Association of Environmental Factors in the Taiwan Strait with Distributions and Habitat Characteristics of Three Swimming Crabs
Abstract
1. Introduction
2. Materials and Methods
2.1. Swimming Crab Fishery Data
2.2. Marine Environmental Data
2.3. Spatial and Temporal Statistical Models of Swimming Crab Catch Rates
2.4. Predictions of Swimming Crab Catch Rates
3. Results
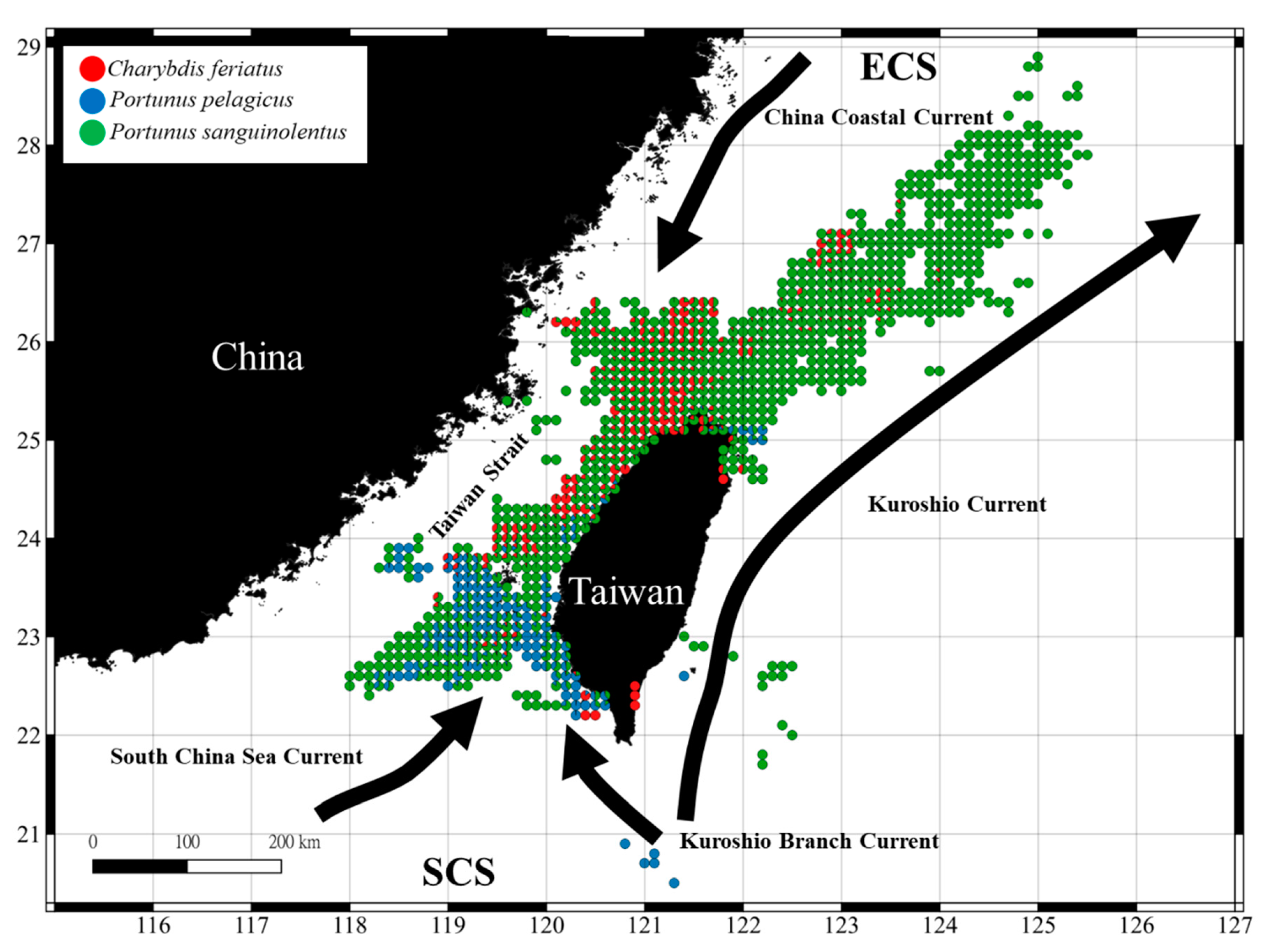
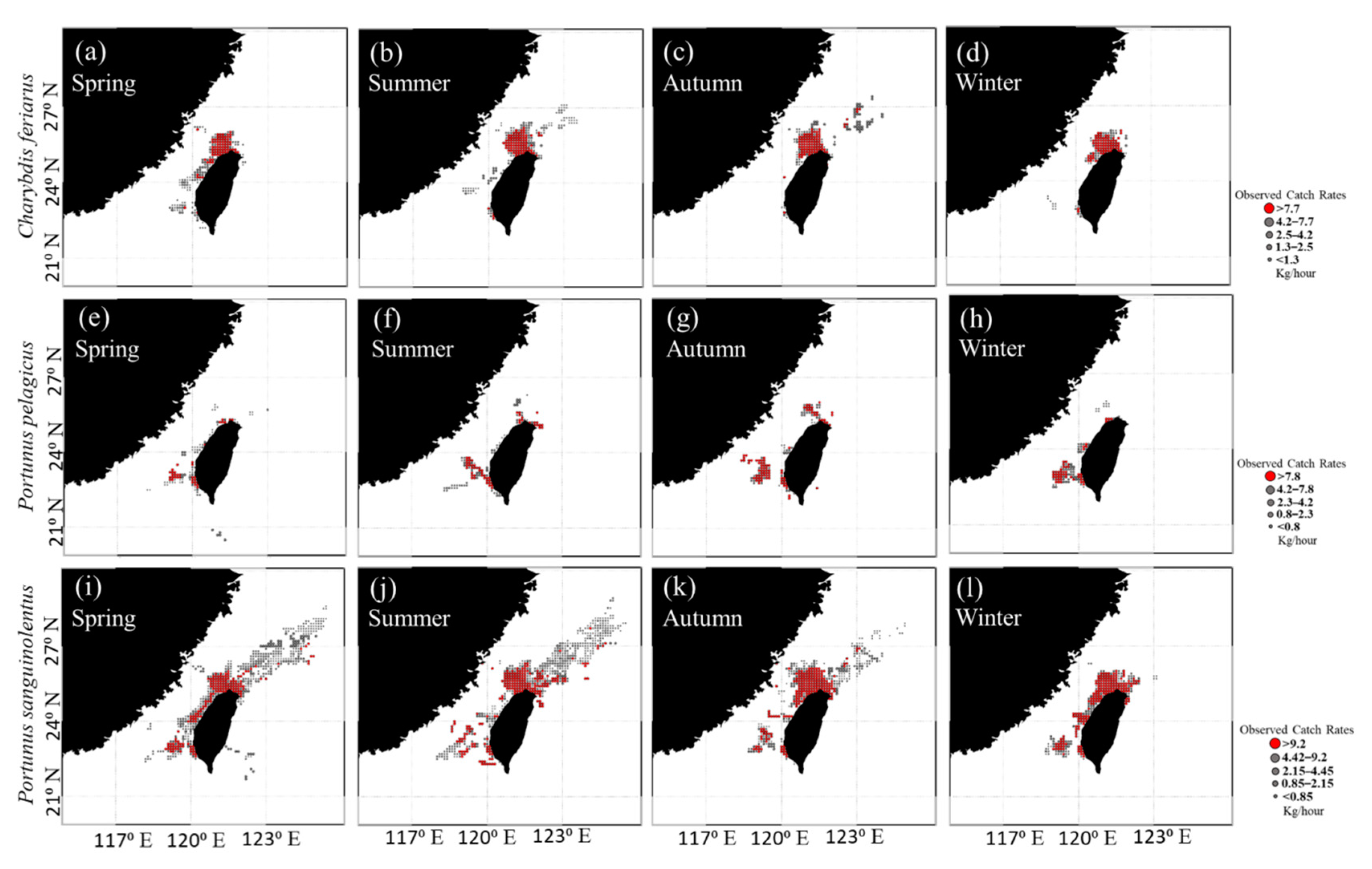
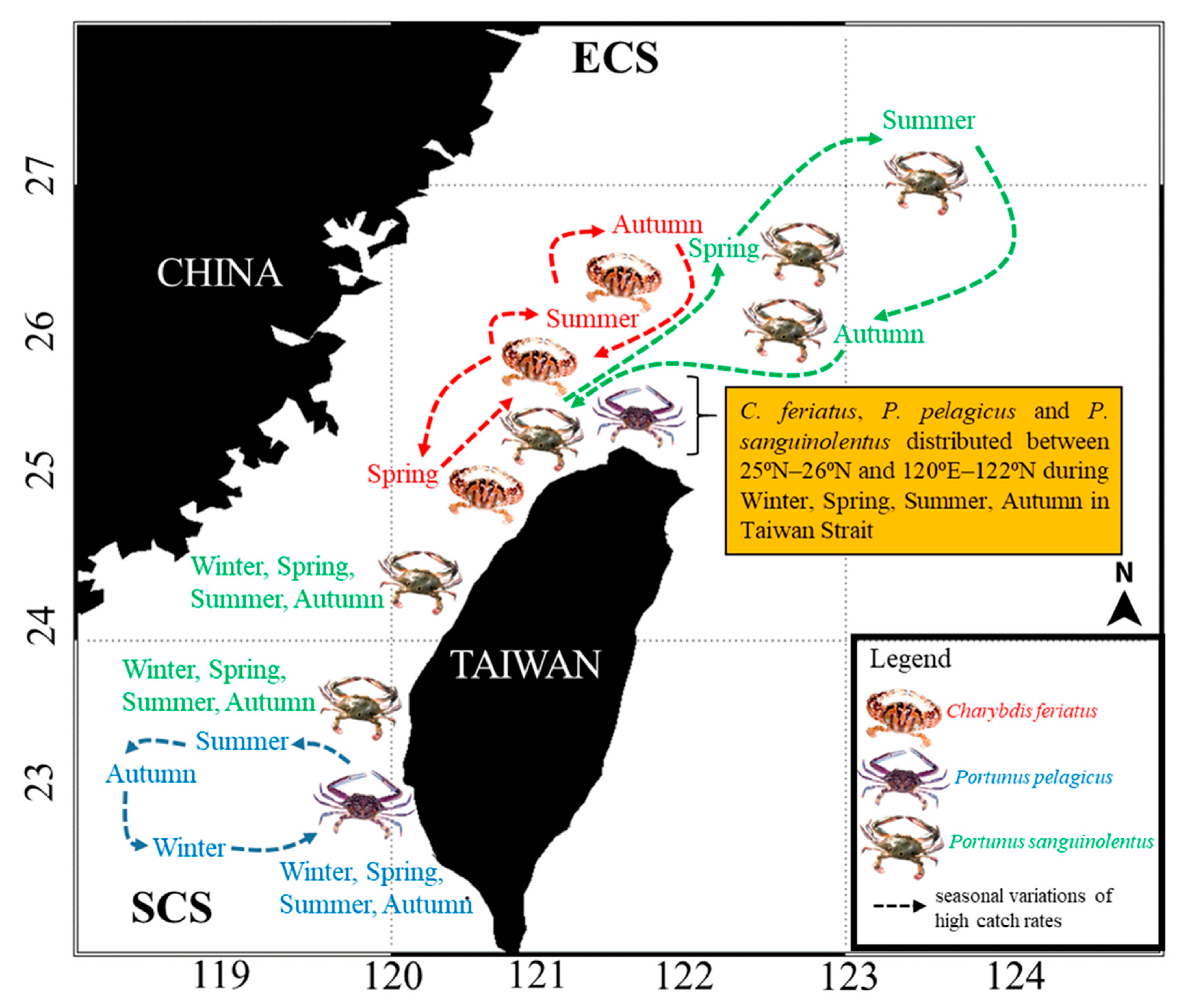
3.1. Spatial and Temporal Distribution of Three Crab Species in the TS
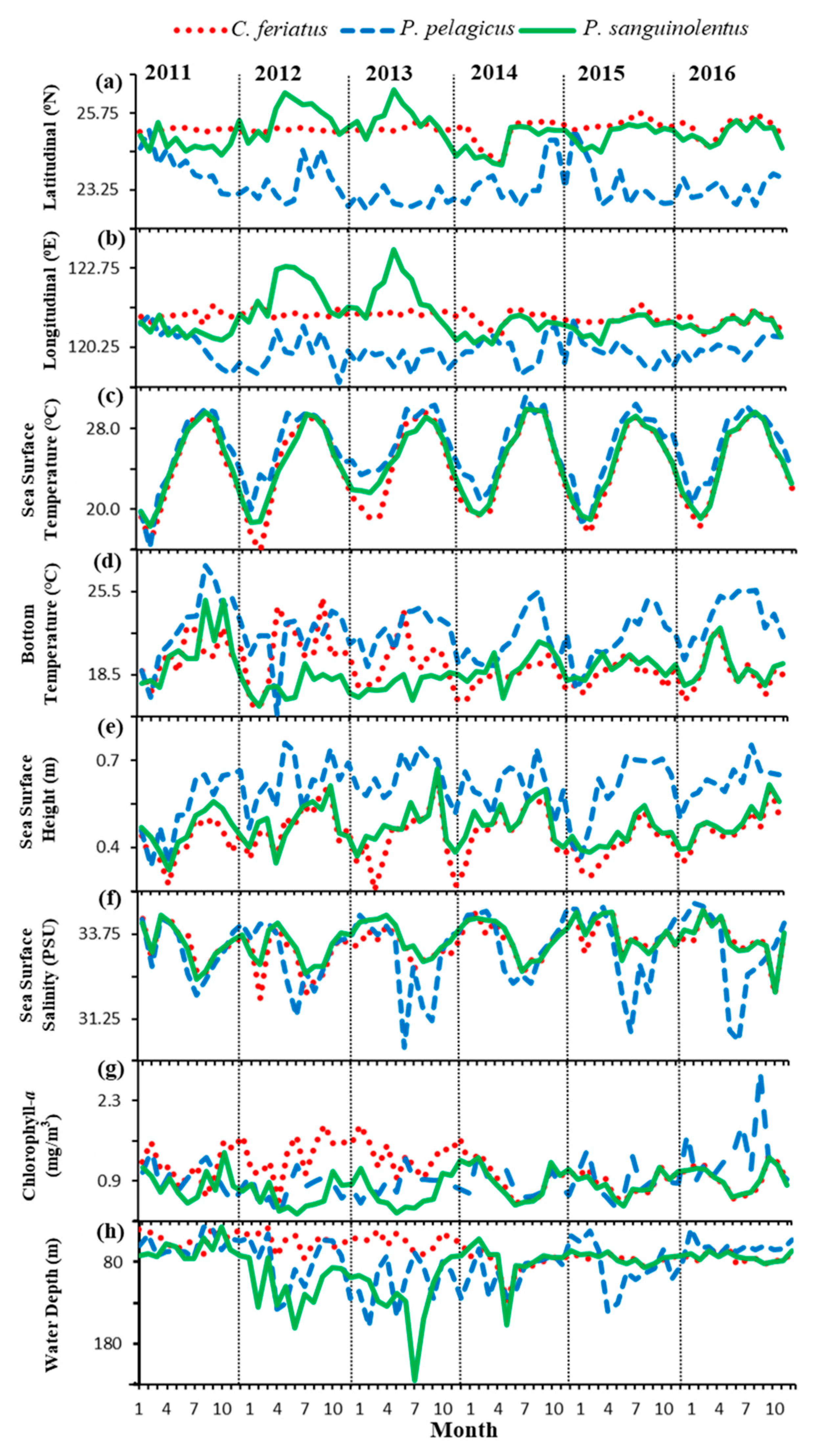
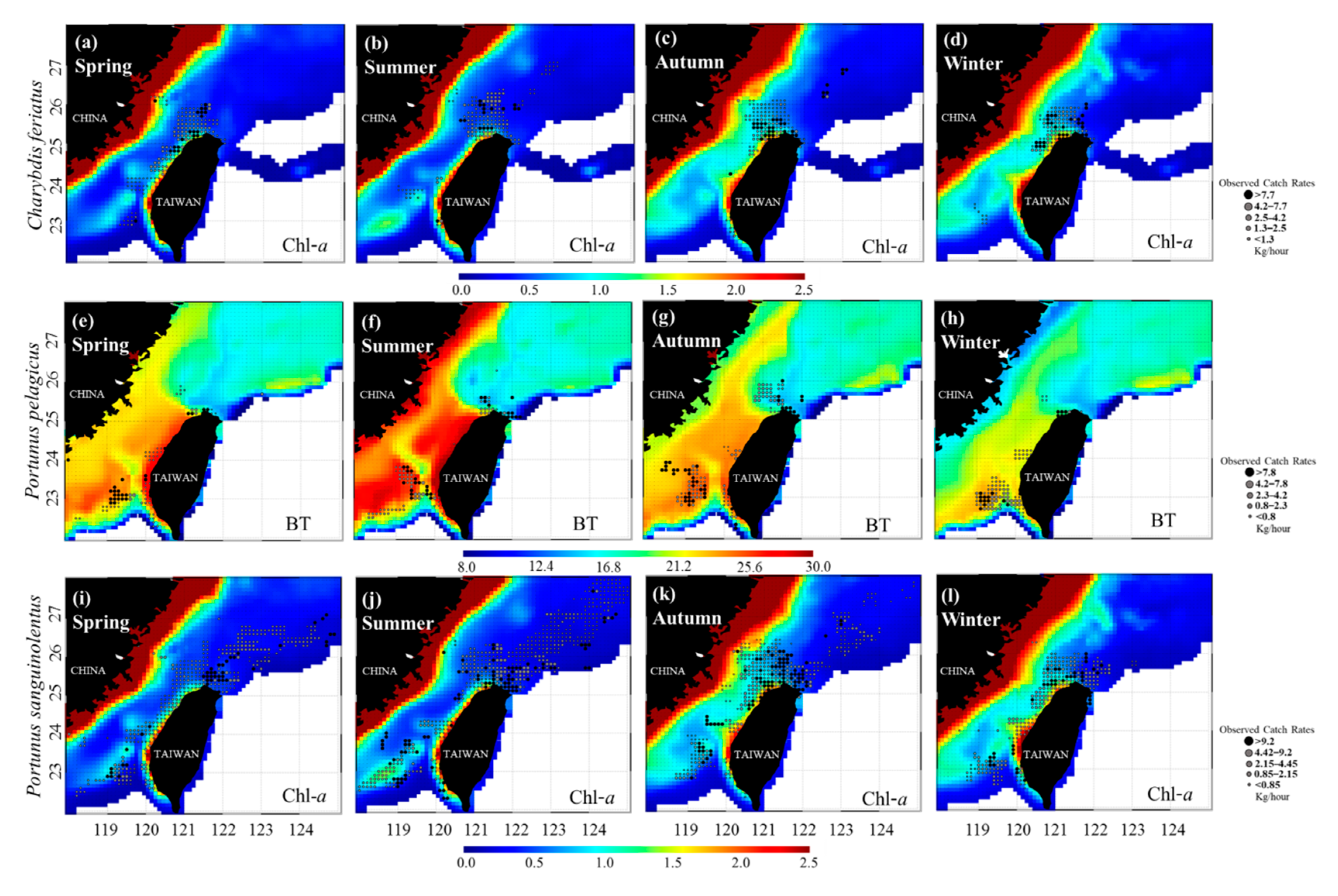
3.2. Environmental Effect on Swimming Crab Catch Rates
3.3. Predicted Spatial Distributions of the Three Crab Species
4. Discussion
4.1. Portunid Crab Distribution in the TS
4.2. Effects of Ocean Environmental Variables on the Swimming Crabs
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kruse, G.H.; Zheng, J.; Stram, D.L. Recovery of the Bristol Bay stock of red king crabs under a rebuilding plan. ICES J. Mar. Sci. 2010, 67, 1866–1874. [Google Scholar] [CrossRef][Green Version]
- Szuwalski, C.S.; Punt, A.E. Fisheries management for regime-based ecosystems: A management strategy evaluation for the snow crab fishery in the eastern Bering Sea. ICES J. Mar. Sci. 2013, 70, 955–967. [Google Scholar] [CrossRef]
- Kunsook, C.; Gajaseni, N.; Paphavasit, N. A stock assessment of the blue swimming crab Portunus pelagicus (Linnaeus, 1758) for sustainable management in Kung Krabaen Bay, Gulf of Thailand. Trop. Life Sci. Res. 2014, 25, 41. [Google Scholar]
