A New Method for Crop Row Detection Using Unmanned Aerial Vehicle Images
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
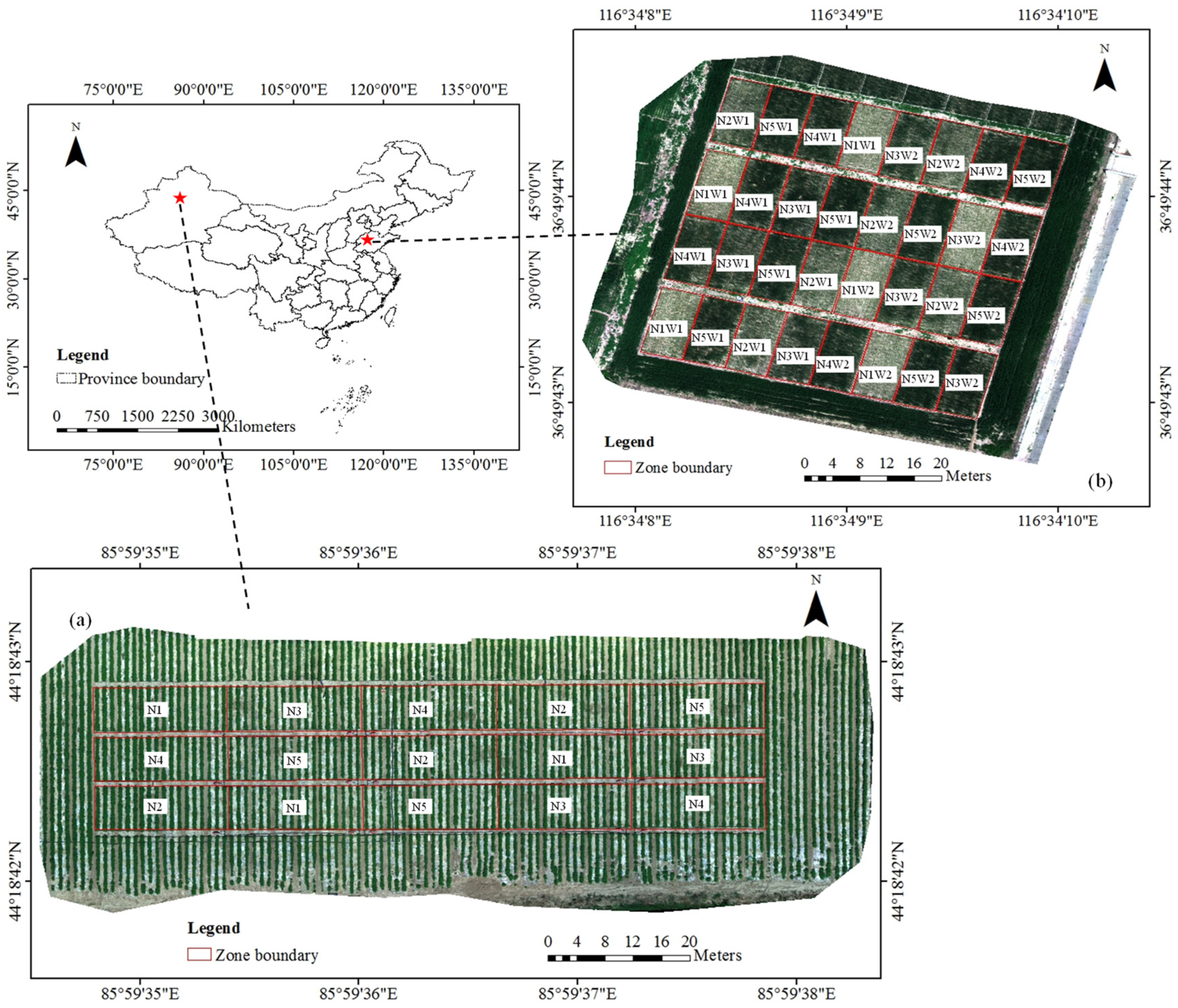
2.1.1. Nitrogen Field Experiment Involving Cotton
2.1.2. Nitrogen and Water Field Experiment Involving Wheat
2.2. Data Acquisition and Preprocessing
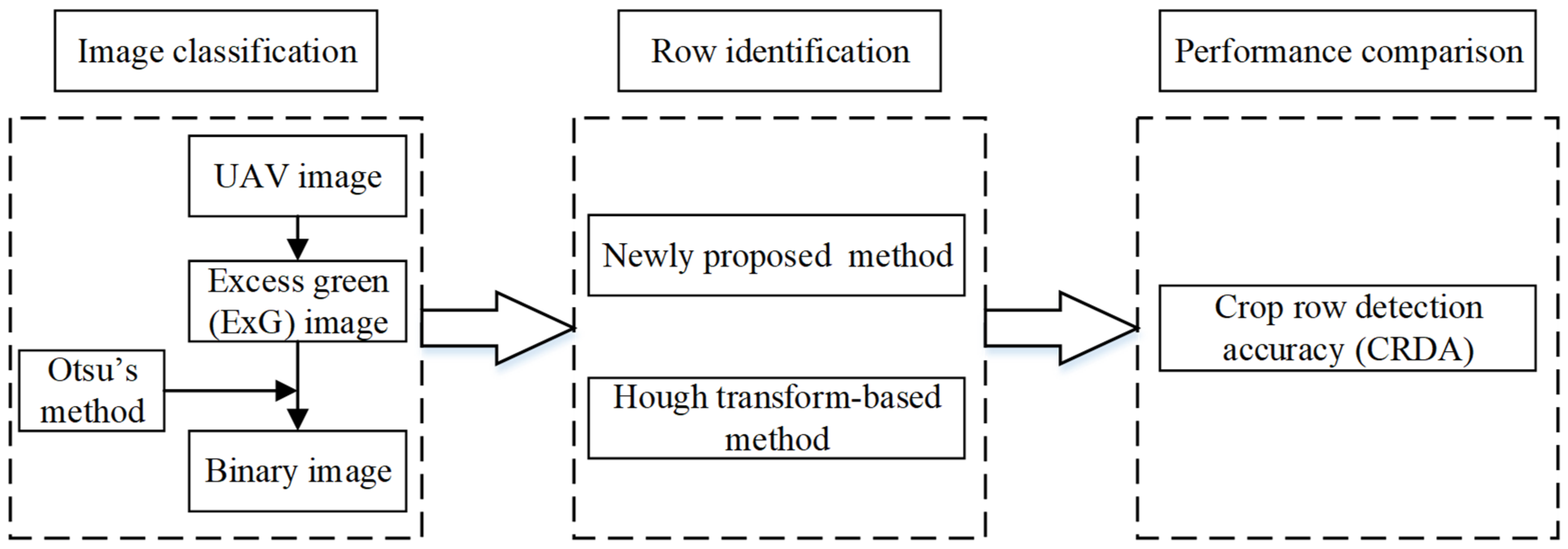
2.3. Data Analysis Method
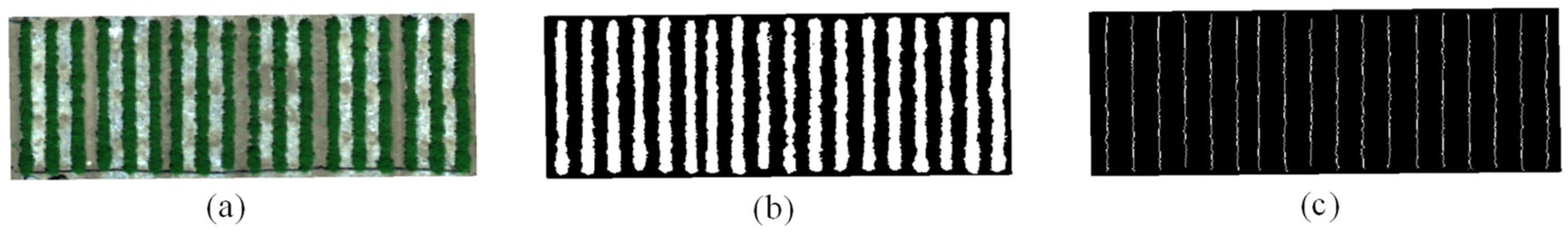
2.3.1. Vegetation/Soil Classification
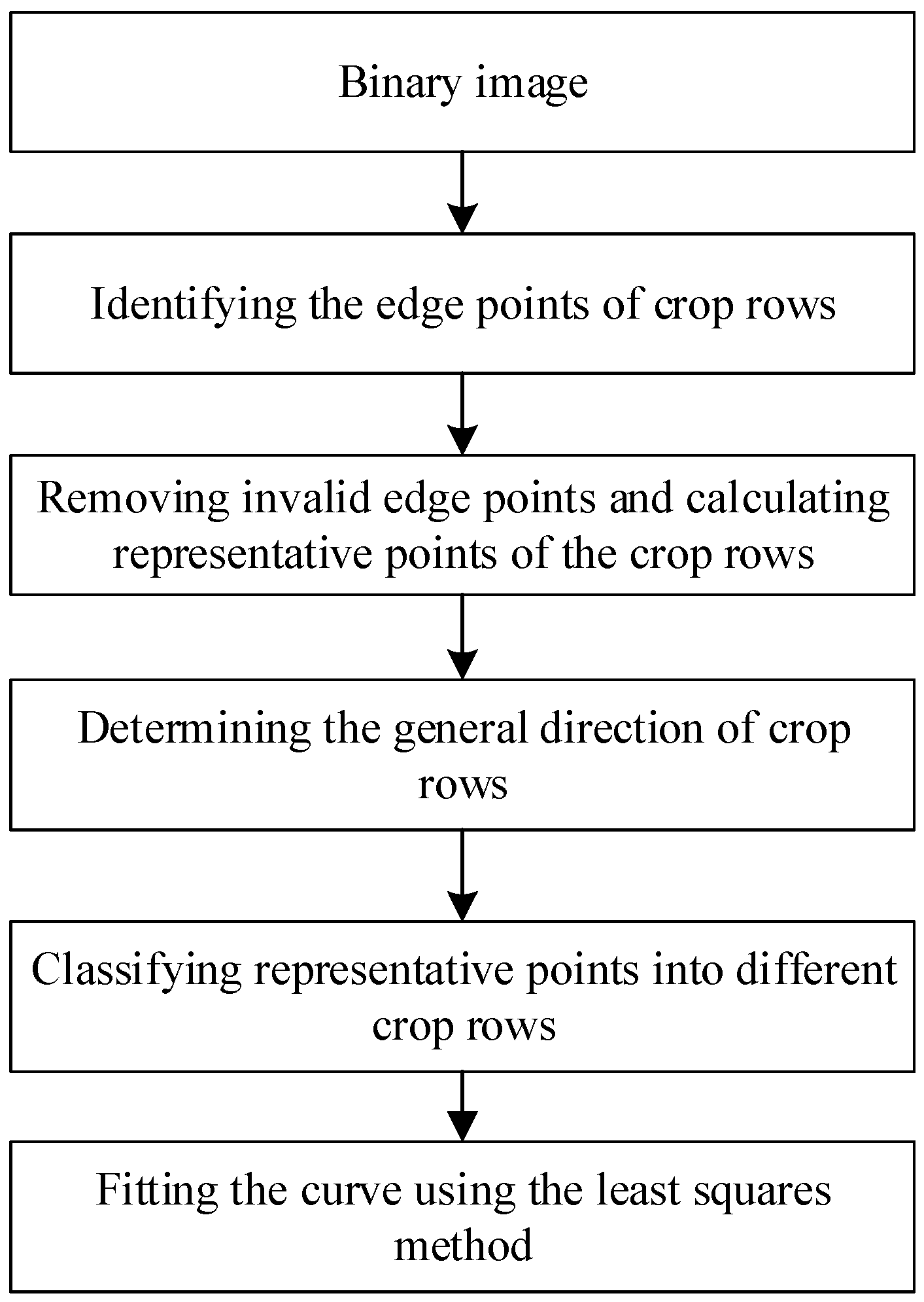
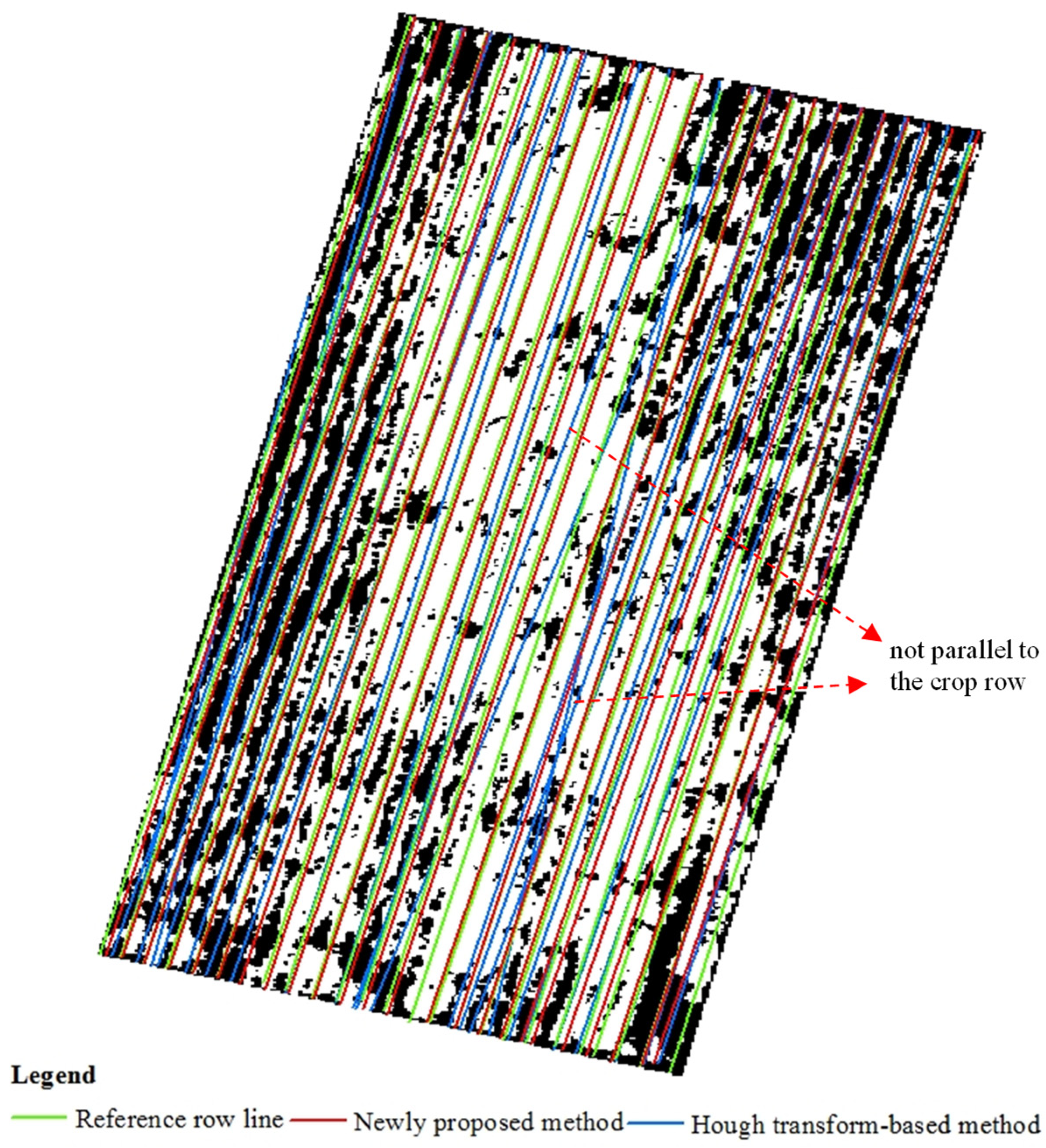
2.3.2. Crop Row Identification Using Different Methods
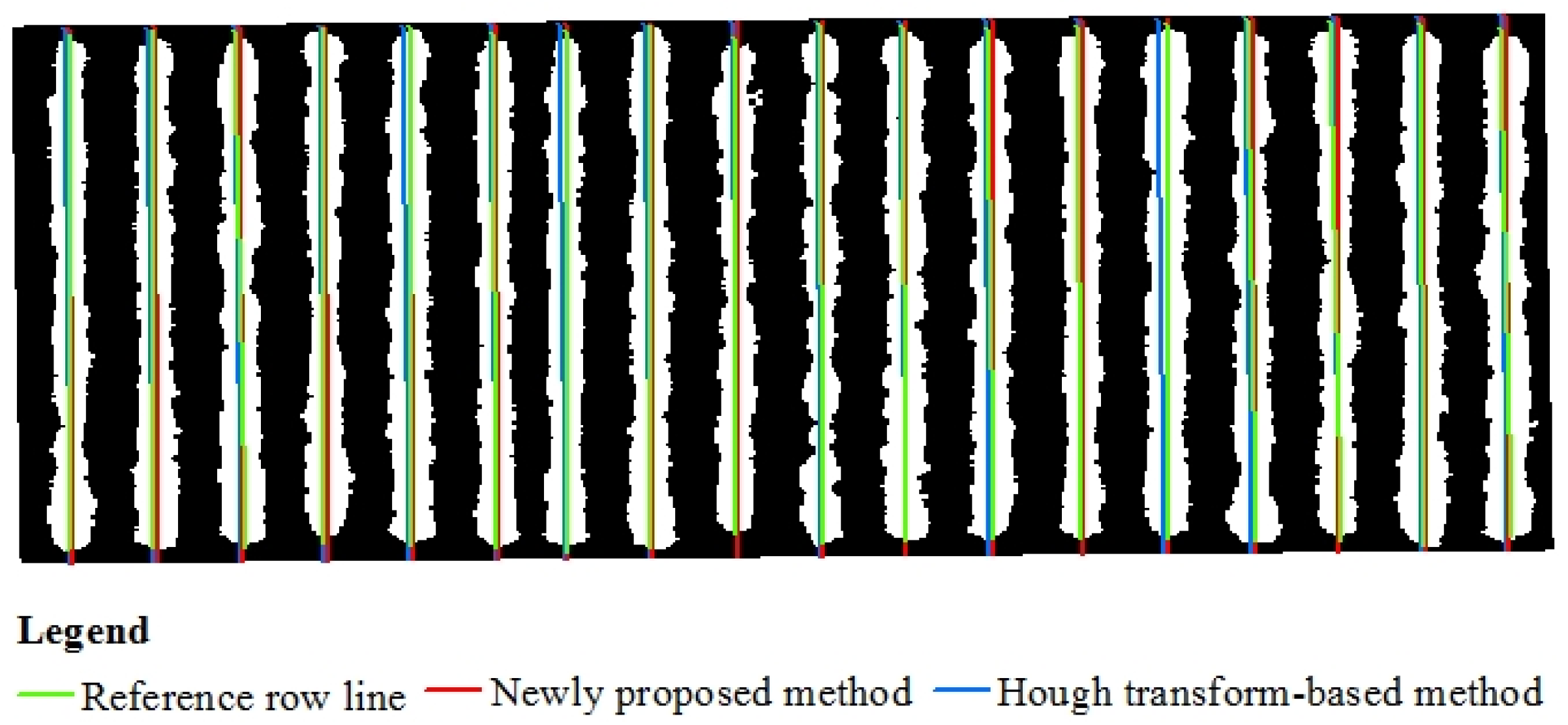
2.3.3. Evaluation of the Crop Row Detection Accuracy for Different Methods
3. Results
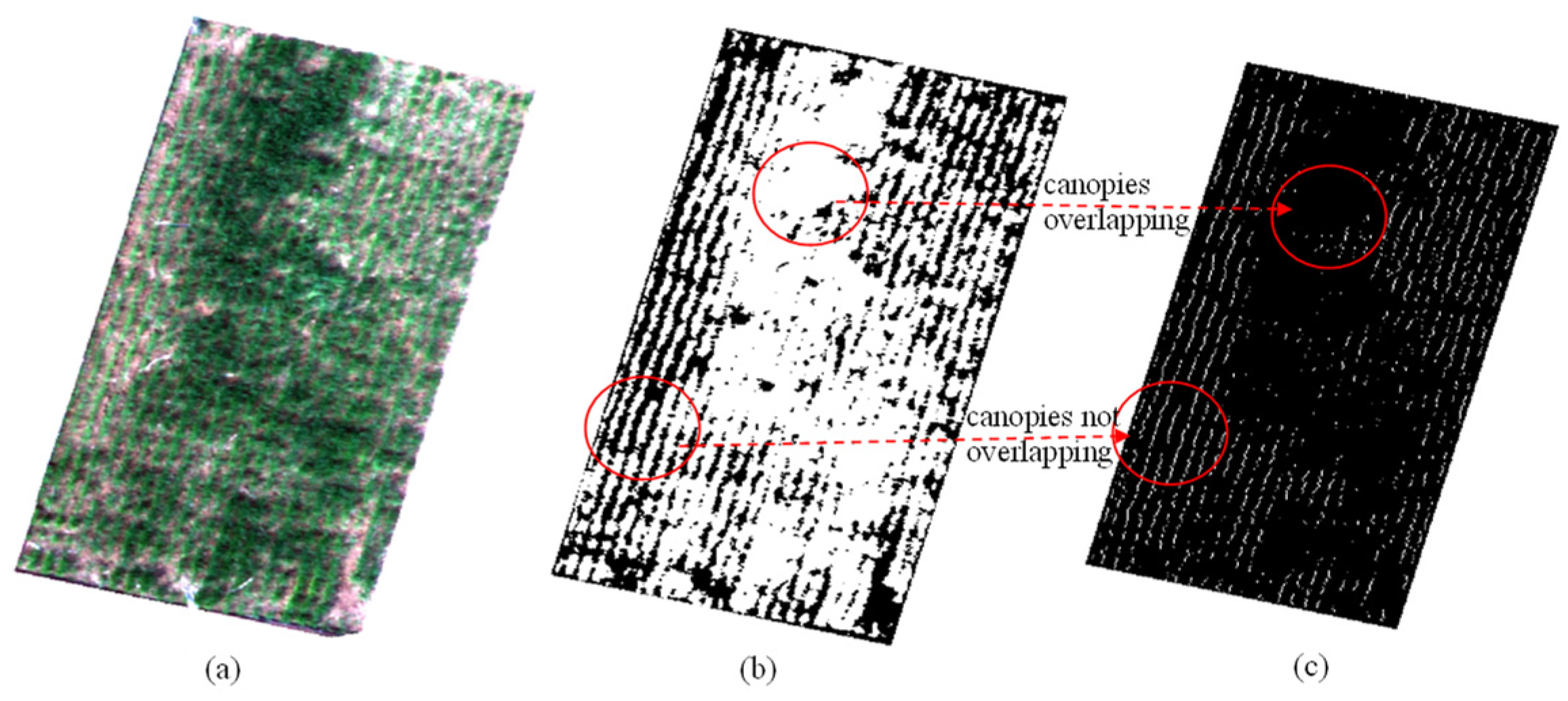
3.1. Wheat and Cotton Rows in Different Scenarios
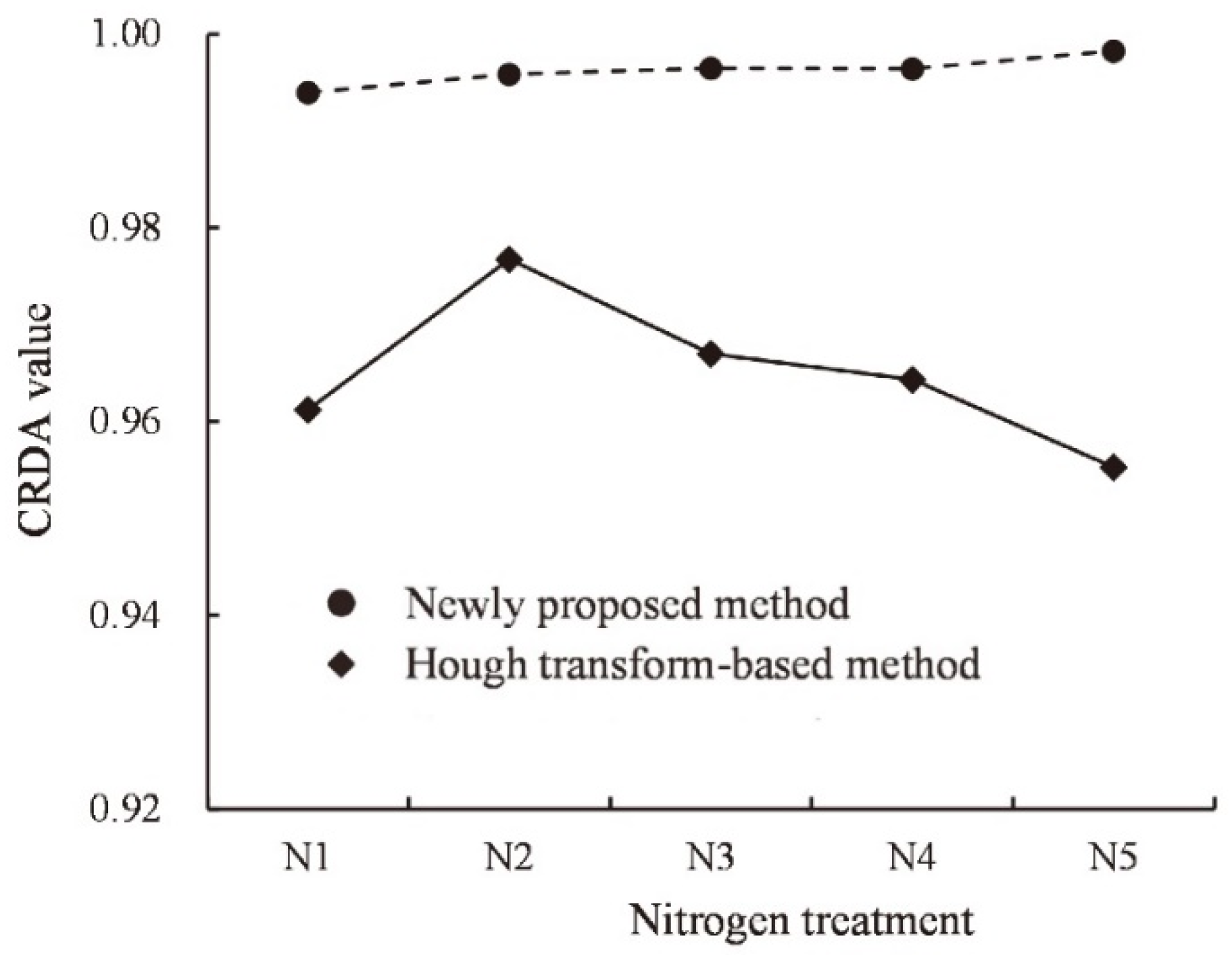
3.2. Results of Cotton Row Detection
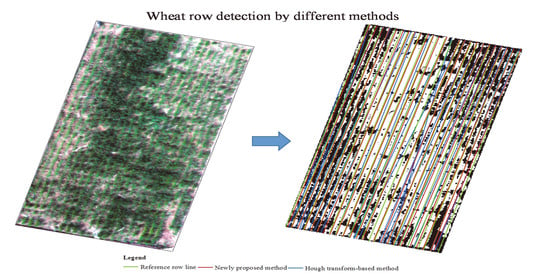
3.3. Results of Wheat Row Detection
4. Discussion
4.1. Advantages of the Proposed Method over Previous Crop Planting Row Detection Methods
