469. [Google Scholar] [CrossRef]
- Houghton, R.A. Balancing the Global Carbon Budget. Annu. Rev. Earth Planet. Sci. 2007, 35, 313–347. [Google Scholar] [CrossRef]
- Lal, R. Accelerated soil erosion as a source of atmospheric CO2. Soil Tillage Res. 2019, 188, 35–40. [Google Scholar] [CrossRef]
- Raiesi, F. The quantity and quality of soil organic matter and humic substances following dry-farming and subsequent restoration in an upland pasture. Catena 2021, 202, 105249. [Google Scholar] [CrossRef]
- Fontana, A.; Anjos, L.H.C.; Sallés, J.M.; Pereira, M.C.; Rossiello, R.O.P. Carbono orgânico e fracionamento químico da matéria orgânica em solos da Sierra de Ánimas—Uruguai. Floresta Ambiente 2005, 12, 36–43. [Google Scholar]
- Lal, R. Enhancing crop yields in the developing countries through restoration of the soil organic carbon pool in agricultural lands. Land Degrad. Dev. 2006, 17, 197–209. [Google Scholar] [CrossRef]
- Kibblewhite, M.G.; Ritz, K.; Swift, M.J. Soil health in agricultural systems. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 685–701. [Google Scholar] [CrossRef]
- Rossel, R.V.; Lee, J.; Behrens, T.; Luo, Z.; Baldock, J.; Richards, A. Continental-scale soil carbon composition and vulnerability modulated by regional environmental controls. Nat. Geosci. 2019, 12, 547–552. [Google Scholar] [CrossRef]
- Goldin, A. Reassessing the use of loss-on-ignition for estimating organic matter content in noncalcareous soils. Commun. Soil Sci. Plant Anal. 1987, 18, 1111–1116. [Google Scholar] [CrossRef]
- Apesteguia, M.; Plante, A.F.; Virto, I. Methods assessment for organic and inorganic carbon quantification in calcareous soils of the mediterranean region. Geoderma Reg. 2018, 12, 39–48. [Google Scholar] [CrossRef]
- Xiaoju, N.; Tongqian, Z.; Yanyan, S. Fossil fuel carbon contamination impacts soil organic carbon estimation incropland. Catena 2021, 196, 104889. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Carmo, D.L.; Silva, C.A. Métodos de quantificação de carbono e matéria orgânica em resíduos orgânicos. Rev. Bras. Ciênc. Solo 2012, 36, 1211–1220. [Google Scholar] [CrossRef]
- Vitti, C.; Stellacci, A.M.; Leogrande, R.; Mastrangelo, M.; Cazzato, E.; Ventrella, D. Assessment of organic carbon in soils: A comparison between the springer–klee wet digestion and the dry combustion methods in mediterranean soils (Southern Italy). Catena 2016, 137, 113–119. [Google Scholar] [CrossRef]
- Sithole, N.J.; Ncama, K.; Magwaza, L. Robust VIS-NIRS models for rapid assessment of soil organic carbon and nitrogen in Feralsols Haplic soils from different tillage management practices. Comput. Electron. Agric. 2018, 153, 295–301. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Neumann, C.; Chabrillat, S.; van Wesemael, B.; Castaldi, F.; Borůvka, L.; Sanderman, J.; Klement, A.; Hohmann, C. Soil organic carbon estimation using VNIR–SWIR spectroscopy: The effect of multiple sensors and scanning conditions. Soil Tillage Res. 2021, 211, 105–117. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, X.; Sun, X.; Sun, Y.; Cen, Y. Predicting nickel concentration in soil using reflectance spectroscopy associated with organic matter and clay minerals. Geoderma 2018, 327, 25–35. [Google Scholar] [CrossRef]
- Benedet, L.; Faria, W.M.; Silva, S.H.G.; Mancini, M.; Demattê, J.A.M.; Guilherme, L.R.G.; Curi, N. Soil texture prediction using portable X-ray fluorescence spectrometry and visible near-infrared diffuse reflectance spectroscopy. Geoderma 2020, 376, 114553. [Google Scholar] [CrossRef]
- Demattê, J.A.M.; Epiphanio, J.C.N.; Formaggio, A.R. Influência da matéria orgânica e de formas de ferro na reflectância de solos tropicais. Bragantia 2003, 62, 451–464. [Google Scholar] [CrossRef]
- Vasava, H.B.; Gupta, A.; Arora, R.; Das, B.S. Assessment of soil texture from spectral reflectance data of bulk soil samples and their dry-sieved aggregate size fractions. Geoderma 2019, 337, 914–926. [Google Scholar] [CrossRef]
- Pudelko, A.; Chodak, M. Estimation of total nitrogen and organic carbon contents in mine soils with NIR reflectance spectroscopy and various chemometric methods. Geoderma 2020, 368, 114306. [Google Scholar] [CrossRef]
- Gomez, C.; Viscarra Rossel, R.A.; McBratney, A.B. Soil organic carbon prediction by hyperspectral remote sensing and field VIS-NIR spectroscopy: An Australian case study. Geoderma 2008, 146, 403–411. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Carmon, N.; Klement, A.; Ben-Dor, E.; Borůvka, L. Agricultural soil spectral response and properties assessment: Effects of measurement protocol and data mining technique. Remote Sens. 2017, 9, 1078. [Google Scholar] [CrossRef]
- Chen, S.; Xu, D.; Li, S.; Ji, W.; Yang, M.; Zhou, Y.; Hu, B.; Xu, H.; Shi, Z. Monitoring soil organic carbon in alpine soils using in situ vis-NIR spectroscopy and a multilayer perceptron. Land Degrad. Dev. 2020, 31, 1026–1038. [Google Scholar] [CrossRef]
- Conforti, M.; Matteucci, G.; Buttafuoco, G. Using laboratory Vis-NIR spectroscopy for monitoring some forest soil properties. J. Soils Sediments 2018, 18, 1009–1019. [Google Scholar] [CrossRef]
- Demattê, J.A.M.; Dotto, A.C.; Bedin, L.G.; Sayão, V.M.; Souza, A.B. Soil analytical quality control by traditional and spectroscopy techniques: Constructing the future of a hybrid laboratory for low environmental impact. Geoderma 2019, 337, 111–121. [Google Scholar] [CrossRef]
- Xu, M.; Chu, X.; Fu, Y.; Wang, C.; Wu, S. Improving the accuracy of soil organic carbon content prediction based on visible and near-infrared spectroscopy and machine learning. Environ. Earth Sci. 2021, 80, 1–10. [Google Scholar] [CrossRef]
- Biney, J.K.M.; Borůvka, L.; Chapman Agyeman, P.; Němeček, K.; Klement, A. Comparison of field and laboratory wet soil spectra in the Vis-NIR range for soil organic carbon prediction in the absence of laboratory dry measurements. Remote Sens. 2020, 12, 3082. [Google Scholar] [CrossRef]
