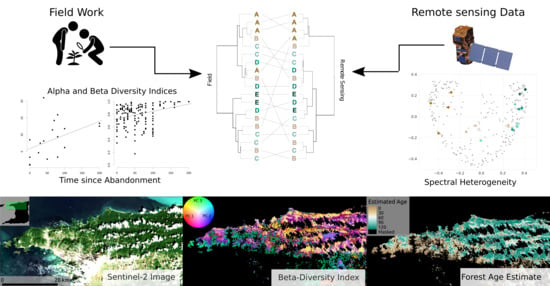
A Remote Sensing Approach to Understanding Patterns of Secondary Succession in Tropical Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Field Plot Network
2.2. Survey Methods
2.3. Satellite Imaging Surveys
2.4. Diversity Indices Computed from Ground Inventories
2.4.1. Alpha-Diversity
2.4.2. Beta-Diversity
2.4.3. Remote Sensing Analyses
2.5. Comparison between Ground Observations and Remotely Sensed Information
2.5.1. Spatial Sampling of Satellite Images
2.5.2. Alpha and Beta-Diversity Analyses
2.5.3. Mapping Forest Age
3. Results
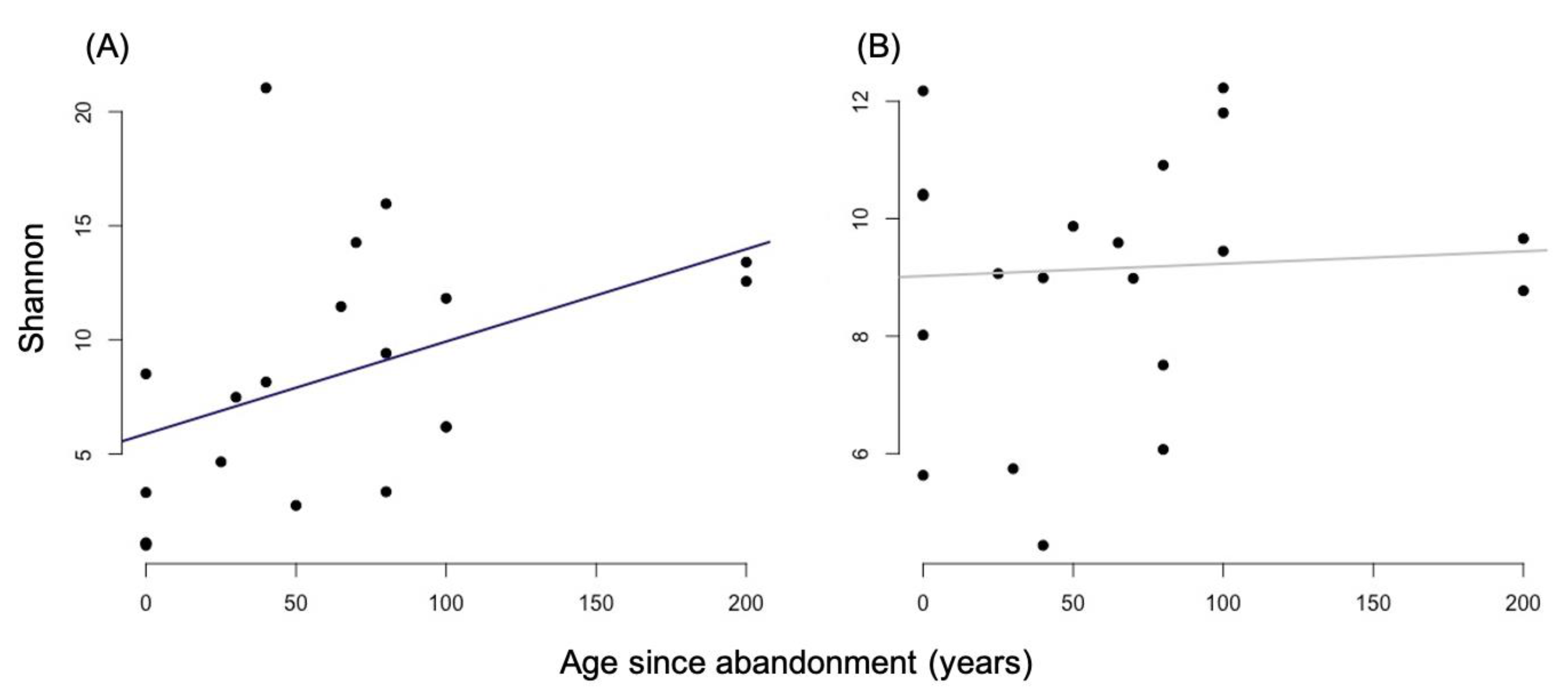
3.1. Alpha Diversity
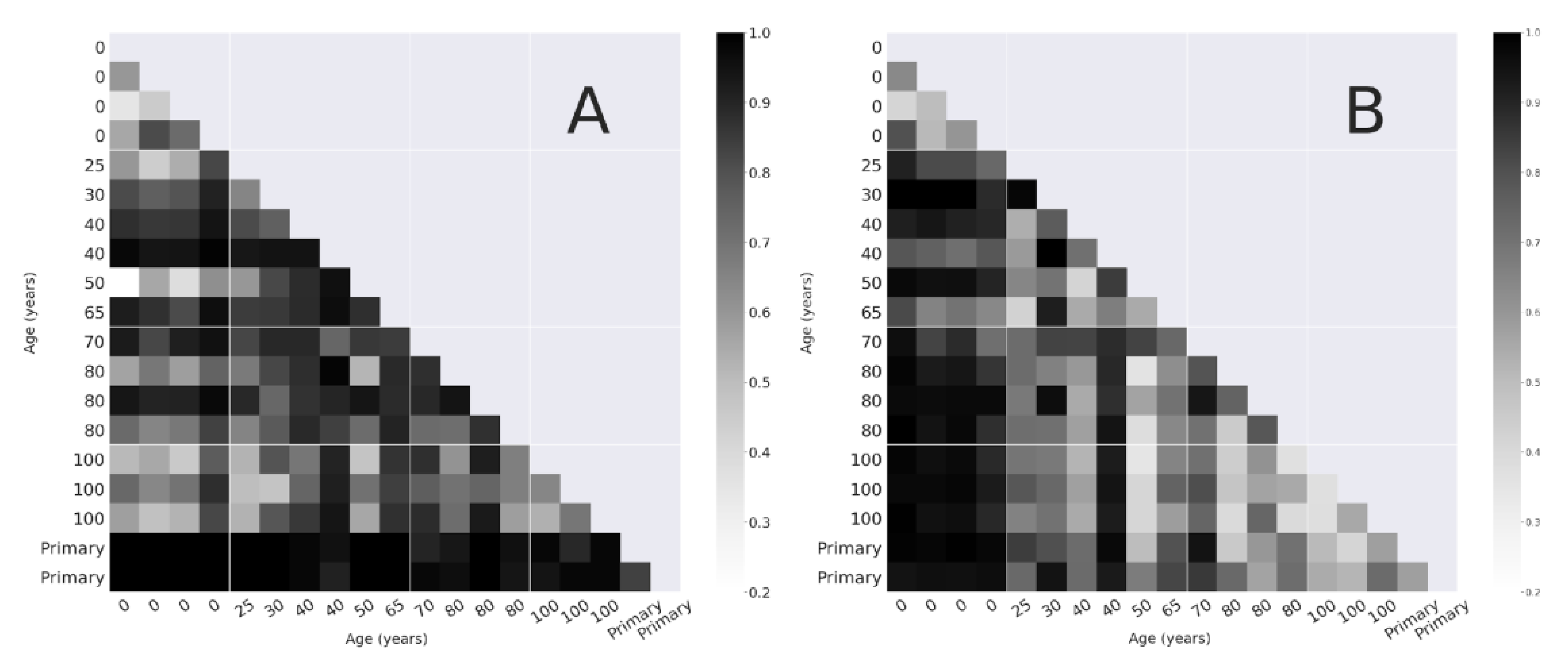
3.2. Beta Diversity
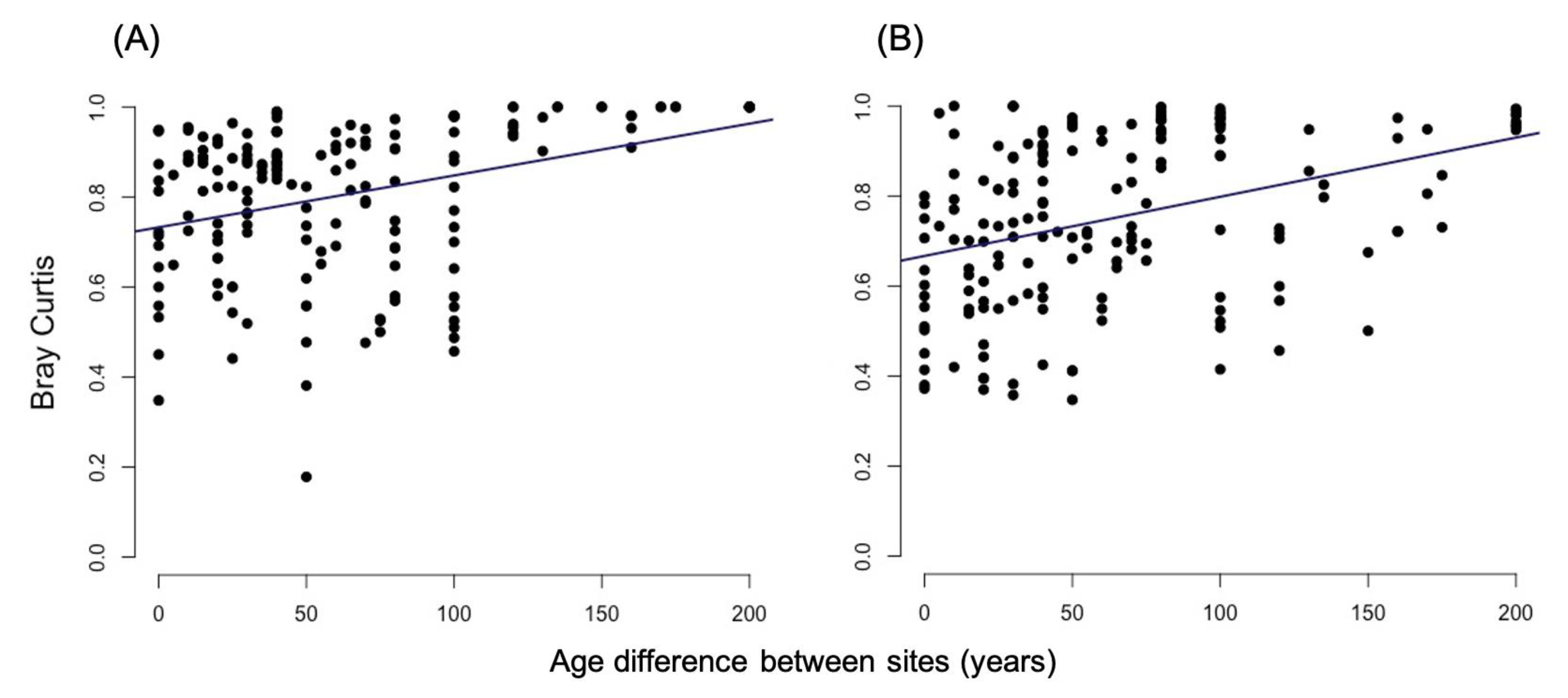
3.2.1. Bray-Curtis Dissimilarity
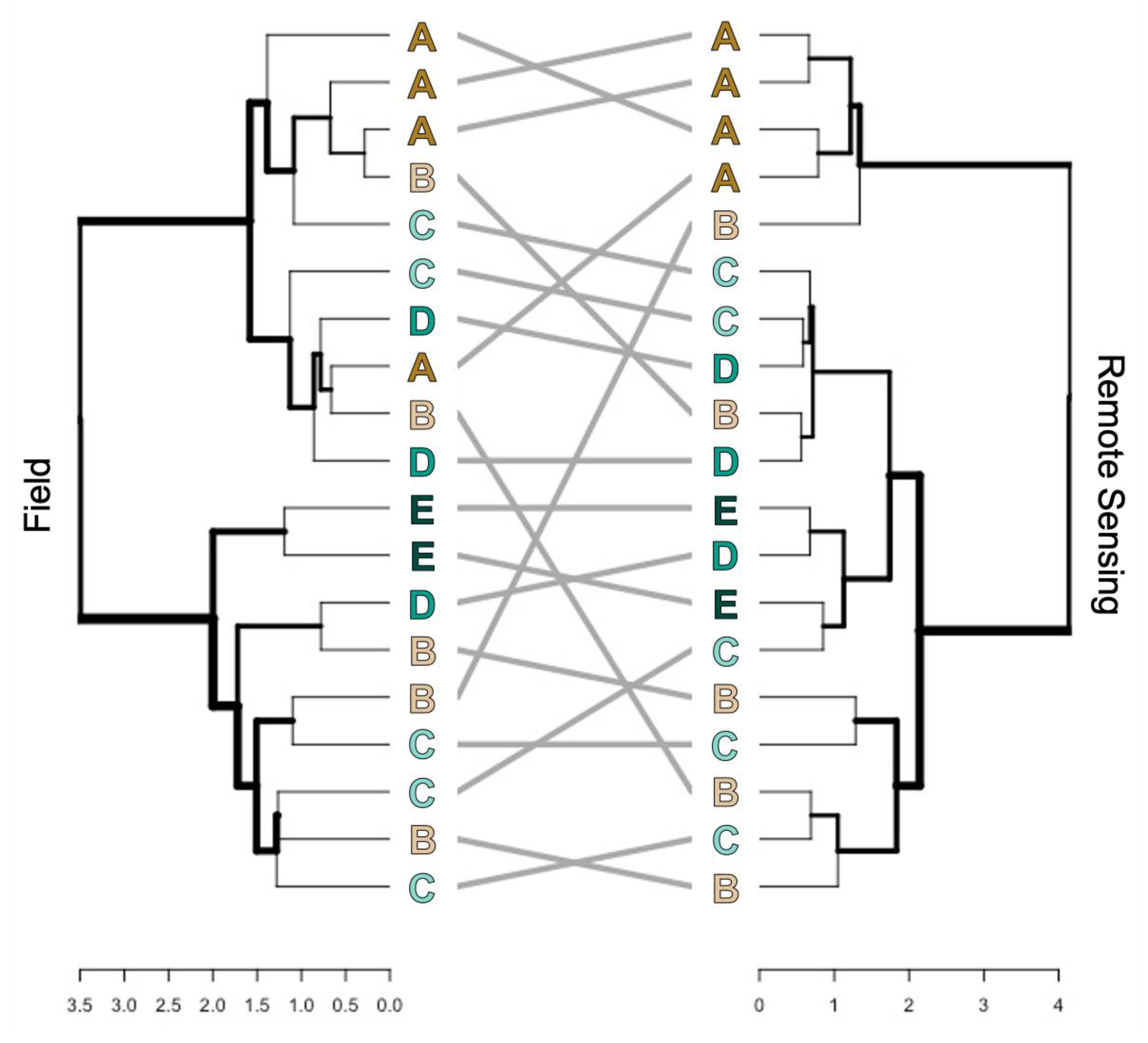
3.2.2. Ordination of Bray Curtis Dissimilarity Computed from Sentinel-2
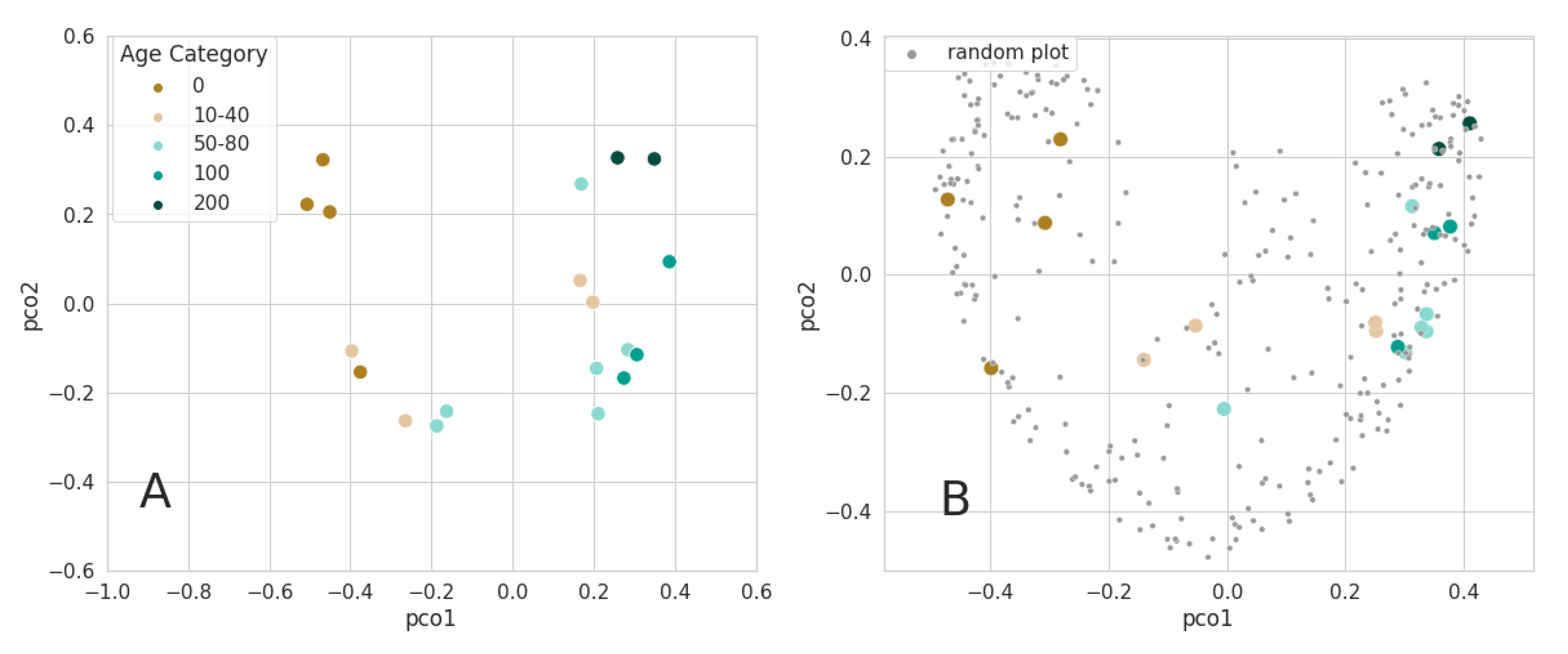
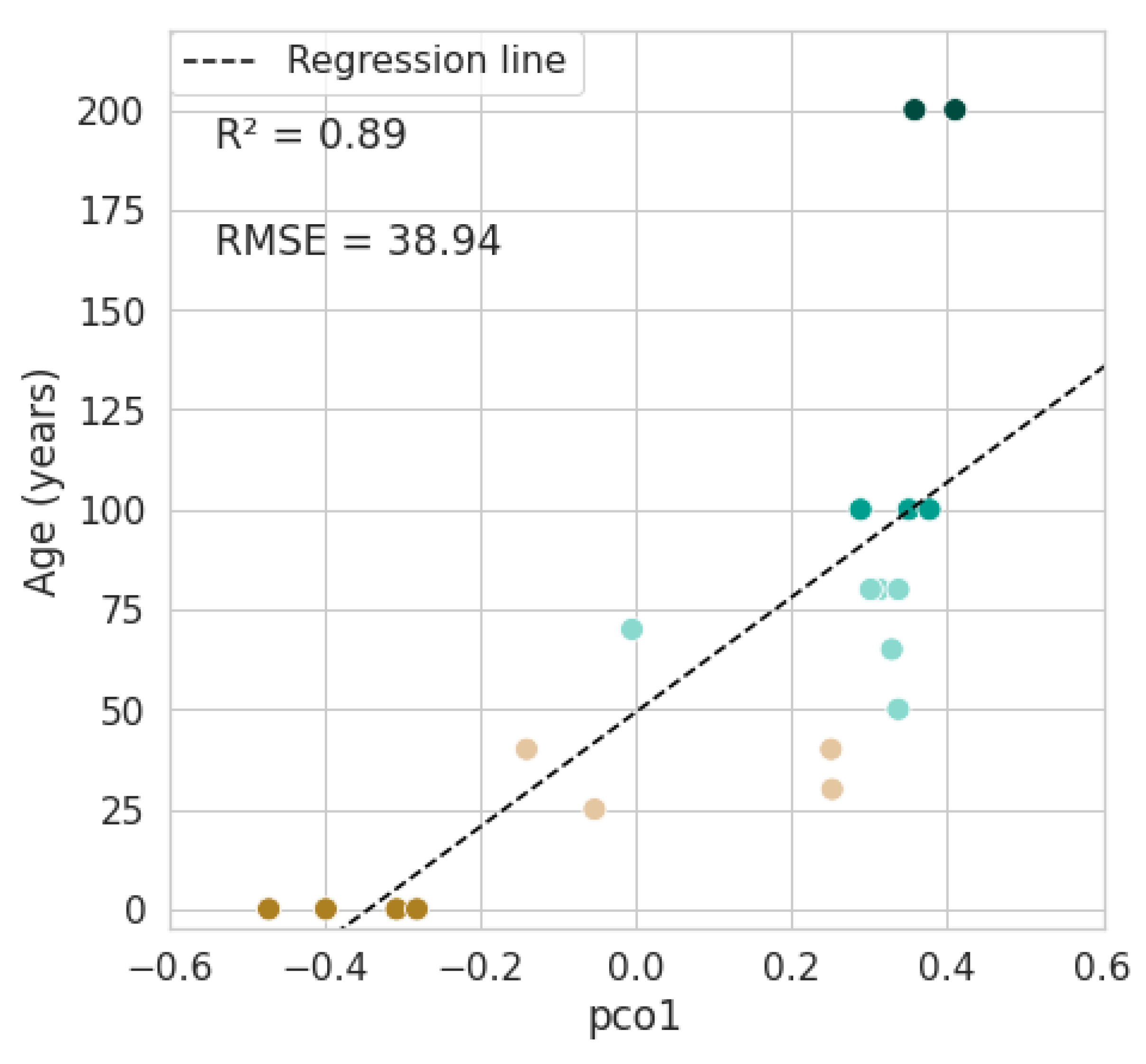
3.3. Mapping Forest Age
4. Discussion
4.1. Alpha and Beta-Diversity Comparison
4.2. Mapping Forest Age
