Quantifying Citrus Tree Health Using True Color UAV Images
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
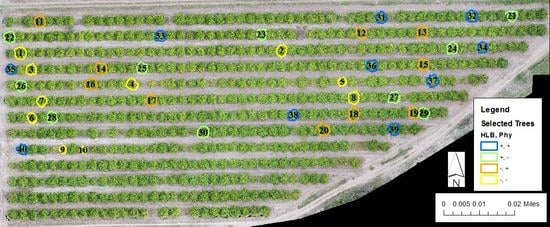
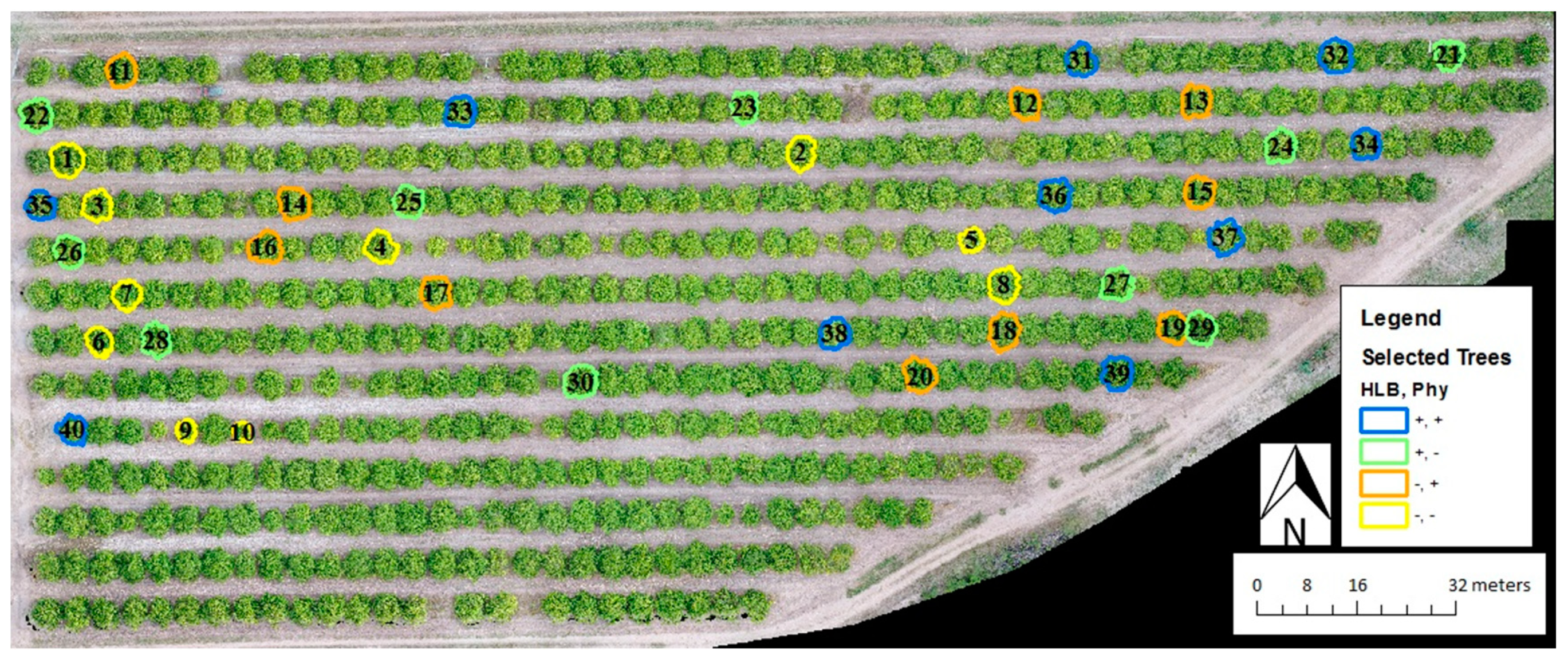
2.2. Experimental Design
2.3. Field Measurements
2.3.1. Nutrient Analysis and Leaf Area
2.3.2. SPAD
2.4. Image Acquisition and Analysis
2.4.1. Acquisition
2.4.2. Image Processing and Analysis
2.5. Data Analyses
3. Results
3.1. Nutritional Analysis
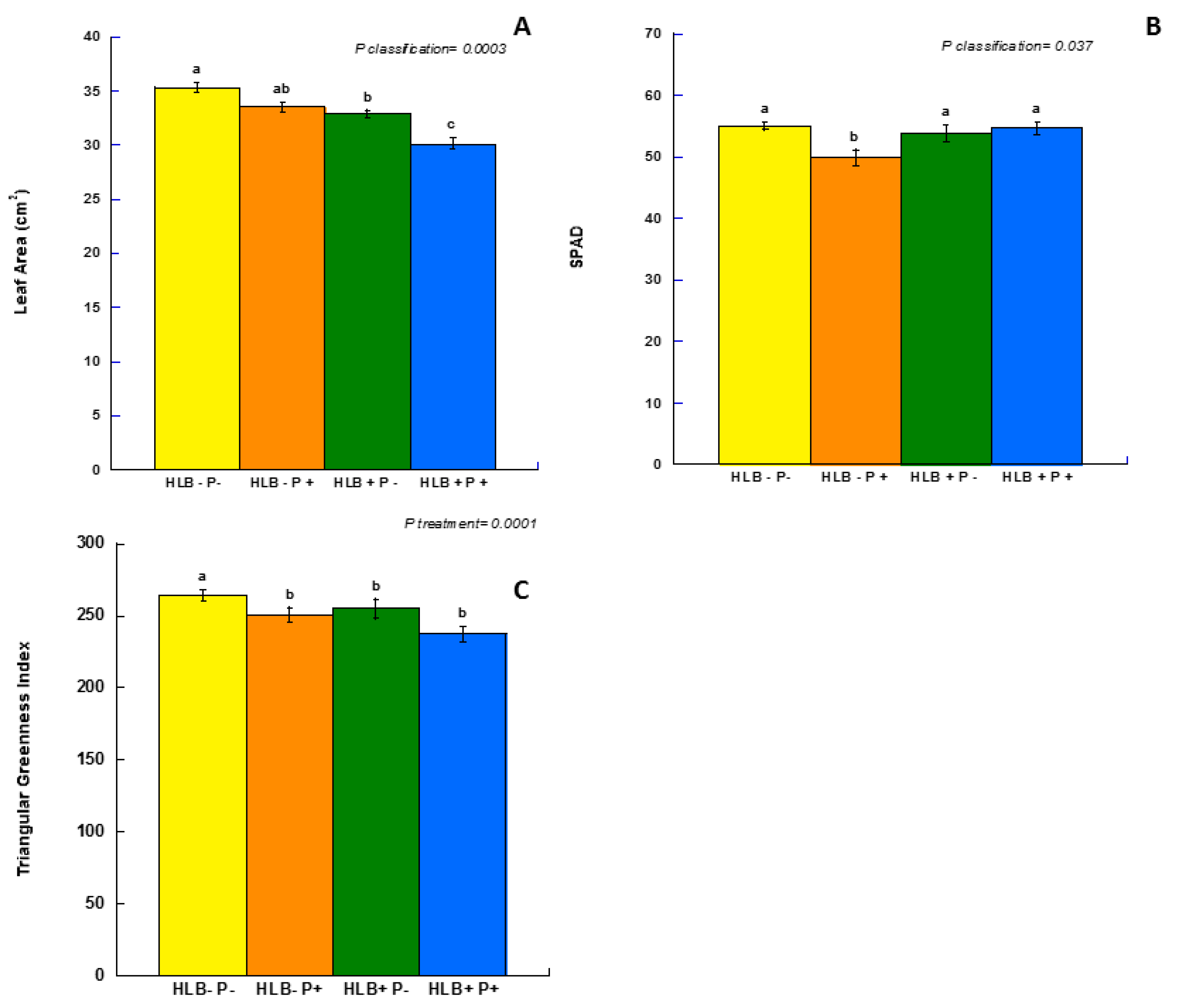
3.2. Field Measurements and Triangular Greenness Index (TGI)
3.3. Relationship Analysis
3.3.1. Correlation of Measured Factors
3.3.2. Stepwise Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simmons, D. Citrus Fruits: Production, Consumption and Health Benefits; Nova Science Publishers: New York, NY, USA, 2016. [Google Scholar]
- USDA-NASS. Texas Citrus Production. 2018, 9992, 1–2. Available online: https://www.nass.usda.gov/Publications/Todays_Reports/reports/cfrt0818.pdf (accessed on 30 December 2019).
