Using Sentinel-2 Multispectral Images to Map the Occurrence of the Cossid Moth (Coryphodema tristis) in Eucalyptus Nitens Plantations of Mpumalanga, South Africa
Abstract
1. Introduction
2. Materials and Methods
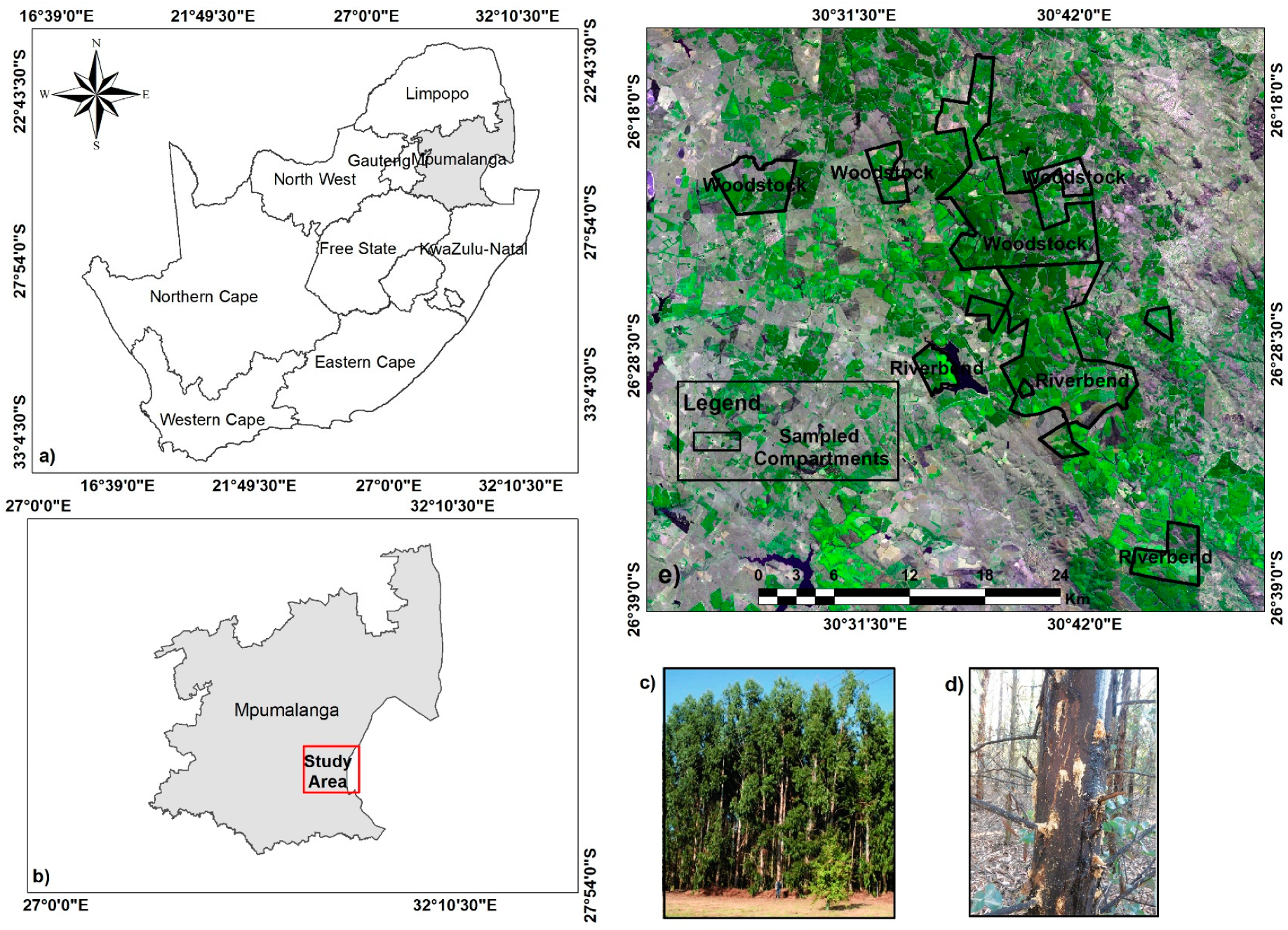
2.1. Study Area
2.2. Image Acquisition
2.3. Image Processing and Analysis
2.4. Field Data Collection
