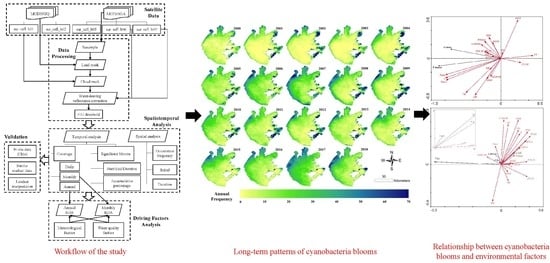
Long-Term Spatial and Temporal Monitoring of Cyanobacteria Blooms Using MODIS on Google Earth Engine: A Case Study in Taihu Lake
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Satellite Data
2.3. Data Processing
2.3.1. Resampling
2.3.2. Land Masking
2.3.3. Cloud Masking
2.3.4. Water-Leaving Reflectance Correction
2.3.5. FAI Threshold
2.4. Spatiotemporal Analysis of Cyanobacteria Blooms
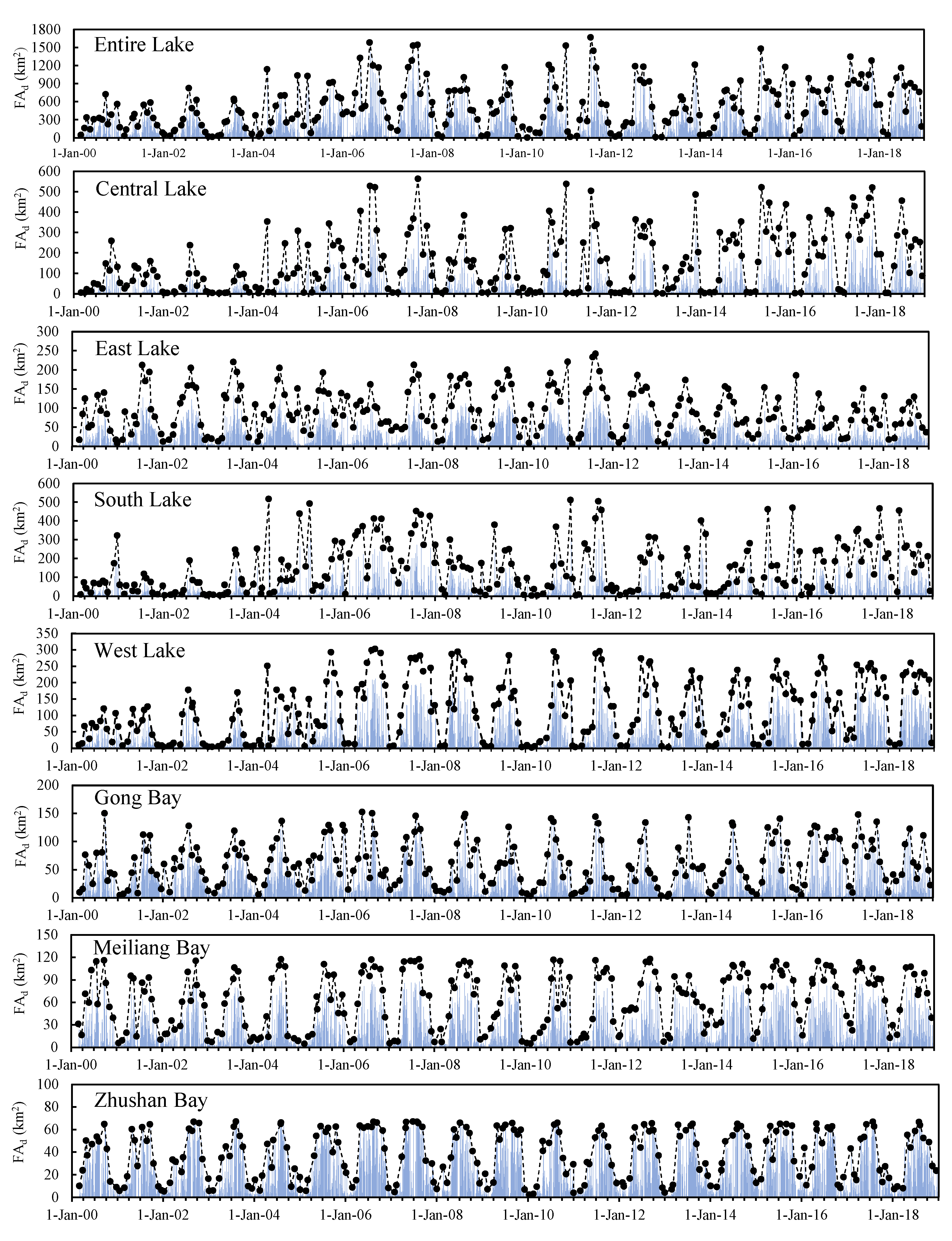
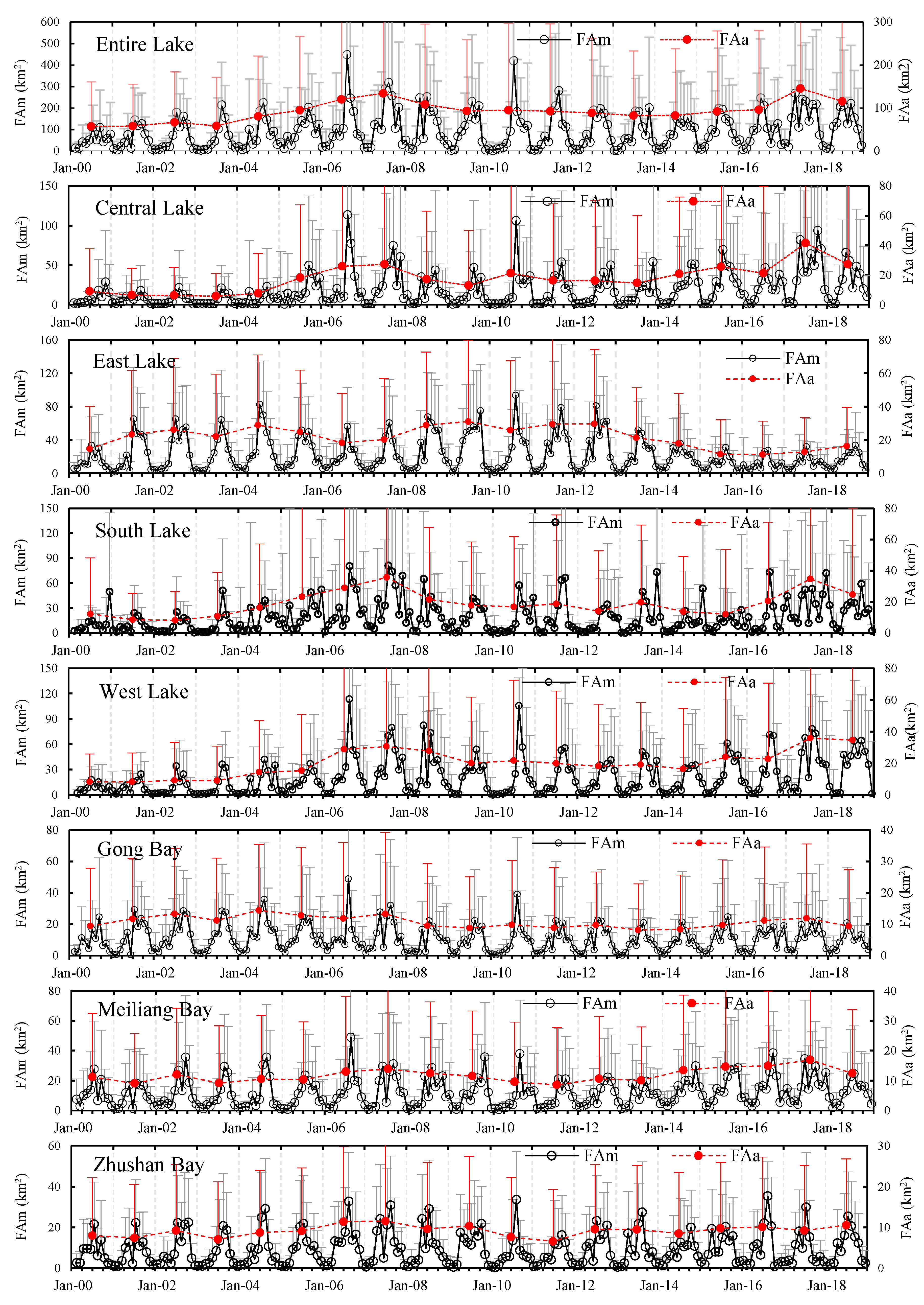
2.4.1. Temporal Analysis
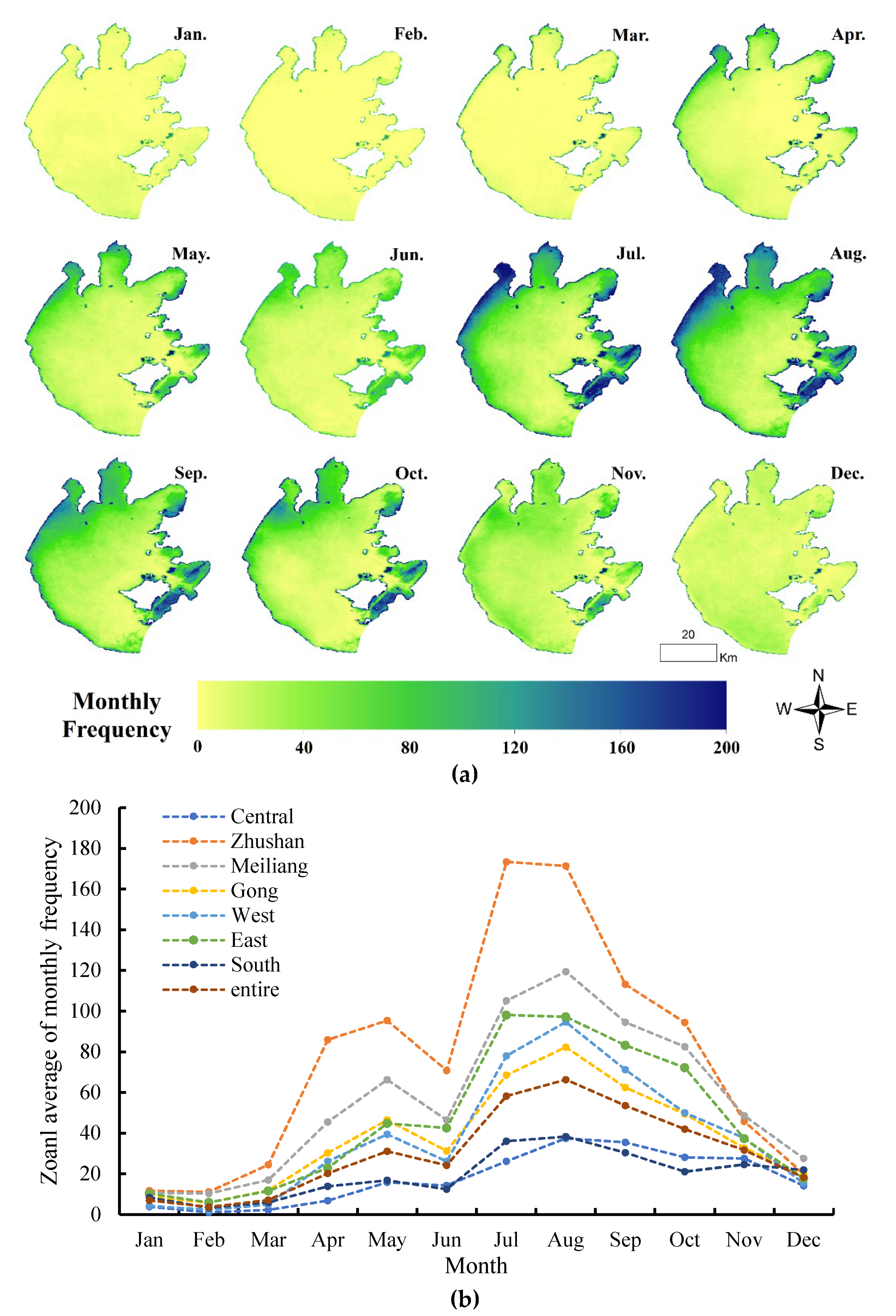
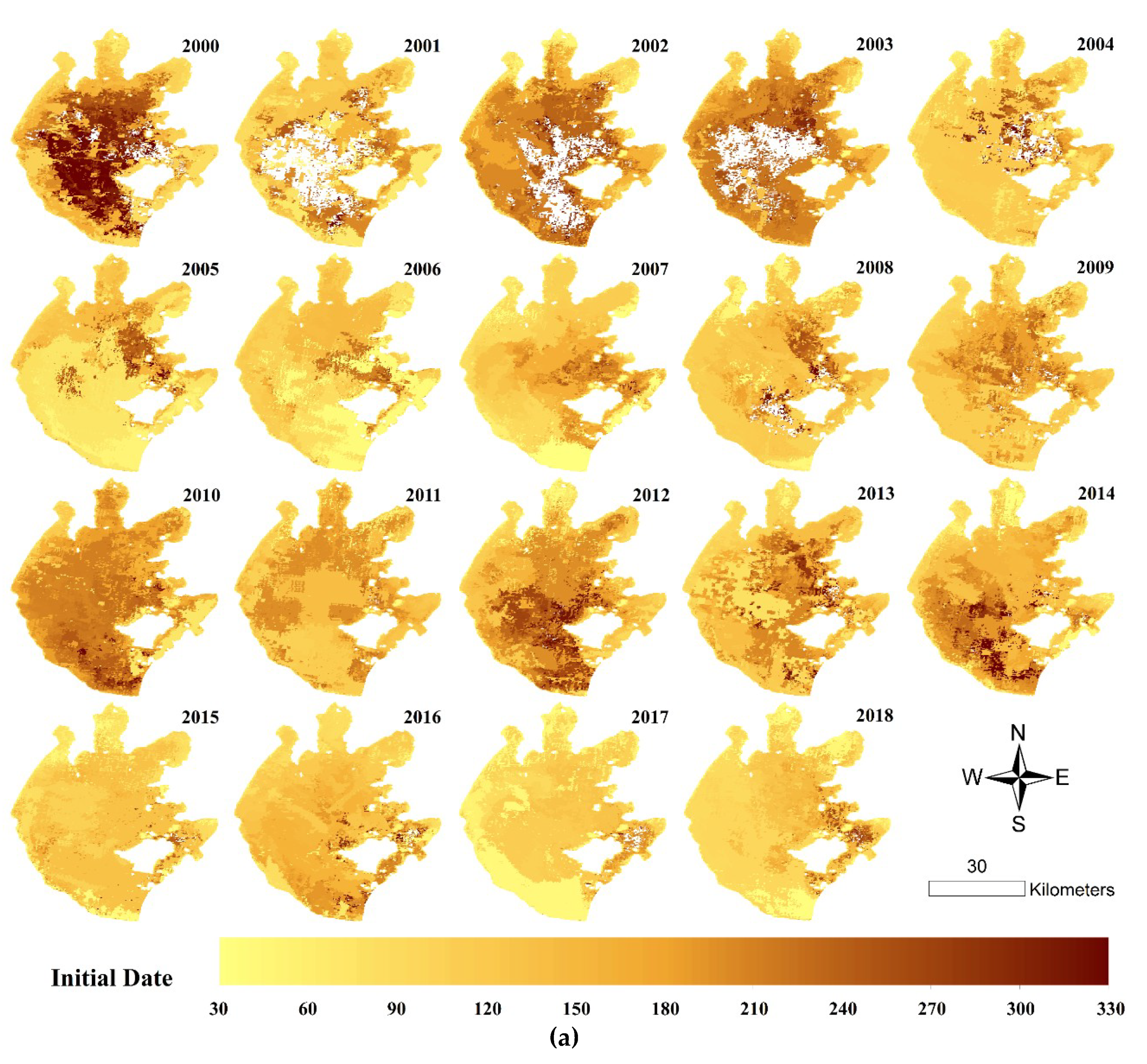
2.4.2. Spatial Analysis
2.5. Validation
2.6. Analysis of the Relationship between Environmental Driving Factors and Cyanobacteria Blooms
3. Results
3.1. Validation of Workflow Accuracy
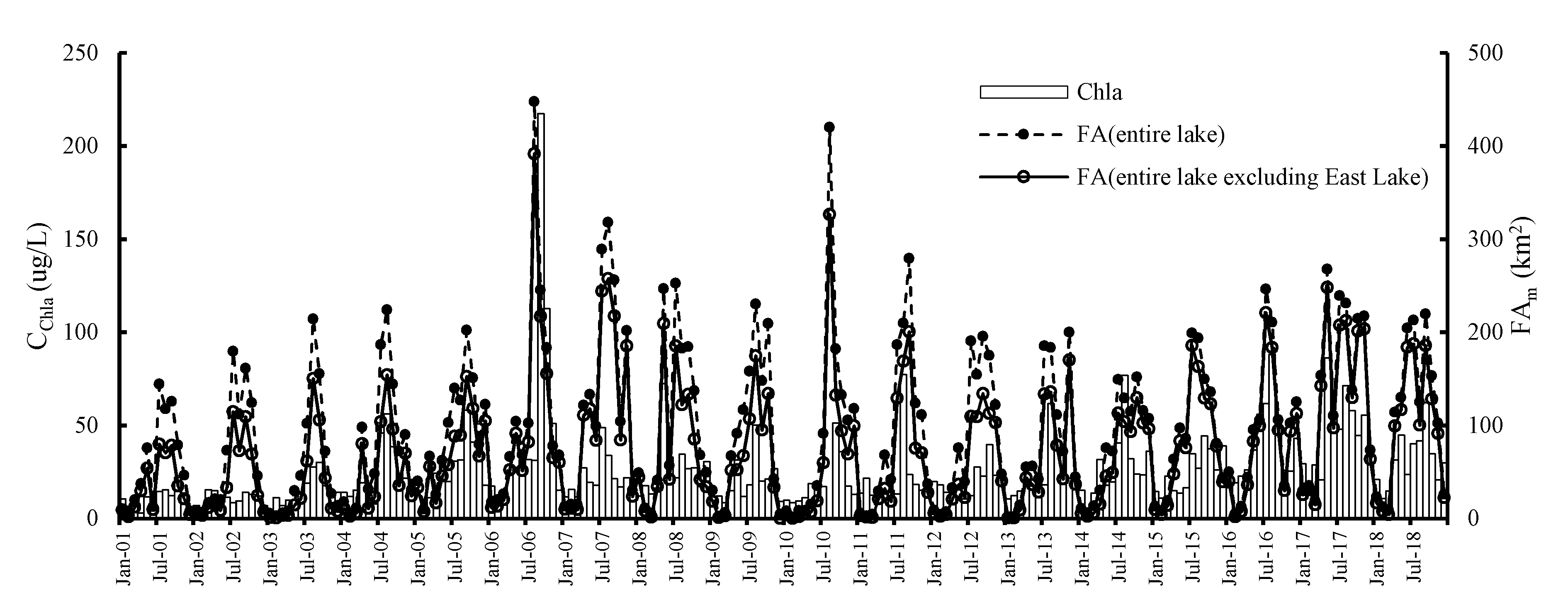
3.1.1. Validation Using In-Situ Data (Chlorophyll-a)
3.1.2. Validation Using the Results of Other Studies
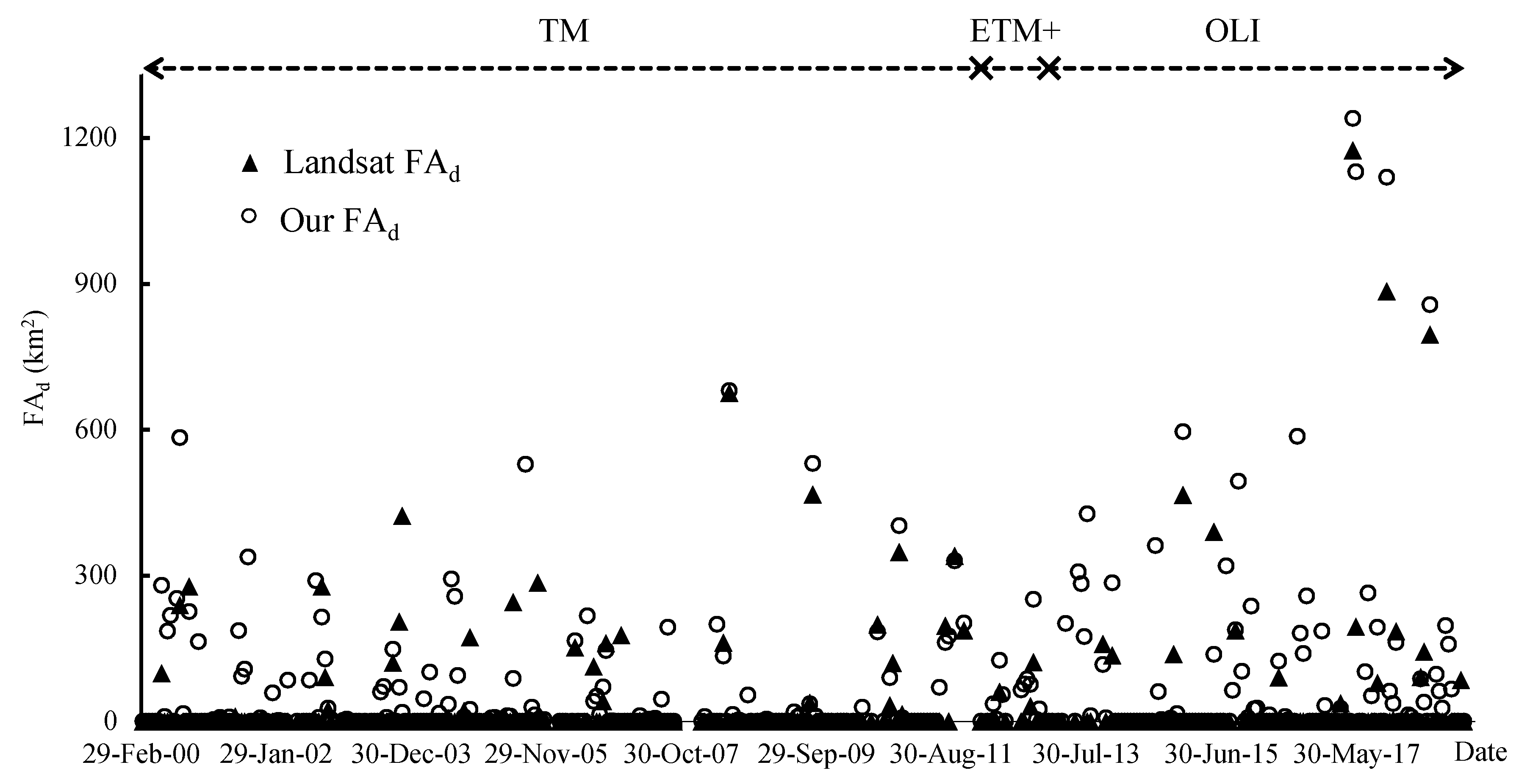
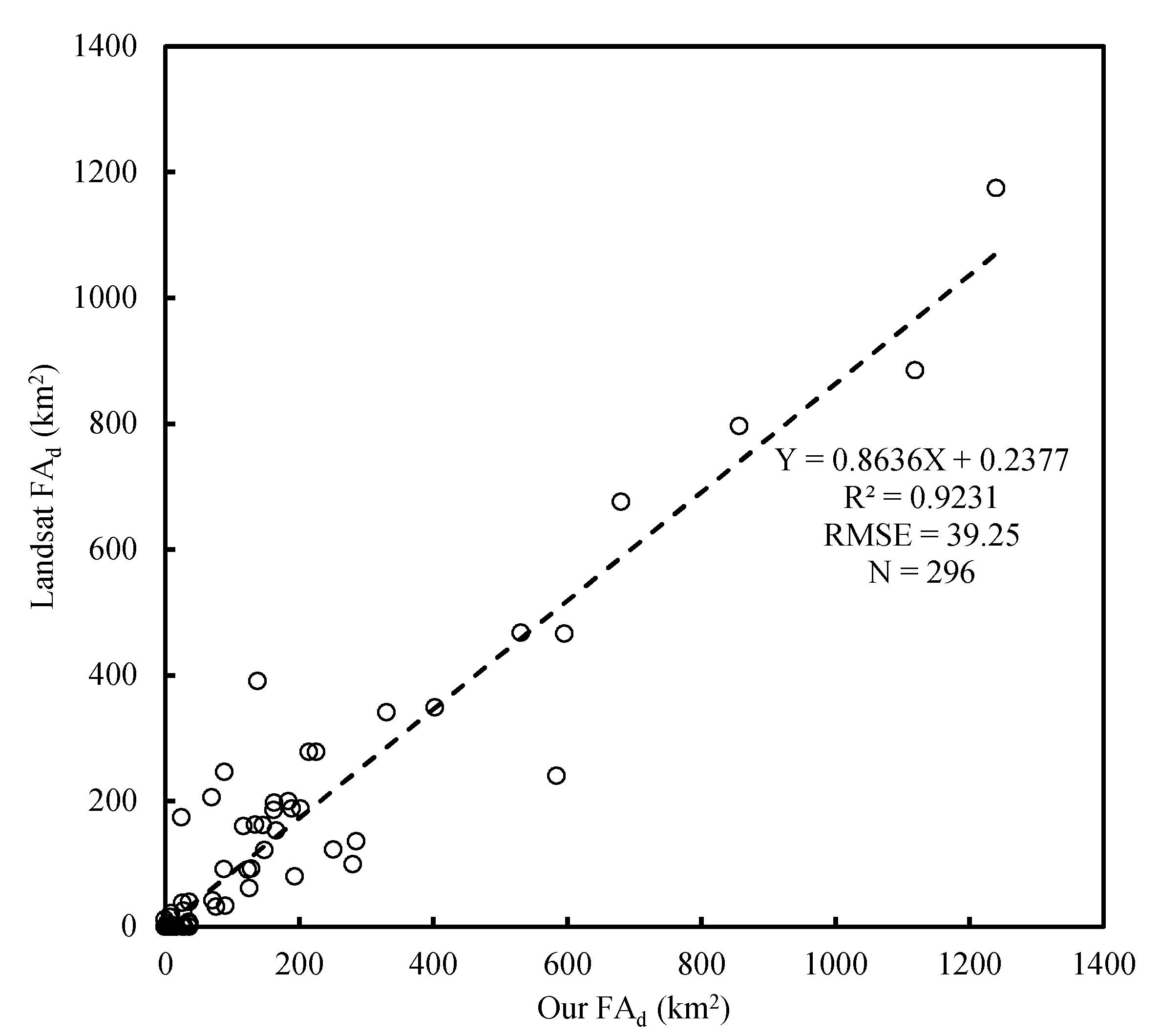
3.1.3. Validation Using Landsat Interpretation Data
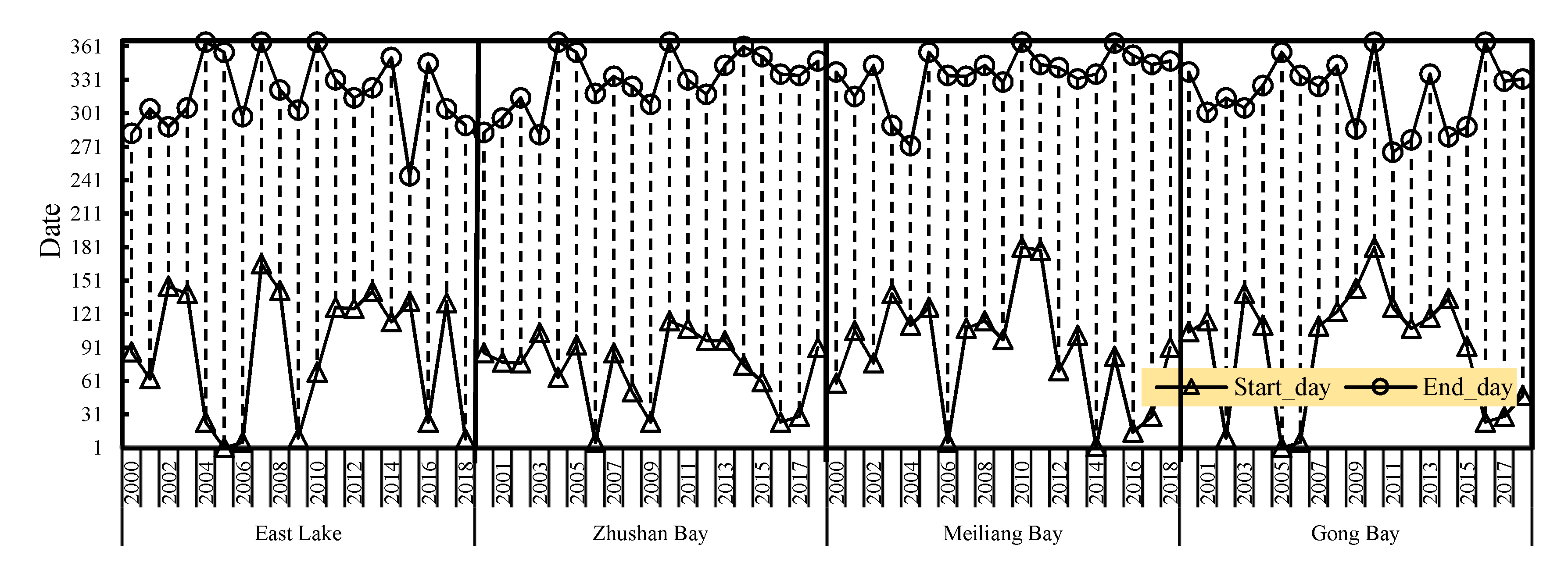
3.2. Temporal Coverage Patterns
3.3. Spatial Distributions of Annual and Monthly Occurrence Frequency
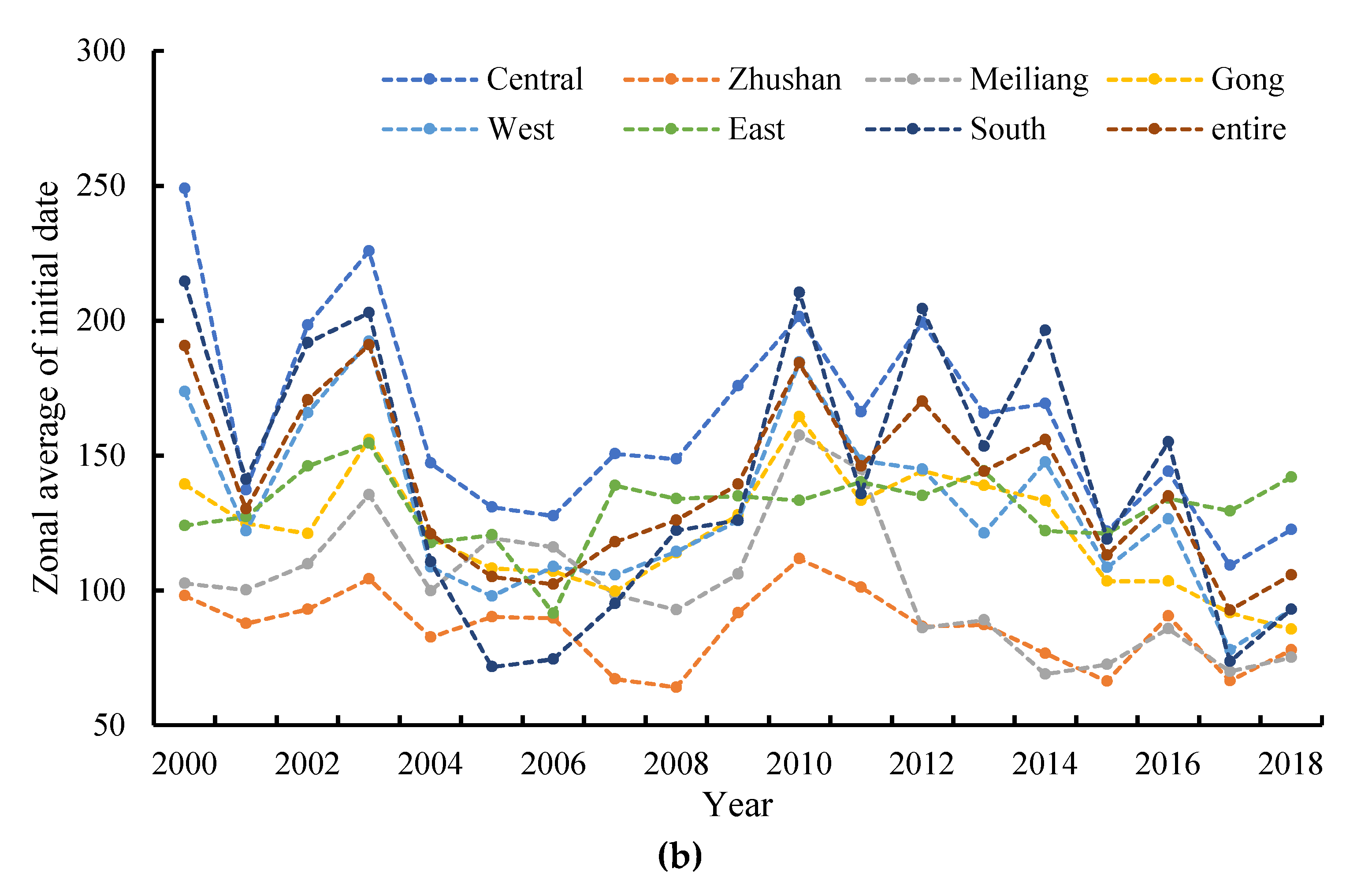
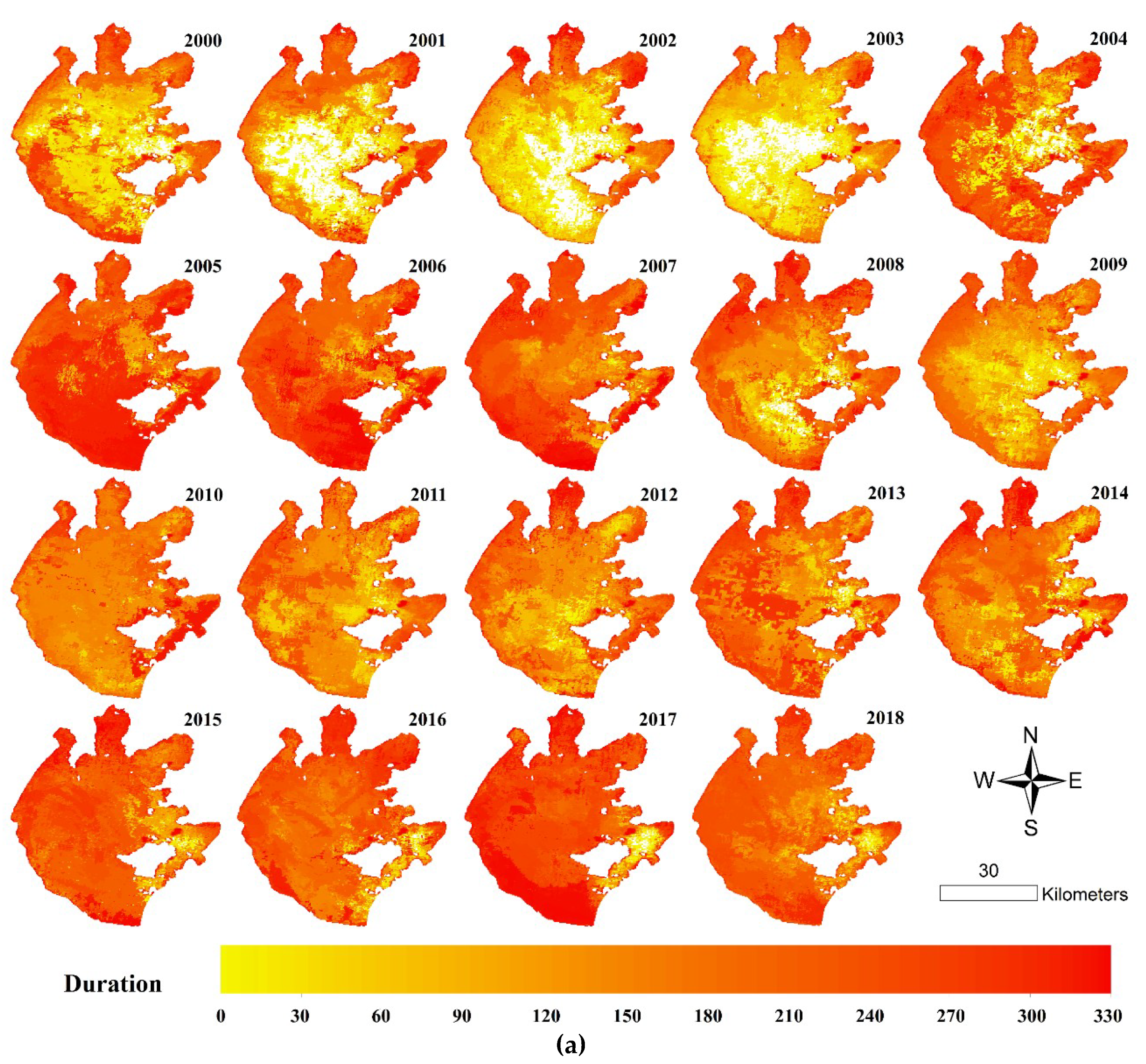
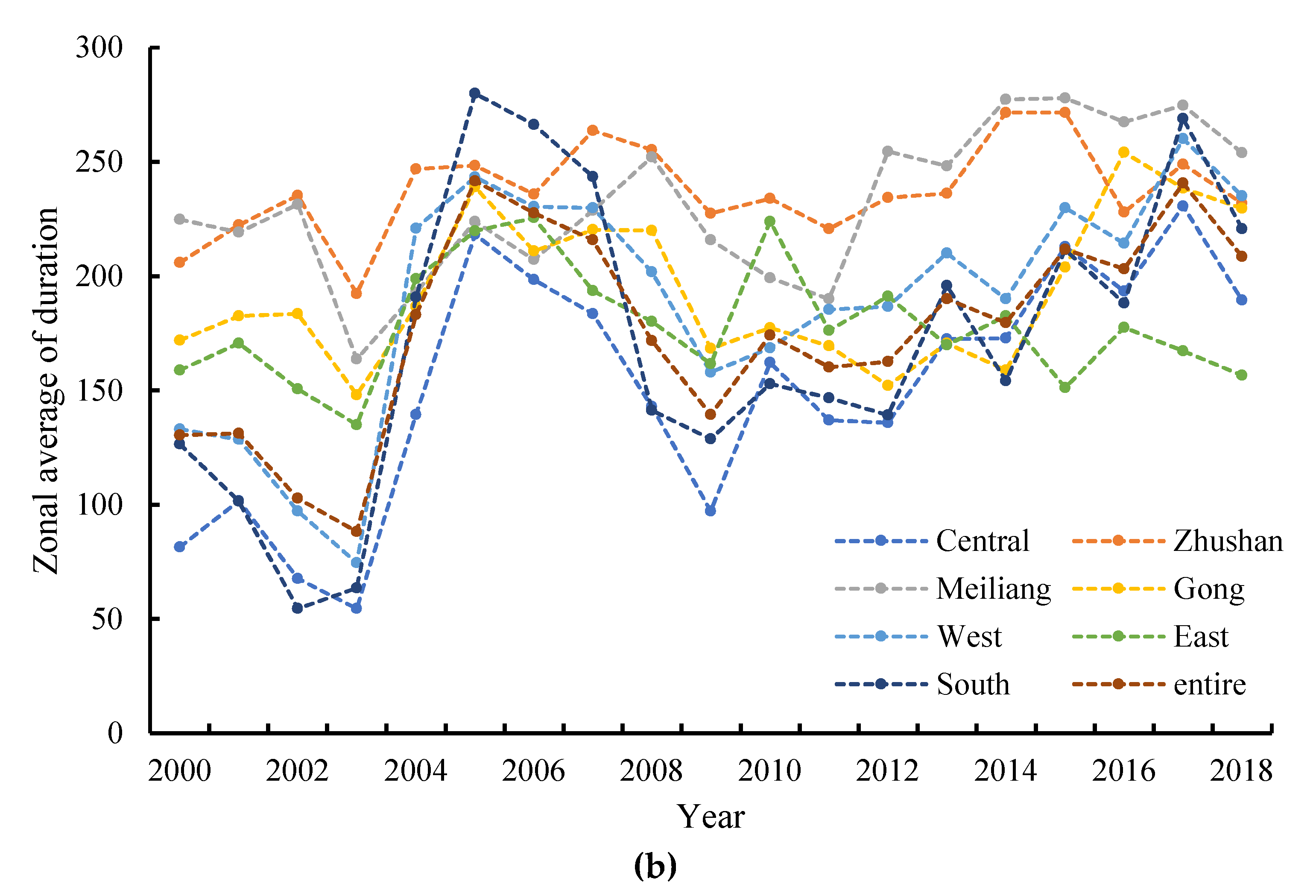
3.4. Spatial Distributions of Annual Initial Date and Duration
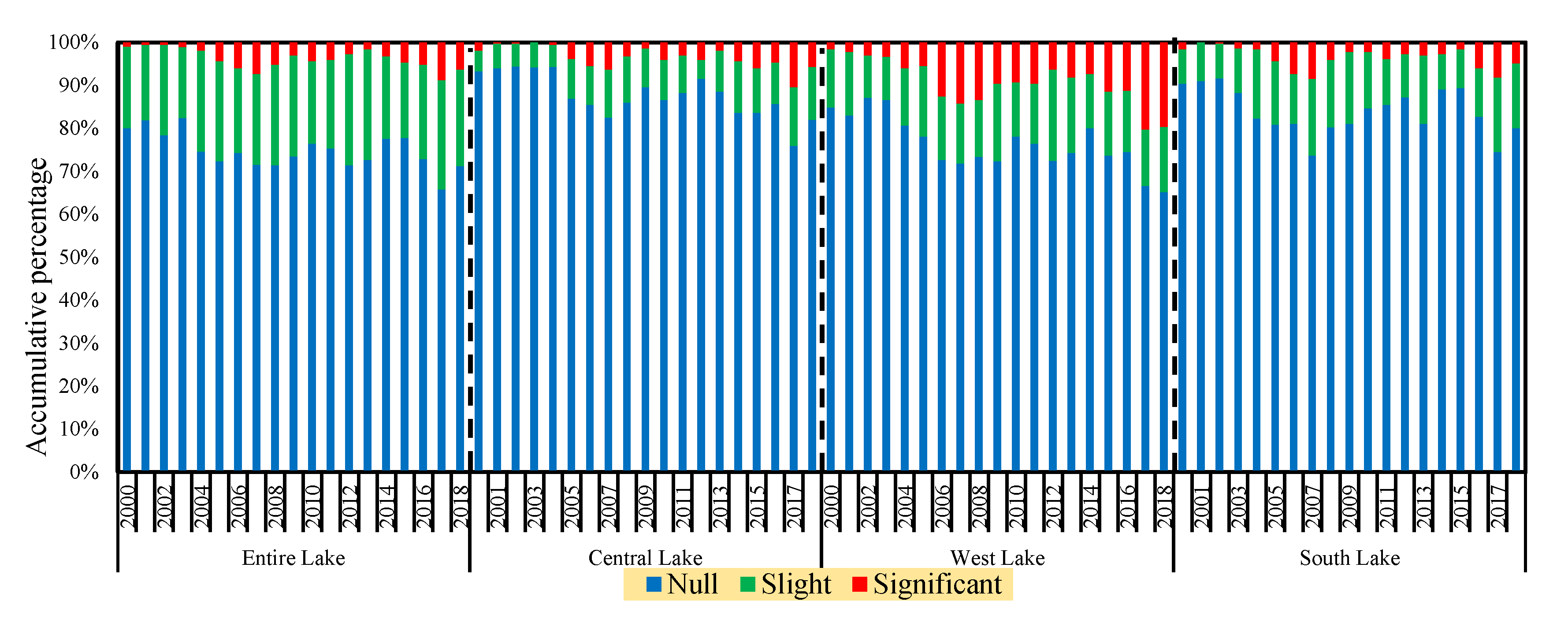
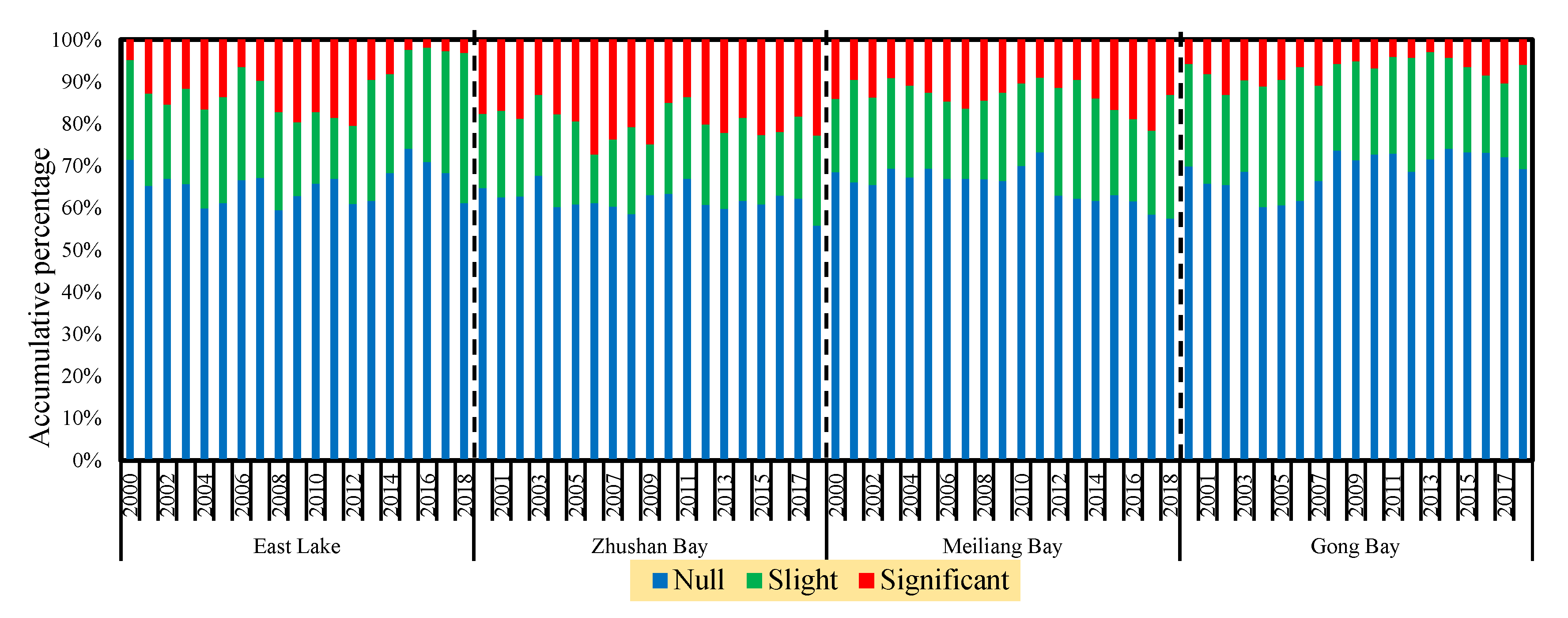
3.5. Temporal Characteristics of Significant Cyanobacteria Blooms
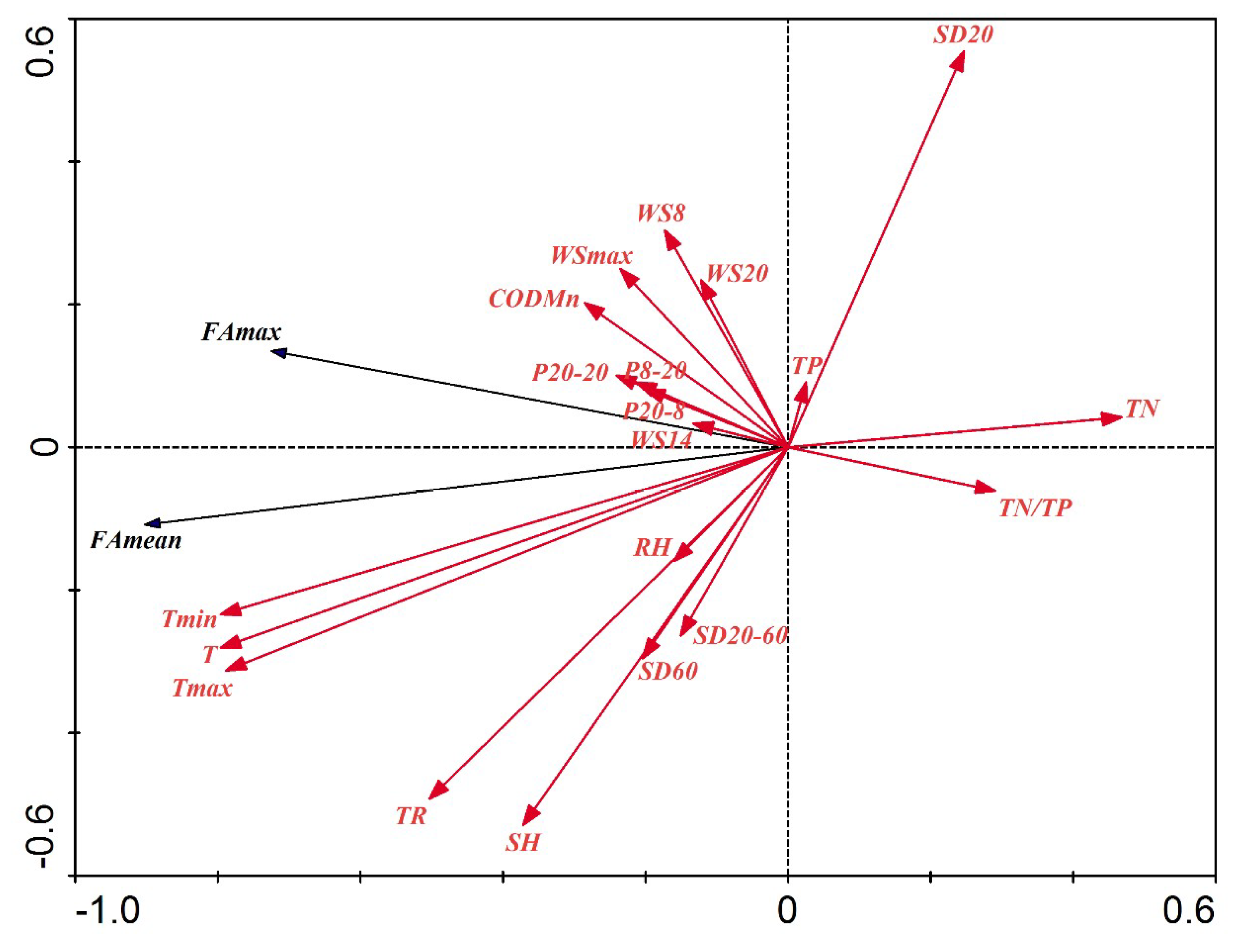
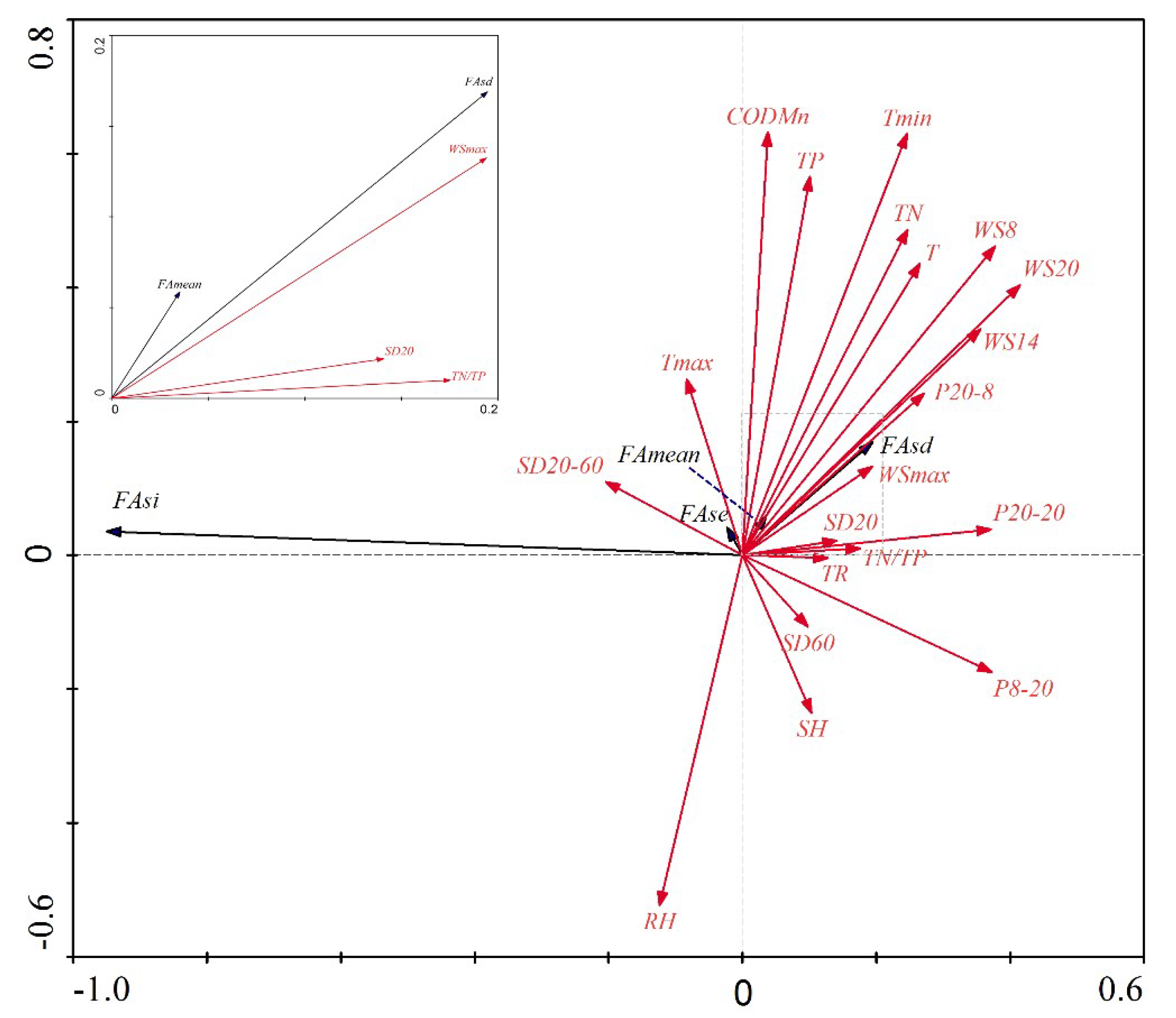
3.6. Redundancy Analysis (RDA) between Environmental Driving Factors and Cyanobacteria Bloom Characteristics
4. Discussion
4.1. Accuracy Deviation Sources in Our Workflow
4.2. Environmental Driving Factors of Taihu Lake’s Severe Cyanobacteria Blooms around 2017
4.3. Broader Applications of Our Workflow
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sellner, K.G.; Doucette, G.J.; Kirkpatrick, G.J. Harmful algal blooms: Causes, impacts and detection. J. Ind. Microbiol. Biotechnol. 2003, 30, 383–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shi, W.; Tang, J. Water property monitoring and assessment for China’s inland Lake Taihu from MODIS-Aqua measurements. Remote Sens. Environ. 2011, 115, 841–854. [Google Scholar] [CrossRef]
- Kudela, R.M.; Palacios, S.L.; Austerberry, D.C.; Accorsi, E.K.; Guild, L.S.; Torres-Perez, J. Application of hyperspectral Remote Sensing to cyanobacterial blooms in inland waters. Remote Sens. Environ. 2015, 167, 196–205. [Google Scholar] [CrossRef]
- Blondeau-Patissier, D.; Gower, J.F.R.; Dekker, A.G.; Phinn, S.R.; Brando, V.E. A review of ocean color Remote Sensing methods and statistical techniques for the detection, mapping and analysis of phytoplankton blooms in coastal and open oceans. Progr. Oceanogr. 2014, 123, 123–144. [Google Scholar] [CrossRef]
