Abstract
The article summarizes the previous experience and results from the study of wild rodents using the NIRS method. The importance and role of rodents in ecosystems and the specifics associated with their research using the NIRS method are briefly mentioned. The results of previous partial studies are mentioned and discussed. The NIRS method proved to be a useful tool to determine the amount of a particular food ingredient in the diet from faeces or chyme and to estimate the food quality (i.e., diversity of nitrogenous substances in chyme). On this basis, other possible directions of research using the NIRS method in wild rodents are proposed. These could help to better understand both the relationship between rodents and their environment and practical management in sectors where rodents interfere with human interests, especially in agriculture and forestry.
1. Brief Introduction to Near-Infrared Spectroscopy (NIRS)
Near-infrared spectroscopy is a spectroscopic method that uses the near-infrared region (i.e., 1000–2500 nm) from the electromagnetic spectrum [1]. The principle of the method consists of different absorption or reflection of the near-infrared light of different wavelength incidents on the organic material. The reflected spectrum is given by the type and amount of chemical bonds in a particular material. However, since the individual peaks of a spectrum curve are not strictly bond-specific, it is usually necessary to refine the estimate by creating a calibration equation. This is obtained by applying mathematical methods (e.g., partial least squares regression) to the spectra obtained from samples of known composition (for more detail, see [2]).
The NIRS method enables fast, technologically easy, low-cost, and repeatable determination of the composition of a large number of organic material samples [3]. It requires a much smaller sample size than classic analysis. The method has been successfully used for chemical evaluation in nutritional studies of many animal species (ruminants [4], marine mammals [5], koalas [6], giant pandas [7] etc.). The NIRS method can be used to determine the content of basic nutrients (nitrogenous substances, fiber, and water content) [2], to determine the presence or proportion of a particular food [8], as well as to detect changes in the content of substances represented in minimal concentrations (e.g., the level of hormones [9,10] or fatty acids in milk [11]). For almost half a century, the method has been increasingly used, especially in agriculture and pharmacy (typically, where large quantities of very similar samples are routinely processed) [12]. Use in other areas (e.g., medicine, biology, and ecology) followed with some delay [13].
In recent years, with technological progress, possibilities of applying the NIRS method have significantly expanded and have not yet been fully exploited. For example, a miniaturization of sensors [14] has already allowed in-situ analysis of a sample instead of laboratory processing [15]. The use of NIRS for the analysis of liquids, or samples dissolved in liquids, has great potential in ecological and ecophysiological studies, but it has been used only rarely so far [12].
2. The Importance of Rodent Food Studies
Small rodents are among the most adaptable vertebrates, inhabiting almost all biogeographic zones in natural [16] and artificial [17] habitats. Their ecological status is in the middle of the food chain, as they are omnivores consuming plants, seeds, and invertebrates [18]. Rodents are key food for birds of prey, weasels, foxes, etc. [19]. Small body size, short generation interval, high fertility, ability of immediate reproduction in suitable conditions, and taxonomic affiliation to vertebrates/mammals, make rodents excellent bioindicators, quickly reflecting the state and changes in the environmental conditions [20]. Ecological bindings and dependencies in small rodents are usually more distinct than in larger and usually longer-living vertebrates. Rodents are resilient, highly mobile, and relatively intelligent. Therefore, they often cause significant damage to field crops, supplies, and infrastructure; the role of rodents as vectors of zoonoses is also important [21,22,23].
Despite many years of effort, there are still many aspects of the ecology of rodents where our knowledge is weak. One of the most important aspects is foraging ecology. The lack of these studies is caused by the small size of studied individuals and substantially complicated demography of these species, which affect the foraging behaviour and diet structure. Most small mammal species undergo population fluctuations, when age structure, body size, population density, reproduction parameters (age at first reproduction, litter size, and proportion of breeding individuals), social hierarchy, and spatial behaviour change in time and place [24]. These features influence (and are influenced) by diet. Moreover, some food resources are seasonal during a year, other food resources are available once every few years (e.g., forest tree seeds). The parameters mentioned above make food studies extremely demanding, as individuals differ in age, sex, phase of population oscillation, season, habitat, etc. However, the knowledge of diet quality and quantity is necessary for the evaluation of other demographic parameters and changes.
Previous studies generally indicated that estimated damage caused by small rodents in agriculture and forestry can be substantially high [25,26,27]. However, the amount of damage is not always directly proportional to the number of rodents; so, the factors influencing the occurrence of damage are not yet sufficiently known. Understanding when and why rodents cause damage or consume target crops is key to reducing or even preventing the damage.
The exact knowledge about rodent diet is important also because, in some countries, small rodents can be legally poisoned under certain conditions [22]. This poison may affect whole ecosystems; non-target species have been also widely harmed directly by the poison or by consuming poisoned prey [28].
3. Why It Is Appropriate to Use the NIRS Method in Wild Rodents
Small mammal food studies have required using a reliable method for analysing composition of their food. Microscopic analyses of the rodents’ diet are extremely time-consuming; they require detailed knowledge of the food supply and allow only rough analyses of food structures [29,30]. The classic chemical method is not suitable because, for hundreds of samples, it is too expensive and laborious. In addition, chemical methods only allow for determination of the chemical composition of the food [31], not the origin of the components. Moreover, it is usually impossible to obtain a sufficiently large sample in small rodent species.
For these reasons, NIRS has been the most promising method for diet studies of small rodents. The method enables the analysis of a high number of samples (individuals) that sufficiently represent the actual demographic situation in the area, and a consequent analysis allows evaluation of all available parameters (such as species, sex, age, size, habitat, 3+season, etc.). The measured spectra can be used even for later developed calibrations. Creating such data files by classic methods would be extremely expensive and difficult or even impossible due to the laboriousness and small size of individual samples.
4. Specifics of Using the NIRS Method in Wild Rodents
4.1. Analysis of Faeces
There are some specific aspects in small rodent studies which should be considered. Likely, the most problematic point is the small size of these mammals.
