Investigating Detection of Floating Plastic Litter from Space Using Sentinel-2 Imagery
Abstract
1. Introduction
2. Materials and Methods
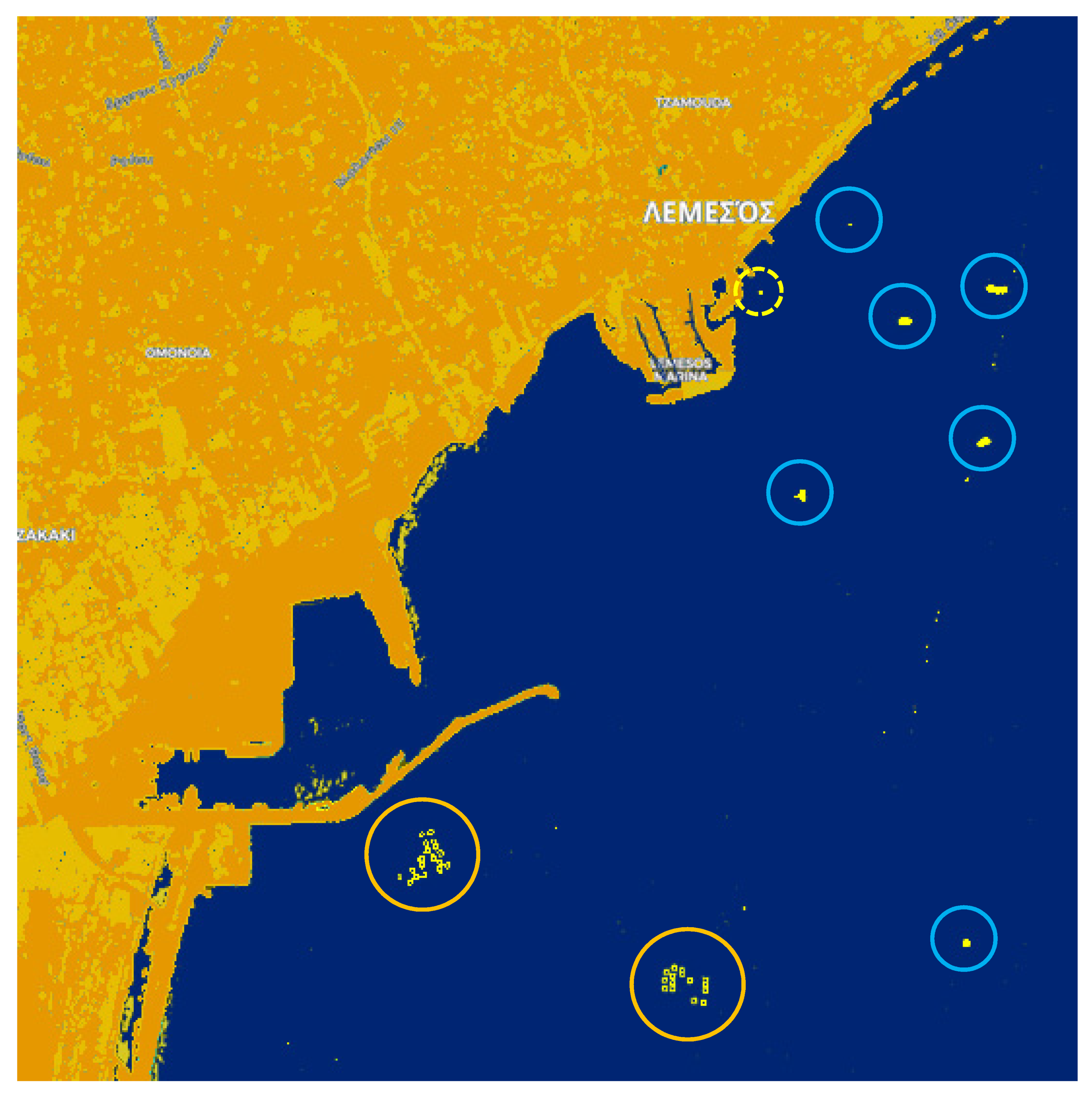
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UNEP—NOAA. Honolulu Strategy—A Global Framework for Prevention and Management of Marine Debris. 2011. Available online: https://repository.library.noaa.gov/view/noaa/10789 (accessed on 21 March 2019).
- Kershaw, P.J. Marine Plastic Debris and Microplastics—Global Lessons and Research to Inspire Action and Guide Policy Change; United Nations Environment Programme: Nairobi, Kenya, 2016; ISBN 978-92-807-3580-6. [Google Scholar]
- Thompson, R.C.; Swan, S.H.; Moore, C.J.; vom Saal, F.S. Our plastic age. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1973–1976. [Google Scholar] [CrossRef]
- Algalita Marine Research Foundation. Annual Report. 2008. Available online: https://algalita.org/ (accessed on 21 March 2019).
