Effect of Dust Deposition on Chlorophyll Concentration Estimation in Urban Plants from Reflectance and Vegetation Indexes
Abstract
:1. Introduction
2. Materials and Methods
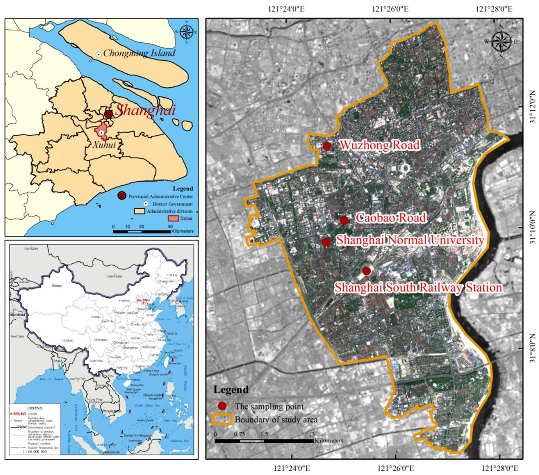
2.1. Sample Collection
2.2. Data Measurement
2.2.1. Reflectance Spectrum Measurement
2.2.2. Dust Deposition Amount Measurement
2.2.3. Pigment Concentration Measurement
2.3. Data Processing
2.4. Accuracy Assessment
3. Results
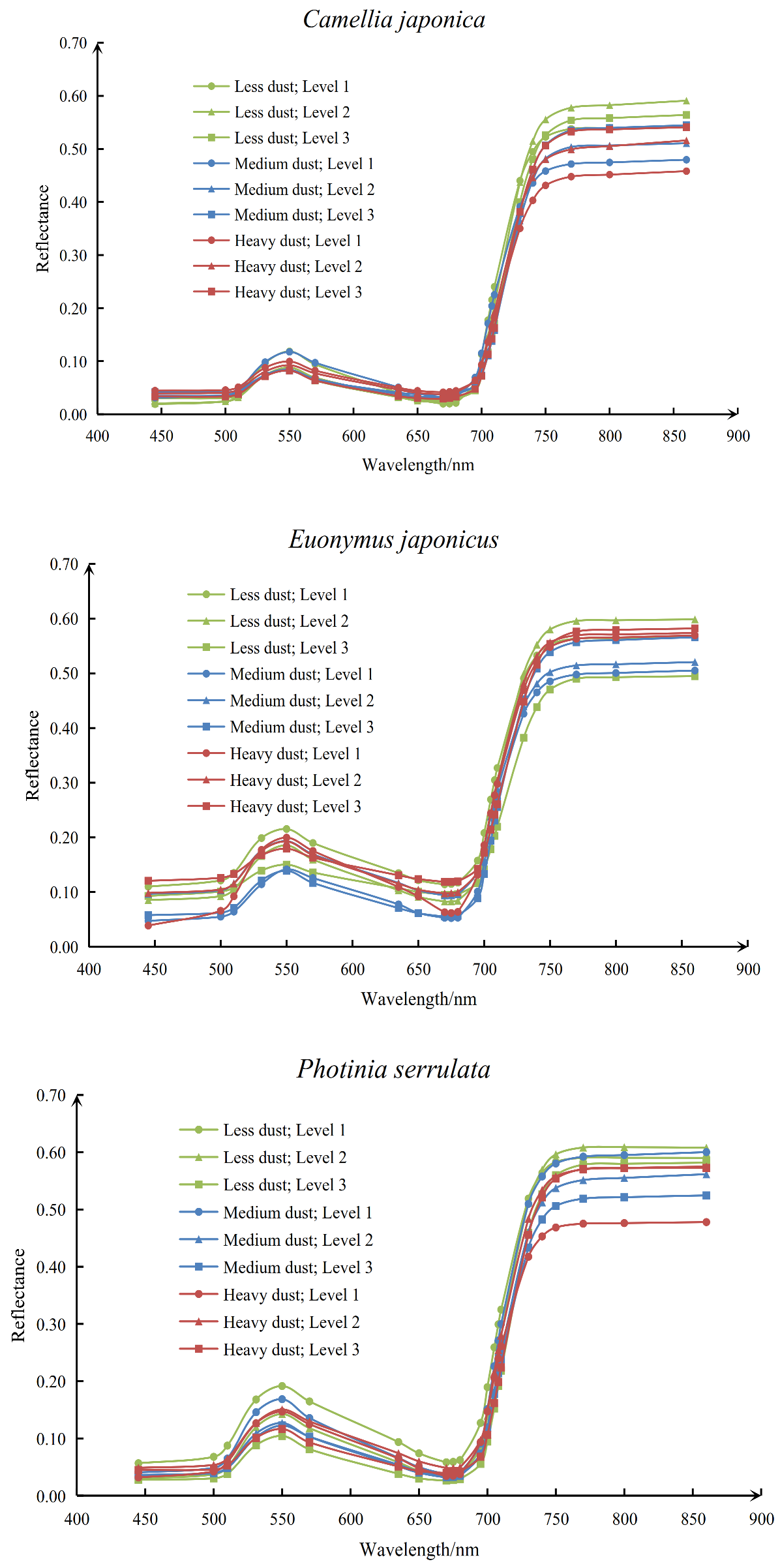
3.1. Influence of Dust Deposition on Reflectance Spectra of Different Pigment Concentrations
3.2. Correlation Analysis between Pigment Concentration and Vegetation Spectrum
3.2.1. Correlation Analysis between Pigment Concentration and Reflectance Spectrum