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Blowes, S.A.; Supp, S.R.; Antão, L.H.; Bates, A.; Bruelheide, H.; Chase, J.M.; Moyes, F.; Magurran, A.; McGill, B.; Myers-Smith, I.H.; et al. The Geography of Biodiversity Change in Marine and Terrestrial Assemblages. Science 2019, 366, 339–345. [Google Scholar] [CrossRef]
- Isbell, F.; Gonzalez, A.; Loreau, M.; Cowles, J.; Díaz, S.; Hector, A.; Mace, G.M.; Wardle, D.A.; O’Connor, M.I.; Duffy, J.E.; et al. Linking the Influence and Dependence of People on Biodiversity across Scales. Nature 2017, 546, 65–72. [Google Scholar] [CrossRef]
- Magurran, A.E.; Dornelas, M. Biological Diversity in a Changing World. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3593–3597. [Google Scholar] [CrossRef]
- Pereira, H.M.; Rosa, I.M.D.; Martins, I.S.; Kim, H.; Leadley, P.; Popp, A.; van Vuuren, D.P.; Hurtt, G.; Anthoni, P.; Arneth, A.; et al. Global Trends in Biodiversity and Ecosystem Services from 1900 to 2050. Biorvix 2020. [Google Scholar] [CrossRef]
- Diamond, J.M. “Normal” extinctions of isolated populations. In Extinctions; University of Chicago Press: Chicago, IL, USA, 1984; pp. 191–246. [Google Scholar]
- Ehrlich, P.R.; Pringle, R.M. Where Does Biodiversity Go from Here? A Grim Business-as-Usual Forecast and a Hopeful Portfolio of Partial Solutions. Proc. Natl. Acad. Sci. USA 2008, 105, 11579–11586. [Google Scholar] [CrossRef] [PubMed]
