Evaluating the Performance of Hyperspectral Leaf Reflectance to Detect Water Stress and Estimation of Photosynthetic Capacities
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Hyperspectral Measurement Processing
2.3. Photosynthetic Measurement
2.4. Extraction of Vegetation Indices
2.5. ANOVA Analysis and Principal Component Analysis (PCA)
2.6. Machine-Learning Algorithms
3. Results
3.1. Photosynthetic Response to Water Stress
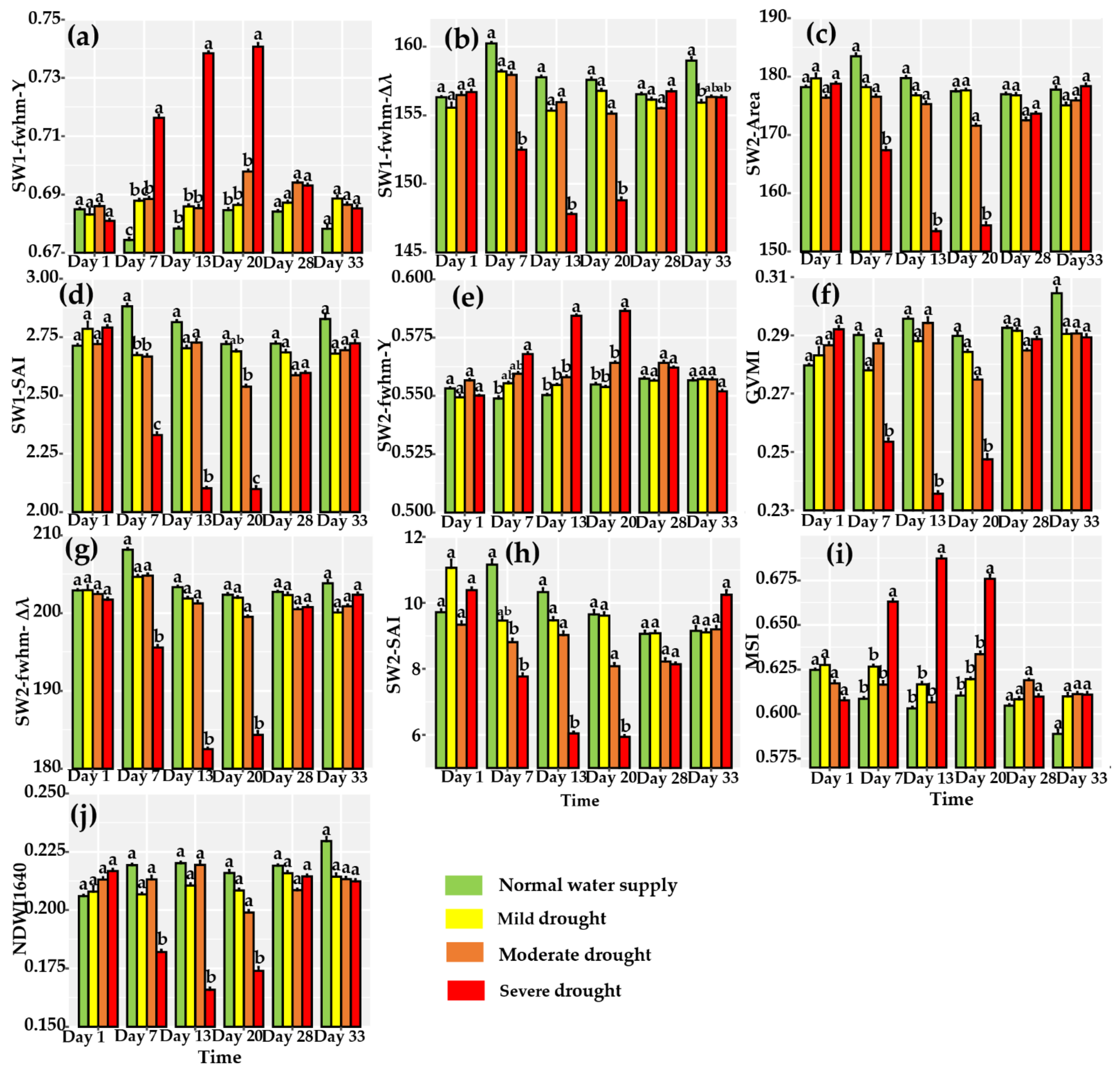
3.2. Variation in Leaf Reflectance Spectra for Different Water Stress and Tracking of Leaf Hyperspectral Reflectance to Water Stress
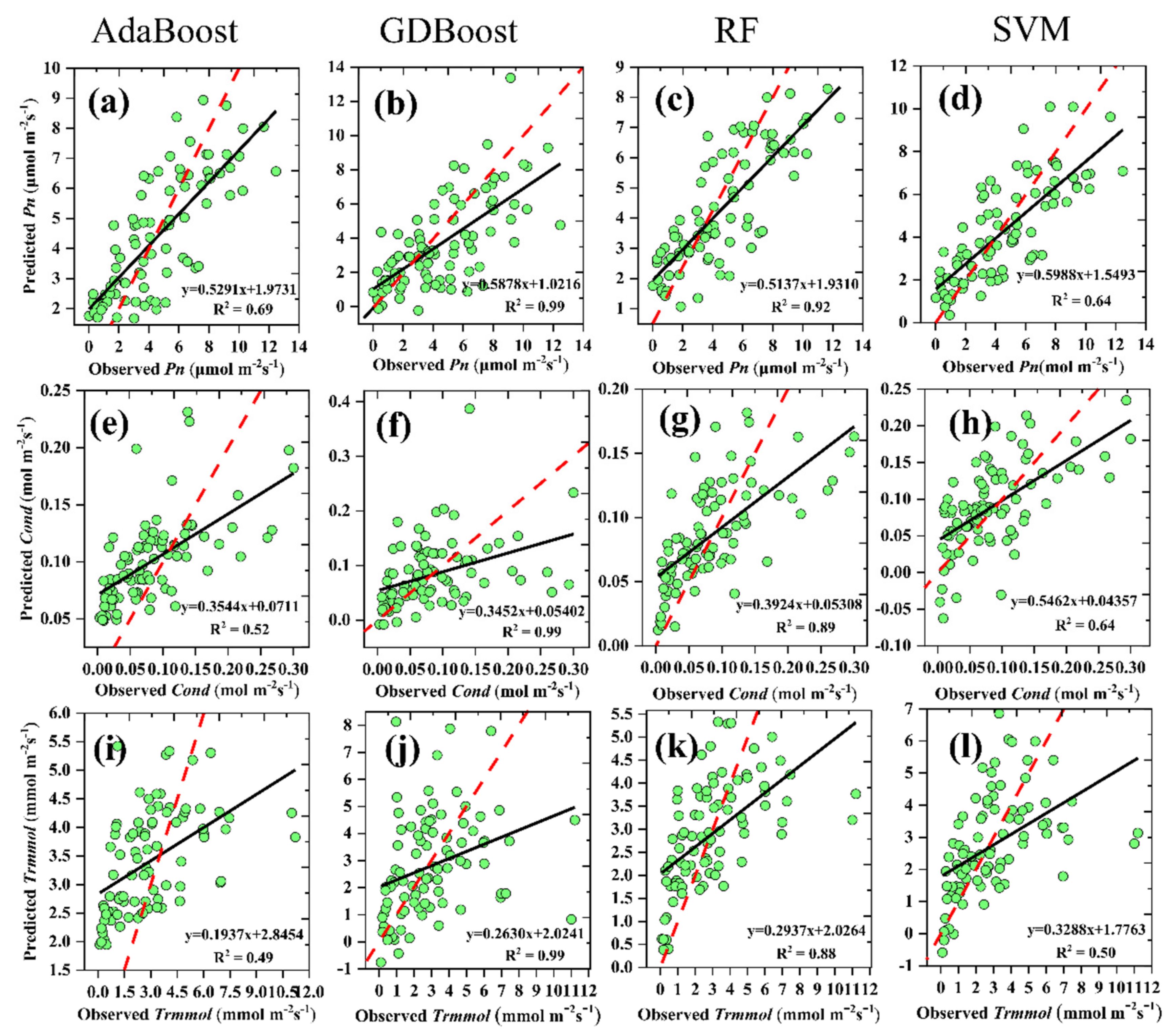
3.3. Machine-Learning Algorithms to Predict Pn, Cond, and Trmmol
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Boretti, A.; Rosa, L. Reassessing the projections of the World Water Development Report. npj Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Samaniego, L.; Thober, S.; Kumar, R.; Wanders, N.; Rakovec, O.; Pan, M.; Zink, M.; Sheffield, J.; Wood, E.F.; Marx, A. Anthropogenic warming exacerbates European soil moisture droughts. Nat. Clim. Chang. 2018, 8, 421–426. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Dai, A.; van der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Morgan, K.T.; Barkataky, S.; Kadyampakeni, D.; Ebel, R.; Roka, F. Effects of Short-term Drought Stress and Mechanical Harvesting on Sweet Orange Tree Health, Water Uptake, and Yield. Hortscience 2014, 49, 835–842. [Google Scholar] [CrossRef]
- Zhu, X.-G.; Long, S.P.; Ort, D.R. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr. Opin. Biotechnol. 2008, 19, 153–159. [Google Scholar] [CrossRef]
- Heckmann, D.; Schlueter, U.; Weber, A.P.M. Machine Learning Techniques for Predicting Crop Photosynthetic Capacity from Leaf Reflectance Spectra. Mol. Plant 2017, 10, 878–890. [Google Scholar] [CrossRef]
- Xiao, M.; Li, Y.; Lu, B. Response of Net Photosynthetic Rate to Environmental Factors under Water Level Regulation in Paddy Field. Pol. J. Environ. Stud. 2019, 28, 1433–1442. [Google Scholar] [CrossRef]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P.; et al. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef]
- Long, S.P.; Marshall-Colon, A.; Zhu, X.-G. Meeting the Global Food Demand of the Future by Engineering Crop Photosynthesis and Yield Potential. Cell 2015, 161, 56–66. [Google Scholar] [CrossRef]
- Osco, L.P.; Marques Ramos, A.P.; Faita Pinheiro, M.M.; Saito Moriya, E.A.; Imai, N.N.; Estrabis, N.; Ianczyk, F.; de Araujo, F.F.; Liesenberg, V.; de Castro Jorge, L.A.; et al. A Machine Learning Framework to Predict Nutrient Content in Valencia-Orange Leaf Hyperspectral Measurements. Remote Sens. 2020, 12, 906. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Yordanov, I.; Velikova, V.; Tsonev, T. Plant responses to drought and stress tolerance. Bulg. J. Plant Physiol. 2003, 187–206. [Google Scholar]
- Chaerle, L.; Van Der Straeten, D. Imaging techniques and the early detection of plant stress. Trends Plant Sci. 2000, 5, 495–501. [Google Scholar] [CrossRef]
- Gerhards, M.; Schlerf, M.; Mallick, K.; Udelhoven, T. Challenges and Future Perspectives of Multi-/Hyperspectral Thermal Infrared Remote Sensing for Crop Water-Stress Detection: A Review. Remote Sens. 2019, 11, 1240. [Google Scholar] [CrossRef]
