Monitoring for Changes in Spring Phenology at Both Temporal and Spatial Scales Based on MODIS LST Data in South Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Data Collection and Pre-Processing
2.4. Indices Calculation
2.5. Statistical Analysis
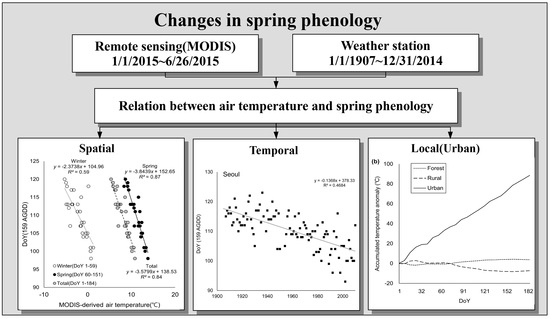
3. Results
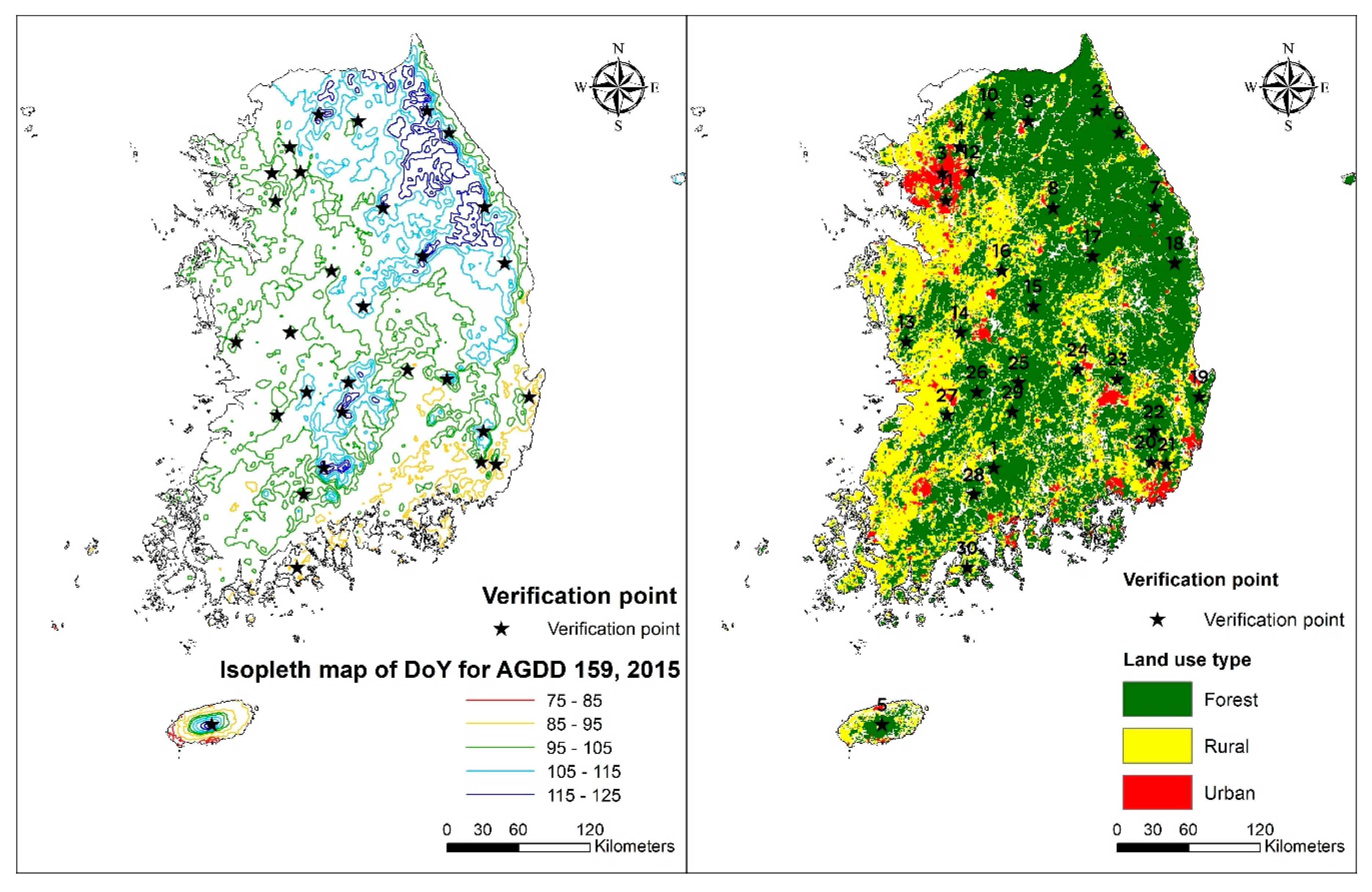
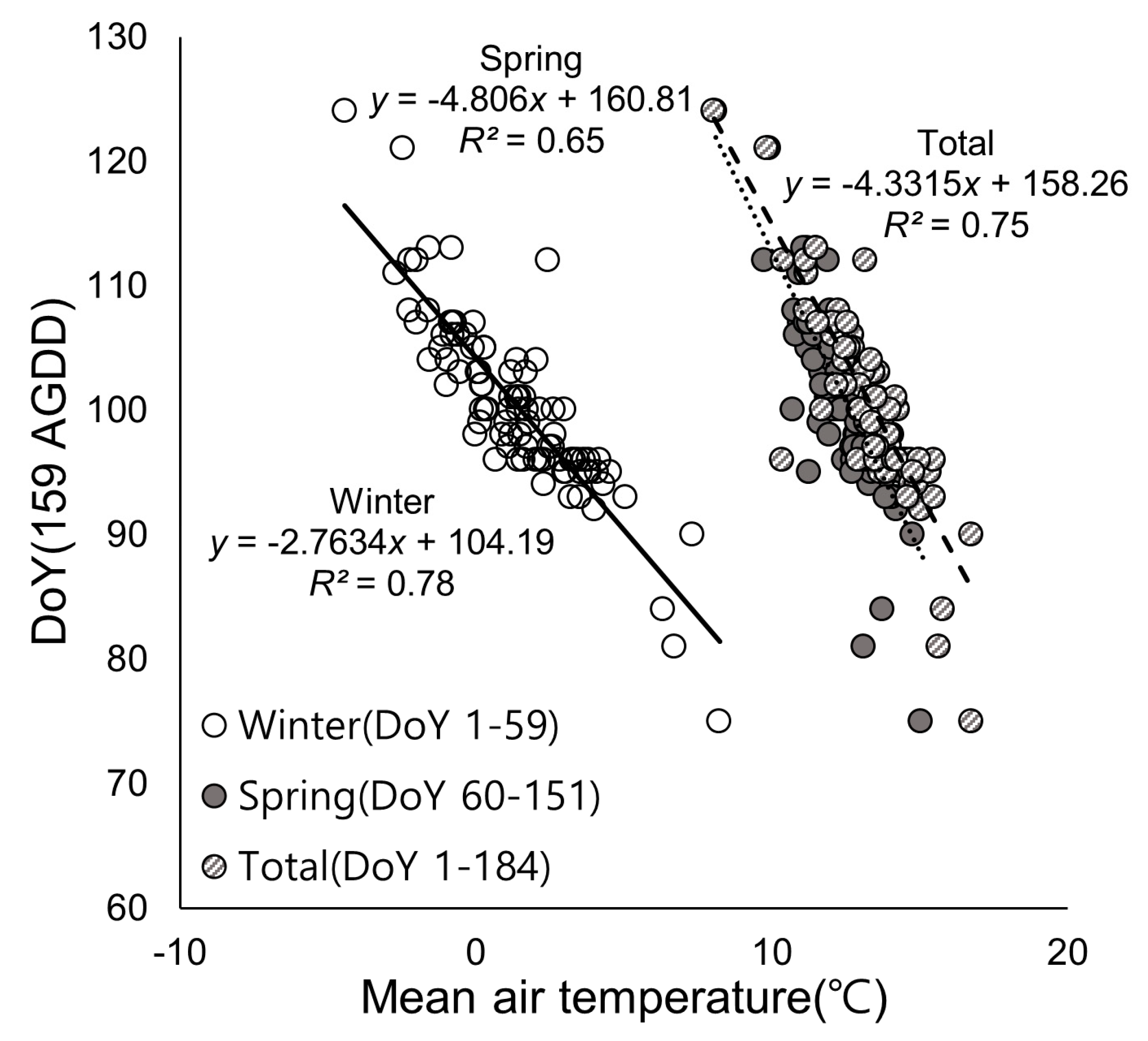
3.1. Spatial Distribution of Isopleth of DoY for AGDD 159
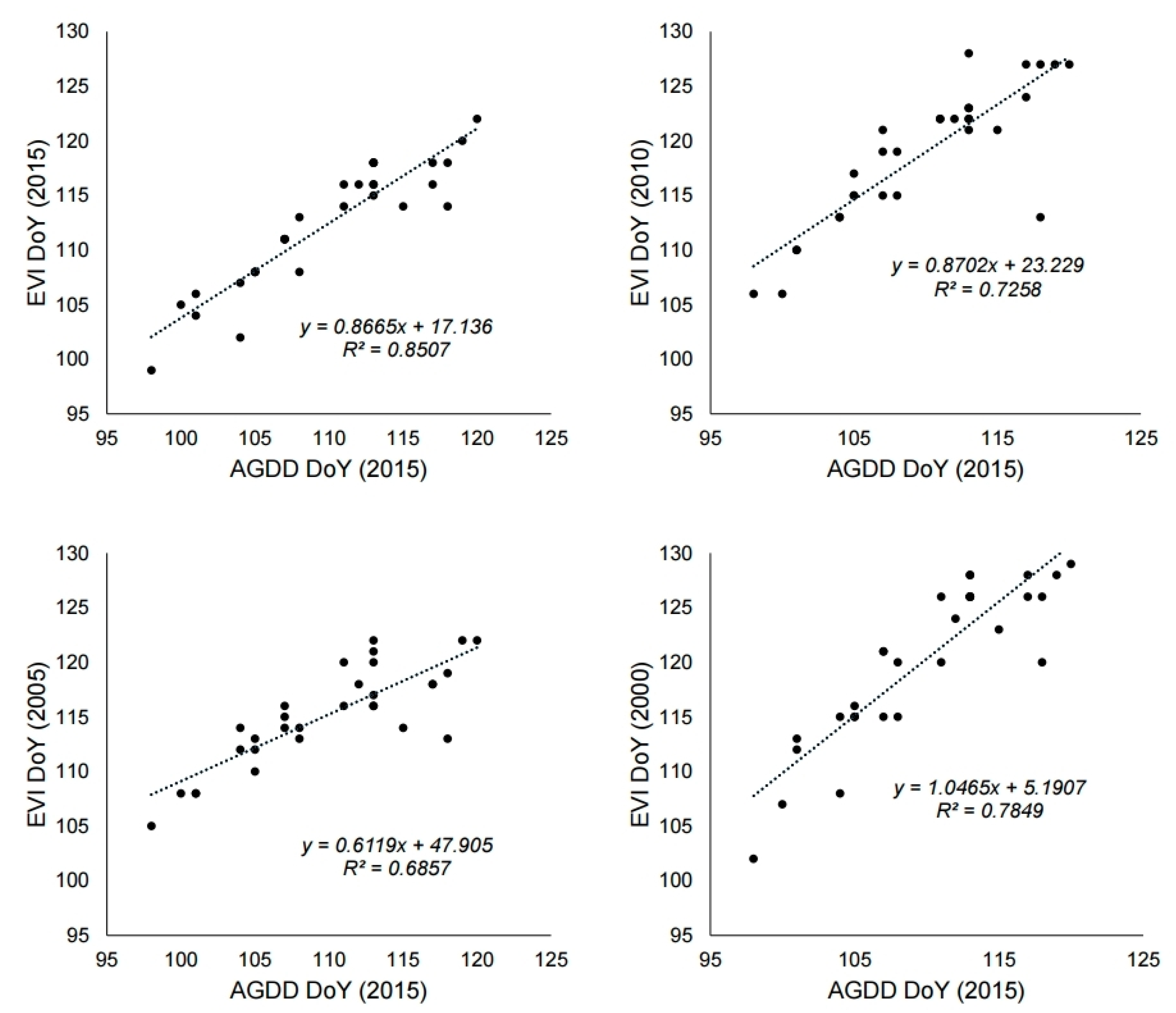
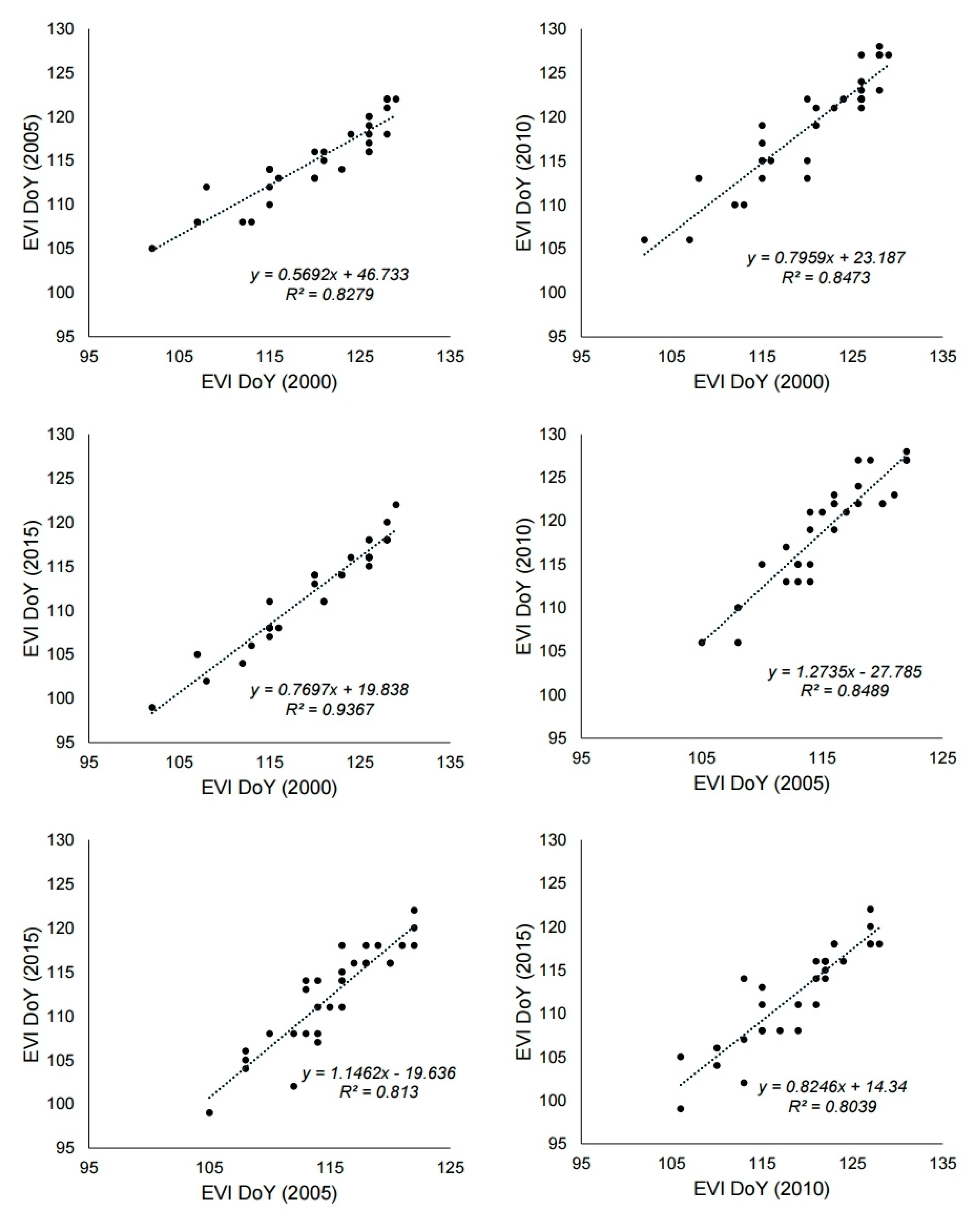
3.2. Relationship Between Air Temperature and Phenological Events
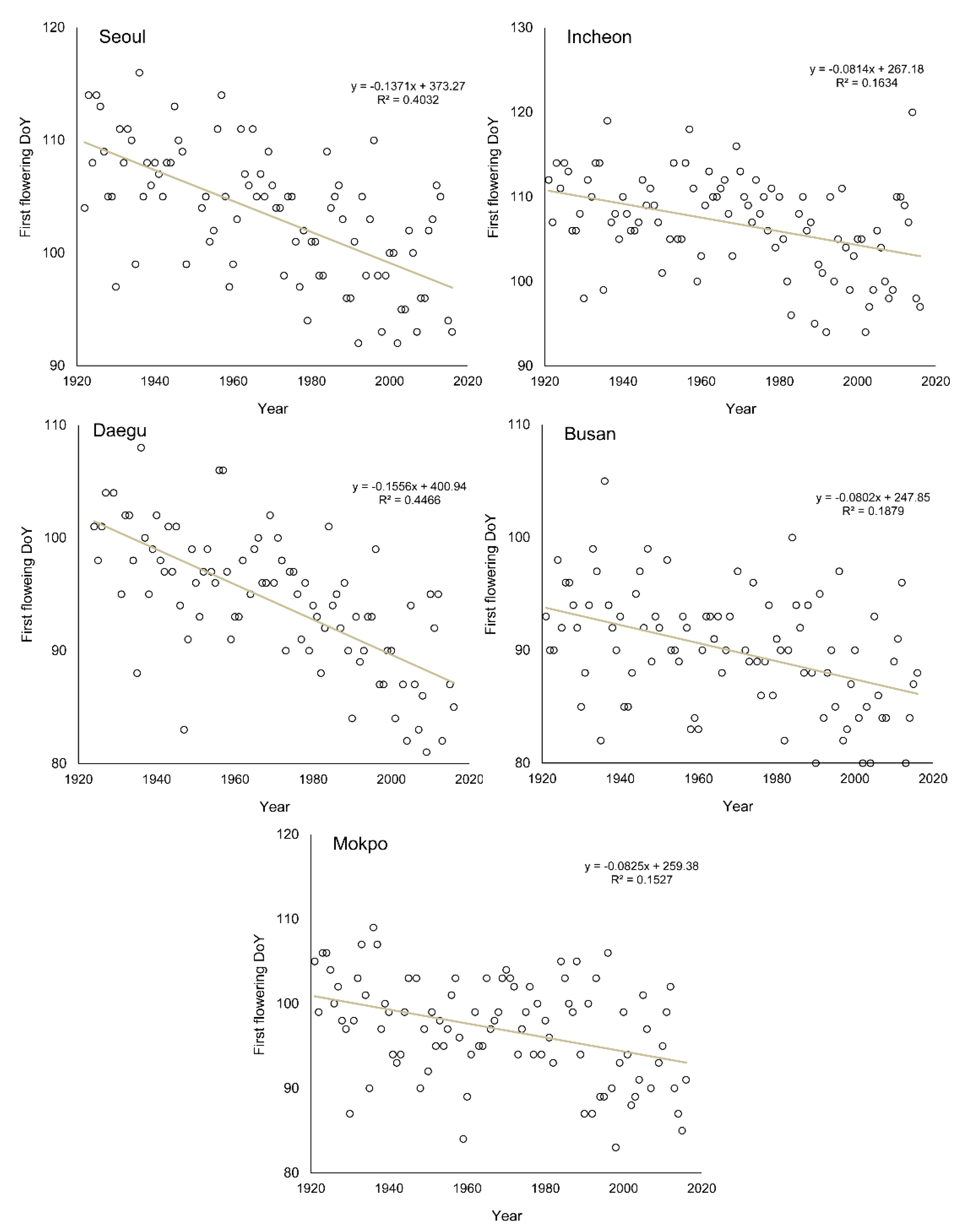
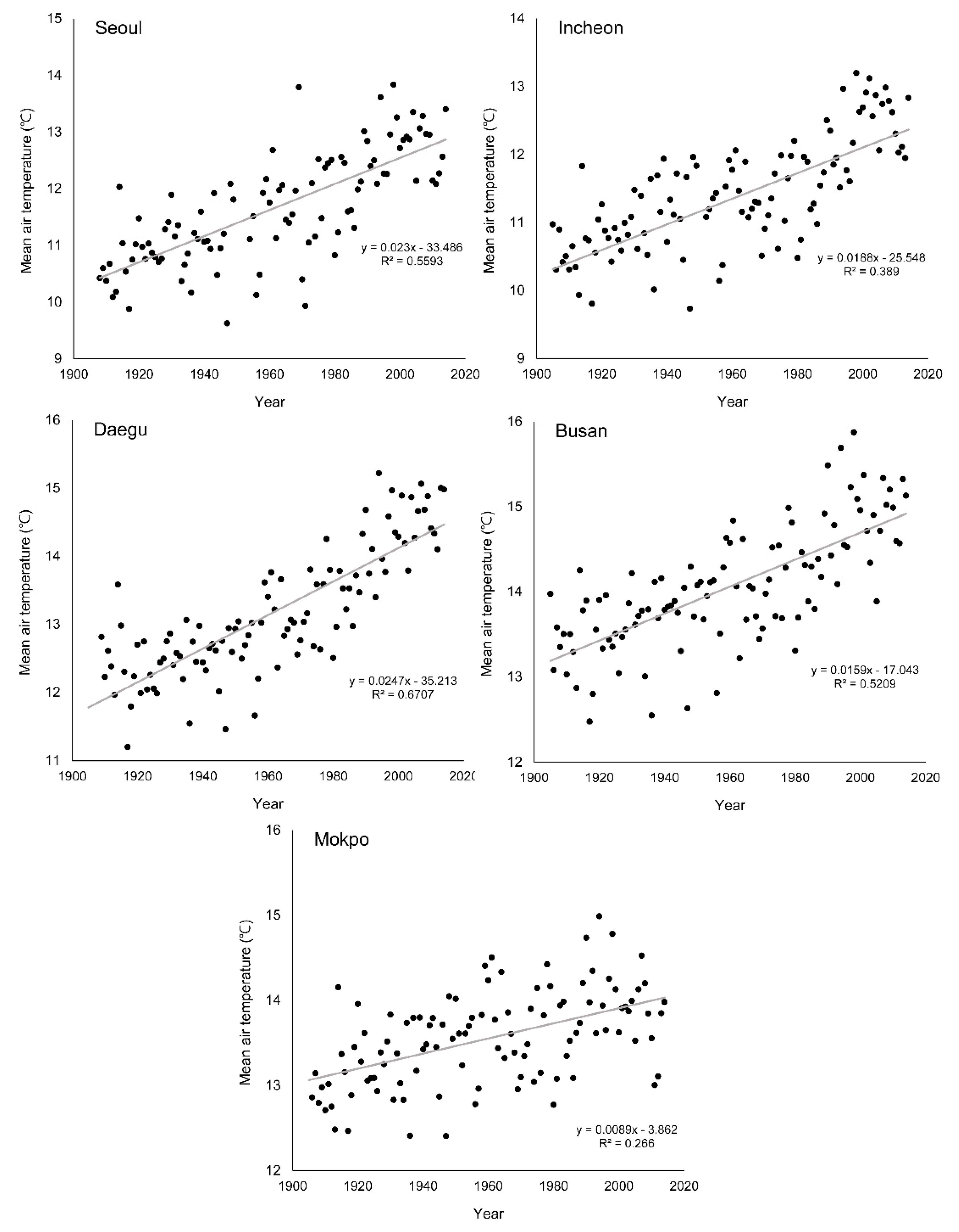
3.3. Change of Green-Up Date during the Past Century
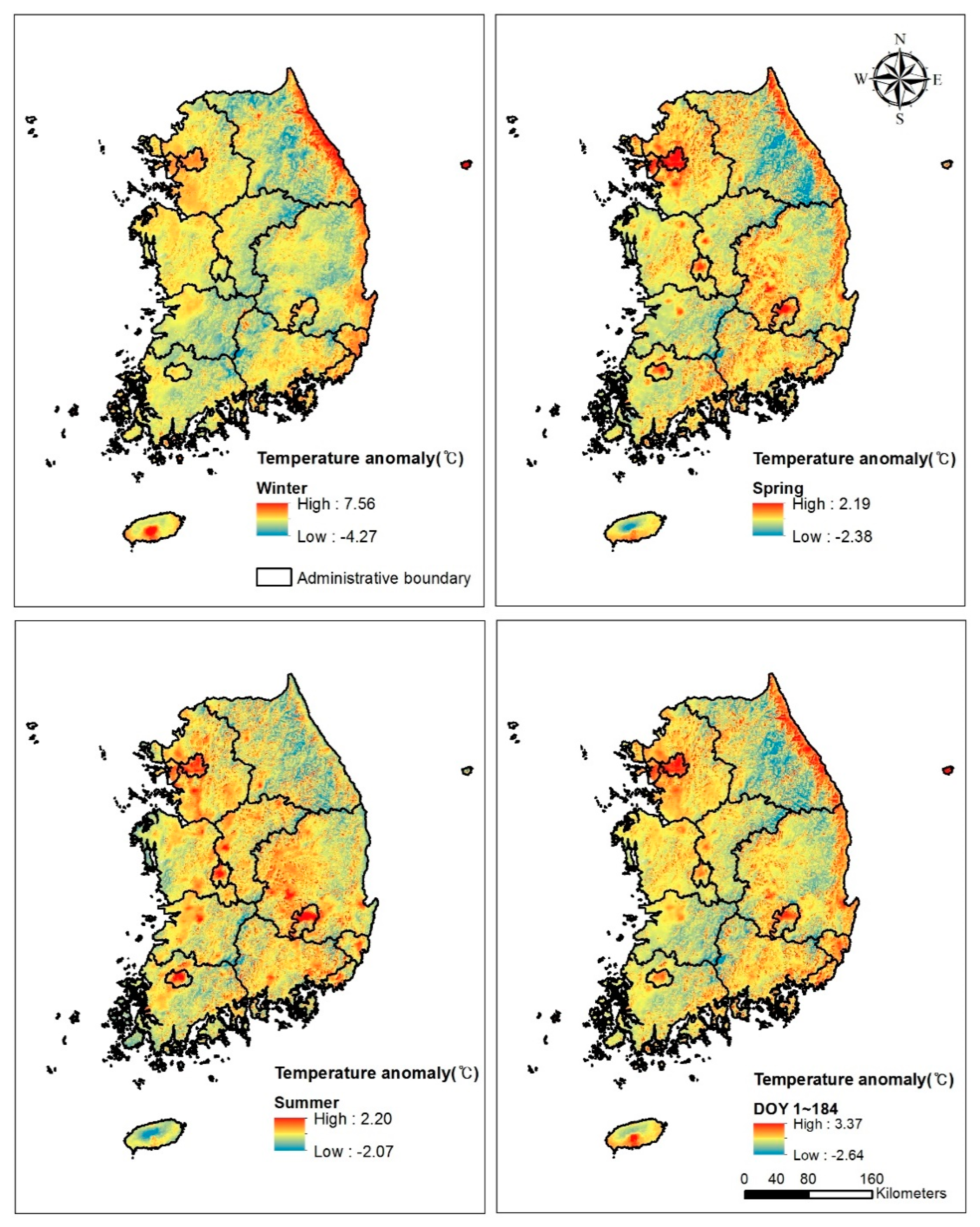
3.4. Temperature Anomaly due to Topography and Land-Use Intensity
4. Discussion
4.1. Utility of MODIS Images as a Tool for Phenology Research
4.2. Climate Change and Vegetation Phenology
4.3. Urban Heat Island Effect and Vegetation Phenology
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GDD | Growing degree days set as 5 °C |
| AGDD | Accumulated growing degree days |
| EVI | Enhanced vegetation index (EVI) |
| MODIS LST | Moderate resolution imaging spectroradiometer land surface temperature |
| DoYAGDD | Day of year needed to reach the AGDD threshold (159 °C·d) |
| DoY (159 AGDD) | Day of year required to reach AGDD 159 °C necessary for the leaf unfolding |
| AGDD 159 °C | Accumulated growing degree days 159 °C necessary for the leaf unfolding |
Appendix A
| No. | Site No. | Lon. | Lat. | Elevation (m) | Aspect (°) | Max. K | DoY for AGDD 159 | Diff. |
|---|---|---|---|---|---|---|---|---|
| 1 | Mt. Jiri | 128.46 | 38.04 | 985 | 90 | 117 | 121 | 4 |
| 2 | Mt. Jeombong | 127.42 | 38.01 | 906 | 78 | 118 | 123 | 5 |
| 3 | Mt. Nam | 127.80 | 37.95 | 141 | 13 | 98 | 98 | 0 |
| 4 | Gwangneung | 128.67 | 37.87 | 230 | 42 | 104 | 107 | 3 |
| 5 | Mt. Halla | 127.16 | 37.75 | 1315 | 317 | 118 | 125 | 7 |
| 6 | Mt. Odae | 127.26 | 37.57 | 690 | 148 | 113 | 116 | 3 |
| 7 | Mt. Deokwang | 126.99 | 37.55 | 878 | 331 | 113 | 119 | 6 |
| 8 | Mt. Chiak | 127.03 | 37.34 | 1090 | 298 | 119 | 123 | 4 |
| 9 | Mt. Majeok | 129.01 | 37.31 | 513 | 2 | 111 | 109 | −2 |
| 10 | Gookmangbong | 128.05 | 37.3 | 1124 | 240 | 113 | 119 | 6 |
| 11 | Mt. Gwanggyo | 128.43 | 36.94 | 429 | 21 | 107 | 108 | 1 |
| 12 | Mt. Yebong | 129.20 | 36.89 | 423 | 54 | 108 | 109 | 1 |
| 13 | Mt. Ami | 127.57 | 36.82 | 306 | 198 | 104 | 107 | 3 |
| 14 | Mt. Gyeryong | 127.88 | 36.56 | 527 | 304 | 107 | 109 | 2 |
| 15 | Mt. Sokri | 127.20 | 36.35 | 907 | 67 | 113 | 115 | 2 |
| 16 | Mt. Doota | 126.69 | 36.27 | 408 | 164 | 105 | 106 | 1 |
| 17 | Mt. Sobaek | 128.29 | 36.08 | 907 | 75 | 120 | 126 | 6 |
| 18 | Mt. Donggo | 128.66 | 36.01 | 900 | 187 | 112 | 116 | 4 |
| 19 | Mt. Moojang | 127.75 | 35.98 | 320 | 90 | 100 | 97 | −3 |
| 20 | Mt. Maebong | 127.36 | 35.9 | 607 | 246 | 105 | 103 | −2 |
| 21 | Mt. Cheongsong | 129.43 | 35.88 | 523 | 225 | 101 | 97 | −4 |
| 22 | Mt. Gaji | 127.69 | 35.76 | 1126 | 316 | 113 | 116 | 3 |
| 23 | Mt. Palgong | 127.09 | 35.72 | 860 | 186 | 107 | 114 | 7 |
| 24 | Mt. Geumoe | 129.01 | 35.62 | 709 | 111 | 108 | 105 | −3 |
| 25 | Mt. Baekwoon | 128.98 | 35.39 | 895 | 203 | 113 | 116 | 3 |
| 26 | Mt. Woonjang | 129.12 | 35.37 | 969 | 235 | 111 | 114 | 3 |
| 27 | Mt. Moak | 127.53 | 35.33 | 721 | 242 | 115 | 109 | −6 |
| 28 | Mt. Birae | 127.35 | 35.13 | 571 | 320 | 105 | 103 | −2 |
| 29 | Mt. Namdeokyou | 127.30 | 34.58 | 1197 | 181 | 117 | 121 | 4 |
| 30 | Mt. Jogye | 126.55 | 33.38 | 266 | 117 | 101 | 97 | −4 |
References
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef]
- Walker, W.H.; Meléndez-Fernández, O.H.; Nelson, R.J.; Reiter, R.J. Global climate change and invariable photoperiods: A mismatch that jeopardizes animal fitness. Ecol. Evol. 2019, 9, 10044–10054. [Google Scholar] [CrossRef] [PubMed]
- De Beurs, K.M.; Henebry, G.M. Land surface phenology, climatic variation, and institutional change: Analyzing agricultural land cover change in Kazakhstan. Remote Sens Environ. 2004, 89, 497–509. [Google Scholar] [CrossRef]
