Variability and Geographical Origin of Five Years Airborne Fungal Spore Concentrations Measured at Saclay, France from 2014 to 2018
Abstract
1. Introduction
2. Materials and Methods
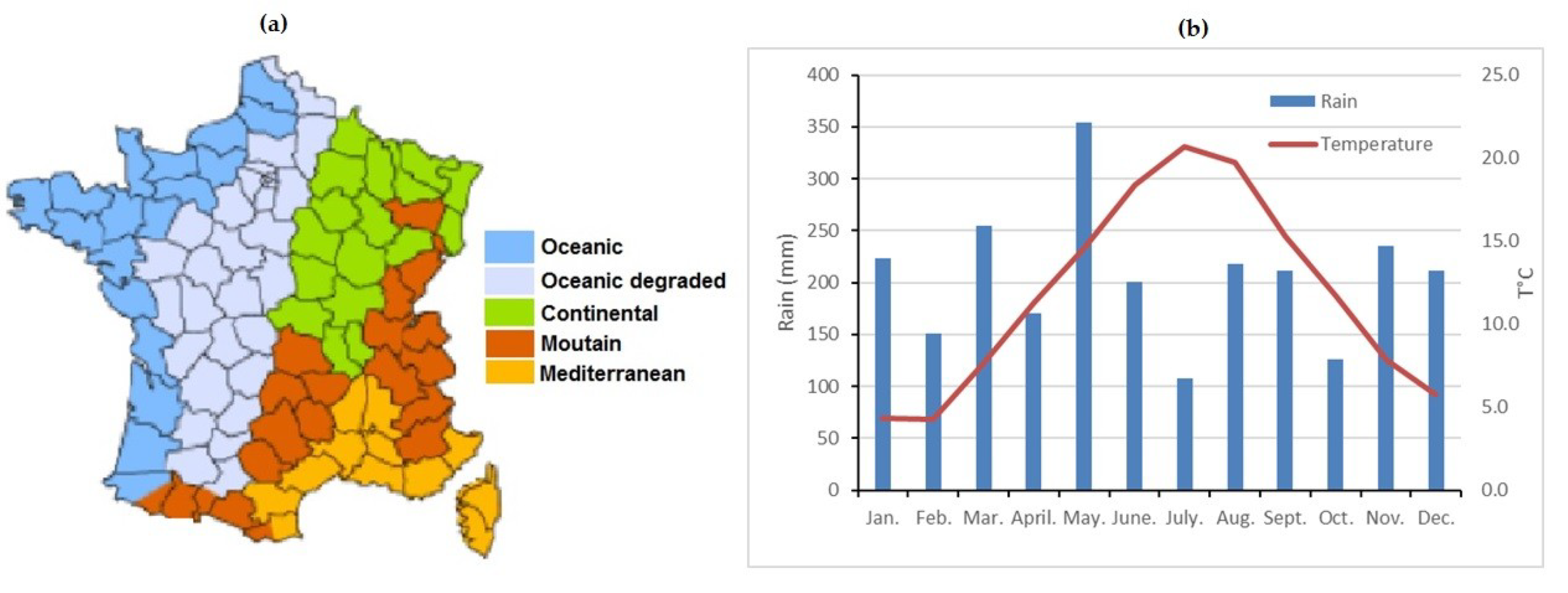
2.1. Experimental Site Description
2.2. Airborne Fungal Spores Monitoring Procedure
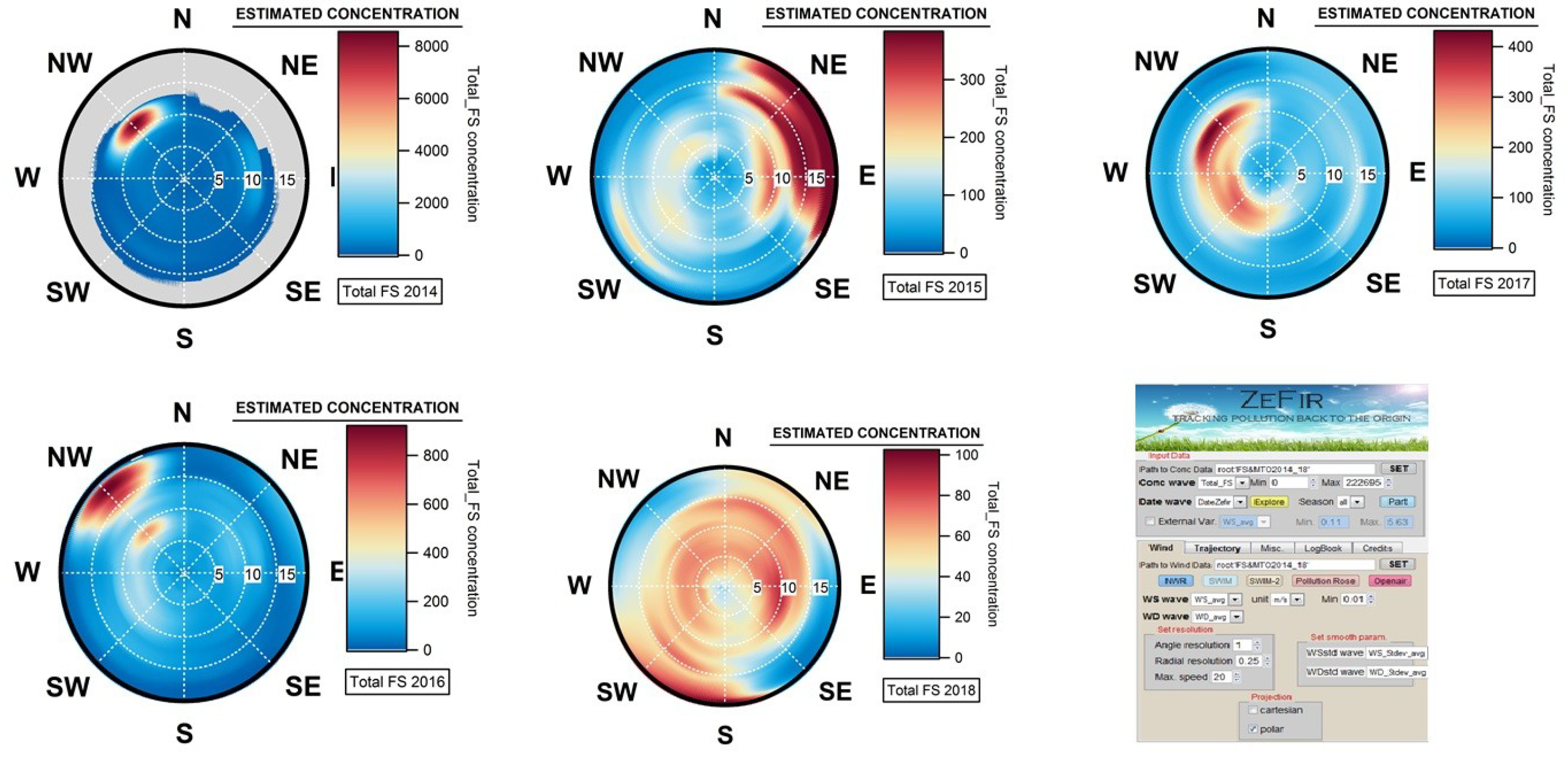
2.3. Investigation of the Geographical Origins of Airborne Fungal Spores
2.4. Meteorological Aspects of the Saclay Ecosystem
2.5. Wind Prevalence at Saclay
3. Results
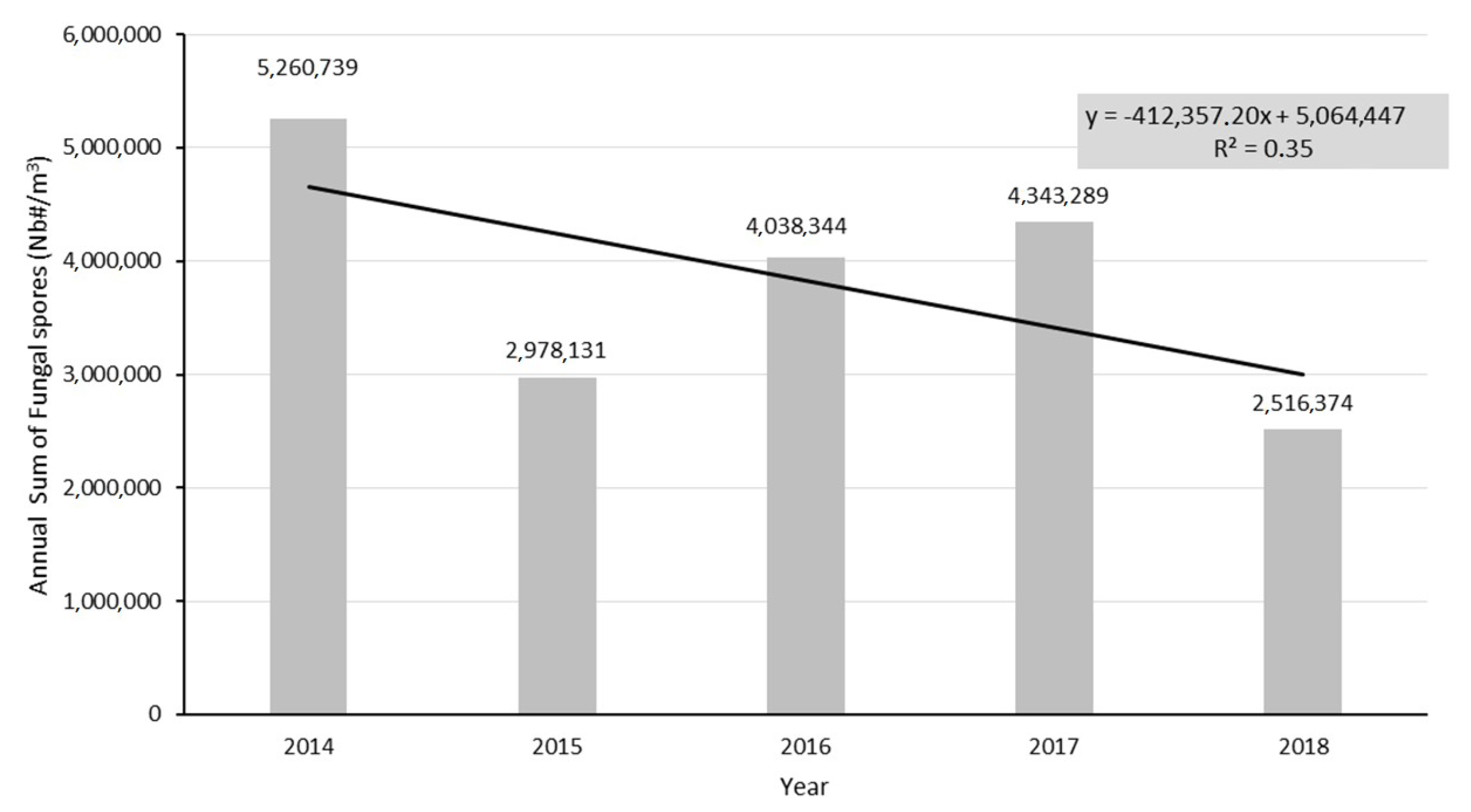
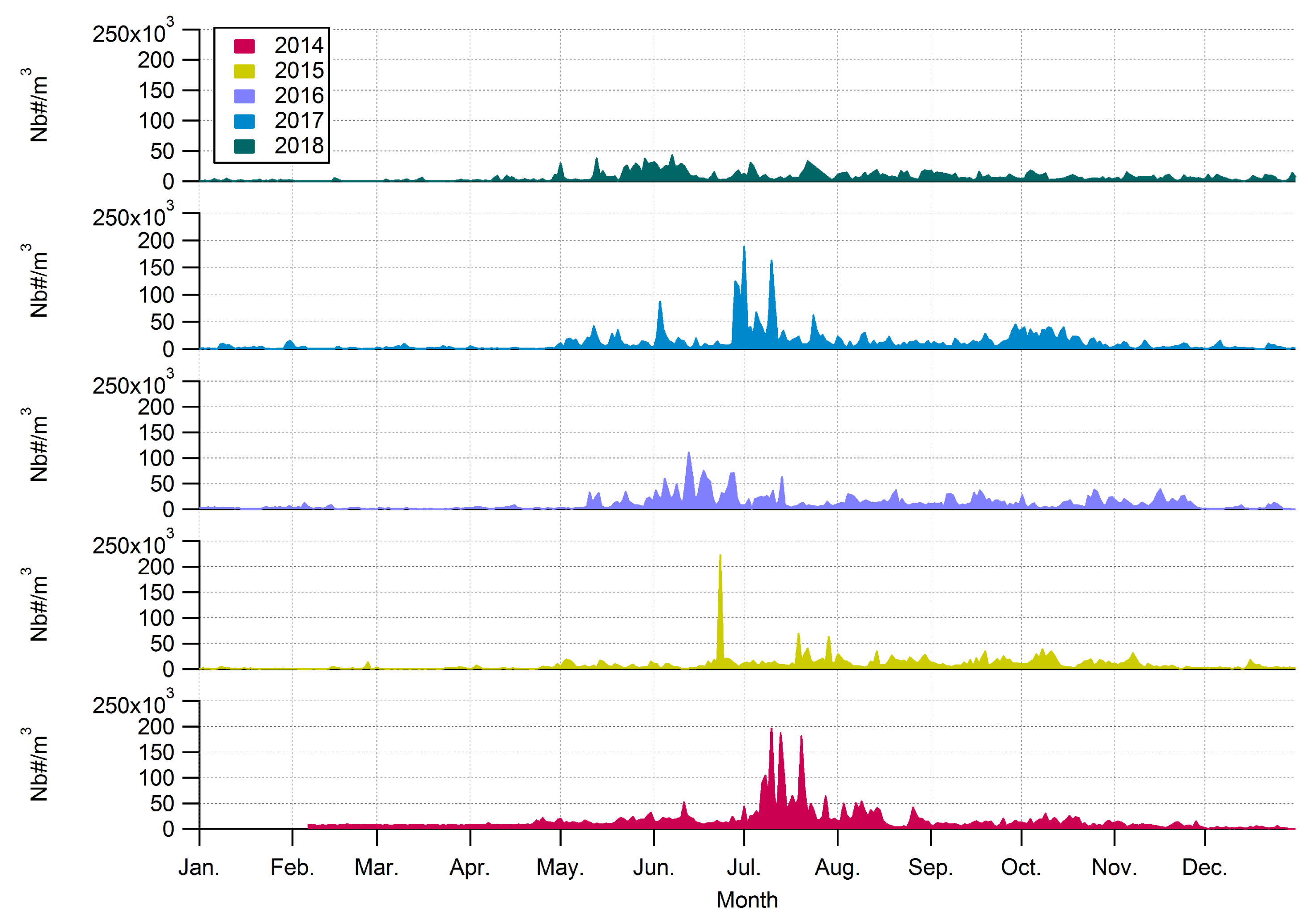
3.1. Interannuality of the Airborne Annual Fungal Spore Integral at Saclay
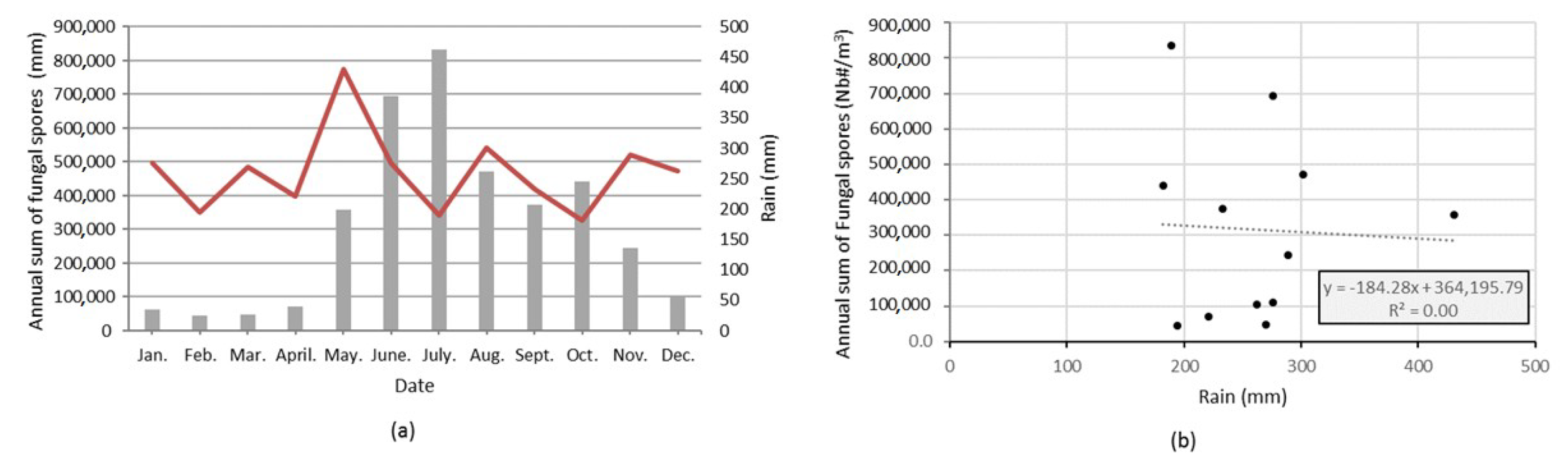
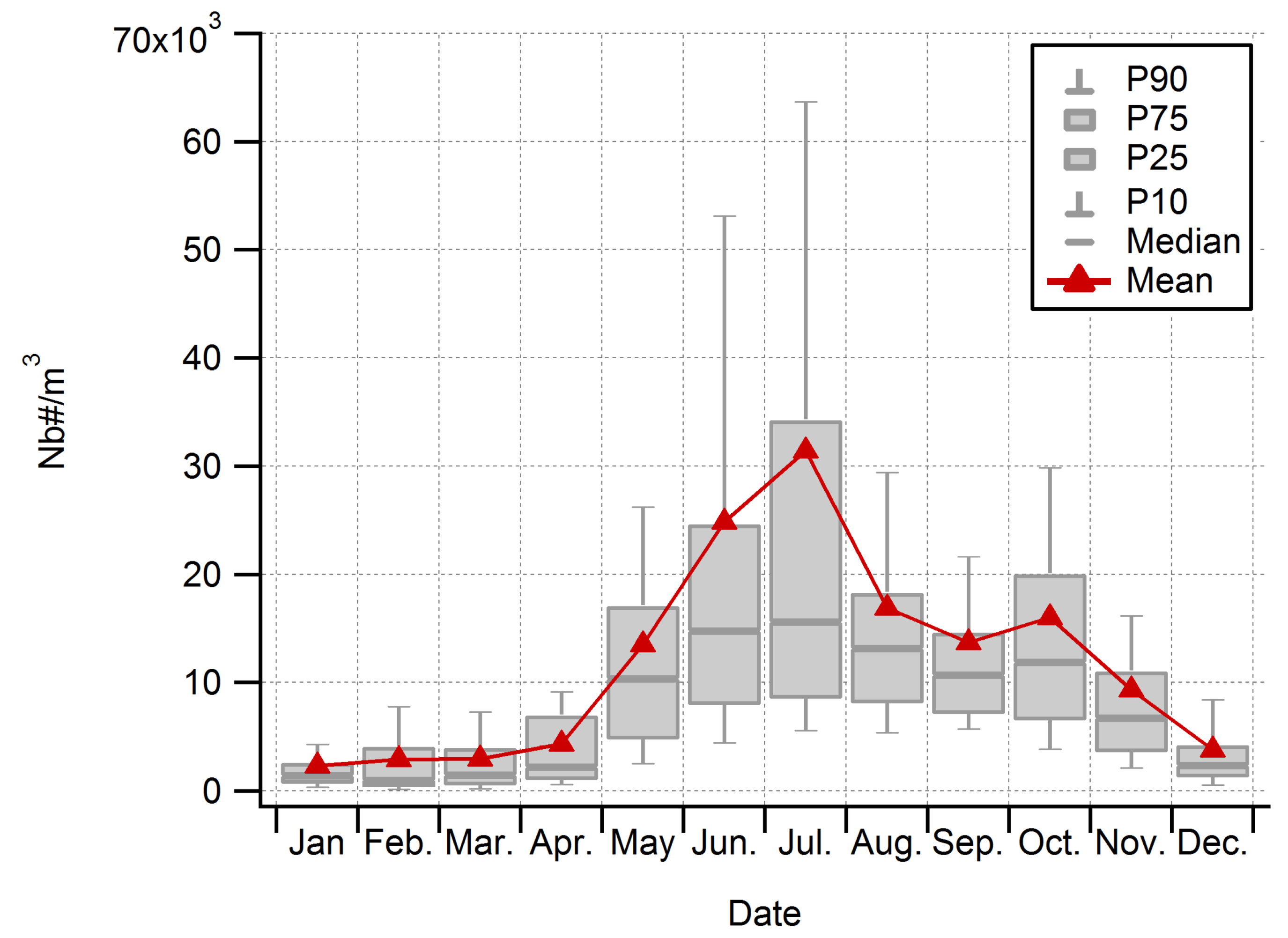
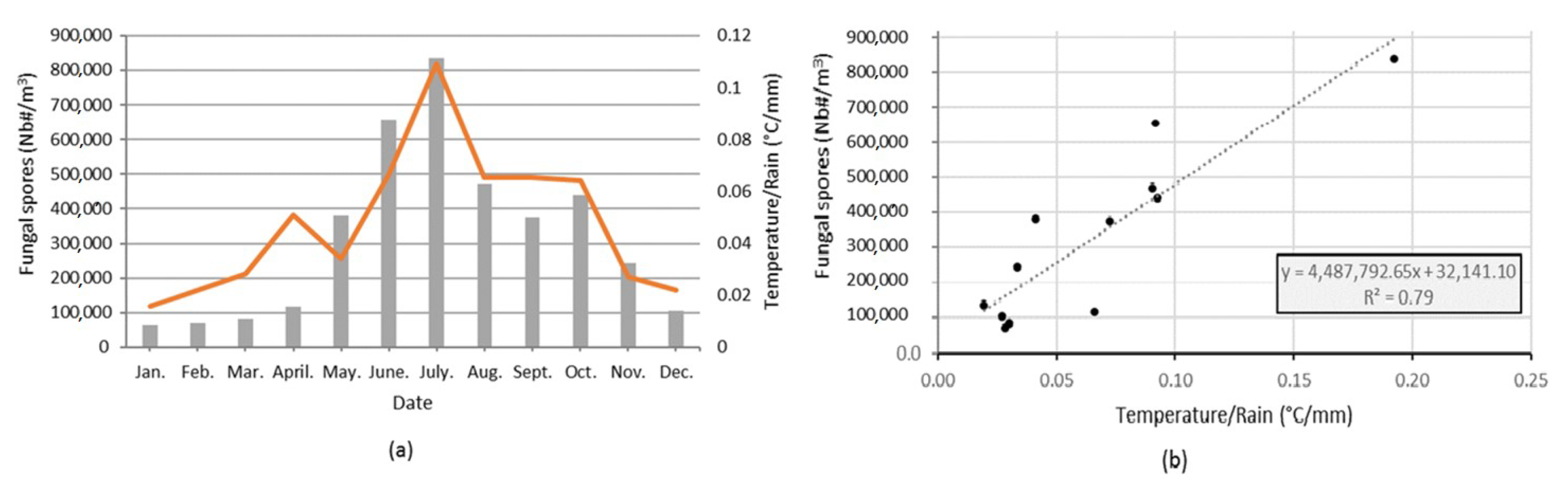
3.2. Seasonality of All Taxa Combined Concentrations at Saclay
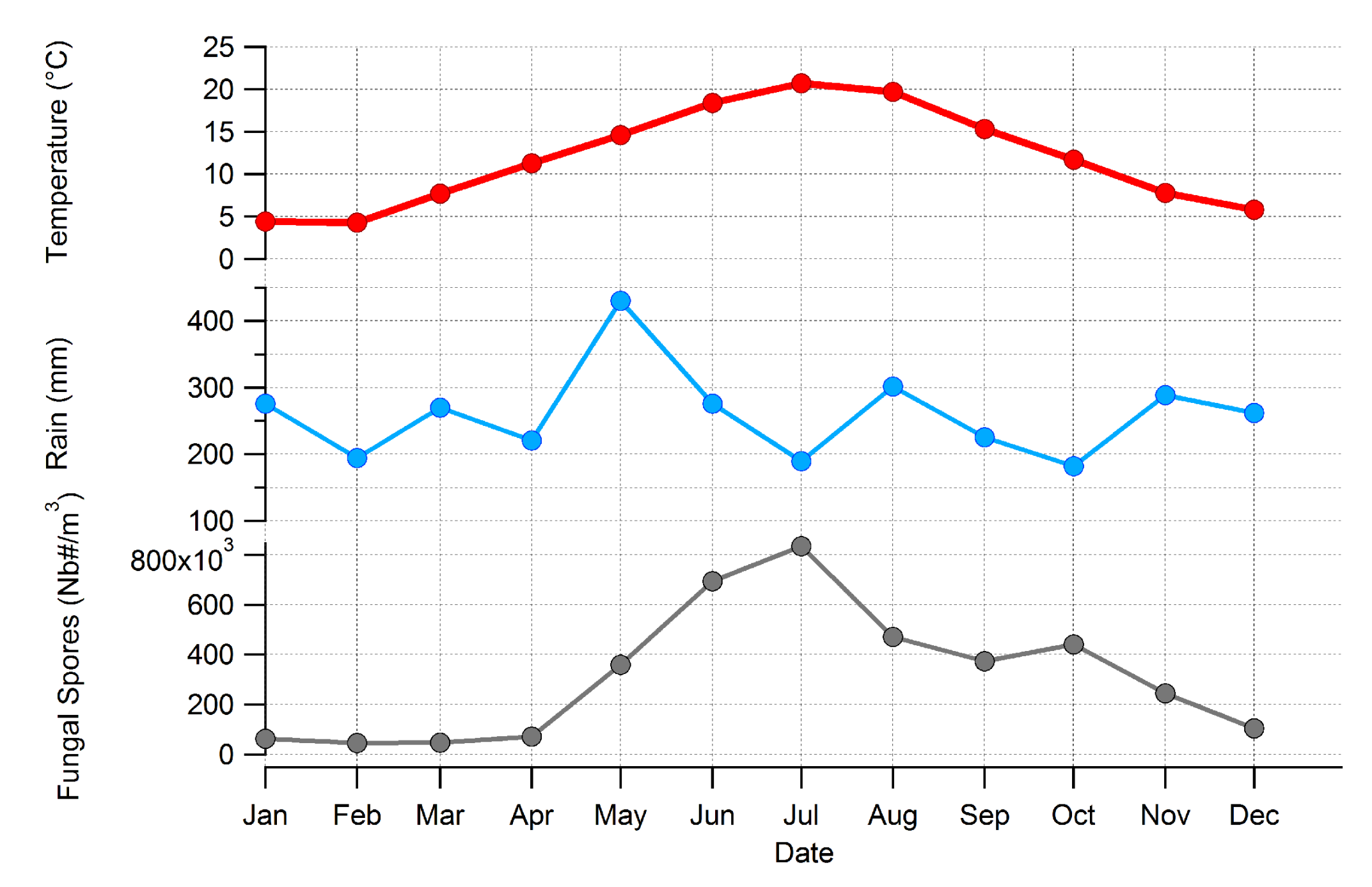
3.3. Major Parameters Driving the Seasonality of AFS Concentrations at Saclay
3.4. Main Spore Season and Daily Variability of Airborne Fungal Spore Concentrations at Saclay
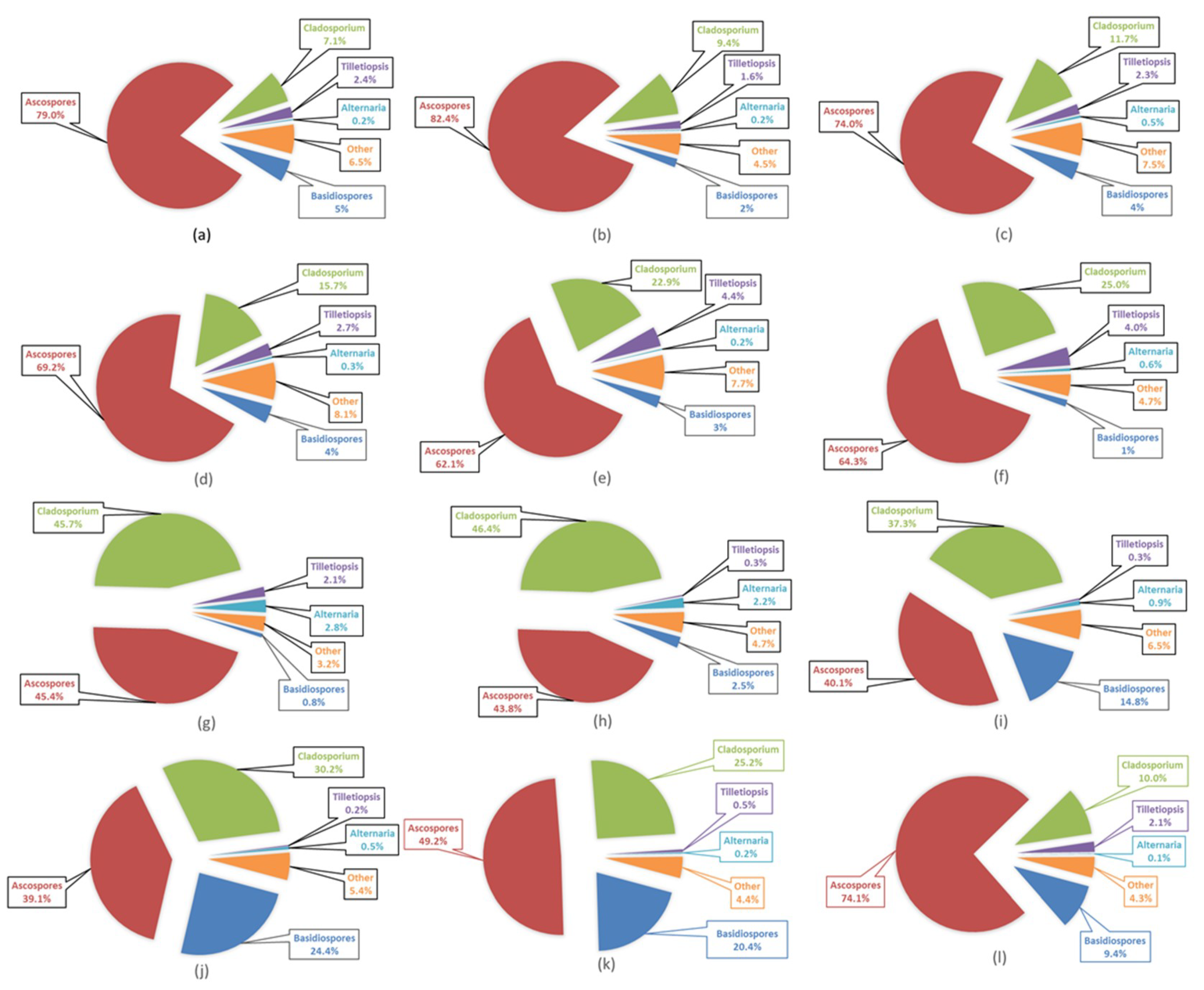
3.5. Airborne Fungal Spore Characteristics at Saclay
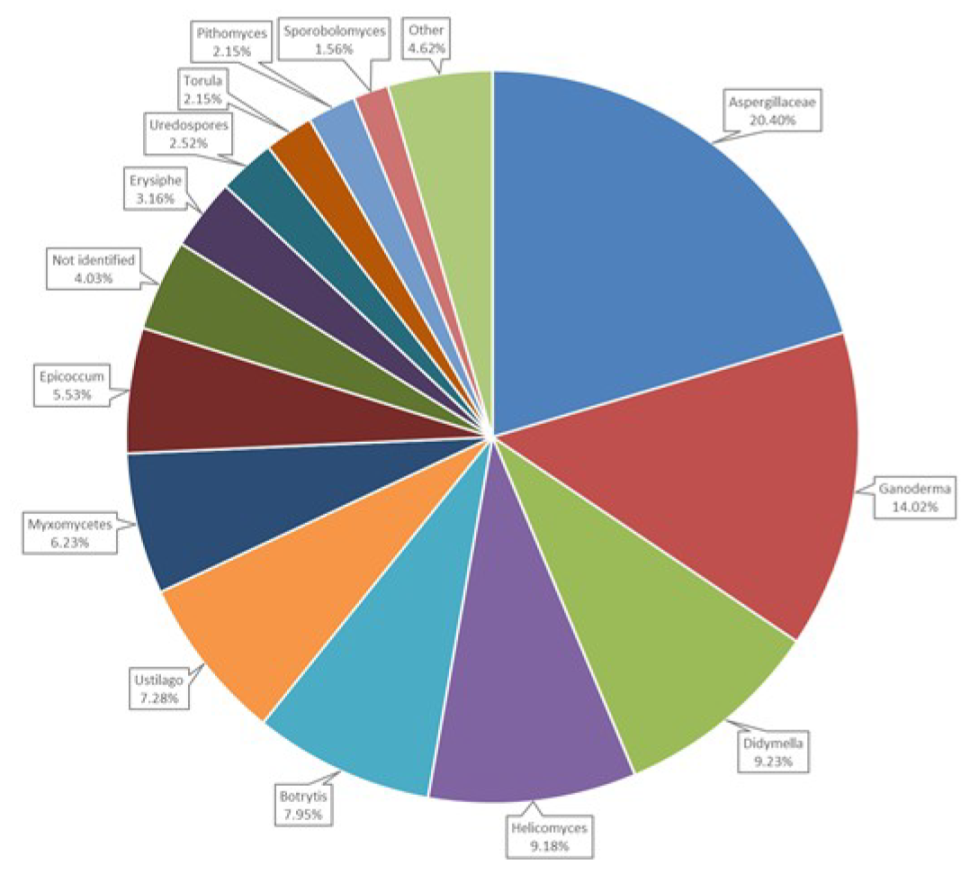
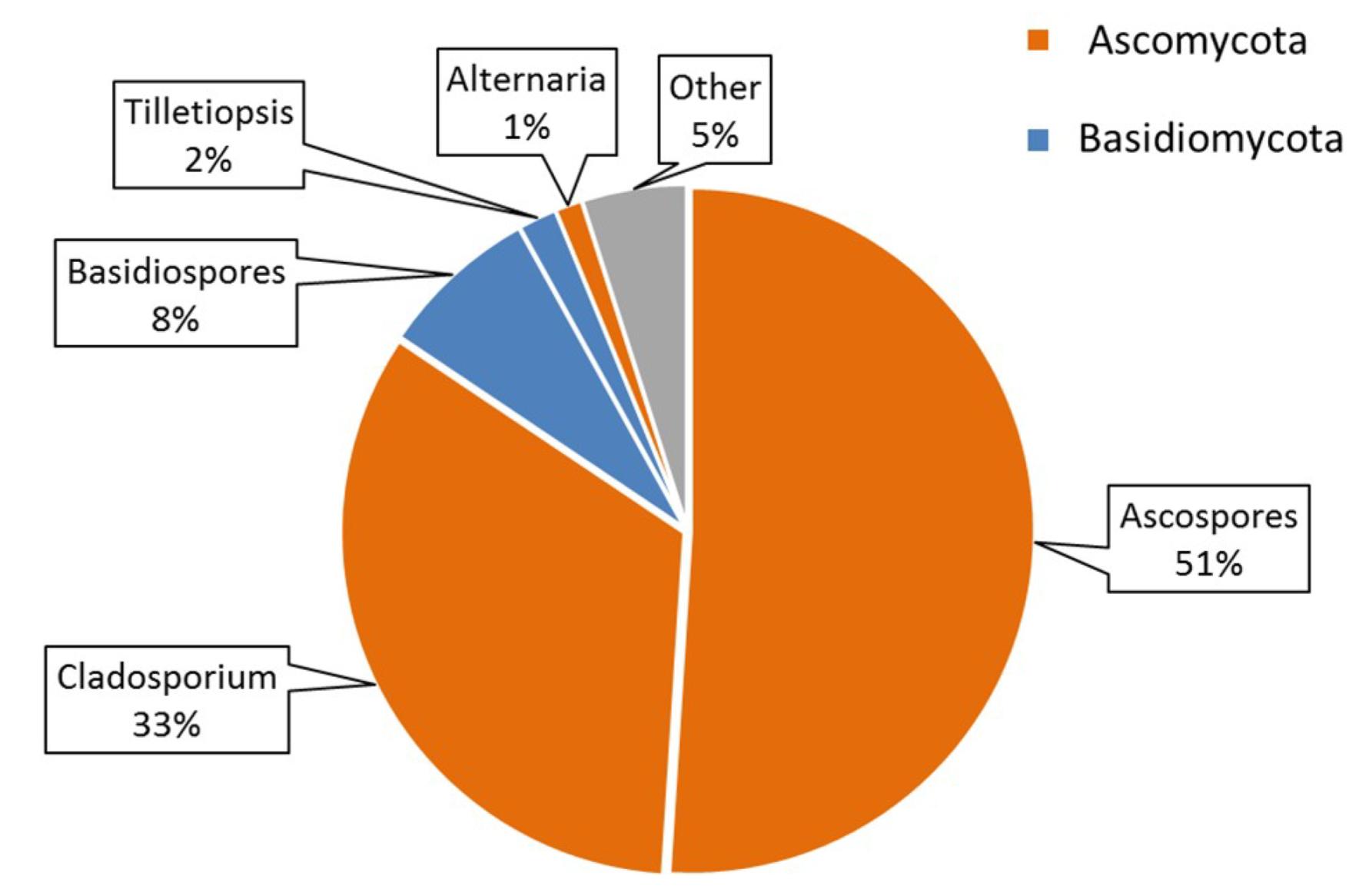
3.6. Airborne Fungal Spore Diversity at Saclay
4. Discussion
4.1. Factors Controling the Airborne Fungal Spore Concentrations
4.2. Interannuality and Global Increase of Specific AFSIn Concentrations
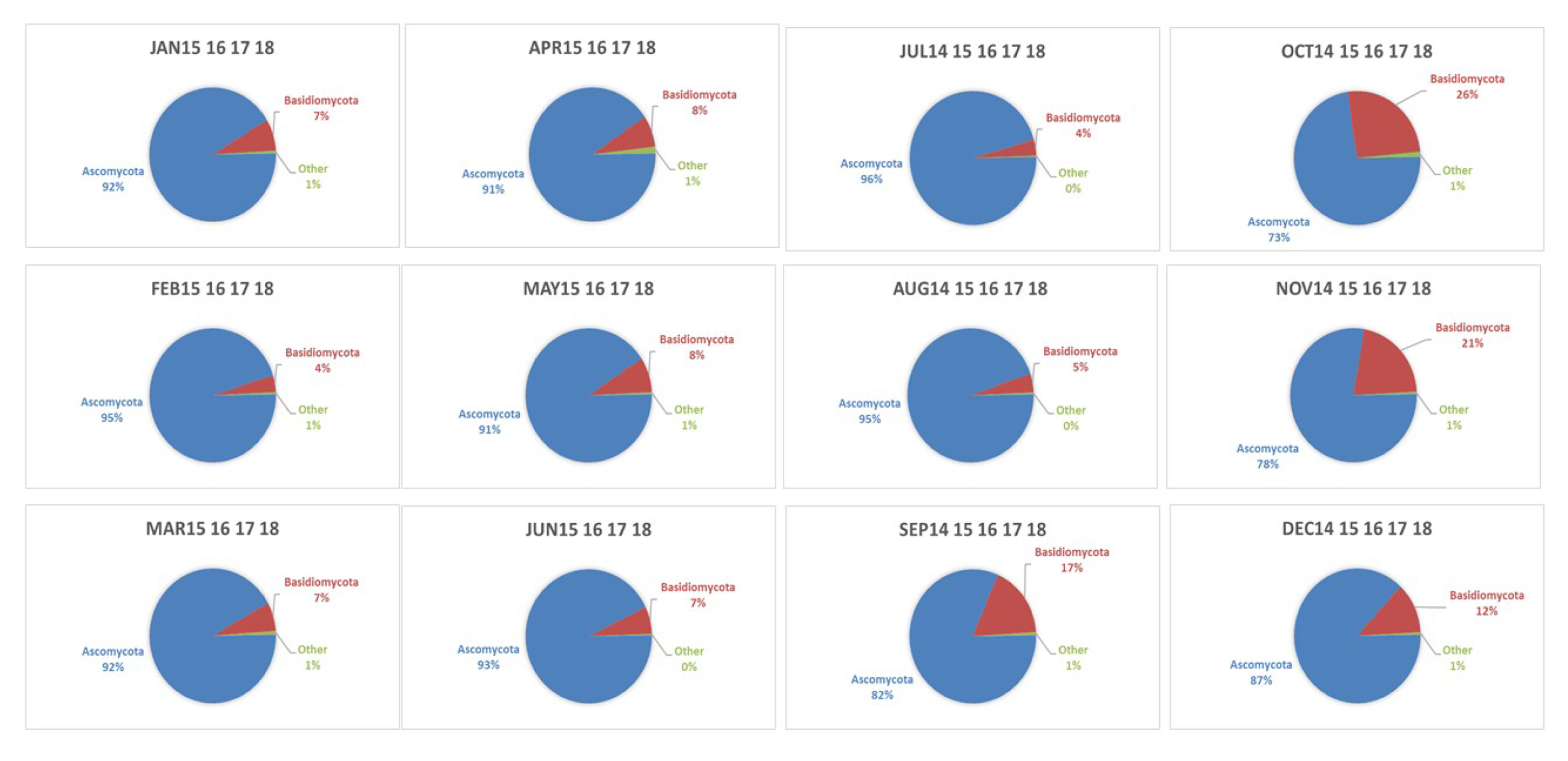
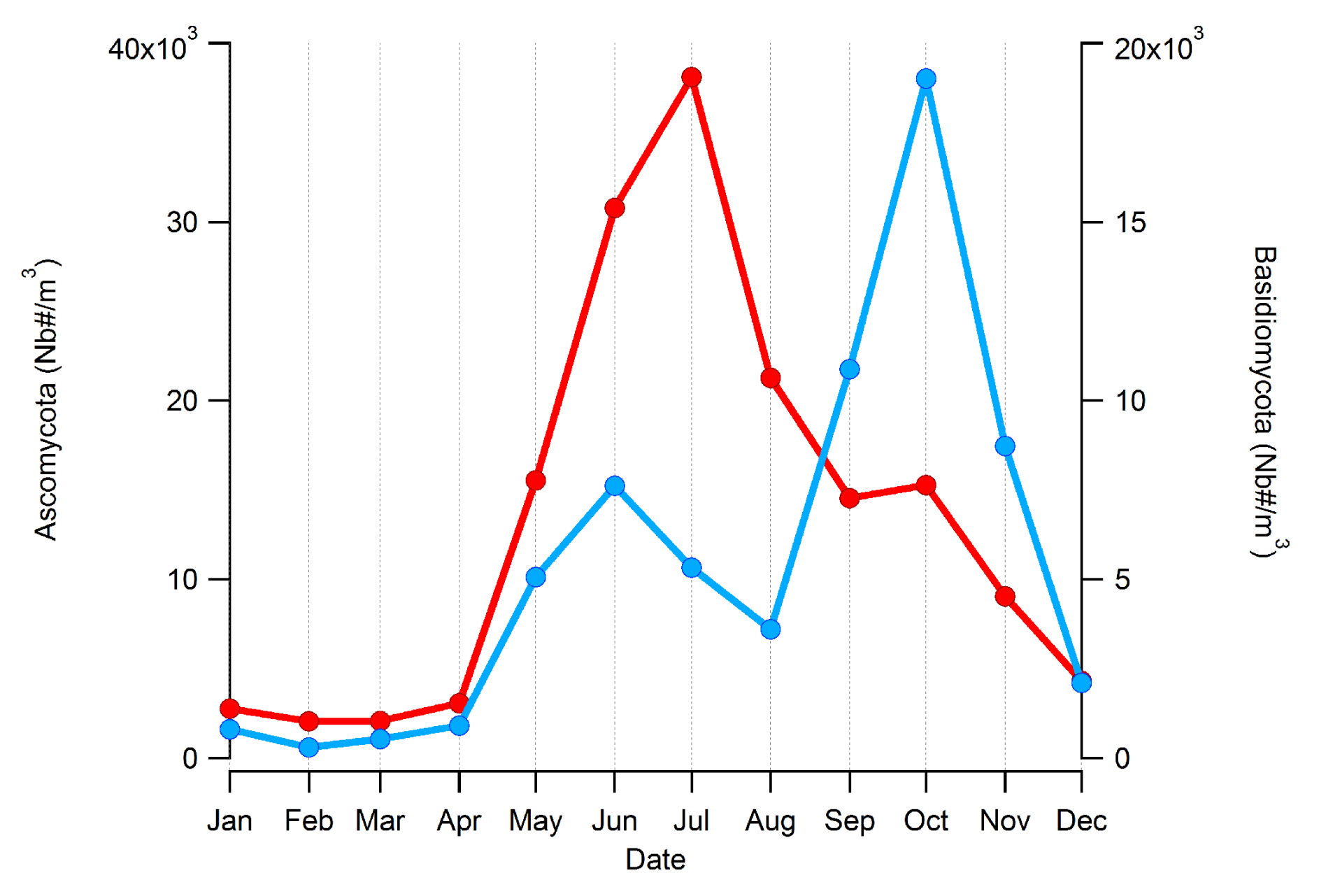
4.3. Seasonality of BMC and AMC
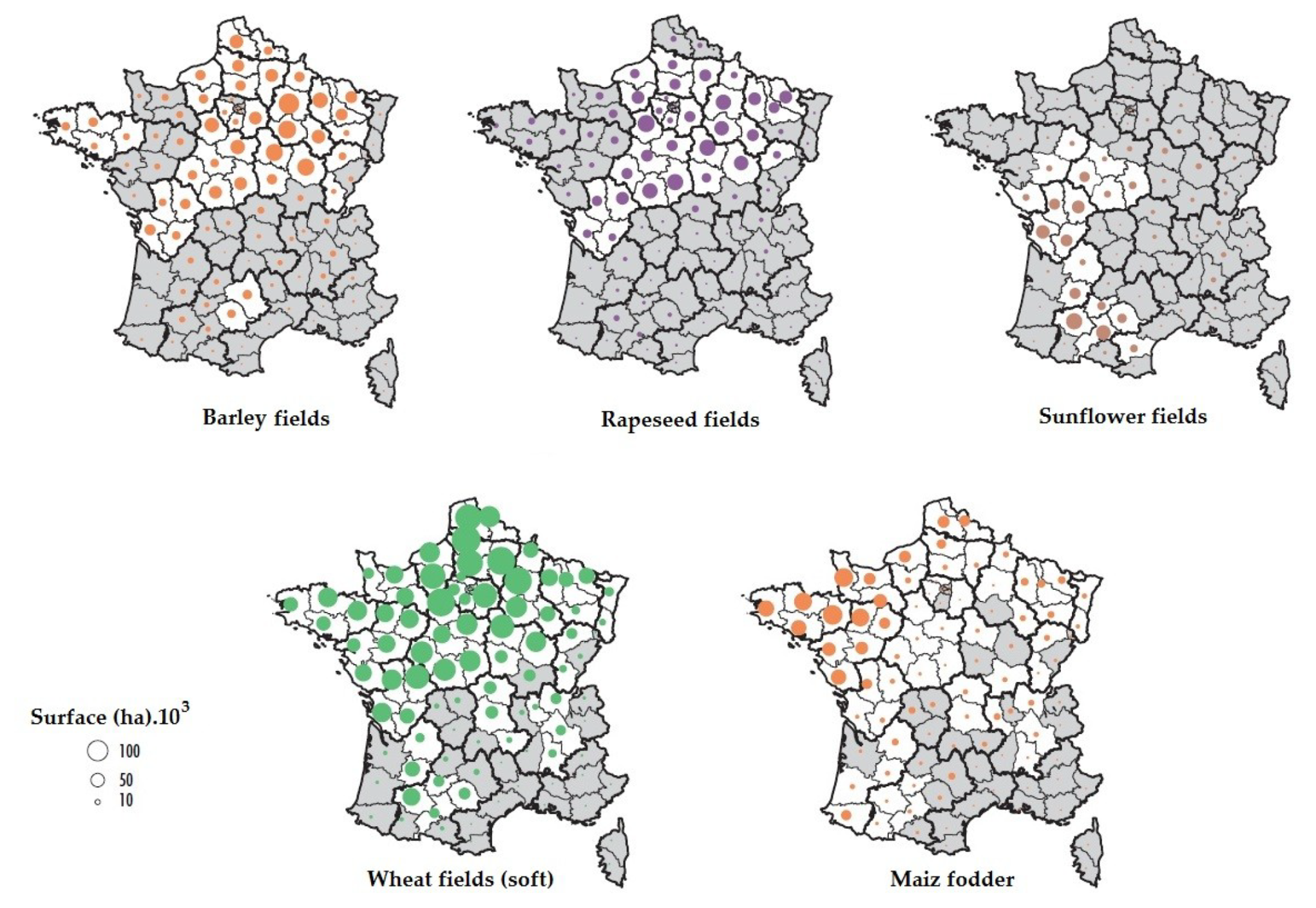
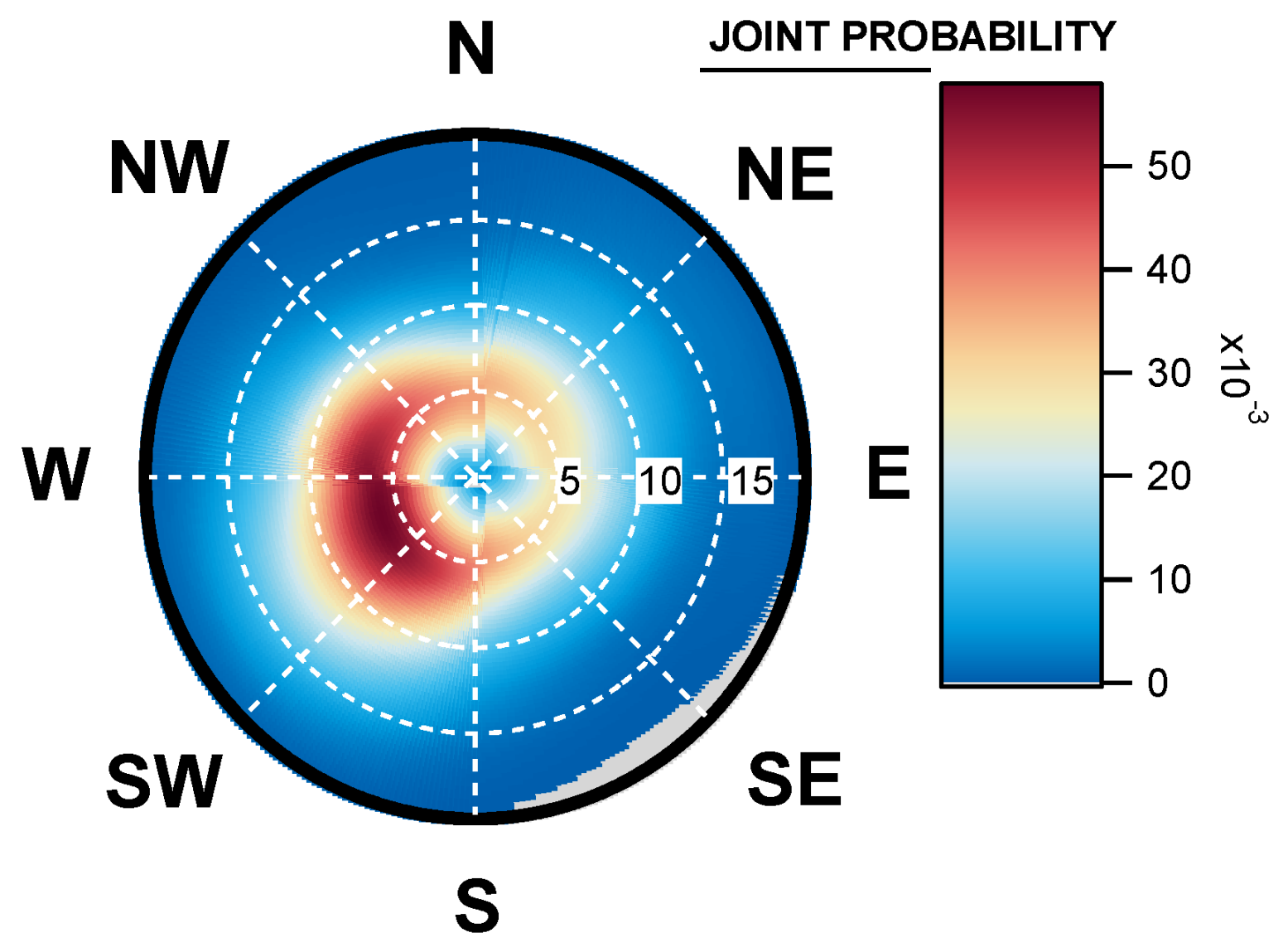
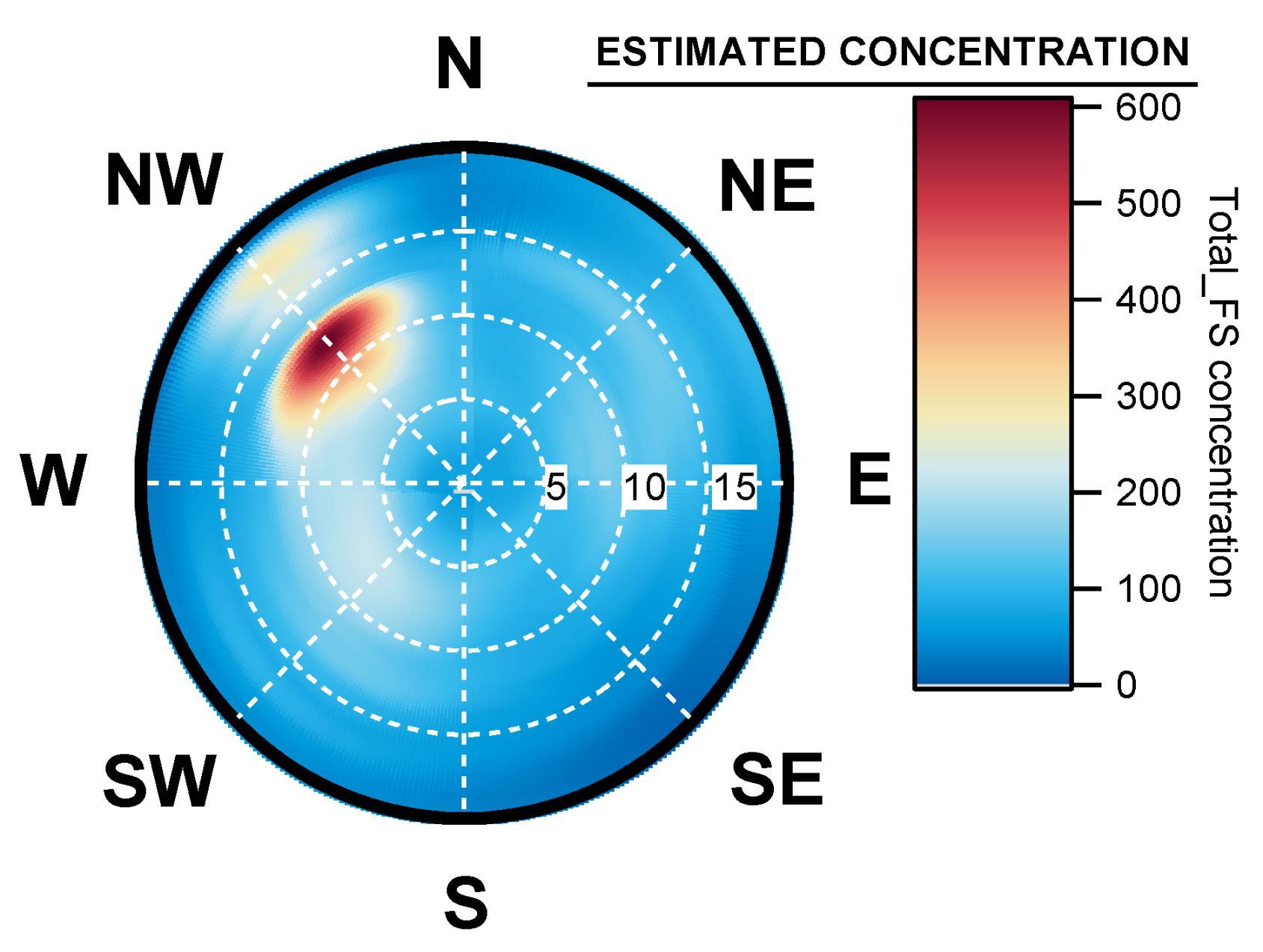
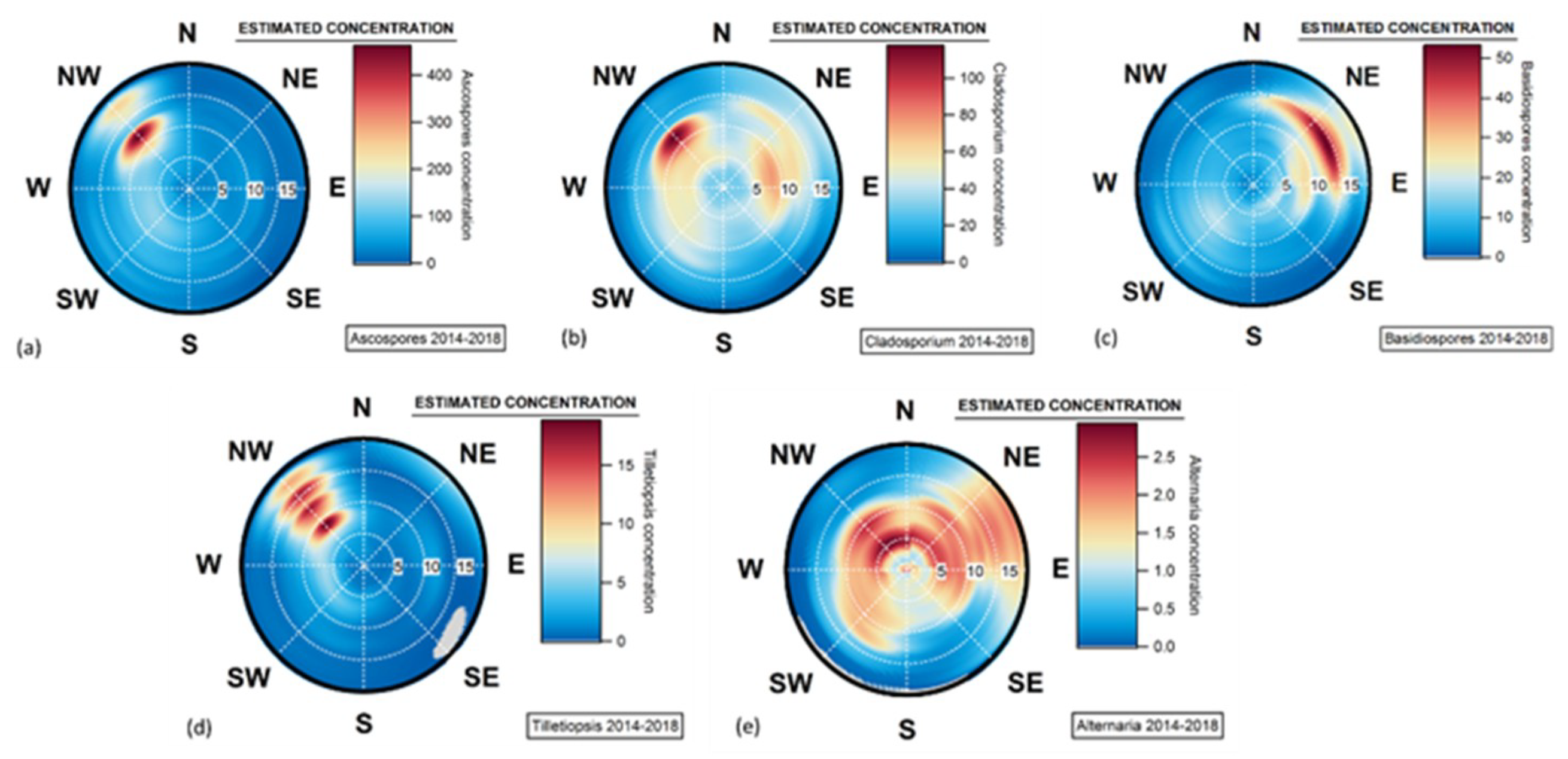
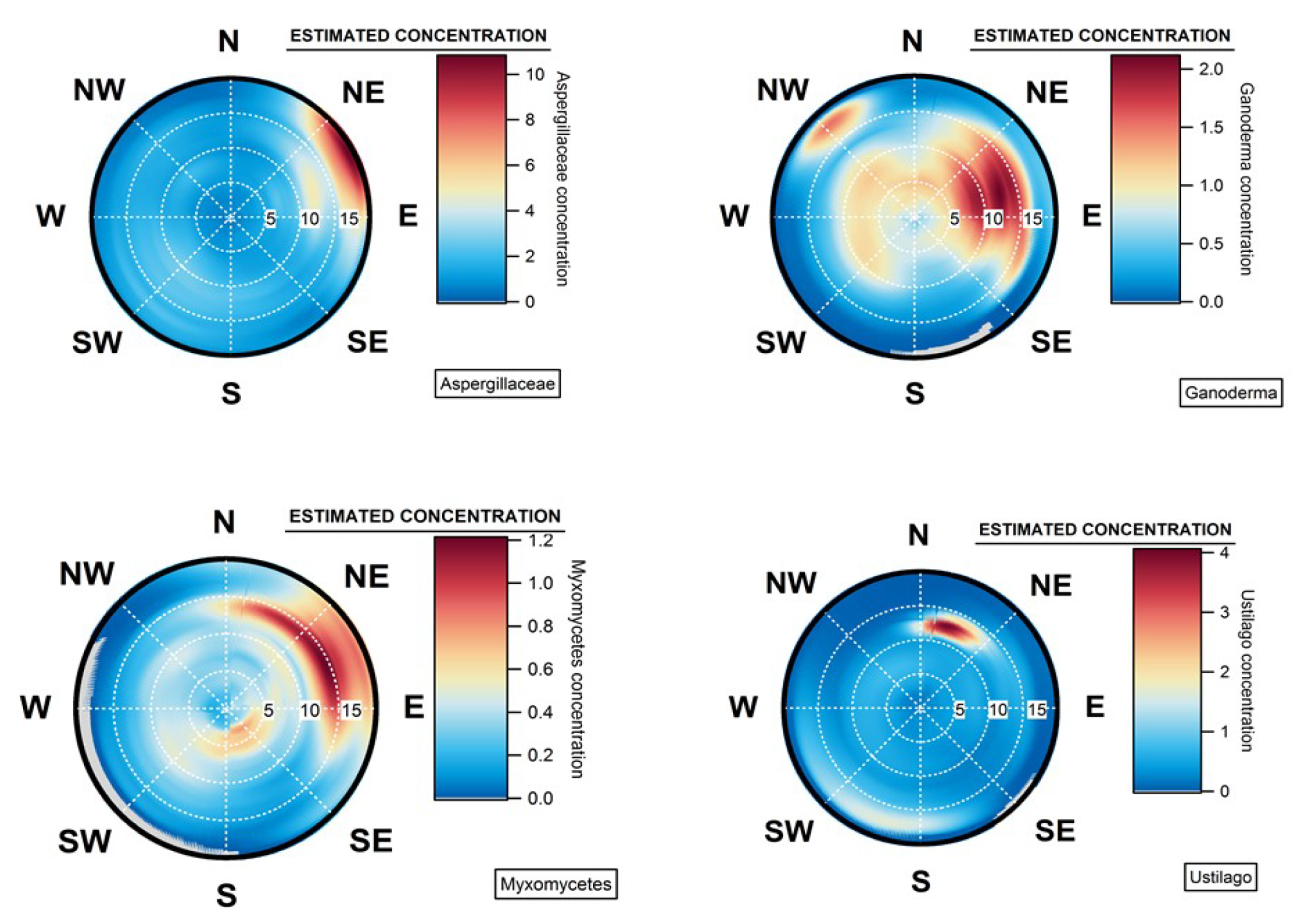
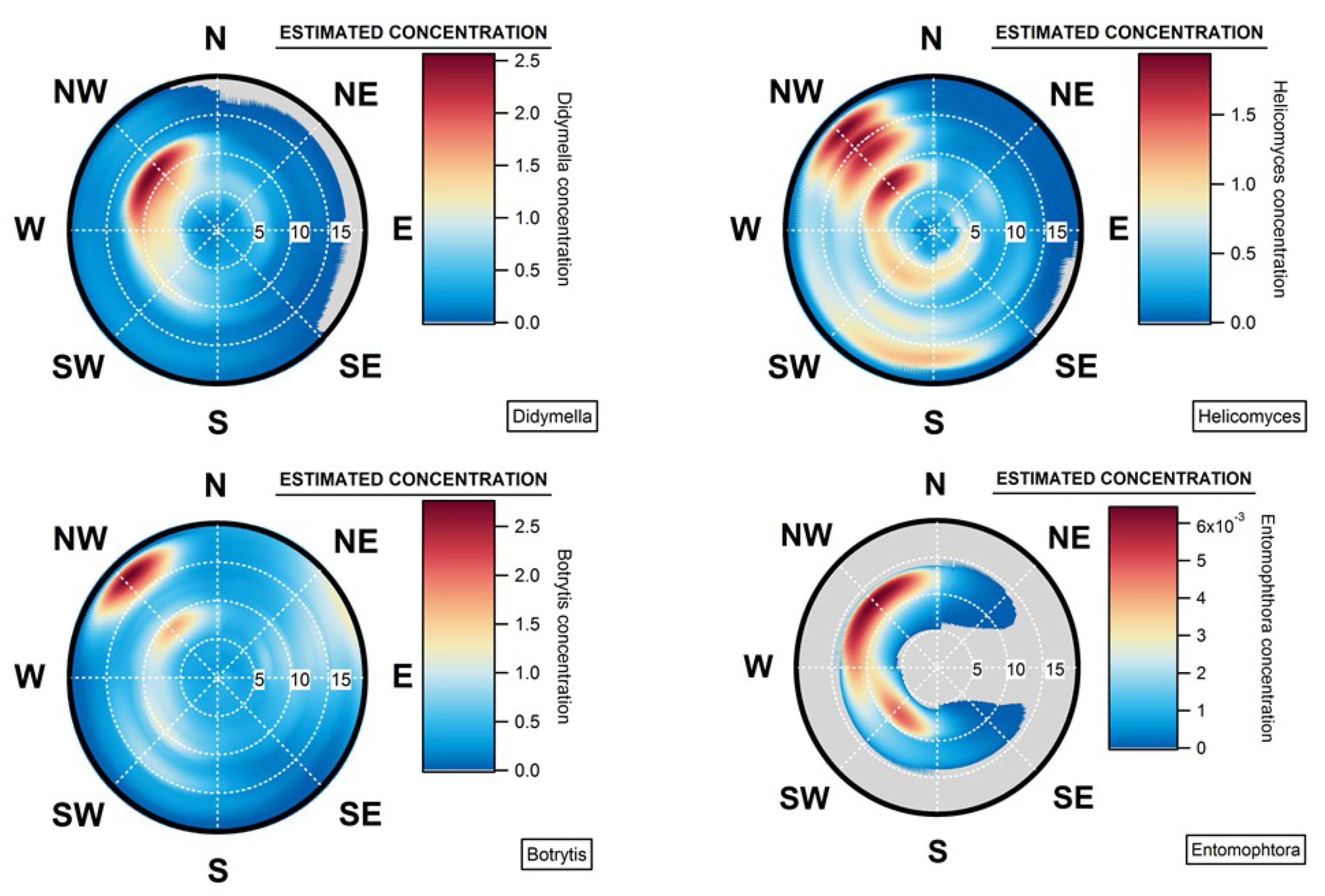
4.4. Geographical Origins of AFS Impacting the Observation Site
4.5. Case of Studies: The Geographical Origins of Plant or Human Pathogens
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| 2014–2018 (Nb#/m3) | |||
|---|---|---|---|
| Month | Mean | Max | Min |
| January | 63,355 | 99,765 | 30,315 |
| February | 71,159 | 174,322 | 18,289 |
| March | 82,535 | 222,756 | 27,654 |
| April | 116,672 | 297,432 | 46,570 |
| May | 380,782 | 469,114 | 235,434 |
| June | 655,789 | 1,170,577 | 446,747 |
| July | 834,069 | 1,838,943 | 247,726 |
| August | 470,549 | 711,466 | 326,907 |
| September | 373,688 | 472,874 | 258,106 |
| October | 440,451 | 678,167 | 251,567 |
| November | 243,986 | 437,597 | 157,354 |
| December | 103,865 | 154,363 | 66,760 |
| 2014–2018 | ||||
|---|---|---|---|---|
| Month | Mean AFS (Nb#/m3) | Mean T(°C) | Total Rain (mm) | Ratio (T/R) |
| January | 63,355 | 4.4 | 276 | 0.0158 |
| February | 71,159 | 4.3 | 194 | 0.0220 |
| March | 82,535 | 7.7 | 270 | 0.0283 |
| April | 116,672 | 11.3 | 221 | 0.0511 |
| May | 380,782 | 14.6 | 430 | 0.0340 |
| June | 655,789 | 18.4 | 276 | 0.0668 |
| July | 834,069 | 20.7 | 189 | 0.1092 |
| August | 470,549 | 19.7 | 302 | 0.0655 |
| September | 373,688 | 15.3 | 233 | 0.0655 |
| October | 440,451 | 11.7 | 182 | 0.0643 |
| November | 243,986 | 7.8 | 289 | 0.0272 |
| December | 103,865 | 5.8 | 262 | 0.0220 |
| 2014 | 2015 | 2016 | 2017 | 2018 | |
|---|---|---|---|---|---|
| Total AFS (Nb#/m3) | 5,395,236 | 2,982,527 | 4,038,344 | 4,351,542 | 2,465,766 |