- Duan, H.; Ma, R.; Xu, X.; Kong, F.; Zhang, S.; Kong, W.; Hao, J.; Shang, L. Two-Decade Reconstruction of Algal Blooms in China’s Lake Taihu. Environ. Sci. Technol. 2009, 43, 3522–3528. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, R.; Duan, H.; Loiselle, S.; Xu, J. Satellite analysis to identify changes and drivers of CyanoHABs dynamics in Lake Taihu. Water Sci. Technol. Water Suppl. 2016, 16, 1451–1466. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, B.; Teubner, K.; Dokulil, M.T. Long-term dynamics of phytoplankton assemblages: Microcystis-domination in Lake Taihu, a large shallow lake in China. J. Plankton Res. 2003, 25, 445–453. [Google Scholar]
- Zhang, M.; Shi, X.; Yang, Z.; Yu, Y.; Shi, L.; Qin, B. Long-term dynamics and drivers of phytoplankton biomass in eutrophic Lake Taihu. Sci. Total Environ. 2018, 645, 876–886. [Google Scholar] [CrossRef]
- Qin, B.; Yang, G.; Ma, J.; Wu, T.; Li, W.; Liu, L.; Deng, J.; Zhou, J. Spatiotemporal Changes of Cyanobacterial Bloom in Large Shallow Eutrophic Lake Taihu, China. Front Microbiol. 2018, 9, 451. [Google Scholar] [CrossRef]
- Peretyatko, A.; Teissier, S.; Backer, S.D.; Triest, L. Assessment of the risk of cyanobacterial bloom occurrence in urban ponds: Probabilistic approach. Annal. Limnolog. Int. J. Limnol. 2010, 46, 121–133. [Google Scholar] [CrossRef]
- Descy, J.P.; Leprieur, F.; Pirlot, S.; Leporcq, B.; Van Wichelen, J.; Peretyatko, A.; Teissier, S.; Codd, G.A.; Triest, L.; Vyverman, W.; et al. Identifying the factors determining blooms of cyanobacteria in a set of shallow lakes. Ecol. Inform. 2016, 34, 129–138. [Google Scholar] [CrossRef]
- Rigosi, A.; Hanson, P.; Hamilton, D.P.; Hipsey, M.; Rusak, J.A.; Bois, J.; Sparber, K.; Chorus, I.; Watkinson, A.J.; Qin, B.; et al. Determining the probablity of cyanobacteria blooms: the application of Bayesian networks in multiple lake systems. Ecol. Appl. 2015, 25, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Oyama, Y.; Fukushima, T.; Matsushita, B.; Matsuzaki, H.; Kamiya, K.; Kobinata, H. Monitoring levels of cyanobacterial blooms using the visual cyanobacteria index (VCI) and floating algae index (FAI). Int. J. Appl. Earth Observ. Geoinform. 2015, 38, 335–348. [Google Scholar] [CrossRef]
- Kuster, T. Quantitative detection of chlorophyll in cynaobacteria bloom by satellite. Remote Sens. Limnol. Oceanogr. 2004, 49, 2179–2189. [Google Scholar]
- Hu, C.; Lee, Z.; Ma, R.; Yu, K.; Li, D.; Shang, S. Moderate Resolution Imaging Spectroradiometer (MODIS) observations of cyanobacteria blooms in Taihu Lake, China. J. Geophys. Res. Oceans 2010, 115. [Google Scholar] [CrossRef]
- Hu, C. A novel ocean color index to detect floating algae in the global oceans. Remote Sens. Environ. 2009, 113, 2118–2129. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, L.; Li, J.; Luo, L.; Yin, Y.; Liu, M.; Li, Y. Seasonal-spatial variation and Remote Sensing of phytoplankton absorption in Lake Taihu, a large eutrophic and shallow lake in China. J. Plankton Res. 2010, 32, 1023–1037. [Google Scholar] [CrossRef]
- Duan, H.; Ma, R.; Hu, C. Evaluation of Remote Sensing algorithms for cyanobacterial pigment retrievals during spring bloom formation in several lakes of East China. Remote Sens. Environ. 2012, 126, 126–135. [Google Scholar] [CrossRef]