3.2.2. Correlation Analysis between Pigment Concentration and Vegetation Indexes
3.3. Prediction Model of Leaf Chlorophyll Concentration Based on Red Edge Vegetation Indexes
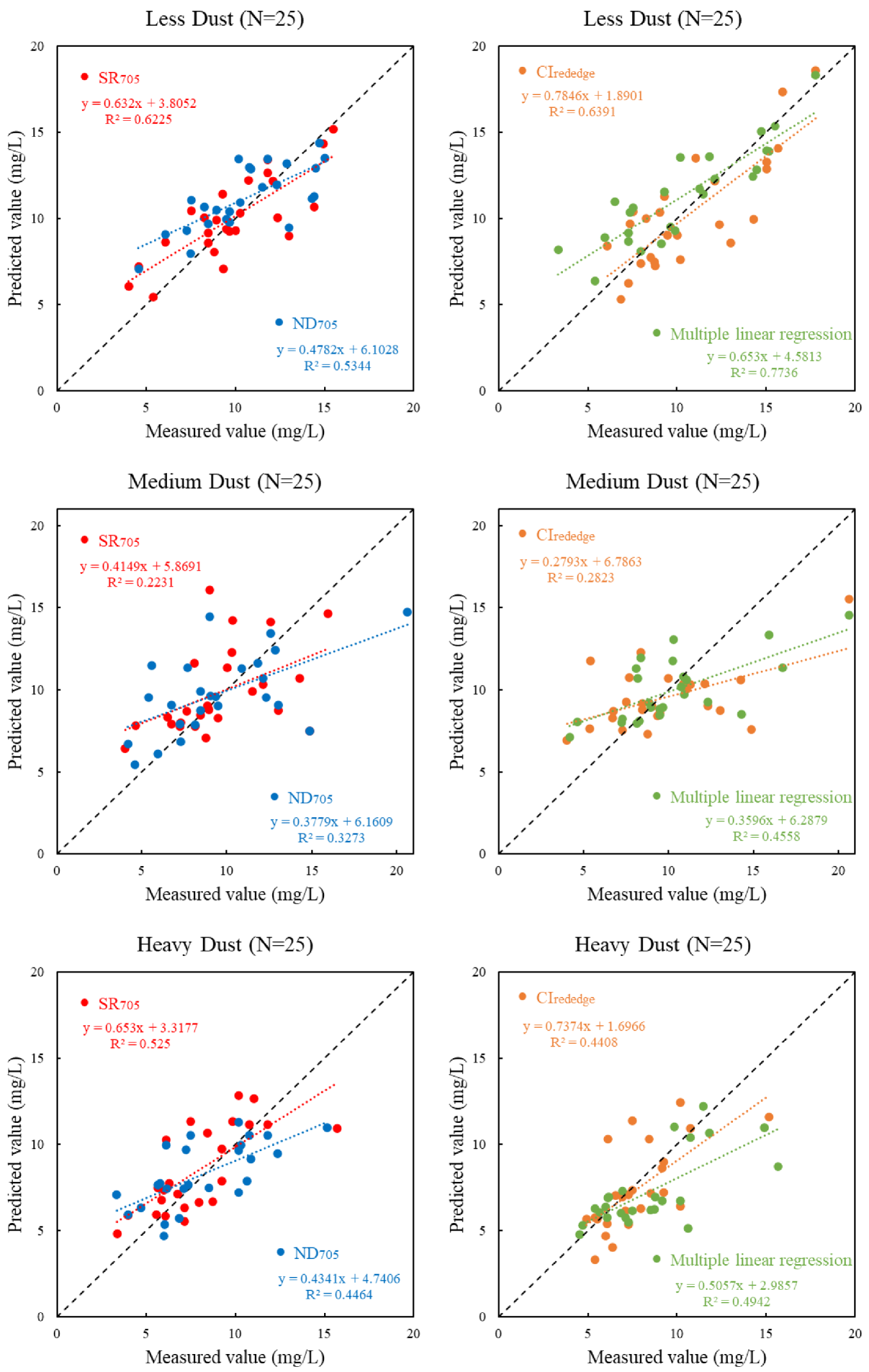
3.3.1. Establishment of the Chlorophyll—A Concentration Prediction Model
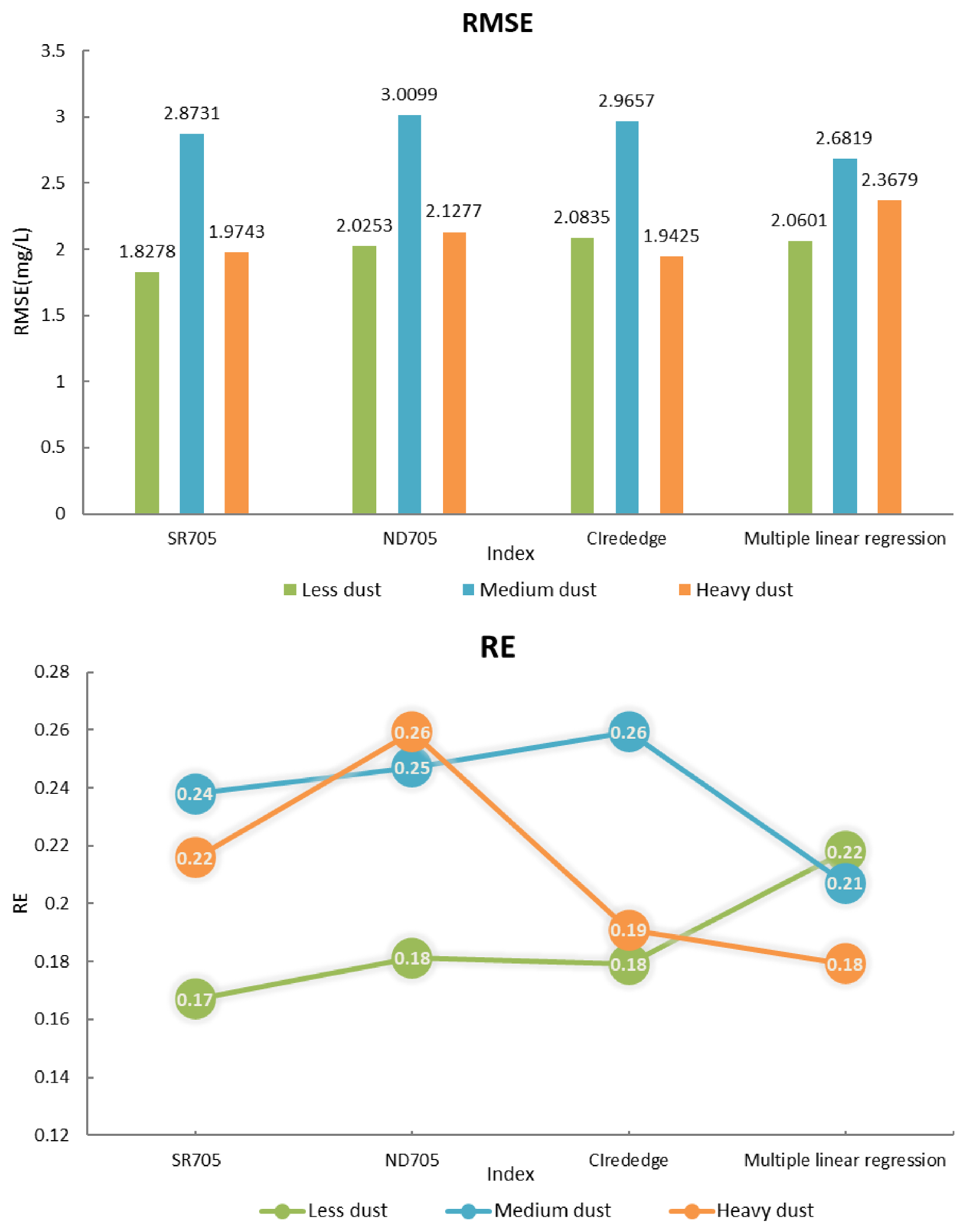
3.3.2. Validation of Chlorophyll—A Concentration Prediction Model
3.4. Influence of Dust Deposition on Pigment Estimation
4. Discussion
5. Conclusions
- Through preliminary guessing, the leaf chlorophyll concentration prediction can be achieved by using reflectance spectroscopy data. After being validated with actual remote sensing data, the appropriate sensitive spectral bands and sensitive vegetation indexes can improve the accuracy of the estimation model.