- Semper-Pascual, A.; Decarre, J.; Baumann, M.; Busso, J.M.; Camino, M.; Gómez-Valencia, B.; Kuemmerle, T. Biodiversity Loss in Deforestation Frontiers: Linking Occupancy Modelling and Physiological Stress Indicators to Understand Local Extinctions. Biol. Conserv. 2019, 236, 281–288. [Google Scholar] [CrossRef]
- Dornelas, M.; Gotelli, N.J.; McGill, B.; Shimadzu, H.; Moyes, F.; Sievers, C.; Magurran, A.E. Assemblage Time Series Reveal Biodiversity Change but Not Systematic Loss. Science 2014, 344, 296–299. [Google Scholar] [CrossRef]
- McGill, B.J.; Dornelas, M.; Gotelli, N.J.; Magurran, A.E. Fifteen Forms of Biodiversity Trend in the Anthropocene. Trends Ecol. Evol. 2015, 30, 104–113. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global Effects of Land Use on Local Terrestrial Biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity Loss and Its Impact on Humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef]
- The Strategic Plan for Biodiversity 2011–2020 and the Aichi Biodiversity Targets. Available online: https://www.cbd.int/sp/ (accessed on 1 March 2021).
- Gaston, K.J. Global Patterns in Biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R.; Honnay, O. Forest Restoration, Biodiversity and Ecosystem Functioning. BMC Ecol. 2011, 11, 29. [Google Scholar] [CrossRef]
- Betts, M.G.; Wolf, C.; Ripple, W.J.; Phalan, B.; Millers, K.A.; Duarte, A.; Butchart, S.H.M.; Levi, T. Global Forest Loss Disproportionately Erodes Biodiversity in Intact Landscapes. Nature 2017, 547, 441–444. [Google Scholar] [CrossRef]
- Morales-Hidalgo, D.; Oswalt, S.N.; Somanathan, E. Status and Trends in Global Primary Forest, Protected Areas, and Areas Designated for Conservation of Biodiversity from the Global Forest Resources Assessment 2015. For. Ecol. Manag. 2015, 352, 68–77. [Google Scholar] [CrossRef]
- Myers, N. The Conversion of Tropical Forests. Environ. Sci. Policy Sustain. Dev. 1980, 22, 6–13. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Shvidenko, A.; McCallum, I.; Nilsson, S. Forest and woodlands systems. In Ecosystems and Human Well-Being: Current State and Trends; Hassan, R., Scholes, R., Ash, N., Eds.; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Global Forest Watch Tree Cover Loss and Gain (Global). Available online: www.globalforestwatch.org (accessed on 20 March 2021).