- Hu, C. An empirical approach to derive MODIS ocean color patterns under severe sun glint. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef]
- Feng, L.; Hu, C.; Chen, X.; Cai, X.; Tian, L.; Gan, W. Assessment of inundation changes of Poyang Lake using MODIS observations between 2000 and 2010. Remote Sens. Environ. 2012, 121, 80–92. [Google Scholar] [CrossRef]
- Hu, C.; Li, D.; Chen, C.; Ge, J.; Muller-Karger, F.E.; Liu, J.; Yu, F.; He, M.-X. On the recurrent Ulva prolifera blooms in the Yellow Sea and East China Sea. J. Geophys. Res. Oceans 2010, 115. [Google Scholar] [CrossRef]
- Huang, C.; Li, Y.; Yang, H.; Sun, D.; Yu, Z.; Zhang, Z.; Chen, X.; Xu, L. Detection of algal bloom and factors influencing its formation in Taihu Lake from 2000 to 2011 by MODIS. Environ. Earth Sci. 2013, 71, 3705–3714. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, R.; Zhang, M.; Duan, H.; Loiselle, S.; Xu, J. Fourteen-Year Record (2000–2013) of the Spatial and Temporal Dynamics of Floating Algae Blooms in Lake Chaohu, Observed from Time Series of MODIS Images. Remote Sens. 2015, 7, 10523–10542. [Google Scholar] [CrossRef]
- Kabenge, M.; Wang, H.; Li, F. Urban eutrophication and its spurring conditions in the Murchison Bay of Lake Victoria. Environ. Sci. Pollut. Res. Int. 2016, 23, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, C. Mapping and quantifying Sargassum distribution and coverage in the Central West Atlantic using MODIS observations. Remote Sens. Environ. 2016, 183, 350–367. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, Y.; Zhou, Y.; Liu, X.; Zhu, G.; Qin, B.; Gao, G. Long-term MODIS observations of cyanobacterial dynamics in Lake Taihu: Responses to nutrient enrichment and meteorological factors. Sci. Rep. 2017, 7, 40326. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Murphy, S.; Wright, R.; Rouwet, D. Color and temperature of the crater lakes at Kelimutu volcano through time. Bull. Volcanol. 2017, 80. [Google Scholar] [CrossRef]
- Griffin, C.G.; McClelland, J.W.; Frey, K.E.; Fiske, G.; Holmes, R.M. Quantifying CDOM and DOC in major Arctic rivers during ice-free conditions using Landsat TM and ETM+ data. Remote Sens. Environ. 2018, 209, 395–409. [Google Scholar] [CrossRef]
- Lin, S.; Novitski, L.N.; Qi, J.; Stevenson, R.J. Landsat TM/ETM+ and Machine-Learning Algorithms for Limnological Studies and algal Bloom Management of Inland Lakes; SPIE: Bellingham, WA, USA, 2018; Volume 12, pp. 1–17. [Google Scholar]
- Traganos, D.; Aggarwal, B.; Poursanidis, D.; Topouzelis, K.; Chrysoulakis, N.; Reinartz, P. Towards Global-Scale Seagrass Mapping and Monitoring Using Sentinel-2 on Google Earth Engine: The Case Study of the Aegean and Ionian Seas. Remote Sens. 2018, 10, 1227. [Google Scholar] [CrossRef]
- Traganos, D.; Poursanidis, D.; Aggarwal, B.; Chrysoulakis, N.; Reinartz, P. Estimating Satellite-Derived Bathymetry (SDB) with the Google Earth Engine and Sentinel-2. Remote Sens. 2018, 10, 859. [Google Scholar] [CrossRef]