- Dust deposition can obviously affect the correlation between vegetation chlorophyll and spectrum. The sensitive spectral bands (680−710 nm) around the red edge had a relatively high negative correlation with the chlorophyll-a concentration, but when the dust deposition level increased to “medium dust” or “heavy dust”, the correlation was significantly reduced. Some sensitive vegetation indexes (SR705, ND705, MC705, MSR705, CIrededge) also showed the same pattern.
- Dust deposition affected the accuracy of the application of reflectance spectroscopy to chlorophyll concentration estimation. Different sensitive vegetation indexes had different effects. SR705 and CIrededge vegetation indexes were suitable to establish a chlorophyll-a concentration prediction model. The prediction effect under the level of “Less dust” was the best, which also proved that the deposition of dust had a significant interference effect on the estimation of chlorophyll-a concentration.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amato-Lourenco, L.F.; Moreira, T.C.L.; de Oliveira Souza, V.C.; Barbosa, F.B.; Saiki, M.; Saldiva, P.; Mauad, T. The influence of atmospheric particles on the elemental content of vegetables in urban gardens of Sao Paulo, Brazil. Environ. Pollut. 2016, 216, 125–134. [Google Scholar] [CrossRef]
- Liang, J.; Fang, H.; Zhang, T.; Wang, X.; Liu, Y. Heavy metal in leaves of twelve plant species from seven different areas in Shanghai, China. Urban For. Urban Green. 2017, 27, 390–398. [Google Scholar] [CrossRef]
- Gillissen, A.; Gessner, C.; Hammerschmidt, S.; Hoheisel, G.; Wirtz, H. Gesundheitliche Bedeutung inhalierter Stäube. DMW-Dtsch. Med. Wochenschr. 2006, 131, 639–644. [Google Scholar] [CrossRef]
- Iwai, K.; Mizuno, S.; Miyasaka, Y.; Mori, T. Correlation between suspended particles in the environmental air and causes of disease among inhabitants: Cross-sectional studies using the vital statistics and air pollution data in Japan. Environ. Res. 2005, 99, 106–117. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, Q.; Zheng, C. Best hyperspectral indices for tracing leaf water status as determined from leaf dehydration experiments. Ecol. Indic. 2015, 54, 96–107. [Google Scholar] [CrossRef]
- Lin, W.; Li, Y.; Du, S.; Zheng, Y.; Gao, J.; Sun, T. Effect of dust deposition on spectrum-based estimation of leaf water content in urban plant. Ecol. Indic. 2019, 104, 41–47. [Google Scholar] [CrossRef]
- Kira, O.; Linker, R.; Gitelson, A. Non-destructive estimation of foliar chlorophyll and carotenoid contents: Focus on informative spectral bands. Int. J. Appl. Earth Obs. Geoinf. 2015, 38, 251–260. [Google Scholar] [CrossRef]
- Freer-Smith, P.; Holloway, S.; Goodman, A. The uptake of particulates by an urban woodland: Site description and particulate composition. Environ. Pollut. 1997, 95, 27–35. [Google Scholar] [CrossRef]
- Shah, K.; Amin, N.U.; Ahmad, I.; Shah, S.; Hussain, K. Dust particles induce stress, reduce various photosynthetic pigments and their derivatives in ficus benjamina: A landscape plant. Int. J. Agric. Biol. 2017, 19, 1469–1474. [Google Scholar] [CrossRef]
- Rai, P.K.; Panda, L.L.S. Dust capturing potential and air pollution tolerance index (APTI) of some road side tree vegetation in Aizawl, Mizoram, India: An Indo-Burma hot spot region. Air Qual. Atmos. Health 2013, 7, 93–101. [Google Scholar] [CrossRef]
- Zafra, C.; Temprano, J.; Tejero, I. The physical factors affecting heavy metals accumulated in the sediment deposited on road surfaces in dry weather: A review. Urban Water J. 2016, 14, 639–649. [Google Scholar] [CrossRef]
- Ram, S.; Kumar, R.; Chaudhuri, P.; Chanda, S.; Santra, S.; Sudarshan, M.; Chakraborty, A. Physico-chemical characterization of street dust and re-suspended dust on plant canopies: An approach for finger printing the urban environment. Ecol. Indic. 2014, 36, 334–338. [Google Scholar] [CrossRef]
- Verrelst, J.; Schaepman, M.; Malenovský, Z.; Clevers, J. Effects of woody elements on simulated canopy reflectance: Implications for forest chlorophyll content retrieval. Remote Sens. Environ. 2010, 114, 647–656. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, G.A.; Ferwerda, J.G. Retrieval of chlorophyll concentration from leaf reflectance spectra using wavelet analysis. Remote Sens. Environ. 2008, 112, 1614–1632. [Google Scholar] [CrossRef]
- Cheng, T.; Rivard, B.; Sanchez-Azofeifa, A.; Feng, J.; Calvo-Polanco, M. Continuous wavelet analysis for the detection of green attack damage due to mountain pine beetle infestation. Remote Sens. Environ. 2010, 114, 899–910. [Google Scholar] [CrossRef]