- Arroyo-Rodríguez, V.; Melo, F.P.L.; Martínez-Ramos, M.; Bongers, F.; Chazdon, R.L.; Meave, J.A.; Norden, N.; Santos, B.A.; Leal, I.R.; Tabarelli, M. Multiple Successional Pathways in Human-Modified Tropical Landscapes: New Insights from Forest Succession, Forest Fragmentation and Landscape Ecology Research. Biol. Rev. 2017, 92, 326–340. [Google Scholar] [CrossRef]
- Derroire, G.; Balvanera, P.; Castellanos-Castro, C.; Decocq, G.; Kennard, D.K.; Lebrija-Trejos, E.; Leiva, J.A.; Odén, P.-C.; Powers, J.S.; Rico-Gray, V.; et al. Resilience of Tropical Dry Forests—A Meta-Analysis of Changes in Species Diversity and Composition during Secondary Succession. Oikos 2016, 125, 1386–1397. [Google Scholar] [CrossRef]
- Lebrija-Trejos, E.; Bongers, F.; Pérez-García, E.A.; Meave, J.A. Successional Change and Resilience of a Very Dry Tropical Deciduous Forest Following Shifting Agriculture. Biotropica 2008, 40, 422–431. [Google Scholar] [CrossRef]
- Martin, P.A.; Newton, A.C.; Bullock, J.M. Carbon Pools Recover More Quickly than Plant Biodiversity in Tropical Secondary Forests. Proc. R. Soc. B Biol. Sci. 2013, 280, 20132236. [Google Scholar] [CrossRef] [PubMed]
- Bernier, P.Y.; Paré, D.; Stinson, G.; Bridge, S.R.J.; Kishchuk, B.E.; Lemprière, T.C.; Thiffault, E.; Titus, B.D.; Vasbinder, W. Moving beyond the Concept of “Primary Forest” as a Metric of Forest Environment Quality. Ecol. Appl. 2017, 27, 349–354. [Google Scholar] [CrossRef]
- Rozendaal, D.M.A.; Bongers, F.; Aide, T.M.; Alvarez-Dávila, E.; Ascarrunz, N.; Balvanera, P.; Becknell, J.M.; Bentos, T.V.; Brancalion, P.H.S.; Cabral, G.A.L.; et al. Biodiversity Recovery of Neotropical Secondary Forests. Sci. Adv. 2019, 5, eaau3114. [Google Scholar] [CrossRef] [PubMed]
- Levrel, H.; Fontaine, B.; Henry, P.-Y.; Jiguet, F.; Julliard, R.; Kerbiriou, C.; Couvet, D. Balancing State and Volunteer Investment in Biodiversity Monitoring for the Implementation of CBD Indicators: A French Example. Ecol. Econ. 2010, 69, 1580–1586. [Google Scholar] [CrossRef]
- Schiller, A.; Hunsaker, C.T.; Kane, M.A.; Wolfe, A.K.; Dale, V.H.; Suter, G.W.; Russell, C.S.; Pion, G.; Jensen, M.H.; Konar, V.C. Communicating Ecological Indicators to Decision Makers and the Public. Conserv. Ecol. 2001, 5, art19. [Google Scholar] [CrossRef]
- Barton, P.S.; Cunningham, S.A.; Manning, A.D.; Gibb, H.; Lindenmayer, D.B.; Didham, R.K. The Spatial Scaling of Beta Diversity: Spatial Scaling of Beta Diversity. Glob. Ecol. Biogeogr. 2013, 22, 639–647. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Gonzalez, A.; Allington, G.R.H.; Loreau, M. Is Local Biodiversity Declining or Not? A Summary of the Debate over Analysis of Species Richness Time Trends. Biol. Conserv. 2018, 219, 175–183. [Google Scholar] [CrossRef]
- Proença, V.; Martin, L.J.; Pereira, H.M.; Fernandez, M.; McRae, L.; Belnap, J.; Böhm, M.; Brummitt, N.; García-Moreno, J.; Gregory, R.D.; et al. Global Biodiversity Monitoring: From Data Sources to Essential Biodiversity Variables. Biol. Conserv. 2017, 213, 256–263. [Google Scholar] [CrossRef]
- Anderson, C.B. Biodiversity Monitoring, Earth Observations and the Ecology of Scale. Ecol. Lett. 2018, 21, 1572–1585. [Google Scholar] [CrossRef]
- Luque, S.; Pettorelli, N.; Vihervaara, P.; Wegmann, M. Improving Biodiversity Monitoring Using Satellite Remote Sensing to Provide Solutions towards the 2020 Conservation Targets. Methods Ecol. Evol. 2018, 9, 1784–1786. [Google Scholar] [CrossRef]
- Mulatu, K.; Mora, B.; Kooistra, L.; Herold, M. Biodiversity Monitoring in Changing Tropical Forests: A Review of Approaches and New Opportunities. Remote Sens. 2017, 9, 1059. [Google Scholar] [CrossRef]
- Rocchini, D.; Salvatori, N.; Beierkuhnlein, C.; Chiarucci, A.; de Boissieu, F.; Förster, M.; Garzon-Lopez, C.X.; Gillespie, T.W.; Hauffe, H.C.; He, K.S.; et al. From Local Spectral Species to Global Spectral Communities: A Benchmark for Ecosystem Diversity Estimate by Remote Sensing. Ecol. Inform. 2021, 61, 101195. [Google Scholar] [CrossRef]
- Wang, R.; Gamon, J.A. Remote Sensing of Terrestrial Plant Biodiversity. Remote Sens. Environ. 2019, 231, 111218. [Google Scholar] [CrossRef]
- Rocchini, D.; Boyd, D.S.; Féret, J.-B.; Foody, G.M.; He, K.S.; Lausch, A.; Nagendra, H.; Wegmann, M.; Pettorelli, N. Satellite Remote Sensing to Monitor Species Diversity: Potential and Pitfalls. Remote Sens. Ecol. Conserv. 2016, 2, 25–36. [Google Scholar] [CrossRef]
- Baldeck, C.A.; Asner, G.P.; Martin, R.E.; Anderson, C.B.; Knapp, D.E.; Kellner, J.R.; Wright, S.J. Operational Tree Species Mapping in a Diverse Tropical Forest with Airborne Imaging Spectroscopy. PLoS ONE 2015, 10, e0118403. [Google Scholar] [CrossRef]
- Clark, M.; Roberts, D.; Clark, D. Hyperspectral Discrimination of Tropical Rain Forest Tree Species at Leaf to Crown Scales. Remote Sens. Environ. 2005, 96, 375–398. [Google Scholar] [CrossRef]
- Féret, J.-B.; Asner, G.P. Tree Species Discrimination in Tropical Forests Using Airborne Imaging Spectroscopy. IEEE Trans. Geosci. Remote Sens. 2013, 51, 73–84. [Google Scholar] [CrossRef]
- Draper, F.C.; Baraloto, C.; Brodrick, P.G.; Phillips, O.L.; Martinez, R.V.; Honorio Coronado, E.N.; Baker, T.R.; Zárate Gómez, R.; Amasifuen Guerra, C.A.; Flores, M.; et al. Imaging Spectroscopy Predicts Variable Distance Decay across Contrasting Amazonian Tree Communities. J. Ecol. 2019, 107, 696–710. [Google Scholar] [CrossRef]
- Vaglio Laurin, G.; Puletti, N.; Hawthorne, W.; Liesenberg, V.; Corona, P.; Papale, D.; Chen, Q.; Valentini, R. Discrimination of Tropical Forest Types, Dominant Species, and Mapping of Functional Guilds by Hyperspectral and Simulated Multispectral Sentinel-2 Data. Remote Sens. Environ. 2016, 176, 163–176. [Google Scholar] [CrossRef]
- Schneider, F.D.; Morsdorf, F.; Schmid, B.; Petchey, O.L.; Hueni, A.; Schimel, D.S.; Schaepman, M.E. Mapping Functional Diversity from Remotely Sensed Morphological and Physiological Forest Traits. Nat. Commun. 2017, 8, 1441. [Google Scholar] [CrossRef] [PubMed]
- Asner, G.P.; Martin, R.E.; Knapp, D.E.; Tupayachi, R.; Anderson, C.B.; Sinca, F.; Vaughn, N.R.; Llactayo, W. Airborne Laser-Guided Imaging Spectroscopy to Map Forest Trait Diversity and Guide Conservation. Science 2017, 355, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Durán, S.M.; Martin, R.E.; Díaz, S.; Maitner, B.S.; Malhi, Y.; Salinas, N.; Shenkin, A.; Silman, M.R.; Wieczynski, D.J.; Asner, G.P.; et al. Informing Trait-Based Ecology by Assessing Remotely Sensed Functional Diversity across a Broad Tropical Temperature Gradient. Sci. Adv. 2019, 5, eaaw8114. [Google Scholar] [CrossRef]
