Spectral Response Assessment of Moss-Dominated Biological Soil Crust Coverage Under Dry and Wet Conditions
Abstract
1. Introduction
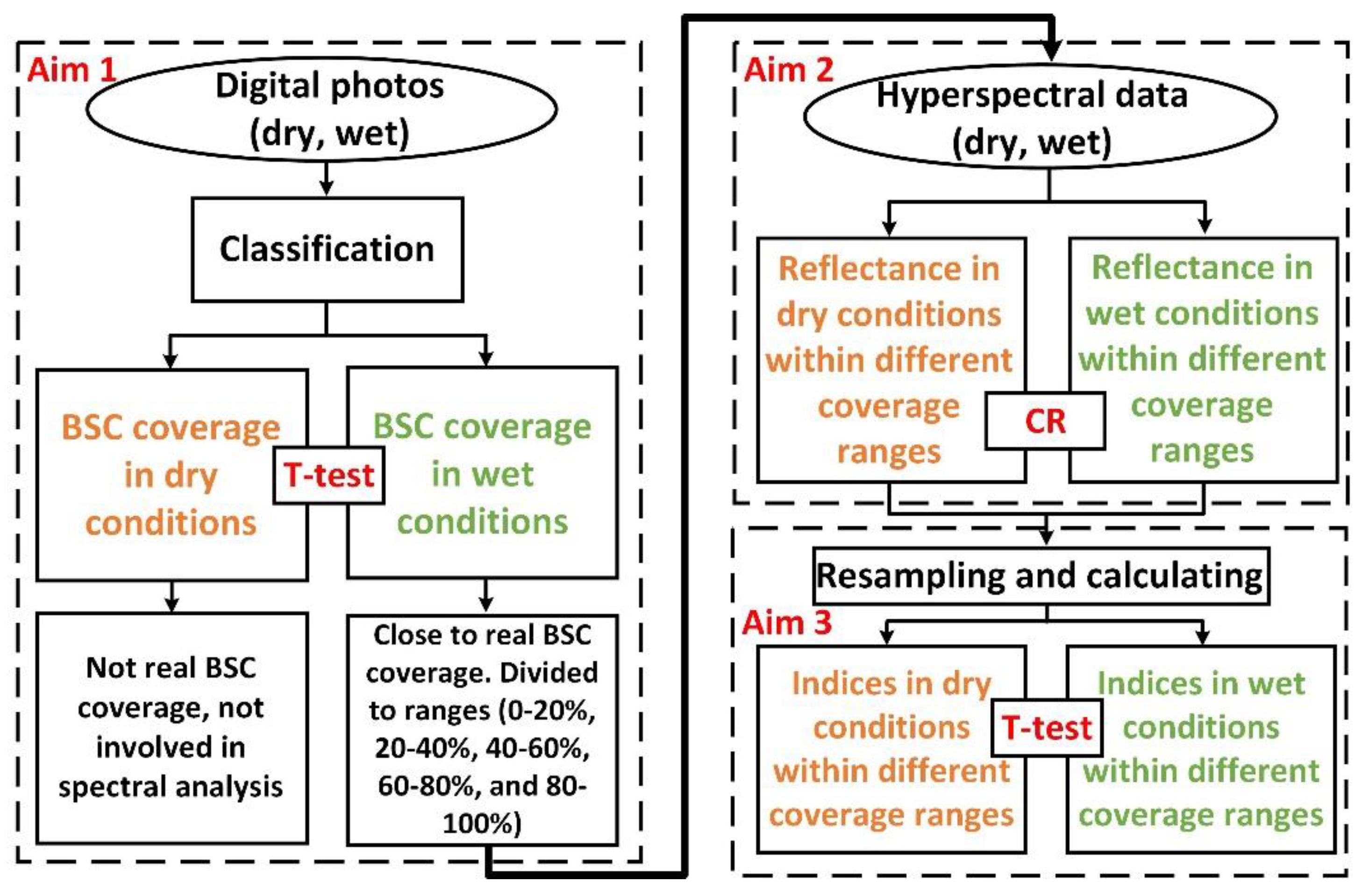
2. Materials and Methods
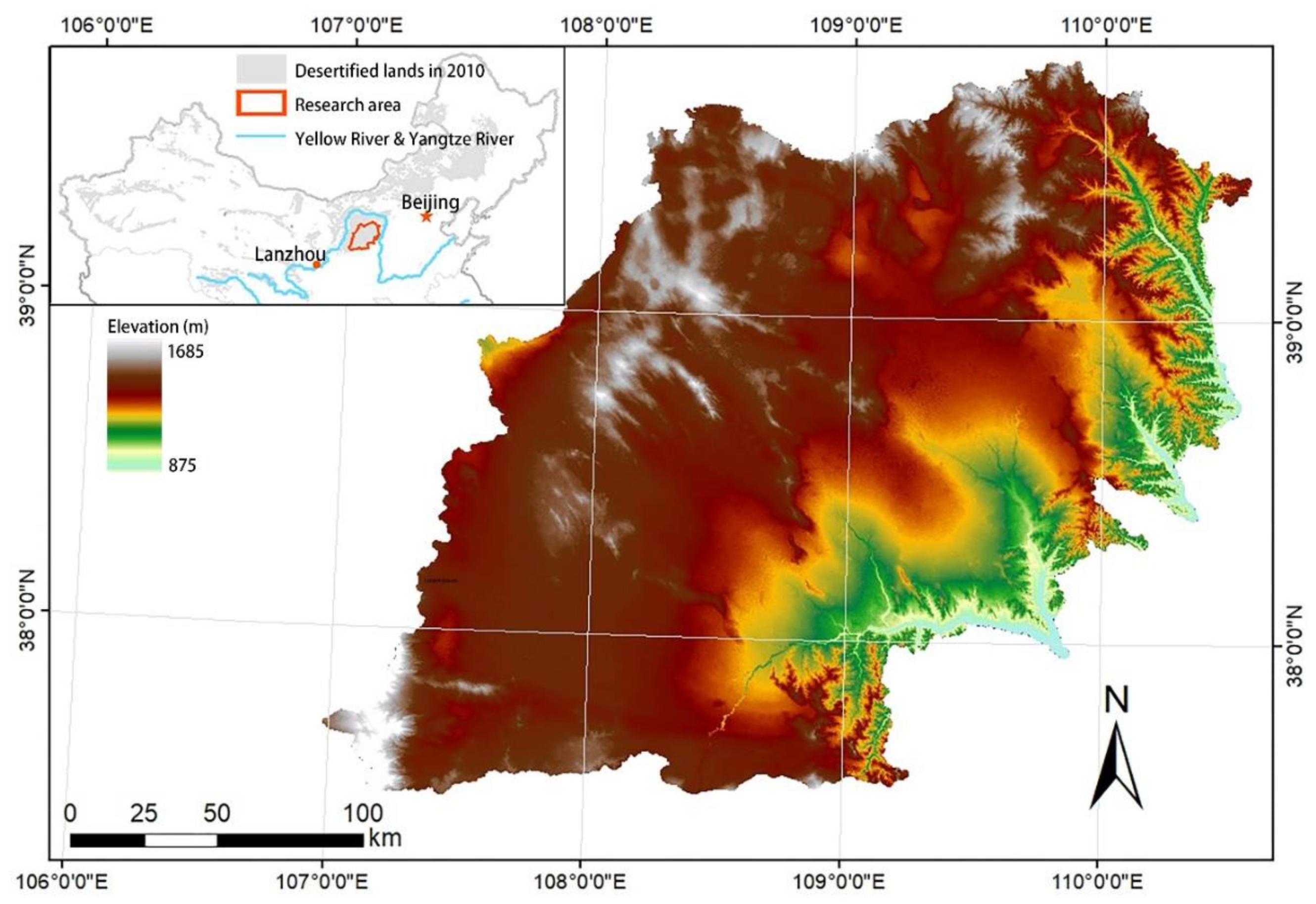
2.1. Study Area
2.2. Datasets
2.2.1. Field Spectra Measurement and Classification of Plots
2.2.2. Pairwise Dry and Wet Biological Soil Crust (BSC) Field Plots
2.2.3. Estimation of BSC Coverage
2.3. Treatment of Spectral Datasets
2.3.1. Continuum Removal (CR)
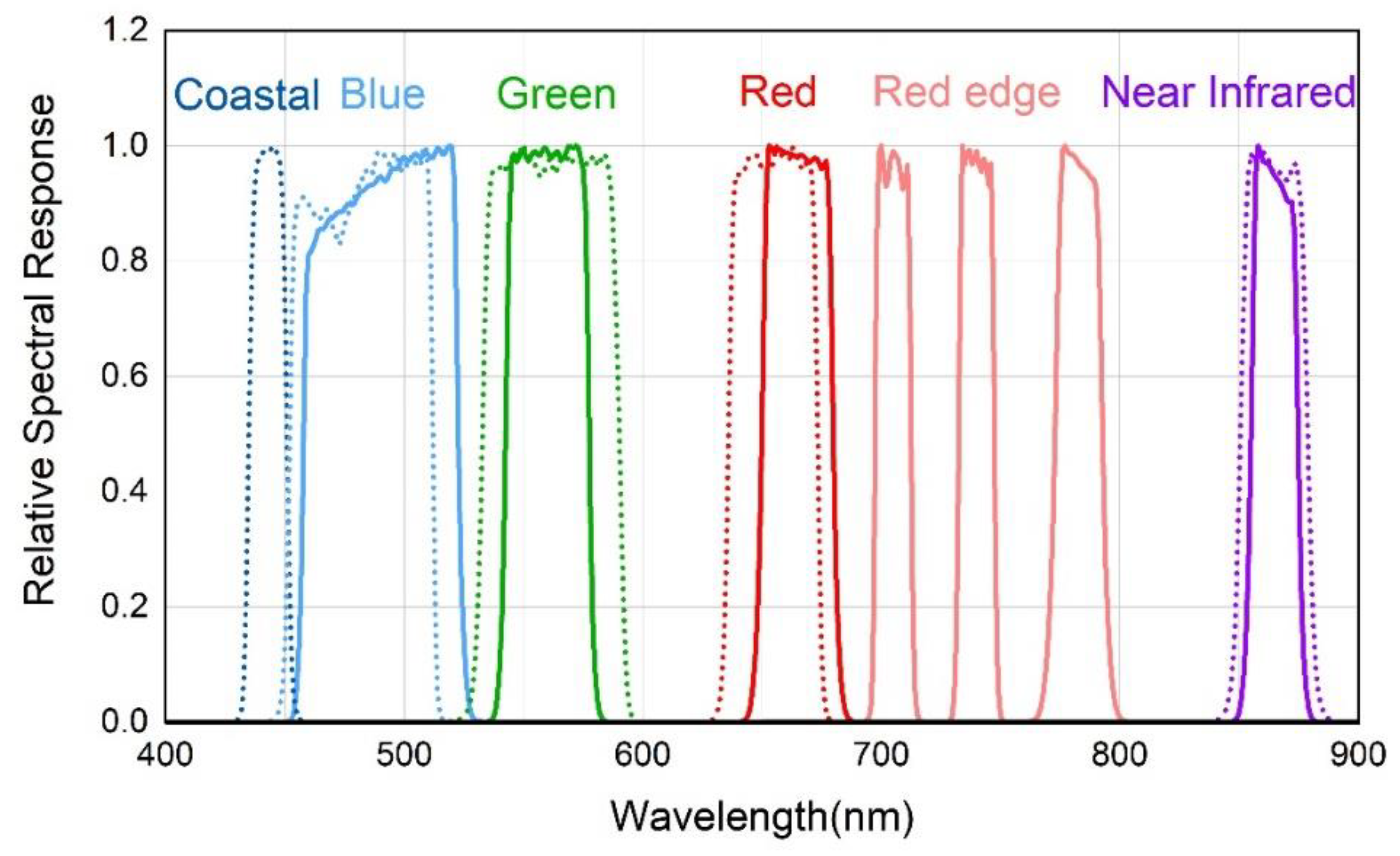
2.3.2. Hyperspectral Resampling
2.3.3. Index Calculations
2.4. Statistical Analysis
3. Results
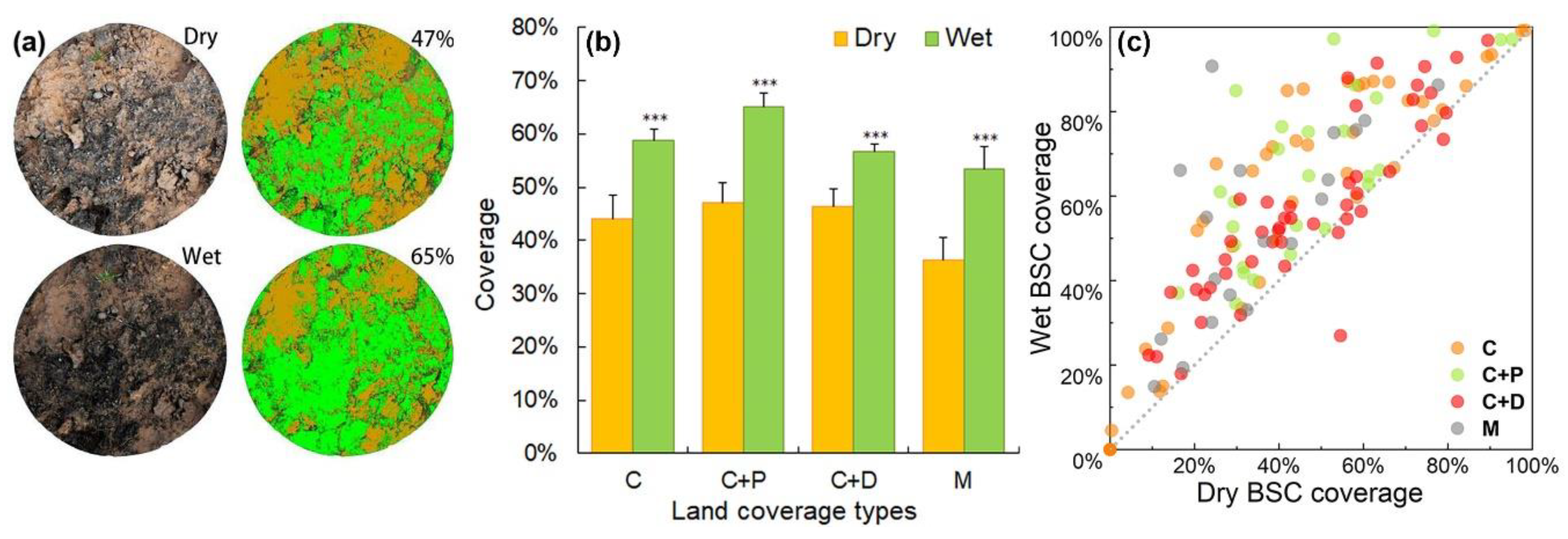
3.1. Influence of Dry and Wet Conditions on BSC Coverage Estimation
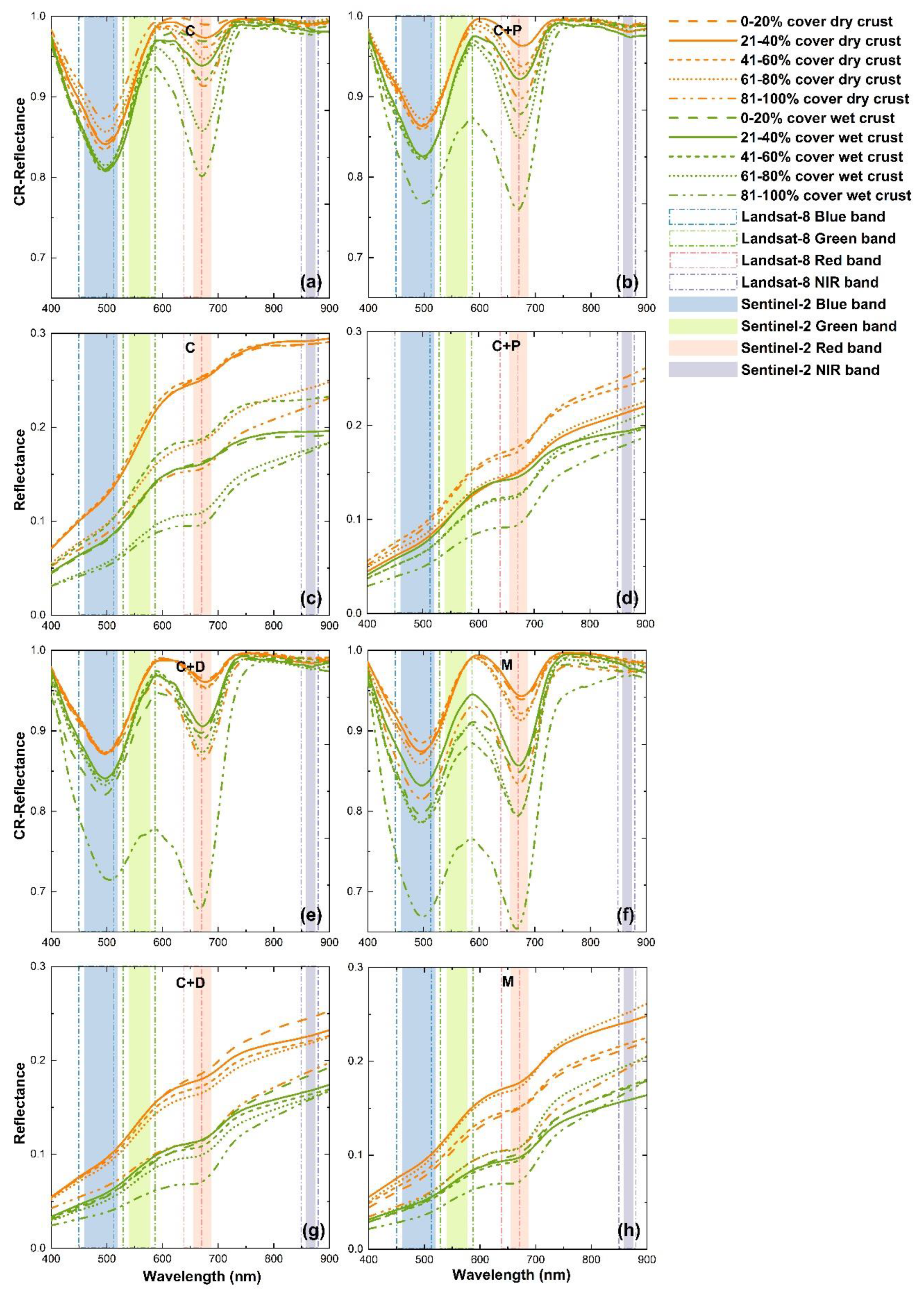
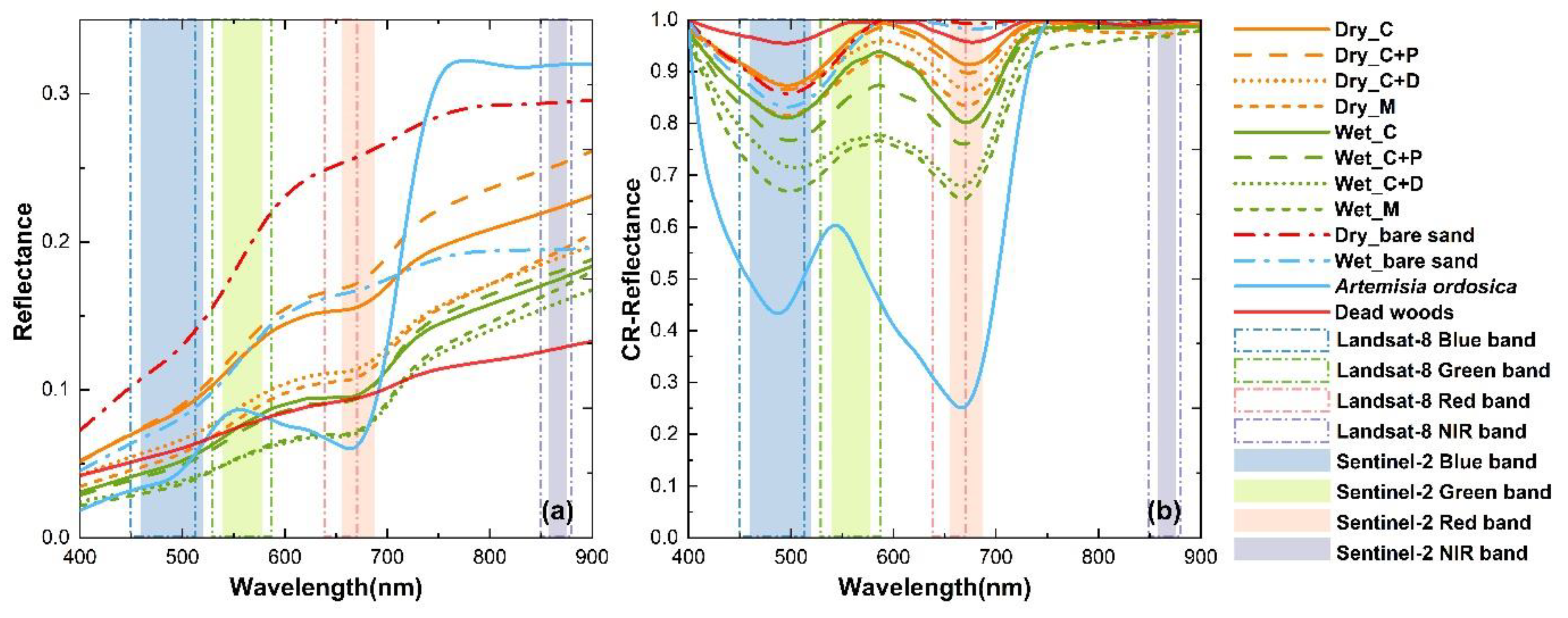
3.2. Response of BSC Spectrum to Dry and Wet Conditions
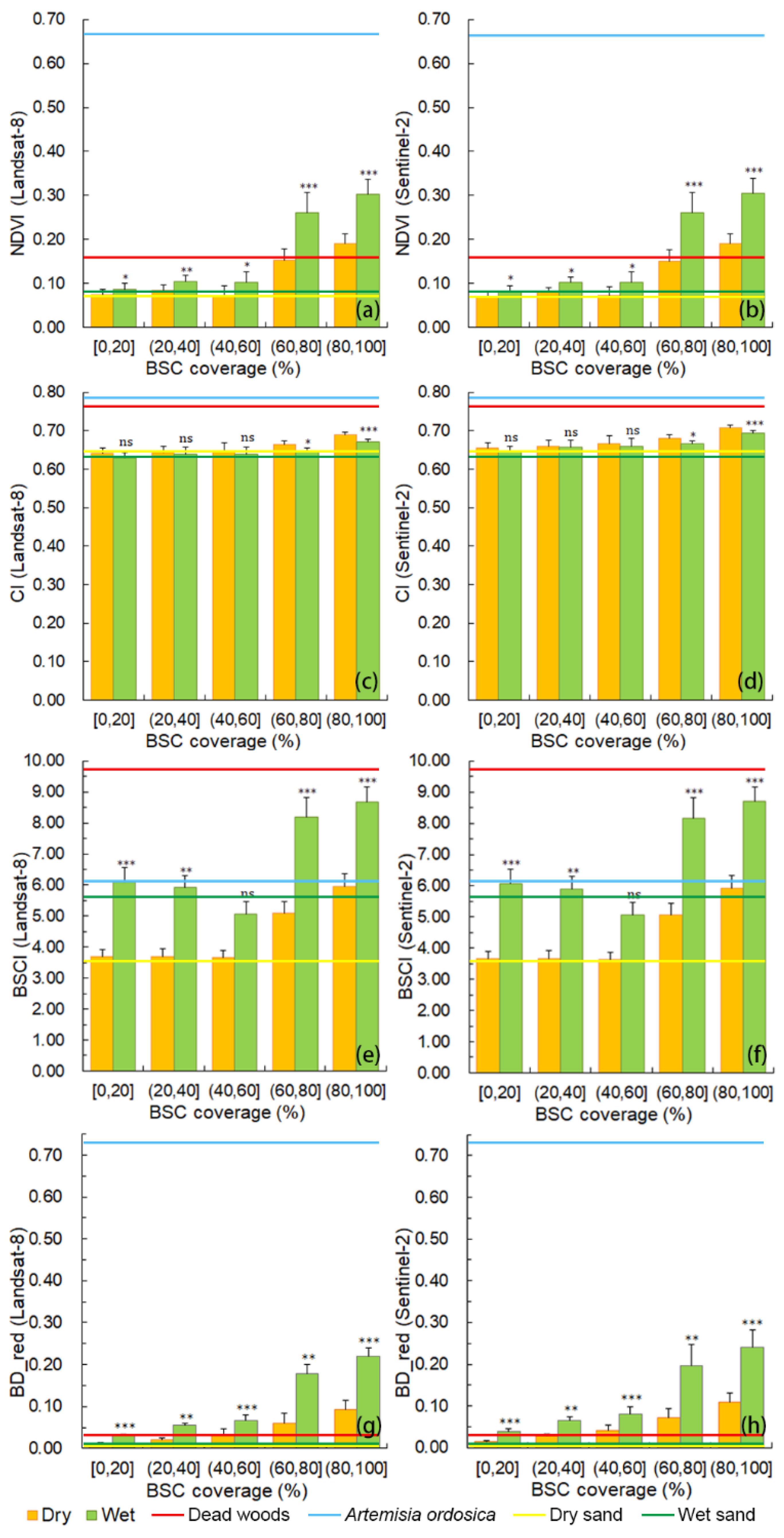
3.3. Normalized Difference Vegetation Index (NDVI), Crust Index (CI), Biological Soil Crust Index (BSCI), and Band Depth of Absorption Feature after Continuum Removal in the Red Band (BD_red) of BSCs with Different Coverage Ranges in Dry and Wet Conditions
4. Discussion
4.1. Influence of Dry and Wet Conditions on BSC Coverage Estimation
4.2. Response of BSC Spectra to Dry and Wet Conditions
4.3. NDVI, CI, BSCI, and BD_red of BSCs with Different Coverage Ranges in Dry and Wet Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Belnap, J.; Lange, O.L. Biological Soil Crusts: Structure, Function, and Management, 2nd ed.; Springer: Berlin, Germany, 2003; pp. 401–471. [Google Scholar]
- Beringer, J.; Lynch, A.; III, F.S.C.; Mack, M.; Bonan, G.B. The representation of arctic soils in the land surface model: The importance of mosses. J. Clim. 2001, 14, 3324–3335. [Google Scholar] [CrossRef]
- Fang, S.B.; Zhang, X.S. Impact of Moss Soil Crust on Vegetation Indexes Interpretation. Spectrosc. Spectr. Anal. 2011, 31, 780–783. [Google Scholar]
- Pointing, S.B.; Belnap, J. Erratum: Microbial colonization and controls in dryland systems. Nat. Rev. Microbiol. 2012, 10, 654. [Google Scholar] [CrossRef]