- Chen, J.; Quan, W.; Zhang, M.; Cui, T. A Simple Atmospheric Correction Algorithm for MODIS in Shallow Turbid Waters: A Case Study in Taihu Lake. IEEE J. Sel. Topics Appl. Earth Observ. Remote Sens. 2013, 6, 1825–1833. [Google Scholar] [CrossRef]
- Duan, H.; Ma, R.; Zhang, Y.; Loiselle, S.A. Are algal blooms occurring later in Lake Taihu? Climate local effects outcompete mitigation prevention. J. Plankton Res. 2014, 36, 866–871. [Google Scholar] [CrossRef]
- Zhu, W.; Tan, Y.; Wang, R.; Feng, G.; Chen, H.; Liu, Y.; Li, M. The trend of water quality variation and analysis in typical area Lake Taihu, 2010-2017. J. Lake Sci. 2018, 30, 296–305. [Google Scholar] [CrossRef]
- Gu, X.; Zeng, Q.; Mao, Z.; Chen, H.; Li, H. Water environment change over the period 2007 - 2016 and the strategy of fishery improve the water quality of Lake Taihu. J. Lake Sci. 2019, 31, 305–318. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, Y.; Zhang, Y.; Li, N.; Qin, B.; Zhu, G.; Zhou, Y. Phenology of Phytoplankton Blooms in a Trophic Lake Observed from Long-Term MODIS Data. Environ. Sci. Technol. 2019, 53, 2324–2331. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Fang, H.; He, G.; Jiang, H.; Wang, C. Effects of internal loading on phosphorus distribution in the Taihu Lake driven by wind waves and lake currents. Environ. Pollut. 2016, 219, 760–773. [Google Scholar] [CrossRef]
- Li, J.; Hu, C.; Shen, Q.; Barnes, B.B.; Murch, B.; Feng, L.; Zhang, M.; Zhang, B. Recovering low quality MODIS-Terra data over highly turbid waters through noise deduction and regional vicarious calibratioin adjustment: A case study in Taihu Lake. Remote Sens. Environ. 2017, 197, 72–84. [Google Scholar] [CrossRef]
- Jiang, G.; Ma, R.; Loiselle, S.A.; Duan, H.; Su, W.; Cai, W.; Huang, C.; Yang, J.; Yu, W. Remote Sensing of particulate organic carbon dynamics in a eutrophic lake (Taihu Lake, China). Sci. Total. Environ. 2015, 532, 245–254. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Shi, K.; Zhou, Y.; Tang, X.; Zhu, G.; Qin, B. Mapping Aquatic Vegetation in a Large, Shallow Eutrophic Lake: A Frequency-Based Approach Using Multiple Years of MODIS Data. Remote Sens. 2015, 7, 10295–10320. [Google Scholar] [CrossRef]
- Wang, S.; Gao, Y.; Li, Q.; Gao, J.; Zhai, S.; Zhou, Y.; Cheng, Y. Long-term and inter-monthly dynamics of aquatic vegetation and its relation with environmental factors in Taihu Lake, China. Sci. Total Environ. 2019, 651, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Vermote, E.W.; Wolfe, R. MOD09GQ MODIS/Terra Surface Reflectance Daily L2G Global 250m SIN Grid V006 [Data set]. NASA EOSDIS LP DAAC 2015. [Google Scholar] [CrossRef]
- Vermote, E.W. MODIS/Terra Surface Reflectance Daily L2G Global 1kmand 500m SIN Grid V006 [Data set]. NASA EOSDIS LP DAAC 2015. [Google Scholar] [CrossRef]
- Wang, S. Tropical state assessment of global inland waters using a MODIS-derived Forel-Ule index. Remote Sens. Environ. 2018. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Y. Generation of new cloud masks from MODIS land surface reflectance products. Remote Sens. Environ. 2013, 133, 21–37. [Google Scholar] [CrossRef]