- Carreiras, J.M.B.; Jones, J.; Lucas, R.M.; Shimabukuro, Y.E. Mapping Major Land Cover Types and Retrieving the Age of Secondary Forests in the Brazilian Amazon by Combining Single-Date Optical and Radar Remote Sensing Data. Remote Sens. Environ. 2017, 194, 16–32. [Google Scholar] [CrossRef]
- Fujiki, S.; Aoyagi, R.; Tanaka, A.; Imai, N.; Kusma, A.D.; Kurniawan, Y.; Lee, Y.F.; Sugau, J.B.; Pereira, J.T.; Samejima, H.; et al. Large-Scale Mapping of Tree-Community Composition as a Surrogate of Forest Degradation in Bornean Tropical Rain Forests. Land 2016, 5, 45. [Google Scholar] [CrossRef]
- Palmer, M.W.; Earls, P.G.; Hoagland, B.W.; White, P.S.; Wohlgemuth, T. Quantitative Tools for Perfecting Species Lists. Environmetrics 2002, 13, 121–137. [Google Scholar] [CrossRef]
- Schweiger, A.K.; Cavender-Bares, J.; Townsend, P.A.; Hobbie, S.E.; Madritch, M.D.; Wang, R.; Tilman, D.; Gamon, J.A. Plant Spectral Diversity Integrates Functional and Phylogenetic Components of Biodiversity and Predicts Ecosystem Function. Nat. Ecol. Evol. 2018, 2, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Cavender-Bares, J.; Gamon, J.A.; Hobbie, S.E.; Madritch, M.D.; Meireles, J.E.; Schweiger, A.K.; Townsend, P.A. Harnessing Plant Spectra to Integrate the Biodiversity Sciences across Biological and Spatial Scales. Am. J. Bot. 2017, 104, 966–969. [Google Scholar] [CrossRef] [PubMed]
- Laliberté, E.; Schweiger, A.K.; Legendre, P. Partitioning Plant Spectral Diversity into Alpha and Beta Components. Ecol. Lett. 2020, 23, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Féret, J.-B.; Asner, G.P. Mapping Tropical Forest Canopy Diversity Using High-fidelity Imaging Spectroscopy. Ecol. Appl. Publ. Ecol. Soc. Am. 2014, 24, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Rocchini, D.; Balkenhol, N.; Carter, G.A.; Foody, G.M.; Gillespie, T.W.; He, K.S.; Kark, S.; Levin, N.; Lucas, K.; Luoto, M.; et al. Remotely Sensed Spectral Heterogeneity as a Proxy of Species Diversity: Recent Advances and Open Challenges. Ecol. Inform. 2010, 5, 318–329. [Google Scholar] [CrossRef]
- Schäfer, E.; Heiskanen, J.; Heikinheimo, V.; Pellikka, P. Mapping Tree Species Diversity of a Tropical Montane Forest by Unsupervised Clustering of Airborne Imaging Spectroscopy Data. Ecol. Indic. 2016, 64, 49–58. [Google Scholar] [CrossRef]
- Rocchini, D.; Luque, S.; Pettorelli, N.; Bastin, L.; Doktor, D.; Faedi, N.; Feilhauer, H.; Féret, J.-B.; Foody, G.M.; Gavish, Y.; et al. Measuring β-Diversity by Remote Sensing: A Challenge for Biodiversity Monitoring. Methods Ecol. Evol. 2018, 9, 1787–1798. [Google Scholar] [CrossRef]
- Torresani, M.; Rocchini, D.; Sonnenschein, R.; Zebisch, M.; Hauffe, H.C.; Heym, M.; Pretzsch, H.; Tonon, G. Height Variation Hypothesis: A New Approach for Estimating Forest Species Diversity with CHM LiDAR Data. Ecol. Indic. 2020, 117, 106520. [Google Scholar] [CrossRef]
- Chazdon, R.L. Second Growth: The Promise of Tropical Forest Regeneration in an Age of Deforestation; University of Chicago Press: Chicago, IL, USA, 2014; ISBN 978-0-226-11807-9. [Google Scholar]
- Egler, F.E. Vegetation Science Concepts I. Initial Floristic Composition, a Factor in Old-Field Vegetation Development with 2 Figs. Veg. Acta Geobot. 1954, 4, 412–417. [Google Scholar] [CrossRef]
- Finegan, B. Pattern and Process in Neotropical Secondary Rain Forests: The First 100 Years of Succession. Trends Ecol. Evol. 1996, 11, 119–124. [Google Scholar] [CrossRef]
- Guariguata, M.R.; Ostertag, R. Neotropical Secondary Forest Succession: Changes in Structural and Functional Characteristics. For. Ecol. Manag. 2001, 148, 185–206. [Google Scholar] [CrossRef]
- Bekele, F. The History of Cocoa Production in Trinidad and Tobago. In Proceedings of the APASTT Seminar, Re-Vitalisation of the Trinidad & Tobago Cocoa Industry, St. Augustine, FL, USA, 20 September 2003; pp. 4–12. [Google Scholar]
- NATT. Honouring Our Industrial Roots: Sugar, Cocoa, Asphalt and Oil; National Archives of Trinidad and Tobago: Port of Spain, Trinidad, 2018. [Google Scholar]
- Kenefick, M.; Restall, R.L.; Hayes, F.E. Birds of Trinidad & Tobago; Bloomsbury Publishing PLC: London, UK, 2011; ISBN 978-1-4729-4152-7. [Google Scholar]
- Arnold, H.; Deacon, A.E.; Hulme, M.F.; Sansom, A.; Jaggernauth, D.; Magurran, A.E. Contrasting Trends in Biodiversity of Birds and Trees during Succession Following Cacao Agroforest Abandonment. J. Appl. Ecol. 2021. [Google Scholar] [CrossRef]
- QGIS Geographic Information System. Open Source Geospatial Foundation. Available online: http://qgis.org (accessed on 1 March 2021).
- ESA SP. Sentinel-2: ESA’s Optical High-Resolution Mission for GMES Operational Services; Fletcher, K., European Space Agency, Eds.; ESA Communications: Noordwijk, The Netherlands, 2012; ISBN 978-92-9221-419-7. [Google Scholar]
- Poilvé, H. Geoland2—BioPar Methods Compendium of MERIS FR Biophysical Products. 2010. Available online: https://www.researchgate.net/publication/265728093_geoland2_-_BioPar_Methods_Compendium_of_MERIS_FR_Biophysical_Products (accessed on 27 May 2021). [CrossRef]
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- MacArthur, R.H. Patterns of Species Diversity. Biol. Rev. 1965, 40, 510–533. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and Extrapolation with Hill Numbers: A Framework for Sampling and Estimation in Species Diversity Studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017.
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- McCarthy, B.C.; Magurran, A.E. Measuring Biological Diversity. J. Torrey Bot. Soc. 2004, 131, 277. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Gamon, J.A.; Townsend, P.A. Remote Sensing of Plant Biodiversity; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 978-3-030-33157-3. [Google Scholar]
- Feret, J.; Boissieu, F. BiodivMapR: An r Package for A- and Β-diversity Mapping Using Remotely Sensed Images. Methods Ecol. Evol. 2020, 11, 64–70. [Google Scholar] [CrossRef]
- Ustin, S.L.; Gamon, J.A. Remote Sensing of Plant Functional Types: Tansley Review. New Phytol. 2010, 186, 795–816. [Google Scholar] [CrossRef] [PubMed]
- Clerc, S.; Team, M. Sentinel-2 Data Quality Report (S2-PDGS-MPC-DQR). ESA Technical Report. 2016. Available online: https://sentinel.esa.int/documents/247904/685211/Sentinel-2+Data+Quality+Report+(DQR)/f42497d3-611f-4165-bcc1-2f81421c646a (accessed on 27 May 2021).