- Barra, I.; Haefele, S.M.; Sakrabani, R.; Kebede, F. Soil spectroscopy with the use of chemometrics, machine learning and pre-processing techniques in soil diagnosis: Recent advances-A review. TrAC Trends Anal. Chem. 2020, 135, 116–166. [Google Scholar] [CrossRef]
- Kawamura, K.; Nishigaki, T.; Tsujimoto, Y.; Andriamananjara, A.; Rabenaribo, M.; Asai, H.; Rakotoson, T.; Razafimbelo, T. Exploring relevant wavelength regions for estimating soil total carbon contents of rice fields in Madagascar from Vis-NIR spectra with sequential application of backward interval PLS. Plant. Prod. Sci. 2021, 24, 1–14. [Google Scholar] [CrossRef]
- Ahmadi, A.; Emami, M.; Daccache, A.; He, L. Soil Properties Prediction for Precision Agriculture Using Visible and Near-Infrared Spectroscopy: A Systematic Review and Meta-Analysis. Agronomy 2021, 11, 433. [Google Scholar] [CrossRef]
- Javadi, S.H.; Munnaf, M.A.; Mouazen, A.M. Fusion of Vis-NIR and XRF spectra for estimation of key soil attributes. Geoderma 2021, 385, 114851. [Google Scholar] [CrossRef]
- Oliveira, M.R.R.; Ribeiro, S.G.; Mas, J.F.; Teixeira, A.S. Advances in hyperspectral sensing in agriculture: A review. Rev. Ciênc. Agron. 2020, 51, 1–12. [Google Scholar] [CrossRef]
- Liu, J.; Han, J.; Xie, J.; Wang, H.; Tong, W.; Ba, Y. Assessing heavy metal concentrations in earth-cumulic-orthicanthrosols soils using VIS-NIR spectroscopy transform coupled with chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 226, 117639. [Google Scholar] [CrossRef]
- Carioca, A.C.; Costa, G.M.; Barrón, V.; Ferreira, C.M.; Torrent, J. Aplicação da espectroscopia de reflectância difusa na quantificação dos constituintes de bauxita e de minério de ferro. Rev. Esc. Minas 2011, 64, 199–204. [Google Scholar] [CrossRef]
- Curcio, D.; Ciraolo, G.; D′asaro, F.; Minacapilli, M. Prediction of soil texture distributions using VNIR-SWIR reflectance spectroscopy. Procedia Environ. Sci. 2013, 19, 494–503. [Google Scholar] [CrossRef]
- Hutengs, C.; Seidel, M.; Oertel, F.; Ludwig, B.; Vohland, M. In situ and laboratory soil spectroscopy with portable visible-to-near infrared and mid-infrared instruments for the assessment of organic carbon in soils. Geoderma 2019, 355, 113900. [Google Scholar] [CrossRef]
- Pinheiro, E.F.M.; Ceddia, M.B.; Clingensmith, C.M.; Grunwald, S.; Vasques, G.M. Prediction of Soil Physical and Chemical Properties by Visible and Near-Infrared Diffuse Reflectance Spectroscopy in the Central Amazon. Remote Sens. 2017, 9, 293. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, Y.; Guo, L.; Fei, T.; Qi, K. Estimating soil organic carbon of cropland soil at different levels of soil moisture using VIS-NIR spectroscopy. Remote Sens. 2016, 8, 755. [Google Scholar] [CrossRef]
- Morellos, A.; Pantazi, X.E.; Moshou, D.; Alexandridis, T.; Whetton, R.; Tziotzios, G.; Wiebensohn, J.; Bill, R.; Mouazen, A.M. Machine learning based prediction of soil total nitrogen, organic carbon and moisture content by using VIS-NIR spectroscopy. Biosyst. Eng. 2016, 152, 104–116. [Google Scholar] [CrossRef]
- Mahajan, G.R.; Das, B.; Gaikwad, B.; Murgaonkar, D.; Desai, A.; Morajkar, S.; Patel, K.P.; Kulkarni, R.M. Monitoring properties of the salt-affected soils by multivariate analysis of the visible and near-infrared hyperspectral data. Catena 2021, 198, 105041. [Google Scholar] [CrossRef]
- Almeida, E.L. Sensoriamento Remoto Hiperespectral na Estimativa da Granulometria de Horizontes Superficiais de Solos. Ph.D. Thesis, Federal University of Ceara, Fortaleza, Brazil, 2020. in press. [Google Scholar]
- FUNCEME. Fundação Cearense de Meteorologia E Recursos Hídricos. Available online: www.funceme.br (accessed on 3 August 2021).
- Jacomine, P.K.T.; Almeida, J.C.; Medeiros, L.A.R. Levantamento Exploratório: Reconhecimento de solos do estado do Ceará. Bol. Técnico 28 1973, 1, 376. [Google Scholar]
- Ferreyra, F.F.H.; Silva, F.R. Identificação mineralógica das frações areia e argila dos solos aluviais do perímetro K do projeto de irrigação de Morada Nova, Ceará. Rev. Ciênc. Agron. 1991, 22, 29–37. [Google Scholar]
- Colares, D.S. Análise Técnico-Econômica do Cultivo do Arroz no Perímetro Irrigado Morada Nova. Master’s Thesis, Federal University of Ceara, Fortaleza, Brazil, 2004. [Google Scholar]
- Cunha, C.S.M. Relação Entre Solos Afetados por Sais e Concentração de Metais Pesados em Quatro Perímetros Irrigados no Ceará. Master’s Thesis, Federal University of Ceara, Fortaleza, Brazil, 2013. [Google Scholar]
- Cunha, T.J.F.; Petrere, V.G.; Silva, D.J.; Mendes, A.M.S.; de MELO, R.F.; de Oliveira Neto, M.B.; da Silva, M.S.L.; Alvarez, I.A. Principais solos do semiárido tropical brasileiro: Caracterização, potencialidades, limitações, fertilidade e manejo. In Semiárido Brasileiro: Pesquisa, Desenvolvimento e Inovação; Sa, I.B., da Silva, P.C.G., Eds.; Embrapa Semiárido: Petrolina, Brazil, 2010; pp. 50–87. [Google Scholar]
- Mota, J.C.A.; Assis Junir, R.N.; Amaro Fiho, J.; Romero, R.E.; Mota, F.O.B.; Libardi, P.L. Atributos mineralógicos de três solos explorados com a cultura do melão na chapada do Apodi-RN. Rev. Bras. Ciênc. Solo 2007, 31, 445–454. [Google Scholar] [CrossRef]
- Corado Neto, F.C.; Sampaio, F.M.T.; Veloso, M.E.C.; Matias, S.S.R.; Andrade, F.R.; Lobato, M.G.R. Variabilidade espacial dos agregados e carbono orgânico total em Neossolo Litólico Eutrófico no município de Gilbués, PI. Rev. Ciênc. Agrár. 2015, 58, 75–83. [Google Scholar] [CrossRef][Green Version]
- Amaro Filho, J.; Assis Júnior, R.N.; Mota, J.C.A. Física Do Solo: Conceitos e Aplicações; Imprensa Universitária: Fortaleza, Brazil, 2008; p. 290. [Google Scholar]
- Analytical Spectral Devices: ASD Technical Guide; Analytical Spectral Devices Inc.: Boulder, CO, USA, 1999.