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Gall, S.C.; Thompson, R.C. The impact of debris on marine life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.R.; Reid, K.; Arnould, J.P.Y.; Croxall, J.P. Marine debris surveys at Bird Island, South Georgia 1990–1995. Mar. Pollut. Bull. 1997, 34, 61–65. [Google Scholar] [CrossRef]
- Walker, T.R.; Grant, J.; Archambault, M.C. Accumulation of marine debris on an intertidal beach in an urban park (Halifax Harbour, Nova Scotia). Water Qual. Res. J. Can. 2006, 41, 256–262. [Google Scholar] [CrossRef]
- Derraik, J.G.B. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Sánchez, P.; Mercedes, M.; Saez, R.; Juan, S.; Muntadas, A.; Demestre, A. Baseline study of the distribution of marine debris on soft-bottom habitats associated with trawling grounds in the northern Mediterranean. Sci. Mar. 2013, 77, 247–255. [Google Scholar] [CrossRef]
- Thompson, R.C. Plastic debris in the marine environment: Consequences and solutions. In Marine Nature Conservation in Europe; Krause, J.C., Nordheim, H., Bräger, S., Eds.; Federal Agency for Nature Conservation: Stralsund, Germany, 2006; pp. 107–115. [Google Scholar]
- Munari, C.; Corbau, C.; Simeoni, U.; Mistri, M. Marine litter on Mediterranean shores: Analysis of composition, spatial distribution and sources in north-western Adriatic beaches. Waste Manag. 2015, 49, 483–490. [Google Scholar] [CrossRef]
- Laist, D.W. Overview of the Biological Effects of Lost and Discarded Plastic Debris in the Marine Environment. Mar. Pollut. Bull. 1987, 18, 319–326. [Google Scholar] [CrossRef]
- Ivar Do Sul, J.A.; Costa, M.F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 80, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Nelms, S.E.; Duncan, E.M.; Broderick, A.C.; Galloway, T.S.; Godfrey, M.H.; Hamann, M.; Lindeque, P.K.; Godley, B.J. Plastic and marine turtles: A review and call for research. ICES J. Mar. Sci. 2016, 73, 165–181. [Google Scholar] [CrossRef]
- Pettipas, S.; Bernier, M.; Walker, T. A Canadian policy framework to mitigate plastic marine pollution. Mar. Policy 2016, 68, 117–122. [Google Scholar] [CrossRef]
- UNEP. Marine Litter: An Analytical Overview. Intergovernmental Oceanographic Commission of The United Nations Educational, Scientific and Cultural Organisation. 2005. Available online: http://wedocs.unep.org/bitstream/handle/20.500.11822/8348/-Marine%20Litter%2c%20an%20analytical%20overview-20053634.pdf?sequence=3&isAllowed=y (accessed on 1 March 2019).
- Law, K.L.; Moret-Ferguson, S.; Maximenko, N.A.; Proskurowski, G.; Peacock, E.E.; Hafner, J.; Reddy, C.M. Plastic accumulation in the North Atlantic Subtropical Gyre. Science 2010, 329, 1185–1188. [Google Scholar] [CrossRef]
- Gallo, F.; Fossi, C.; Weber, R.; Santillo, D.; Sousa, J.; Ingram, I.; Nadal, A.; Romano, D. Marine litter plastics and microplastics and their toxic chemicals components: The need for urgent preventive measures. Environ. Sci. Eur. 2018, 30, 13. [Google Scholar] [CrossRef]
- McKinsey Center for Business and Environment. Stemming the Tide: Land-Based Strategies for a Plastic-Free Ocean; McKinsey & Company and Ocean Conservancy: New York, NY, USA, 2015; Available online: https://oceanconservancy.org/wp-content/uploads/2017/04/full-report-stemming-the.pdf (accessed on 7 April 2019).
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef]
- Ellen Macarthur Foundation. The new Plastics Economy: Rethinking the Future of Plastics. Ellen Macarthur Foundation, Cowes. 2016. Available online: https://www.ellenmacarthurfoundation.org/publications/the-new-plastics-economy-rethinking-the-future-of-plastics (accessed on 5 April 2019).
- Carlson, D.F.; Suaria, G.; Aliani, S.; Fredj, E.; Fortibuoni, T.; Griffa, A.; Russo, A.; Melli, V. Combining litter observations with a regional ocean model to identify sources and sinks of floating debris in a semi-enclosed basin: The Adriatic Sea. Front. Mar. Sci. 2017, 4, 78. [Google Scholar] [CrossRef]
- Corbin, C.J.; Singh, J.G. Marine debris contamination of beaches in St. Lucia and Dominica. Mar. Pollut. Bull. 1993, 26, 325–328. [Google Scholar] [CrossRef]
- Kubota, M. A mechanism for the accumulation of floating marine debris north of Hawaii. J. Phys. Oceanogr. 1994, 5, 1059–1064. [Google Scholar] [CrossRef]
- Kubota, M.; Takayama, K.; Namimoto, D. Pleading for the use of biodegradable polymers in favor of marine environments and to avoid an asbestos-like problem for the future. Appl. Microbiol. Biotechnol. 2005, 67, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Biermann, L.; Clewley, D.; Martinez-Vicente, V.; Topouzelis, K. Finding plastic patches in coastal waters using optical satellite data. Sci. Rep. 2020, 10, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Napper, I.E.; Thompson, R.C. Marine plastic pollution: Other than microplastic. In Waste; Elsevier: Amsterdam, The Netherlands, 2019; pp. 425–442. [Google Scholar] [CrossRef]
- D’Asaro, E.A.; Shcherbina, A.Y.; Klymak, J.M.; Molemaker, J.; Novelli, G.; Guigand, C.M.; Haza, A.C.; Haus, B.K.; Ryan, E.H.; Jacobs, G.A.; et al. Ocean convergence and the dispersion of flotsam. Proc. Natl. Acad. Sci. USA 2018, 115, 1162–1167. [Google Scholar] [CrossRef]
- Möhlenkamp, P.; Purser, A.; Thomsen, L. Plastic microbeads from cosmetic products: An experimental study of their hydrodynamic behaviour, vertical transport and resuspension in phytoplankton and sediment aggregates. Elem. Sci. Anth. 2018, 6, 61. [Google Scholar] [CrossRef]
- Brooks, M.T.; Coles, V.J.; Coles, W.C. Inertia influences pelagic sargassum advection and distribution. Geophys. Res. Lett. 2019, 46, 2610–2618. [Google Scholar] [CrossRef]