2.5. Maxent Modelling Approach
2.6. Model Accuracy Assessment
2.7. Mapping C. tristis Occurrence
3. Results
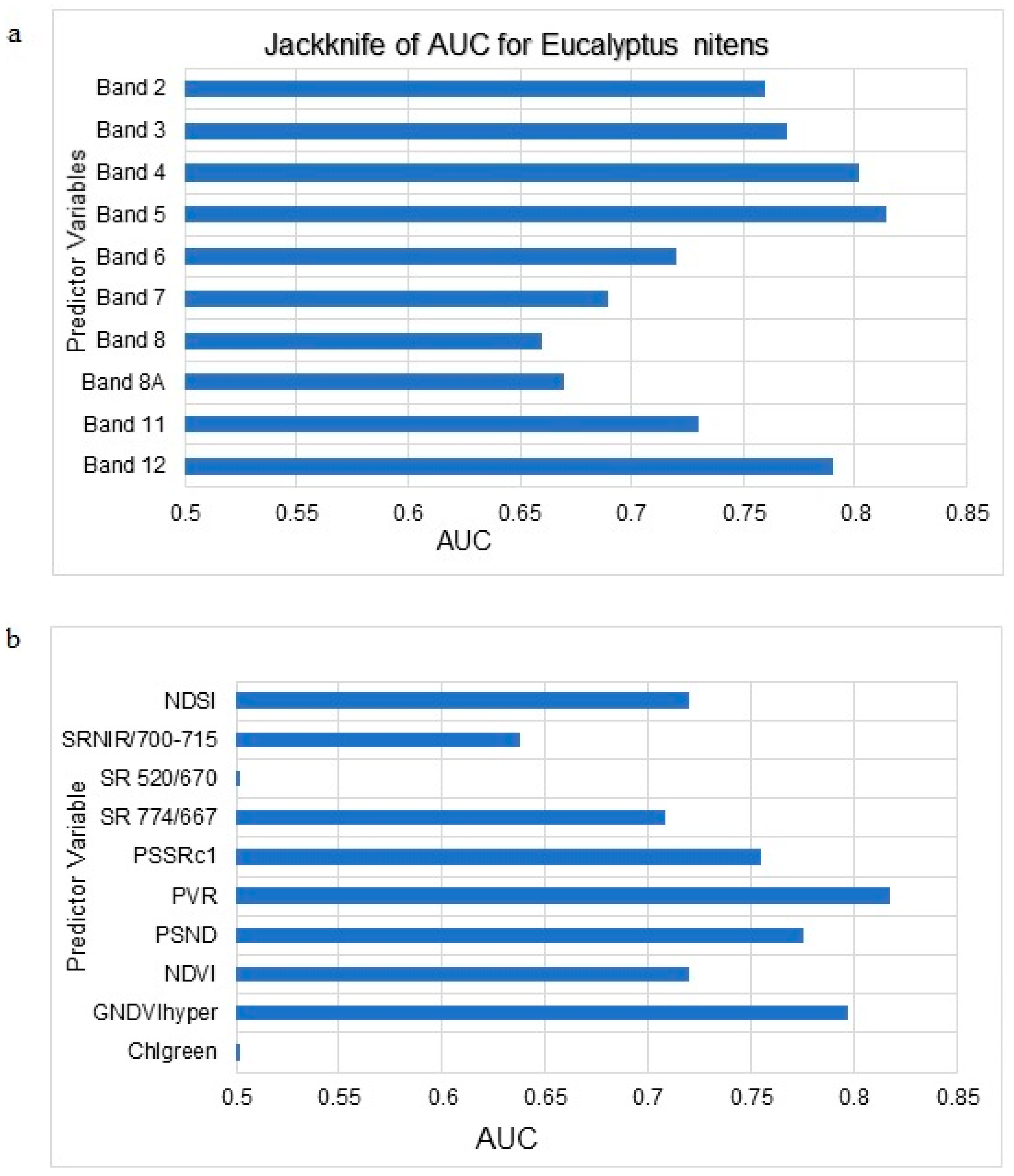
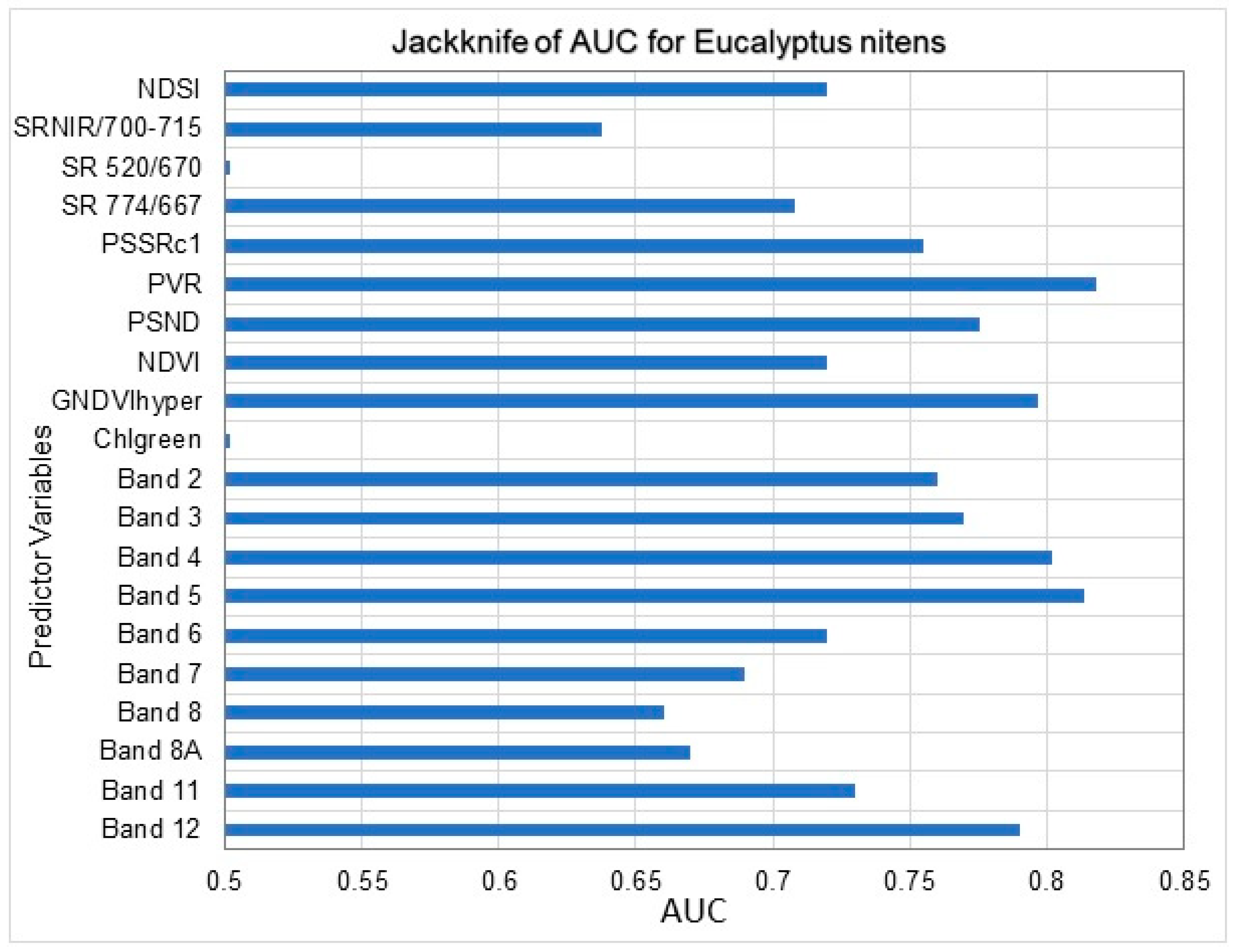
3.1. Maxent Modelling of C. tristis Occurrence
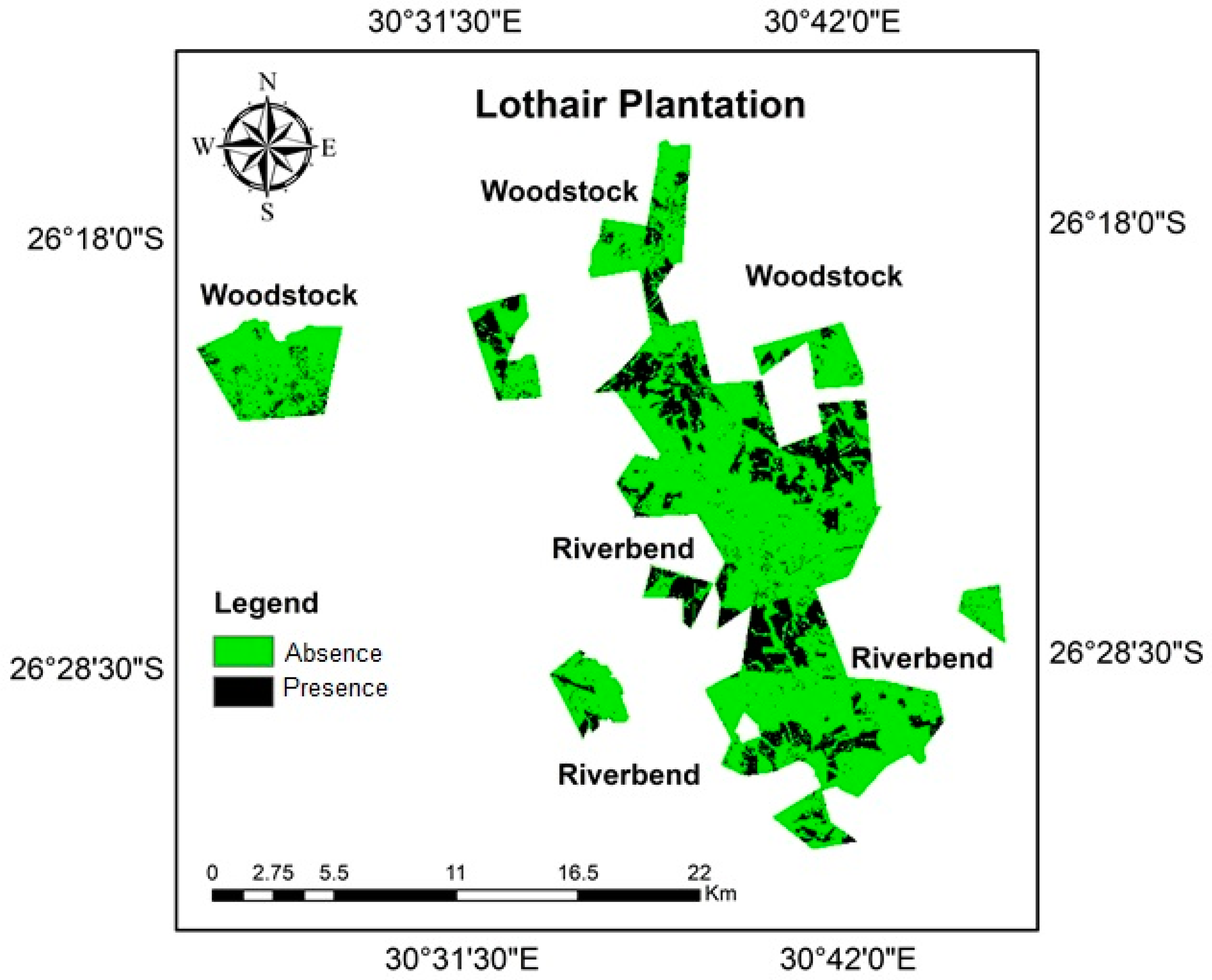
3.2. C. tristis Spatial Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wingfield, M.J.; Roux, J.; Coutinho, T.; Govender, P.; Wingfield, B.D. Plantation disease and pest management in the next century. S. Afr. For. J. 2001, 190, 67–71. [Google Scholar] [CrossRef]
- DAFF. A Profile of the South African Forestry Market Value Chain. 2015. Available online: http://www.nda.agric.za/doaDev/sideMenu/Marketing/Annual Publications/Commodity Profiles/field crops/Forestry Market Value Chain Profile 2016.pdf (accessed on 25 June 2017).
- DAFF. Forestry Regulation & Oversight. 2017. Available online: https://www.daff.gov.za/daffweb3/Branches/Forestry-Natural-Resources-Management/Forestry-Regulation-Oversight/Facts-and-Figures/plantationsmore (accessed on 12 August 2017).