- Acevedo, E.; Hsiao, T.C.; Henderson, D.W. Immediate and subsequent growth responses of maize leaves to changes in water status. Plant Physiol. 1971, 48, 631–636. [Google Scholar] [CrossRef]
- Sobejano-Paz, V.; Mikkelsen, T.N.; Baum, A.; Mo, X.; Liu, S.; Koppl, C.J.; Johnson, M.S.; Gulyas, L.; Garcia, M. Hyperspectral and Thermal Sensing of Stomatal Conductance, Transpiration, and Photosynthesis for Soybean and Maize under Drought. Remote Sens. 2020, 12, 3182. [Google Scholar] [CrossRef]
- Zaher-Ara, T.; Boroomand, N.; Sadat-Hosseini, M. Physiological and morphological response to drought stress in seedlings of ten citrus. Trees-Struct. Funct. 2016, 30, 985–993. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Huang, Z.; Su, X.; Siu, A.; Song, Y.; Zhang, D.; Fang, Q. Machine learning models for net photosynthetic rate prediction using poplar leaf phenotype data. PLoS ONE 2020, 15. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Peron, R.; Tuinstra, M.R.; Mickelbart, M.V.; Couture, J.J. Spectral Phenotyping of Physiological and Anatomical Leaf Traits Related with Maize Water Status. Plant Physiol. 2020, 184, 1363–1377. [Google Scholar] [CrossRef]
- Long, S.P.; Bernacchi, C.J. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J. Exp. Bot. 2003, 54, 2393–2401. [Google Scholar] [CrossRef]
- Fu, P.; Meacham-Hensold, K.; Guan, K.; Bernacchi, C.J. Hyperspectral Leaf Reflectance as Proxy for Photosynthetic Capacities: An Ensemble Approach Based on Multiple Machine Learning Algorithms. Front. Plant Sci. 2019, 10, 730. [Google Scholar] [CrossRef]
- Fu, P.; Meacham-Hensold, K.; Guan, K.; Wu, J.; Bernacchi, C. Estimating photosynthetic traits from reflectance spectra: A synthesis of spectral indices, numerical inversion, and partial least square regression. Plant Cell Environ. 2020, 43, 1241–1258. [Google Scholar] [CrossRef]
- Yendrek, C.R.; Tomaz, T.; Montes, C.M.; Cao, Y.; Morse, A.M.; Brown, P.J.; McIntyre, L.M.; Leakey, A.D.B.; Ainsworth, E.A. High-Throughput Phenotyping of Maize Leaf Physiological and Biochemical Traits Using Hyperspectral Reflectance. Plant Physiol. 2017, 173, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Asner, G.P. Biophysical and biochemical sources of variability in canopy reflectance. Remote Sens. Environ. 1998, 64, 234–253. [Google Scholar] [CrossRef]
- Gamon, J.A.; Somers, B.; Malenovsky, Z.; Middleton, E.M.; Rascher, U.; Schaepman, M.E. Assessing Vegetation Function with Imaging Spectroscopy. Surv. Geophys. 2019, 40, 489–513. [Google Scholar] [CrossRef]
- Streher, A.S.; da Silva Torres, R.; Cerdeira Morellato, L.P.; Freire Silva, T.S. Accuracy and limitations for spectroscopic prediction of leaf traits in seasonally dry tropical environments. Remote Sens. Environ. 2020, 244, 111828. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Couture, J.J.; Cavender-Bares, J.; Kingdon, C.C.; Fallon, B.; Pilz, G.; Pellegrini, E.; Nali, C.; Townsend, P.A. Using foliar spectral properties to assess the effects of drought on plant water potential. Tree Physiol. 2017, 37, 1582–1591. [Google Scholar] [CrossRef]
- Santoso, H.; Tani, H.; Wang, X.; Segah, H. Predicting oil palm leaf nutrient contents in kalimantan, indonesia by measuring reflectance with a spectroradiometer. Int. J. Remote Sens. 2019, 40, 7581–7602. [Google Scholar] [CrossRef]
