Estimating the Suitability for the Reintroduced Arabian Oryx (Oryx leucoryx, Pallas 1777) of Two Desert Environments by NIRS-Aided Fecal Chemistry
Abstract
1. Introduction
2. Materials and Methods
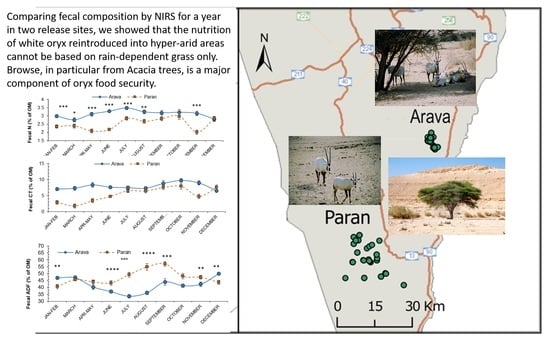
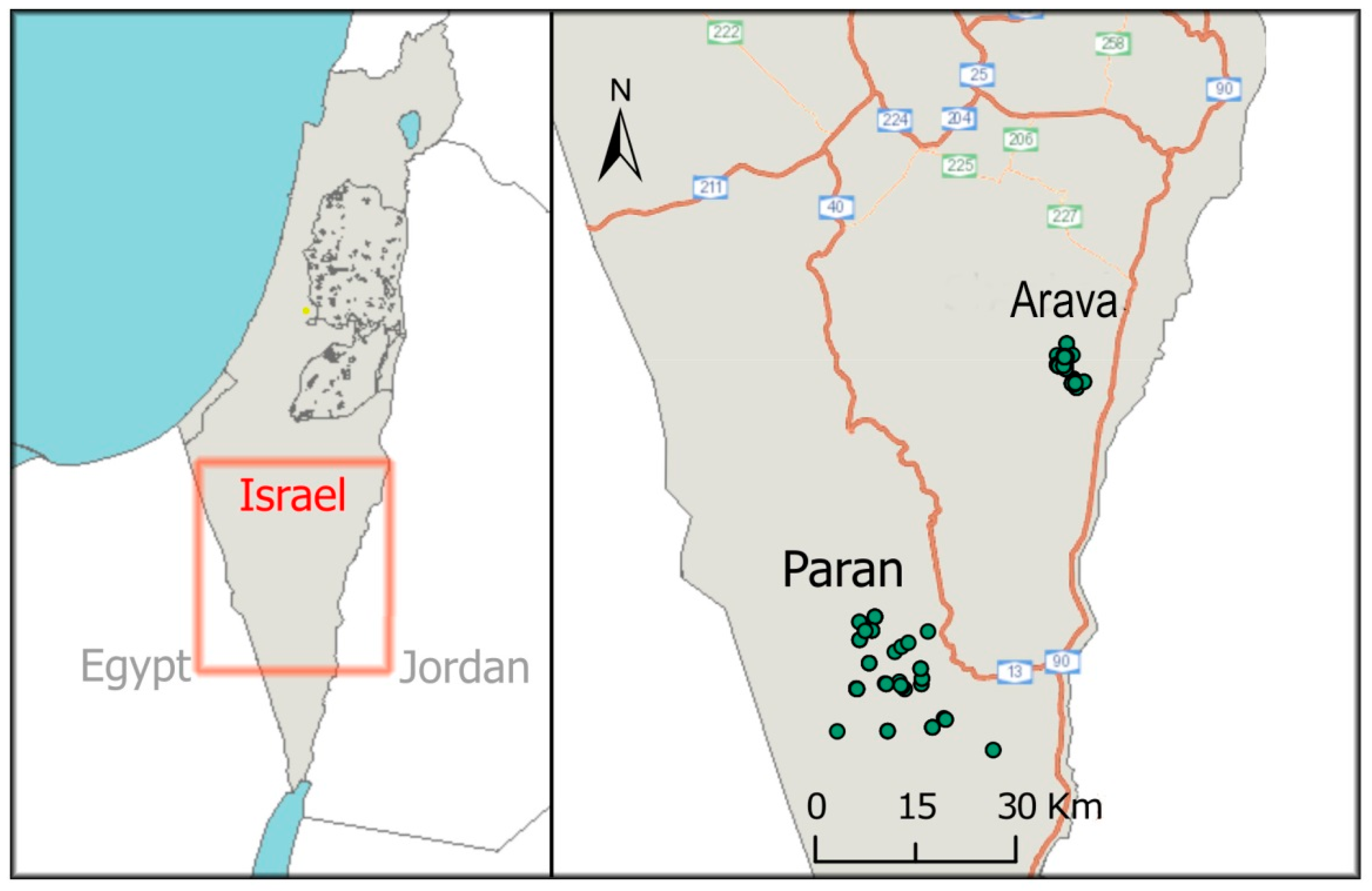
2.1. Study Area