- Colesie, C.; Green, T.G.; Haferkamp, I.; Budel, B. Habitat stress initiates changes in composition, CO2 gas exchange and C-allocation as life traits in biological soil crusts. ISME J. 2014, 8, 2104–2115. [Google Scholar] [CrossRef]
- Elbert, W.; Weber, B.; Burrows, S.; Steinkamp, J.; Büdel, B.; Andreae, M.O.; Pöschl, U. Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat. Geosci. 2012, 5, 459–462. [Google Scholar] [CrossRef]
- Rodríguez-Caballero, E.; Paul, M.; Tamm, A.; Caesar, J.; Budel, B.; Escribano, P.; Hill, J.; Weber, B. Biomass assessment of microbial surface communities by means of hyperspectral remote sensing data. Sci. Total Environ. 2017, 586, 1287–1297. [Google Scholar] [CrossRef]
- Rodríguez-Caballero, E.; Escribano, P.; Cantón, Y. Advanced image processing methods as a tool to map and quantify different types of biological soil crust. ISPRS-J. Photogramm. Remote Sens. 2014, 90, 59–67. [Google Scholar]
- Karnieli, A. Development and implementation of spectral crust index over dune sands. Int. J. Remote Sens. 1997, 18, 1207–1220. [Google Scholar] [CrossRef]
- Chen, J.; Yuan Zhang, M.; Wang, L.; Shimazaki, H.; Tamura, M. A new index for mapping lichen-dominated biological soil crusts in desert areas. Remote Sens. Environ. 2005, 96, 165–175. [Google Scholar] [CrossRef]
- Chamizo, S.; Stevens, A.; Cantón, Y.; Miralles, I.; Domingo, F.; Van Wesemael, B. Discriminating soil crust type, development stage and degree of disturbance in semiarid environments from their spectral characteristics. Eur. J. Soil Sci. 2012, 63, 42–53. [Google Scholar] [CrossRef]
- Weber, B.; Olehowski, C.; Knerr, T.; Hill, J.; Deutschewitz, K.; Wessels, D.C.J.; Eitel, B.; Büdel, B. A new approach for mapping of Biological Soil Crusts in semidesert areas with hyperspectral imagery. Remote Sens. Environ. 2008, 112, 2187–2201. [Google Scholar] [CrossRef]