- Ryu, C.; Suguri, M.; Umeda, M. Multivariate analysis of nitrogen content for rice at the heading stage using reflectance of airborne hyperspectral remote sensing. Field Crop. Res. 2011, 122, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Gray, S.B.; Dermody, O.; DeLucia, E.H. Spectral reflectance from a soybean canopy exposed to elevated CO2 and O3. J. Exp. Bot. 2010, 61, 4413–4422. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Reddy, K.R.; Kakani, V.G.; Read, J.J.; Koti, S. Canopy reflectance in cotton for growth assessment and lint yield prediction. Eur. J. Agron. 2007, 26, 335–344. [Google Scholar] [CrossRef]
- Cabungcag, L.; Madroñal, M.H.; Olila, J.B.A.; Quilatan, D.P.; Galarpe, V.R.K.R. Dust and Chlorophyll Contents of Selected Plant Species Along the Highway in Cagayan de Oro City, Philippines. Adv. Sci. Eng. Med. 2017, 9, 725–730. [Google Scholar] [CrossRef]
- Zulfa, A.; Norizah, K.; Hamdan, O.; Zulkifly, S.; Faridah-Hanum, I.; Rhyma, P. Discriminating trees species from the relationship between spectral reflectance and chlorophyll contents of mangrove forest in Malaysia. Ecol. Indic. 2019, 111, 106024. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, C.; Lu, S.; Wang, P.; Qiu, G.Y.; Li, R. Estimation of chlorophyll content in intertidal mangrove leaves with different thicknesses using hyperspectral data. Ecol. Indic. 2019, 106, 105511. [Google Scholar] [CrossRef]
- Delegido, J.; Van Wittenberghe, S.; Verrelst, J.; Ortiz, V.; Veroustraete, F.; Valcke, R.; Samson, R.; Rivera, J.P.; Tenjo, C.; Moreno, J. Chlorophyll content mapping of urban vegetation in the city of Valencia based on the hyperspectral NAOC index. Ecol. Indic. 2014, 40, 34–42. [Google Scholar] [CrossRef]
- Shah, K.; Amin, N.U.; Ahmad, I.; Ara, G. Impact assessment of leaf pigments in selected landscape plants exposed to roadside dust. Environ. Sci. Pollut. Res. 2018, 25, 23055–23073. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Amin, N.U.; Ahmad, I.; Ara, G.; Ren, X.; Xing, L. Effects of Chronic Dust Load On Leaf Pigments of the Landscape Plant Murraya Paniculata. Gesunde Pflanz. 2019, 71, 249–258. [Google Scholar] [CrossRef]
- Prusty, B.A.K.; Mishra, P.; Azeez, P. Dust accumulation and leaf pigment content in vegetation near the national highway at Sambalpur, Orissa, India. Ecotoxicol. Environ. Saf. 2005, 60, 228–235. [Google Scholar] [CrossRef]
- Lin, W.; Sun, Y.; Wang, D.; Li, Y.; Yu, X. Estimation model of dust deposition capacity of common vegetation based on spectral characteristics in Shanghai, China. Sustain. Cities Soc. 2021, 70, 102915. [Google Scholar] [CrossRef]
- Brackx, M.; Van Wittenberghe, S.; Verhelst, J.; Scheunders, P.; Samson, R. Hyperspectral leaf reflectance of Carpinus betulus L. saplings for urban air quality estimation. Environ. Pollut. 2017, 220, 159–167. [Google Scholar] [CrossRef]
- Zajec, L.; Gradinjan, D.; Klančnik, K.; Gaberščik, A. Limestone dust alters the optical properties and traits of Fagus sylvatica leaves. Trees 2016, 30, 2143–2152. [Google Scholar] [CrossRef]
- Zhao, Y.; Lei, S.; Yang, X.; Gong, C.; Wang, C.; Cheng, W.; Li, H.; She, C. Study on Spectral Response and Estimation of Grassland Plants Dust Retention Based on Hyperspectral Data. Remote Sens. 2020, 12, 2019. [Google Scholar] [CrossRef]
- Chen, J.M. Evaluation of Vegetation Indices and a Modified Simple Ratio for Boreal Applications. Can. J. Remote Sens. 1996, 22, 229–242. [Google Scholar] [CrossRef]
- Nichol, C.J.; Grace, J. Determination of leaf pigment content in Calluna vulgaris shoots from spectral reflectance. Int. J. Remote Sens. 2010, 31, 5409–5422. [Google Scholar] [CrossRef]
- Han, Y.; Hao, T.; Li, Z.; Li, Y. Inversion of the fluorescence spectral information of vegetation chlorophyll based on the inverted Gaussian model. J. Quant. Spectrosc. Radiat. Transf. 2019, 242, 106761. [Google Scholar] [CrossRef]
- Ali, A.M.; Darvishzadeh, R.; Skidmore, A.; Gara, T.; O’Connor, B.; Roeoesli, C.; Heurich, M.; Paganini, M. Comparing methods for mapping canopy chlorophyll content in a mixed mountain forest using Sentinel-2 data. Int. J. Appl. Earth Obs. Geoinfor. 2019, 87, 102037. [Google Scholar] [CrossRef]
- Darvishzadeh, R.; Skidmore, A.; Schlerf, M.; Atzberger, C. Inversion of a radiative transfer model for estimating vegetation LAI and chlorophyll in a heterogeneous grassland. Remote Sens. Environ. 2008, 112, 2592–2604. [Google Scholar] [CrossRef]
- Qi, H.; Zhu, B.; Kong, L.; Yang, W.; Zou, J.; Lan, Y.; Zhang, L. Hyperspectral Inversion Model of Chlorophyll Content in Peanut Leaves. Appl. Sci. 2020, 10, 2259. [Google Scholar] [CrossRef] [Green Version]
- Zarco-Tejada, P.; Hornero, A.; Beck, P.; Kattenborn, T.; Kempeneers, P.; Hernández-Clemente, R. Chlorophyll content estimation in an open-canopy conifer forest with Sentinel-2A and hyperspectral imagery in the context of forest decline. Remote Sens. Environ. 2019, 223, 320–335. [Google Scholar] [CrossRef]