- Visser, M.E.; Both, C. Shifts in phenology due to global climate change: The need for a yardstick. Proc. R. Soc. B 2005, 272, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.D.; Ahas, R.; Aasa, A. Onset of spring starting earlier across the Northern Hemisphere. Glob. Chang. Biol. 2006, 12, 343–351. [Google Scholar] [CrossRef]
- Primack, R.; Higuchi, H. Climate change and cherry tree blossom festivals in Japan. Arnoldia 2007, 65, 14–22. [Google Scholar]
- Jeong, S.J.; Ho, C.H.; Choi, S.D.; Kim, J.; Lee, E.J.; Gim, H.J. Satellite Data-Based Phenological Evaluation of the Nationwide Reforestation of South Korea. PLoS ONE 2013, 8, e58900. [Google Scholar] [CrossRef]
- Richardson, A.D.; Keenan, T.F.; Migliavacca, M.; Ryu, Y.; Sonnentag, O.; Toomey, M. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric. For. Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Liu, Q.; Piao, S.; Janssens, I.A.; Fu, Y.; Peng, S.; Lian, X.; Ciais, P.; Myneni, R.B.; Peñuelas, J.; Wang, T. Extension of the growing season increases vegetation exposure to frost. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Menzel, A. Plant Phenological Anomalies in Germany and their Relation to Air Temperature and NAO. Clim. Chang. 2003, 57, 243–263. [Google Scholar] [CrossRef]
- Estrella, N.; Menzel, A. Responses of leaf colouring in four deciduous tree species to climate and weather in Germany. Clim. Res. 2006, 32, 253–267. [Google Scholar] [CrossRef]
- Fu, Y.H.; Piao, S.; Op de Beeck, M.; Cong, N.; Zhao, H.; Zhang, Y.; Menzel, A.; Janssens, I.A. Recent spring phenology shifts in western Central Europe based on multiscale observations. Glob. Ecol. Biogeogr. 2014, 23, 1255–1263. [Google Scholar] [CrossRef]
- Keenan, T.F.; Richardson, A.D. The timing of autumn senescence is affected by the timing of spring phenology: Implications for predictive models. Glob. Chang. Biol. 2015, 21, 2634–2641. [Google Scholar] [CrossRef] [PubMed]
- Ju, R.; Gao, L.; Wei, S.; Li, B. Spring warming increases the abundance of an invasive specialist insect: Links to phenology and life history. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.; Shen, M.; Zhu, X. Plant phenology and global climate change: Current progresses and challenges. Glob. Chang. Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.D.; Reiter, B.E. Changes in North American spring. Int. J. Climatol. 2000, 20, 929–932. [Google Scholar] [CrossRef]
- Richardson, A.D.; Hollinger, D.Y.; Dail, D.B.; Lee, J.T.; Munger, J.W.; O’keefe, J. Influence of spring phenology on seasonal and annual carbon balance in two contrasting New England forests. Tree Physiol. 2009, 29, 321–331. [Google Scholar] [CrossRef]
- Jung, S.H.; Kim, A.R.; An, J.H.; Lim, C.H.; Lee, H.; Lee, C.S. Abnormal shoot growth in Korean red pine as a response to microclimate changes due to urbanization in Korea. Int. J. Biometeorol. 2020, 64, 571–584. [Google Scholar] [CrossRef]
- Xu, M.; Wang, H.; Wen, X.; Zhang, T.; Di, Y.; Wang, Y.; Wang, J.; Cheng, C.; Zhang, W. The full annual carbon balance of a subtropical coniferous plantation is highly sensitive to autumn precipitation. Sci. Rep. 2017, 7, 10025. [Google Scholar] [CrossRef]
- Gu, L.; Post, W.M.; Baldocchi, D.; Andy Black, T.; Verma, S.B.; Vesala, T.; Wofsy, S.C. Phenology of Vegetation Photosynthesis. In Phenology: An Integrative Environmental Science; Schwartz, M.D., Ed.; Springer: Dordrecht, The Netherlands, 2003; pp. 467–485. [Google Scholar]
- Fantinato, E.; Vecchio, S.D.; Giovanetti, M.; Alicia Teresa Rosario Acosta, A.; Buffa, G. New insights into plants co-existence in species-rich communities: The pollination interaction perspective. J. Veg. Sci. 2018, 29, 6–14. [Google Scholar] [CrossRef]
- Inouye, D. Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 2008, 89, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Allstadt, A.J.; Vavrus, S.J.; Heglund, P.J.; Pidgeon, A.M.; Thogmartin, W.E.; Radeloff, V.C. Spring plant phenology and false springs in the conterminous US during the 21st century. Environ. Res. Lett. 2015, 10, 104008. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Miller-Rushing, A.J.; Høye, T.T.; Inouye, D.W.; Post, E. The effects of phenological mismatches on demography. Philos. Trans. R. Soc. B 2010, 365, 3177–3186. [Google Scholar] [CrossRef]