For diet studies, the most usual type of sample is faeces. Faeces can be used also for middle-size animals [6,32]. However, the usual body size of rodents ranged from 7 to 35 grams, thus a piece of faeces is too small not only for a classic chemical analysis but even for the NIRS analysis (Figure 1). Theoretically, more faeces can be collected in one place for NIRS analysis of one single mixed sample. Dozens of fresh faeces from one place should be used for one sample; unfortunately, these are very difficult to find in larger quantities. In addition, most rodent species live socially and more individuals share burrows and living space [33]. Therefore, one sample would be composed of faeces of several individuals. This means that the results of the NIRS analysis cannot be linked to a specific individual of a specific age, sex, size, etc., sometimes not even to a specific species. Demographic studies of wild rodents based on such faecal analysis are therefore practically impossible. However, analyses of rodent faeces can be a useful tool in laboratory conditions, where a sample can be reliably assigned to a specific individual (e.g., for food preference tests [34]).

Figure 1.
Rodent faeces. Their collection is limited by defecation rate of the individual species. It also depends on food. While on a grain diet and water, herbivorous voles (Microtus sp.) produce about 1 g of faeces a day, the granivorous Apodemus sp. produce 1 g of faeces within 4 to 5 days.
4.2. Analysis of Stomach Contents
A more promising type of rodent sampling involves stomachs; some stomachs contain sufficient chyme to perform NIRS analysis. For the analysis of nutrients from stomach content, NIRS is probably the only possible analysing method, as stomachs are only rarely sufficiently large for traditional chemical analysis. Stomach NIRS analysis has been successfully used in ruminants [35] and dugongs [36].
For stomach contents analysis, material obtained from research into the spread of zoonoses in small mammal populations was used. Whole stomachs were taken from snap-trapped animals. Each individual was determined by species, sex, and age, measured, and weighed. Stomachs were dried in a kiln for 4 hours in 50–60 °C. Rodent tissue was then removed, and the remaining stomach contents were preserved in a standard freezer at a temperature of −20 °C. The following processing is described in Section 5.2. Important steps of the processing are shown in Figure 2.
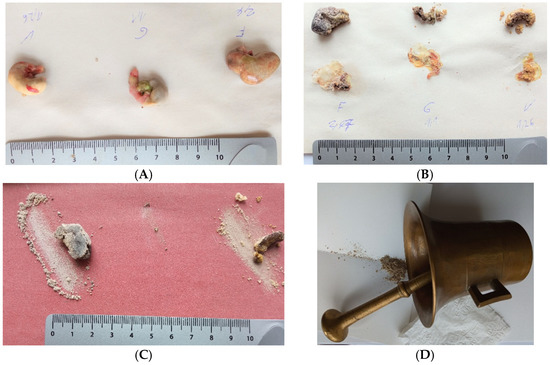
Figure 2.
Steps of stomach content samples processing. Fresh samples show the difference between the light cardiac and dark pyloric part (A). Nitrogen NIRS analysis however showed similar results for both parts of the stomach. Samples are marked with letters (identification of a specific individual); the number indicates the weight of a fresh sample. After drying, the rodent tissue was removed, and the stomach content (chyme) was prepared (B). It can be seen in the same figure that the samples contained different amounts of water; the residual dry matter in the middle sample (letter G) is at the limit of processability. An example of processing samples by abrasion with sandpaper is in (C), grinding in a mortar in (D). Most samples were processed by abrasion because it is significantly faster.
4.3. Calibration
The NIRS analysis of stomach content has a specific requirement, building a calibration curve that is usually based on samples analysed both by NIRS and by a classic chemical analysis. Results of traditional analysis and of NIRS spectrums are then incorporated into a NIRS calibration model. However, a vast majority of stomachs are too small for a chemical analysis and cannot be used for building a NIRS calibration model. The size of a stomach is mainly affected by the species, the size of an animal, and the food consumed. Stomachs of herbivorous species tend to be smaller, and their chyme contains significantly lower amounts of dry matter than in granivorous species [37,38]. These differences have been caused both by the type of food consumed and the way it is ingested [39].
Another issue is the variability of rodent food. To make the calibration curve as reliable as possible, a wide range of types of food samples should be included, preferably an entire spectrum. However, this is almost impossible with omnivorous rodents. They consume both plant and animal food in very variable proportions, sometimes also rather unexpected substances (for example, ash or clay) [29]. NIRS calibrations generally less accurately predict the chemical composition of compound materials, compared to raw materials [40]. In varied diets, most compounds can occur in more types of materials and chemical bonds (for example nitrogen in vertebrate muscles, seeds, vegetation tissues, etc.) [41]. Despite an accurate calibration curve based on many different types of diet, some samples deviate extremely from the calibration model. These circumstances limit the possibility of creating a precise calibration and make NIRS results for omnivores weaker than results for food specialists [42,43]. The NIRS results should be interpreted regarding some specific features of species and natural conditions. One of them is the spatial behaviour of rodent species; the home range of voles is usually much smaller than areas used by mice [44]. Unlike voles, mice can be caught in a different environment than the one in which they feed. Another feature is that the grass and herbal diet in the stomachs of herbivores (voles) is more susceptible to damage in hot weather than the seed food in the stomachs of mice; this can lead to inaccuracies in some samples.
A sample of the untransformed spectra of stomach contents is presented in Figure 3; the NIRS calibration equation is presented in Table 1; the fit between real and NIRS predicted values are presented in Figure 4.
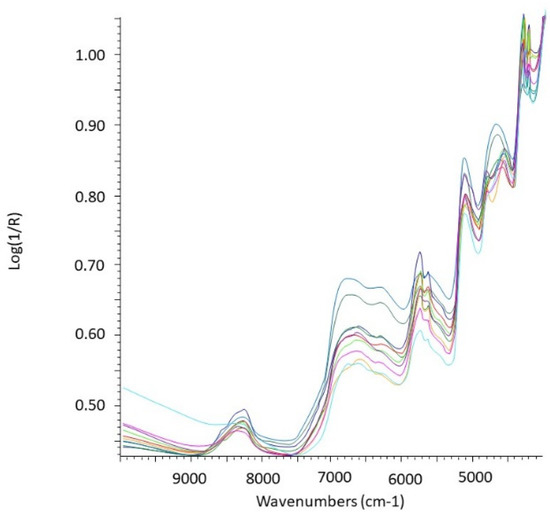
Figure 3.
Example of untransformed NIR spectra of rodent stomach contents (n = 10).

Table 1.
Near-infrared spectroscopy (NIRS) calibration performance for the prediction of the nitrogenous substances amount of rodent stomach content including the number of samples used in calibrations (N), mathematical treatment (DER, 1st or 2nd derivative), standard error of calibration (SEC), and cross-validation (SECV), and the coefficients of determination of calibration (R2cal) and cross-validation (R2cv).