- Demattê, J.A.M.; Sousa, A.A.; Nanni, M.R. Avaliação espectral de amostras de solos e argilo-minerais em função de diferentes níveis de hidratação. Simpósio Bras. Sens. Remoto 1998, 9, 1295–1298. [Google Scholar]
- Lobell, D.B.; Asner, G.P. Moisture effects on Soil Reflectance. Soil Sci. Soc. Am. J. 2002, 66, 722–727. [Google Scholar] [CrossRef]
- Tian, J.; Yue, J.; Philpot, W.D.; Dong, X.; Tian, Q. Soil moisture content estimate with drying process segmentation using shortwave infrared bands. Remote Sens. Environ. 2021, 263, 112552. [Google Scholar] [CrossRef]
- Patel, K.F.; Myers-Pigg, A.; Bond-Lamberty, B.; Fansler, S.J.; Norris, C.G.; McKever, S.A.; Zheng, J.; Rod, K.A.; Bailey, V.L. Soil carbon dynamics during drying vs. rewetting: Importance of antecedent moisture conditions. Soil Biol. Biochem. 2021, 156, 108165. [Google Scholar] [CrossRef]
- Ge, Z.; Gao, L.; Ma, N.; Hu, E.; Li, M. Variation in the content and fluorescent composition of dissolved organic matter in soil water during rainfall-induced wetting and extract of dried soil. Sci. Total Environ. 2021, 791, 148296. [Google Scholar] [CrossRef] [PubMed]
- O’Haver, T.C. An introduction to signal processing in chemical measurement. J. Chem. Educ. 1991, 68, A147. [Google Scholar] [CrossRef]
- Rudorff, C.M.; Novo, E.M.L.M.; Galvão, L.S.; Pereira Filho, W. Análise derivativa de dados hiperespectrais medidos em nível de campo e orbital para caracterizar a composição de águas opticamente complexas na Amazônia. Acta Amaz. 2007, 37, 269–280. [Google Scholar] [CrossRef][Green Version]
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Wold, H. Soft modelling by latent variables: The Non-Linear Iterative Partial Least Squares (NIPALS) approach. J. Appl. Probab. 1975, 12, 117–142. [Google Scholar] [CrossRef]
- Massy, W.F. Principal components regression in exploratory statistical research. J. Am. Stat. Assoc. 1965, 60, 234–246. [Google Scholar] [CrossRef]
- Forster, M.A. Principal Components Regression Analysis for Plant Physiologists. Edaphic Scientific: Environmental Research & Monitoring Equipment . Available online: https://edaphic.com.au (accessed on 24 May 2021).
- Bushong, J.T.; Norman, R.J.; Slaton, N.A. Near-infrared reflectance spectroscopy as a method for determining organic carbon concentrations in soil. Commun. Soil Sci. Plant Anal. 2015, 46, 1791–1801. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Moorman, T.B.; Novak, J.M.; Parkin, T.B.; Karlen, D.L.; Turco, R.F.; Konopka, A.E. Field-scale variability of soil properties in central Iowa soils. Soil Sci. Soc. Am. J. 1994, 58, 1501–1511. [Google Scholar] [CrossRef]
- Oliveira, J.C., Jr.; Souza, L.C.P.; Melo, V.F. Variabilidade de atributos físicos e químicos de solos da Formação Guabirotuba em diferentes unidades de amostragem. Rev. Bras. Ciênc. Solo 2010, 34, 1491–1502. [Google Scholar] [CrossRef]
- Petrucci, E.; Oliveira, L.A. Coeficientes de assimetria e curtose nos dados de vazão média mensal da bacia do Rio Preto-BA. Os Desafios Geogr. Física Front. Conhecimento 2017, 1, 158–170. [Google Scholar]
- Girão, R.O.; Moreira, L.J.S.; Girão, A.L.A.; Romero, R.E.; Ferreira, T.O. Soil genesis and iron nodules in a karst environment of the Apodi Plateau. Rev. Ciênc. Agron. 2014, 45, 683–695. [Google Scholar] [CrossRef]