- Pinter, P.J.; Hatfield, J.L.; Schepers, J.S.; Barnes, E.M.; Moran, M.S.; Daughtry, C.S.T.; Upchurch, D.R. Remote sensing for crop management. Photogramm. Eng. Remote Sens. 2003, 69, 647–664. [Google Scholar] [CrossRef]
- Chen, J.; Li, F.; Wang, R.; Fan, Y.; Raza, M.A.; Liu, Q.; Wang, Z.; Cheng, Y.; Wu, X.; Yang, F.; et al. Estimation of nitrogen and carbon content from soybean leaf reflectance spectra using wavelet analysis under shade stress. Comput. Electron. Agric. 2019, 156, 482–489. [Google Scholar] [CrossRef]
- Bruning, B.; Liu, H.; Brien, C.; Berger, B.; Lewis, M.; Garnett, T. The Development of Hyperspectral Distribution Maps to Predict the Content and Distribution of Nitrogen and Water in Wheat (Triticum aestivum). Front. Plant Sci. 2019, 10, 1380. [Google Scholar] [CrossRef] [PubMed]
- Sonobe, R.; Hirono, Y.; Oi, A. Quantifying chlorophyll-aandbcontent in tea leaves using hyperspectral reflectance and deep learning. Remote Sens. Lett. 2020, 11, 933–942. [Google Scholar] [CrossRef]
- Sonobe, R.; Yamashita, H.; Mihara, H.; Morita, A.; Ikka, T. Estimation of Leaf Chlorophyll a, b and Carotenoid Contents and Their Ratios Using Hyperspectral Reflectance. Remote Sens. 2020, 12, 3265. [Google Scholar] [CrossRef]
- Yamashita, H.; Sonobe, R.; Hirono, Y.; Morita, A.; Ikka, T. Dissection of hyperspectral reflectance to estimate nitrogen and chlorophyll contents in tea leaves based on machine learning algorithms. Sci. Rep. 2020, 10, 17360. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Yang, Q.; Han, J.-Y.; Lin, L.; Shi, L.-S. Estimation of Winter Wheat Leaf Water Content Based on Leaf and Canopy Hyperspectral Data. Spectrosc. Spectr. Anal. 2020, 40, 891–897. [Google Scholar] [CrossRef]
- Kong, W.; Huang, W.; Zhou, X.; Mortimer, H.; Ma, L.; Tang, L.; Li, C. Estimating leaf water content at the leaf scale in soybean inoculated with arbuscular mycorrhizal fungi from in situ spectral measurements. Int. J. Agric. Biol. Eng. 2019, 12, 149–155. [Google Scholar] [CrossRef]
- Zhou, X.; Jun, S.; Yan, T.; Bing, L.; Hang, Y.; Quansheng, C. Hyperspectral technique combined with deep learning algorithm for detection of compound heavy metals in lettuce. Food Chem. 2020, 321, 126503. [Google Scholar] [CrossRef]
- Manevski, K.; Manakos, I.; Petropoulos, G.P.; Kalaitzidis, C. Discrimination of common Mediterranean plant species using field spectroradiometry. Int. J. Appl. Earth Obs. Geoinf. 2011, 13, 922–933. [Google Scholar] [CrossRef]
- Serbin, S.P.; Singh, A.; McNeil, B.E.; Kingdon, C.C.; Townsend, P.A. Spectroscopic determination of leaf morphological and biochemical traits for northern temperate and boreal tree species. Ecol. Appl. 2014, 24, 1651–1669. [Google Scholar] [CrossRef]
- Silva-Perez, V.; Molero, G.; Serbin, S.P.; Condon, A.G.; Reynolds, M.P.; Furbank, R.T.; Evans, J.R. Hyperspectral reflectance as a tool to measure biochemical and physiological traits in wheat. J. Exp. Bot. 2018, 69, 483–496. [Google Scholar] [CrossRef]
- Watt, M.S.; Buddenbaum, H.; Leonardo, E.M.C.; Estarija, H.J.; Bown, H.E.; Gomez-Gallego, M.; Hartley, R.J.L.; Pearse, G.D.; Massam, P.; Wright, L.; et al. Monitoring biochemical limitations to photosynthesis in N and P-limited radiata pine using plant functional traits quantified from hyperspectral imagery. Remote Sens. Environ. 2020, 248, 112003. [Google Scholar] [CrossRef]