- Chen, P.Y.; Srinivasan, R.; Fedosejevs, G.; Narasimhan, B. An automated cloud detection method for daily NOAA-14 AVHRR data for Texas, USA. Int. J. Remote Sens. 2002, 23, 2939–2950. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, C.; Muller-Karger, F. Monitoring turbidity in Tampa Bay using MODIS/Aqua 250-m imagery. Remote Sens. Environ. 2007, 109, 207–220. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Zhang, B.; Shen, Q.; Zhang, F.; Lu, Z. A simple correction method for the MODIS surface reflectance product over typical inland waters in China. Int. J. Remote Sens. 2016, 37, 6076–6096. [Google Scholar] [CrossRef]
- Feng, L.; Hu, C.; Li, J. Can MODIS Land Reflectance Products be Used for Estuarine and Inland Waters? Water Resour. Res. 2018, 54, 3583–3601. [Google Scholar] [CrossRef]
- Wang, S.; Yang, M.; Li, J.; Shen, Q.; Zhang, F. MODIS surface reflectance product (MOD09) validation for typical inland waters in China. SPIE Proc. 2014, 9261, 92610F. [Google Scholar] [CrossRef]
- Moradi, M. Comparison of the efficacy of MODIS and MERIS data for detecting cyanobacterial blooms in the southern Caspian Sea. Mar. Pollut. Bull. 2014, 87, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Legendre, P.; Anderson, M.J. Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecol. Monogr. 1999, 69, 1–24. [Google Scholar] [CrossRef]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, W.; Zhang, J.; Jeppesen, E.; Liu, Z.; Kociolek, J.P.; Xu, X.; Wang, L. Local habitat heterogeneity determines the differences in benthic diatom metacommunities between different urban river types. Sci. Total. Environ. 2019, 669, 711–720. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, J. Coupled effects of land use pattern and hydrological regime on composition and diversity of riverine eukaryotic community in a coastal watershed of Southeast China. Sci. Total. Environ. 2019, 660, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, R.; Duan, H.; Loiselle, S.A.; Xu, J.; Ma, M. A novel algorithm to estimate algae bloom coverage to subpixel resolution in Taihu Lake. IEEE J. Sel. Topics Appl. Earth Observ. Remote Sens. 2014, 7, 3060–3068. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, Y.; Ma, R.; Loiselle, S.; Li, J.; Hu, M. A MODIS-Based Novel Method to Distinguish Surface Cyanobacterial Scums and Aquatic Macrophytes in Lake Taihu. Remote Sens. 2017, 9, 133. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Bloom Like It Hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Visser, P.M.; Verspagen, J.M.H.; Sandrini, G.; Stal, L.J.; Matthijs, H.C.P.; Davis, T.W.; Paerl, H.W.; Huisman, J. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 2016, 54, 145–159. [Google Scholar] [CrossRef]
- Cao, H.-S.; Kong, F.-X.; Luo, L.-C.; Shi, X.-L.; Yang, Z.; Zhang, X.-F.; Tao, Y. Effects of Wind and Wind-Induced Waves on Vertical Phytoplankton Distribution and Surface Blooms ofMicrocystis aeruginosain Lake Taihu. J. Freshw. Ecol. 2006, 21, 231–238. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Z.; Shi, X. Expansion and drivers of cyanobacteria blooms in Lake Taihu. J. Lake Sci. 2019, 31, 336–344. [Google Scholar] [CrossRef]