- Rozenstein, O.; Karnieli, A. Identification and characterization of Biological Soil Crusts in a sand dune desert environment across Israel–Egypt border using LWIR emittance spectroscopy. J. Arid. Environ. 2015, 112, 75–86. [Google Scholar] [CrossRef]
- Karnieli, A.; Shachak, M.; Tsoar, H.; Zaady, E.; Kaufman, Y.; Danin, A.; Porter, W. The effect of microphytes on the spectral reflectance of vegetation in semiarid regions. Remote Sens. Environ. 1996, 57, 88–96. [Google Scholar] [CrossRef]
- O’Neill, A.L. Reflectance spectra of microphytic soil crusts in semi-arid Australia. Int. J. Remote Sens. 1994, 15, 675–681. [Google Scholar] [CrossRef]
- Weber, B.; Büdel, B.; Belnap, J. Biological Soil Crusts: An Organizing Principle in Drylands, 1st ed.; Springer: Cham, Switzerland, 2016; pp. 37–236. [Google Scholar]
- Chen, X.; Wang, T.; Liu, S.; Peng, F.; Tsunekawa, A.; Kang, W.; Guo, Z.; Feng, K. A New Application of Random Forest Algorithm to Estimate Coverage of Moss-Dominated Biological Soil Crusts in Semi-Arid Mu Us Sandy Land, China. Remote Sens. 2019, 11, 1286. [Google Scholar] [CrossRef]
- Burgheimer, J.; Wilske, B.; Maseyk, K.; Karnieli, A.; Zaady, E.; Yakir, D.; Kesselmeier, J. Relationships between Normalized Difference Vegetation Index (NDVI) and carbon fluxes of biologic soil crusts assessed by ground measurements. J. Arid. Environ. 2006, 64, 651–669. [Google Scholar] [CrossRef]
- Rodríguez-Caballero, E.; Escribano, P.; Olehowski, C.; Chamizo, S.; Hill, J.; Cantón, Y.; Weber, B. Transferability of multi- and hyperspectral optical biocrust indices. ISPRS-J. Photogramm. Remote Sens. 2017, 126, 94–107. [Google Scholar]
- Panigada, C.; Tagliabue, G.; Zaady, E.; Rozenstein, O.; Garzonio, R.; Mauro, B.D.; Amicis, M.D.; Colombo, R.; Cogliati, S.; Miglietta, F.; et al. A new approach for biocrust and vegetation monitoring in drylands using multi-temporal Sentinel-2 images. Prog. Phys. Geog. 2019, 43, 496–520. [Google Scholar] [CrossRef]
- Clark, R.N.; Roush, T.L. Reflectance spectroscopy: Quantitative analysis techniques for remote sensing applications. J. Geophys. Res.-Solid Earth 1984, 89, 6329–6340. [Google Scholar] [CrossRef]