- Xu, M.; Liu, R.; Chen, J.M.; Liu, Y.; Shang, R.; Ju, W.; Wu, C.; Huang, W. Retrieving leaf chlorophyll content using a matrix-based vegetation index combination approach. Remote Sens. Environ. 2019, 224, 60–73. [Google Scholar] [CrossRef]
- Lv, J.; Yan, Z.G. Retrieval of Chlorophyll Content from Leaf Reflectance Spectra Using Support Vector Machine. Appl. Mech. Mater. 2014, 602, 2313–2316. [Google Scholar] [CrossRef]
- Lunagaria, M.M.; Patel, H.R. Evaluation of PROSAIL inversion for retrieval of chlorophyll, leaf dry matter, leaf angle, and leaf area index of wheat using spectrodirectional measurements. Int. J. Remote Sens. 2018, 40, 8125–8145. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Tripathi, B.D. Seasonal Variation of Leaf Dust Accumulation and Pigment Content in Plant Species Exposed to Urban Particulates Pollution. J. Environ. Qual. 2008, 37, 865–870. [Google Scholar] [CrossRef]
- Ma, B.; Li, X.; Liang, A.; Chen, Y.; Che, D. Experimental and Numerical Investigation of Dustfall Effect on Remote Sensing Retrieval Accuracy of Chlorophyll Content. Sensors 2019, 19, 5530. [Google Scholar] [CrossRef] [Green Version]
- Goetz, A.F.; Vane, G.; Solomon, J.E.; Rock, B.N. Imaging Spectrometry for Earth Remote Sensing. Science 1985, 228, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Lin, W.; Li, Y.; Guo, P.; Zeng, Y. Effect of Different Dust Weight Levels on Unban Canopy Reflectance Spectroscopy. Spectrosc. Spectr. Anal. 2017, 37, 2539–2545. [Google Scholar]
- Jouraeva, V.A.; Johnson, D.L.; Hassett, J.P.; Nowak, D.J. Differences in accumulation of PAHs and metals on the leaves of Tilia×euchlora and Pyrus calleryana. Environ. Pollut. 2002, 120, 331–338. [Google Scholar] [CrossRef]
- Wessman, C.A.; Aber, J.D.; Peterson, D.L.; Melillo, J.M. Foliar analysis using near infrared reflectance spectroscopy. Can. J. For. Res. 1988, 18, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Hansen, P.; Schjoerring, J. Reflectance measurement of canopy biomass and nitrogen status in wheat crops using normalized difference vegetation indices and partial least squares regression. Remote Sens. Environ. 2003, 86, 542–553. [Google Scholar] [CrossRef]
- Haboudane, D. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Lu, T.; Lin, X.; Chen, J.; Huang, D.; Li, M. Atmospheric particle retention capacity and photosynthetic responses of three common greening plant species under different pollution levels in Hangzhou. Glob. Ecol. Conserv. 2019, 20, e00783. [Google Scholar] [CrossRef]
- Chai, Y.; Zhu, N.; Han, H. Dust removal effect of urban tree species in Harbin. J. Appl. Ecol. 2002, 13, 1121–1126. [Google Scholar] [CrossRef]
- Xu, J.H.; Yu, J.T. Air Dustfall Impact on Spectrum of Ficus Microcarpa’s Leaf. Adv. Mater. Res. 2013, 655, 813–815. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. [34] Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1987; pp. 350–382. [Google Scholar] [CrossRef]
- Hu, B.; Huang, H.; Ji, Y.; Zhao, X.; Qi, J.; Zhang, H.; Zhang, G. Evaluation of the optimum concentration of chlorophyll extract for determination of chlorophyll content by spectrophotometry. Pratacultural Sci. 2018, 35, 1965–1974. [Google Scholar] [CrossRef]
- Rai, P.K.; Panda, L.L.S. Leaf dust deposition and its impact on biochemical aspect of some roadside plants in Aizawl, Mizoram, North-east India. Int. Res. J. Environ. Sci. 2014, 3, 14–19. [Google Scholar]
- Zhu, J.; Yu, Q.; Zhu, H.; He, W.; Xu, C.; Liao, J.; Zhu, Q.; Su, K. Response of dust particle pollution and construction of a leaf dust deposition prediction model based on leaf reflection spectrum characteristics. Environ. Sci. Pollut. Res. 2019, 26, 36764–36775. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, X.; He, W.; Yan, X.; Yu, Q.; Xu, C.; Jiang, Q.; Huang, H.; Wang, R. Response of plant reflectance spectrum to simulated dust deposition and its estimation model. Sci. Rep. 2020, 10, 15803. [Google Scholar] [CrossRef] [PubMed]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. In Proceedings of the Third Earth Resources Technology Satellite-1 Symposium, Washington, DC, USA, 10–14 December 1973; pp. 310–317. [Google Scholar]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; de Colstoun, E.B.; McMurtrey, J.E. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Wu, C.; Niu, Z.; Tang, Q.; Huang, W. Estimating chlorophyll content from hyperspectral vegetation indices: Modeling and validation. Agric. For. Meteorol. 2008, 148, 1230–1241. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-destructive optical detection of pigment changes during leaf senescence and fruit ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Penuelas, J.; Filella, I.; Gamon, J. Assessment of photosynthetic radiation-use efficiency with spectral reflectance. New Phytol. 1995, 131, 291–296. [Google Scholar] [CrossRef]