- Thiel, M.; Hinojosa, I.A.; Joschko, T.; Gutow, L. Spatio-temporal distribution of floating objects in the german bight (North Sea). J. Sea Res. 2011, 65, 368–379. [Google Scholar] [CrossRef]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef]
- Chu, S.; Wang, J.; Leong, G.; Woodward, L.A.; Letcher, R.J.; Li, Q.X. Perfluoroalkyl sulfonates and carboxylic acids in liver, muscle and adipose tissues of black-footed albatross (Phoebastria nigripes) from Midway Island, North Pacific Ocean. Chemosphere 2015, 138, 60–66. [Google Scholar] [CrossRef][Green Version]
- Pasquini, G.; Ronchi, F.; Strafella, P.; Scarcella, G.; Fortibuoni, T. Seabed litter composition, distribution and sources in the Northern and Central Adriatic Sea (Mediterranean). Waste Manag. 2016, 58, 41–51. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Corcoran, P.L.; Biesinger, M.C.; Grifi, M. Plastics and beaches: A degrading relationship. Mar. Pollut. Bull. 2009, 58, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.R.; Andrady, A.L. Plastics in the marine environment. In Plastics and the Environment; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 379–402. [Google Scholar]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.R. Environmental implications of plastic debris in marine settings entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc. B 2009, 364, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Browne, M.A.; Underwood, A.J.; van Franeker, J.A.; Thompson, R.C. The ecological impacts of marine debris: Unraveling the demonstrated evidence from what is perceived. Ecology 2015, 97, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Moore, C.J.; vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B 2009, 364, 2153–2166. [Google Scholar] [CrossRef]
- Kwon, B.G.; Koizumi, K.; Chung, S.-Y.; Kodera, Y.; Kim, J.-O.; Saido, K. Global styrene oligomers monitoring as new chemical contamination from polystyrene plastic marine pollution. J. Hazard Mater. 2015, 300, 359–367. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “Plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Wilcox, C.; van Sebille, E.; Hardesty, B.D. Threat of plastic pollution to seabirds is global, pervasive, and increasing. Proc. Natl. Acad. Sci. USA 2015, 112, 11899–11904. [Google Scholar] [CrossRef]
- Schuyler, Q.A.; Wilcox, C.; Townsend, K.A.; Wedemeyer-Strombel, K.R.; Balazs, G.; van Sebille, E.; Hardesty, B.D. Risk analysis reveals global hotspots for marine debris ingestion by sea turtles. Glob. Chang. Biol. 2016, 22, 567–576. [Google Scholar] [CrossRef]
- Sherman, P.; van Sebille, E. Modeling marine surface microplastic transport to assess optimal removal locations. Environ. Res. Lett. 2016, 11, 014006. [Google Scholar] [CrossRef]
- UN Environment (2016) Regional Seas Programme. Regional Seas Strategic Directions (2017–2020); Regional Seas Reports and Studies No.201; UN Environment Regional Seas Programme: Nairobi, Kenya, 2016; Available online: www.unep.org/regionalseas (accessed on 25 April 2019).
- Notten, P. Addressing Marine Plastics: A Systemic Approach; United Nations Environment Programme: Nairobi, Kenya, 2019; Available online: http://wedocs.unep.org/bitstream/handle/20.500.11822/26746/marine_plastics.pdf?sequence=1&isAllowed=y (accessed on 18 March 2019).
- GESAMP. Guidelines for the Monitoring and Assessment of Plastic Litter in the Ocean; Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection: London, UK, 2019; Available online: http://www.gesamp.org/publications/guidelines-for-the-monitoring-and-assessment-of-plastic-litter-in-the-ocean (accessed on 18 March 2019).
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment—A Global Assessment (Part 1); Joint Group of Experts on the Scientific, Aspects of Marine Environmental Protection, 2015. Available online: http://www.gesamp.org/publications/reports-and-studies-no-90 (accessed on 1 April 2019).
- UNEP/MAP. Report Meeting of MED POL Focal Points Mediterranean Action Plan; MED POL, WG. 334/8; UNEP/MAP: Kalamata, Greece, 29 July 2009. [Google Scholar]
- Cozar, A.; Sanz-Martin, M.; Marti, E.; Gonzalez-Gordillo, J.I.; Ubeda, B.; Galvez, J.A.; Irigoien, X.; Duarte, C.M. Plastic accumulation in the Mediterranean Sea. PLoS ONE 2015, 10, e0121762. [Google Scholar] [CrossRef]
- Fossi, M.C.; Romeo, T.; Baini, M.; Panti, C.; Marsili, L.; Campani, T.; Canese, S.; Galgani, F.; Druon, J.N.; Airoldi, S.; et al. Plastic debris occurrence, convergence areas and Fin Whales feeding ground in the Mediterranean Marine Protected Area Pelagos Sanctuary: A modelling approach. Front. Mar. Sci. 2017, 4, 167. [Google Scholar] [CrossRef]
- van Sebille, E.; Wilcox, C.; Lebreton, L.C.M.; Maximenko, N.A.; Hardesty, B.D.; van Franeker, J.A.; Eriksen, M.; Siegel, D.; Galgani, F.; Law, K.L. A global inventory of small floating plastic debris. Environ. Res. Lett. 2015, 10, 124006. [Google Scholar] [CrossRef]
- Alomar, C.; Estarellas, F.; Deudero, S. Microplastics in the Mediterranean Sea: Deposition in coastal shallow sediments, spatial variation and preferential grain size. Mar. Environ. Res. 2016, 115, 1–10. [Google Scholar] [CrossRef]
- Suaria, G.; Avio, C.G.; Mineo, A.; Lattin, G.L.; Magaldi, M.G.; Belmonte, G.; Moore, C.J.; Regoli, F.; Aliani, S. The Mediterranean Plastic Soup: Synthetic polymers in Mediterranean surface waters. Sci. Rep. 2016, 6, 37551. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, G.; Zviely, D.; Assaf, A.; Spanier, E.; Ribic, C. Message in a bottle—The story of floating plastic in the eastern Mediterranean Sea. Waste Manag. 2018, 77, 67–77. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef]
- Hecht, A.; Pinardi, N.; Robinson, A.R.; Hecht, A.; Pinardi, N.; Robinson, A.R. Currents, water masses, eddies and jets in the Mediterranean Levantine Basin. J. Phys. Oceanogr. 1988, 18, 1320–1353. [Google Scholar] [CrossRef]