- Escribano, P.; Palacios-Orueta, A.; Oyonarte, C.; Chabrillat, S. Spectral properties and sources of variability of ecosystem components in a Mediterranean semiarid environment. J. Arid. Environ. 2010, 74, 1041–1051. [Google Scholar] [CrossRef]
- Lehnert, L.; Jung, P.; Obermeier, W.; Büdel, B.; Bendix, J. Estimating Net Photosynthesis of Biological Soil Crusts in the Atacama Using Hyperspectral Remote Sensing. Remote Sens. 2018, 10, 891. [Google Scholar] [CrossRef]
- Schofield, W.B.; Pojar, J.; Mackinnon, A. Plants of the Pacific Northwest Coast. The Bryologist 1994, 102, 775. [Google Scholar] [CrossRef][Green Version]
- Fang, S.; Yu, W.; Qi, Y. Spectra and vegetation index variations in moss soil crust in different seasons, and in wet and dry conditions. Int. J. Appl. Earth Obs. Geoinf. 2015, 38, 261–266. [Google Scholar] [CrossRef]
- Green, T.G.A.; Proctor, M.C.F. Physiology of Photosynthetic Organisms Within Biological Soil Crusts: Their Adaptation, Flexibility, and Plasticity. In Biological Soil Crusts: An Organizing Principle in Drylands; Springer: Cham, Switzerland, 2016; pp. 347–381. [Google Scholar]
- Karnieli, A.; Kidron, G.; Glaesser, C. Spectral Characteristics of Cyanobacteria Soil Crust in Semiarid Environments. Remote Sens. Environ 1999, 69, 67–75. [Google Scholar] [CrossRef]
- Rodríguez-Caballero, E.; Knerr, T.; Weber, B. Importance of biocrusts in dryland monitoring using spectral indices. Remote Sens. Environ. 2015, 170, 32–39. [Google Scholar] [CrossRef]
- Blanco-Sacristán, J.; Panigada, C.; Tagliabue, G.; Gentili, R.; Colombo, R.; Ladrón de Guevara, M.; Maestre, F.T.; Rossini, M. Spectral Diversity Successfully Estimates the α-Diversity of Biocrust-Forming Lichens. Remote Sens. 2019, 11, 2942. [Google Scholar] [CrossRef]
- Román, J.R.; Rodríguez-Caballero, E.; Rodríguez-Lozano, B.; Roncero-Ramos, B.; Chamizo, S.; Águila-Carricondo, P.; Cantón, Y. Spectral Response Analysis: An Indirect and Non-Destructive Methodology for the Chlorophyll Quantification of Biocrusts. Remote Sens. 2019, 11, 1350. [Google Scholar]