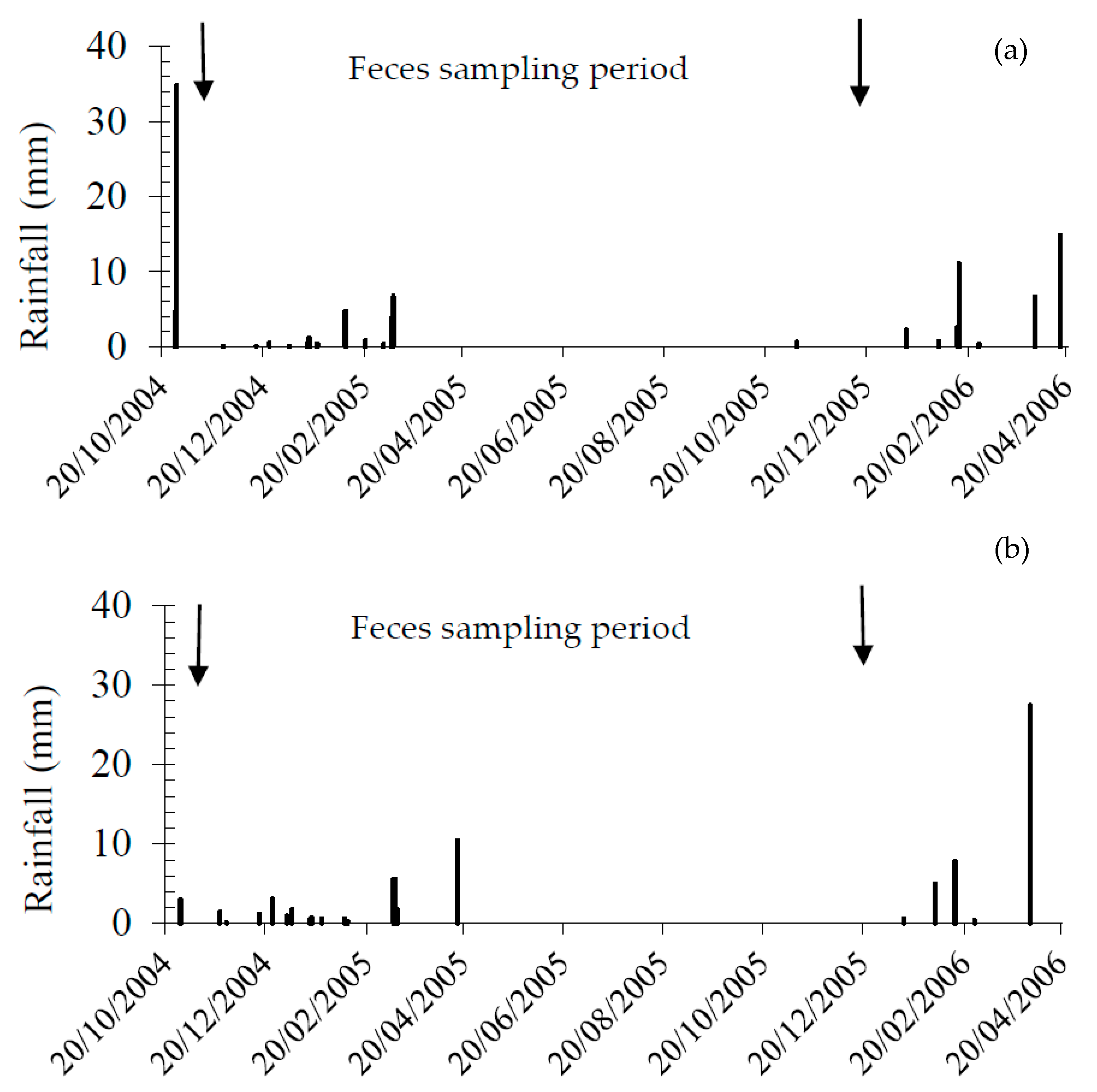
2.2. Fecal Sampling
2.3. Chemical Analyses
2.4. Statistical Analyses
3. Results
3.1. Habitat and Rainfall
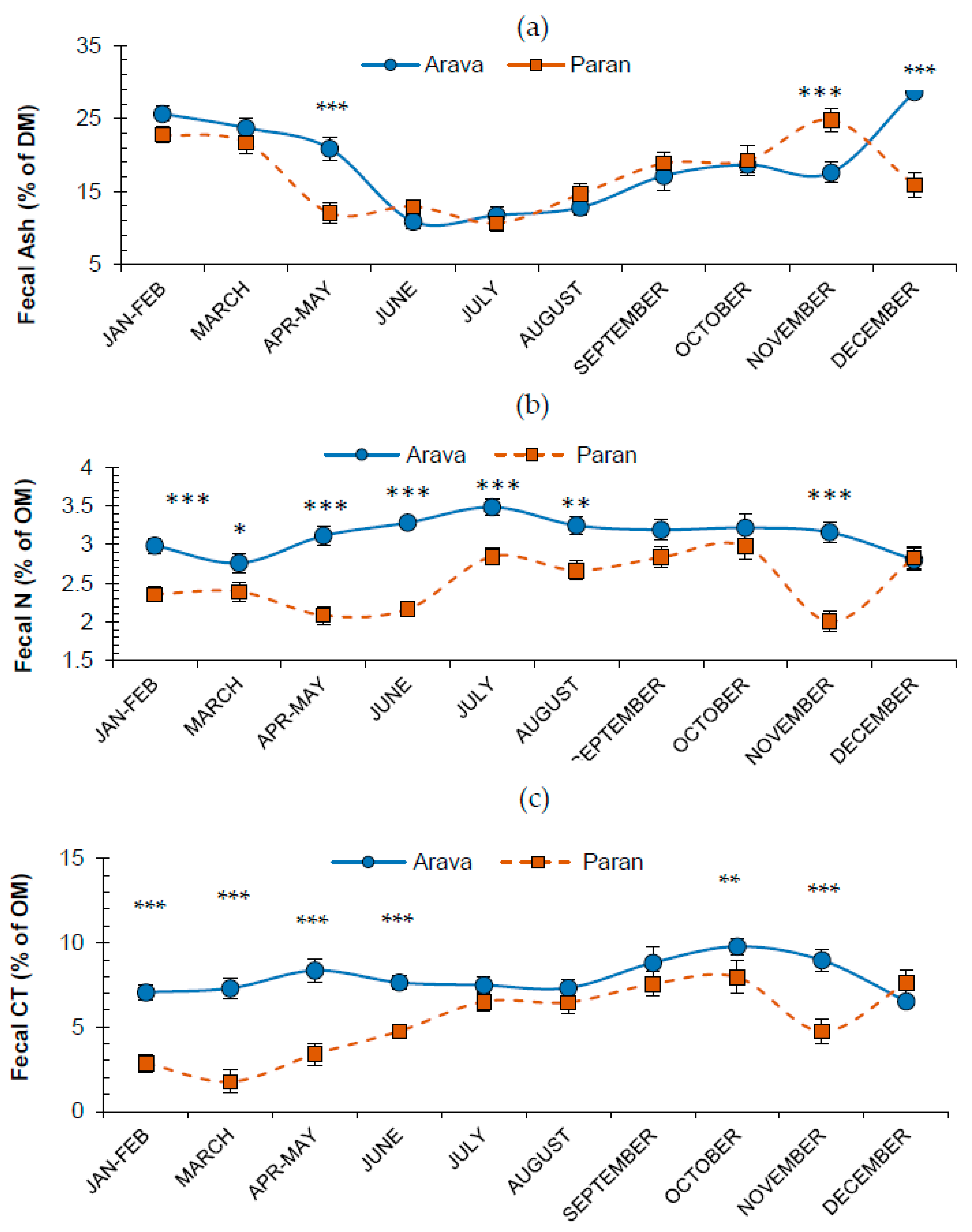
3.2. Effects of Release Site and Month on Fecal Attributes
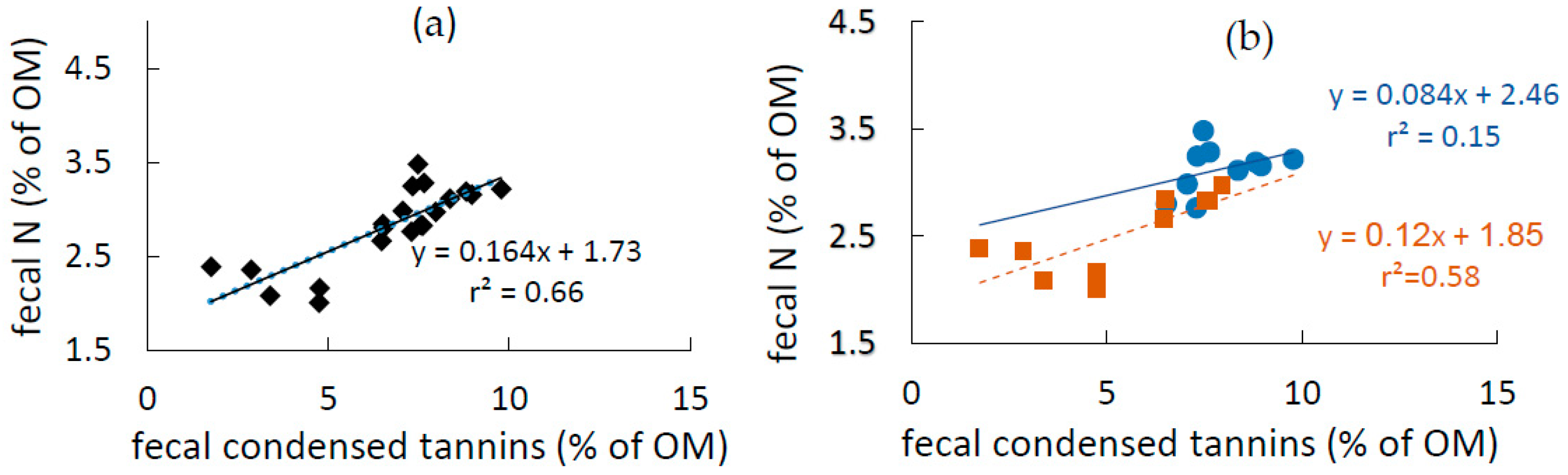
3.3. Fecal N and Tannins