- Albaugh, J.M.; Dye, P.J.; King, J.S. Eucalyptus and water use in South Africa. Int. J. For. Res. 2013, 2013, 852540. [Google Scholar]
- Swain, T.-L.; Gardner, R.A.W. A Summary of Current Knowledge of Cold Tolerant Eucalypt Species (CTE’s) Grown in South Africa; University of Natal, Institute for Commercial Forestry Research: Pietermartizburg, South Africa, 2003. [Google Scholar]
- Wingfield, M.J.; Slippers, B.; Hurley, B.P.; Coutinho, T.A.; Wingfield, B.D.; Roux, J. Eucalypt pests and diseases: Growing threats to plantation productivity. South. For. J. For. Sci. 2008, 70, 139–144. [Google Scholar] [CrossRef]
- Seta. A Profile of the Forestry and Wood Products Sub-Sector. Available online: http://www.fpmseta.org.za/downloads/FPM_sub-sector_forestry_wood_final.pdf (accessed on 12 August 2017).
- Adam, E.; Mutanga, O.; Ismail, R. Determining the susceptibility of Eucalyptus nitens forests to Coryphodema tristis (cossid moth) occurrence in Mpumalanga, South Africa. Int. J. Geogr. Inf. Sci. 2013, 27, 1924–1938. [Google Scholar] [CrossRef]
- Boreham, G.R. A survey of cossid moth attack in Eucalyptus nitens on the Mpumalanga Highveld of South Africa. S. Afr For. J. 2006, 206, 23–26. [Google Scholar]
- Gebeyehu, S.; Hurley, B.P.; Wingfield, M.J. A new lepidopteran insect pest discovered on commercially grown Eucalyptus nitens in South Africa: Research in action. S. Afr. J. Sci. 2005, 101, 26–28. [Google Scholar]
- Bouwer, M.C.; Slippers, B.; Degefu, D.; Wingfield, M.J.; Lawson, S.; Rohwer, E.R. Identification of the sex pheromone of the tree infesting Cossid moth Coryphodema tristis (Lepidoptera: Cossidae). PLoS ONE 2015, 10, e0118575. [Google Scholar] [CrossRef]
- FAO. Overview of Forest Pests South Africa; FAO: Rome Italy, 2007. [Google Scholar]
- Xing, Z.; Zhang, L.; Wu, S.; Yi, H.; Gao, Y.; Lei, Z. Niche comparison among two invasive leafminer species and their parasitoid Opius biroi: Implications for competitive displacement. Sci. Rep. 2017, 7, 4246. [Google Scholar] [CrossRef]
- Pause, M.; Schweitzer, C.; Rosenthal, M.; Keuck, V.; Bumberger, J.; Dietrich, P.; Heurich, M.; Jung, A.; Lausch, A. In situ/remote sensing integration to assess forest health—A review. Remote Sens. 2016, 8, 471. [Google Scholar] [CrossRef]
- Pietrzykowski, E.; Sims, N.; Stone, C.; Pinkard, L.; Mohammed, C. Predicting Mycosphaerella leaf disease severity in a Eucalyptus globulus plantation using digital multi-spectral imagery. South. Hemisph. For. J. 2007, 69, 175–182. [Google Scholar] [CrossRef]
- Germishuizen, I.; Peerbhay, K.; Ismail, R. Modelling the susceptibility of pine stands to bark stripping by Chacma baboons (Papio ursinus) in the Mpumalanga Province of South Africa. Wildl. Res. 2017, 44, 298–308. [Google Scholar] [CrossRef]
- Donatelli, M.; Magarey, R.D.; Bregaglio, S.; Willocquet, L.; Whish, J.P.M.; Savary, S. Modelling the impacts of pests and diseases on agricultural systems. Agric. Syst. 2017, 155, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Lottering, R.; Mutanga, O. Optimising the spatial resolution of WorldView-2 pan-sharpened imagery for predicting levels of Gonipterus scutellatus defoliation in KwaZulu-Natal, South Africa. ISPRS J. Photogramm. Remote Sens. 2016, 112, 13–22. [Google Scholar] [CrossRef]
- Oumar, Z.; Mutanga, O. Using WorldView-2 bands and indices to predict bronze bug (Thaumastocoris peregrinus) damage in plantation forests. Int. J. Remote Sens. 2013, 34, 2236–2249. [Google Scholar] [CrossRef]
- Adelabu, S.; Mutanga, O.; Adam, E.; Sebego, R. Spectral discrimination of insect defoliation levels in mopane woodland using hyperspectral data. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens 2014, 7, 177–186. [Google Scholar] [CrossRef]