- Li, H. Citrus Tree Abiotic and Biotic Stress and Implication of Simulation and Modeling Tools in Tree Management. Tree For. Sci. Biotechnol. 2009, 3, 66–78. [Google Scholar]
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Chaudhary, S. Identification and characterization of Phytophthora isolates from citrus orchards of South Texas. In Proceedings of the American Phytopathological Society Annual Meeting, At San Antonio, TX, USA, 31 January 2017. [Google Scholar]
- Kunta, M.; Setamou, M.; Skaria, M.; Rascoe, J.; Li, W.; Nakhla, M.; daGraca, J. First report of citrus huanglongbing in Texas. Phytopathology 2012, 102, 66. [Google Scholar]
- Home, J.; Picks, E.; Health, P.; Health, P. Home|Log In Citrus Huanglongbing: The Pathogen and Its Impact. Plant Health Prog. 2018, 1–46. [Google Scholar] [CrossRef]
- Graham, J.H.; Timmer, L.W. Phytophthora Diseases of Citrus; University of Florida IFAS Extesion: Gainesville, FL, USA, 2003. [Google Scholar]
- Ciancio, A.; Mukerji, K.G. (Eds.) Management of citrus diseases caused by Phytopthora spp. In Integrated Management of Diseases Caused by Fungi, Phytoplasma and Bacteria; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Hunt, E.R.; Perry, E.M.; Doraiswamy, P.C.; McMurtrey, J.E.; Daughtry, C.S.; Akhmedov, B. A visible band index for remote sensing leaf chlorophyll content at the canopy scale. Int. J. Appl. Earth Obs. Geoinf. 2013, 21, 103–112. [Google Scholar] [CrossRef]
- Sankaran, S.; Ehsani, R. Visible-near infrared spectroscopy based citrus greening detection: Evaluation of spectral feature extraction techniques. Crop Prot. 2011, 30, 1508–1513. [Google Scholar] [CrossRef]
- Sankaran, S.; Mishra, A.; Maja, J.M.; Ehsani, R. Visible-near infrared spectroscopy for detection of Huanglongbing in citrus orchards. Comput. Electron. Agric. 2011, 77, 127–134. [Google Scholar] [CrossRef]
- Mishra, A.; Karimi, D.; Ehsani, R.; Albrigo, L.G. Evaluation of an active optical sensor for detection of Huanglongbing (HLB) disease. Biosyst. Eng. 2011, 110, 302–309. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Enciso, J.; Avila, C.; Jung, J.; Elsayed-Farag, S.; Chang, A.; Yeom, J.; Landivar, J.; Maeda, M.; Chavez, J. Validation of agronomic UAV and field measurements for tomato varieties. Comput. Electron. Agric. 2019, 15, 278–283. [Google Scholar] [CrossRef]
- Louhaichi, M.; Borman, M.M.; Johnson, D.E. Spatially located platform and aerial photography for documentation of grazing impacts on wheat. Geocarto Int. 2001, 16, 65–70. [Google Scholar] [CrossRef]
- Hunt, R.E.; Daughtry, C.S.T.; Eitel, J.U.H.; Long, D.S. Remote sensing leaf chlorophyll content using a visible band index. Agron. J. Abstr. 2011, 103, 1090–1099. [Google Scholar] [CrossRef]
- Garcia-Ruiz, F.; Sankaran, S.; Maja, J.M.; Lee, S.; Rasmussen, J.; Ehsani, R. Comparison of two aerial imaging platforms for identification of Huanglongbing-infected citrus trees. Comput. Electron. Agric. 2013, 91, 106–115. [Google Scholar] [CrossRef]