- Gutiérrez, N.L.; Masello, A.; Uscudun, G.; Defeo, O. Spatial distribution patterns in biomass and population structure of the deep sea red crab Chaceon notialis in the Southwestern Atlantic Ocean. Fish. Res. 2011, 110, 59–66. [Google Scholar] [CrossRef]
- Frid, A.; McGreer, M.; Stevenson, A. Rapid recovery of Dungeness crab within spatial fishery closures declared under indigenous law in British Columbia. Glob. Ecol. Conserv. 2016, 6, 48–57. [Google Scholar] [CrossRef]
- Masello, A.; Defeo, O. The deep-sea red crab Chaceon notialis (Geryonidae) in the southwestern Atlantic Ocean: Spatial patterns and long-term effects of fishing. Fish. Res. 2016, 183, 254–262. [Google Scholar] [CrossRef]
- Morris, A.S.; Wilson, S.M.; Dever, E.F.; Chambers, R.M. A test of bycatch reduction devices on commercial crab pots in a tidal marsh creek in Virginia. Estuar. Coasts 2011, 34, 386–390. [Google Scholar] [CrossRef]
- Spencer, D.M.; Brown, I.W.; Doubell, M.J.; Brown, C.J.; Redondo Rodriguez, A.; Lee, S.Y.; Zhang, H.; Lemckert, C.J. Bottom boundary layer cooling and wind-driven upwelling enhance the catchability of spanner crab (Ranina ranina) in South-East Queensland, Australia. Fish. Oceanogr. 2019, 28, 317–326. [Google Scholar] [CrossRef]
- Spencer, D.M.; Doubell, M.J.; Brown, I.W.; Rodriguez, A.R.; Lee, S.Y.; Lemckert, C.J. Environmental indices for spanner crab (Ranina ranina) catch rates depend on regional oceanographic features. Estuar. Coast. Shelf Sci. 2019, 228, 106–361. [Google Scholar] [CrossRef]