- Mansui, J.; Molcard, A.; Ourmières, Y. Modelling the transport and accumulation of floating marine debris in the Mediterranean basin. Mar. Pollut. Bull. 2015, 91, 249–257. [Google Scholar] [CrossRef]
- Zambianchi, E.; Trani, M.; Falco, P. Lagrangian transport of marine litter in the Mediterranean Sea. Front. Environ. Sci. 2017, 5, 5. [Google Scholar] [CrossRef]
- Driedger, A.; Dürr, H.; Mitchell, K.; Van Cappellen, P. Plastic debris in the Laurentian Great Lakes: A review. J. Great Lakes Res. 2015, 41, 9–19. [Google Scholar] [CrossRef]
- Hafeez, S.; Wong, M.; Abbas, S.; Nichol, J.; Kwok, C. Detection and Monitoring of Marine Pollution Using Remote Sensing Technologies. In Monitoring of Marine Pollution; Fouzia, H.B., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Aoyama, T. Extraction of marine debris in the Sea of Japan using high-spatial-resolution satellite images. In Remote Sensing of the Oceans and Inland Waters: Techniques, Applications, and Challenges; Frouin, R.J., Shenoi, S.C., Rao, K.H., Eds.; Proceedings of SPIE; SPIE: Bellingham, WA, USA, 2016; Volume 9878, p. 987817. [Google Scholar]
- Garaba, S.; Aitken, J.; Slat, B.; Dierssem, H.; Lebreton, L.; Zielinski, O.; Reisser, J. Sensing ocean plastics with an airborne hyperspectral shortwave infrared imager. Environ. Sci. Technol. 2018, 52, 11699–11707. [Google Scholar] [CrossRef] [PubMed]
- Goddijn-Murphy, L.; Peters, S.; Van Sebille, E.; James, N.A.; Gibb, S. Concept for a hyperspectral remote sensing algorithm for floating marine macro plastics. Mar. Pollut. Bull. 2018, 126, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Maximenko, N.; Arvesen, J.; Asner, G.; Carlton, J.; Castrence, M.; Centurioni, L.; Chao, Y.; Chapman, J.; Chirayath, V.; Corradi, P.; et al. Remote Sensing of Marine Debris to Study Dynamics, Balances and Trends. In Community White Paper Produced at the Workshop on Mission Concepts for Marine Debris Sensing. 2016. Available online: https://pdfs.semanticscholar.org/fab0/8714d6f6b12bbd7645582f03b386b019cd14.pdf (accessed on 18 March 2019).
- Pichel, W.G.; Veenstra, T.S.; Churnside, J.H.; Arabini, E.; Friedman, K.S.; Foley, D.G.; Brainard, R.E.; Kiefer, D.; Ogle, S.; Clemente-Coloón, P.; et al. GhostNet marine debris survey in the Gulf of Alaska—Satellite guidance and aircraft observations. Mar. Pollut. Bull. 2012, 65, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Davaasuren, N.; Marino, A.; Boardman, C.; Alparone, M.; Nunziata, F.; Ackermann, N.; Hajnsek, I. Detecting microplastics pollution in world oceans using SAR remote sensing. In Proceedings of the International Geoscience and Remote Sensing Symposium (IEEE), Valencia, Spain, 22–27 July 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 938–941. [Google Scholar] [CrossRef]
- Howe, K.L.; Dean, C.W.; John Kluge, J.; Soloviev, A.V.; Tartar, A.; Shivji, M.; Lehner, S.; Shen, H.; Perrie, W. Relative abundance of Bacillus spp., surfactant-associated bacterium present in a natural sea slick observed by satellite SAR imagery over the Gulf of Mexico. Elem. Sci. Anth. 2018, 6, 8. [Google Scholar] [CrossRef]
- Nazeer, M.; Nichol, J.E. Combining landsat TM/ETM+ and HJ-1 A/B CCD sensors for monitoring coastal water quality in Hong Kong. IEEE Geosci. Remote Sens. Lett. 2015, 12, 1898–1902. [Google Scholar] [CrossRef]
- Khorram, S.; Cheshire, H.; Geraci, A.L.; La Rosa, G. Water quality mapping of Augusta Bay, Italy from Landsat-TM data. Int. J. Remote Sens. 1991, 12, 803–808. [Google Scholar] [CrossRef]
- Baban, S.M.J. Detecting water quality parameters in the Norfolk Broads, UK, using Landsat imagery. Int. J. Remote Sens. 1993, 14, 1247–1267. [Google Scholar] [CrossRef]
- Pattiaratchi, C.; Lavery, P.; Wyllie, A.; Hick, P. Estimates of water quality in coastal waters using multi-date Landsat Thematic Mapper data. Int. J. Remote Sens. 1994, 15, 1571–1584. [Google Scholar] [CrossRef]
- Lim, J.; Choi, M. Assessment of water quality based on Landsat 8 operational land imager associated with human activities in Korea. Environ. Monit. Assess. 2015, 187, 384. [Google Scholar] [CrossRef]
- Liubartseva, S.; Coppini, G.; Lecci, R.; Creti, S. Regional approach to modeling the transport of floating plastic debris in the Adriatic Sea. Mar. Pollut. Bull. 2016, 103, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Topouzelis, K.; Papakonstantinou, A.; Graba, S. Detection of floating plastics from satellite and unmanned aerial systems (Plastic Litter Project 2018). Int. J. Appl. Earth Obs. Geoinf. 2019, 79, 175–183. [Google Scholar] [CrossRef]
- Hörig, B.; Kühn, F.; Oschütz, F.; Lehmann, F. HyMap hyperspectral remote sensing to detect hydrocarbons. Int. J. Remote Sens. 2001, 22, 1413–1422. [Google Scholar] [CrossRef]
- Martin, C.; Parkes, S.; Zhang, Q.; Zhang, X.; McCabe, M.F.; Duarte, C.M. Use of unmanned aerial vehicles for efficient beach litter monitoring. Mar. Pollut. Bull. 2018, 131, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Moy, K.; Neilson, B.; Chung, A.; Meadows, A.; Castrence, M.; Ambagis, S.; Davidson, K. Mapping coastal marine debris using aerial imagery and spatial analysis. Mar. Pollut. Bull. 2017, 132, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vicente, V.; Clark, J.R.; Corradi, P.; Aliani, S.; Arias, M.; Bochow, M.; Bonnery, G.; Cole, M.; Cózar, A.; Donnelly, R.; et al. Measuring Marine Plastic Debris from Space: Initial Assessment of Observation Requirements. Remote Sens. 2019, 11, 2443. [Google Scholar] [CrossRef]
- Ocean Conservancy. 30th Anniversary of International Coastal Cleanup; Annual Report; Ocean Conservancy: Washington, DC, USA, 2016; 24p, Available online: https://oceanconservancy.org/wp-content/uploads/2017/04/2016-Ocean-Conservancy-ICC-Report.pdf (accessed on 15 April 2019).