- Dadras Javan, F.; Samadzadegan, F.; Seyed Pourazar, S.H.; Fazeli, H. UAV-based multispectral imagery for fast Citrus Greening detection. J. Plant Dis. Prot. 2019, 126, 307–318. [Google Scholar] [CrossRef]
- Salgadoe, A.; Robson, A.; Lamb, D.; Dann, E.; Searle, C. Quantifying the severity of phytophthora root rot disease in avocado trees using image analysis. Remote Sens. 2018, 10, 226. [Google Scholar] [CrossRef]
- Mekonen, M.; Ayalew, A.; Weldetsadik, K.; Seid, A. Assessing and Measuring of Citrus gummosis (Phytophthora spp.) in Major Citrus Growing Areas of Ethiopia. J. Hortic. 2015, 2, 2–5. [Google Scholar]
- Li, W.; Hartung, J.S.; Levy, L. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus huanglongbing. J. Microbiol. Methods 2006, 66, 104–115. [Google Scholar] [CrossRef]
- Kunta, M.; Viloria, Z.; del Rio, H.S.; Louzada, E.S. Diverse DNA extraction methods and PCR primers for detection of Huanglongbing-associated bacteria from roots of ‘Valencia’ sweet orange on sour orange rootstock. Sci. Hortic. 2014, 178, 23–30. [Google Scholar] [CrossRef]
- Jifon, J.L.; Syvertsen, J.P.; Whaley, E. Growth environment and leaf anatomy affect nondestructive estimates of chlorophyll and nitrogen in Citrus sp. leaves. J. Am. Soc. Hortic. Sci. 2005, 130, 152–158. [Google Scholar] [CrossRef]
- Su, T.; Chou, H. Application of multispectral sensors carried on unmanned aerial vehicle (UAV) to trophic state mapping of small reservoirs: A case study of Tain-Pu reservoir in Kinmen, Taiwan. Remote Sens. 2015, 7, 10078–10097. [Google Scholar] [CrossRef]
- Oppelt, N.; Mauser, W. Hyperspectral monitoring of physiological parameters of wheat during a vegetation period using AVIS data. Int. J. Remote Sens. 2004, 25, 145–157. [Google Scholar] [CrossRef]
- Congalton, R.G. A review of assessing the accuracy of classifications of remotely sensed data. Remote Sens. Environ. 1991, 37, 35–46. [Google Scholar] [CrossRef]
- Morales-Barquero, L.; Lyons, M.B.; Phinn, S.R.; Roelfsema, C.M. Trends in Remote Sensing Accuracy Assessment Approaches in the Context of Natural Resources. Remote Sens. 2019, 11, 2305. [Google Scholar] [CrossRef]
- Little, T.M. Agricultural Experimentation: Design and Analysis; Wiley & Sons: New York, NY, USA, 1978. [Google Scholar]
- Foster, A.J.; Kakani, V.G.; Ge, J.; Gregory, M.; Mosali, J.; Gopal Kakani, V. Discriminant analysis of nitrogen treatments in switchgrass and high biomass sorghum using leaf and canopy-scale reflectance spectroscopy Discriminant analysis of nitrogen treatments in switchgrass and high biomass. Int. J. Remote Sens. 2016, 37, 2252–2279. [Google Scholar] [CrossRef]
- Sancho-Adamson, M.; Trillas, M.; Bort, J.; Fernandez-Gallego, J.; Romanyà, J. Use of RGB Vegetation Indexes in Assessing Early Effects of Verticillium Wilt of Olive in Asymptomatic Plants in High and Low Fertility Scenarios. Remote Sens. 2019, 11, 607. [Google Scholar] [CrossRef]
- Ayala-Silva, T.; Beyl, C.A. Changes in spectral reflectance of wheat leaves in response to specific macronutrient deficiency. Adv. Space Res. 2005, 35, 305–317. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Smith, P. A case of sodium toxicity in citrus. Proc. Fla. State Hort. Soc. 1962, 120–124. [Google Scholar]