- Zhang, P.-F.; Yin, J.-Q.; Bao, A.-M.; Yao, F.; Liu, J.-P. Using hyperspectral indices to measure the effect of mine dust on the growth of three typical desert plants. Guang Pu Xue Yu Guang Pu Fen Xi 2014, 34, 2162–2168. [Google Scholar] [PubMed]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.; Merzlyak, M.N. Spectral Reflectance Changes Associated with Autumn Senescence of Aesculus hippocastanum L. and Acer platanoides L. Leaves. Spectral Features and Relation to Chlorophyll Estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Datt, B. Remote Sensing of Chlorophyll a, Chlorophyll b, Chlorophyll a+b, and Total Carotenoid Content in Eucalyptus Leaves. Remote Sens. Environ. 1998, 66, 111–121. [Google Scholar] [CrossRef]
- Pearson, K. Notes on the History of Correlation. Biometrika 1920, 13, 25–45. [Google Scholar] [CrossRef]
- Gutman, G.; Skakun, S.; Gitelson, A. Revisiting the use of red and near-infrared reflectances in vegetation studies and numerical climate models. Sci. Remote Sens. 2021, 4, 100025. [Google Scholar] [CrossRef]
- Gitelson, A.; Arkebauer, T.; Viña, A.; Skakun, S.; Inoue, Y. Evaluating plant photosynthetic traits via absorption coefficient in the photosynthetically active radiation region. Remote Sens. Environ. 2021, 258, 112401. [Google Scholar] [CrossRef]
- Inoue, Y.; Guérif, M.; Baret, F.; Skidmore, A.; Gitelson, A.; Schlerf, M.; Darvishzadeh, R.; Olioso, A. Simple and robust methods for remote sensing of canopy chlorophyll content: A comparative analysis of hyperspectral data for different types of vegetation. Plant Cell Environ. 2016, 39, 2609–2623. [Google Scholar] [CrossRef] [Green Version]
| Index | Formula | Source |
|---|---|---|
| SR680 | R800/R680 | Sims and Gamon [56] |
| SR705 | R750/R705 | Sims and Gamon [56] |
| mSR705 | (R705 − R445)/(R705 + R445) | Sims and Gamon [56] |
| ND680 | (R800 − R680)/(R800 + R680) | Sims and Gamon [56] |
| ND705 | (R750 − R705)/(R750 + R705) | Sims and Gamon [56] |
| mND705 | (R750 − R705)/(R750 + R705−2 R445) | Sims and Gamon [56] |
| NDVI670 | (R800 − R670)/(R800 + R670) | Rouse et al. [57] |
| MSR670 | [(R800/R670) − 1]/sqrt[(R800/R670) + 1] | Chen [30] |
| MC670 | [(R700 − R670) − 0.2 × (R700 − R550)](R700/R670) | Daughtry et al. [58] |
| MC/OS670 | [(R700 − R670) − 0.2 × (R700 − R550)](R700/R670)/[(1 + 0.16)(R800 − R670)/(R800 + R670 + 0.16)] | Daughtry et al. [58] |
| MSR705 | [(R750/R705) − 1]/sqrt R750/R705) + 1] | Wu et al. [59] |
| MC705 | R750 − R705) − 0.2 × (R750 − R550) R750/R705) | Wu et al. [59] |
| MC/OS705 | [(R750 − R705) − 0.2 × (R750 − R550)](R750/R705)/[(1 + 0.16)(R750 − R705)/(R750 + R705 + 0.16)] | Wu et al. [59] |
| PRI | (R531 − R570)/(R531 + R570) | Wu et al. [59] |
| SIPI | (R800 − R445)/(R800 − R680) | Merzlyak et al. [60] |
| PSRI | (R680 − R500)/R750 | Penuelas et al. [61] |
| RARSa | R675/R700 | Zhang et al. [62] |
| RSSRb | R800/R635 | Zhang et al. [62] |
| PSNDb | (R800 − R635)/(R800 + R635) | Zhang et al. [62] |
| RARSb | R675/R650 × R700 | Zhang et al. [62] |
| CIrededge | (R770/R710) − 1 | Gitelson et al. [63] |
| CRIrededge | (R510)−1 − (R730)−1 × R770 | Gitelson et al. [63] |
| G1 | (R800 − R700)/(R800 + R700) | Gitelson and Merzlyak [64] |
| D | (R860/R708)(R700) | Datt [65] |
| G2 | (R750 − R800)/((R695 − R740) − 1) | Gitelson et al. [63] |
| Wave-Length (nm) | Less Dust (N = 75) | Medium Dust (N = 75) | Heavy Dust (N = 75) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | Cb | C | Cc | Pigment | Ca | Cb | C | Cc | Pigment | Ca | Cb | C | Cc | Pigment | |
| 445 | −0.507 ** | −0.380 ** | −0.486 ** | −0.303 ** | −0.498 ** | −0.381 ** | −0.212 | −0.332 ** | −0.312 ** | −0.352 ** | −0.371 ** | −0.321 ** | −0.382 ** | −0.204 | −0.386 ** |
| 500 | −0.548 ** | −0.374 ** | −0.507 ** | −0.358 ** | −0.526 ** | −0.437 ** | −0.249 * | −0.383 ** | −0.333 ** | −0.401 ** | −0.465 ** | −0.355 ** | −0.458 ** | −0.287 * | −0.470 ** |
| 510 | −0.577 ** | −0.370 ** | −0.523 ** | −0.395 ** | −0.546 ** | −0.494 ** | −0.280 * | −0.432 ** | −0.376 ** | −0.452 ** | −0.542 ** | −0.371 ** | −0.513 ** | −0.368 ** | −0.535 ** |
| 531 | −0.634 ** | −0.360 ** | −0.553 ** | −0.469 ** | −0.585 ** | −0.588 ** | −0.331 ** | −0.514 ** | −0.463 ** | −0.540 ** | −0.667 ** | −0.390 ** | −0.600 ** | −0.504 ** | −0.639 ** |
| 550 | −0.651 ** | −0.370 ** | −0.568 ** | −0.470 ** | −0.599 ** | −0.600 ** | −0.346 ** | −0.528 ** | −0.442 ** | −0.550 ** | −0.679 ** | −0.39 * | −0.609 ** | −0.505 ** | −0.648 ** |
| 570 | −0.648 ** | −0.374 ** | −0.568 ** | −0.460 ** | −0.597 ** | −0.593 ** | −0.344 ** | −0.523 ** | −0.411 ** | −0.539 ** | −0.669 ** | −0.397 ** | −0.605 ** | −0.482 ** | −0.639 ** |