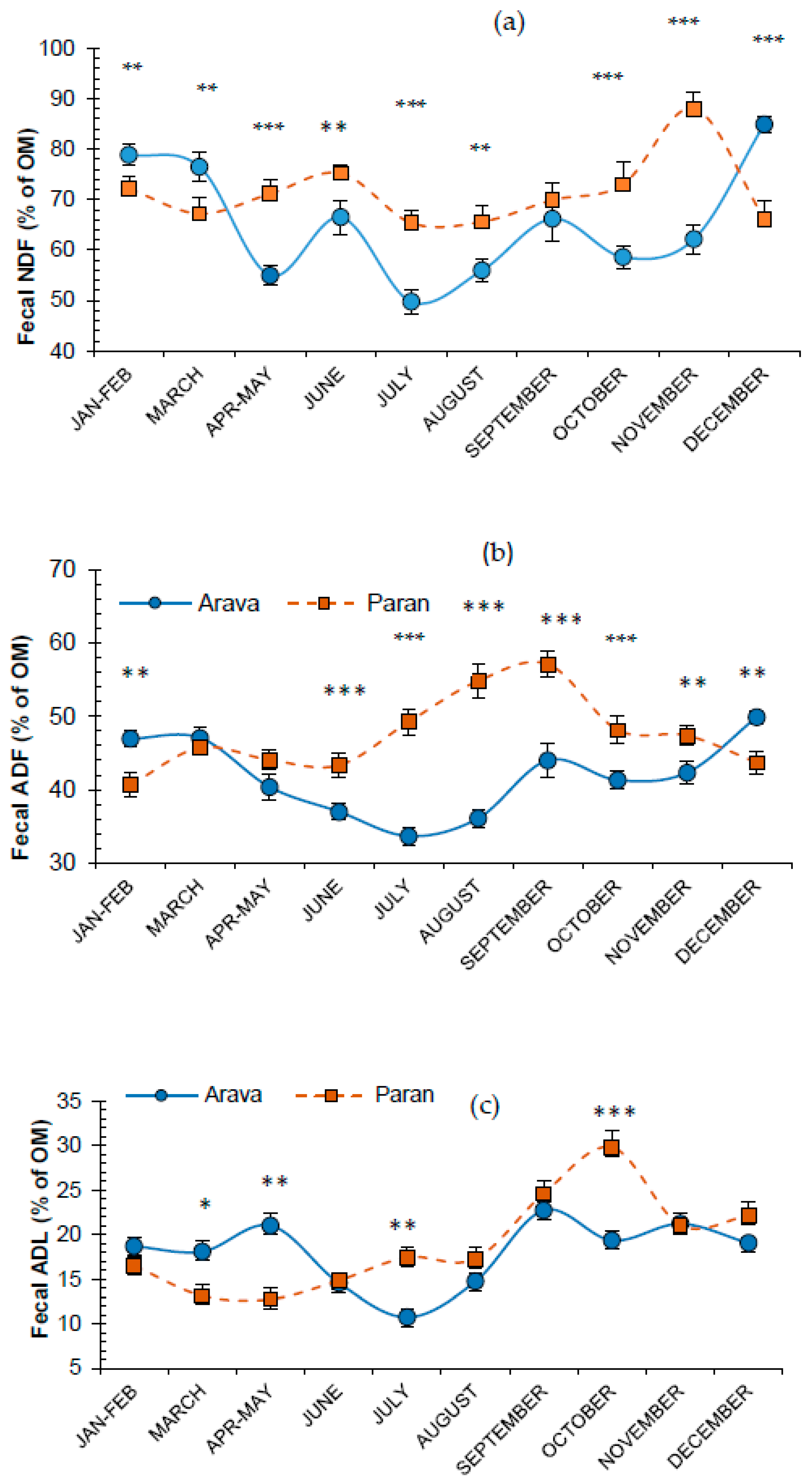
3.4. Fecal Fiber Attributes
3.5. Chemical Composition of Vegetation
4. Discussion
4.1. Fecal Nitrogen as an Estimate of Nutritional Status
4.2. Grass versus Browse: The Versatility of Oryx Feeding Behavior
4.3. Migration or Browsing as Means to Mitigate Food Depletion
4.4. Nutritional Status and Population Dynamics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- IUCN SSC Antelope Specialist Group. Oryx Leucoryx. The IUCN Red List of Threatened Species. 2017. Available online: http://dx.doi.org/10.2305/IUCN.UK.2017-2.RLTS.T15569A50191626.en (accessed on 9 March 2021).