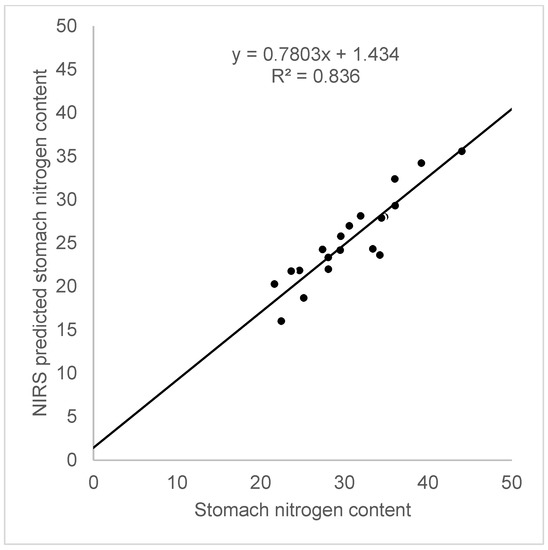
Figure 4.
The fit between the nitrogen content in the rodent stomachs determined by a classic chemical analysis and predicted NIRS values.
5. What Has Already Been Found?
As far as we know, little attention has been paid to the study of rodents by the NIRS method so far. There is a methodology for the preparation and processing of samples for a NIRS analysis from stomach contents. Several case studies have been performed to determine the amount of a particular crop in the diet from faeces or chyme. So far, the greatest attention has been paid to the food quality (i.e., diversity of nitrogenous substances in chyme).
All studies known to us were performed on the most numerous species of rodents in the Czech Republic. The species spectrum of the studied rodents includes mice of the genus Apodemus (A. agrarius, A. flavicollis, A. sylvaticus, and A. uralensis), common voles (Microtus arvalis), and bank voles (Clethrionomys glareolus).
5.1. NIRS Analysis of Rodent Dietary Preferences
The first case study tested the possibility of using NIRS to determine the number of cereals and acorns in the stomach content. The subject of the study was the food preferences of Apodemus flavicollis, depending on the yield of acorns and the availability of supplementary food in a pheasantry. The applicability of the method for this type of work was verified on 94 samples. The results confirmed that A. flavicollis prefer cereals over the common available food (e.g., acorns). In the “seed year” (a year with a high harvest of acorns), there was a higher proportion of acorns in the diet of A. flavicollis [45].
To the best of our knowledge, the analysis of rodent faeces using NIRS has been used only once, to predict the proportion of two types of cereals (wheat and barley) in the diet of common rodents occurring in the arable landscape of Moravia. The calibration equation was developed by measuring samples obtained by mixing two types of faeces in various known ratios. Each type of faeces was collected in a laboratory experiment from a wood mouse (Apodemus sylvaticus) fed either barley or wheat. The percentage diet preferences were determined by comparing faeces from preference tests with a calibration equation. The calibration was shown to be sufficiently accurate also for the pygmy field mouse (A. uralensis) and black-striped mouse (A. agrarius) (R2 = 0.99, p < 0.001; SECV = 1.78). However, estimates for the common voles’ faeces varied substantially. The reason is probably taxonomic and/or physiological differences in the process of digestion [39]. Hence, a special calibration equation had to be developed for the common vole. Results based on the NIRS method showed that all Apodemus species preferred wheat over barley, while the common vole showed no preference for any crop [34].
NIRS analysis of stomachs and faeces proved to be useful for determining the content of a specific food in omnivorous and herbivorous rodents. However, it has also been shown that the use of calibrations can be taxonomically or ecophysiologically limited.
5.2. Rodent Stomach Sample Preparation for the NIRS Analysis
During the above-mentioned research, it was found that, for samples from stomach contents, it would be useful to verify the influence of chyme composition in different stomach parts as well as the methodology of sample preparation on the results. There were two main reasons; first, rodents chew different foods differently and the food can then progress through the stomach at different speeds [46]. Second, homogenization of whole samples by grinding is much more time consuming than abrasion of a sample part with sandpaper and subsequent analysis of the ground part. Therefore, it had to be verified that none of these reasons affected the outcome of the NIRS analysis. It was found that there are no differences in the nitrogen content between the pyloric and cardiac chyme or between the sample prepared by abrasion and grinding [47].
After verifying the methodology, the nitrogen content was selected as an ideal indicator of food quality for further study. Samples from forest and field habitats were available. Because they differ both in potential food sources and, to some extent, in the species spectrum of rodents, both groups of habitats were examined separately.
5.3. NIRS Analysis of Rodents Food Quality in Forest Habitats
The NIRS method was used to compare the food quality (i.e., nitrogen content) of four species of the most numerous forest rodents in the Czech Republic (Apodemus flavicollis, A. sylvaticus, Clethrionomys glareolus, and Microtus arvalis) [48]. Both Apodemus species showed higher variability and higher mean food quality compared to M. arvalis (Figure 5; [48]). Results for C. glareolus were intermediate for both variability and mean value. Differences in diet composition (or ratio of nitrogenous substance) in different biotopes was demonstrated only for the C. glareolus. Moreover, the C. glareolus diet varied within the same biotope over different years. C. glareolus was the only species to display differences in diet between males and females, with males having higher nitrogen concentrations. In addition, for A. sylvaticus, changes in food quality during the year were intensively monitored [49]. The highest quality as well as variability in food quality was found outside the growing season (i.e., through the winter).
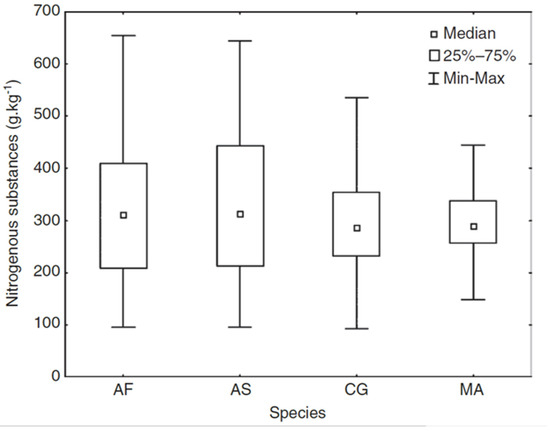
Figure 5.
Box plot indicating differences in the amount of nitrogenous substances in stomachs of four small terrestrial mammal species. (AF—Apodemus flavicollis, AS—Apodemus sylvaticus, CG—Clethrionomys glareolus, MA—Microtus arvalis).