| 5% | 160 | 149,126 | 201,917 | 217,577 | 123,288 |
| 95% | 4,500,000 | 2,833,401 | 3,836,427 | 4,133,965 | 2,342,478 |
| Start Season | 5 Apr | 30 Apr | 05 Apr | 23 Mar | 01 May |
| End Season | 15 Nov | 19 Nov | 21 Nov | 16 Nov | 27 Dec |
| Name | Phylum | Spore Type | Optical Spore Size (μm) | Habitat |
|---|---|---|---|---|
| Alternaria | Ascomycota | C | 20–80 | Soil, plants, and vegetables, mainly develop on decaying plants, especially cereals and hay. |
| Ascospores | Ascomycota | A | 15–40 | All the sexually produced fungal spores formed within an ascus. |
| Aspergillaceae | Ascomycota | A | 2–10 | Everywhere in nature (e.g., soil, decaying organic debris, and compost), in grains storage areas. |
| Basidiospores | Basidiomycota | B | 5–15 | Mainly in forests and woodlands, especially in the autumn. |
| Botrytis | Ascomycota | C | 5–10 | Ubiquitous, polyphagous, and necrotrophic, also abundant in soil. |
| Cercospora | Ascomycota | C | 2–5 × 50–325 | Leaf parasites of chard, sugar beet, carrot, lettuce, and maize. |
| Chaetomium | Ascomycota | A | 10 | On plant debris, soil, straw, and dung, also on wooden products. |
| Cladosporium | Ascomycota | C | 4–11 | Saprophyte, on soil, plants, and cereals, colonizes very varied substrates (foodstuff, paper, textile, etc.). |
| Didymella | Ascomycota | A | 3–15 × 1–4 | On the leaves of barley and wheat, berries and vegetable cultivation (e.g., peas, tomatoes, gherkins, and cucumbers). |
| Entomophthora | Zygomycota | C | 11–18 × 8–15 | On flies, causes a fatal disease. |
| Epicoccum | Ascomycota | C | 20 | On soil and senescent, dying or dead plants, especially cereals, beans, potatoes, peas, and peaches. |
| Erysiphe | Ascomycota | C | 30–60 | On plants, pathogens which cause powdery mildew. |
| Fusarium | Ascomycota | C | 25–68 × 3–6 | Mainly in cereal crops (grains, straw, and hay), also invade fresh fruits and vegetables. |
| Fusicladium | Ascomycota | A | On plants, and most are pathogens. | |
| Ganoderma | Basidiomycota | B | 6–10 | At the base and on stumps of deciduous trees, (oak, beech, and poplar), also on the roots of some fruit trees. |
| Helicomyces | Ascomycota | A and C | 70–140 × 2–3 and 14–21 | Most are aquatic, on marsh plants, on dead leaves, on grass stems, or on shelled wood. |
| Helminthosporium | Ascomycota | C | 40–118 × 11–20 | In humid areas, on grasses (above all barley and corn), also on dead branches and fallen branches of most trees and shrubs. |
| Myxomycetes | Mycetozoa | M | 5–24 | In open forests, on deadwood, the bark of living trees, rotting plant material, soil and animal excrements. |
| Nigrospora | Ascomycota | C | 13–15 × 10–13, 18–21 × 14–15, 18–24 | In air, soil, various decaying plants, and some cereal grains; it is rarely found growing indoors. |
| Peronospora | Chromista | S | 15–35 | Plant pathogens of herbaceous dicotyledonous plants, (mildew). |
| Pithomyces | Ascomycota | C | 15–25 | Ubiquitous on soil, also on dead leaves and fodder grasses, occasionally in indoor environment. |
| Pleospora | Ascomycota | A | 30–33 × 14–15 | A plant pathogen with a cosmopolitan distribution, infecting all kinds of herbaceous debris and crops. |
| Polythrincium | Ascomycota | C | 5 × 1.5 | On leaves, especially on leaves of red clover |