- Driever, S.M.; Lawson, T.; Andralojc, P.J.; Raines, C.A.; Parry, M.A.J. Natural variation in photosynthetic capacity, growth, and yield in 64 field-grown wheat genotypes. J. Exp. Bot. 2014, 65, 4959–4973. [Google Scholar] [CrossRef]
- Meacham-Hensold, K.; Montes, C.M.; Wu, J.; Guan, K.; Fu, P.; Ainsworth, E.A.; Pederson, T.; Moore, C.E.; Brown, K.L.; Raines, C.; et al. High-throughput field phenotyping using hyperspectral reflectance and partial least squares regression (PLSR) reveals genetic modifications to photosynthetic capacity. Remote Sens. Environ. 2019, 231, 111176. [Google Scholar] [CrossRef]
- Garriga, M.; Romero-Bravo, S.; Estrada, F.; Escobar, A.; Matus, I.A.; del Pozo, A.; Astudillo, C.A.; Lobos, G.A. Assessing Wheat Traits by Spectral Reflectance: Do We Really Need to Focus on Predicted Trait-Values or Directly Identify the Elite Genotypes Group? Front. Plant Sci. 2017, 8, 280. [Google Scholar] [CrossRef]
- Shen, J.; Xiao, Q.; Qiu, H.; Chen, C.; Chen, H. Integrative effect of drought and low temperature on litchi (Litchi chinensis Sonn.) floral initiation revealed by dynamic genome-wide transcriptome analysis. Sci. Rep. 2016, 6, 32005. [Google Scholar] [CrossRef]
- Shah, S.H.; Angel, Y.; Houborg, R.; Ali, S.; McCabe, M.F. A Random Forest Machine Learning Approach for the Retrieval of Leaf Chlorophyll Content in Wheat. Remote Sens. 2019, 11, 920. [Google Scholar] [CrossRef]
- He, J.; Qin, J.; Long, L.; Ma, Y.; Li, H.; Li, K.; Jiang, X.; Liu, T.; Polle, A.; Liang, Z.; et al. Net cadmium flux and accumulation reveal tissue-specific oxidative stress and detoxification in Populus x canescens. Physiol. Plant. 2011, 143, 50–63. [Google Scholar] [CrossRef]
- Rousel, J.; Haas, R.; Schell, J.; Deering, D. Monitoring vegetation systems in the great plains with ERTS. In Proceedings of the Third Earth Resources Technology Satellite Symposium, Washington, DC, USA, 10–14 December 1973; pp. 309–317. [Google Scholar]
- Jordan, C.F.J.E. Derivation of leaf-area index from quality of light on the forest floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Huete, A.; Justice, C.; Liu, H. Development of vegetation and soil indices for MODIS-EOS. Remote Sens. Environ. 1994, 49, 224–234. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Berjón, A.; López-Lozano, R.; Miller, J.R.; Martín, P.; Cachorro, V.; González, M.; De Frutos, A. Assessing vineyard condition with hyperspectral indices: Leaf and canopy reflectance simulation in a row-structured discontinuous canopy. Remote Sens. Environ. 2005, 99, 271–287. [Google Scholar] [CrossRef]
- Mehdaoui, R.; Anane, M. Exploitation of the red-edge bands of Sentinel 2 to improve the estimation of durum wheat yield in Grombalia region (Northeastern Tunisia). Int. J. Remote Sens. 2020, 41, 8984–9006. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-band model for noninvasive estimation of chlorophyll, carotenoids, and anthocyanin contents in higher plant leaves. Geophys. Res. Lett. 2006, 33, L11402. [Google Scholar] [CrossRef]
- Fitzgerald, G.; Rodriguez, D.; O’Leary, G. Measuring and predicting canopy nitrogen nutrition in wheat using a spectral index-The canopy chlorophyll content index (CCCI). Field Crop. Res. 2010, 116, 318–324. [Google Scholar] [CrossRef]