- Kleiman, D.G.; Stanley Price, M.R.; Beck, B.B. Criteria for reintroductions. In Creative Conservation: Interactive Management of Wild and Captive Animals; Onley, P.J.S., Mace, G.M., Feistner, A.T.C., Eds.; Springer: Dordrecht, The Netherlands, 1994; pp. 287–303. [Google Scholar]
- Mésochina, P.; Bedin, E.; Ostrowski, S. Reintroducing antelopes into arid areas: Lessons learnt from the oryx in Saudi Arabia. Comptes Rendus Biol. 2003, 326, 158–165. [Google Scholar] [CrossRef]
- Ginat, H.; Shlomi, Y.; Batarseh, S.; Vogel, J. Reduction in precipitation levels in the Arava Valley (southern Israel and Jordan), 1949–2009. J. Dead Sea Arava Res. 2011, 1, 1–7. Available online: http://www.adssc.org/sites/default/files/Journal2011.pdf (accessed on 9 March 2021).
- Saltz, D. A long-term systematic approach to reintroductions: The Persian fallow deer and Arabian oryx in Israel. Anim. Conserv. 1998, 1, 245–252. [Google Scholar]
- Gilad, O.; Grant, W.E.; Saltz, D. Simulated dynamics of Arabian oryx (Oryx leucoryx) in the Israeli Negev: Effects of migration corridors and post-reintroduction changes in natality on population viability. Ecol. Model. 2008, 210, 169–178. [Google Scholar]
- Maoz, N. Impact of Repeated Release on the Space Use Patterns and Social Organization of Arabian Oryx (Oryx leucoryx) Reintroduced in the Central Negev, Israel. Master’s Thesis, Ben-Gurion University of the Negev, Beer Sheva, Israel, 2003. [Google Scholar]
- Stanley Price, M.R. Animal Re-Introductions: The Arabian Oryx in Oman. Ph.D. Thesis, University of Aberdeen, Cambridge, UK, 1989. [Google Scholar]
- Spalton, J.A. Effects of Rainfall on the Reproduction and Mortality of the Arabian oryx Oryx leucoryx (Pallas) in the Sultanate of Oman. Ph.D. Thesis, University of Aberdeen, Aberdeen, UK, 1995. [Google Scholar]
- Ismail, K.; Kamal, K.; Plath, M.; Wronski, T. Effects of an exceptional drought on daily activity patterns, reproductive behaviour, and reproductive success of reintroduced Arabian oryx (Oryx leucoryx). J. Arid Environ. 2011, 75, 125–131. [Google Scholar] [CrossRef]
- Spalton, J.A. The food supply of Arabian oryx (Oryx leucoryx) in the desert of Oman. J. Zool. 1999, 248, 433–441. [Google Scholar]
- Lancaster, R.J. Estimation of digestibility of grazed pasture from faeces nitrogen. Nature 1949, 163, 330–331. [Google Scholar]
- Leslie, D.M.; Bowyer, R.T. Facts from feces: Nitrogen still measures up as a nutritional index for mammalian herbivores. J. Wildl. Manag. 2008, 72, 1420–1433. [Google Scholar] [CrossRef]
- Wehausen, J.D. Fecal measures of diet quality in wild and domestic ruminants. J. Wildl. Manag. 1995, 59, 816–823. [Google Scholar]
- Floate, M.J.S. Decomposition of organic materials from hill soils and pastures: IV. The effects of moisture content on the mineralization of carbon, nitrogen and phosphorus from plant materials and sheep faeces. Soil Biol. Biochem. 1970, 2, 275–283. [Google Scholar]
- Jianzhang, M.; Junsheng, L.; Zhaowen, J.; Mingbo, G. Nitrogen and fiber concentration in rumen contents and feces contents of Mongolian gazelles. J. For. Res. 1999, 10, 103–106. [Google Scholar] [CrossRef]
- Jean, P.O.; Bradley, R.L.; Giroux, M.A.; Tremblay, J.P.; Côté, S.D. Near infrared spectroscopy and fecal chemistry as predictors of the diet composition of white-tailed deer. Rangel. Ecol. Manag. 2014, 67, 154–159. [Google Scholar]
- Landau, S.Y.; Dvash, L.; Yehuda, Y.; Muklada, H.; Peleg, G.; Henkin, Z.; Voet, H.; Ungar, E.D. Impact of animal density on cattle nutrition in dry Mediterranean rangelands: A faecal near-IR spectroscopy-aided study. Animal 2018, 12, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Rohner, C.; Ward, D. Chemical and mechanical defenses against herbivory in two sympatric species of desert Acacia. J. Veg. Sci. 1997, 8, 717–726. [Google Scholar] [CrossRef]