- Kehrberger, S.; Holzschuh, A. Warmer temperatures advance flowering in a spring plant more strongly than emergence of two solitary spring bee species. PLoS ONE 2019, 14, e0218824. [Google Scholar] [CrossRef]
- Kudo, G.; Ida, T.Y. Early onset of spring increases the phenological mismatch between plants and pollinators. Ecology 2013, 94, 2311–2320. [Google Scholar] [CrossRef]
- Berndes, G.; Bird, N.; Cowie, A. Bioenergy, Land Use Change and Climate Change Mitigation; IEA Bioenergy: Rotorua, New Zealand, 2013; Available online: https://www.ieabioenergy.com/wp-content/uploads/2013/10/Bioenergy-Land-Use-Change-and-Climate-Change-Mitigation-Background-Technical-Report.pdf (accessed on 10 August 2020).
- Zhang, X.; Friedl, M.A.; Schaaf, C.B.; Strahler, A.H.; Schneider, A. The footprint of urban climates on vegetation phenology. Geophys. Res. Lett. 2004, 31, 1–4. [Google Scholar] [CrossRef]
- Yao, X.; Wang, Z.; Wang, H. Impact of Urbanization and Land-Use Change on Surface Climate in Middle and Lower Reaches of the Yangtze River, 1988–2008. Adv. Meteorol. 2015, 2015, 10. [Google Scholar] [CrossRef]
- Li, X.X.; Tieh-Yong Koh, T.Y.; Panda, J.; Leslie, K.; Norford, L.K. Impact of urbanization patterns on the local climate of a tropical city, Singapore: An ensemble study. J. Geophys. Res. Atmos. 2016, 121, 4386–4403. [Google Scholar] [CrossRef]
- Li, J.; Zou, C.; Li, Q.; Xu, X.; Zhao, Y.; Yang, W.; Zhang, Z.; Liu, L. Effects of urbanization on productivity of terrestrial ecological systems based on linear fitting: A case study in Jiangsu, eastern China. Sci. Rep. 2019, 9, 17140. [Google Scholar] [CrossRef]
- Argüeso, D.; Evans, J.P.; Fita, L.; Bormann, K.J. Temperature response to future urbanization and climate change. Clim. Dyn. 2014, 42, 2183–2199. [Google Scholar] [CrossRef]
- Krehbiel, C.; Henebry, G.M. A Comparison of Multiple Datasets for Monitoring Thermal Time in Urban Areas over the U.S. Upper Midwest. Remote. Sens. 2016, 8, 297. [Google Scholar] [CrossRef]
- Yang, Z.; Dominguez, F.; Gupta, H.; Zeng, X.; Norman, L. Urban Effects on Regional Climate: A Case Study in the Phoenix and Tucson “Sun Corridor”. Earth Interact 2016, 20, 1–25. [Google Scholar] [CrossRef]
- Miller, J.D.; Hutchins, M. The impacts of urbanisation and climate change on urban flooding and urban water quality: A review of the evidence concerning the United Kingdom. Journal of Hydrology. Reg. Stud. 2017, 12, 345–362. [Google Scholar] [CrossRef]
- Imhoff, M.L.; Zhang, P.; Wolfe, R.E.; Bounoua, L. Remote sensing of the urban heat island effect across biomes in the continental USA. Remote Sens. Environ. 2010, 114, 504–513. [Google Scholar] [CrossRef]
- Huang, Q.; Lu, Y. The Effect of Urban Heat Island on Climate Warming in the Yangtze River Delta Urban Agglomeration in China. Int. J. Environ. Res. Public Health 2015, 12, 8773–8789. [Google Scholar] [CrossRef]
- Olsson, L.; Barbosa, H.; Bhadwal, S.; Cowie, A.; Delusca, K.; Flores-Renteria, D.; Hermans, K.; Jobbagy, E.; Kurz, W.; Li, D.; et al. Land Degradation. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; IPCC: Geneva, Switzerland, 2019; pp. 345–436. [Google Scholar]
- Lee, K.; Kim, Y.; Sung, H.C.; Ryu, J.; Jeon, S.W. Trend analysis of urban heat island intensity according to urban area change in Asian mega cities. Sustainability 2020, 12, 112. [Google Scholar] [CrossRef]
- McCarthy, M.P.; Best, M.J.; Betts, R.A. Climate change in cities due to global warming and urban effects. Geophys. Res. Lett. 2010, 37, 1–5. [Google Scholar] [CrossRef]
- Reich, P.B.; Borchert, R. Water Stress and Tree Phenology in a Tropical Dry Forest in the Lowlands of Costa Rica. J. Ecol. 1984, 72, 61–74. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavská, O.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Global. Chang. Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Richardson, A.D.; Braswell, B.H.; Hollinger, D.Y.; Jenkins, J.P.; Ollinger, S.V. Near-surface remote sensing of spatial and temporal variation in canopy phenology. Ecol. Appl. 2009, 19, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Ide, R.; Oguma, H. Use of digital cameras for phenological observations. Ecol. Inform. 2010, 5, 339–347. [Google Scholar] [CrossRef]