- Barbosa, C.C.F. Sensoriamento Remoto da Dinâmica da Circulação da Água do Sistema Planície de Curuai/Rio Amazonas. Ph.D. Thesis, National Institute for Space Research, São José dos Campos, Brazil, 2005. [Google Scholar]
- Ennes, R.; Galo, M.D.L.B.T.; Tachibana, V.M. Caracterização espectral da água do reservatório de Itupararanga, SP, a partir de imagens hiperespectrais Hyperion e análise derivativa. Bol. Ciênc. Geod. 2010, 16, 86–104. [Google Scholar]
- Ben-Dor, E.; Inbar, Y.; Chen, Y. The Reflectance Spectra of Organic Matter in the Visible Near-Infrared and Short-Wave Infrared Region (400–2500 nm) during a Controlled Decomposition Process. Remote Sens. Environ. 1997, 61, 1–15. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Walvoort, D.J.J.; Mcbratney, A.B.; Janik, L.J.; Skjemstad, J.O. Visible, near infrared, mid infrared or combined diffuse reflectance spectroscopy for simultaneous assessment of various soil properties. Geoderma 2006, 131, 59–75. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; McGlynn, R.N.; McBratney, A.B. Determining the composition of mineral-organic mixes using UV–vis–NIR diffuse reflectance spectroscopy. Geoderma 2006, 137, 70–82. [Google Scholar] [CrossRef]
- Rocha Neto, O.C.D.; Teixeira, A.D.S.; Leão, R.A.D.O.; Moreira, L.C.J.; Galvão, L.S. Hyperspectral remote sensing for detecting soil salinization using prospectir-vs aerial imagery and sensor simulation. Remote Sens. 2017, 9, 42. [Google Scholar] [CrossRef]
- Pearlshtien, D.H.; Ben-Dor, E. Effect of organic matter content on the spectral signature of iron oxides across the VIS–NIR spectral region in artificial mixtures: An example from a red soil from Israel. Remote Sens. 2020, 12, 1960. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Behrens, T. Using data mining to model and interpret soil diffuse reflectance spectra. Geoderma 2010, 158, 46–54. [Google Scholar] [CrossRef]
- Romagnoli, F.; Nanni, M.R.; Gasparotto, A.C.; Silva Junior, C.A.; Cezar, E.; da Silva, A.A.; Sacioto, M. Predição do carbono do solo por meio de analise multivariada e sensoriamento remoto. Simpósio Bras. Sens. Remoto 2015, 17, 1169–1175. [Google Scholar]
- Campanha, M.M.; Nogueira, R.S.; Oliveira, T.S.; Teixeira, A.S.; Romero, R.E. Teores e Estoques de Carbono no Solo de Sistemas Agroflorestais e Tradicionais no Semiárido Brasileiro. Embrapa Caprinos Ovinos 2009, 1, 13. [Google Scholar]
- Mousavi, F.; Abdi, E.; Ghalandarzadeh, A.; Bahrami, H.A.; Majnourian, B.; Ziadi, N. Diffuse reflectance spectroscopy for rapid estimation of soil Atterberg limits. Geoderma 2020, 361, 114083. [Google Scholar] [CrossRef]
- Chang, C.W.; Laird, D.A.; Mausbach, M.J.; Hurburgh, C.R., Jr. Near-infrared reflectance spectroscopy–principal components regression analyses of soil properties. Soil Sci. Soc. Am. J. 2001, 65, 480–490. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, J.; Zhu, C.; Wang, J.; Ma, G.; Ge, X.; Li, Z.; Han, L. Strategies for the efficient estimation of soil organic matter in salt-affected soils through Vis-NIR spectroscopy: Optimal band combination algorithm and spectral degradation. Geoderma 2021, 382, 114–129. [Google Scholar] [CrossRef]