| Sporobolomyces | Basidiomycota | C | 5–25 | Yeast in air, on humans, mammals, birds, the environment, and plants. |
| Stemphylium | Ascomycota | C | 22–35 | In the agricultural environment, on dead tissues, animal or plant fibers (straw), also parasitic plants. |
| Tilletiopsis | Basidiomycota | T | 1–3 | Parasites of flowering plants. |
| Torula | Ascomycota | C | 3–4 | Yeast, on stems of dead herbaceous plants and on the leaves of barley and mature wheat. |
| Trichothecium | Ascomycota | C | 8–10 × 12–18 | Cosmopolitan, in various habitats ranging from leaf litter to fruit crops. |
| Uredospores | Basidiomycota | U | 15–25 | Parasite of many plant families, cereals are the most common hosts. |
| Ustilago | Basidiomycota | T | 5–10 | Parasite of herbs (Poaceae), mainly cereals (maize and teosinte). |
| 2014–2018 | 2014 | 2015 | 2016 | 2017 | 2018 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Avg (Nb#/m3) | (%) | Avg (Nb#/m3) | (%) | Avg (Nb#/m3) | (%) | Avg (Nb#/m3) | (%) | Avg (Nb#/m3) | (%) | Avg (Nb#/m3) | (%) | |
| Alternaria | 257 | 2.377 | 277 | 1.386 | 63 | 0.746 | 106 | 0.958 | 202 | 1.696 | 115 | 1.604 |
| Ascospores | 5568 | 51.548 | 11,560 | 57.881 | 3418 | 40.519 | 6888 | 62.082 | 5784 | 48.474 | 2686 | 37.407 |
| Aspergillaceae | 181 | 1.679 | 124 | 0.620 | 87 | 1.031 | 104 | 0.942 | 145 | 1.217 | 88 | 1.222 |
| Unidentified | 118 | 1.097 | 51 | 0.254 | 36 | 0.422 | 21 | 0.190 | 12 | 0.098 | 3 | 0.046 |
| Basidiospores | 960 | 8.887 | 1284 | 6.427 | 1099 | 13.030 | 449 | 4.049 | 1243 | 10.413 | 213 | 2.969 |
| Botrytis | 109 | 1.011 | 56 | 0.282 | 17 | 0.200 | 98 | 0.885 | 27 | 0.227 | 20 | 0.284 |
| Cercospora | 52 | 0.480 | 6 | 0.030 | 6 | 0.069 | 11 | 0.099 | 11 | 0.090 | 6 | 0.081 |
| Chaetomium | 33 | 0.306 | 0 | 0.001 | 1 | 0.012 | 1 | 0.006 | 0 | 0.003 | 1 | 0.016 |
| Cladosporium | 3731 | 34.544 | 5795 | 29.015 | 3289 | 38.989 | 2784 | 25.089 | 3888 | 32.585 | 3644 | 50.744 |
| Didymella | 190 | 1.760 | 4 | 0.022 | 31 | 0.364 | 56 | 0.501 | 89 | 0.744 | 47 | 0.657 |
| Entomophthora | 52 | 0.481 | 0 | 0.000 | 0 | 0.000 | 0 | 0.003 | 0 | 0.000 | 0 | 0.000 |
| Epicoccum | 80 | 0.743 | 77 | 0.384 | 26 | 0.305 | 24 | 0.217 | 28 | 0.238 | 17 | 0.239 |
| Erysiphe | 65 | 0.602 | 19 | 0.096 | 26 | 0.302 | 22 | 0.199 | 10 | 0.085 | 9 | 0.130 |
| Fusarium | 36 | 0.337 | 0 | 0.001 | 3 | 0.034 | 2 | 0.019 | 1 | 0.012 | 0 | 0.002 |
| Fusicladium | 34 | 0.314 | 3 | 0.017 | 4 | 0.045 | 1 | 0.010 | 2 | 0.018 | 2 | 0.025 |
| Ganoderma | 131 | 1.212 | 141 | 0.704 | 65 | 0.772 | 64 | 0.575 | 74 | 0.619 | 68 | 0.948 |
| Helicomyces | 225 | 2.084 | 15 | 0.077 | 38 | 0.453 | 58 | 0.523 | 61 | 0.510 | 60 | 0.837 |
| Helminthosporium | 32 | 0.297 | 1 | 0.007 | 1 | 0.010 | 0 | 0.002 | 1 | 0.008 | 0 | 0.006 |
| Myxomycetes | 92 | 0.854 | 44 | 0.220 | 19 | 0.220 | 28 | 0.252 | 40 | 0.334 | 44 | 0.609 |
| Nigrospora | 29 | 0.266 | 1 | 0.007 | 1 | 0.016 | 0 | 0.001 | 1 | 0.006 | 0 | 0.006 |
| Peronospora | 45 | 0.420 | 16 | 0.078 | 2 | 0.021 | 2 | 0.017 | 5 | 0.040 | 2 | 0.022 |
| Pithomyces | 136 | 1.260 | 21 | 0.103 | 5 | 0.055 | 12 | 0.107 | 11 | 0.095 | 15 | 0.203 |
| Pleospora | 52 | 0.481 | 0 | 0.000 | 0 | 0.004 | 0 | 0.002 | 0 | 0.000 | 0 | 0.000 |
| Polythrincium | 40 | 0.373 | 22 | 0.109 | 3 | 0.030 | 3 | 0.029 | 6 | 0.048 | 2 | 0.026 |
| Sporobolomyces | 160 | 1.477 | 43 | 0.218 | 15 | 0.179 | 0 | 0.000 | 0 | 0.001 | 0 | 0.000 |