- De Lestang, S.; Bellchambers, L.M.; Caputi, N.; Thomson, A.W.; Pember, M.B.; Johnston, D.J.; Harris, D.C. Stock–recruitment–environment relationship in a Portunus pelagicus fishery in Western Australia. In Biology and Management of Exploited Crab Populations under Climate Change; Kruse, G.H., Eckert, G.L., Foy, R.J., Lipcius, R.N., Sainte-Marie, B., Stram, D.L., Woodby., D., Eds.; Alaska Sea Grant, University of Alaska Fairbanks: Fairbanks, AK, USA, 2010; pp. 443–460. [Google Scholar] [CrossRef]
- Lima, P.A.; Andrade, L.S.; Alencar, C.E.R.D.; Pereira, R.T.; Teixeira, G.M.; Fransozo, A. Two species of swimming crabs of the genus Achelous (Crustacea, Brachyura): Environmental requirements determining the niche. Hydrobiologia 2014, 727, 197–207. [Google Scholar] [CrossRef]
- Andrade, L.S.; Frameschi, I.F.; Costa, R.C.; Castilho, A.L.; Fransozo, A. The assemblage composition and structure of swimming crabs (Portunoidea) in continental shelf waters of southeastern Brazil. Cont. Shelf Res. 2015, 94, 8–16. [Google Scholar] [CrossRef]
- Johnston, D.; Harris, D.; Caputi, N.; Thomson, A. Decline of a blue swimmer crab (Portunus pelagicus) fishery in Western Australia-History, contributing factors and future management strategy. Fish. Res. 2011, 109, 119–130. [Google Scholar] [CrossRef]
- Szuwalski, C.; Punt, A.E. Regime shifts and recruitment dynamics of snow crab, Chionoecetes opilio, in the eastern Bering Sea. Fish. Oceanogr. 2013, 22, 345–354. [Google Scholar] [CrossRef]
- Andrade, L.S.; Frameschi, I.F.; Castilho, A.L.; Costa, R.C.; Fransozo, A. Can the pattern of juvenile recruitment and population structure of the speckled swimming crab Arenaeus cribrarius (Decapoda: Brachyura) be determined by geographical variations? Mar. Ecol. 2015, 36, 950–958. [Google Scholar] [CrossRef]