Analysis by the NIRS method highlighted differences in food quality between species with different foraging ecology. For example, omnivorous mouse species have eaten food with higher and more variable content of nitrogenous substances than the herbivorous common vole. Additionally, food quality changes throughout the year (at least in A. sylvaticus).
5.4. NIRS Analysis of Rodents Food Quality in Arable Landscape
Three articles dealt with the study of food quality by the NIRS method of the most numerous rodents in Central European agroecosystems. In the first article, changes in the quality of food were observed in two populations of Microtus arvalis (the most numerous rodents in this habitat). A higher proportion of nitrogenous substances was found in breeding females at the highest population densities [50]. The second article compared the food quality of A. sylvaticus and M. arvalis in extensively and intensively agriculturally used localities. The effect of population density, crop, and season on the food quality was found in both species [51]. The third article sought to verify whether the knowledge found in previous works on food of the genus Apodemus can be applied to A. uralensis; it is the dominant species in some areas together with the M. arvalis. A. uralensis’ food was shown to have, on average, a lower proportion of nitrogenous substances and higher variability; the food quality was influenced by the season. In M. arvalis, the most important factor was the overall abundance of rodents [52].
A strong correlation was found between food quality and population density in the common vole and wood mouse [51,52]. This finding clearly confirms that food quality is related to rodent population dynamics. In arable ecosystems, the amount of nitrogen in consumed food differs in various crops in relation to their nitrogen content [51]. In forest and arable habitats, reproduction and season of year were also reflected in changes in food quality [49,50] in contrast to sex, body size, and age.
6. Suggestions for Further Research
A large group of scientists, agronomists, and epidemiologists would benefit from a wider use of NIRS in small mammal research. Small rodents are the most abundant group of mammals [23,53] and are intensively studied as important zoological and ecological subjects, pest species, and carriers of a large number of zoonosis. The great advantage of the NIRS method is that more components (such as diet composition, hormones, pathogens, etc.) can be estimated in only one scanned spectrum. With larger use of the method, newly established calibration models can be additionally used on the once processed samples.
The miniaturization of portable sensors enabling scanning of NIRS spectra is very promising [14]. The NIRS analysis was already performed on living animals [54,55]. If this method becomes reliable when applied in natural conditions and almost in real time, it might be an ideal solution in all aspects, even for rodents and small mammals in general. The main issue, a small size of samples from small rodents, could be resolved by using another type of sample. Probably, the use of solutions made by mixed or dissolved faeces can be suitable for wider use of NIRS analysis in more disciplines. Liquid samples for NIRS analysis have been used for studying fatty acids in milk [11], fiber digestibility in rumen fluid [56], or hormones in blood and urine [9,10,57]. As far as faecal analysis in small rodents is concerned, it should be considered that other “informative” substances are added to faecal samples with urine [58,59]. This would also provide additional information on “anonymous faeces”: the determination of species, sex, or breeding status by hormones in the urine.
The NIRS method used on faecal solutions would be non-invasive, fast for sampling and it would allow many samples and components to be analysed. Faeces contain a wide range of organic and inorganic substances taken into the body from the environment or produced by the body in response to environmental conditions, and their amount reflects the state of an individual, population, and environment [60].
There are some topics that can be studied or monitored using faecal NIRS analysis:
- -
- Diseases. Small rodents are hosts and inter-hosts of a huge number of animal diseases and zoonoses, such as Francisella tularensis [61,62], Borrelia sp. [63], Yersinia sp. [64], Brucella sp. [65], Toxoplasma gondii [66], viruses and many others [67,68,69,70,71]. A reliable methodology for detecting pathogens (similar to [70,72]) from rodent faecal samples could help to estimate occurrence of these pathogens and make preventive screenings.
- -
- Estimation of environmental pollution. Small mammals, as consumers of vegetation, accumulate natural and artificial substances, such as pesticides, heavy metals, etc. Monitoring could help prevent damage to the environment and public health [73,74].
- -
- Food quality changes and rodent population oscillations (especially population declines). For ecologists, the study of rodent seasonal oscillation is one of the unresolved topics. The role of diet has been widely discussed but we have not obtained clear conclusions yet [31,75,76]. NIRS makes it possible to detect the diet of many individuals in different populations during the oscillating cycle and it can help to clarify the role of food quality and quantity on population fluctuations.
- -
- Hormonal changes during population oscillation. According to recently published knowledge, gastrointestinal microbiota produce hormones, which affect hosts health and behaviour, such as cortisol and catecholamines (e.g., adrenaline) [77,78]. The levels of these stress hormones change according to population oscillation [79]. These hormones or their precursors might be detectable in faeces. The estimation of stress hormones levels would help to predict an increase/decrease in population and excessive use of poisons could be prevented. The study of hormone levels from faeces may be a promising method for many other types of studies, such as physiological, environmental, or agrotechnical studies.
- -
- Field, orchard, and forest management. In arable landscapes, NIRS can ascertain the real impact of small rodents on crops. Although these mammals sometimes cause great damage in agriculture, it is not clear when and what they eat in the fields; sometimes there are many rodents, even in crops that are not suitable food for them (for example, corn, wheat, or barley for common voles) [80,81]. Weeds growing in fields with these crops may be a more suitable food for rodents [82]. NIRS can help answer the question: what is the proportion of crops and weeds in the diet of rodents? Of course, this proportion can change during the growing season or the ripening of the crop [83]. The NIRS method could help determine how much and when (at what stage of the population cycle, crop ripening, or season) rodents consume the target crops. With this knowledge, interventions against rodent damage could be more precise. Similarly in orchards [84,85] and forests [86,87]: what proportion of rodents’ diet is made up of the bark and sap of young trees? When do rodents consume them? Answers to such questions would allow more accurate crop protection and lower the use of rodenticides.
- -
- Studies of Norway rats in cities. Although rats are the most common mammalian health threat in cities, exhaustive information about their ecology is lacking [88]. Their faeces are easy to find and continuous monitoring of their health status (zoonoses occurrence, poison levels, and stress hormones) and population parameters (breeding etc.) could be helpful to planning their reduction.