- Zhang, J.; Wu, B.; Li, Y.; Yang, W.; Lei, Y.; Han, H.; He, J. Biological soil crust distribution in Artemisia ordosica communities along a grazing pressure gradient in Mu Us Sandy Land, Northern China. J. Arid Land 2013, 5, 172–179. [Google Scholar] [CrossRef]
- Cheng, X.; An, S.; Liu, S.; Li, G. Micro-scale spatial heterogeneity and the loss of carbon, nitrogen and phosphorus in degraded grassland in Ordos Plateau, northwestern China. Plant Soil 2004, 259, 29–37. [Google Scholar] [CrossRef]
- Wu, B.; Ci, L.J. Landscape change and desertification development in the Mu Us Sandland, Northern China. J. Arid. Environ. 2002, 50, 429–444. [Google Scholar] [CrossRef]
- Li, X. Eco-physiology of biological soil crusts in desert regions of China, 1st ed.; Higher Education Press: Beijing, China, 2016; pp. 1–141. [Google Scholar]
- Rajeev, L.; da Rocha, U.N.; Klitgord, N.; Luning, E.G.; Fortney, J.; Axen, S.D.; Shih, P.M.; Bouskill, N.J.; Bowen, B.P.; Kerfeld, C.A.; et al. Dynamic cyanobacterial response to hydration and dehydration in a desert biological soil crust. ISME J. 2013, 7, 2178–2191. [Google Scholar] [CrossRef]
- Proctor, M.C.F.; Smirnoff, N. Rapid recovery of photosystems on rewetting desiccation-tolerant mosses: chlorophyll fluorescence and inhibitor experiments. J. Exp. Bot. 2000, 51, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.; Guo, F.; Zhang, W.; Cheng, G. Effect of transplantation time on yield and quality of Astragalus membranaceus var. mongholicus. J. Desert Res. 2016, 36, 406–414. [Google Scholar]
- Qian, T.; Tsunekawa, A.; Peng, F.; Masunaga, T.; Wang, T.; Li, R. Derivation of salt content in salinized soil from hyperspectral reflectance data: A case study at Minqin Oasis, Northwest China. J. Arid Land 2019, 11, 111–122. [Google Scholar] [CrossRef]
- Huang, Z.; Turner, B.J.; Dury, S.J.; Wallis, I.R.; Foley, W.J. Estimating foliage nitrogen concentration from HYMAP data using continuum removal analysis. Remote Sens. Environ. 2004, 93, 18–29. [Google Scholar] [CrossRef]