- De Anda-Montañez, J.A.; Martínez-Aguilar, S.; Balart, E.F.; Zenteno-Savín, T.; Méndez-Rodríguez, L.; Amador-Silva, E.; Figueroa-Rodríguez, M. Spatio-temporal distribution and abundance patterns of red crab Pleuroncodes planipes related to ocean temperature from the Pacific coast of the Baja California Peninsula. Fish. Sci. 2016, 82, 1–15. [Google Scholar] [CrossRef]
- Thorson, J.T. Measuring the impact of oceanographic indices on species distribution shifts: The spatially varying effect of cold-pool extent in the eastern Bering Sea. Limnol. Oceanogr. 2019, 64, 2632–2645. [Google Scholar] [CrossRef]
- Liang, W.D.; Tang, T.Y.; Yang, Y.J.; Ko, M.T.; Chuang, W.S. Upper-ocean currents around Taiwan. Deep Sea Res. Part Ii Top. Stud. Oceanogr. 2003, 50, 1085–1105. [Google Scholar] [CrossRef]
- Huh, C.A.; Lin, H.L.; Lin, S.; Huang, Y.W. Modern accumulation rates and a budget of sediment off the Gaoping (Kaoping) River, SW Taiwan: A tidal and flood dominated depositional environment around a submarine canyon. J. Mar. Syst. 2009, 76, 405–416. [Google Scholar] [CrossRef]
- Huh, C.A.; Chen, W.; Hsu, F.H.; Su, C.C.; Chiu, J.K.; Lin, S.; Liu, C.S.; Huang, B.J. Modern (<100 years) sedimentation in the Taiwan Strait: Rates and source-to-sink pathways elucidated from radionuclides and particle size distribution. Cont. Shelf Res. 2011, 31, 47–63. [Google Scholar]
- Ng, P.K.; Wang, C.H.; Ho, P.H.; Shih, H.T. An annotated checklist of brachyuran crabs from Taiwan (Crustacea: Decapoda); National Taiwan Museum: Taipei, Taiwan, 2001; Volume 11.