- Galvão, L.S.; Pizarro, M.A.; Epiphanio, J.C.N. Variations in Reflectance of Tropical Soils: Spectral-Chemical Composition Relationships from AVIRIS data. Remote Sens. Environ. 2001, 75, 245–255. [Google Scholar] [CrossRef]
- Madeira Netto, J.S.; Baptista, G.M.M. Reflectância espectral de solos. Embrapa Cerrados 2000, 1, 55. [Google Scholar]
- Vaidyanathan, S.; Mcneil, B.; Macaloney, G. Fundamental investigations on the near-infrared spectra of microbial biomass as applicable to bioprocess monitoring. Analyst 1999, 124, 157–162. [Google Scholar] [CrossRef]
- Fidêncio, P.H.; Poppi, R.J.; Andrade, J.C.; Cantarella, H. Determination of organic matter in soil using near-infrared spectroscopy and partial least squares regression. Commun. Soil Sci. Plant Anal. 2002, 33, 1607–1615. [Google Scholar] [CrossRef]
- Isaaks, E.H.; Srivastava, M.R. Applied Geostatistics, 1st ed.; Oxford University Press: New York, NY, USA, 1989; p. 561. [Google Scholar]
- Lopes, T.C.S. Atributos Estruturais e Mineralógicos em Classes de Solos na Chapada do Apodi. Ph.D. Thesis, Rural Federal University of the Semiarid, Mossoró, Brazil, 2018; p. 100. [Google Scholar]
- Dalmolin, R.S.D.; Gonçalves, C.N.; Klamt, E.; Dick, D.P. Relação entre os constituintes do solo e seu comportamento espectral. Ciênc. Rural 2005, 35, 481–489. [Google Scholar] [CrossRef]
- Mulder, V.L.; De Bruin, S.; Schaepman, M.E.; Mayr, T.R. The use of remote sensing in soil and terrain mapping—A review. Geoderma 2011, 162, 1–19. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, Y.; Chen, Y.; Liu, Y.; Yu, L.; Liu, Y.; Cheng, H. Application of fractional-order derivative in the quantitative estimation of soil organic matter content through visible and near-infrared spectroscopy. Geoderma 2019, 337, 758–769. [Google Scholar] [CrossRef]
- Demattê, J.A.M.; Horák-Terra, I.; Beirigo, R.M.; Terra, F.S.; Marques, K.P.P.; Fongaro, C.T.; Silva, A.C.; Vidal-Torrado, P. Genesis and properties of wetland soils by VIS-NIR-SWIR as a technique for environmental monitoring. J. Environ. Manag. 2017, 197, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Gosh, A.K.; Das, B.S.; Reddy, N. Application of VIS-NIR spectroscopy for estimation of soil organic carbon using different spectral preprocessing techniques and multivariate methods in the middle Indo-Gangetic plains of India. Geoderma Reg. 2020, 23, e00349. [Google Scholar]
- Vohland, M.; Ludwig, M.; Thiele-Bruhn, S.; Ludwig, B. Determination of soil properties with visible to near- and mid-infrared spectroscopy: Effects of spectral variable selection. Geoderma 2014, 223, 88–96. [Google Scholar] [CrossRef]
- Rinnan, R.; Rinnan, A. Application of near infrared reflectance (NIR) and fluorescence spectroscopy to analysis of microbiological and chemical properties of arctic soil. Soil Biol. Biochem. 2007, 39, 1664–1673. [Google Scholar] [CrossRef]
- Terra, F.D.S.; Demattê, J.A.M.; Rossel, R.V. Discriminação de solos baseada em espectroscopia de reflectância VIS-NIR. XVI Simpósio Bras. Sens. Remoto 2013, 1, 9224–9232. [Google Scholar]