- Landsat 8 Surface Reflectance Code (Lasrc) Product Guide. Available online: https://www.usgs.gov/media/files/landsat-8-surface-reflectance-code-lasrc-product-guide (accessed on 17 December 2018).
- Sentinel-2 User Handbook. Available online: https://sentinels.copernicus.eu/web/sentinel/user-guides/document-library/-/asset_publisher/xlslt4309D5h/content/sentinel-2-user-handbook (accessed on 24 July 2015).
- Schell, J.A. Monitoring Vegetation Systems in the Great Plains with ERTS. In Proceedings of the Third Earth Resources Technology Satellite (ERTS) Symposium, Washington, DC, USA, 10–14 December 1973; pp. 309–317. [Google Scholar]
- Goffinet, B.; Show, A. Bryophyte Biology, 2nd ed.; Cambridge University Press: New York, NY, USA, 2008. [Google Scholar]
- Proctor, M.C.F.; Oliver, M.; Wood, A.; Alpert, P.; Stark, L.; Cleavitt, N.; Mishler, B. Desiccation-tolerance in bryophytes: A review. Bryologist 2007, 110, 595–621. [Google Scholar] [CrossRef]
- Buitink, J.; Hemmings, M.; Hoekstra, F. Is there a role for oligosaccharides in seed longevity? An assessment of intracellular glass stability. Plant Physiol. 2000, 122, 1217–1224. [Google Scholar] [CrossRef]
- Danin, A.; Ganor, E. Trapping of airborne dust by mosses in the Negev Desert, Israel. Earth Surf. Process. Landf. 1991, 16, 153–162. [Google Scholar] [CrossRef]
- Gilchrist, A.; Jacobsen, A. Perception of lightness and illumination in a world of one reflectance. Perception 1984, 13, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.; Tamm, A.; Wu, D.; Caesar, J.; Grube, M.; Weber, B. Photoautotrophic organisms control microbial abundance, diversity, and physiology in different types of biological soil crusts. ISME J. 2018, 12, 1032–1046. [Google Scholar] [CrossRef]
- Olarra, J.A. Biological Soil Crusts in Forested Ecosystems of Southern Oregon: Presence, Abundance and Distribution across Climate Gradients. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 14 December 2012. [Google Scholar]