| Stemphylium | 29 | 0.272 | 2 | 0.010 | 1 | 0.008 | 1 | 0.007 | 1 | 0.010 | 0 | 0.006 |
| Tilletiopsis | 393 | 3.640 | 291 | 1.457 | 103 | 1.221 | 306 | 2.758 | 231 | 1.932 | 98 | 1.360 |
| Torula | 49 | 0.456 | 23 | 0.115 | 9 | 0.112 | 12 | 0.105 | 12 | 0.103 | 7 | 0.101 |
| Trichothecium | 49 | 0.453 | 1 | 0.004 | 0 | 0.000 | 1 | 0.007 | 0 | 0.001 | 0 | 0.000 |
| Uredospores | 64 | 0.595 | 44 | 0.221 | 8 | 0.093 | 15 | 0.133 | 8 | 0.069 | 8 | 0.109 |
| Ustilago | 120 | 1.109 | 51 | 0.256 | 62 | 0.737 | 26 | 0.234 | 39 | 0.324 | 25 | 0.343 |
| Alternaria | Ascospores | Basidiospores | Cladosporium | Tilletiopsis | |
|---|---|---|---|---|---|
| 17/06/2015 | 0 | 746 | 52 | 3907 | 26 |
| 18/06/2015 | 0 | 1311 | 78 | 2313 | 257 |
| 19/06/2015 | 78 | 6194 | 0 | 5989 | 1106 |
| 20/06/2015 | 78 | 1157 | 103 | 5166 | 155 |
| 21/06/2015 | 103 | 6888 | 180 | 7068 | 2699 |
| 22/06/2015 | 26 | 5397 | 257 | 5269 | 206 |
| 23/06/2015 | 0 | 205,755 | 78 | 13,133 | 1799 |
| 24/06/2015 | 0 | 1208 | 155 | 16,037 | 309 |
| 25/06/2015 | 78 | 1311 | 129 | 18,068 | 52 |
| 26/06/2015 | 257 | 1028 | 52 | 16,962 | 0 |
| 27/06/2015 | 52 | 360 | 26 | 13,005 | 0 |
Appendix B



References
- Fröhlich-Nowoisky, J.; Kampf, C.J.; Weber, B.; Huffman, J.A.; Pöhlker, C.; Andreae, M.O.; Lang-Yona, N.; Burrows, S.M.; Gunthe, S.S.; Elbert, W. Bioaerosols in the Earth system: Climate, health, and ecosystem interactions. Atmos. Res. 2016, 182, 346–376. [Google Scholar] [CrossRef]
- Romsdahl, J.; Blachowicz, A.; Chiang, A.J.; Singh, N.; Stajich, J.E.; Kalkum, M.; Venkateswaran, K.; Wang, C.C. Characterization of Aspergillus niger Isolated from the International Space Station. MSystems 2018, 3, e00112-18. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.H.; Si, M.; Chou, C.; Irish, V.E.; Dickie, R.; Elizondo, P.; Wong, R.; Brintnell, M.; Elsasser, M.; Lassar, W.M.; et al. Size-resolved measurements of ice-nucleating particles at six locations in North America and one in Europe. Atmos. Chem. Phys. 2016, 16, 1637–1651. [Google Scholar] [CrossRef]
- Núñez, A.; Amo de Paz, G. Monitoring of the airborne biological particles in outdoor atmosphere. Part 1: Importance, variability and ratios. Int. Microbiol. 2016, 19, 1–13. [Google Scholar]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186. [Google Scholar] [CrossRef]
- Eduard, W. Fungal spores: A critical review of the toxicological and epidemiological evidence as a basis for occupational exposure limit setting. Crit. Rev. Toxicol. 2009, 39, 799–864. [Google Scholar] [CrossRef] [PubMed]
- Vélez-Pereira, A.M.; De Linares, C.; Delgado, R.; Belmonte, J. Temporal trends of the airborne fungal spores in Catalonia (NE Spain), 1995–2013. Aerobiologia 2016, 32, 23–37. [Google Scholar] [CrossRef]
- Poschl, U.; Martin, S.T.; Sinha, B.; Chen, Q.; Gunthe, S.S.; Huffman, J.A.; Borrmann, S.; Farmer, D.K.; Garland, R.M.; Helas, G.; et al. Rainforest aerosols as biogenic nuclei of clouds and precipitation in the Amazon. Science 2010, 329, 1513–1516. [Google Scholar] [CrossRef]
- Iannone, R.; Chernoff, D.I.; Pringle, A.; Martin, S.T.; Bertram, A.K. The ice nucleation ability of one of the most abundant types of fungal spores found in the atmosphere. Atmos. Chem. Phys. Discuss. 2010, 10, 24621–24650. [Google Scholar] [CrossRef]