- Pasternak, G.; Zviely, D.; Ribic, C.; Assaf, A.; Spanier, E. Sources, composition and spatial distribution of marine debris along the Mediterranean coast of Israel. Mar. Pollut. Bull. 2017, 114, 1036–1045. [Google Scholar] [CrossRef]
- Galgani, F.; Hanke, G.; Maes, T. Global Distribution, Composition and Abundance of Marine Litter. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Berlin, Germany, 2015; pp. 29–56. [Google Scholar]
- García-Rivera, S.; Lizaso, J.L.S.; Bellido, J.M.B. Composition, spatial distribution and sources of macro-marine litter on the Gulf of Alicante seafloor (Spanish Mediterranean). Mar. Pollut. Bull. 2017, 121, 249–259. [Google Scholar] [CrossRef]
- Rokni, K.; Ahmad, A.; Selamat, A.; Hazini, S. Water Feature Extraction and Change Detection Using Multitemporal Landsat Imagery. Remote Sens. 2014, 6, 4173–4189. [Google Scholar] [CrossRef]
- McFeeters, S. The Use of Normalized Difference Water Index (NDWI) in the Delineation of Open Water Features. Int. J. Remote Sens. 1996, 17, 1425–1432. [Google Scholar] [CrossRef]
- Shen, L.; Li, C. Water body extraction from Landsat ETM+ imagery using adaboost algorithm. In Proceedings of the 18th International Conference on Geoinformatics, Beijing, China, 18–20 June 2010. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. In Proceedings of the Third Earth Resources Technology Satellite—1 Symposium; NASA SP-351, Washington, DC, USA, 31 December 1973; pp. 309–317. [Google Scholar]
- Feyisa, G.L.; Meilby, H.; Fensholt, R.; Proud, S.R. Automated Water Extraction Index: A new technique for surface water mapping using Landsat imagery. Remote Sens. Environ. 2014, 140, 23–35. [Google Scholar] [CrossRef]
- Xu, H. Modification of normalised difference water index (NDWI) to enhance open water features in remotely sensed imagery. Int. J. Remote Sens. 2006, 27, 3025–3033. [Google Scholar] [CrossRef]
- Wilson, E.H.; Sader, S.A. Detection of forest harvest type using multiple dates of Landsat TM imagery. Remote Sens. Environ. 2002, 80, 385–396. [Google Scholar] [CrossRef]
- Ustinov, E.A. Sensitivity Analysis in Remote Sensing; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-319-15840-2. [Google Scholar] [CrossRef]
- Masoumi, H.; Safavi, S.M.; Khani, Z. Identification and classification of plastic resins using near infrared reflectance spectroscopy. Int. J. Mech. Ind. Eng. 2012, 6, 213–220. [Google Scholar]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Phil. Trans. R. Soc. B 2009, 364, 2115–2126. [Google Scholar] [CrossRef]