- Guo, C.; Zhu, G.; Paerl, H.W.; Zhu, M.; Yu, L.; Zhang, Y.; Liu, M.; Zhang, Y.; Qin, B. Extreme weather event may induce Microcystis blooms in the Qiantang River, Southeast China. Environ. Sci. Pollut. Res. Int. 2018, 25, 22273–22284. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, K.; Liu, J.; Deng, J.; Qin, B.; Zhu, G.; Zhou, Y. Meteorological and hydrological conditions driving the formation and disappearance of black blooms, an ecological disaster phenomena of eutrophication and algal blooms. Sci. Total. Environ. 2016, 569–570, 1517–1529. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, Y.; Xu, H.; Zhu, G.; Qin, B.; Huang, C.; Liu, X.; Zhou, Y.; Lv, H. Long-Term Satellite Observations of Microcystin Concentrations in Lake Taihu during Cyanobacterial Bloom Periods. Environ. Sci. Technol. 2015, 49, 6448–6456. [Google Scholar] [CrossRef]
- Feng, L.; Hou, X.; Li, J.; Zheng, Y. Exploring the potential of Rayleigh-corrected reflectance in coastal and inland water applications: A simple aerosol correction method and its merits. ISPRS J. Photogramm. Remote Sens. 2018, 146, 52–64. [Google Scholar] [CrossRef]
- Matthews, M.W.; Odermatt, D. Improved algorithm for routine monitoring of cyanobacteria and eutrophication in inland and near-coastal waters. Remote Sens. Environ. 2015, 156, 374–382. [Google Scholar] [CrossRef]
- Santer, R.; Carrere, V.; Dubuisson, P.; Roger, J.C. Atmospheric correction over land for MERIS. Int. J. Remote Sens. 1999, 20, 1819–1840. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Huang, T.; Yang, H.; Li, Y.; Zhang, Z.; He, M.; Hu, Z.; Song, T.; Zhu, A.X. Long-term variation of phytoplankton biomass and physiology in Taihu lake as observed via MODIS satellite. Water Res. 2019, 153, 187–199. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, H.; Wang, R.; Feng, G.; Xue, Z.; Hu, S. Analysis on the reasons for the large bloom area of Lake Taihu in 2017. J. Lake Sci. 2019, 31, 621–632. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Huang, Q.; Gao, J. When and where to reduce nutrient for controlling harmful algal blooms in large eutrophic lake Chaohu, China? Ecol. Indicat. 2018, 89, 808–817. [Google Scholar] [CrossRef]
- Huo, S.; He, Z.; Ma, C.; Zhang, H.; Xi, B.; Xia, X.; Xu, Y.; Wu, F. Stricter nutrient criteria are required to mitigate the impact of climate change on harmful cyanobacterial blooms. J. Hydrol. 2019, 569, 698–704. [Google Scholar] [CrossRef]
- Yang, L.; Yang, X.; Ren, L.; Qian, X.; Xiao, L. Mechanism and control strategy of cyanobacteria bloom in Lake Taihu. J. Lake Sci. 2019, 31, 18–27. [Google Scholar] [CrossRef][Green Version]