- Hojas-Gascon, L.; Belward, A.; Eva, H.; Ceccherini, G.; Hagolle, O.; Garcia, J.; Cerutti, P. Potential improvement for forest cover and forest degradation mapping with the forthcoming Sentinel-2 program. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2015, 7, W3. [Google Scholar] [CrossRef]
- Immitzer, M.; Vuolo, F.; Atzberger, C. First experience with Sentinel-2 data for crop and tree species classifications in central Europe. Remote Sens. 2016, 8, 166. [Google Scholar] [CrossRef]
- Ng, W.-T.; Rima, P.; Einzmann, K.; Immitzer, M.; Atzberger, C.; Eckert, S. Assessing the Potential of Sentinel-2 and Pléiades Data for the Detection of Prosopis and Vachellia spp. in Kenya. Remote Sens. 2017, 9, 74. [Google Scholar] [CrossRef]
- Gascon, F.; Bouzinac, C.; Thépaut, O.; Jung, M.; Francesconi, B.; Louis, J.; Lonjou, V.; Lafrance, B.; Massera, S.; Gaudel-Vacaresse, A. Copernicus Sentinel-2A calibration and products validation status. Remote Sens. 2017, 9, 584. [Google Scholar] [CrossRef]
- Radoux, J.; Chomé, G.; Jacques, D.; Waldner, F.; Bellemans, N.; Matton, N.; Lamarche, C.; D’Andrimont, R.; Defourny, P. Sentinel-2’s Potential for Sub-Pixel Landscape Feature Detection. Remote Sens. 2016, 8, 488. [Google Scholar] [CrossRef]
- Blackburn, G.A. Spectral indices for estimating photosynthetic pigment concentrations: A test using senescent tree leaves. Int. J. Remote Sens. 1998, 19, 657–675. [Google Scholar] [CrossRef]
- Carter, G.A. Ratios of leaf reflectances in narrow wavebands as indicators of plant stress. Int. J. Remote Sens. 1994, 15, 697–703. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Noland, T.L.; Mohammed, G.H.; Sampson, P.H. Scaling-up and model inversion methods with narrow-band optical indices for chlorophyll content estimation in closed forest canopies with hyperspectral data. IEEE Trans. Geosci. Remote Sens. 2001, 39, 1491–1507. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Lichtenthaler, H.K. Detection of Red Edge Position and Chlorophyll Content by Reflectance Measurements Near 700 nm. J. Plant Physiol. 1996, 148, 501–508. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-band model for noninvasive estimation of chlorophyll, carotenoids, and anthocyanin contents in higher plant leaves. Geophys. Res. Lett. 2006, 33, L11402. [Google Scholar] [CrossRef]
- Metternicht, G. Vegetation indices derived from high-resolution airborne videography for precision crop management. Int. J. Remote Sens. 2003, 24, 2855–2877. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Ndlovu, P.; Mutanga, O.; Sibanda, M.; Odindi, J.; Rushworth, I. Modelling potential distribution of bramble (rubus cuneifolius) using topographic, bioclimatic and remotely sensed data in the KwaZulu-Natal Drakensberg, South Africa. Appl. Geogr. 2018, 99, 54–62. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel Methods Improve Prediction of Species’ Distributions from Occurrence Data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Biber-Freudenberger, L.; Ziemacki, J.; Tonnang, H.E.Z.; Borgemeister, C. Future Risks of Pest Species under Changing Climatic Conditions. PLoS ONE 2016, 11, e0153237. [Google Scholar] [CrossRef] [PubMed]
- Matawa, F.; Murwira, A.; Zengeya, F.M.; Atkinson, P.M. Modelling the spatial-temporal distribution of tsetse (Glossina pallidipes) as a function of topography and vegetation greenness in the Zambezi Valley of Zimbabwe. Appl. Geogr. 2016, 76, 198–206. [Google Scholar] [CrossRef]
- Yi, Y.; Cheng, X.; Yang, Z.-F.; Zhang, S.-H. Maxent modeling for predicting the potential distribution of endangered medicinal plant (H. riparia Lour) in Yunnan, China. Ecol. Eng. 2016, 92, 260–269. [Google Scholar] [CrossRef]