- Kim, M.S.; Daughtry, C.; Chappelle, E.; McMurtrey, J.; Walthall, C. The Use of High Spectral Resolution Bands for Estimating Absorbed Photosynthetically Active Radiation (APAR). In Proceedings of the 6th International Symposium on Physical Measurement and Signatures in Remote Sensing, Val-D’Isere, France, 17–21 January 1994; pp. 299–306. [Google Scholar]
- Dash, J.; Curran, P. The MERIS Terrestrial Chlorophyll Index. Int. J. Remote Sens. 2004, 25, 5003–5013. [Google Scholar] [CrossRef]
- Gamon, J.; Penuelas, J.; Field, C. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Hunt, E.R.; Rock, B.N. Detection of changes in leaf water-content using near-infrared and middle-infrared reflectances. Remote Sens. Environ. 1989, 30, 43–54. [Google Scholar] [CrossRef]
- Penuelas, J.; Pinol, J.; Ogaya, R.; Filella, I. Estimation of plant water concentration by the reflectance water index WI (R900/R970). Int. J. Remote Sens. 1997, 18, 2869–2875. [Google Scholar] [CrossRef]
- Ceccato, P.; Gobron, N.; Flasse, S.; Pinty, B.; Tarantola, S. Designing a spectral index to estimate vegetation water content from remote sensing data: Part 1: Theoretical approach. Remote Sens. Environ. 2002, 82, 188–197. [Google Scholar] [CrossRef]
- Gao, B.C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Pu, R.; Ge, S.; Kelly, N.M.; Gong, P. Spectral absorption features as indicators of water status in coast live oak (Quercus agrifolia) leaves. Int. J. Remote Sens. 2003, 24, 1799–1810. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Zhang, C.; Denka, S.; Cooper, H.; Mishra, D.R. Quantification of sawgrass marsh aboveground biomass in the coastal Everglades using object-based ensemble analysis and Landsat data. Remote Sens. Environ. 2018, 204, 366–379. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, S.; Zhao, F.; Tian, L.; Zhao, Z. Comparison of machine learning algorithms for forest parameter estimations and application for forest quality assessments. For. Ecol. Manag. 2019, 434, 224–234. [Google Scholar] [CrossRef]
- Wang, H.; Lu, K.; Pu, R. Mapping Robinia pseudoacacia forest health in the Yellow River delta by using high-resolution IKONOS imagery and object-based image analysis. J. Appl. Remote Sens. 2016, 10, 045022. [Google Scholar] [CrossRef]
- Mountrakis, G.; Im, J.; Ogole, C. Support vector machines in remote sensing: A review. Isprs J. Photogramm. Remote Sens. 2011, 66, 247–259. [Google Scholar] [CrossRef]
- Were, K.; Bui, D.T.; Dick, O.B.; Singh, B.R. A comparative assessment of support vector regression, artificial neural networks, and random forests for predicting and mapping soil organic carbon stocks across an Afromontane landscape. Ecol. Indic. 2015, 52, 394–403. [Google Scholar] [CrossRef]
- Rodriguez-Galiano, V.; Sanchez-Castillo, M.; Chica-Olmo, M.; Chica-Rivas, M. Machine learning predictive models for mineral prospectivity: An evaluation of neural networks, random forest, regression trees and support vector machines. Ore Geol. Rev. 2015, 71, 804–818. [Google Scholar] [CrossRef]
- Freund, Y.; Schapire, R.E. A decision-theoretic generalization of on-line learning and an application to boosting. J. Comput. Syst. Sci. 1997, 55, 119–139. [Google Scholar] [CrossRef]
- Ashraf, M. Inducing drought tolerance in plants: Recent advances. Biotechnol. Adv. 2010, 28, 169–183. [Google Scholar] [CrossRef]