- Hsu, C.C.; Chang, H.C.; Liu, H.C. Sex-variant morphometrics of the swimming Crab, Portunus sanguinolentus (Herbst), from the. J. Fish. Soc. Taiwan 2000, 27, 175–185. [Google Scholar]
- Hsueh, P.W.; Ng, P.K.; Hung, H.T. Brachyuran crab assemblages in subtidal soft-bottom habitats of Taiwan. J. Fish. Soc. Taiwan 2006, 33, 28–294. [Google Scholar]
- Hsueh, P.W.; Hung, H.T. Temporal and spatial reproductive patterns of subtidal brachyuran crabs in coastal waters of Taiwan. Crustaceana 2009, 449–465. [Google Scholar]
- Huang, Z.R. Species composition and quantitative distribution of crabs in the northern continental shelf of South China Sea. J. Dalian Fish. Univ. 2009, 6, 14. [Google Scholar]
- Sumpton, W.D.; Smith, G.S.; Potter, M.A. Notes on the biology of the portunid crabs, Portunus sanguinolentus (Herbst) in subtropical Queensland waters. Aust. J. Mar. Freshw. Res. 1989, 40, 711–717. [Google Scholar] [CrossRef]
- Potter, M.A.; Sumpoton, W.D.; Smith, G.S. Movement, fishing sector impact and factors affecting the recapture rate of tagged crabs, Portunus pelagicus (L.) in Moreton Bay, Queensland. Aust. J. Mar. Freshw. Res. 1991, 42, 751–760. [Google Scholar] [CrossRef]
- Ikhwanuddin, M.; Nurfaseha, A.H.; Abol-Munafi, A.B.; Shabdin, M.L. Movement patterns of blue swimming crab, Portunus pelagicus in the Sarawak coastal water, South China Sea. J. Sustain. Sci. Manag. 2012, 7, 1–8. [Google Scholar]
- Carr, S.D.; Tankersley, R.A.; Hench, J.L.; Forward, R.B., Jr.; Luettich, R.A., Jr. Movement patterns and trajectories of ovigerous blue crabs Callinectes sapidus during the spawning migration. Estuar. Coast. Shelf Sci. 2004, 60, 567–579. [Google Scholar] [CrossRef]
- Luan, J.; Zhang, C.; Xu, B.; Xue, Y.; Ren, Y. Modelling the spatial distribution of three Portunidae crabs in Haizhou Bay, China. PLoS ONE 2018, 13, e0207457. [Google Scholar] [CrossRef]
- Spencer, D.M.; Brown, I.W.; Doubell, M.J.; McGarvey, R.; Lee, S.Y.; Lemckert, C.J. Bottom Currents Affect Spanner Crab Catch Rates in Southern Queensland, Australia. Mar. Coast. Fish. 2019, 11, 248–257. [Google Scholar] [CrossRef]
- Furlan, M.; Castilho, A.L.; Fernandes-Goes, L.C.; Fransozo, V.; Bertini, G.; Costa, R.C. Effect of environmental factors on the abundance of decapod crustaceans from soft bottoms off southeastern Brazil. An. Da Acad. Bras. De Ciências 2013, 85, 1345–1356. [Google Scholar] [CrossRef]
- Bertini, G.; Fransozo, A. Bathymetric distribution of brachyuran crab (Crustacea, Decapoda) communities on coastal soft bottoms off southeastern Brazil. Mar. Ecol. Prog. Ser. 2004, 279, 193–200. [Google Scholar] [CrossRef]
- De la Barra, P.; Svendsen, G.; Romero, M.A.; Avaca, M.S.; Narvarte, M. Predicting the distribution of a portunid crab in Patagonian coastal waters. Mar. Ecol. Prog. Ser. 2020, 638, 95–105. [Google Scholar] [CrossRef]
- Chou, W.R.; Lai, S.H.; Fang, L.S. Benthic crustacean communities in waters of southwestern Taiwan and their relationships to environmental characteristics. Acta Zool. Taiwanica 1999, 10, 25–33. [Google Scholar]
- Abelló, P.; Hispano, C. The capture of the Indo-Pacific crab Charybdis feriata (Linnaeus, 1758) (Brachyura: Portunidae) in the Mediterranean Sea. Aquat. Invasions 2006, 1, 13–16. [Google Scholar] [CrossRef]
- Edgar, G.J. Predator-prey interactions in seagrass beds. II. Distribution and diet of the blue manna crab Portunus pelagicus Linnaeus at Cliff Head, Western Australia. J. Exp. Mar. Biol. Ecol. 1990, 139, 23–32. [Google Scholar] [CrossRef]
- Hosseini, M.; Vazirizade, A.; Parsa, Y.; Mansori, A. Sex ratio, size distribution and seasonal abundance of blue swimming crab, Portunus pelagicus (Linnaeus, 1758) in Persian Gulf Coasts, Iran. World Appl. Sci. J. 2012, 17, 919–925. [Google Scholar]
- Lee, H.H.; Hsu, C.C. Population biology of the swimming crab Portunus sanguinolentus in the waters off northern Taiwan. J. Crustac. Biol. 2003, 23, 691–699. [Google Scholar] [CrossRef]
- Rasheed, S.; Mustaquim, J. Size at sexual maturity, breeding season and fecundity of three-spot swimming crab Portunus sanguinolentus (Herbst, 1783) (Decapoda, Brachyura, Portunidae) occurring in the coastal waters of Karachi, Pakistan. Fish. Res. 2010, 103, 56–62. [Google Scholar] [CrossRef]
- Monk, J. How long should we ignore imperfect detection of species in the marine environment when modelling their distribution? Fish Fish. 2014, 15, 352–358. [Google Scholar] [CrossRef]
- Dell, J.; Wilcox, C.; Hobday, A.J. Estimation of yellowfin tuna (Thunnus albacares) habitat in waters adjacent to Australia’s East Coast: Making the most of commercial catch data. Fish. Oceanogr. 2011, 20, 383–396. [Google Scholar] [CrossRef]
- Compton, T.J.; Leathwick, J.R.; Inglis, G.J. Thermogeography predicts the potential global range of the invasive European green crab (Carcinus maenas). Divers. Distrib. 2010, 16, 243–255. [Google Scholar] [CrossRef]
- Shanks, A.; Roegner, G.C.; Miller, J. Using megalopae abundance to predict future commercial catches of Dungeness crabs (Cancer magister) in Oregon. Rep. Calif. Coop. Ocean. Fish. Investig. 2010, 51, 106–118. [Google Scholar]
- Lan, K.W.; Shimada, T.; Lee, M.A.; Su, N.J.; Chang, Y. Using remote-sensing environmental and fishery data to map potential yellowfin tuna habitats in the tropical Pacific Ocean. Remote Sens. 2017, 9, 444. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS 3.6 Noosa. 2019. Available online: https://qgis.org/en/site (accessed on 30 September 2019).