7. Conclusions
The NIRS method has been used only rarely to study small mammals; this is a shame, especially in connection with the invention of microsensors that have great potential. NIR spectroscopy can help find answers to questions that are difficult to answer by other methods because of time, technology, and financial requirements; these are issues of both basic and applied research. Basic research could use NIRS for monitoring physiological variables (e.g., type and quality of food, occurrence of pathogens, environmental pollution, etc.). The applied area could use the acquired knowledge for population monitoring (e.g., frequency of pathogens or environmental pollution), and subsequently to manage rodent populations more precisely (detection and prediction of conditions under which damage occurs facilitates better timing of interventions to minimize the damage). The main limitation of using the NIRS method is likely the high demand on technological equipment and expertise in a number of different branches, such as experimental design, spectrum removal, calibration, and the interpretation of results (i.e., the need to create a team of people of very different specializations).
Author Contributions
Writing—original draft preparation, E.J. and L.Č.; writing—review and editing, M.H. and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the anonymous reviewers and the editor for their inspiring comments, which significantly improved the quality of the paper. Eva Čepelková helped to improve the English.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Givens, D.I.; De Boever, J.L.; Deaville, E.R. The principles, practices and some future applications of near infrared spectroscopy for predicting the nutritive value of foods for animals and humans. Nutr. Res. Rev. 1997, 10, 83–84. [Google Scholar] [CrossRef]
- Foley, W.J.; McIlwee, A.; Lawler, I.R.; Aragones, L.; Woolnough, A.P.; Berding, N. Ecological applications of near infrared reflectance spectroscopy—A tool for rapid, cost-effective prediction of composition of plant and animal tissues and aspects of animal performance. Oecologia 1998, 116, 293–305. [Google Scholar] [CrossRef]
- Shenk, J.S.; Westerhaus, M.O. The Application of near Infrared Reflectance Spectroscopy (NIRS) to Forage Analysis. In Forage Quality, Evaluation and Utilization; American Society of Agronomy: Madison, WI, USA, 1994; pp. 406–449. [Google Scholar]
- Tolleson, D.R.; Angerer, J.P. The application of near infrared spectroscopy to predict faecal nitrogen and phosphorus in multiple ruminant herbivore species. Rangel. J. 2021, 42, 415–423. [Google Scholar] [CrossRef]
- Kaneko, H.; Lawler, I.R. Can Near Infrared Spectroscopy be used to improve assessment of marine mammal diets via fecal analysis? Mar. Mammal Sci. 2006, 22, 261–275. [Google Scholar] [CrossRef]
- Moore, B.D.; Lawler, I.R.; Wallis, I.R.; Beale, C.M.; Foley, W.J. Palatability mapping: A koala’s eye view of spatial variation in habitat quality. Ecology 2010, 91, 3165–3176. [Google Scholar] [CrossRef] [PubMed]
- Wiedower, E.E.; Kouba, A.J.; Vance, C.K.; Hansen, R.L.; Stuth, J.W.; Tolleson, D.R. Fecal Near Infrared Spectroscopy to Discriminate Physiological Status in Giant Pandas. PLoS ONE 2012, 7, e38908. [Google Scholar] [CrossRef] [PubMed]
- Counsell, K.R.; Vance, C.K. Recent Advances of near Infrared Spectroscopy in Wildlife and Ecology Studies. NIR News 2016, 27, 29–32. [Google Scholar] [CrossRef]
- Kinoshita, K.; Miyazaki, M.; Morita, H.; Vassileva, M.; Tang, C.; Li, D.; Ishikawa, O.; Kusunoki, H.; Tsenkova, R. Spectral pattern of urinary water as a biomarker of estrus in the giant panda. Sci. Rep. 2012, 2, 7. [Google Scholar] [CrossRef]
- Kinoshita, K.; Kuze, N.; Kobayashi, T.; Miyakawa, E.; Narita, H.; Inoue-Murayama, M.; Idani, G.; Tsenkova, R. Detection of urinary estrogen conjugates and creatinine using near infrared spectroscopy in Bornean orangutans (Pongo Pygmaeus). Primates 2016, 57, 51–59. [Google Scholar] [CrossRef]
- Iverson, S.J.; Arnould, J.P.Y.; Boyd, I.L. Milk fatty acid signatures indicate both major and minor shifts in the diet of lactating Antarctic fur seals. Can. J. Zool. 1997, 75, 188–197. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Huck, C.W. Near-Infrared Spectroscopy in Bio-Applications. Molecules 2020, 25, 2948. [Google Scholar] [CrossRef]
- Vance, C.K.; Tolleson, D.R.; Kinoshita, K.; Rodriguez, J.; Foley, W.J. Near Infrared Spectroscopy in Wildlife and Biodiversity. J. Near Infrared Spectrosc. 2016, 24, 1–25. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Huck, C.W. Principles and Applications of Miniaturized Near-Infrared (NIR) Spectrometers. Chem. A Eur. J. 2021, 27, 1514–1532. [Google Scholar] [CrossRef]
- Pereira, J.F.Q.; Silva, C.S.; Vieira, M.J.L.; Pimentel, M.F.; Braz, A.; Honorato, R.S. Evaluation and identification of blood stains with handheld NIR spectrometer. Microchem. J. 2017, 133, 561–566. [Google Scholar] [CrossRef]
- Dickman, C.R. Rodent-Ecosystem Relationships: A Review. In Ecologically-Based Management of Rodent Pests; Singleton, G.R., Hinds, L.A., Leirs, H., Zhang, Z., Eds.; Australian Centre for International Agricultural Research: Canberra, Australia, 1999; Volume 59, pp. 113–133. [Google Scholar]