- Terrill, T.H.; Waghorn, G.C.; Woolley, D.J.; McNabb, W.C.; Barry, T.N. Assay and digestion of 14C-labelled condensed tannins in the gastro-intestinal tract of sheep. Br. J. Nutr. 1994, 72, 467–477. [Google Scholar] [CrossRef]
- Landau, S.Y.; Dvash, L.; Ryan, P.; Saltz, D.; Deutch, T.; Rosen, S.A. Faecal pellets, rock shelters, and seasonality: The chemistry of stabling in the Negev of Israel in late prehistory. J. Arid Environ. 2020, 181, 104219. [Google Scholar] [CrossRef]
- Purnomoadi, A.; Kurihara, M.; Nishida, T.; Shibata, M.; Abe, A.; Kameoka, K. Application of near infrared reflectance spectroscopy to predict faecal composition and its use for digestibility estimation. Anim. Sci. Technol. 1996, 67, 851–862. [Google Scholar]
- Dixon, R.; Coates, D. Near infrared spectroscopy of faeces to evaluate the nutrition and physiology of herbivores. J. Near Infrared Spectr. 2009, 17, 1–31. [Google Scholar] [CrossRef]
- Shmida, A.; Aronson, J.A. Sudanian elements in the flora of Israel. Ann. Mo. Bot. Garden 1986, 73, 1–28. [Google Scholar] [CrossRef]
- Isler, I. Using Fecal Analysis to Assess Nutritional Differences between Three Herds of Arabian Oryx (Oryx leucoryx) Reintroduced in Different Areas. Master’s Thesis, Ben-Gurion University of the Negev, Beer Sheva, Israel, 2007. [Google Scholar]
- AOAC. Official Methods of Analysis, 16th ed.; AOAC International: Arlington, MA, USA, 1995. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses (Apparatus, Reagents, Procedures, and Some Applications); Agriculture Handbook No. 379; ARS-USDA: Washington, DC, USA, 1970.
- Landau, S.; Dvash, L.; Decandia, M.; Cabiddu, A.; Shapiro, F.; Molle, G.; Silanikove, N. Determination of poly(ethylene glycol)-binding to browse foliage, as an assay of tannin, by near-infrared reflectance spectroscopy. J. Agric. Food Chem. 2004, 52, 638–642. [Google Scholar] [CrossRef]
- Makkar, H.P. Quantification of Tannins in Tree and Shrub Foliage: A Laboratory Manual; Springer Science & Business Media, Kluwer Academic Pub: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Tilley, J.M.A.; Terry, R.A. A two-stage technique for the in vitro digestion of forage crops. J. Br. Grassl. Soc. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Landau, S.; Glasser, T.; Dvash, L. Monitoring nutrition in small ruminants with the aid of near infrared reflectance spectroscopy (NIRS) technology: A review. Small Rumin. Res. 2006, 61, 1–11. [Google Scholar] [CrossRef]
- Shenk, J.S.; Westerhaus, M.O. Population definition, sample selection, and calibration procedures for near-infrared reflectance spectroscopy. Crop Sci. 1991, 31, 469–474. [Google Scholar] [CrossRef]
- SAS. SAS/STAT Guide; Version 6.12; SAS Institute: Cary, NC, USA, 1989. [Google Scholar]
- Silanikove, N.; Tagari, H.; Shkolnik, A. Gross energy digestion and urea recycling in the desert black Bedouin goat. Comp. Biochem. Physiol. Part A Physiol. 1980, 67, 215–218. [Google Scholar] [CrossRef]
- Choshniak, I.; Arnon, H.; Shkolnik, A. Digestive efficiency in a wild goat: The Nubian ibex. Can. J. Anim. Sci. 1984, 64, 160–162. [Google Scholar] [CrossRef]
- Maloiy, G.M.O.; Rugangazi, B.M.; Clemens, E.T. Nitrogen metabolism and renal function in the dik-dik antelope (Rhynchotragus kirkii). Small Rumin. Res. 2000, 37, 243–248. [Google Scholar] [CrossRef]
- Van Soeste, P.J. Nutritional Ecology of the Ruminant; O&B Books: Corvallis, OR, USA, 1982. [Google Scholar]
- Knight, M.H. Ecology of the Gemsbok Oryx Gazella Gazella (Linnaeus) and Blue Wildebeest Connochaetes Taurinus (Burchell) in the Southern Kalahari. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2008. Available online: https://repository.up.ac.za/bitstream/handle/2263/24345/11appendices.pdf?sequence=12 (accessed on 9 May 2021).