- Lehodey, P.; Bertignac, M.; Hampton, J.; Lewis, A.; Picaut, J. El Niño Southern Oscillation and tuna in the western Pacific. Nature 1997, 389, 715–718. [Google Scholar] [CrossRef]
- Lan, K.W.; Evans, K.; Lee, M.A. Effects of climate variability on the distribution and fishing conditions of yellowfin tuna (Thunnus albacares) in the western Indian Ocean. Clim. Chang. 2013, 119, 63–77. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R; Chapman and Hall/CRC: Boca Raton, FL, USA, 2017. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: http://www.R-project.org (accessed on 30 September 2019).
- Shaohua, F. Crabs from central and northern Taiwan Strait. J. Oceanogr. Taiwan Strait 1991, 4. [Google Scholar]
- Ye, S.Z.; Zhang, Z.L.; Ye, Q.T. Species composition and characteristics of crab distribution in south East China Sea. J. Oceanogr. Taiwan Strait 2006, 25, 387. [Google Scholar]
- Ye, S.Z. Species composition and distribution characteristics of crab on Minnan-Taiwan bank fishing grounds. Mar. Tieheries 2004, 4, 1. [Google Scholar]
- Huang, P.-M. The amount distribution and biological characteristics of Charybdis feriatus in the southern part of East China Sea. J. Fujian Fish. 2006, 1, 4. [Google Scholar]
- Potter, I.C.; de Lestang, S. Blue swimmer crab Portunus pelagicus in Leschenault Estuary and Koombana Bay, south-western Australia. J. R. Soc. West. Aust. 2000, 83, 443–458. [Google Scholar]
- Polity, I.A.; Muhamad, J.H.; Long, S.M.; Bolong, A.M.A. Fecundity of blue swimming crab, Portunus pelagicus Linnaeus, 1758 from Sematan fishing district, Sarawak coastal water of South China Sea. Borneo J. Resour. Sci. Technol. 2011, 1, 46–51. [Google Scholar] [CrossRef]
- Baylon, J.; Suzuki, H. Effects of changes in salinity and temperature on survival and development of larvae and juveniles of the crucifix crab Charybdis feriatus (Crustacea: Decapoda: Portunidae). Aquaculture 2007, 269, 390–401. [Google Scholar] [CrossRef]
- Qari, S.; Aljarari, R. The effect of season and acclimation on the heat and cold tolerance of the Red Sea Crab, Portunus pelagicus. Life Sci. J. 2014, 11, 145–148. [Google Scholar]
- Chande, A.I.; Mgaya, Y.D. The fishery of Portunus pelagicus and species diversity of portunid crabs along the Coast of Dar es Salaam, Tanzania. West. Indian Ocean J. Mar. Sci. 2003, 2, 75–84. [Google Scholar]
- Hisam, F.; Hajisamae, S.; Ikhwanuddin, M.; Pradit, S. Distribution pattern and habitat shifts during ontogeny of the blue swimming crab, Portunus pelagicus (Linnaeus, 1758) (Brachyura, Portunidae). Crustaceana 2020, 93, 17–32. [Google Scholar] [CrossRef]
- Samuel, N.J.; Soundarapandian, P. Fishery Potential of Commercially Important Crab Portuns sanguinolentus (Herbst) along Parangipettai Coast, South East Cost of India. Int. J. Anim. Veter. Adv. 2009, 1, 99–104. [Google Scholar]
- Huang, J.R.; Brown, C.L.; Yang, T.B. Spatio-temporal patterns of crab fisheries in the main bays of Guangdong Province, China. Iran. J. Fish. Sci. 2011, 10, 425–436. [Google Scholar]