- Shabani, F.; Kumar, L.; Ahmadi, M. A comparison of absolute performance of different correlative and mechanistic species distribution models in an independent area. Ecol. Evol. 2016, 6, 5973–5986. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Efron, B.; Stein, C. The jackknife estimate of variance. Ann. Stat. 1981, 9, 586–596. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Hageer, Y.; Esperón-Rodríguez, M.; Baumgartner, J.B.; Beaumont, L.J. Climate, soil or both? Which variables are better predictors of the distributions of Australian shrub species? PeerJ 2017, 5, e3446. [Google Scholar] [CrossRef]
- Molloy, S.W.; Davis, R.A.; van Etten, E.J.B. Incorporating field studies into species distribution and climate change modelling: A case study of the koomal Trichosurus vulpecula hypoleucus (Phalangeridae). PLoS ONE 2016, 11, e0154161. [Google Scholar] [CrossRef] [PubMed]
- Tabet, S.; Belhemra, M.; Francois, L.; Arar, A. Evaluation by prediction of the natural range shrinkage of Quercus ilex L. in eastern Algeria. İstanbul Üniversitesi Orman Fakültesi Dergisi 2018, 68, 7–15. [Google Scholar] [CrossRef]
- Wang, R.; Li, Q.; He, S.; Liu, Y.; Wang, M.; Jiang, G. Modeling and mapping the current and future distribution of Pseudomonas syringae pv. actinidiae under climate change in China. PLoS ONE 2018, 13, e0192153. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, H.; Jones, G. Ground validation of presence-only modelling with rare species: A case study on barbastelles Barbastella barbastellus (Chiroptera: Vespertilionidae). J. Appl. Ecol. 2010, 47, 410–420. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Berthon, K.; Esperon-Rodriguez, M.; Beaumont, L.J.; Carnegie, A.J.; Leishman, M.R. Assessment and prioritisation of plant species at risk from myrtle rust (Austropuccinia psidii) under current and future climates in Australia. Biol. Conserv. 2018, 218, 154–162. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Minařík, R.; Langhammer, J. Use of a multispectral UAV photogrammetry for detection and tracking of forest disturbance dynamics. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2016, XLI-B8, 711–718. [Google Scholar]
- Hart, S.J.; Veblen, T.T. Detection of spruce beetle-induced tree mortality using high- and medium-resolution remotely sensed imagery. Remote Sens. Environ. 2015, 168, 134–145. [Google Scholar] [CrossRef]
- Sanchez-Azofeifa, A.; Oki, Y.; Wilson Fernandes, G.; Ball, R.A.; Gamon, J. Relationships between endophyte diversity and leaf optical properties. Trees 2012, 26, 291–299. [Google Scholar] [CrossRef]
- Matawa, F.; Murwira, K.S.; Shereni, W. Modelling the distribution of suitable Glossina Spp. habitat in the North Western parts of Zimbabwe using remote sensing and climate data. Geoinform. Geostast. Overv. 2013. [Google Scholar] [CrossRef]
- Eitel, J.U.H.; Vierling, L.A.; Litvak, M.E.; Long, D.S.; Schulthess, U.; Ager, A.A.; Krofcheck, D.J.; Stoscheck, L. Broadband, red-edge information from satellites improves early stress detection in a New Mexico conifer woodland. Remote Sens. Environ. 2011, 115, 3640–3646. [Google Scholar] [CrossRef]
- Murfitt, J.; He, Y.; Yang, J.; Mui, A.; De Mille, K. Ash decline assessment in emerald ash borer infested natural forests using high spatial resolution images. Remote Sens. 2016, 8, 256. [Google Scholar] [CrossRef]
- Senf, C.; Seidl, R.; Hostert, P. Remote sensing of forest insect disturbances: Current state and future directions. Int. J. Appl. Earth Obs. Geoinf. 2017, 60, 49–60. [Google Scholar] [CrossRef]