- Goldreich, Y.; Karni, O. Climate and precipitation regime in the Arava Valley, Israel. Israel J. Earth Sci. 2001, 50, 53–59. [Google Scholar] [CrossRef]
- Kafle, H.K.; Bruins, H.J. Climatic trends in Israel 1970–2002: Warmer and increasing aridity inland. Clim. Chang. 2009, 96, 63–77. [Google Scholar] [CrossRef]
- Sharon, D. The spottiness of rainfall in a desert area. J. Hydrol. 1972, 17, 161–175. [Google Scholar] [CrossRef]
- Corp, N.; Spalton, A.; Golrman, M.L. The influence of rainfall in a female desert ungulate: The Arabian oryx (Oryx leucoryx) in the Sultanate of Oman. J. Zool. Lond. 1998, 246, 369–377. [Google Scholar] [CrossRef]
- Van Heezik, Y.; Ismail, K.; Seddon, P.J. Shifting spatial distribution of Arabian Oryx in relation to sporadic water provision and artificial shade. Oryx 2003, 37, 295–303. [Google Scholar] [CrossRef]
- Tear, T.H.; Mosley, J.C.; Ables, E.D. Landscape-scale foraging decisions by re-introduced Arabian oryx. J. Wildl. Manag. 1997, 61, 1142–1154. [Google Scholar] [CrossRef]
- Rohner, C.; Ward, D. Large mammalian herbivores and the conservation of arid Acacia stands in the Middle East. Conserv. Biol. 1999, 13, 1162–1171. [Google Scholar] [CrossRef]
- Stavi, I.; Zinnes, T.A.; Joseph, A.; Solowey, E.; Groner, E. The role of large herbivores in recruitment of Acacia trees via endozoochory in the Arava Valley, Israel. Eur. J. Wildl. Res. 2015, 61, 775–781. [Google Scholar] [CrossRef]
- Arieli, A.; Naim, E.; Benjamin, R.W.; Pasternak, D. The effect of feeding saltbush and sodium chloride on energy metabolism in sheep. Anim. Prod. 1989, 49, 451–457. [Google Scholar] [CrossRef]
- Saltz, D.; White, G.C.; Rubenstein, D. Does increased variance from global change threaten wildlife? Predicting the future dynamics of Asiatic wild ass under environmental stochasticity. Conserv. Biol. 2006, 20, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Gunn, A.; Irvine, R.J. Subclinical parasitism and ruminant foraging strategies—A review. Wildl. Soc. Bul. 2003, 31, 117–126. [Google Scholar]
- Goossens, E.; Dorny, P.; Vercammen, F.; Vercruysse, J. Field evaluation of the efficacy of fenbendazole in captive wild ruminants. Vet. Rec. 2005, 157, 582–586. [Google Scholar] [CrossRef]
- Githiori, J.B.; Athanasiadou, S.; Thamsborg, S.M. Use of plants in novel approaches for control of gastrointestinal helminths in livestock with emphasis on small ruminants. Vet. Parasitol. 2006, 139, 308–320. [Google Scholar] [CrossRef] [PubMed]
| Constituent | N | Mean | SD | SEC | R2cal | SECV | R2cv | SD/SECV | DER |
|---|---|---|---|---|---|---|---|---|---|
| Feces | |||||||||
| FN | 103 | 2.18 | 0.46 | 0.088 | 0.96 | 0.10 | 0.95 | 4.60 | 1 |
| FNDF | 59 | 52.4 | 7.37 | 2.29 | 0.90 | 2.87 | 0.85 | 2.57 | 2 |
| FADF | 55 | 35.1 | 5.31 | 1.26 | 0.94 | 1.62 | 0.91 | 3.28 | 1 |
| FADL | 58 | 13.1 | 3.83 | 1.31 | 0.88 | 1.68 | 0.81 | 2.28 | 2 |
| FASH | 100 | 21.7 | 8.10 | 1.06 | 0.98 | 1.51 | 0.97 | 5.36 | 1 |
| Forage | |||||||||
| N | 425 | 2.11 | 0.80 | 0.17 | 0.95 | 0.20 | 0.94 | 4.00 | 1 |
| NDF | 422 | 45.71 | 12.67 | 3.69 | 0.92 | 4.35 | 0.88 | 2.91 | 1 |
| ADF | 418 | 29.00 | 7.63 | 2.59 | 0.88 | 2.88 | 0.86 | 2.65 | 1 |
| ADL | 204 | 2.58 | 1.53 | 0.48 | 0.90 | 0.79 | 0.85 | 1.94 | 1 |
| IVDMD | 145 | 66.09 | 14.03 | 2.46 | 0.97 | 2.97 | 0.96 | 4.72 | 1 |