- Pelicice, F.M.; Agostinho, A.A.; Thomaz, S.M. Fish assemblages associated with Egeria in a tropical reservoir: Investigating the effects of plant biomass and diel period. Acta Oecolog. 2005, 27, 9–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, X.; Qin, B. A critical review of the development, current hotspots, and future directions of Lake Taihu research from the bibliometrics perspective. Environ. Sci. Pollut Res. Int. 2016, 23, 12811–12821. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, Y.; Li, Y.; Li, L.; Lv, H.; Liu, X. Remote estimation of cyanobacteria-dominance in inland waters. Water Res. 2015, 68, 217–226. [Google Scholar] [CrossRef]
- Palmer, S.C.J.; Kutser, T.; Hunter, P.D. Remote Sensing of inland waters: Challenges, progress and future directions. Remote Sens. Environ. 2015, 157, 1–8. [Google Scholar] [CrossRef]






| Products | Revisit | Band information (only related bands listed) | |||
|---|---|---|---|---|---|
| Name | Spatial Resolution (m) | Wavelength (nm) | Description | ||
| MOD09GQ.006 Terra Surface Reflectance Daily L2G Global 250 m | Daily | sur_refl_b01 | 250 | 620–670 | Surface reflectance for red band |
| sur_refl_b02 | 250 | 841–876 | Surface reflectance for near-IR (NIR) band | ||
| MOD09GA.006 Terra Surface Reflectance Daily L2G Global 1 km and 500 m | Daily | sur_refl_b05 | 500 | 1230–1250 | Surface reflectance for SWIR1 band |
| sur_refl_b06 | 500 | 1628–1652 | Surface reflectance for SWIR2 band | ||
| sur_refl_b07 | 500 | 2105–2055 | Surface reflectance for SWIR3 band | ||
| Entire Lake | Entire Lake Excluding East Lake | |||||
|---|---|---|---|---|---|---|
| 2001–2008 (N = 93) | 2009–2018 (N = 118) | 2001–2018 (N = 211) | 2001–2008 (N = 93) | 2009–2018 (N = 118) | 2001–2018 (N = 211) | |
| Pearson coefficient | 0.4810 | 0.6299 | 0.5401 | 0.5082 | 0.6898 | 0.5833 |
| Reference | Indicator/Proxy | Spatial Extent | Temporal Stage |
|---|---|---|---|
| Hu et al. [15] | Cyanobacteria bloom frequency/ initial/duration/area/specific date | Entire lake | 2000–2008 |
| Duan et al. [5,34] | Cyanobacteria bloom initial date | Entire lake | 1987–2011 |
| Liu et al. [41] | Aquatic vegetation spatial/temporal dynamic | Entire lake | 2003–2013 |
| Wang et al. [2] | Chla monthly spatial dynamic | Entire lake | 2002–2008 |
| Shi et al. [26] | Chla spatial/temporal dynamic | Entire lake | 2003–2013 |
| Zhang et al. [6] | Annual and monthly cyanobacteria bloom frequency/initial date/duration | Entire lake excluding East Lake | 2001-–2013 |
| Type | Item | Abbreviation | Dimension | Monthly Permutation Test | Annual permutation Test | Individual Explanation | |||
|---|---|---|---|---|---|---|---|---|---|
| P-Value 1 | F-Value | P-Value | F-Value | Monthly | Annual | ||||
| Meteorological factors | Monthly mean of daily mean temperature | T | °C | 0.002 ** | 104.364 | 0.374 | 0.831 | 0.273 | 0.168 |
| Monthly mean of daily maximum temperature | Tmax | °C | 0.002 ** | 101.183 | 0.838 | 0.101 | 0.265 | 0.010 | |
| Monthly mean of daily minimum temperature | Tmin | °C | 0.002 ** | 104.251 | 0.406 | 0.791 | 0.283 | 0.212 | |
| Monthly mean of daily mean relative humidity | RH | % | 0.140 | 2.525 | 0.644 | 0.256 | 0.015 | 0.117 | |
| Monthly cumulative precipitation from 20:00 to 08:00 | P20-8 | mm | 0.046 * | 3.928 | 0.292 | 0.784 | 0.029 | 0.167 | |