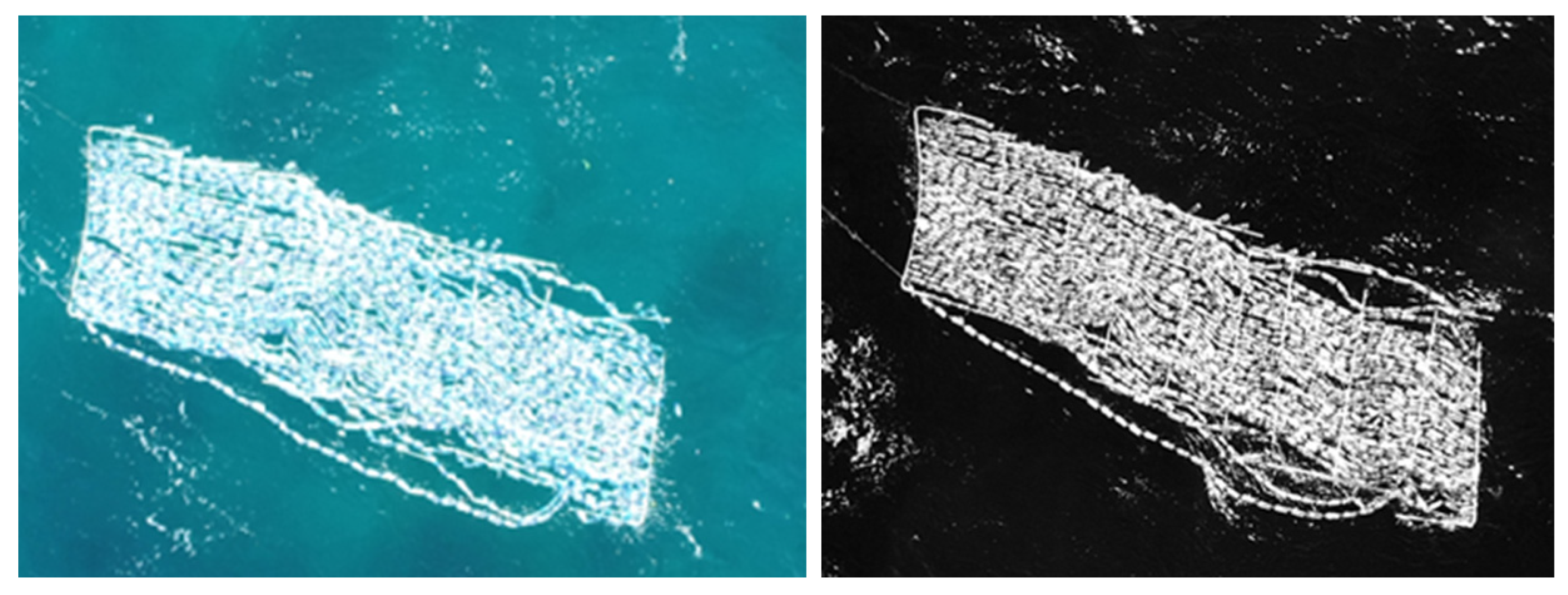
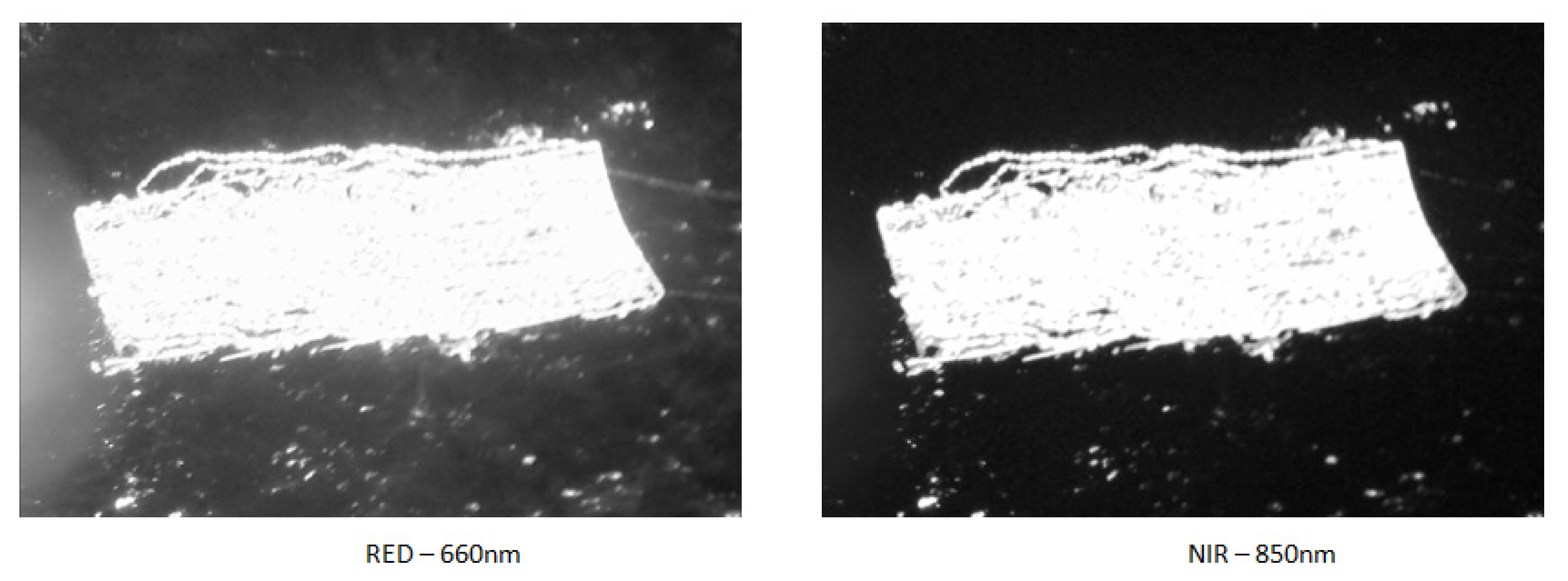
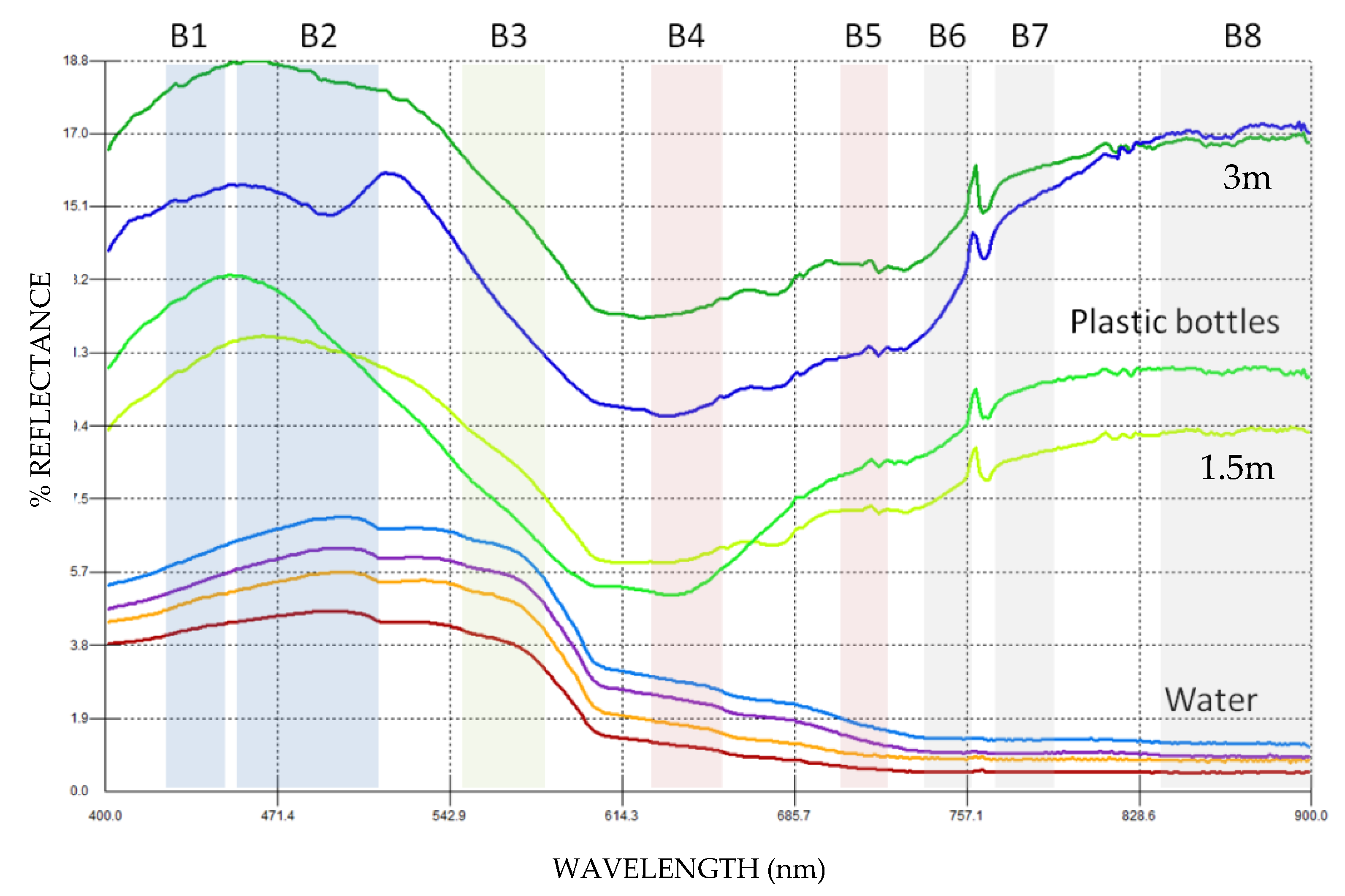

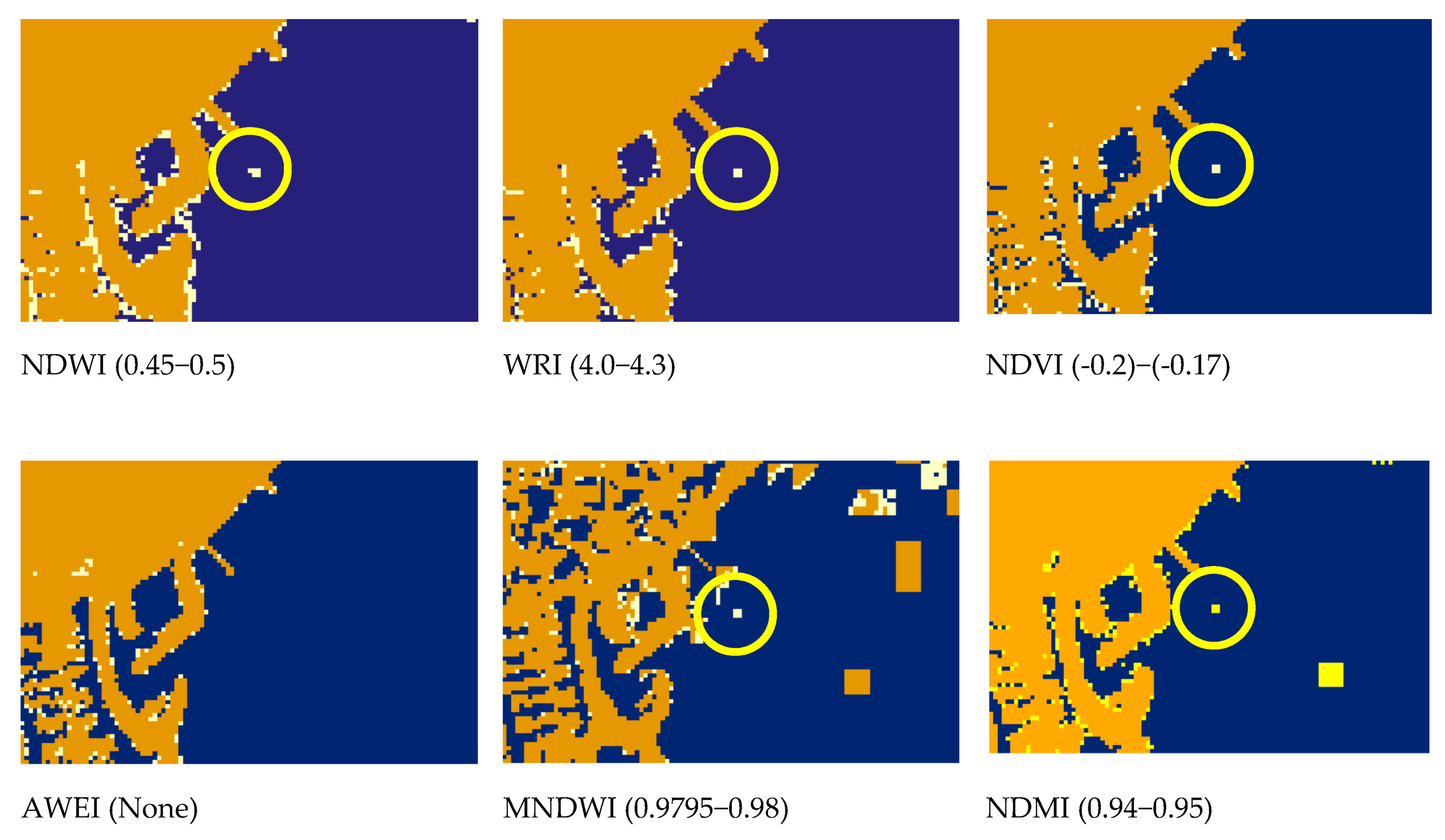


| Band Name | Spatial Resolution (m) | Central Wavelength (nm) | Bandwidth (nm) |
|---|---|---|---|
| B01 | 60 | 443 | 20 |
| B02 | 10 | 490 | 65 |
| B03 | 10 | 560 | 35 |
| B04 | 10 | 665 | 30 |
| B05 | 20 | 705 | 15 |
| B06 | 20 | 740 | 15 |
| B07 | 20 | 783 | 20 |
| B08 | 10 | 842 | 115 |
| B08A | 20 | 865 | 21 |
| B9 | 60 | 945 | 20 |
| B10 | 60 | 1375 | 30 |
| B11 | 20 | 1610 | 90 |
| B12 | 20 | 2190 | 180 |
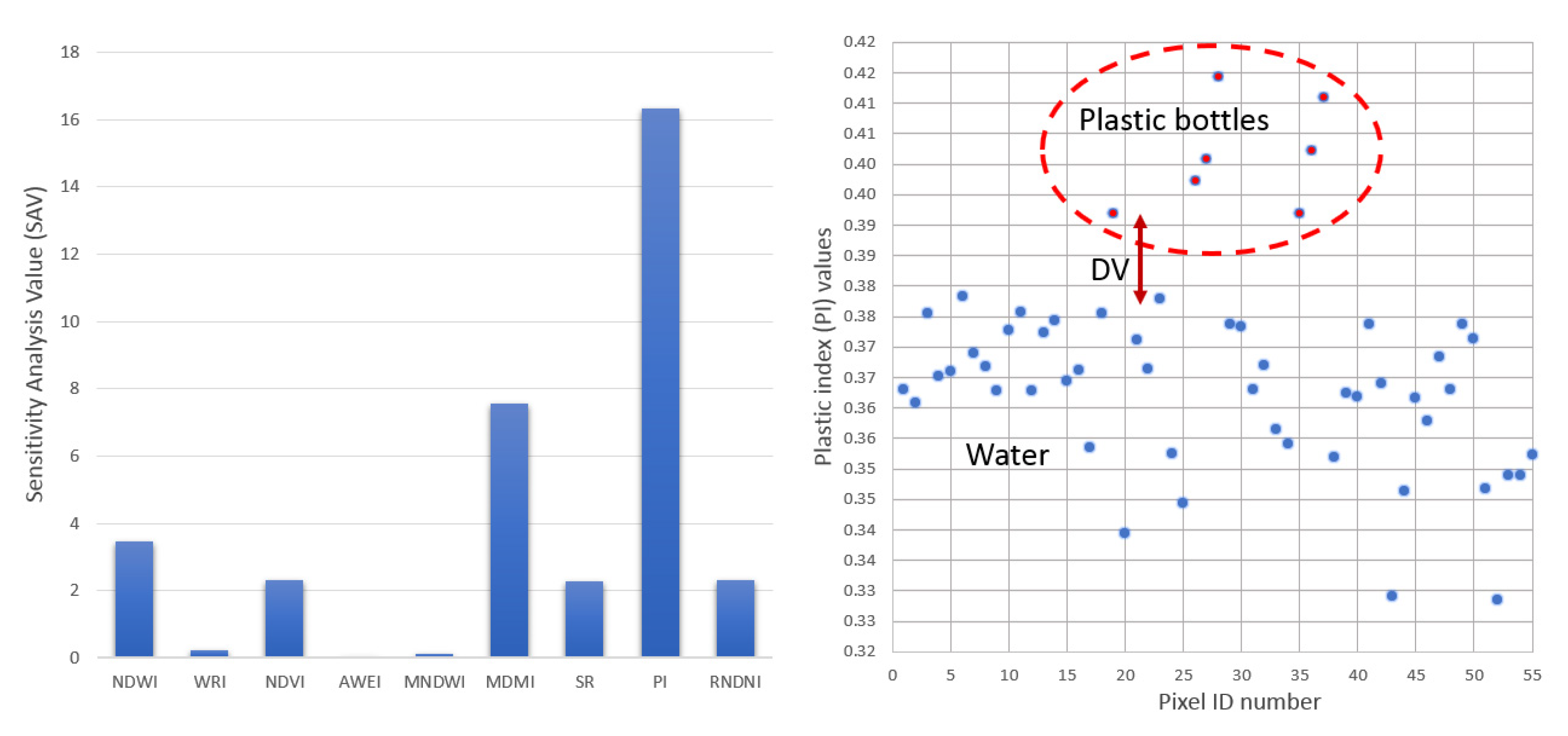
| Indices | Index MIN Value (Dmin) | Index MAX Value (Dmax) | Number of Plastic Pixels Detected (NPD) | Plastic Index Value (PIV) | Water Index Value (WIV) | Discriminative Value (DV) | Sensitivity Analysis Value (SAV) |
|---|---|---|---|---|---|---|---|
| NDWI | 0.4612 | 0.6049 | 5 | 0.45–0.50 | 0.50–0.60 | 0.1 | 3.4795 |
| WRI | 4.0135 | 5.749 | 4 | 4.0–4.3 | 4.3–6.25 | 0.1 | 0.2305 |
| NDVI | −0.3428 | −0.1712 | 4 | −0.2–−0.17 | −0.37–−0.2 | 0.1 | 2.3310 |
| AWEI | 1241 | 2160 | 0 | - | 1241–2160 | 0 | 0.0000 |
| MNDWI | 0.9722 | 0.9797 | 4 | 0.97–0.98 | 0.96–0.97 | 0.0002 | 0.1067 |