- Mondal, B.P.; Sekhon, B.S.; Sahoo, R.N.; Paul, P. VIS-NIR reflectance spectroscopy for assessment of soil organic carbon in a rice-wheat field of Ludhiana district of Punjab. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2019, XLII-3/W6, 417–422. [Google Scholar] [CrossRef]
- Vasques, G.M.; Grunwald, S.; Sickman, J.O. Comparison of multivariate methods for inferential modeling of soil carbon using visible/near-infrared spectra. Geoderma 2008, 146, 14–25. [Google Scholar] [CrossRef]
- Aichi, H.; Fouad, Y.; Walter, C.; Viscarra Rossel, R.A.; Chabaane, Z.L.; Sanaa, M. Regional predictions of soil organic carbon content from spectral reflectance measurements. Biosyst. Eng. 2009, 104, 442–446. [Google Scholar] [CrossRef]
- Inda, A.V., Jr.; Bayer, C.; Conceição, P.C.; Boeni, M.; Salton, J.C.; Tonin, A.T. Variáveis relacionadas à estabilidade de complexos organo-minerais em solos tropicais e subtropicais brasileiros. Ciênc. Rural 2007, 37, 1301–1307. [Google Scholar] [CrossRef]
- Rakhsh, F.; Golchin, A.; Al Agha, A.B.; Nelson, P.N. Mineralization of organic carbon and formation of microbial biomass in soil: Effects of clay content and composition and the mechanisms involved. Soil Biol. Biochem. 2020, 151, 108036. [Google Scholar] [CrossRef]
- Stenberg, B.; Viscarra-Rossel, R.A.; Mouazen, A.M.; Wetterlind, J. Visible and near-infrared spectroscopy in soil science. Adv. Agron. 2010, 107, 163–215. [Google Scholar]
- Gmur, S.; Vogt, D.; Zabowski, D.; Moskal, L.M. Hyperspectral Analysis of Soil Nitrogen, Carbon, Carbonate, and Organic Matter Using Regression Trees. Sensors 2012, 12, 10639–10658. [Google Scholar] [CrossRef] [PubMed]
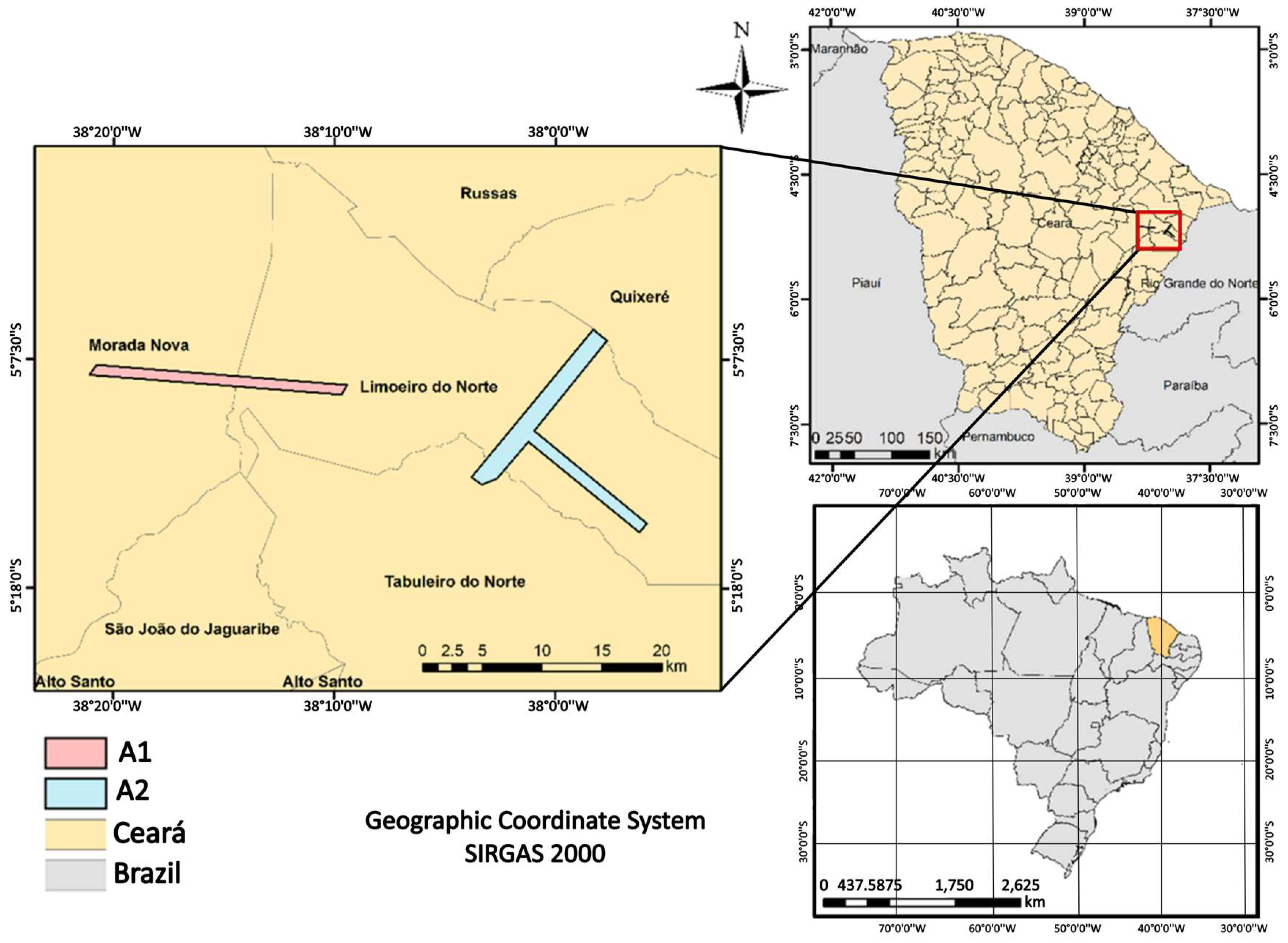
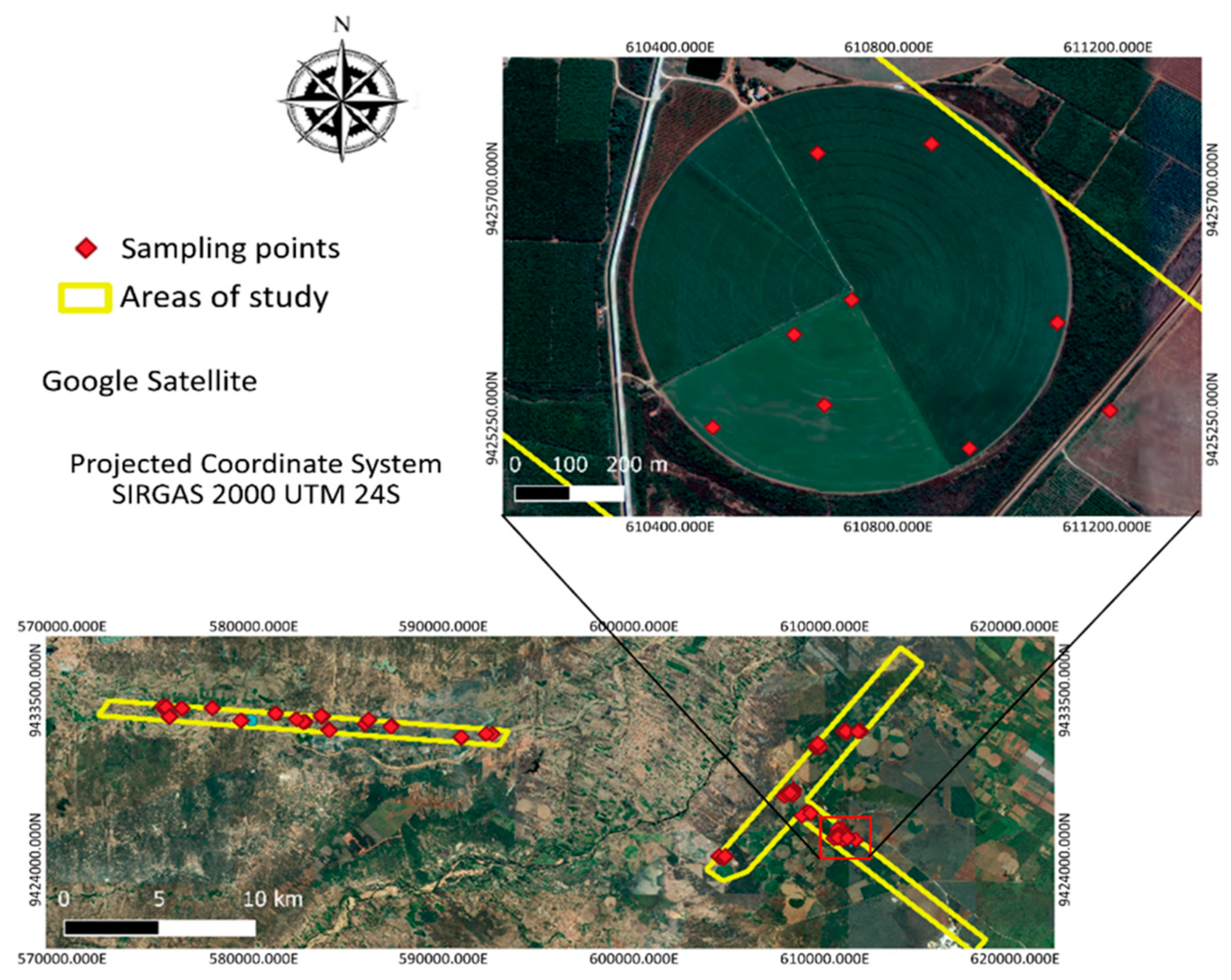

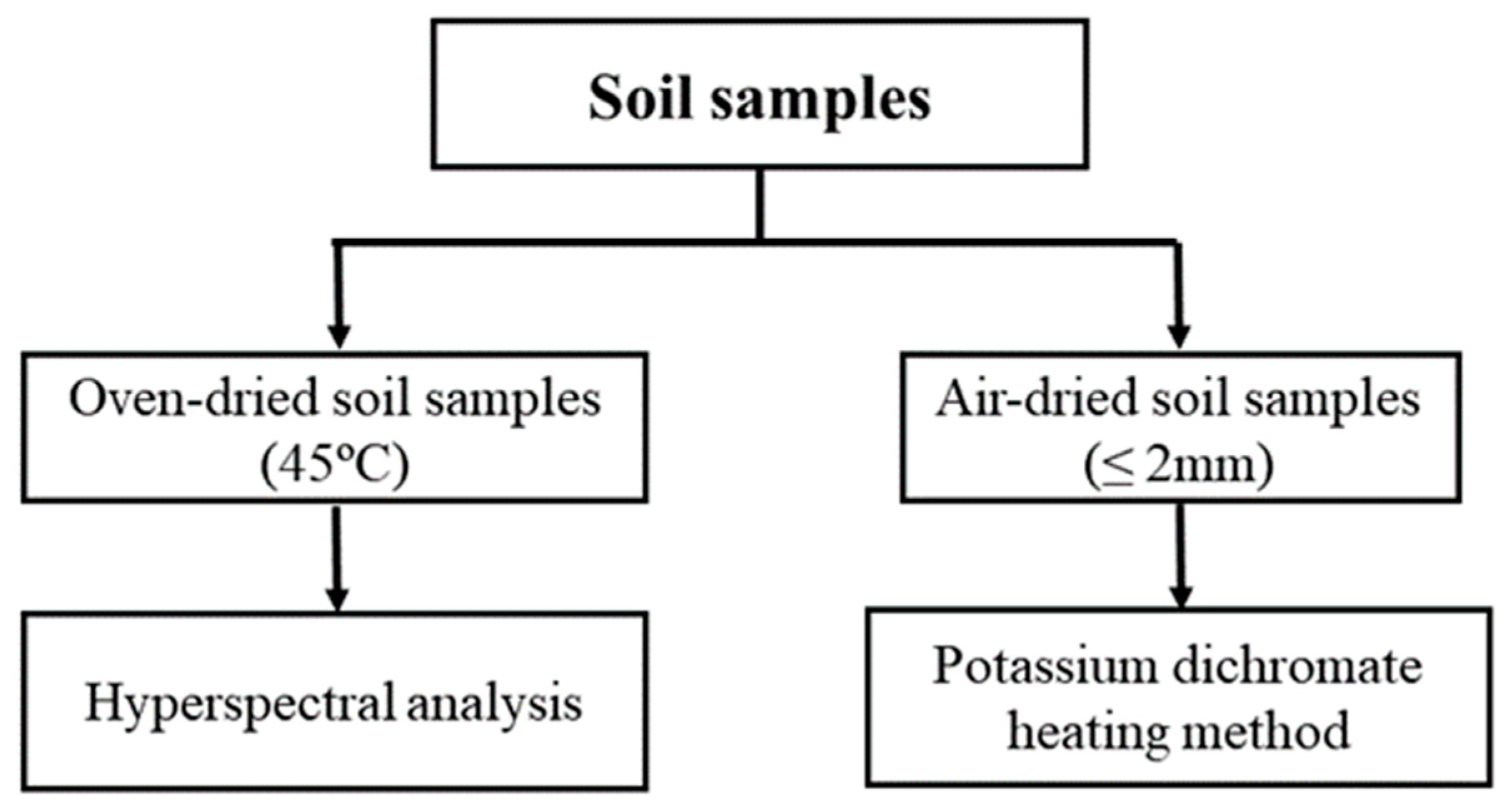

| Attribute | A1 | A2 |
|---|---|---|
| Municipality | Morada Nova, Ceará | Limoeiro do Norte, Ceará |
| Hydrographic basin | Banabuiú | Lower Jaguaribe |
| area | 18.22 km2 | 37.65 km2 |
| Predominant soil class | Fluvic Neosols (Fluvents) | Haplic Cambisols (Typic Dystrudept) |
| Predominant textural classes | Sandy-loam to Silt-clay-loam | Sandy-loam to Clay |
| Mean particle size (sand-silt-clay) [45] | 44%-35%-21% | 48%-22%-30% |
| Number of samples collected | 29 | 36 |
| Soil Organic Carbon (g/kg) | |||
|---|---|---|---|
| Statistical Parameters | A1 | A2 | A1 & A2 |
| Mean | 16.86 | 23.30 | 20.43 |
| Standard error | 1.64 | 0.91 | 0.97 |
| Median | 16.77 | 23.71 | 20.72 |
| Standard deviation | 8.84 | 5.48 | 7.81 |
| Coefficient of variation (%) | 52.42 | 23.50 | 38.23 |
| Sample variance | 78.16 | 29.98 | 60.99 |
| Curtosis | 2.19 | −0.52 | 0.49 |
| Assimetry | 1.07 | 0.03 | 0.16 |
| Amplitude | 39.46 | 22.95 | 39.46 |
| Minimum | 5.74 | 13.41 | 5.74 |
| Maximum | 45.20 | 36.36 | 45.20 |
| Kolmogorov-Smirnov (p-value) | 0.561 | 0.693 | 0.510 |
| Normality | Normal | Normal | Normal |
| Count | 29 | 36 | 65 |
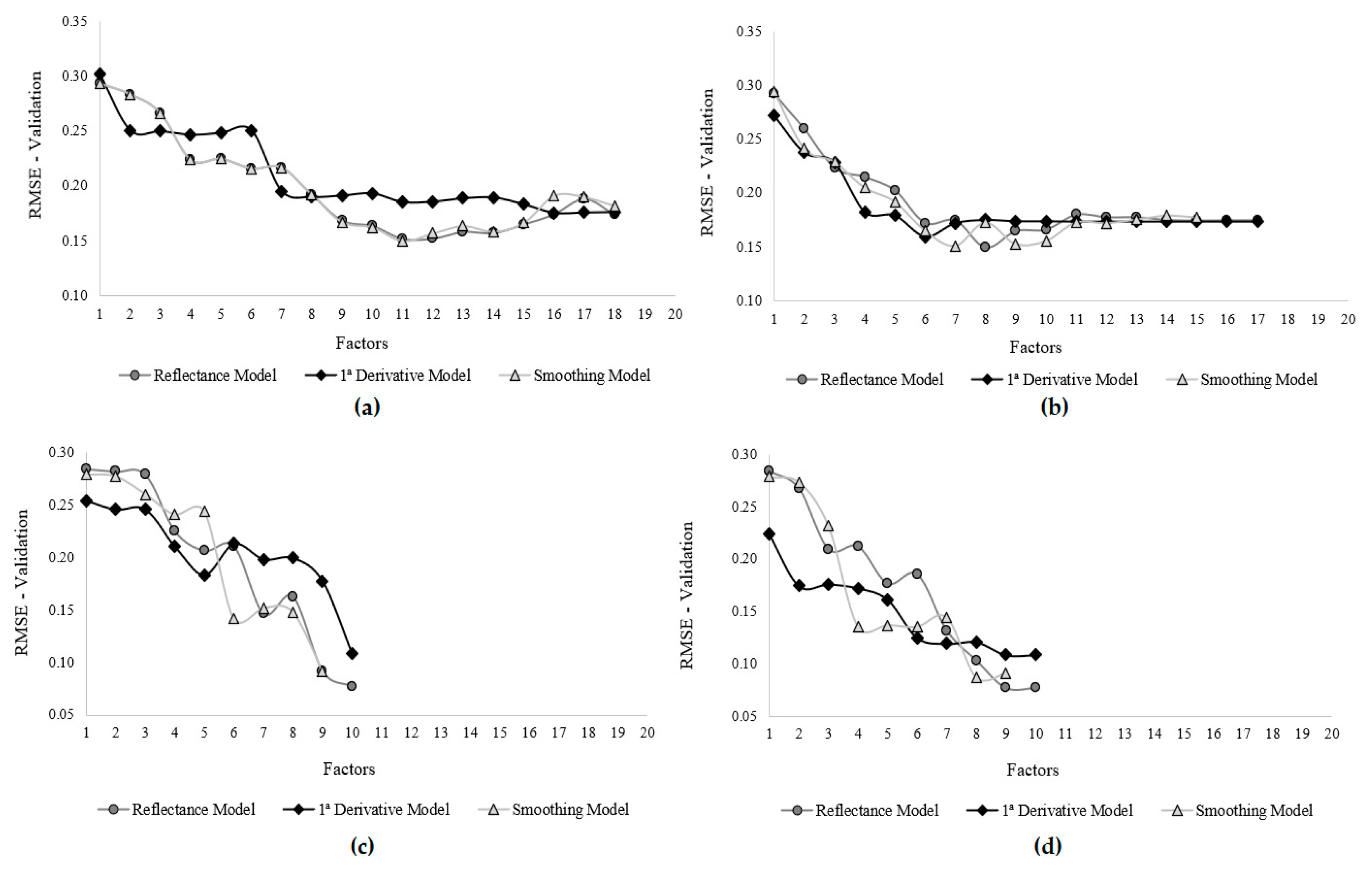
| Sample | Spectral Data | Nbr of Factors | R2 Calib. | R2 Valid. | Adj. R2 Valid. | RMSE (Norm) | Standard Dev. | RPD |
|---|---|---|---|---|---|---|---|---|
| A1 (20/9) | Untransformed | 11 | 0.78 | 0.86 | 0.84 | 0.152 | 0.31 | 2.03 |
| First derivative | 17 | 0.99 | 0.91 | 0.90 | 0.176 | 0.31 | 1.76 | |