| 635 | −0.605 ** | −0.374 ** | −0.542 ** | −0.413 ** | −0.566 ** | −0.524 ** | −0.302 ** | −0.461 ** | −0.337 ** | −0.471 ** | −0.577 ** | −0.389 ** | −0.543 ** | −0.379 ** | −0.564 ** |
| 650 | −0.572 ** | −0.372 ** | −0.521 ** | −0.381 ** | −0.542 ** | −0.473 ** | −0.272 * | −0.416 ** | −0.308 ** | −0.426 ** | −0.522 ** | −0.379 ** | −0.504 ** | −0.330 ** | −0.520 ** |
| 670 | −0.541 ** | −0.368 ** | −0.501 ** | −0.355 ** | −0.519 ** | −0.395 ** | −0.224 | −0.346 ** | −0.271 * | −0.357 ** | −0.449 ** | −0.362 ** | −0.451 ** | −0.258 * | −0.458 ** |
| 675 | −0.537 ** | −0.364 ** | −0.496 ** | −0.354 ** | −0.515 ** | −0.386 ** | −0.216 | −0.337 ** | −0.275 * | −0.350 ** | −0.447 ** | −0.362 ** | −0.449 ** | −0.257 * | −0.456 ** |
| 680 | −0.537 ** | −0.361 ** | −0.495 ** | −0.358 ** | −0.514 ** | −0.385 ** | −0.213 | −0.335 ** | −0.284 * | −0.349 ** | −0.455 ** | −0.366 ** | −0.456 ** | −0.266 * | −0.464 ** |
| 695 | −0.641 ** | −0.376 ** | −0.565 ** | −0.455 ** | −0.593 ** | −0.556 ** | −0.316 ** | −0.487 ** | −0.372 ** | −0.501 ** | −0.635 ** | −0.403 ** | −0.586 ** | −0.435 ** | −0.614 ** |
| 700 | −0.678 ** | −0.385 ** | −0.592 ** | −0.483 ** | −0.623 ** | −0.593 ** | −0.344 ** | −0.523 ** | −0.390 ** | −0.536 ** | −0.691 ** | −0.410 ** | −0.624 ** | −0.485 ** | −0.658 ** |
| 705 | −0.688 ** | −0.399 ** | −0.604 ** | −0.473 ** | −0.633 ** | −0.595 ** | −0.355 ** | −0.528 ** | −0.383 ** | −0.540 ** | −0.703 ** | −0.411 ** | −0.632 ** | −0.494 ** | −0.667 ** |
| 708 | −0.683 ** | −0.405 ** | −0.604 ** | −0.453 ** | −0.630 ** | −0.585 ** | −0.356 ** | −0.523 ** | −0.373 ** | −0.533 ** | −0.697 ** | −0.409 ** | −0.628 ** | −0.489 ** | −0.662 ** |
| 710 | −0.675 ** | −0.409 ** | −0.601 ** | −0.437 ** | −0.625 ** | −0.576 ** | −0.355 ** | −0.517 ** | −0.366 ** | −0.527 ** | −0.690 ** | −0.406 ** | −0.622 ** | −0.485 ** | −0.656 ** |
| 730 | −0.474 ** | −0.354 ** | −0.453 ** | −0.206 | −0.454 ** | −0.405 ** | −0.268 * | −0.372 ** | −0.297 ** | −0.385 ** | −0.554 ** | −0.353 ** | −0.512 ** | −0.415 ** | −0.543 ** |
| 740 | −0.330 ** | −0.283 * | −0.333 ** | −0.091 | −0.325 ** | −0.282 * | −0.184 | −0.258 * | −0.257 * | −0.276 * | −0.490 ** | −0.332 ** | −0.462 ** | −0.379 ** | −0.491 ** |
| 750 | −0.227 * | −0.231 * | −0.246 * | −0.014 | −0.232 * | −0.192 | −0.122 | −0.174 | −0.22 | −0.194 | −0.452 ** | −0.321 ** | −0.433 ** | −0.354 ** | −0.460 ** |
| 770 | −0.16 | −0.201 | −0.191 | 0.042 | −0.173 | −0.13 | −0.084 | −0.118 | −0.187 | −0.138 | −0.427 ** | −0.316 ** | −0.415 ** | −0.334 ** | −0.440 ** |
| 800 | −0.153 | −0.202 | −0.188 | 0.052 | −0.169 | −0.124 | −0.082 | −0.114 | −0.179 | −0.133 | −0.423 ** | −0.317 ** | −0.413 ** | −0.329 ** | −0.437 ** |
| 860 | −0.15 | −0.208 | −0.189 | 0.061 | −0.168 | −0.118 | −0.083 | −0.11 | −0.169 | −0.128 | −0.421 ** | −0.320 ** | −0.414 ** | −0.326 ** | −0.437 ** |
| Index | Less Dust (N = 75) | Medium Dust (N = 75) | Heavy Dust (N = 75) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | Cb | C | Cc | Pig-Ment | Ca | Cb | C | Cc | Pig-Ment | Ca | Cb | C | Cc | Pig-Ment | |
| SR680 | 0.477 ** | 0.325 ** | 0.441 ** | 0.316 ** | 0.458 ** | 0.313 ** | 0.164 | 0.268 * | 0.177 | 0.271 * | 0.473 ** | 0.387 ** | 0.478 ** | 0.226 | 0.476 ** |
| SR705 | 0.816 ** | 0.460 ** | 0.710 ** | 0.593 ** | 0.749 ** | 0.643 ** | 0.418 ** | 0.586 ** | 0.327 ** | 0.582 ** | 0.736 ** | 0.377 ** | 0.637 ** | 0.502 ** | 0.673 ** |
| mSR705 | 0.011 | 0.141 | 0.073 | −0.07 | 0.058 | −0.124 | −0.123 | −0.132 | 0.034 | −0.112 | −0.066 | 0.12 | 0.015 | −0.142 | −0.013 |
| ND680 | 0.541 ** | 0.334 ** | 0.485 ** | 0.404 ** | 0.511 ** | 0.370 ** | 0.191 | 0.316 ** | 0.241 * | 0.324 ** | 0.415 ** | 0.320 ** | 0.409 ** | 0.234 * | 0.416 ** |
| ND705 | 0.786 ** | 0.419 ** | 0.673 ** | 0.596 ** | 0.715 ** | 0.637 ** | 0.378 ** | 0.566 ** | 0.352 ** | 0.568 ** | 0.718 ** | 0.359 ** | 0.617 ** | 0.500 ** | 0.654 ** |
| mND705 | 0.779 ** | 0.374 ** | 0.648 ** | 0.614 ** | 0.694 ** | 0.607 ** | 0.367 ** | 0.542 ** | 0.312 ** | 0.539 ** | 0.732 ** | 0.304 ** | 0.600 ** | 0.543 ** | 0.647 ** |
| NDVI670 | 0.546 ** | 0.342 ** | 0.491 ** | 0.398 ** | 0.517 ** | 0.380 ** | 0.202 | 0.327 ** | 0.230 * | 0.333 ** | 0.411 ** | 0.317 ** | 0.406 ** | 0.228 * | 0.411 ** |
| MSR670 | 0.516 ** | 0.347 ** | 0.476 ** | 0.343 ** | 0.494 ** | 0.350 ** | 0.183 | 0.299 ** | 0.198 | 0.302 ** | 0.453 ** | 0.359 ** | 0.452 ** | 0.224 | 0.453 ** |
| MC670 | −0.468 ** | −0.163 | −0.360 ** | −0.406 ** | −0.395 ** | −0.493 ** | −0.291 * | −0.437 ** | −0.350 ** | −0.452 ** | −0.453 ** | −0.098 | −0.329 ** | −0.445 ** | −0.382 ** |
| TC/OS670 | −0.682 ** | −0.339 ** | −0.573 ** | −0.527 ** | −0.611 ** | −0.618 ** | −0.356 ** | −0.544 ** | −0.424 ** | −0.560 ** | −0.731 ** | −0.339 ** | −0.616 ** | −0.581 ** | −0.668 ** |