- Hufkens, K.; Friedl, M.; Sonnentag, O.; Braswell, B.H.; Milliman, T.; Richardson, A.D. Linking near-surface and satellite remote sensing measurements of deciduous broadleaf forest phenology. Remote Sens. Environ. 2012, 117, 307–321. [Google Scholar] [CrossRef]
- Klosterman, S.T.; Hufkens, K.; Gray, J.M.; Melaas, E.; Sonnentag, O.; Lavine, I.; Mitchell, L.; Norman, R.; Friedl, M.A.; Richardson, A.D. Evaluating remote sensing of deciduous forest phenology at multiple spatial scales using PhenoCam imagery. Biogeosci. Discuss. 2014, 11, 4305–4320. [Google Scholar] [CrossRef]
- Richardson, A.D.; Koen Hufkens, K.; Tom Milliman, T.; Aubrecht, D.M.; Chen, M.; Gray, J.M.; Johnston, M.R.; Keenan, T.F.; Klosterman, S.T.; Kosmala, M.; et al. Tracking vegetation phenology across diverse North American biomes using PhenoCam imagery. Sci. Data 2018, 5, 180028. [Google Scholar] [CrossRef]
- Fisher, J.I.; Mustard, J.F. Cross-scalar satellite phenology from ground, Landsat, and MODIS data. Remote Sens. Environ. 2007, 109, 261–273. [Google Scholar] [CrossRef]
- Zipper, S.C.; Schatz, J.; Singh, A.; Kucharik, C.J.; Townsend, P.A.; Loheide, S.P. Urban heat island impacts on plant phenology: Intra-urban variability and response to land cover. Environ. Res. Lett. 2016, 11, 054023. [Google Scholar] [CrossRef]
- Weber, M.; Dalei Hao, D.; Asrar, G.R.; Zhou, Y.; Li, X.; Chen, M. Exploring the use of DSCOVR/EPIC satellite observations to monitor vegetation phenology. Remote Sens. 2020, 12, 2384. [Google Scholar] [CrossRef]
- Fitchett, J.M.; Grab, S.W.; Thompson, D.I. Plant phenology and climate change: Progress in methodological approaches and application. Prog. Phys. Geog. 2015, 39, 460–482. [Google Scholar] [CrossRef]
- Wang, S.; Yang, B.; Yang, Q.; Lu, L.; Wang, X.; Peng, Y. Temporal Trends and Spatial Variability of Vegetation Phenology over the Northern Hemisphere during 1982–2012. PLoS ONE 2016, 11, e0157134. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhu, X.; Wu, J.; Shen, M.; Chen, J. Coarse-resolution satellite images overestimate urbanization effects on vegetation spring phenology. Remote Sens. 2020, 12, 117. [Google Scholar] [CrossRef]
- Zhang, X.; Friedl, M.A.; Schaaf, C.B.; Strahler, A.H.; Hodges, J.C.F.; Gao, F.; Reed, B.C.; Huete, A. Monitoring vegetation phenology using MODIS. Remote Sens. Environ. 2003, 84, 471–475. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, J.; Guo, R.; Li, X.; Sun, W.; Wang, X. Spatio-temporal reconstruction of air temperature maps and their application to estimate rice growing season heat accumulation using multi-temporal MODIS data. J. Zhejiang Univ. Sci. B 2013, 14, 144–161. [Google Scholar] [CrossRef]
- Peng, J.; Loew, A.; Merlin, O.; Verhoest, N.E.C. A review of spatial downscaling of satellite remotely sensed soil moisture. Rev. Geophys. 2017, 55, 341–366. [Google Scholar] [CrossRef]
- Neteler, M. Estimating Daily Land Surface Temperatures in Mountainous Environments by Reconstructed MODIS LST Data. Remote Sens. 2010, 2, 333–351. [Google Scholar] [CrossRef]
- Lim, C.H.; Jung, S.H.; Kim, N.S.; Lee, C.S. Deduction of a meteorological phenology indicator from reconstructed MODIS LST imagery. J. For. Res. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Segal-Rozenhaimer, M.; Li, A.; Das, K.; Chirayath, V. Cloud detection algorithm for multi-modal satellite imagery using convolutional neural-networks (CNN). Remote Sens. Environ. 2020, 237, 111446. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, B.; Zhang, M.; Zeng, H. Crop phenology detection using high spatio-temporal resolution data fused from SPOT5 and MODIS Products. Sensors 2016, 16, 2099. [Google Scholar] [CrossRef]