- Carpenter, K.E.; Krupp, F.; Jones, D.A.; Zajonz, U. FAO species identification field guide for fishery purpose. In The Living Marine Resources of Kuwait, Eastern Saudi Arabia, Bahrain, Qatar, and the United Arab Emirates; FAO: Rome, Italy, 1997; Volume VII, pp. 1–293. [Google Scholar]
- Yang, C.P.; Li, H.X.; Li, L.; Xu, J.; Yan, Y. Population Structure, Morphometric Analysis and Reproductive Biology of Portunus Sanguinolentus (Decapoda: Brachyura: Portunidae) in Honghai Bay, South China Sea. J. Crustacean Biol. 2014, 34, 722–730. [Google Scholar] [CrossRef]
- Carvalho, F.L.; Carvalho, E.A.S.; Couto, E.C.G. Comparative analysis of the distribution and morphological sexual maturity of Persephona lichtensteinii and P. punctata (Brachyura, Leucosiidae) in Ilhe´us, BA, Brazil. Nauplius 2010, 18, 109–115. [Google Scholar]
- Signa, G.; Cartes, J.E.; Solé, M.; Serrano, A.; Sánchez, F. Trophic ecology of the swimming crab Polybius henslowii Leach, 1820 in Galician and Cantabrian Seas: Influences of natural variability and the Prestige oil spill. Cont. Shelf Res. 2008, 28, 2659–2667. [Google Scholar] [CrossRef]
- Van Couwelaar, M.; Angel, M.Y.; Madin, L.P. The distribution and biology of the swimming crab Charybdis smithii (McLeay,1938) (Crustacea; Brachyura; Portunidae) in the NW Indian Ocean. Deep-Sea Res. Ii 1997, 44, 1251–1280. [Google Scholar] [CrossRef]
- Robinson, C.J.; Anislado, V.; Lopez, A. The pelagic red crab (Pleuroncodes planipes) related to active upwelling sites in the California current off the west coast of Baja California. Deep-Sea Res. Ii 2004, 51, 753–766. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X.H. Progress on upwelling studies in the China seas. Rev. Geophys. 2016, 54, 653–673. [Google Scholar] [CrossRef]
- Rome, M.S.; Young-Williams, A.C.; Davis, G.R.; Hines, A.H. Linking temperature and salinity tolerance to winter mortality of Chesapeake Bay blue crabs (Callinectes sapidus). J. Exp. Mar. Biol. Ecol. 2005, 319, 129–145. [Google Scholar] [CrossRef]
- Batoy, C.B.; Sarmago, J.F.; Pilapil, B.C. Breeding season, sexual maturity and fecundity of the blue crab, Portunus pelagicus (L.) in selected coastal waters in Leyte and vicinity, Philippines. Ann. Trop. Res. 1987, 9, 157–177. [Google Scholar]
- Campbell, G.R. Size at sexual maturity and occurrence of ovigerous females in three species of commercially exploited portunid crabs in SE Queensland. Proc. R. Soc. Qld. 1986, 97, 79–87. [Google Scholar]
- Carvalho, F.L.; Couto, E.C.G. Environmental variables influencing the Callinectes (Crustacea: Brachyura: Portunidae) species distribution in a tropical estuary Cachoeira River (Bahia, Brazil). J. Mar. Biol. Assoc. UK 2011, 91, 793–800. [Google Scholar] [CrossRef]
- Jan, S.; Wang, J.; Chern, C.S.; Chao, S.Y. Seasonal variation of the circulation in the Taiwan Strait. J. Mar. Syst. 2002, 35, 249–268. [Google Scholar] [CrossRef]
- Soundarapandian, P.; Varadharajan, D.; Boopathi, A. Reproductive Biology of the Commercially Important Portunid Crab, Portunus sanguinolentus (Herbst). J. Mar. Sci. Res. Dev. 2013, 3, 124. [Google Scholar]