- Ismail, R.; Mutanga, O.; Bob, U. Forest health and vitality: The detection and monitoring of Pinus patula trees infected by Sirex noctilio using digital ultispectral imagery (DMSI). South. Hemisph. For. J. 2007, 69, 39. [Google Scholar] [CrossRef]
| Spatial Resolution (m) | Sentinel-2 Bands | Wavelength (nm) | Bandwidth (nm) |
|---|---|---|---|
| Band 2—Blue | 496.6 | 98 | |
| 10 | Band 3—Green | 560.0 | 45 |
| Band 4—Red | 664.5 | 38 | |
| Band 8—NIR | 835.1 | 145 | |
| Band 5—Vegetation Red Edge | 703.9 | 19 | |
| Band 6—Vegetation Red Edge Band 7—Vegetation Red Edge Band 8a—Narrow NIR | 740.2 782.5 864.8 | 18 28 33 | |
| 20 | Band 11—SWIR (2) | 1613.7 | 143 |
| Band 12—SWIR (3) | 2202.4 | 242 |
| Vegetation Indices | Abbreviation | Equation | Index Equation | Reference |
|---|---|---|---|---|
| Simple Ratio 800/500 Pigment specific simple ratio C1 | PSSRc1 | [26] | ||
| Simple Ratio 520/670 | SR520/670 | [27] | ||
| Simple Ratio 774/677 | SR774/677 | [28] | ||
| Simple Ratio NIR/700–715 | SRNir/700–715 | [29] | ||
| Normalized Difference Vegetation Index | NDVI | [30] | ||
| Normalized Difference 780/550 Green NDVI hyper | GNDVIhyper | [31] | ||
| Normalized Difference Salinity Index | NDSI | [32] | ||
| Normalized Difference 800/470 Pigment specific normalized difference C2 | PSNDc2 | [26] | ||
| Chlorophyll Green | Chlgreen | [33] | ||
| Normalized Difference 550/650 Photosynthetic vigour ratio | PVR | [34] |
| Model Scenarios | Sentinel-2A Bands and Indices | List of Variables Used |
|---|---|---|
| 1 | Spectral bands | Band 2, Band 3, Band 4, Band 5, Band 6, Band 7, Band 8, Band 8A, Band 11 and Band 12 |
| 2 | Vegetation indices | PSSRc1, SR520/670, SR774/677, SRNir/700-715, NDVI, GNDVIhyper, NDSI, PSNDc2, Chlgreen and PVR |
| 3 | Combined variables | Bands and vegetation indices |
| 4 | The most influential bands | Band 5, Band 4, Band 3, Band 12, Band 2, PVR, GNDVIhyper, PSND, SR774/667 and NDSI |
| Predictor Variables | Model Accuracy | ||
|---|---|---|---|
| AUC | TSS | ||
| Training | Testing | ||
| Bands | 0.891 | 0.898 | 0.282 |
| Vegetation indices | 0.875 | 0.872 | 0.324 |
| Combined variables | 0.900 | 0.890 | 0.344 |
| Predictor Variables | Model Accuracy | ||
|---|---|---|---|
| AUC | TSS | ||
| Training | Testing | ||
| Bands | 0.858 | 0.849 | 0.482 |
| Vegetation indices | 0.868 | 0.867 | 0.564 |
| Combined variables | 0.869 | 0.872 | 0.581 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumbula, S.T.; Mafongoya, P.; Peerbhay, K.Y.; Lottering, R.T.; Ismail, R. Using Sentinel-2 Multispectral Images to Map the Occurrence of the Cossid Moth (Coryphodema tristis) in Eucalyptus Nitens Plantations of Mpumalanga, South Africa. Remote Sens. 2019, 11, 278. https://doi.org/10.3390/rs11030278
Kumbula ST, Mafongoya P, Peerbhay KY, Lottering RT, Ismail R. Using Sentinel-2 Multispectral Images to Map the Occurrence of the Cossid Moth (Coryphodema tristis) in Eucalyptus Nitens Plantations of Mpumalanga, South Africa. Remote Sensing. 2019; 11(3):278. https://doi.org/10.3390/rs11030278
Chicago/Turabian StyleKumbula, Samuel Takudzwa, Paramu Mafongoya, Kabir Yunus Peerbhay, Romano Trent Lottering, and Riyad Ismail. 2019. "Using Sentinel-2 Multispectral Images to Map the Occurrence of the Cossid Moth (Coryphodema tristis) in Eucalyptus Nitens Plantations of Mpumalanga, South Africa" Remote Sensing 11, no. 3: 278. https://doi.org/10.3390/rs11030278
APA StyleKumbula, S. T., Mafongoya, P., Peerbhay, K. Y., Lottering, R. T., & Ismail, R. (2019). Using Sentinel-2 Multispectral Images to Map the Occurrence of the Cossid Moth (Coryphodema tristis) in Eucalyptus Nitens Plantations of Mpumalanga, South Africa. Remote Sensing, 11(3), 278. https://doi.org/10.3390/rs11030278