- Dufrêne, Y.F. Direct characterization of the physicochemical properties of fungal spores using functionalized AFM probes. Biophys. J. 2000, 78, 3286–3291. [Google Scholar] [CrossRef]
- Bauer, H.; Schueller, E.; Weinke, G.; Berger, A.; Hitzenberger, R.; Marr, I.L.; Puxbaum, H. Significant contributions of fungal spores to the organic carbon and to the aerosol mass balance of the urban atmospheric aerosol. Atmos. Environ. 2008, 42, 5542–5549. [Google Scholar] [CrossRef]
- Dales, R.E.; Cakmak, S.; Burnett, R.T.; Judek, S.; Coates, F.; Brook, J.R. Influence of ambient fungal spores on emergency visits for asthma to a regional children’s hospital. Am. J. Respir. Crit. Care Med. 2000, 162, 2087–2090. [Google Scholar] [CrossRef] [PubMed]
- Dauvillier, J.; Woort, F.; van Erck-Westergren, E. Fungi in respiratory samples of horses with inflammatory airway disease. J. Vet. Intern. Med. 2019, 33, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Gioulekas, D.; Damialis, A.; Mpalafoutis, C.; Papakosta, D.; Giouleka, P.; Patakas, D. Allergenic fungal spore records (15 years) and relationship with meteorological parameters in Thessaloniki, Greece. Allergy Clin. Immunol. Int. J. World Allergy Organ. 2004, 16, 52–59. [Google Scholar] [CrossRef]
- Fröhlich-Nowoisky, J.; Burrows, S.M.; Xie, Z.; Engling, G.; Solomon, P.A.; Fraser, M.P.; Mayol-Bracero, O.L.; Artaxo, P.; Begerow, D.; Conrad, R.; et al. Biogeography in the air: Fungal diversity over land and oceans. Biogeosciences 2012, 9, 1125–1136. [Google Scholar] [CrossRef]
- Spracklen, D.V.; Heald, C.L. The contribution of fungal spores and bacteria to regional and global aerosol number and ice nucleation immersion freezing rates. Atmos. Chem. Phys. 2014, 14, 9051–9059. [Google Scholar] [CrossRef]
- Bauer, H.; Claeys, M.; Vermeylen, R.; Schueller, E.; Weinke, G.; Berger, A.; Puxbaum, H. Arabitol and mannitol as tracers for the quantification of airborne fungal spores. Atmos. Environ. 2008, 42, 588–593. [Google Scholar] [CrossRef]
- Elbert, W.; Taylor, P.E.; Andreae, M.O.; Pöschl, U. Contribution of fungi to primary biogenic aerosols in the atmosphere: Wet and dry discharged spores, carbohydrates, and inorganic ions. Atmos. Chem. Phys. 2007, 7, 4569–4588. [Google Scholar] [CrossRef]
- Heald, C.L.; Spracklen, D.V. Atmospheric budget of primary biological aerosol particles from fungal spores. Geophys. Res. Lett. 2009, 36, L09806. [Google Scholar] [CrossRef]
- Di Filippo, P.; Pomata, D.; Riccardi, C.; Buiarelli, F.; Perrino, C. Fungal contribution to size-segregated aerosol measured through biomarkers. Atmos. Environ. 2013, 64, 132–140. [Google Scholar] [CrossRef]
- Hirst, J.M. An automatic volumetric spore trap. Ann. Appl. Biol. 1952, 39, 257–265. [Google Scholar] [CrossRef]
- Kellogg, C.A.; Griffin, D.W. Aerobiology and the global transport of desert dust. Trends Ecol. Evol. 2006, 21, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Almaguer-Chávez, M.; Aira, M.; Rojas, T.-I.; Fernández-González, M.; Rodríguez-Rajo, F.-J. New findings of airborne fungal spores in the atmosphere of Havana, Cuba, using aerobiological non-viable methodology. Ann. Agric. Environ. Med. 2018, 25, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.W. Atmospheric movement of microorganisms in clouds of desert dust and implications for human health. Clin. Microbiol. Rev. 2007, 20, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Ouédraogo, S.J.; Bayala, J.; Dembélé, C.; Kaboré, A.; Kaya, B.; Niang, A.; Somé, A.N. Establishing jujube trees in sub-Saharan Africa: Response of introduced and local cultivars to rock phosphate and water supply in Burkina Faso, West Africa. Agrofor. Syst. 2006, 68, 69–80. [Google Scholar] [CrossRef]