4.2. Comparison of Crop Row Detection Results with Those of Other Studies Using UAV Images
4.3. Application of the Newly Proposed Method and Future Work
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arnó, J.; Casasnovas, M.J.A.; Dasi, R.M.; Rosell, J.R. Review. Precision viticulture. Research topics, challenges and opportunities in site-specific vineyard management. Span. J. Agric. Res. 2009, 7, 779–790. [Google Scholar] [CrossRef] [Green Version]
- Balafoutis, A.; Beck, B.; Fountas, S.; Vangeyte, J.; Wal, T.V.D.; Soto, I.; Gómez-Barbero, M.; Barnes, A.; Eory, V. Precision agriculture technologies positively contributing to GHG emissions mitigation, farm productivity and economics. Sustainability 2017, 9, 1339. [Google Scholar] [CrossRef] [Green Version]
- Hedley, C. The role of precision agriculture for improved nutrient management on farms. J. Sci. Food Agric. 2015, 95, 12–19. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, A.; Huang, W.; Liu, K.; Liu, L.; Wang, J. Evaluation of variable-rate nitrogen recommendation of winter wheat based on SPAD chlorophyll meter measurement. New Zeal. J. Agr. Res. 2007, 50, 735–741. [Google Scholar]
- Mandrini, G.; Bullock, D.S.; Martin, N.F. Modeling the economic and environmental effects of corn nitrogen management strategies in Illinois. Field Crop. Res. 2021, 261, 108000. [Google Scholar] [CrossRef]
- García-Santillán, I.D.; Guerrero, J.M.; Montalvo, M.; Pajares, G. Curved and straight crop row detection by accumulation of green pixels from images in maize fields. Precis. Agric. 2017, 19, 18–41. [Google Scholar] [CrossRef]
- Astrand, B.; Baerveldt, A.J. A vision based row-following system for agricultural field machinery. Mechatronics 2015, 15, 251–269. [Google Scholar] [CrossRef]
- Hague, T.; Tillett, N.D. A bandpass filter-based approach to crop row location and tracking. Mechatronics 2001, 11, 1–12. [Google Scholar] [CrossRef]
- Pang, Y.; Shi, Y.; Gao, S.; Jiang, F.; Veeranampalayam-Sivakumar, A.; Thompson, L.; Luck, J.; Liu, C. Improved crop row detection with deep neural network for early-season maize stand count in UAV imagery. Comput. Electron. Agric. 2020, 178, 105766. [Google Scholar] [CrossRef]