| MDMI | 0.893 | 0.9459 | 4 | 0.86–0.94 | 0.94–0.95 | 0.1 | 7.5614 |
| SR | 0.4894 | 0.7076 | 5 | 0.65–0.8 | 0.45–0.65 | 0.1 | 2.2915 |
| PI | 0.3285 | 0.4143 | 7 | 0.39–0.42 | 0.31–0.37 | 0.2 | 16.3170 |
| RNDVI | 0.1712 | 0.3428 | 4 | 0.17–0.2 | 0.2–0.35 | 0.1 | 2.3310 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Themistocleous, K.; Papoutsa, C.; Michaelides, S.; Hadjimitsis, D. Investigating Detection of Floating Plastic Litter from Space Using Sentinel-2 Imagery. Remote Sens. 2020, 12, 2648. https://doi.org/10.3390/rs12162648
Themistocleous K, Papoutsa C, Michaelides S, Hadjimitsis D. Investigating Detection of Floating Plastic Litter from Space Using Sentinel-2 Imagery. Remote Sensing. 2020; 12(16):2648. https://doi.org/10.3390/rs12162648
Chicago/Turabian StyleThemistocleous, Kyriacos, Christiana Papoutsa, Silas Michaelides, and Diofantos Hadjimitsis. 2020. "Investigating Detection of Floating Plastic Litter from Space Using Sentinel-2 Imagery" Remote Sensing 12, no. 16: 2648. https://doi.org/10.3390/rs12162648
APA StyleThemistocleous, K., Papoutsa, C., Michaelides, S., & Hadjimitsis, D. (2020). Investigating Detection of Floating Plastic Litter from Space Using Sentinel-2 Imagery. Remote Sensing, 12(16), 2648. https://doi.org/10.3390/rs12162648