- Lim, C.H.; An, J.H.; Jung, S.H.; Nam, G.B.; Cho, Y.C.; Kim, N.S.; Lee, C.S. Ecological consideration for several methodologies to diagnose vegetation phenology. Ecol. Res. 2018, 33, 363–377. [Google Scholar] [CrossRef]
- Zhang, S.; Pavelsk, T.M. Remote Sensing of Lake Ice Phenology across a Range of Lakes Sizes, ME, USA. Remote Sens. 2019, 11, 1718. [Google Scholar] [CrossRef]
- Franch, B.; Vermote, E.F.; Roger, J.-C.; Murphy, E.; Becker-Reshef, I.; Justice, C.; Claverie, M.; Nagol, J.; Csiszar, I.; Meyer, D.; et al. A 30+ Year AVHRR Land Surface Reflectance Climate Data Record and Its Application to Wheat Yield Monitoring. Remote Sens. 2017, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, S.; Frederic, B.; Verger, A.; Neveux, P.; Weiss, M. A comparison of methods for smoothing and gap filling time series of remote sensing observations: Application to MODIS LAI products. Biogeosciences 2013, 10, 4055–4071. [Google Scholar] [CrossRef]
- Wang, T.; Shi, J.; Letu, H.; Ma, Y.; Li, X.; Zheng, Y. Detection and Removal of Clouds and Associated Shadows in Satellite Imagery Based on Simulated Radiance Fields. J. Geophys. Res. Atmos. 2019, 124, 7207–7225. [Google Scholar] [CrossRef]
- McMaster, G.S.; Wilhelm, W. Growing degree-days: One equation, two interpretations. Agric. For. Meteorol. 1997, 87, 291–300. [Google Scholar] [CrossRef]
- Diamond, S.E.; Cayton, H.; Wepprich, T.; Jenkins, C.N.; Dunn, R.R.; Haddad, N.M.; Ries, L. Unexpected phenological responses of butterflies to the interaction of urbanization and geographic temperature. Ecology 2014, 95, 2613–2621. [Google Scholar] [CrossRef]
- Parry, M.L.; Carter, T.R. The effect of climatic variations on agricultural risk. Clim. Chang. 1985, 7, 95–110. [Google Scholar] [CrossRef]
- Bonhomme, R. Bases and limits to using ‘degree day’ units. Eur. J. Agron. 2000, 13, 1–10. [Google Scholar] [CrossRef]
- Ozelkan, E.; Bagis, S.; Ozelkan, E.C.; Ustundag, B.B.; Yucel, M.; Ormeci, C. Spatial interpolation of climatic variables using land surface temperature and modified inverse distance weighting. Int. J. Remote Sens. 2015, 36, 1000–1025. [Google Scholar] [CrossRef]
- Mostovoy, G.V.; King, R.L.; Reddy, K.R.; Kakani, V.G.; Filippova, M.G. Statistical estimation of daily maximum and minimum air temperatures from MODIS LST data over the state of Mississippi. GIScience Remote Sens. 2006, 43, 78–110. [Google Scholar] [CrossRef]
- Hassan, Q.K.; Bourque, C.P.; Meng, F.R.; Richards, W. Spatial mapping of growing degree days: An application of MODIS-based surface temperatures and enhanced vegetation index. J. Appl. Remote Sens. 2007, 1, 013511. [Google Scholar] [CrossRef]
- Duncan, J.; Dash, J.; Atkinson, P.M. The potential of satellite-observed crop phenology to enhance yield gap assessments in smallholder landscapes. Front. Environ. Sci. 2015, 3, 56. [Google Scholar] [CrossRef]
- Ahas, R.; Aasa, A. The effects of climate change on the phenology of selected Estonian plant, bird and fish populations. Int. J. Biometeorol. 2006, 51, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L. Biological consequences of global warming: Is the signal already apparent? Trends Ecol. Evol. 2000, 15, 56–61. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I. Responses to a warming world. Science 2001, 294, 793–795. [Google Scholar] [CrossRef]
- Donoso, I.; Stefanescu, C.; Martínez-Abraín, A.; Traveset, A. Phenological asynchrony in plant–butterfly interactions associated with climate: A community-wide perspective. Oikos 2016, 125, 1434–1444. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 399, 579–583. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef]
- Menzel, A.; Fabian, P. Growing season extended in Europe. Nature 1999, 397, 659. [Google Scholar] [CrossRef]
- Fitter, A.H.; Fitter, R.S.R. Rapid changes in flowering time in British plants. Science 2002, 296, 1689–1691. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Comas, P. Changed plant and animal life cycles from 1952 to 2000 in the Mediterranean region. Glob. Chang. Biol. 2002, 8, 531–544. [Google Scholar] [CrossRef]