- Zhang, C.; Kovacs, J.M. The application of small unmanned aerial systems for precision agriculture: A review. Precis. Agric. 2012, 13, 693–712. [Google Scholar] [CrossRef]
- Basso, M.; de Freitas, E.P. A UAV guidance system using crop row detection and line follower algorithms. J. Intell. Robot. Syst. 2020, 97, 605–621. [Google Scholar] [CrossRef]
- Hough, P.V.C. Method and Means for Recognizing Complex Patterns. US Patent 306954 18 December 1962. [Google Scholar]
- Duda, R.O.; Hart, P.E. Use of the Hough transformation to detect lines and curves in pictures. Commun. ACM 1972, 15, 11–15. [Google Scholar] [CrossRef]
- Pérez-Ortiz, M.; Peña, J.M.; Gutiérrez, P.A.; Torres-Sánchez, J.; Hervás-Martínez, C.; López-Granados, F. A semi-supervised system for weed mapping in sunflower crops using unmanned aerial vehicles and a crop row detection method. Appl. Soft Comput. 2015, 37, 533–544. [Google Scholar] [CrossRef]
- Weiss, M.; Baret, F. Using 3D point clouds derived from UAV RGB imagery to describe vineyard 3D macro-structure. Remote Sens. 2017, 9, 111. [Google Scholar] [CrossRef] [Green Version]
- Su, W.; Jiang, K.; Yan, A.; Liu, Z.; Zhang, M.; Wang, W. Monitoring of planted lines for breeding corn using UAV remote sensing image. Trans. CSAE 2018, 34, 92–98, (In Chinese with English Abstract). [Google Scholar]
- Basak, J. Learning Hough transform: A neural network model. Neural Comput. 2001, 13, 651–676. [Google Scholar] [CrossRef]
- Tenhunen, H.; Pahikkala, T.; Nevalainen, O.; Teuhola, J.; Mattila, H.; Tyystjärvi, E. Automatic detection of cereal rows by means of pattern recognition techniques. Comput. Electron. Agric. 2019, 162, 677–688. [Google Scholar] [CrossRef]
- Billingsley, J.; Schoenfisch, M. The successful development of a vision guidance system for agriculture. Comput. Electron. Agric. 1997, 16, 147–163. [Google Scholar] [CrossRef]
- Søgaard, H.T.; Olsen, H.J. Determination of crop rows by image analysis without segmentation. Comput. Electron. Agric. 2003, 38, 141–158. [Google Scholar] [CrossRef]