- Middleton, N.J.; Goudie, A.S. Saharan dust: Sources and trajectories. Trans. Inst. Br. Geogr. 2001, 26, 165–181. [Google Scholar] [CrossRef]
- Kaufman, Y.J. Dust transport and deposition observed from the Terra-Moderate Resolution Imaging Spectroradiometer (MODIS) spacecraft over the Atlantic Ocean. J. Geophys. Res. 2005, 110, D10S12. [Google Scholar] [CrossRef]
- Brefort, T.; Doehlemann, G.; Mendoza-Mendoza, A.; Reissmann, S.; Djamei, A.; Kahmann, R. Ustilago maydis as a pathogen. Annu. Rev. Phytopathol. 2009, 47, 423–445. [Google Scholar] [CrossRef]
- Simon-Nobbe, B.; Denk, U.; Pöll, V.; Rid, R.; Breitenbach, M. The spectrum of fungal allergy. Int. Arch. Allergy Immunol. 2008, 145, 58–86. [Google Scholar] [CrossRef]
- Borrego, S.F.; Molina, A. Determination of viable allergenic fungi in the documents repository environment of the national archive of Cuba. Austin J. Public Health Epidemiol. 2018, 5, 1077. [Google Scholar]
- Sarda-Estève, R.; Roux, J. Physico chemical qualification and refinements of a new portable bio aerosol collector. Institute of Physics (IOP). In Proceedings of the Charged Aerosols Conference, London, UK, 20 November 2013. [Google Scholar]
- Van Leuken, J.P.G.; Swart, A.N.; Havelaar, A.H.; Van Pul, A.; Van der Hoek, W.; Heederik, D. Atmospheric dispersion modelling of bioaerosols that are pathogenic to humans and livestock–A review to inform risk assessment studies. Microb. Risk Anal. 2016, 1, 19–39. [Google Scholar] [CrossRef]
- Douglas, P.; Hayes, E.T.; Williams, W.B.; Tyrrel, S.F.; Kinnersley, R.P.; Walsh, K.; O’Driscoll, M.; Longhurst, P.J.; Pollard, S.J.T.; Drew, G.H. Use of dispersion modelling for Environmental Impact Assessment of biological air pollution from composting: Progress, problems and prospects. Waste Manag. 2017, 70, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Ritenberga, O.; Sofiev, M.; Siljamo, P.; Saarto, A.; Dahl, A.; Ekebom, A.; Sauliene, I.; Shalaboda, V.; Severova, E.; Hoebeke, L.; et al. A statistical model for predicting the inter-annual variability of birch pollen abundance in Northern and North-Eastern Europe. Sci. Total Environ. 2018, 615, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Grinn-Gofroń, A.; Nowosad, J.; Bosiacka, B.; Camacho, I.; Pashley, C.; Belmonte, J.; De Linares, C.; Ianovici, N.; Manzano, J.M.M.; Sadyś, M.; et al. Airborne Alternaria and Cladosporium fungal spores in Europe: Forecasting possibilities and relationships with meteorological parameters. Sci. Total Environ. 2019, 653, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Oteros, J.; García-Mozo, H.; Alcázar, P.; Belmonte, J.; Bermejo, D.; Boi, M.; Cariñanos, P.; Díaz de la Guardia, C.; Fernández-González, D.; González-Minero, F.; et al. A new method for determining the sources of airborne particles. J. Environ. Manag. 2015, 155, 212–218. [Google Scholar] [CrossRef]
- Feeney, P.; Rodríguez, S.F.; Molina, R.; McGillicuddy, E.; Hellebust, S.; Quirke, M.; Daly, S.; O’Connor, D.; Sodeau, J. A comparison of on-line and off-line bioaerosol measurements at a biowaste site. Waste Manag. 2018, 76, 323–338. [Google Scholar] [CrossRef]