- Polgar, C.A.; Primack, R.B. Leaf-out phenology of temperate woody plants: From trees to ecosystems. New Phytol. 2011, 191, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Gallinat, A.S.; Primack, R.B.; Wagner, D.L. Autumn, the neglected season in climate change research. Trends Ecol. Evol. 2015, 30, 169–176. [Google Scholar] [CrossRef]
- Thompson, R.; Clark, R.M. Is spring starting earlier? Holocene 2008, 18, 95–104. [Google Scholar] [CrossRef]
- Richardson, A.D.; Bailey, A.S.; Denny, E.G.; Martin, C.W.; O’Keee, J. Phenology of a northern hardwood forest canopy. Glob. Chang. Biol. 2006, 12, 1174–1188. [Google Scholar] [CrossRef]
- Vitasse, Y.; Delzon, S.; Dufrêne, E.; Pontailler, J.Y.; Louvet, J.M.; Kremer, A.; Michalet, R. Leaf phenology sensitivity to temperature in European trees: Do within-species populations exhibit similar responses? Agric. For. Meteorol. 2009, 149, 735–744. [Google Scholar] [CrossRef]
- Jeong, S.J.; Ho, C.H.; Gim, H.J.; Brown, M.E. Phenology shifts at start vs. end of growing season in temperate vegetation over the Northern Hemisphere for the period 1982–2008. Glob. Chang. Biol. 2011, 17, 2385–2399. [Google Scholar] [CrossRef]
- Linkosalo, T.; Hakkinen, R.; Terhivuo, J.; Tuomenvirta, H.; Hari, P. The time series of flowering and leaf bud burst of boreal trees (1846–2005) support the direct temperature observations of climatic warming. Agric. For. Meteorol. 2009, 149, 453–461. [Google Scholar] [CrossRef]
- Delbart, N.; Picard, G.; Le Toans, T.; Kergoat, L.; Quegan, S.; Woodward, I.; Dye, D.; Fedotova, V. Spring phenology in boreal Eurasia over a nearly century time scale. Glob. Chang. Biol. 2008, 14, 603–614. [Google Scholar] [CrossRef]
- Nordli, O.; Wielgolaski, F.E.; Bakken, A.K.; Hjeltnes, S.H.; Mage, F.; Sivle, A.; Skre, O. Regional trends for bud burst and flowering of woody plants in Norway as related to climate change. Int. J. Biometeorol. 2008, 52, 625–639. [Google Scholar] [CrossRef]
- Pudas, E.; Leppala, M.; Tolvanen, A.; Poikolainen, J.; Venalainen, A.; Kubin, E. Trends in phenology of Betula pubescens across the boreal zone in Finland. Int. J. Biometeorol. 2008, 52, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Gordo, O.; Sanz, J.J. Long-term temporal changes of plant phenology in the Western Mediterranean. Glob. Chang. Biol. 2009, 15, 1930–1948. [Google Scholar] [CrossRef]
- Reich, P.B. Phenology of tropical forests: Patterns, causes and consequences. Can. J. Bot. 1995, 73, 164–174. [Google Scholar] [CrossRef]
- Wright, S.J.; Van Schaik, C.P. Light and the phenology of tropical trees. Am. Nat. 1994, 143, 192–199. [Google Scholar] [CrossRef]
- Xiao, X.; Hagen, S.; Zhang, Q.; Keller, M.; Moore, B. Leaf phenology of seasonally moist tropical forests in South America with multi-temporal MODIS images. Remote Sens. Environ. 2006, 103, 456–473. [Google Scholar] [CrossRef]
- Doughty, C.E.; Goulden, M. Seasonal patterns of tropical forest leaf area index and CO2 exchange. J. Geophys. Res. 2008, 113, 1–12. [Google Scholar] [CrossRef]
- Bradley, A.V.; Gerard, G.G.; Barbier, N.; Weedon, G.P.; Anderson, L.O.; Huntingford, C.; Aragao, L.E.O.C.; Zelazowski, P.; Arai, E. Relationships between phenology, radiation and precipitation in the Amazon region. Glob. Chang. Biol. 2011, 17, 2245–2260. [Google Scholar] [CrossRef]
- Zimmerman, J.K.; Wright, S.J.; Calderón, O.; Pagan, M.A.; Paton, S. Flowering and fruiting phenologies of seasonal and aseasonal neotropical forests: The role of annual changes in irradiance. J. Trop. Ecol. 2007, 23, 231–251. [Google Scholar] [CrossRef]
- Zalamea, M.; González, G. Leaffall phenology in a subtropical wet forest in Puerto Rico: From species to community patterns. Biotropica 2008, 40, 295–304. [Google Scholar] [CrossRef]
- Zhang, X.; Tarpley, D.; Sullivan, J.T. Diverse responses of vegetation phenology to a warming climate. Geophys. Res. Lett. 2007, 34, 1–5. [Google Scholar] [CrossRef]
- Morin, X.; Lechowicz, M.J.; Augspurger, C.; O’ Keefe, J.; Viner, D.; Chuine, I. Leaf phenology in 22 North American tree species during the 21st century. Glob. Chang. Biol. 2009, 15, 961–975. [Google Scholar] [CrossRef]
- Korner, C.; Basler, D. Phenology under global warming. Science 2010, 327, 1461–1462. [Google Scholar] [CrossRef] [PubMed]