- Montalvo, M.; Pajares, G.; Guerrero, J.M.; Romeo, J.; Guijarro, M.; Ribeiro, A.; Ruz, J.J.; Cruz, J.M. Automatic detection of crop rows in maize fields with high weeds pressure. Expert Syst. Appl. 2012, 39, 11889–11897. [Google Scholar] [CrossRef] [Green Version]
- Jiang, G.; Wang, Z.; Liu, H. Automatic detection of crop rows based on multi-ROIs. Expert Syst. Appl. 2015, 42, 2429–2441. [Google Scholar] [CrossRef]
- Chen, P.; Wang, F. New textural indicators for assessing above-ground cotton biomass extracted from optical imagery obtained via unmanned aerial vehicle. Remote Sens. 2020, 12, 4170. [Google Scholar] [CrossRef]
- Pilon, C. Physiological Responses of Cotton Genotypes to Water-Deficit Stress during Reproductive Development. Ph.D. Thesis, University of Arkansas, Fayetteville, AR, USA, 2015. [Google Scholar]
- Zhao, C.; Chen, P.; Huang, W.; Wang, J.; Wang, Z.; Jiang, A. Effects of two kinds of variable-rate nitrogen application strategies on the production of winter wheat (Triticum aestivum). N. Z. J. Crop. Hortic. Sci. 2009, 37, 149–155. [Google Scholar] [CrossRef]