- Boochs, F.; Kupfer, G.; Dockter, K.; Kuhbauch, W. Shape of the red edge as vitality indicator for plants. Int. J. Remote Sens. 1990, 11, 1741–1753. [Google Scholar] [CrossRef]
- Zovko, M.; Zibrat, U.; Knapic, M.; Kovacic, M.B.; Romic, D. Hyperspectral remote sensing of grapevine drought stress. Precis. Agric. 2019, 20, 335–347. [Google Scholar] [CrossRef]
- Liu, K.; Wang, J.; Zeng, W.; Song, J. Comparison and Evaluation of Three Methods for Estimating Forest above Ground Biomass Using TM and GLAS Data. Remote Sens. 2017, 9, 341. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Ballester, C.; Zarco-Tejada, P.J.; Nicolas, E.; Alarcon, J.J.; Fereres, E.; Intrigliolo, D.S.; Gonzalez-Dugo, V. Evaluating the performance of xanthophyll, chlorophyll and structure-sensitive spectral indices to detect water stress in five fruit tree species. Precis. Agric. 2018, 19, 178–193. [Google Scholar] [CrossRef]
- Gerhards, M.; Schlerf, M.; Rascher, U.; Udelhoven, T.; Juszczak, R.; Alberti, G.; Miglietta, F.; Inoue, Y. Analysis of Airborne Optical and Thermal Imagery for Detection of Water Stress Symptoms. Remote Sens. 2018, 10, 1139. [Google Scholar] [CrossRef]
- Sun, X.P.; Yan, H.L.; Kang, X.Y.; Ma, F.W. Growth, gas exchange, and water-use efficiency response of two young apple cultivars to drought stress in two scion-one rootstock grafting system. Photosynthetica 2013, 51, 404–410. [Google Scholar] [CrossRef]
- Dong, T.; Xi, L.; Xiong, B.; Qiu, X.; Huang, S.; Xu, W.; Wang, J.; Wang, B.; Yao, Y.; Duan, C.; et al. Drought resistance in Harumi tangor seedlings grafted onto different rootstocks. Funct. Plant Biol. 2021, 48, 529–541. [Google Scholar] [CrossRef]
- Govender, M.; Dye, P.J.; Weiersbye, I.M.; Witkowski, E.T.F.; Ahmed, F. Review of commonly used remote sensing and ground-based technologies to measure plant water stress. Water SA 2009, 35, 741–752. [Google Scholar] [CrossRef]
- Panigada, C.; Rossini, M.; Meroni, M.; Cilia, C.; Busettoa, L.; Amaducci, S.; Boschetti, M.; Cogliati, S.; Picchi, V.; Pinto, F.; et al. Fluorescence, PRI and canopy temperature for water stress detection in cereal crops. Int. J. Appl. Earth Obs. Geoinf. 2014, 30, 167–178. [Google Scholar] [CrossRef]
- Suarez, L.; Zarco-Tejada, P.J.; Berni, J.A.J.; Gonzalez-Dugo, V.; Fereres, E. Modelling PRI for water stress detection using radiative transfer models. Remote Sens. Environ. 2009, 113, 730–744. [Google Scholar] [CrossRef]
- Zhao, Y. Principles and Methods of Remote Sensing Application Analysis; Science Press: Beijing, China, 2003; p. 367. [Google Scholar]
- Arbona, V.; Iglesias, D.J.; Jacas, J.; Primo-Millo, E.; Talon, M.; Gomez-Cadenas, A. Hydrogel substrate amendment alleviates drought effects on young citrus plants. Plant Soil 2005, 270, 73–82. [Google Scholar] [CrossRef]
- Osco, L.P.; Marques Ramos, A.P.; Pereira, D.R.; Saito Moriya, E.A.; Imai, N.N.; Matsubara, E.T.; Estrabis, N.; de Souza, M.; Marcato Junior, J.; Goncalves, W.N.; et al. Predicting Canopy Nitrogen Content in Citrus-Trees Using Random Forest Algorithm Associated to Spectral Vegetation Indices from UAV-Imagery. Remote Sens. 2019, 11, 2925. [Google Scholar] [CrossRef]
- Ma, L.; Liu, Y.; Zhang, X.; Ye, Y.; Yin, G.; Johnson, B.A. Deep learning in remote sensing applications: A meta-analysis and review. Isprs J. Photogramm. Remote Sens. 2019, 152, 166–177. [Google Scholar] [CrossRef]