| MC/OS670 | −0.540 ** | −0.204 | −0.423 ** | −0.461 ** | −0.462 ** | −0.618 ** | −0.356 ** | −0.544 ** | −0.424 ** | −0.560 ** | −0.731 ** | −0.339 ** | −0.616 ** | −0.581 ** | −0.668 ** |
| MSR705 | 0.808 ** | 0.444 ** | 0.698 ** | 0.598 ** | 0.739 ** | 0.644 ** | 0.403 ** | 0.581 ** | 0.339 ** | 0.579 ** | 0.730 ** | 0.371 ** | 0.631 ** | 0.502 ** | 0.667 ** |
| MC705 | 0.799 ** | 0.406 ** | 0.675 ** | 0.643 ** | 0.723 ** | 0.596 ** | 0.395 ** | 0.548 ** | 0.262 * | 0.536 ** | 0.598 ** | 0.288 * | 0.508 ** | 0.360 ** | 0.530 ** |
| TC/OS705 | −0.779 ** | −0.439 ** | −0.678 ** | −0.567 ** | −0.716 ** | −0.661 ** | −0.421 ** | −0.599 ** | −0.383 ** | −0.603 ** | −0.724 ** | −0.410 ** | −0.644 ** | −0.511 ** | −0.681 ** |
| MC/OS705 | 0.766 ** | 0.375 ** | 0.640 ** | 0.636 ** | 0.690 ** | −0.661 ** | −0.421 ** | −0.599 ** | −0.383 ** | −0.603 ** | −0.724 ** | −0.410 ** | −0.644 ** | −0.511 ** | −0.681 ** |
| PRI | 0.437 ** | 0.310 ** | 0.411 ** | 0.137 | 0.404 ** | 0.244 * | 0.215 | 0.248 * | −0.149 | 0.196 | 0.188 | 0.142 | 0.184 | −0.031 | 0.162 |
| SIPI | −0.314 ** | 0.033 | −0.175 | −0.439 ** | −0.226 | 0.022 | 0.023 | 0.024 | 0.175 | 0.052 | −0.328 ** | −0.179 | −0.289 * | −0.236 * | −0.307 ** |
| PSRI | 0.353 ** | 0.289 * | 0.349 ** | 0.179 | 0.353 ** | 0.438 ** | 0.280 * | 0.398 ** | 0.375 ** | 0.421 ** | 0.179 | −0.017 | 0.104 | 0.222 | 0.136 |
| RARSa | 0.073 | −0.065 | 0.013 | 0.104 | 0.027 | 0.265 * | 0.198 | 0.253 * | 0.105 | 0.245 * | 0.045 | −0.159 | −0.047 | 0.145 | −0.016 |
| RSSRb | 0.649 ** | 0.410 ** | 0.585 ** | 0.435 ** | 0.610 ** | 0.511 ** | 0.306 ** | 0.455 ** | 0.274 * | 0.455 ** | 0.609 ** | 0.382 ** | 0.560 ** | 0.372 ** | 0.579 ** |
| PSNDb | 0.631 ** | 0.362 ** | 0.552 ** | 0.471 ** | 0.584 ** | 0.534 ** | 0.296 ** | 0.465 ** | 0.310 ** | 0.470 ** | 0.556 ** | 0.341 ** | 0.508 ** | 0.368 ** | 0.530 ** |
| RARSb | −0.663 ** | −0.394 ** | −0.587 ** | −0.469 ** | −0.616 ** | −0.558 ** | −0.319 ** | −0.490 ** | −0.385 ** | −0.505 ** | −0.607 ** | −0.410 ** | −0.572 ** | −0.388 ** | −0.592 ** |
| CIrededge | 0.813 ** | 0.471 ** | 0.714 ** | −0.567 ** | 0.749 ** | 0.633 ** | 0.422 ** | 0.583 ** | 0.306 ** | 0.575 ** | 0.737 ** | 0.362 ** | 0.630 ** | 0.507 ** | −0.668 ** |
| CRIrededg | 0.565 ** | 0.372 ** | 0.517 ** | 0.390 ** | 0.539 ** | −0.113 | −0.052 | −0.094 | −0.338 ** | −0.143 | 0.108 | 0.13 | 0.129 | −0.167 | 0.086 |
| G1 | 0.753 ** | 0.393 ** | 0.640 ** | 0.587 ** | 0.683 ** | 0.632 ** | 0.361 ** | 0.554 ** | 0.372 ** | 0.561 ** | 0.696 ** | 0.360 ** | 0.603 ** | 0.491 ** | 0.640 ** |
| D | −0.465 ** | −0.316 ** | −0.430 ** | −0.279 * | −0.442 ** | −0.364 ** | −0.203 | −0.317 ** | −0.321 ** | −0.340 ** | −0.593 ** | −0.417 ** | −0.567 ** | −0.428 ** | −0.595 ** |
| G2 | 0.713 ** | 0.306 ** | 0.576 ** | 0.589 ** | 0.623 ** | 0.634 ** | 0.378 ** | 0.563 ** | 0.400 ** | 0.574 ** | 0.511 ** | 0.183 | 0.405 ** | 0.425 ** | 0.448 ** |
| Dust Deposition Condition | Index | Regression Equation | R2 |
|---|---|---|---|
| Less dust | SR705 | y = 3.5774 *SR705 − 0.9945 | 0.6689 |
| ND705 | y = 30.5050 *ND705 − 4.9257 | 0.6552 | |
| CIrededge | y = 6.0072 *CIrededge + 1.6574 | 0.6848 | |
| SR705 + ND705 + CIrededge | y = 0.9905 + 3.9483 *SR705−6.5772 *ND705 + 0.3109 *CIrededge | 0.6379 | |
| Medium dust | SR705 | y = 3.3428 *SR705 − 1.1278 | 0.5115 |
| ND705 | y = 29.6969 *ND705 − 5.5891 | 0.4551 | |
| CIrededge | y = 4.3246 *CIrededge + 3.2881 | 0.4653 | |
| SR705 + ND705 + CIrededge | y = −1.3681 + 0.7468 *SR705 + 14.6317 *ND705 + 0.6737 *CIrededge | 0.3915 | |
| Heavy dust | SR705 | y = 4.3232 *SR705 − 3.4567 | 0.5653 |
| ND705 | y = 26.2446 *ND705 − 3.7136 | 0.5476 | |
| CIrededge | y = 7.103 *CIrededge − 0.0795 | 0.4408 | |
| SR705 + ND705 + CIrededge | y = −6.7285 + 15.8466 *SR705 − 36.6609 *ND705 − 10.6375 *CIrededge | 0.5486 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.; Yu, X.; Xu, D.; Sun, T.; Sun, Y. Effect of Dust Deposition on Chlorophyll Concentration Estimation in Urban Plants from Reflectance and Vegetation Indexes. Remote Sens. 2021, 13, 3570. https://doi.org/10.3390/rs13183570
Lin W, Yu X, Xu D, Sun T, Sun Y. Effect of Dust Deposition on Chlorophyll Concentration Estimation in Urban Plants from Reflectance and Vegetation Indexes. Remote Sensing. 2021; 13(18):3570. https://doi.org/10.3390/rs13183570
Chicago/Turabian StyleLin, Wenpeng, Xumiao Yu, Di Xu, Tengteng Sun, and Yue Sun. 2021. "Effect of Dust Deposition on Chlorophyll Concentration Estimation in Urban Plants from Reflectance and Vegetation Indexes" Remote Sensing 13, no. 18: 3570. https://doi.org/10.3390/rs13183570
APA StyleLin, W., Yu, X., Xu, D., Sun, T., & Sun, Y. (2021). Effect of Dust Deposition on Chlorophyll Concentration Estimation in Urban Plants from Reflectance and Vegetation Indexes. Remote Sensing, 13(18), 3570. https://doi.org/10.3390/rs13183570