- Petit, J.-E.; Favez, O.; Albinet, A.; Canonaco, F. A user-friendly tool for comprehensive evaluation of the geographical origins of atmospheric pollution: Wind and trajectory analyses. Environ. Model. Softw. 2017, 88, 183–187. [Google Scholar] [CrossRef]
- Sarda-Estève, R.; Baisnée, D.; Guinot, B.; Petit, J.-E.; Sodeau, J.; O’Connor, D.; Besancenot, J.-P.; Thibaudon, M.; Gros, V. Temporal variability and geographical origins of airborne pollen grains concentrations from 2015 to 2018 at Saclay, France. Remote Sens. 2018, 10, 1932. [Google Scholar] [CrossRef]
- Chakraborty, P.; Ghosal, K.; Sengupta, K.; Karak, P.; Sarkar, E. Airborne fungal spores in a suburban area of Eastern India with reference to their allergenic potential and effect on asthma related hospitalisation. J. Palynol. Vol. 2018, 54, 76. [Google Scholar]
- Lastra, C.C.L.; Siri, A.; García, J.J.; Eilenberg, J.; Humber, R.A. Entomophthora ferdinandii (Zygomycetes: Entomophthorales) causing natural infections of Musca domestica (Diptera: Muscidae) in Argentina. Mycopathologia 2006, 161, 251–254. [Google Scholar] [CrossRef]
- Sarda-Esteve, R.; Gallagher, M.; Huffman, J.; Poeschl, U.; Su, H.; Kiselev, D.; Saari, S.; Sodeau, J.; O’Connor, D.; Mcmeeking, G. International inter-comparison of laser/light-induced fluorescence (L/LIF) methods for the real-time detection of bioaerosols: BIODETECT 2014 campaign at CEA/LSCE ACTRIS SUPERSITE (Saclay, France).; 2014. In Proceedings of the American Association for Aerosol Research (AAAR) Annual Conference, Orlando, FL, USA, 20–24 October 2014. [Google Scholar]
- Crenn, V.; Sciare, J.; Croteau, L.; Verlhac, S.; Fröhlich, R.; Belis, A.; Aas, W.; Äijälä, M.; Alastuey, A.; Artiñano, B. ACTRIS ACSM intercomparison–Part 1: Reproducibility of concentration and fragment results from 13 individual quadrupole aerosol chemical speciation monitors (Q-ACSM) and consistency with co-located instruments. Atmos. Meas. Tech. 2014, 8, 5063–5087. [Google Scholar] [CrossRef]
- LANZONI Volumetric Pollen and Particle Sampler, VPPS 2000, User’s Manual. pp. 1–19. Available online: https://www.lanzoni.it/campionatore-pollini (accessed on 30 November 2018).
- Käpylä, M.; Penttinen, A. An evaluation of the microscopical counting methods of the tape in Hirst-Burkard pollen and spore trap. Grana 1981, 20, 131–141. [Google Scholar] [CrossRef]
- Galán, C.; Smith, M.; Thibaudon, M.; Frenguelli, G.; Oteros, J.; Gehrig, R.; Berger, U.; Clot, B.; Brandão, R. EAS QC working group Pollen monitoring: Minimum requirements and reproducibility of analysis. Aerobiologia 2014, 30, 385–395. [Google Scholar]
- Galán, C.; Ariatti, A.; Bonini, M.; Clot, B.; Crouzy, B.; Dahl, A.; Fernandez-González, D.; Frenguelli, G.; Gehrig, R.; Isard, S.; et al. Recommended terminology for aerobiological studies. Aerobiologia 2017, 33, 293–295. [Google Scholar] [CrossRef]
- Henry, R.; Norris, G.A.; Vedantham, R.; Turner, J.R. Source region identification using kernel smoothing. Environ. Sci. Technol. 2009, 43, 4090–4097. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.A.; Vedantham, R.; Norris, G.A.; Brown, S.G.; Roberts, P. Determining source impacts near roadways using wind regression and organic source markers. Atmos. Environ. 2012, 47, 261–268. [Google Scholar] [CrossRef]