| Monthly cumulative precipitation from 08:00 to 20:00 | P8-20 | mm | 0.040 * | 4.436 | 0.228 | 1.567 | 0.010 | 0.125 | |
| Monthly cumulative precipitation from 20:00 to 20:00 | P20-20 | mm | 0.014 * | 5.725 | 0.178 | 1.531 | 0.024 | 0.186 | |
| Monthly amount of total radiation | TR | MJ/m2 | 0.002 ** | 29.516 | 0.752 | 0.161 | 0.078 | 0.010 | |
| Monthly amount of sunshine hours | SH | h | 0.002 ** | 15.073 | 0.778 | 0.129 | 0.034 | 0.008 | |
| Monthly number of days with sunshine time greater than 60% in a day | SD60 | days | 0.028 * | 4.239 | 0.848 | 0.098 | 0.006 | 0.002 | |
| Monthly number of days with sunshine time less than 20% in a day | SD20 | days | 0.014 * | 6.696 | 0.694 | 0.199 | 0.012 | 0.037 | |
| Monthly number of days with sunshine time greater than 20% but less than 60% in a day | SD20-60 | days | 0.112 | 2.317 | 0.448 | 0.421 | 0.010 | 0.007 | |
| Monthly maximum wind speed | WSmax | m/s | 0.028 * | 5.566 | 0.524 | 0.380 | 0.018 | 0.079 | |
| Monthly mean wind speed at 08:00 | WS8 | m/s | 0.058 | 3.078 | 0.210 | 1.672 | 0.008 | 0.292 | |
| Monthly mean wind speed at 14:00 | WS14 | m/s | 0.196 | 1.690 | 0.226 | 1.426 | 0.001 | 0.245 | |
| Monthly mean wind speed at 20:00 | WS20 | m/s | 0.216 | 1.533 | 0.136 | 2.059 | 0.006 | 0.286 | |
| Water quality factors | Total nitrogen | TN | mg/L | 0.002 ** | 24.194 | 0.388 | 0.730 | 0.146 | 0.135 |
| Total phosphorus | TP | mg/L | 0.838 | 0.077 | 0.658 | 0.217 | 0.001 | 0.135 | |
| Ratio of total nitrogen to total phosphorus | TN/TP | - | 0.008 ** | 8.432 | 0.634 | 0.318 | 0.079 | 0.021 | |
| Chemical oxygen demand | CODMn | mg/L | 0.010 ** | 8.247 | 0.748 | 0.168 | 0.066 | 0.110 | |
| Cyanobacteria bloom characteristics | Monthly/annual mean area of floating cyanobacteria | FAmean | km2 | ||||||
| Monthly maximum area of floating cyanobacteria | FAmax | km2 | |||||||
| Initial date of significant floating cyanobacteria | FAsi | day | |||||||
| End date of significant floating cyanobacteria | FAse | day | |||||||
| Duration of significant floating cyanobacteria | FAsd | days | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, T.; Zhang, X.; Dong, R. Long-Term Spatial and Temporal Monitoring of Cyanobacteria Blooms Using MODIS on Google Earth Engine: A Case Study in Taihu Lake. Remote Sens. 2019, 11, 2269. https://doi.org/10.3390/rs11192269
Jia T, Zhang X, Dong R. Long-Term Spatial and Temporal Monitoring of Cyanobacteria Blooms Using MODIS on Google Earth Engine: A Case Study in Taihu Lake. Remote Sensing. 2019; 11(19):2269. https://doi.org/10.3390/rs11192269
Chicago/Turabian StyleJia, Tianxia, Xueqi Zhang, and Rencai Dong. 2019. "Long-Term Spatial and Temporal Monitoring of Cyanobacteria Blooms Using MODIS on Google Earth Engine: A Case Study in Taihu Lake" Remote Sensing 11, no. 19: 2269. https://doi.org/10.3390/rs11192269
APA StyleJia, T., Zhang, X., & Dong, R. (2019). Long-Term Spatial and Temporal Monitoring of Cyanobacteria Blooms Using MODIS on Google Earth Engine: A Case Study in Taihu Lake. Remote Sensing, 11(19), 2269. https://doi.org/10.3390/rs11192269