| Smoothed reflectance | 11 | 0.78 | 0.86 | 0.84 | 0.150 | 0.31 | 2.06 | |
| A2 (25/11) | Untransformed | 19 | 0.96 | 0.40 | 0.34 | 0.217 | 0.28 | 1.30 |
| First derivative | 5 | 0.59 | 0.13 | 0.03 | 0.257 | 0.28 | 1.10 | |
| Smoothed reflectance | 20 | 0.95 | 0.39 | 0.32 | 0.221 | 0.28 | 1.28 | |
| A1 & A2 (45/20) | Untransformed | 26 | 0.89 | 0.77 | 0.76 | 0.106 | 0.22 | 2.11 |
| First derivative | 1 | 0.55 | 0.17 | 0.13 | 0.292 | 0.22 | 0.77 | |
| Smoothed reflectance | 20 | 0.84 | 0.73 | 0.72 | 0.116 | 0.22 | 1.93 |
| Sample | Spectral Data | Nbr of Factors | R2 Calib. | R2 Valid. | Adj. R2 Valid. | RMSE (Norm) | Standard Dev. | RPD |
|---|---|---|---|---|---|---|---|---|
| A1 (20/9) | Untransformed | 8 | 0.87 | 0.81 | 0.78 | 0.150 | 0.31 | 2.07 |
| First derivative | 6 | 0.98 | 0.88 | 0.86 | 0.160 | 0.31 | 1.93 | |
| Smoothed reflectance | 7 | 0.78 | 0.84 | 0.81 | 0.151 | 0.31 | 2.04 | |
| A2 (25/11) | Untransformed | 11 | 0.98 | 0.44 | 0.38 | 0.223 | 0.28 | 1.27 |
| First derivative | 1 | 0.98 | 0.07 | 0.02 | 0.292 | 0.28 | 0.97 | |
| Smoothed reflectance | 11 | 0.96 | 0.47 | 0.41 | 0.218 | 0.28 | 1.29 | |
| A1 & A2 (45/20) | Untransformed | 12 | 0.89 | 0.73 | 0.71 | 0.119 | 0.22 | 1.88 |
| First derivative | 1 | 0.61 | 0.19 | 0.14 | 0.302 | 0.22 | 0.74 | |
| Smoothed reflectance | 13 | 0.88 | 0.76 | 0.75 | 0.112 | 0.22 | 1.99 |
| Sample | Spectral Data | Nbr of Factors | R2 Calib. | R2 Valid. | Adj. R2 Valid. | RMSE (Norm) | Standard Dev. | RPD |
|---|---|---|---|---|---|---|---|---|
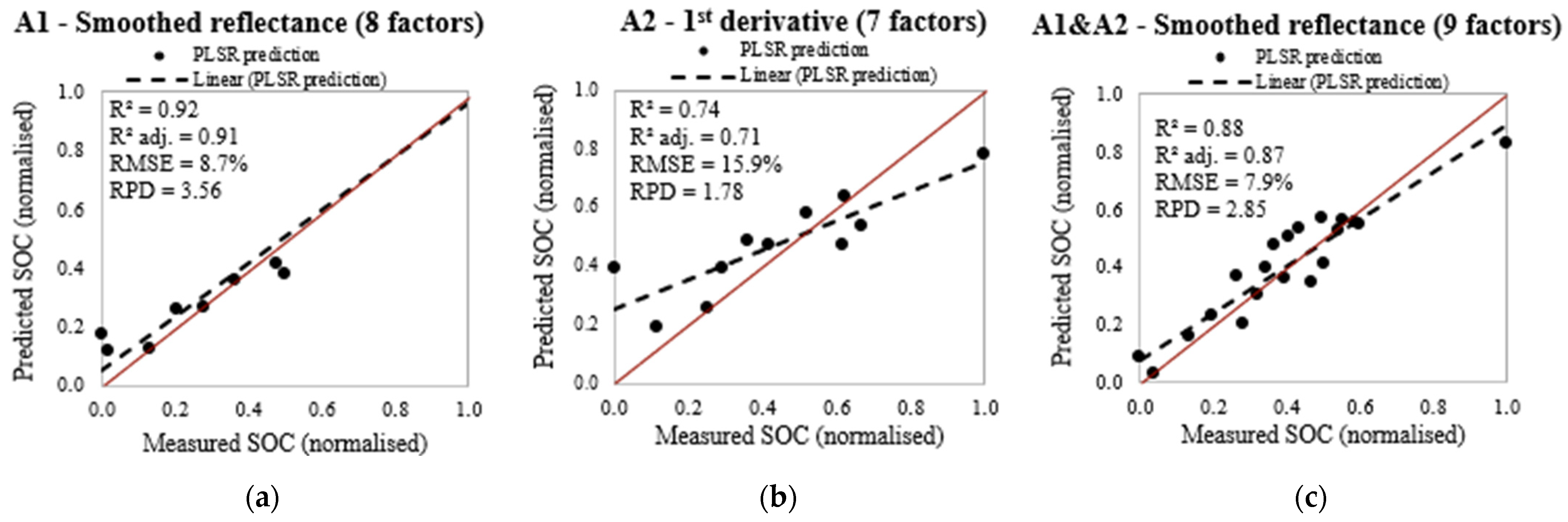
| A1 (20/9) | Untransformed | 10 | 0.97 | 0.96 | 0.96 | 0.117 | 0.31 | 2.65 |
| First derivative | 10 | 0.72 | 0.90 | 0.89 | 0.108 | 0.31 | 2.85 | |
| Smoothed reflectance | 9 | 0.90 | 0.91 | 0.90 | 0.092 | 0.31 | 3.38 | |
| A2 (25/11) | Untransformed | 5 | 0.62 | 0.61 | 0.57 | 0.181 | 0.28 | 1.56 |
| First derivative | 10 | 0.82 | 0.75 | 0.72 | 0.159 | 0.28 | 1.77 | |
| Smoothed reflectance | 4 | 0.46 | 0.61 | 0.56 | 0.202 | 0.28 | 1.40 | |
| A1 & A2 (45/20) | Untransformed | 6 | 0.74 | 0.71 | 0.70 | 0.118 | 0.22 | 1.90 |
| First derivative | 11 | 0.86 | 0.82 | 0.81 | 0.102 | 0.22 | 2.19 | |
| Smoothed reflectance | 13 | 0.86 | 0.88 | 0.87 | 0.077 | 0.22 | 2.90 |
| Sample | Spectral Data | Best SOC Prediction Models | Adj. R2 |
|---|---|---|---|
| A1 | Smoothed reflectance | Y = 33.62032 + 1005.37 (ρ 1876 nm) − 2749.841 (ρ 2047 nm) + 3447.796 (ρ 369 nm) − 3087.381 (ρ 354 nm) − 1961.023 (ρ 361 nm) − 2416.18 (ρ 366 nm) + 2568.7 (ρ 355 nm) + 1783.143 (ρ 2037 nm) + 1103.126 (ρ 375 nm) | 0.90 |
| A2 | First derivative (ρ’) | Y = 7.1693 + 42,200.559 (ρ’ 1813 nm) + 26,103.8192 (ρ’ 1840 nm) − 7700.9938 (ρ’ 419 nm) – 17,267.2974 (ρ’ 1719 nm) + 32,662.4702 (ρ’ 1273 nm) + 14,296.5261 (ρ’ 406 nm) + 2574.3248 (ρ’ 1685 nm) – 18,546.4386 (ρ’ 1757 nm) + 3891.96 (ρ’ 380 nm) + 5449.9437 (ρ’ 1728 nm) | 0.72 |
| A1 & A2 | Smoothed reflectance | Y = 15.3110 − 816.0873 (ρ 2057 nm) + 4177.451 (ρ 370 nm) + 408.1595 (ρ 605 nm) + 770.9059 (ρ 1876 nm) + 2420.435 (ρ 390 nm) − 758.8688 (ρ 362 nm) + 317.3015 (ρ 1406 nm) + 288.1545 (ρ 2018 nm) − 1810.411 (ρ 371 nm) − 776.0894 (ρ 691 nm) − 2994.089 (ρ 388 nm) − 958.5162 (ρ 381 nm) + 297.9998 (ρ 708 nm) − 442.805 (ρ 1880 nm) | 0.87 |