- Bernstein, L.; Hayward, H.E. Physiology of salt tolerance. Annu. Rev. Plant Physiol. 1958, 9, 25–46. [Google Scholar] [CrossRef]
- Obreza, T.; Alva, A.; Hanlon, E.; Rouse, R. Citrus Grove Leaf Tissue and Soil Testing: Sampling, Analysis, and Interpretation; University of Florida IFAS Extesion: Gainesville, FL, USA, 1992. [Google Scholar]
- Nebiker, S.; Lack, N.; Abächerli, M.; Läderach, S. Light-weight multispectral uav sensors and their capabilities for predicting grain yield and detecting plant diseases. In Proceedings of the 2016XXIII ISPR Congress on International Archives of Photogrammetry, Remote Sensing and Spatial Information Sciences, Prague, Crech, 12–19 July 2016; Volume XLI-B1. [Google Scholar]
- Pourreza, A.; Lee, W.S.; Ehsani, R.; Schueller, J.K.; Raveh, E. An optimum method for real-time in-field detection of Huanglongbing disease using a vision sensor. Comput. Electron. Agric. 2015, 110, 221–232. [Google Scholar] [CrossRef]
- Mahlein, A.; Rumpf, T.; Welke, P.; Dehne, H.; Plümer, L.; Steiner, U.; Oerke, E. Development of spectral indices for detecting and identifying plant diseases. Remote Sens. Environ. 2013, 128, 21–30. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2015; ISBN 978-1-60535-255-8. [Google Scholar]
- Stover, E.; Shatters, R.G.; Gruber, B.; Kumar, P.; Moore, G.A. Influence of Photoperiod Duration and Phloem Disruption through Scoring on Growth, Disease Symptoms, and Bacterial Titer in Citrus Graft Inoculated with Candidatus Liberibacter asiaticus. HortScience 2016, 51, 1215–1219. [Google Scholar] [CrossRef]
- Battie-Laclau, P.; Laclau, J.; Piccolo, M.; Arenque, B.C.; Beri, C.; Mietton, L.; Muniz, M.R.A.; Jordan-Meille, L.; Buckeridge, M.S.; Nouvellon, Y.; et al. Influence of potassium and sodium nutrition on leaf area components in Eucalyptus grandis trees. Plant Soil 2013, 371, 19–35. [Google Scholar] [CrossRef]
| Disease Class | N (%) | P (ppm) | K (ppm) | Ca (ppm) | Mg (ppm) | Na (ppm) | Zn (ppm) | Fe (ppm) | Cu (ppm) | Mn (ppm) | S (ppm) | B (ppm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HLB − Phy − | 1.92 | 1663.73 | 16,050.71 | 66,935.56 | 3450.68 | 919.89 b | 54.92 | 82.85 | 5.20 | 36.17 | 5730.17 | 169.98 |
| HLB − Phy + | 2.01 | 1777.40 | 14,700.20 | 68,689.00 | 3100.90 | 1056.5 b | 54.10 | 85.60 | 5.70 | 36.30 | 5829.80 | 182.50 |
| HLB + Phy − | 2.09 | 1914.30 | 16,051.60 | 65,815.30 | 3351.70 | 1216.1 b | 59.80 | 89.20 | 5.60 | 38.90 | 6148.50 | 173.60 |
| HLB + Phy + | 2.09 | 1777.10 | 14,304.50 | 65,309.40 | 3288.20 | 1685.4 a | 55.00 | 87.90 | 6.60 | 38.00 | 6208.70 | 200.20 |
| p classification | 0.334 | 0.607 | 0.636 | 0.977 | 0.172 | 0.001 | 0.647 | 0.813 | 0.228 | 0.529 | 0.349 | 0.430 |
| CLas Titer | Foot Rot Disease | Leaf Area | SPAD | N% | P | K | Ca | Mg | Na | Zn | Fe | Cu | Mn | S | B | TGI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLas titer | 1.0000 | ||||||||||||||||
| Foot rot Disease | 0.0704 | 1.0000 | |||||||||||||||