- Fischer, C.; Gayer, C.; Kurucz, K.; Riesch, F.; Tscharntke, T.; Batáry, P. Ecosystem services and disservices provided by small rodents in arable fields: Effects of local and landscape management. J. Appl. Ecol. 2018, 55, 548–558. [Google Scholar] [CrossRef]
- Verde Arregoitia, L.D.; D’Elía, G. Classifying rodent diets for comparative research. Mamm. Rev. 2021, 51, 51–65. [Google Scholar] [CrossRef]
- Hansson, L. Intraspecific Variation in Dynamics: Small Rodents between Food and Predation in Changing Landscapes. Oikos 1999, 86, 159–169. [Google Scholar] [CrossRef]
- Ieradi, L.A.; Moreno, S.; Bolívar, J.P.; Cappai, A.; Di Benedetto, A.; Cristaldi, M. Free-living rodents as bioindicators of genetic risk in natural protected areas. Environ. Pollut. 1998, 102, 265–268. [Google Scholar] [CrossRef]
- Singleton, G.R.; Jacob, J.; Krebs, C.J.; Monadjem, A. A meeting of mice and men: Rodent impacts on food security, human diseases and wildlife conservation; Ecosystem benefits; Fascinating biological models. Wildl. Res. 2015, 42, 83–85. [Google Scholar] [CrossRef][Green Version]
- Jacob, J.; Imholt, C.; Caminero-Saldaña, C.; Couval, G.; Giraudoux, P.; Herrero-Cófreces, S.; Horváth, G.; Luque-Larena, J.J.; Tkadlec, E.; Wymenga, E. Europe-wide outbreaks of common voles in 2019. J. Pest Sci. 2020, 93, 703–709. [Google Scholar] [CrossRef]
- Wilson, D.E.; Lacher, T.E.; Mittermeier, R.A. Handbook of the Mammals of the World, Volume 6 and 7: Lagomorphs and Rodents I and II.; Wilson, D.E., Lacher, T.E., Mittermeier, R.A., Eds.; Lynx: Barcelona, Spain, 2017. [Google Scholar]
- Singleton, G.R.; Belmain, S.R.; Brown, P.R.; Hardy, B. Rodent Outbreaks: Ecology and Impacts; Singleton, G.R., Belmain, S., Brown, P.R., Hardy, B., Eds.; International Rice Research Institute: Los Baños, Philippines, 2010; ISBN 9789712202575. [Google Scholar]
- Meerburg, B.G.; Singleton, G.R.; Leirs, H. The year of the rat ends—Time to fight hunger! Pest Manag. Sci. 2009, 65, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Tkadlec, E. Rodent outbreaks in Europe: Dynamics and damage. In Rodent Outbreaks—Ecology and Impacts; Singleton, G.R., Belmain, S., Brown, P.R., Hardy, B., Eds.; International Rice Research Institute: Los Baños, Philippines, 2010; pp. 207–223. ISBN 9789712202575. [Google Scholar]
- Wood, B.J.; Singleton, G.R. Rodents in agriculture and forestry. In Rodent Pests and Their Control, 2nd ed.; University of Reading: Reading, UK, 2015; pp. 33–80. ISBN 9781845938178. [Google Scholar]
- Hansen, S.C.; Stolter, C.; Jacob, J. The smell to repel: The effect of odors on the feeding behavior of female rodents. Crop Prot. 2015, 78, 270–276. [Google Scholar] [CrossRef]
- Heroldová, M. Diet of four rodent species from Robinia pseudo-acacia stands in South Moravia. Acta Theriol. 1994, 39, 333–337. [Google Scholar] [CrossRef]
- Holišová, V. The food of Clethrionomys glareolus at different population densities. Acta Sci. Nat. Brno 1971, 5, 1–43. [Google Scholar]
- Butet, A. Does food quality Drive Cycle in Microtus arvalis? Study on a French Atlantic Marsh Population. Proc. I Eur. Congr. Mammal. 1996, 177, 177–188. [Google Scholar]
- Windley, H.R.; Wallis, I.R.; DeGabriel, J.L.; Moore, B.D.; Johnson, C.N.; Foley, W.J. A faecal index of diet quality that predicts reproductive success in a marsupial folivore. Oecologia 2013, 173, 203–212. [Google Scholar] [CrossRef]
- Wolff, J.O.; Sherman, P.W. Rodent Societies: An Ecological and Evolutionary Perspective; The University Chicago Press: Chicago, IL, USA; London, UK, 2007; ISBN 9780226905372. [Google Scholar]
- Heroldová, M.; Čižmár, D.; Tkadlec, E. Predicting rodent impact in crop fields by near-infrared reflectance spectroscopy analysis of their diet preferences. Crop Prot. 2010, 29, 773–776. [Google Scholar] [CrossRef]
- Lean, I.J.; Golder, H.M.; Black, J.L.; King, R.; Rabiee, A.R. In vivo indices for predicting acidosis risk of grains in cattle: Comparison with in vitro methods. J. Anim. Sci. 2013, 91, 2823–2835. [Google Scholar] [CrossRef]
- André, J.; Lawler, I.R. Near infrared spectroscopy as a rapid and inexpensive means of dietary analysis for a marine herbivore, dugong Dugong dugon. Mar. Ecol. Prog. Ser. 2003, 257, 259–266. [Google Scholar] [CrossRef][Green Version]
- Chivers, D.J.; Langer, P. The Digestive System in Mammals: Food, Form and Function; Cambridge University Press: Cambridge, UK, 1994; ISBN 0-521-44016-5. [Google Scholar]
- Langer, P. The digestive tract and life history of small mammals. Mamm. Rev. 2002, 32, 107–131. [Google Scholar] [CrossRef]
- Butet, A.; Delettre, Y.R. Diet differentiation between European arvicoline and murine rodents. Acta Theriol. 2011, 56, 297–304. [Google Scholar] [CrossRef]
- Givens, D.I.; Deaville, E.R. The current and future role of near infrared reflectance spectroscopy in animal nutrition: A review. Aust. J. Agric. Res. 1999, 50, 1131–1145. [Google Scholar] [CrossRef]
- Steyaert, S.M.; Hütter, F.J.; Elfström, M.; Zedrosser, A.; Hackländer, K.; Lê, M.H.; Windisch, W.M.; Swenson, J.E.; Isaksson, T. Faecal spectroscopy: A practical tool to assess diet quality in an opportunistic omnivore. Wildl. Biol. 2012, 18, 431–438. [Google Scholar] [CrossRef]
- Zijlstra, R.T.; Swift, M.L.; Wang, L.F.; Scott, T.A.; Edney, M.J. Short communication: Near infrared reflectance spectroscopy accurately predicts the digestible energy content of barley for pigs. Can. J. Anim. Sci. 2011, 91, 301–304. [Google Scholar] [CrossRef]