- Rohlf, F.J. An Empirical Comparison of Three Ordination Techniques in Numerical Taxonomy. Syst. Biol. 1972, 21, 271–280. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Koenker, R.; Leorato, S.; Peracchi, F. Distributional vs. Quantile Regression. SSRN Electron. J. 2013, 300, 1–45. [Google Scholar] [CrossRef]
- Cade, B.S.; Noon, B.R. A Gentle Introduction to Quantile Regression for Ecologists. Front. Ecol. Environ. 2003, 1, 412–420. [Google Scholar] [CrossRef]
- John, O. Robustness of Quantile Regression to Outliers. Am. J. Appl. Math. Stat. 2015, 3, 86–88. [Google Scholar] [CrossRef]
- Galili, T. Dendextend: An R Package for Visualizing, Adjusting and Comparing Trees of Hierarchical Clustering. Bioinformatics 2015, 31, 3718–3720. [Google Scholar] [CrossRef]
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K. Cluster: Cluster Analysis Basics and Extensions. Available online: https://CRAN.R-project.org/package=cluster (accessed on 1 March 2021).
- Helmer, E.H.; Ruzycki, T.S.; Benner, J.; Voggesser, S.M.; Scobie, B.P.; Park, C.; Fanning, D.W.; Ramnarine, S. Detailed Maps of Tropical Forest Types Are within Reach: Forest Tree Communities for Trinidad and Tobago Mapped with Multiseason Landsat and Multiseason Fine-Resolution Imagery. For. Ecol. Manag. 2012, 279, 147–166. [Google Scholar] [CrossRef]
- Lebrija-Trejos, E.; Meave, J.A.; Poorter, L.; Pérez-García, E.A.; Bongers, F. Pathways, Mechanisms and Predictability of Vegetation Change during Tropical Dry Forest Succession. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 267–275. [Google Scholar] [CrossRef]
- Schoonmaker, P.; McKee, A. Species Composition and Diversity During Secondary Succession of Coniferous Forests in the Western Cascade Mountains of Oregon. For. Sci. 1988, 34, 960–979. [Google Scholar] [CrossRef]
- Toky, O.P.; Ramakrishnan, P.S. Secondary Succession Following Slash and Burn Agriculture in North-Eastern India: I. Biomass, Litterfall and Productivity. J. Ecol. 1983, 71, 735–745. [Google Scholar] [CrossRef]
- Wang, R.; Gamon, J.A.; Cavender-Bares, J.; Townsend, P.A.; Zygielbaum, A.I. The Spatial Sensitivity of the Spectral Diversity-Biodiversity Relationship: An Experimental Test in a Prairie Grassland. Ecol. Appl. 2018, 28, 541–556. [Google Scholar] [CrossRef]
- Aguirre-Gutiérrez, J.; Rifai, S.; Shenkin, A.; Oliveras, I.; Bentley, L.P.; Svátek, M.; Girardin, C.A.J.; Both, S.; Riutta, T.; Berenguer, E.; et al. Pantropical Modelling of Canopy Functional Traits Using Sentinel-2 Remote Sensing Data. Remote Sens. Environ. 2021, 252, 112122. [Google Scholar] [CrossRef]
- Ma, X.; Mahecha, M.D.; Migliavacca, M.; van der Plas, F.; Benavides, R.; Ratcliffe, S.; Kattge, J.; Richter, R.; Musavi, T.; Baeten, L.; et al. Inferring Plant Functional Diversity from Space: The Potential of Sentinel-2. Remote Sens. Environ. 2019, 233, 111368. [Google Scholar] [CrossRef]
- Sandor, M.E.; Chazdon, R.L. Remnant Trees Affect Species Composition but Not Structure of Tropical Second-Growth Forest. PLoS ONE 2014, 9, e83284. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.I.B.; Brehm, G.; Donoso, D.A.; Fiedler, K.; Homeier, J.; Paulsch, D.; Süßenbach, D.; Tiede, Y.; Brandl, R.; Farwig, N.; et al. Remote Sensing Improves Prediction of Tropical Montane Species Diversity but Performance Differs among Taxa. Ecol. Indic. 2017, 83, 538–549. [Google Scholar] [CrossRef]
- García-Montiel, D.C.; Scatena, F.N. The Effect of Human Activity on the Structure and Composition of a Tropical Forest in Puerto Rico. For. Ecol. Manag. 1994, 63, 57–78. [Google Scholar] [CrossRef]
- Riswan, S.; Kenworthy, J.B.; Kartawinata, K. The Estimation of Temporal Processes in Tropical Rain Forest: A Study of Primary Mixed Dipterocarp Forest in Indonesia. J. Trop. Ecol. 1985, 1, 171–182. [Google Scholar] [CrossRef]
- Guariguata, M.R.; Chazdon, R.L.; Denslow, J.S.; Dupuy, J.M.; Anderson, L. Structure and Floristics of Secondary and Old-Growth Forest Stands in Lowland Costa Rica. Plant Ecol. 1997, 132, 107–120. [Google Scholar] [CrossRef]