- Lan, S.; Ouyang, H.; Wu, L.; Zhang, D.; Hu, C. Biological soil crust community types differ in photosynthetic pigment composition, fluorescence and carbon fixation in Shapotou region of China. Appl. Soil. Ecol. 2017, 111, 9–16. [Google Scholar] [CrossRef]
- Kershaw, K.A.; Webber, M.R. Seasonal changes in the chlorophyll content and quantum efficiency of the moss Brachythecium rutabulum. J. Bryol. 1986, 14, 151–158. [Google Scholar] [CrossRef]
- Price, J.C. Estimating Leaf Area Index from Satellite Data. IEEE Trans. Geosci. Remote Sens. 1993, 31, 727–734. [Google Scholar] [CrossRef]
- Farrar, T.J.; Nicholson, S.E.; Lare, A.R. The influence of soil type on the relationships between NDVI, rainfall, and soil moisture in semiarid Botswana. II. NDVI response to soil moisture. Remote Sens. Environ. 1994, 50, 121–133. [Google Scholar] [CrossRef]
- Lan, S.; Wu, L.; Zhang, D.; Hu, C. Analysis of environmental factors determining development and succession in biological soil crusts. Sci. Total Environ. 2015, 538, 492–499. [Google Scholar] [CrossRef]
- Weber, B.; Hill, J. Remote Sensing of Biological Soil Crusts at Different Scales. In Biological Soil Crusts: An Organizing Principle in Drylands, 1st ed.; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; Volume 226, pp. 215–234. [Google Scholar]

| Cyanobacteria | Lichen | Moss |
|---|---|---|
| Anabaena azotica Ley (++) Chroococcus minutus (Kuetz.) Näg. (+++) Diatoma vulgare Bory (++) Lyngbya digueti Gom. (++) Microcoleus vaginatus (Vauch.) Gom. (++) Penium cruciferum (DeBary) Wittr. (++) Scytonema incrasstum Jao (++) Sc. stuposum (Kuetz.) Born. (++) Sc. javanicum (Kuetz.) Born. (++) Synechocystis crassa Woronichin (++) | Collema coccophorum Tuck. (+++) C. tenax (Sw.) Ach. Em. Degel. (+++) Diploschistes muscorum (Scop.) R. Sant (++) Psora decipiens (Hedw.) Hoffm. (+++) | Aloina brevirostris (Hook. and Grev.) Kindb. (++) Bryum argenteum Hedw. (+++) Bryum caespiticium L. ex Hedw. (++) Ceratodon purpureus var. purpureus (Hedw.) Brid. (++) Crossidium crassinerve (De Not.) Jur. (++) Cr. squamigerum (Viv.) Jur. (++) Dicranella varia (Hedw.) Schimp. (++) Didymodon constrictus (Mitt.) Saito (++) Did. icmadophilus (Schimp. ex C. Müll.) Saito (++) Did. nigrescens (Mitt.) Saito (+++) Did. perobtusus Broth. (++) Funaria hygrometrica Hedw. (++) Hilpertia velenovskyi (Schiffn.) Zand. (++) Microbryum rectum (With.) Zand. (++) Pterygoneurum ovatum (Hedw.) Dix. (++) Tortula atrovirens (Sm.) Lindb. (+++) T. cernua (Hueb.) Lindb. (++) T. desertorum Broth. (++) |
| Landsat-8 OLI | Sentinel-2 MSI | ||||
|---|---|---|---|---|---|
| Band | Range [nm] | Resolution [m] | Band | Range [nm] | Resolution [m] |
| Band1 (Coastal Aerosol) | 430–450 | 30 | Band2 (Blue) | 457–523 | 20 |
| Band2 (Blue) | 450–510 | 30 | Band3 (Green) | 543–578 | 20 |
| Band3 (Green) | 530–590 | 30 | Band4 (Red) | 653–683 | 20 |
| Band4 (Red) | 640–670 | 30 | Band5 (Red-edge) | 698–713 | 20 |
| Band5 (Near-infrared, NIR) | 850–880 | 30 | Band6 (Red-edge) | 732–748 | 20 |
| Band7 (Red-edge) | 773–793 | 20 | |||
| Band8A (NIR) | 855–875 | 20 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wang, T.; Liu, S.; Peng, F.; Kang, W.; Guo, Z.; Feng, K.; Liu, J.; Tsunekawa, A. Spectral Response Assessment of Moss-Dominated Biological Soil Crust Coverage Under Dry and Wet Conditions. Remote Sens. 2020, 12, 1158. https://doi.org/10.3390/rs12071158
Chen X, Wang T, Liu S, Peng F, Kang W, Guo Z, Feng K, Liu J, Tsunekawa A. Spectral Response Assessment of Moss-Dominated Biological Soil Crust Coverage Under Dry and Wet Conditions. Remote Sensing. 2020; 12(7):1158. https://doi.org/10.3390/rs12071158
Chicago/Turabian StyleChen, Xiang, Tao Wang, Shulin Liu, Fei Peng, Wenping Kang, Zichen Guo, Kun Feng, Jia Liu, and Atsushi Tsunekawa. 2020. "Spectral Response Assessment of Moss-Dominated Biological Soil Crust Coverage Under Dry and Wet Conditions" Remote Sensing 12, no. 7: 1158. https://doi.org/10.3390/rs12071158
APA StyleChen, X., Wang, T., Liu, S., Peng, F., Kang, W., Guo, Z., Feng, K., Liu, J., & Tsunekawa, A. (2020). Spectral Response Assessment of Moss-Dominated Biological Soil Crust Coverage Under Dry and Wet Conditions. Remote Sensing, 12(7), 1158. https://doi.org/10.3390/rs12071158