- Olson, D.; Chatterjee, A.; Franzen, D.W.; Day, S.S. Relationship of drone-based vegetation indices with corn and sugarbeet yields. Agron. J. 2019, 11, 2545–2557. [Google Scholar] [CrossRef]
- Pérez-Ortiz, M.; Peña, J.M.; Gutiérrez, P.A.; Torres-Sánchez, J.; Hervás-Martínez, C.; López-Granados, F. Selecting patterns and features for between- and within- crop-row weed mapping using UAV-imagery. Expert Syst. Appl. 2016, 47, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1975, 11, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Woebbecke, D.M.; Meyer, G.E.; Von Bargen, K.; Mortensen, D.A. Color indices for weed identification under various soil, residue, and lighting conditions. Trans. ASAE 1995, 38, 259–269. [Google Scholar] [CrossRef]
- Vidović, I.; Cupec, R.; Hocenski, Ž. Crop row detection by global energy minimization. Pattern Recognit. 2016, 55, 68–86. [Google Scholar] [CrossRef]
- García-Santillán, I.D.; Montalvo, M.; Guerrero, J.M.; Pajares, G. Automatic detection of curved and straight crop rows from images in maize fields. Biosyst. Eng. 2017, 156, 61–79. [Google Scholar] [CrossRef]
- Bah, M.D.; Hafiane, A.; Canals, A.R. Crownet: Deep network for crop row detection in UAV images. IEEE Access 2020, 8, 5189–5200. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Ma, X.; Wang, F.; Li, J. A New Method for Crop Row Detection Using Unmanned Aerial Vehicle Images. Remote Sens. 2021, 13, 3526. https://doi.org/10.3390/rs13173526
Chen P, Ma X, Wang F, Li J. A New Method for Crop Row Detection Using Unmanned Aerial Vehicle Images. Remote Sensing. 2021; 13(17):3526. https://doi.org/10.3390/rs13173526
Chicago/Turabian StyleChen, Pengfei, Xiao Ma, Fangyong Wang, and Jing Li. 2021. "A New Method for Crop Row Detection Using Unmanned Aerial Vehicle Images" Remote Sensing 13, no. 17: 3526. https://doi.org/10.3390/rs13173526
APA StyleChen, P., Ma, X., Wang, F., & Li, J. (2021). A New Method for Crop Row Detection Using Unmanned Aerial Vehicle Images. Remote Sensing, 13(17), 3526. https://doi.org/10.3390/rs13173526