- Tseng, H.C.; You, W.L.; Huang, W.; Chung, C.C.; Tsai, A.Y.; Chen, T.Y.; Lan, K.W.; Gong, G.C. Seasonal Variations of Marine Environment and Primary Production in the Taiwan Strait. Front. Mar. Sci. 2020, 7, 38. [Google Scholar] [CrossRef]
- Maestrini, S.Y.; Graneli, E. Environmental-conditions and ecophysiological mechanisms which led to the 1988 chrysochromulina-polylepis bloom-an hypothesis. Oceanol. Acta 1991, 14, 397–413. [Google Scholar]
- Jan, S.; Tseng, Y.H.; Dietrich, D.E. Sources of water in the Taiwan Strait. J. Oceanogr. 2010, 66, 211–221. [Google Scholar] [CrossRef]
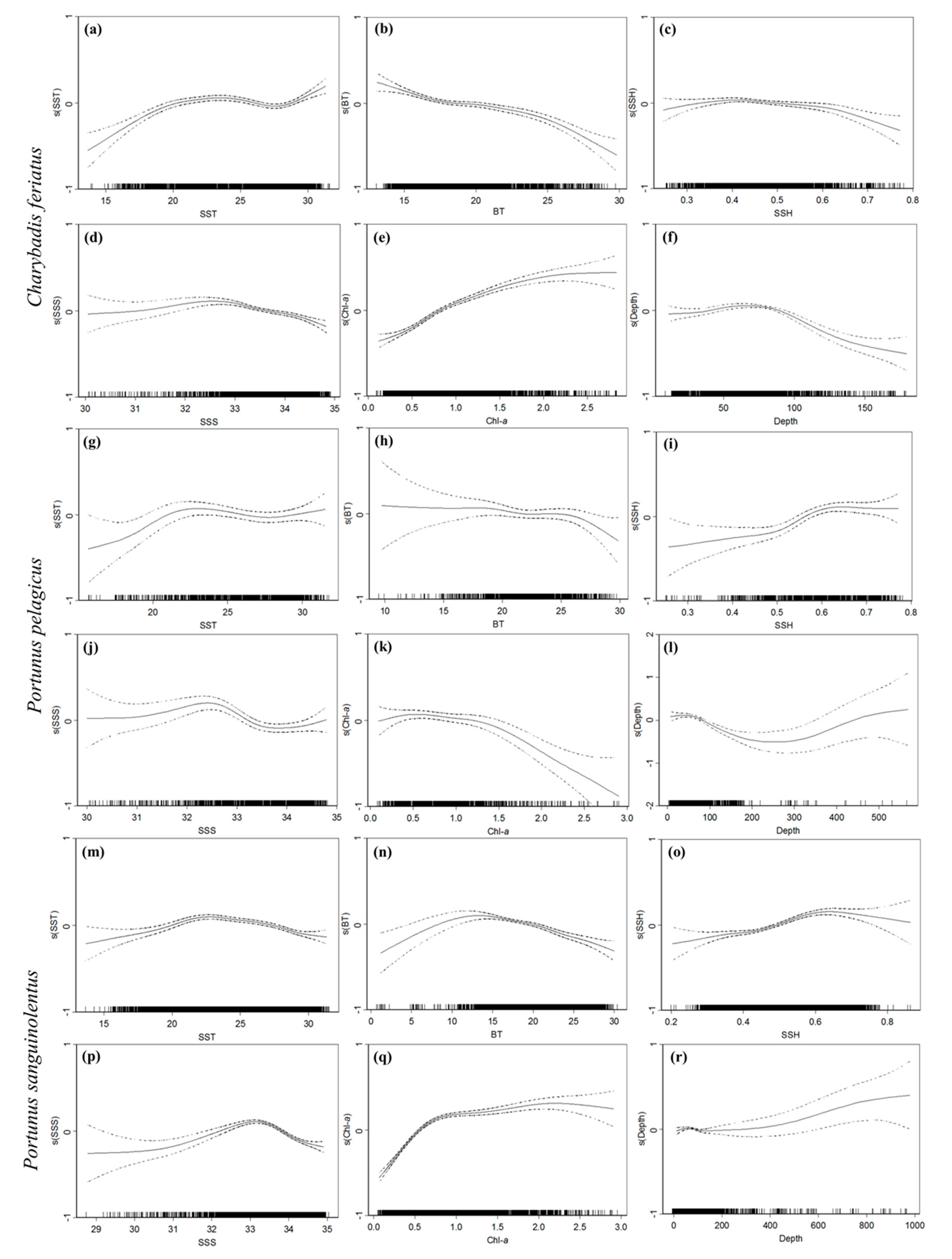
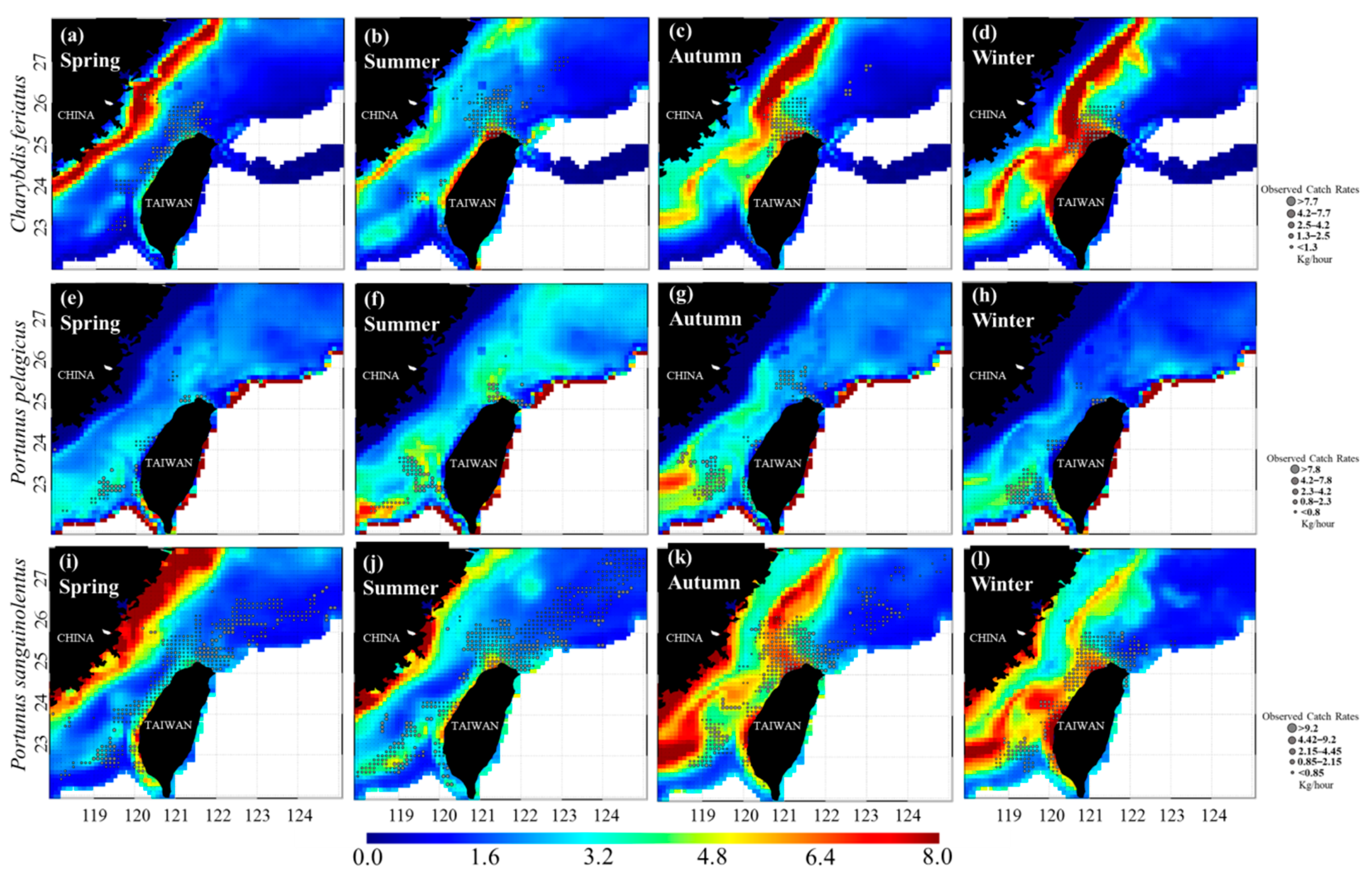
| Variable | C. feriatus | P. pelagicus | P. sanguinolentus | |||
|---|---|---|---|---|---|---|
| EV, % | AIC | EV, % | AIC | EV, % | AIC | |
| +s (SST) | 11.0 | 5983.908 | 1.80 | 3698.498 | 2.55 | 18392.500 |
| +s (BT) | 2.33 | 5950.341 | 7.27 | 3653.093 | 2.32 | 18284.730 |
| +s (SSH) | 3.64 | 5838.423 | 3.09 | 3631.735 | 0.68 | 18221.980 |
| +s (SSS) | 2.20 | 5708.007 | 5.34 | 3562.240 | 2.15 | 17966.830 |
| +s (Chl-a) | 22.6 | 5403.713 | 2.45 | 3551.349 | 12.1 | 17371.340 |
| +s (Depth) | 14.7 | 5340.132 | 6.55 | 3509.710 | 6.13 | 17326.040 |
| Total variance explained (%) | 38.1 | 24.0 | 21.7 | |||
| r2 | 0.34 | 0.18 | 0.19 | |||
| Season | C. feriatus | P. pelagicus | P. sanguinolentus | ||||||
|---|---|---|---|---|---|---|---|---|---|
| O | P | RMSE | O | P | RMSE | O | P | RMSE | |
| Spring | 53 | 14 | 0.48 | 42 | 32 | 0.49 | 131 | 77 | 0.54 |
| Summer | 127 | 28 | 0.47 | 25 | 16 | 0.78 | 66 | 16 | 0.60 |
| Autumn | 177 | 119 | 0.37 | 16 | 6 | 0.84 | 186 | 156 | 0.38 |
| Winter | 84 | 47 | 0.44 | 22 | 17 | 0.45 | 160 | 75 | 0.52 |
| Year | 438 | 205 | 0.43 | 75 | 39 | 0.64 | 488 | 261 | 0.49 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naimullah, M.; Lan, K.-W.; Liao, C.-H.; Hsiao, P.-Y.; Liang, Y.-R.; Chiu, T.-C. Association of Environmental Factors in the Taiwan Strait with Distributions and Habitat Characteristics of Three Swimming Crabs. Remote Sens. 2020, 12, 2231. https://doi.org/10.3390/rs12142231
Naimullah M, Lan K-W, Liao C-H, Hsiao P-Y, Liang Y-R, Chiu T-C. Association of Environmental Factors in the Taiwan Strait with Distributions and Habitat Characteristics of Three Swimming Crabs. Remote Sensing. 2020; 12(14):2231. https://doi.org/10.3390/rs12142231
Chicago/Turabian StyleNaimullah, Muhamad, Kuo-Wei Lan, Cheng-Hsin Liao, Po-Yuan Hsiao, Yen-Rong Liang, and Ting-Chen Chiu. 2020. "Association of Environmental Factors in the Taiwan Strait with Distributions and Habitat Characteristics of Three Swimming Crabs" Remote Sensing 12, no. 14: 2231. https://doi.org/10.3390/rs12142231
APA StyleNaimullah, M., Lan, K.-W., Liao, C.-H., Hsiao, P.-Y., Liang, Y.-R., & Chiu, T.-C. (2020). Association of Environmental Factors in the Taiwan Strait with Distributions and Habitat Characteristics of Three Swimming Crabs. Remote Sensing, 12(14), 2231. https://doi.org/10.3390/rs12142231