- Rivero-Marcotegui, A.; Olivera-Olmedo, J.E.; Valverde-Visus, F.S.; Palacios-Sarrasqueta, M.; Grijalba-Uche, A.; García-Merlo, S. Water, fat, nitrogen, and sugar content in feces: Reference intervals in children. Clin. Chem. 1998, 44, 1540–1544. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Bjørnstad, O.N.; Stenseth, N.C. Density Dependence in Voles and Mice: A Comparative Study. Ecology 1999, 80, 638–650. [Google Scholar] [CrossRef]
- Suchomel, J.; Heroldová, M.; Mlček, J.; Šustová, K.; Růžičková, J.; Remeš, M. Winter Diet Preferences of Apodemus flavicollis Under Influence of Supplementary Food in Pheasantry. In Proceedings of the 9th International Mammalogical Congress, The Scientific Council of Japan, The Mammalogical Society of Japan, Sapporo, Japan, 31 July 2005; p. 301. [Google Scholar]
- Cox, P.G.; Rayfield, E.J.; Fagan, M.J.; Herrel, A.; Pataky, T.C.; Jeffery, N. Functional evolution of the feeding system in rodents. PLoS ONE 2012, 7, e36299. [Google Scholar] [CrossRef]
- Čepelka, L.; Heroldová, M.; Jánová, E.; Suchomel, J.; Čižmár, D. Rodent stomach sample preparation for nitrogen NIRS analysis. Mamm. Biol. 2017, 87, 13–16. [Google Scholar] [CrossRef]
- Čepelka, L.; Heroldová, M.; Jánová, E.; Suchomel, J. The Dynamics of Nitrogenous Substances in Rodent Diet in a Forest Environment. Mammalia 2014, 78, 327–333. [Google Scholar] [CrossRef]
- Čepelka, L.; Heroldová, M.; Jánová, E.; Suchomel, J. Dynamics of nitrogenous substance content in the diet of the wood mouse (Apodemus sylvaticus). Acta Univ. Agric. Silvic. Mendel. Brun. 2013, 61, 1–7. [Google Scholar] [CrossRef]
- Jánová, E.; Bryja, J.; Čižmár, D.; Čepelka, L.; Heroldová, M. A new method for assessing food quality in common vole (Microtus arvalis) populations. Eur. J. Wildl. Res. 2015, 61, 57–62. [Google Scholar] [CrossRef]
- Jánová, E.; Heroldová, M.; Čepelka, L. Rodent food quality and its relation to crops and other environmental and population parameters in an agricultural landscape. Sci. Total Environ. 2016, 562, 164–169. [Google Scholar] [CrossRef]
- Heroldová, M.; Jánová, E. Feeding strategy of two rodent species in a set-Aside field and its influence on alimentary tract morphometry. Mammalia 2019, 83, 34–40. [Google Scholar] [CrossRef]
- Burgin, C.J.; Colella, J.P.; Kahn, P.L.; Upham, N.S. How many species of mammals are there? J. Mammal. 2018, 99, 1–14. [Google Scholar] [CrossRef]
- Vance, C.K.; Kouba, A.J.; Willard, S.T. Near Infrared Spectroscopy Applications in Amphibian Ecology and Conservation: Gender and Species Identification. NIR News 2014, 25, 10–15. [Google Scholar] [CrossRef]
- Tolleson, D.R.; Schafer, D.W. Evaluation of non-invasive bioforensic techniques for determining the age of hot-iron brand burn scars in cattle. Transl. Anim. Sci. 2021, 1–10. [Google Scholar] [CrossRef]
- Goeser, J.P.; Hoffman, P.C.; Combs, D.K. Modification of a rumen fluid priming technique for measuring in vitro neutral detergent fiber digestibility. J. Dairy Sci. 2009, 92, 3842–3848. [Google Scholar] [CrossRef]
- Gagnon, D.D.; Hancock, C.; Mccue, A.; Beckett-Brown, N.; Gagnon, J.; Williams, L.; Marsh, D.; Munten, S. Muscle cooling modulates tissue oxidative and biochemical responses but not energy metabolism during exercise. Eur. J. Appl. Physiol. 2020, 120, 1761–1775. [Google Scholar] [CrossRef]
- Drozdz, A. Digestibility and Assimilation of Natural Foods in Small Rodents. Acta Theriol. 1968, 13, 367–389. [Google Scholar] [CrossRef]
- Kurien, B.T.; Everds, N.E.; Scofield, R.H. Experimental animal urine collection: A review. Lab. Anim. 2004, 38, 333–361. [Google Scholar] [CrossRef]
- Rose, C.; Parker, A.; Jefferson, B.; Cartmell, E. The characterization of feces and urine: A review of the literature to inform advanced treatment technology. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1827–1879. [Google Scholar] [CrossRef] [PubMed]
- Rossow, H.; Sissonen, S.; Koskela, K.A.; Kinnunen, P.M.; Hemmilä, H.; Niemimaa, J.; Huitu, O.; Kuusi, M.; Vapalahti, O.; Henttonen, H.; et al. Detection of Francisella tularensis in Voles in Finland. Vector Borne Zoonotic Dis. 2014, 14, 193–198. [Google Scholar] [CrossRef]
- Jeske, K.; Tomaso, H.; Imholt, C.; Schulz, J.; Beerli, O.; Suchomel, J.; Heroldová, M.; Jacob, J.; Staubach, C.; Ulrich, R.G. Detection of Francisella tularensis in three vole species in Central Europe. Transbound. Emerg. Dis. 2019, 66, 1029–1032. [Google Scholar] [CrossRef] [PubMed]
- Hanincova, K.; Schäfer, S.M.; Etti, S.; Sewell, H.S.; Taragelová, V.; Ziak, D.; Labuda, M.; Kurtenbach, K. Association of Borrelia afzelii with rodents in Europe. Parasitology 2003, 126, 11–20. [Google Scholar] [CrossRef]
- Backhans, A.; Fellström, C.; Lambertz, S.T. Occurrence of pathogenic Yersinia enterocolitica and Yersinia pseudotuberculosis in small wild rodents. Epidemiol. Infect. 2011, 139, 1230–1238. [Google Scholar] [CrossRef]
- Zheludkov, M.M.; Tsirelson, L.E. Reservoirs of Brucella infection in nature. Biol. Bull. 2010, 37, 709–715. [Google Scholar] [CrossRef]
- Gotteland, C.; Chaval, Y.; Villena, I.; Galan, M.; Geers, R.; Aubert, D.; Poulle, M.L.; Charbonnel, N.; Gilot-Fromont, E. Species or local environment, what determines the infection of rodents by Toxoplasma gondii? Parasitology 2014, 141, 259–268. [Google Scholar] [CrossRef]