- Zhou, J.; Dian, Y.; Wang, X.; Yao, C.; Jian, Y.; Li, Y.; Han, Z. Comparison of GF2 and SPOT6 Imagery on Canopy Cover Estimating in Northern Subtropics Forest in China. Forests 2020, 11, 407. [Google Scholar] [CrossRef]

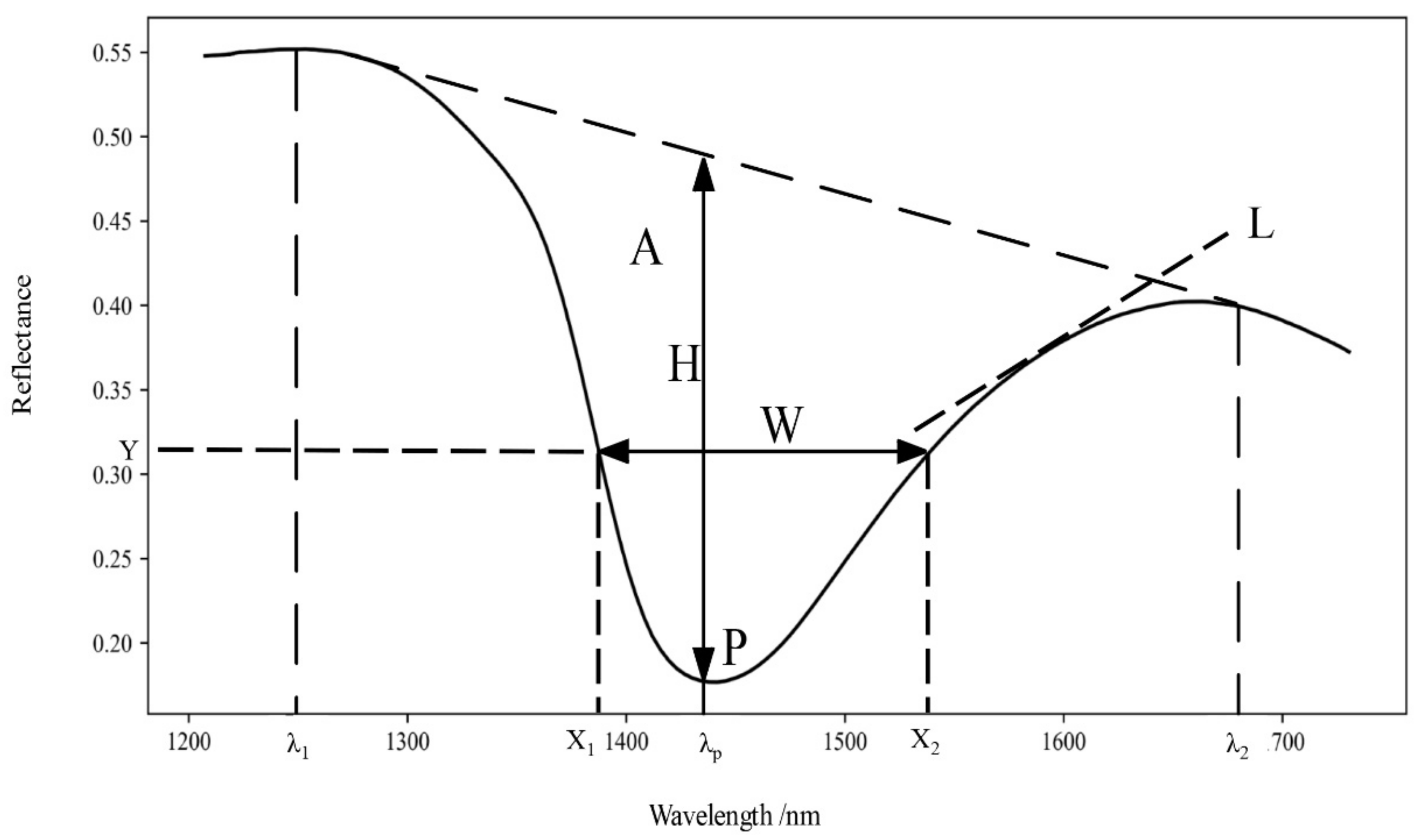

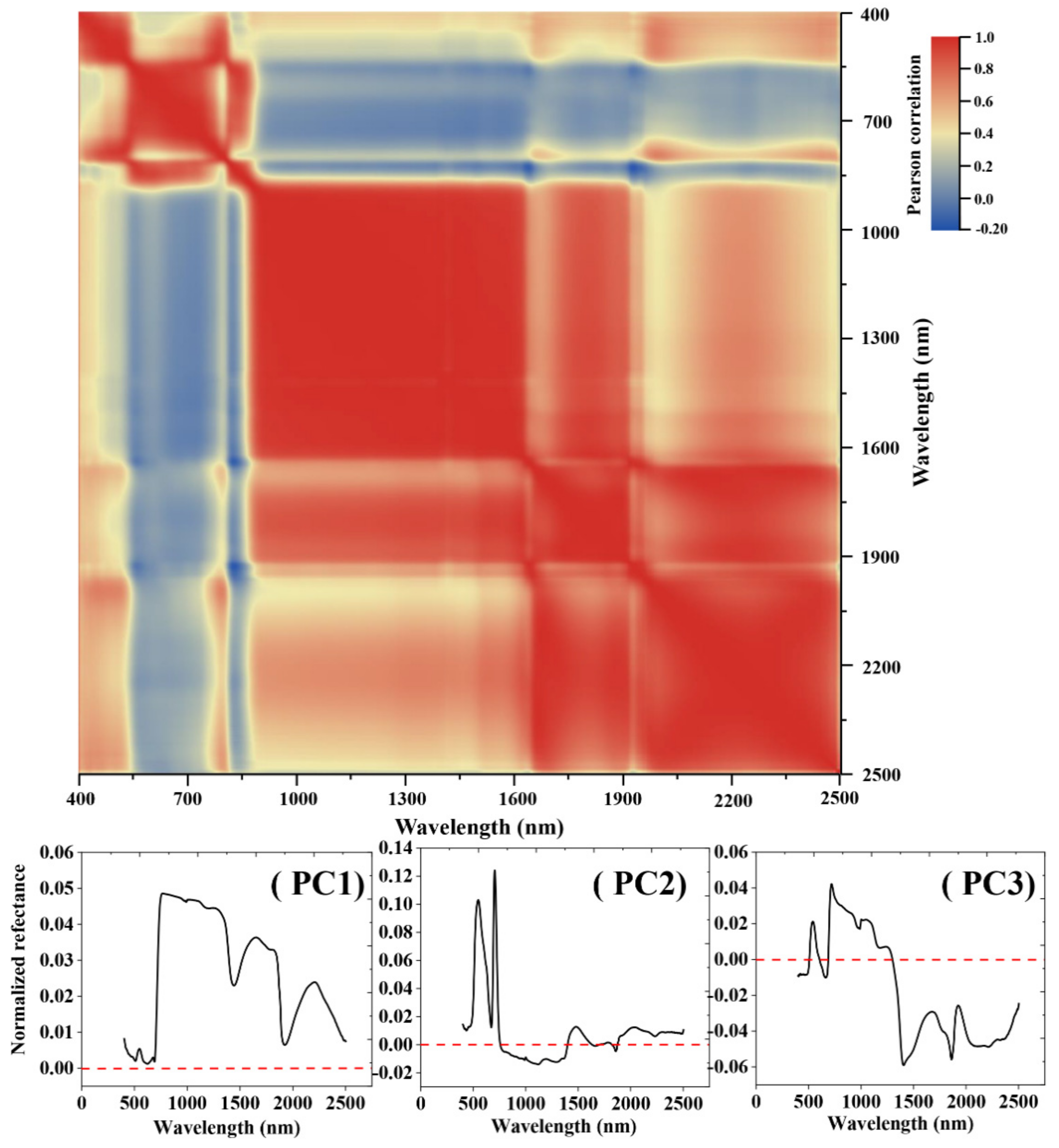
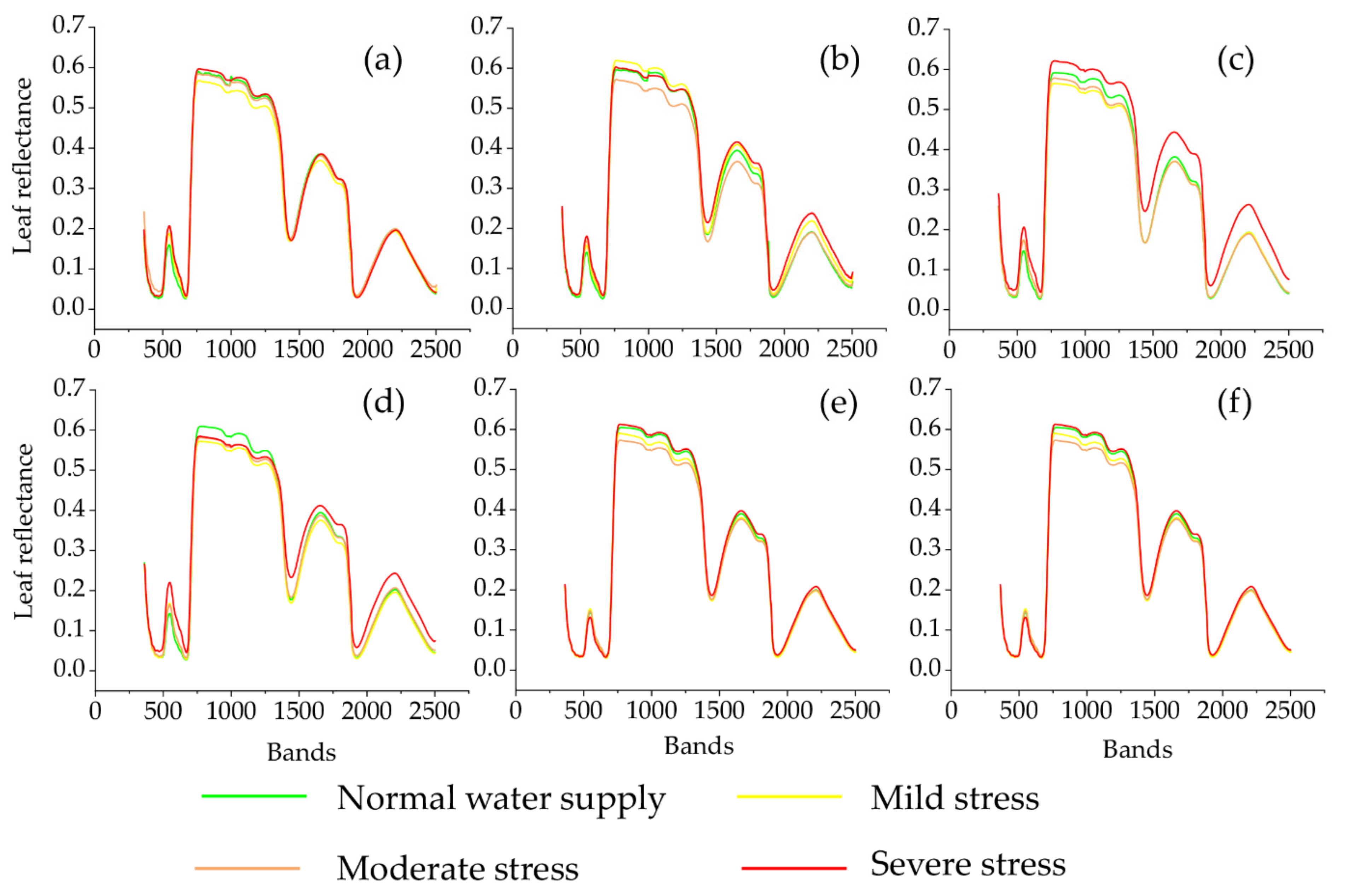
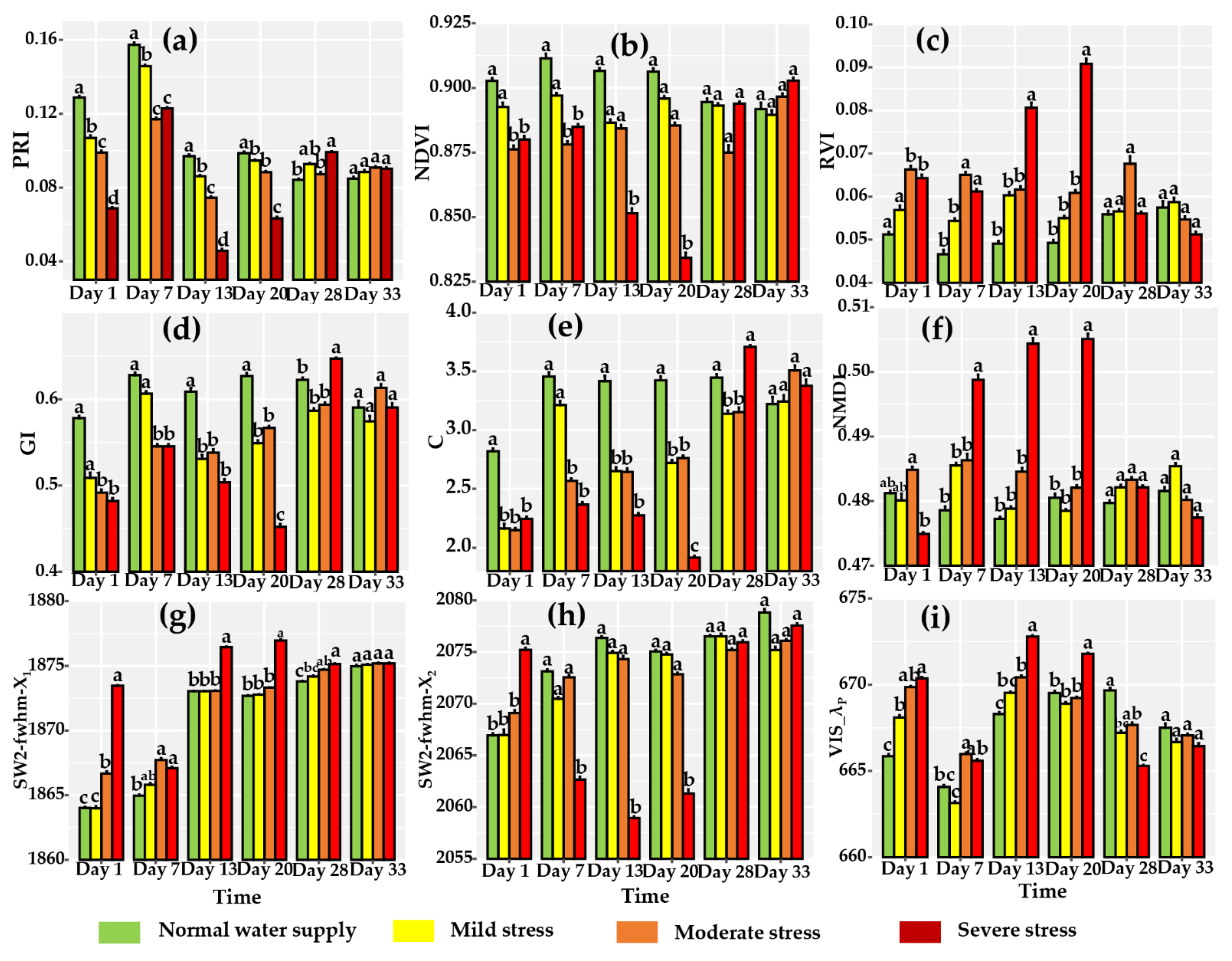
| NO. | Spectral Vegetation Indices | Description | Reference |
|---|---|---|---|
| 1 | Normalized difference vegetation index, NDVI = (R800 − R670)/(R800 + R670) | Structure: greenness, vegetation cover, biomass, LAI and fraction of photosynthetic active radiation | [48] |
| 2 | Ratio vegetation index, RVI = R800/R670 | [49] | |
| 3 | Enhanced Vegetation Index, EVI = 2.5 × (R800 − R680)/(R800 + 6 × R680 − 7.5 × R450 + 1) | [50] | |
| 4 | Greenness Index, GI = R554/R667 | Pigments: Chlorophyll, carotenoids, and anthocyanin. | [51] |
| 5 | Red Edge model, CI730 = R800/R730 − 1.0 | [52] | |
| 6 | Red Edge model, CI709 = R800/R709 − 1.0 | ||
| 7 | Chrollophy Index at Green band, ch1green = R800/R550 − 1.0 | [53] | |
| 8 | Normalized Difference Red Edge, NDRE = (R790 − R720)/(R790 + R720) | [54] | |
| 9 | Red and Green Vegetation Index, RGVI = R550/R670 | [49] | |
| 10 | CARI_a = (R700 − R550)/150 CARI _b = R550 − CARIa × 500 | [55] | |
| Chlorophyll Absorption Ratio Index, CARI = CAR × R700/R670 | |||
| 11 | MERIS Terrestrial Chlorophyll Index, MTCI = (R754 − R709)/(R709 − R681) | [56] | |
| 12 | Photochemical Reflectance Index, PRI = (R570 − R531)/(R570 + R531) | Photosynthetic activity | [57] |
| 13 | Photochemical Reflectance Index Improved, PRI2 = (R528 − R567)/(R528 + R567) | ||
| 14 | Moisture Stress Index, MSI = R1600/R820 | Water content | [58] |
| 15 | Water Index, WI = R900/R970 | [59] | |
| 16 | Normalized Multi-band Drought Index, NMDI = (R860 − R1640 + R2130)/(R860 + R1640 − R2130) | [54] | |
| 17 | Global Vegetation Moisture Index, GVMI = (R820 + 0.1 − R1600 − 0.02)/(R820 + 0.1 + R1600 + 0.02) | [60] | |
| 18 | Normalized Difference Water Index, NDWI1200 = (R886 − R1200)/(R886 − R1200) | [61] | |
| 19 | Normalized Difference Water Index, NDWI1240 = (R886 − R1240)/(R886 − R1240) | [61] | |
| 20 | Normalized Difference Water Index, NDWI1640 = (R886 − R1640)/(R886 − R1640) | [61] |
| Parameter | Normal Water Supply | Mild Stress | Moderate Stress | Severe Stress |
|---|---|---|---|---|
| GI | 0.6228 ± 0.0027 b | 0.5870 ± 0.0027 b | 0.5937 ± 0.0028 b | 0.6473 ± 0.0013 a |
| CI730 | 0.2040 ± 0.0020 a | 0.1617 ± 0.0021 c | 0.1742 ± 0.0016 bc | 0.2024 ± 0.0011 ab |
| CI709 | 1.1799 ± 0.012 ab | 0.9835 ± 0.011 c | 1.0303 ± 0.0010 bc | 1.2230 ± 0.0067 a |
| CIG | 3.3888 ± 0.038 ab | 2.9082 ± 0.031 b | 3.0010 ± 0.036 b | 3.6980 ± 0.022 a |
| NDRE | 0.09232 ± 0.00082 a | 0.07453 ± 0.00091 c | 0.07998 ± 0.00065 bc | 0.09182 ± 0.00047 ab |
| rg | 0.1418 ± 0.00011 ab | 0.1539 ± 0.00086 a | 0.1478 ± 0.00012 ab | 0.1329 ± 0.00082 b |
| CARI | 1.1168 ± 0.024 ab | 1.2758 ± 0.018 a | 1.0828 ± 0.017 ab | 0.9431 ± 0.0072 b |
| MCTI | 1.3550 ± 0.015 ab | 1.1412 ± 0.013 c | 1.2204 ± 0.012 bc | 1.4350 ± 0.0075 a |
| PRI | 0.08426 ± 0.00059 b | 0.09272 ± 0.00063 ab | 0.08426 ± 0.00059 b | 0.09925 ± 0.00035 a |
| VIS-λ2 | 745.7833 ± 0.073 a | 743.9444 ± 0.1051 c | 744.3944 ± 0.076 bc | 745.4222 ± 0.043 ab |