- Migliavacca, M.; Sonnentag, O.; Keenan, T.F.; Cescatti, A.; O’Keefe, J.; Richardson, A.D. On the uncertainty of phenological responses to climate change, and implications for a terrestrial biosphere model. Biogeosciences 2012, 9, 2063–2083. [Google Scholar] [CrossRef]
- Wenden, B.; Mariadassou, M.; Chmielewski, F.M.; Vitasse, Y. Shifts in the temperature-sensitive periods for spring phenology in European beech and pedunculate oak clones across latitudes and over recent decades. Glob. Chang. Biol. 2020, 26, 1808–1819. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; He, H.S.; Zhao, J.; Larsen, D.R.; Zhang, H.; Sunde, M.G.; Duan, S. Climate and Spring Phenology Effects on Autumn Phenology in the Greater Khingan Mountains, Northeastern China. Remote Sens. 2018, 10, 449. [Google Scholar] [CrossRef]
- Buermann, W.; Bikash, P.R.; Jung, M.; Burn, D.H.; Reichstein, M. Earlier springs decrease peak summer productivity in North American boreal forests. Environ. Res. Lett. 2013, 8, 024027. [Google Scholar] [CrossRef]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global change and the ecology of cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef]
- Matlack, G.R. Microenvironment variation within and among forest edge sites in the eastern United States. Biol. Conserv. 1993, 66, 185–194. [Google Scholar] [CrossRef]
- McDonnell, M.J.; Pickett, S.T.A.; Groffman, P.; Bohlen, P.; Pouyat, R.V.; Zipperer, W.C.; Parmelee, R.W.; Carreiro, M.M.; Medley, K. Ecosystem processes along an urban-to-rural gradient. Urban Ecosyst. 1997, 1, 21–36. [Google Scholar] [CrossRef]
- Kaye, J.P. Carbon fluxes, nitrogen cycling, and soil microbial communities in adjacent urban, native and agricultural ecosystems. Glob. Chang. Biol. 2005, 11, 575–587. [Google Scholar] [CrossRef]
- Coomes, D.A.; Simonson, W.D.; Burslem, D.F. Forests and Global Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Jiang, Y.; Fu, P.; Weng, Q. Assessing the Impacts of Urbanization-Associated Land Use/Cover Change on Land Surface Temperature and Surface Moisture: A Case Study in the Midwestern United States. Remote Sens. 2015, 7, 4880. [Google Scholar] [CrossRef]
- Cleugh, H.A.; Oke, T.R. Suburban-rural energy balance comparisons in summer for Vancouver, BC. Bound. Layer Meteorol. 1986, 36, 351–369. [Google Scholar] [CrossRef]
- Peña, M.A. Examination of the land surface temperature response for Santiago, Chile. Photogramm. Eng. Remote Sens. 2009, 75, 1191–1200. [Google Scholar] [CrossRef]
- Henry, J.A.; Dicks, S.E.; Marotz, G.A. Urban and rural humidity distributions: Relationships to surface materials and land use. Int. J. Climatol. 1985, 5, 53–62. [Google Scholar] [CrossRef]
- Henry, J.A.; Dicks, S.E. Association of urban temperatures with land use and surface materials. Landsc. Urban Plan. 1987, 14, 21–29. [Google Scholar] [CrossRef]
- Bonan, G. The microclimates of a suburban Colorado (USA) landscapes and implications for planning and design. Landsc. Urban Plan. 2000, 49, 97–114. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Oke, T.R.; Johnson, G.T.; Steyn, D.G.; Watson, I.D. Simulation of surface urban heat islands under ‘ideal’ conditions at night. Part 2: Diagnosis of causation. Bound. Layer Meteorol. 1991, 56, 339–358. [Google Scholar] [CrossRef]
- Grimmond, C.S.B. The suburban energy balance: Methodological considerations and results for a mid-latitude west coast city under winter and spring conditions. Int. J. Climatol. 1992, 12, 481–497. [Google Scholar] [CrossRef]
- Roth, M.; Oke, T.R. Relative efficiencies of turbulent transfer of heat, mass, and momentum over a patchy urban surface. J. Atmos. Sci. 1995, 52, 1863–1874. [Google Scholar] [CrossRef]
- Oke, T.R. Boundary Layer Climates, 2nd, ed.; Routledge: London, UK, 1987. [Google Scholar]
- Stone, B. Urban and rural temperature trends in proximity to large US cities: 1951–2000. Int. J. Climatol. 2007, 27, 1801–1807. [Google Scholar] [CrossRef]