- Obiegala, A.; Heuser, E.; Ryll, R.; Imholt, C.; Fürst, J.; Prautsch, L.M.; Plenge-Bönig, A.; Ulrich, R.G.; Pfeffer, M. Norway and black rats in Europe: Potential reservoirs for zoonotic arthropod-borne pathogens? Pest Manag. Sci. 2019, 75, 1556–1563. [Google Scholar] [CrossRef]
- Mrochen, D.M.; Schulz, D.; Fischer, S.; Jeske, K.; El Gohary, H.; Reil, D.; Imholt, C.; Trübe, P.; Suchomel, J.; Tricaud, E.; et al. Wild rodents and shrews are natural hosts of Staphylococcus aureus. Int. J. Med. Microbiol. 2018, 308, 590–597. [Google Scholar] [CrossRef]
- Heroldová, M.; Pejčoch, M.; Bryja, J.; Jánová, E.; Suchomel, J.; Tkadlec, E. Tula Virus in Populations of Small Terrestrial Mammals in a Rural Landscape. Vector Borne Zoonotic Dis. 2010, 10, 599–603. [Google Scholar] [CrossRef]
- Sakudo, A.; Suganuma, Y.; Kobayashi, T.; Onodera, T.; Ikuta, K. Near-infrared spectroscopy: Promising diagnostic tool for viral infections. Biochem. Biophys. Res. Commun. 2006, 341, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Hardestam, J.; Karlsson, M.; Falk, K.I.; Olsson, G.; Klingström, J.; Lundkvist, Å. Puumala hantavirus excretion kinetics in bank voles (Myodes glareolus). Emerg. Infect. Dis. 2008, 14, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.M.B.; Mutsaers, M.; Garcia, G.A.; David, M.R.; Pavan, M.G.; Petersen, M.T.; Corrêa-Antônio, J.; Couto-Lima, D.; Maes, L.; Dowell, F.; et al. High throughput estimates of Wolbachia, Zika and chikungunya infection in Aedes aegypti by near-infrared spectroscopy to improve arbovirus surveillance. Commun. Biol. 2021, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vávrová, M.; Gargošová, Z.H.; Šucman, E.; Večerek, V.; Kořinek, P.; Zukal, J.; Zejda, J.; Sebestiánová, N.; Kubištová, I. Game animals and small terrestrial mammals—Suitable bioindicators for the pollution assessment in agrarian ecosystems. Fresenius Environ. Bull. 2003, 12, 165–172. [Google Scholar]
- Casale, M.; Bagnasco, L.; Giordani, P.; Mariotti, M.G.; Malaspina, P. NIR spectroscopy as a tool for discriminating between lichens exposed to air pollution. Chemosphere 2015, 134, 355–360. [Google Scholar] [CrossRef]
- Yoccoz, N.G.; Stenseth, N.C.; Henttonen, H.; Prévot-Julliard, A.-C. Effects of food addition on the seasonal density dependent structure of bank vole Clethrionomys glareolus populations. J. Anim. Ecol. 2001, 70, 713–720. [Google Scholar] [CrossRef]
- White, T.C.R. The role of food, weather and climate in limiting the abundance of animals. Biol. Rev. 2008, 83, 227–248. [Google Scholar] [CrossRef]
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, 1288–1295. [Google Scholar] [CrossRef]
- Boonstra, R.; Hik, D.; Singleton, G.R.; Tinnikov, A. The Impact of Predator-Induced Stress on the Snowshoe Hare Cycle. Ecol. Monogr. 1998, 68, 371–394. [Google Scholar] [CrossRef]
- Korpimäki, E.; Brown, P.R.; Jacob, J.; Pech, R.P. The Puzzles of Population Cycles and Outbreaks of Small Mammals Solved? Bioscience 2004, 54, 1071–1079. [Google Scholar] [CrossRef]
- Jánová, E.; Heroldová, M.; Konecny, A.; Bryja, J.; Konečný, A. Traditional and diversified crops in South Moravia (Czech Republic): Habitat preferences of common vole and mice species. Mamm. Biol. 2011, 76, 570–576. [Google Scholar] [CrossRef]
- Dell’Agnello, F.; Natali, C.; Bertolino, S.; Fattorini, L.; Fedele, E.; Foggi, B.; Martini, M.; Pisani, C.; Riga, F.; Sgarlata, A.; et al. Assessment of seasonal variation of diet composition in rodents using DNA barcoding and Real-Time PCR. Sci. Rep. 2019, 9, 14124. [Google Scholar] [CrossRef] [PubMed]
- Htwe, N.M.; Singleton, G.R.; Johnson, D.E. Interactions between rodents and weeds in a lowland rice agro-ecosystem: The need for an integrated approach to management. Integr. Zool. 2019, 14, 396–409. [Google Scholar] [CrossRef]
- Heroldová, M.; Zejda, J.; Zapletal, M.; Obdržálková, D.; Jánová, E.; Bryja, J.; Tkadlec, E. Foraging strategy of small rodents in winter rape. Plant Soil Environ. 2004, 50, 175–181. [Google Scholar] [CrossRef]
- Bertolino, S.; Asteggiano, L.; Saladini, M.A.; Giordani, L.; Vittone, G.; Alma, A. Environmental factors and agronomic practices associated with Savi’s pine vole abundance in Italian apple orchards. J. Pest Sci. 2015, 88, 135–142. [Google Scholar] [CrossRef]
- Suchomel, J.; Šipoš, J.; Čepelka, L.; Heroldová, M. The impact of Microtus arvalis and Lepus europaeus on apple trees by trunk bark gnawing. Plant Prot. Sci. 2019, 55, 142–147. [Google Scholar] [CrossRef]
- Krojerová-Prokešová, J.; Homolka, M.; Heroldová, M.; Barančeková, M.; Baňař, P.; Kamler, J.; Modlinger, R.; Purchart, L.; Zejda, J.; Suchomel, J. Patterns of vole gnawing on saplings in managed clearings in Central European forests. For. Ecol. Manag. 2018, 408, 137–147. [Google Scholar] [CrossRef]
- Leiva, M.J.; Díaz-Maqueda, A. Fast-growing seeds and delayed rodent predatory activity in the seeding season: A combined mechanism to escape and survive rodent predation in Quercus ilex subsp. ballota L. acorns and seedlings. For. Ecol. Manag. 2016, 380, 23–30. [Google Scholar] [CrossRef]
- Byers, K.A.; Lee, M.J.; Patrick, D.M.; Himsworth, C.G. Rats about town: A systematic review of rat movement in urban ecosystems. Front. Ecol. Evol. 2019, 7, 1–12. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).