| Leaf area | 0.3757 | −0.2524 | 1.0000 | ||||||||||||||
| SPAD | −0.1611 | −0.0777 | −0.3439 | 1.0000 | |||||||||||||
| N% | −0.2291 | 0.1195 | −0.1853 | −0.1448 | 1.0000 | ||||||||||||
| P | −0.1035 | −0.0246 | 0.1962 | −0.5175 | 0.5717 | 1.000 | |||||||||||
| K | 0.0454 | −0.2011 | 0.2696 | −0.4438 | 0.4045 | 0.7259 | 1.0000 | ||||||||||
| Ca | 0.0805 | 0.0107 | −0.0270 | 0.3793 | −0.6928 | −0.7315 | −0.6367 | 1.0000 | |||||||||
| Mg | 0.0079 | −0.3724 | 0.2816 | −0.1046 | −0.0631 | −0.1509 | 0.0145 | 0.0884 | 1.0000 | ||||||||
| Na | −0.4518 | 0.2100 | −0.5099 | 0.3714 | 0.0458 | −0.2512 | −0.5069 | 0.2761 | −0.1347 | 1.0000 | |||||||
| Zn | −0.1023 | −0.1374 | −0.0459 | −0.4025 | 0.3994 | 0.5290 | 0.4332 | −0.3840 | −0.0074 | −0.1376 | 1.0000 | ||||||
| Fe | −0.2801 | 0.0566 | −0.3671 | 0.1487 | 0.2478 | −0.0189 | 0.0132 | −0.2583 | −0.1090 | 0.2600 | 0.0417 | 1.0000 | |||||
| Cu | −0.2679 | 0.2290 | −0.2595 | −0.0463 | 0.4263 | 0.5119 | 0.3593 | −0.3941 | −0.4532 | 0.2233 | 0.1638 | 0.4524 | 1.000 | ||||
| Mn | −0.3009 | −0.0686 | −0.0862 | −0.2604 | 0.0197 | 0.3288 | 0.2234 | −0.2688 | −0.0836 | −0.0632 | 0.6782 | 0.2160 | 0.2935 | 1.0000 | |||
| S | −0.2636 | 0.0134 | 0.1215 | −0.0213 | −0.3273 | 0.0457 | −0.0106 | 0.3020 | 0.0598 | 0.1314 | −0.0358 | −0.0568 | 0.0688 | 0.1286 | 1.0000 | ||
| B | −0.2124 | 0.1755 | −0.3423 | 0.4051 | −0.5240 | −0.4842 | −0.4996 | 0.5715 | −0.3526 | 0.5306 | −0.2970 | 0.1129 | 0.0783 | 0.0987 | 0.4907 | 1.0000 | |
| TGI | 0.3351 | −0.3649 | 0.4512 | −0.0737 | −0.1664 | 0.0829 | 0.3792 | 0.1482 | 0.1520 | −0.5441 | 0.1012 | −0.4513 | −0.2358 | −0.1024 | 0.0088 | −0.2043 | 1.0000 |
| Summary of Stepwise Selection | |||||||
|---|---|---|---|---|---|---|---|
| Step | Variable | Variable Number | Partial R-Square | Model R-Square | C(p) | F Value | Pr > F |
| 1 | Na | 1 | 0.2960 | 0.2960 | 14.3571 | 15.98 | 0.0003 |
| 2 | Fe | 2 | 0.1022 | 0.3982 | 9.0461 | 6.28 | 0.0167 |
| 3 | Foot rot disease | 3 | 0.0650 | 0.4632 | 6.3998 | 4.36 | 0.0440 |
| 4 | Ca | 4 | 0.0388 | 0.5020 | 5.6229 | 2.73 | 0.1075 |
| 5 | K | 5 | 0.1107 | 0.6127 | −0.2970 | 9.72 | 0.0037 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garza, B.N.; Ancona, V.; Enciso, J.; Perotto-Baldivieso, H.L.; Kunta, M.; Simpson, C. Quantifying Citrus Tree Health Using True Color UAV Images. Remote Sens. 2020, 12, 170. https://doi.org/10.3390/rs12010170
Garza BN, Ancona V, Enciso J, Perotto-Baldivieso HL, Kunta M, Simpson C. Quantifying Citrus Tree Health Using True Color UAV Images. Remote Sensing. 2020; 12(1):170. https://doi.org/10.3390/rs12010170
Chicago/Turabian StyleGarza, Blanca N., Veronica Ancona, Juan Enciso, Humberto L. Perotto-Baldivieso, Madhurababu Kunta, and Catherine Simpson. 2020. "Quantifying Citrus Tree Health Using True Color UAV Images" Remote Sensing 12, no. 1: 170. https://doi.org/10.3390/rs12010170
APA StyleGarza, B. N., Ancona, V., Enciso, J., Perotto-Baldivieso, H. L., Kunta, M., & Simpson, C. (2020). Quantifying Citrus Tree Health Using True Color UAV Images. Remote Sensing, 12(1), 170. https://doi.org/10.3390/rs12010170