| Sample | Spectral Data | Nbr of Factors | R2 Calib. | R2 Valid. | Adj. R2 Valid. | RMSE (norm) | Standard Dev. | RPD |
|---|---|---|---|---|---|---|---|---|
| A1 (20/9) | Untransformed | 9 | 0.95 | 0.95 | 0.95 | 0.141 | 0.31 | 2.19 |
| First derivative | 9 | 0.72 | 0.90 | 0.89 | 0.109 | 0.31 | 2.85 | |
| Smoothed reflectance | 8 | 0.90 | 0.92 | 0.91 | 0.087 | 0.31 | 3.56 | |
| A2 (25/11) | Untransformed | 5 | 0.62 | 0.61 | 0.57 | 0.182 | 0.28 | 1.56 |
| First derivative | 7 | 0.81 | 0.74 | 0.71 | 0.159 | 0.28 | 1.78 | |
| Smoothed reflectance | 4 | 0.46 | 0.61 | 0.56 | 0.202 | 0.28 | 1.40 | |
| A1 & A2 (45/20) | Untransformed | 6 | 0.74 | 0.71 | 0.70 | 0.118 | 0.22 | 1.90 |
| First derivative | 7 | 0.86 | 0.86 | 0.85 | 0.097 | 0.22 | 2.30 | |
| Smoothed reflectance | 9 | 0.83 | 0.88 | 0.87 | 0.079 | 0.22 | 2.85 |
| Sample | Spectral Data | Best SOC Prediction Models | Adj. R2 |
|---|---|---|---|
| A1 | Smoothed reflectance | Y = 33.11747 + 1056.97 (ρ 1876 nm) − 2634.573 (ρ 2047 nm) + 3390.864 (ρ 369 nm) − 3108.625 (ρ 354 nm) − 2379.895 (ρ 361 nm) − 2363.19 (ρ 366 nm) + 2709.004 (ρ 355 nm) + 1617.641 (ρ 2037 nm) + 1393.959(ρ 375 nm) | 0.91 |
| A2 | First derivative (ρ’) | Y = 7.0083 + 42,065.0622 (ρ’ 1813 nm) + 25,818.0458 (ρ’ 1840 nm) − 7725.9588 (ρ’ 419 nm) − 17,941.3756 (ρ’ 1719 nm) + 31,823.2575 (ρ’1273 nm) + 15,099.1473 (ρ’ 406 nm) + 1880.134 (ρ’1685 nm) – 16,964.3115 (ρ’ 1757 nm) + 3299.873 (ρ’ 380 nm) + 5051.7084 (ρ’ 1728 nm) | 0.71 |
| A1 & A2 | Smoothed reflectance | Y = 17.0995 − 741.6441 (ρ2057 nm) + 4471.37 (ρ370 nm) + 364.3325 (ρ605 nm) + 639.766 (ρ1876 nm) + 2839.064 (ρ390 nm) − 932.5751 (ρ362 nm) + 294.2628 (ρ1406 nm) + 163.4081 (ρ2018 nm) –2184.5980 (ρ371 nm) − 738.3680 (ρ691 nm) − 3583.7460 (ρ388 nm) –549.9565 (ρ381 nm) + 306.6913 (ρ708 nm) − 248.5569 (ρ1880 nm) | 0.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, S.G.; Teixeira, A.d.S.; de Oliveira, M.R.R.; Costa, M.C.G.; Araújo, I.C.d.S.; Moreira, L.C.J.; Lopes, F.B. Soil Organic Carbon Content Prediction Using Soil-Reflected Spectra: A Comparison of Two Regression Methods. Remote Sens. 2021, 13, 4752. https://doi.org/10.3390/rs13234752
Ribeiro SG, Teixeira AdS, de Oliveira MRR, Costa MCG, Araújo ICdS, Moreira LCJ, Lopes FB. Soil Organic Carbon Content Prediction Using Soil-Reflected Spectra: A Comparison of Two Regression Methods. Remote Sensing. 2021; 13(23):4752. https://doi.org/10.3390/rs13234752
Chicago/Turabian StyleRibeiro, Sharon Gomes, Adunias dos Santos Teixeira, Marcio Regys Rabelo de Oliveira, Mirian Cristina Gomes Costa, Isabel Cristina da Silva Araújo, Luis Clenio Jario Moreira, and Fernando Bezerra Lopes. 2021. "Soil Organic Carbon Content Prediction Using Soil-Reflected Spectra: A Comparison of Two Regression Methods" Remote Sensing 13, no. 23: 4752. https://doi.org/10.3390/rs13234752
APA StyleRibeiro, S. G., Teixeira, A. d. S., de Oliveira, M. R. R., Costa, M. C. G., Araújo, I. C. d. S., Moreira, L. C. J., & Lopes, F. B. (2021). Soil Organic Carbon Content Prediction Using Soil-Reflected Spectra: A Comparison of Two Regression Methods. Remote Sensing, 13(23), 4752. https://doi.org/10.3390/rs13234752