| Feces and forage | |||||||||
| CT | 100 | 7.93 | 5.64 | 1.10 | 0.86 | 1.33 | 0.84 | 4.24 | 1 |
| Species | Type | Part | Month | Percentage of Dry Matter | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ash | CP | CT | NDF | ADF | ADL | IVDMD | ||||
| Acacia pachyceras | tree | leaves | AYR * | 6.5 | 13.5 | 11.2 | 37.8 | 26.5 | 13.0 | 43.9 |
| tree | seedless pods | AYR * | 6.7 | 10.1 | 8.6 | 42.9 | 27.4 | 7.8 | 45.3 | |
| Acacia raddiana | tree | leaves | AYR * | 8.8 | 15.6 | 8.7 | 32.3 | 19.5 | 5.8 | 56.7 |
| tree | pods on tree | June–August | 6.2 | 17.2 | 8.7 | 38.5 | 24.8 | 7.6 | 61.4 | |
| tree | pods on ground | AYR * | 9.2 | 15.6 | 7.1 | 38.5 | 25.6 | 5.3 | 60.4 | |
| Acacia tortilis | tree | leaves | AYR * | 6.6 | 21.1 | 11.3 | 34.9 | 24.5 | 7.8 | 53.3 |
| tree | pods on ground | July-August | 10.5 | 16.5 | 6.7 | 39.6 | 27.7 | 5.2 | 58.1 | |
| Atriplex halimus | bush | twig | AYR * | 11.9 | 9.0 | 1.1 | 48.6 | 20.9 | 5.7 | 67.2 |
| Lycium shawii | bush | leaves | AYR * | 14.5 | 12.3 | 2.2 | 37.1 | 18.5 | 4.2 | 72.1 |
| Nitraria retusa | bush | leaves | AYR * | 8.5 | 16.5 | 7.2 | 40.7 | 25.6 | 7.1 | 54.1 |
| Ochradenus baccatus | bush | fruit | May–July, October, December | 6.4 | 8.4 | 5.5 | 52.1 | 29.1 | 7.4 | 62.2 |
| bush | twig | AYR * | 5.9 | 11.9 | 2.4 | 57.8 | 36.3 | 9.3 | 47.5 | |
| Plicosepalus acaciae | parasite | leaves | AYR * | 2.7 | 7.6 | 21.7 | 39.8 | 22.7 | 11.3 | 28.4 |
| Tamarix nilotica | bush | twig | AYR * | 7.4 | 6.0 | 13.9 | 38.8 | 26.4 | 10.3 | 38.1 |
| Tamarix aphylla | bush | twig | AYR * | 9.3 | 10.4 | 11.0 | 40.6 | 24.5 | 6.3 | 43.9 |
| Zilla spinosa | bush | leaves | AYR * | 7.9 | 9.5 | 1.7 | 59.8 | 37.6 | 7.9 | 44.3 |
| Annual herbaceous | ||||||||||
| (young) | grass | Whole plant | December–January | 13.0 | 22.4 | <1 | 44.6 | 24.8 | 2.2 | 72.1 |
| (pre-bloom) | grass | Whole plant | February–March | 11.0 | 15.5 | <1 | 57.8 | 31.9 | 2.5 | 67.3 |
| (dry) | grass | Whole plant | April | 9.3 | 5.6 | <1 | 73.4 | 46.4 | 4.0 | 37.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landau, S.Y.; Isler, I.; Dvash, L.; Shalmon, B.; Arnon, A.; Saltz, D. Estimating the Suitability for the Reintroduced Arabian Oryx (Oryx leucoryx, Pallas 1777) of Two Desert Environments by NIRS-Aided Fecal Chemistry. Remote Sens. 2021, 13, 1876. https://doi.org/10.3390/rs13101876
Landau SY, Isler I, Dvash L, Shalmon B, Arnon A, Saltz D. Estimating the Suitability for the Reintroduced Arabian Oryx (Oryx leucoryx, Pallas 1777) of Two Desert Environments by NIRS-Aided Fecal Chemistry. Remote Sensing. 2021; 13(10):1876. https://doi.org/10.3390/rs13101876
Chicago/Turabian StyleLandau, Serge Yan, Ido Isler, Levana Dvash, Benny Shalmon, Amir Arnon, and David Saltz. 2021. "Estimating the Suitability for the Reintroduced Arabian Oryx (Oryx leucoryx, Pallas 1777) of Two Desert Environments by NIRS-Aided Fecal Chemistry" Remote Sensing 13, no. 10: 1876. https://doi.org/10.3390/rs13101876
APA StyleLandau, S. Y., Isler, I., Dvash, L., Shalmon, B., Arnon, A., & Saltz, D. (2021). Estimating the Suitability for the Reintroduced Arabian Oryx (Oryx leucoryx, Pallas 1777) of Two Desert Environments by NIRS-Aided Fecal Chemistry. Remote Sensing, 13(10), 1876. https://doi.org/10.3390/rs13101876