- Fujibe, F. Urban warming in Japanese cities and its relation to climate change monitoring. Int. J. Climatol. 2011, 31, 162–173. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Lee, C.S.; Song, H.G.; Kim, H.S.; Lee, B.; Pi, J.H.; Cho, Y.C.; Seol, E.S.; Oh, W.S.; Park, S.A.; Lee, S.M. Which Environmental Factors Caused Lammas Shoot Growth of Korean Red Pine? J. Ecol. Environ. 2007, 30, 101–105. [Google Scholar] [CrossRef][Green Version]
- Qiao, D.; Wang, N. Relationship between Winter Snow Cover Dynamics, Climate and Spring Grassland Vegetation Phenology in Inner Mongolia, China. ISPRS Int. J. Geo-Inf. 2019, 8, 42. [Google Scholar] [CrossRef]





| Data type | Original Resolution | Time Period | Utility | Source | |
|---|---|---|---|---|---|
| MODIS product | Air temperature dataset | 8 d, 1000 m | 1/1/2015~6/26/2015 | Deduction of spring green-up date based on AGDD for Mongolian oak | Lim et al. [60] |
| MOD09A1 | 8 d, 500 m | 1/1/2000~6/26/2000 1/1/2005~6/26/2005 1/1/2010~6/26/2010 1/1/2015~6/26/2015 | Deduction of spring green-up date based on EVI for Mongolian oak | NASA Earthdata website | |
| MCD12Q1 | Yearly, 500 m | 2015 | Land-use classification | ||
| Meteorological station data | First flowering date of cherry | Yearly | 1921~2014 | Deduction of spring green-up date for cherry | Korea Meteorological Administration |
| Air temperature record (maximum, minimum, mean) | Daily | 1/1/1907~12/31/2014 | AGDD calculation to extract phenological ascending trend | ||
| 1/1/2015~7/2/2015 | Deduction of relationship between phenological event dates based on AGDD and EVI | ||||
| City | Linear Regression Coefficient (/100 Years) | ||
|---|---|---|---|
| Annual Mean Air Temperature (R2) | DoYAGDD (R2) | First flowering DoY of Cherry (R2) | |
| Seoul | 2.3 (0.56 **) | −13.68 (0.47 **) | −13.71 (0.40 **) |
| Incheon | 1.8 (0.39 *) | −11.56 (0.36 *) | −8.10 (0.16 *) |
| Daegu | 2.5 (0.67 **) | −18.87 (0.56 **) | −15.56 (0.45 **) |
| Busan | 1.6 (0.52 **) | −19.97 (0.38 *) | −8.00 (0.19 *) |
| Mokpo | 0.9 (0.27 *) | −8.65 (0.17 *) | −8.30 (0.15 *) |
| City | N | Coefficient of Pearson’s Correlation | |
|---|---|---|---|
| Annual Mean Air Temperature (°C) | First Flowering DoY of Cherry | ||
| Seoul | 87 | −0.754 *** | 0.913 *** |
| Incheon | 92 | −0.574 *** | 0.731 *** |
| Daegu | 87 | −0.832 *** | 0.854 *** |
| Busan | 91 | −0.789 *** | 0.838 *** |
| Mokpo | 91 | −0.716 *** | 0.756 *** |
| Total | 299 | −0.823 *** | 0.913 *** |
| Sum of Squares | Df | Mean Square | F | Sig. | |
|---|---|---|---|---|---|
| Between Groups | 1049.47 | 2 | 524.73 | 1889.62 | 0.000 *** |
| Within Groups | 23,910.99 | 86,106 | 0.28 | ||
| Total | 24,960.46 | 86,108 |
| CLASS | CLASS | Mean Difference | Std. Error | Sig. | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Forest | Rural | 0.06 * | 0.004 | 0.000 *** | 0.0506 | 0.0689 |
| Urban | −0.46 * | 0.008 | 0.000 *** | −0.4802 | −0.4419 | |
| Rural | Forest | −0.06 * | 0.004 | 0.000 *** | −0.0689 | −0.0506 |
| Urban | −0.52 * | 0.008 | 0.000 *** | −0.5407 | −0.5009 | |
| Urban | Forest | 0.46 * | 0.008 | 0.000 *** | 0.4419 | 0.4802 |
| Rural | 0.52 * | 0.008 | 0.000 *** | 0.5009 | 0.5407 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, C.H.; Jung, S.H.; Kim, A.R.; Kim, N.S.; Lee, C.S. Monitoring for Changes in Spring Phenology at Both Temporal and Spatial Scales Based on MODIS LST Data in South Korea. Remote Sens. 2020, 12, 3282. https://doi.org/10.3390/rs12203282
Lim CH, Jung SH, Kim AR, Kim NS, Lee CS. Monitoring for Changes in Spring Phenology at Both Temporal and Spatial Scales Based on MODIS LST Data in South Korea. Remote Sensing. 2020; 12(20):3282. https://doi.org/10.3390/rs12203282
Chicago/Turabian StyleLim, Chi Hong, Song Hie Jung, A Reum Kim, Nam Shin Kim, and Chang Seok Lee. 2020. "Monitoring for Changes in Spring Phenology at Both Temporal and Spatial Scales Based on MODIS LST Data in South Korea" Remote Sensing 12, no. 20: 3282. https://doi.org/10.3390/rs12203282
APA StyleLim, C. H., Jung, S. H., Kim, A. R., Kim, N. S., & Lee, C. S. (2020). Monitoring for Changes in Spring Phenology at Both Temporal and Spatial Scales Based on MODIS LST Data in South Korea. Remote Sensing, 12(20), 3282. https://doi.org/10.3390/rs12203282