| VIS-λP | 669.6667 ± 0.15 a | 667.2111 ± 0.13 bc | 667.6667 ± 0.14 ab | 665.2833 ± 0.065 c |
| VIS-Area | 118.6700 ± 0.24 ab | 116.47 ± 0.24 ab | 115.10900.4322 ± 0.43 b | 120.87 ± 0.16 a |
| VIS-symmetry | 0.6914 ± 0.0012 a | 0.6800 ± 0.00096 a | 0.6795 ± 0.0010 a | 0.6613 ± 0.00043 b |
| VIS-slope | 0.002289 ± 0.000012 ab | 0.002182 ± 0.000013 b | 0.002120 ± 0.0000081 b | 0.002386 ± 0.0000048 a |
| VIS-fwhm-X1 | 573.3167 ± 0.10 ab | 572.0278 ± 0.11 a | 572.1167 ± 0.13 a | 569.6222 ± 0.039 b |
| VIS-fwhm-Y | 138.0944 ± 0.22 ab | 135.5389 ± 0.24 b | 136.1056 ± 0.22 b | 140.5556 ± 0.077 a |
| SW1-λ1 | 1281.6778 ± 0.10 b | 1282.5222 ± 0.14 b | 1282.5222 ± 0.10 b | 1284.8444 ± 0.088 a |
| SW2-λ1 | 1818.1444 ± 0.085 b | 1818.3333 ± 0.089 b | 1819.8444 ± 0.079 a | 1820.4111 ± 0.076 a |
| SW2-cslope | −0.0003405 ± 1.58 × 10−6 b | −0.0003277 ± 1.6 × 10−6 ab | −0.0003189 ± 7.9 × 10−8 a | −0.0003425 ± 8.2 × 10−7 b |
| SW2-fwhm-X1 | 1838.8 ± 0.045 c | 1874.1833 ± 0.070 bc | 1874.1 ± 0.042 ab | 1875.1444 ± 0.044 a |
| C | 3.4474 ± 0.030 ab | 3.1406 ± 0.028 b | 3.1544 ± 0.039 b | 3.7082 ± 0.015 a |
| λg | 543.4862 ± 0.022 a | 543.0308 ± 0.016 b | 543.1019 ±0.028 b | 542.8853 ± 0.0059 b |
| λ0 | 676.0881 ± 0.051 ab | 675.2125 ± 0.052 c | 6754985 ± 0.045 bc | 676.3048 ± 0.025 c |
| σ | 29.6841 ± 0.053 a | 28.7165 ± 0.063 b | 29.1676 ± 0.041 ab | 29.8467 ± 0.024 a |
| Summary | Pn (μmol CO2 m−2s−1) | Cond (mol HO2 m−2s−1) | Trmmol (mmol HO2 m−2s−1) |
|---|---|---|---|
| Mean | 4.53 | 0.089 | 3.00 |
| SD | 2.85 | 0.069 | 2.21 |
| Median | 4.15 | 0.073 | 2.58 |
| Maximum | 12.51 | 0.36 | 11.61 |
| Minimum | 0.022 | 0.0014 | 0.037 |
| Coefficient Variation | 63.02 | 77.79 | 73.70 |
| AdaBoost | GDBoost | RF | SVM | ||
|---|---|---|---|---|---|
| Pn | R2 | 0.69 | 0.99 | 0.92 | 0.64 |
| RMSE | 1.84 | 2.55 | 1.86 | 1.91 | |
| MAE | 1.53 | 1.95 | 1.51 | 1.53 | |
| Cond | R2 | 0.52 | 0.99 | 0.89 | 0.28 |
| RMSE | 0.056 | 0.068 | 0.049 | 0.055 | |
| MAE | 0.045 | 0.049 | 0.038 | 0.045 | |
| Trmmol | R2 | 0.49 | 0.99 | 0.88 | 0.50 |
| RMSE | 2.078 | 2.48 | 1.88 | 2.06 | |
| MAE | 1.65 | 1.74 | 1.34 | 1.46 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.-J.; Zhang, Y.-H.; Han, Z.-M.; Liu, X.-Y.; Jian, Y.-F.; Hu, C.-G.; Dian, Y.-Y. Evaluating the Performance of Hyperspectral Leaf Reflectance to Detect Water Stress and Estimation of Photosynthetic Capacities. Remote Sens. 2021, 13, 2160. https://doi.org/10.3390/rs13112160
Zhou J-J, Zhang Y-H, Han Z-M, Liu X-Y, Jian Y-F, Hu C-G, Dian Y-Y. Evaluating the Performance of Hyperspectral Leaf Reflectance to Detect Water Stress and Estimation of Photosynthetic Capacities. Remote Sensing. 2021; 13(11):2160. https://doi.org/10.3390/rs13112160
Chicago/Turabian StyleZhou, Jing-Jing, Ya-Hao Zhang, Ze-Min Han, Xiao-Yang Liu, Yong-Feng Jian, Chun-Gen Hu, and Yuan-Yong Dian. 2021. "Evaluating the Performance of Hyperspectral Leaf Reflectance to Detect Water Stress and Estimation of Photosynthetic Capacities" Remote Sensing 13, no. 11: 2160. https://doi.org/10.3390/rs13112160
APA StyleZhou, J.-J., Zhang, Y.-H., Han, Z.-M., Liu, X.-Y., Jian, Y.-F., Hu, C.-G., & Dian, Y.-Y. (2021). Evaluating the Performance of Hyperspectral Leaf Reflectance to Detect Water Stress and Estimation of Photosynthetic Capacities. Remote Sensing, 13(11), 2160. https://doi.org/10.3390/rs13112160