- Norden, N.; Chazdon, R.L.; Chao, A.; Jiang, Y.-H.; Vílchez-Alvarado, B. Resilience of Tropical Rain Forests: Tree Community Reassembly in Secondary Forests. Ecol. Lett. 2009, 12, 385–394. [Google Scholar] [CrossRef]
- Ganivet, E.; Bloomberg, M. Towards Rapid Assessments of Tree Species Diversity and Structure in Fragmented Tropical Forests: A Review of Perspectives Offered by Remotely-Sensed and Field-Based Data. For. Ecol. Manag. 2019, 432, 40–53. [Google Scholar] [CrossRef]
- Araújo-Santos, I.; Morante-Filho, J.C.; Oliveira, S.; Cabral, J.P.; Rocha-Santos, L.; Cassano, C.R.; Faria, D.; Benchimol, M. Seed Rain in Cocoa Agroforests Is Induced by Effects of Forest Loss on Frugivorous Birds and Management Intensity. Agric. Ecosyst. Environ. 2021, 313, 107380. [Google Scholar] [CrossRef]
- Cabral, J.P.; Faria, D.; Morante-Filho, J.C. Landscape Composition Is More Important than Local Vegetation Structure for Understory Birds in Cocoa Agroforestry Systems. For. Ecol. Manag. 2021, 481, 118704. [Google Scholar] [CrossRef]
- Cubiña, A.; Aide, T.M. The Effect of Distance from Forest Edge on Seed Rain and Soil Seed Bank in a Tropical Pasture. Biotropica 2001, 33, 260–267. [Google Scholar] [CrossRef]
- Hooper, E.R.; Legendre, P.; Condit, R. Factors Affecting Community Composition of Forest Regeneration in Deforested, Abandoned Land in Panama. Ecology 2004, 85, 3313–3326. [Google Scholar] [CrossRef]
- Hyatt, L.A.; Casper, B.B. Seed Bank Formation during Early Secondary Succession in a Temperate Deciduous Forest. J. Ecol. 2000, 88, 516–527. [Google Scholar] [CrossRef]
- Rolim, S.G.; Machado, R.E.; Pillar, V.D. Divergence in a Neotropical Forest during 33 Years of Succession Following Clear-Cutting. J. Veg. Sci. 2017, 28, 495–503. [Google Scholar] [CrossRef]
- Franklin, J.F.; Spies, T.A.; Pelt, R.V.; Carey, A.B.; Thornburgh, D.A.; Berg, D.R.; Lindenmayer, D.B.; Harmon, M.E.; Keeton, W.S.; Shaw, D.C.; et al. Disturbances and Structural Development of Natural Forest Ecosystems with Silvicultural Implications, Using Douglas-Fir Forests as an Example. For. Ecol. Manag. 2002, 155, 399–423. [Google Scholar] [CrossRef]
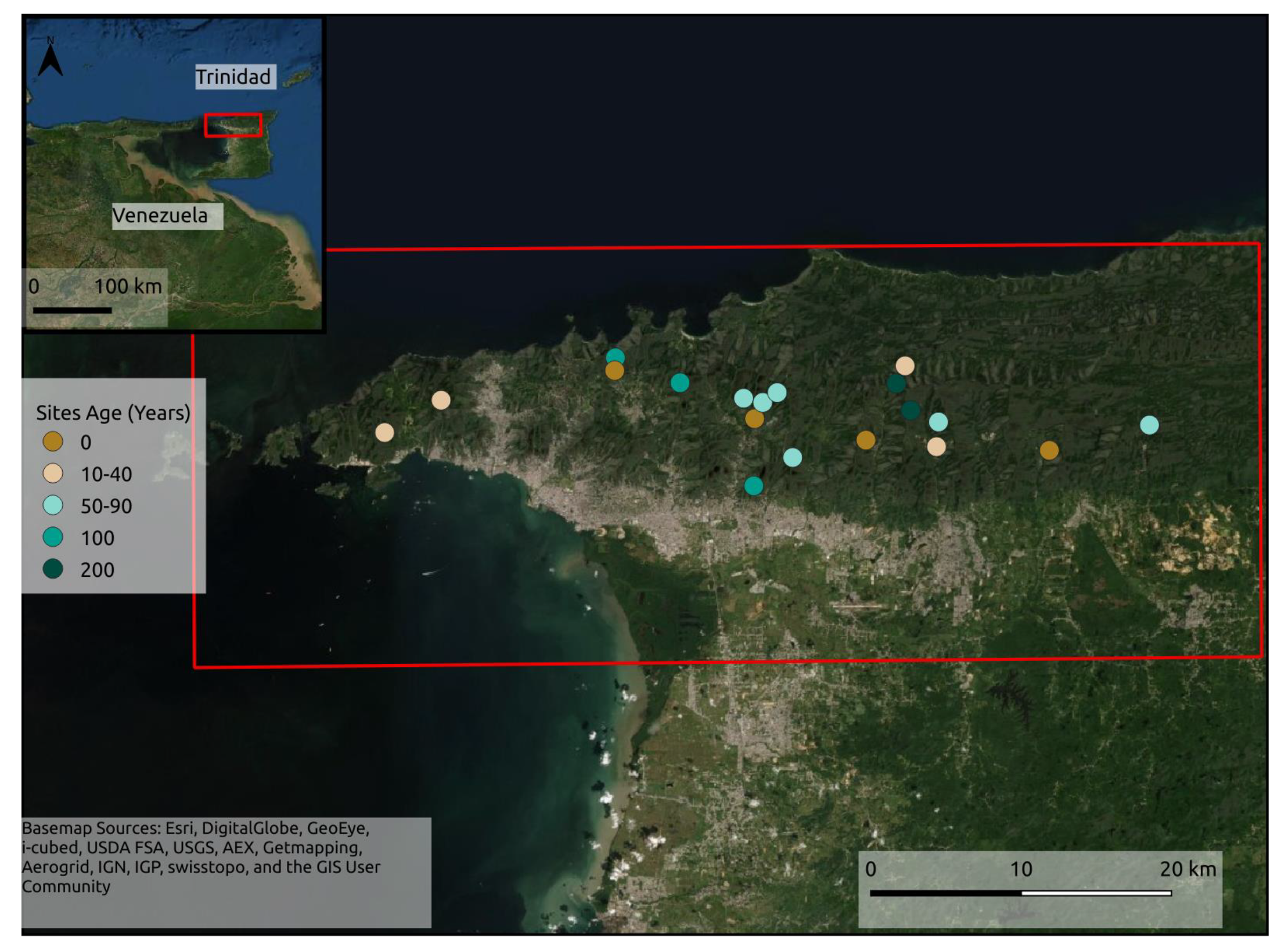


| Site | Age (Years) | Altitude (m) | Site | Age (Years) | Altitude (m) |
|---|---|---|---|---|---|
| AME | 0 | 207 | VCR | 65 | 352 |
| UpLop1 | 0 | 550 | BR1 | 70 | 321 |
| OA | 0 | 100 | UC1 | 75 | 158 |
| SASC | 0 | 92 | OT1 | 80 | 185 |
| ERE | 0 | 6 | MW8 | 80 | 244 |
| Lop1 | 0 | 167 | LAL | 80 | 471 |
| BSC | 0 | 52 | MSB | 100 | 267 |
| Sim1 | 25 | 211 | LHC | 100 | 360 |
| MSBT | 25 | 467 | SCA | 100 | 217 |
| BST | 30 | 100 | GL | 100 | 373 |
| CMA | 40 | 122 | LLP | 200 | 528 |
| CH | 40 | 90 | LALP | 200 | 550 |
| BR2 | 45 | 334 | NRGS | 200 | 297 |
| OT2 | 50 | 221 | VCRP | 200 | 148 |
| C1 | 50 | 149 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chraibi, E.; Arnold, H.; Luque, S.; Deacon, A.; Magurran, A.E.; Féret, J.-B. A Remote Sensing Approach to Understanding Patterns of Secondary Succession in Tropical Forest. Remote Sens. 2021, 13, 2148. https://doi.org/10.3390/rs13112148
Chraibi E, Arnold H, Luque S, Deacon A, Magurran AE, Féret J-B. A Remote Sensing Approach to Understanding Patterns of Secondary Succession in Tropical Forest. Remote Sensing. 2021; 13(11):2148. https://doi.org/10.3390/rs13112148
Chicago/Turabian StyleChraibi, Eric, Haley Arnold, Sandra Luque, Amy Deacon, Anne E. Magurran, and Jean-Baptiste Féret. 2021. "A Remote Sensing Approach to Understanding Patterns of Secondary Succession in Tropical Forest" Remote Sensing 13, no. 11: 2148. https://doi.org/10.3390/rs13112148
APA StyleChraibi, E., Arnold, H., Luque, S., Deacon, A., Magurran, A. E., & Féret, J.-B. (2021). A Remote Sensing Approach to Understanding Patterns of Secondary Succession in Tropical Forest. Remote Sensing, 13(11), 2148. https://doi.org/10.3390/rs13112148