- Yamartino, R.J. A comparison of several “single-pass” estimators of the standard deviation of wind direction. J. Clim. Appl. Meteorol. 1984, 23, 1362–1366. [Google Scholar] [CrossRef]
- Meteo France. Available online: http://www.meteofrance.fr/climat-passe-et-futur/climat-en-france (accessed on 4 March 2019).
- Nikkels, A.; Terstegge, P.; Spieksma, F.T.M. Ten types of microscopically identifiable airborne fungal spores at Leiden, The Netherlands. Aerobiologia 1996, 12, 107–112. [Google Scholar] [CrossRef]
- Damialis, A.; Vokou, D.; Gioulekas, D.; Halley, J.M. Long-term trends in airborne fungal-spore concentrations: A comparison with pollen. Fungal Ecol. 2015, 13, 150–156. [Google Scholar] [CrossRef]
- Herrero, A.D.; Ruiz, S.S.; Bustillo, M.G.; Morales, P.C. Study of airborne fungal spores in Madrid, Spain. Aerobiologia 2006, 22, 135–142. [Google Scholar] [CrossRef]
- Lin, W.-H.; Li, C.-S. Associations of fungal aerosols, air pollutants, and meteorological factors. Aerosol Sci. Technol. 2000, 32, 359–368. [Google Scholar] [CrossRef]
- Grinn-Gofroń, A.; Bosiacka, B. Effects of meteorological factors on the composition of selected fungal spores in the air. Aerobiologia 2015, 31, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.; Persson, S. Tree pollen spectra in the stockholm region (Sweden), 1973–1980. Grana 1981, 20, 179–182. [Google Scholar] [CrossRef]
- Sarda-Estve, R.; Vinarnick, J.; Baisnee, D.; Bohard, C.; Favez, O.; Roux, J.M.; Bossuet, C. On line quantification of anhydrosugars emitted in the atmosphere by High Performance Anion Exchange Chromatography with Pulsed Amperometric Detection (HPAEC-PAD). In Proceedings of the American Association for Aerosol Research (AAAR) Annual Conference, Mineapolis, MN, USA, 12–16 October 2015. [Google Scholar]
- Huffman, J.A.; Prenni, A.; DeMott, P.; Pöhlker, C.; Mason, R.; Robinson, N.; Fröhlich-Nowoisky, J.; Tobo, Y.; Després, V.; Garcia, E. High concentrations of biological aerosol particles and ice nuclei during and after rain. Atmos. Chem. Phys. 2013, 13, 6151. [Google Scholar] [CrossRef]
- Pace, L.; Boccacci, L.; Casilli, M.; Fattorini, S. Temporal variations in the diversity of airborne fungal spores in a Mediterranean high altitude site. Atmos. Environ. 2019, 210, 166–170. [Google Scholar] [CrossRef]
- Hibbett, D.S.; Taylor, J.W. Fungal systematics: Is a new age of enlightenment at hand? Nat. Rev. Microbiol. 2013, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- UBIO. Available online: https://ubio.org/ (accessed on 4 March 2019).
- Jones, E.; Sakayaroj, J.; Suetrong, S.; Somrithipol, S.; Pang, K. Classification of marine Ascomycota, anamorphic taxa and Basidiomycota. Fungal Divers. 2009, 35, 187. [Google Scholar]
- Jones, A.M.; Harrison, R.M. The effects of meteorological factors on atmospheric bioaerosol concentrations—A review. Sci. Total Environ. 2004, 326, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Hirst, J.; Stedman, O. Dry liberation of fungus spores by raindrops. Microbiology 1963, 33, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Chew, F.T.; Binti Mohd Dali, S.D.; Wah Tan, H.T.; Lee, B.W.; Tan, T.K. Outdoor airborne fungal spores in Singapore. Grana 1998, 37, 246–252. [Google Scholar] [CrossRef]
- Sindt, C.; Besancenot, J.-P.; Thibaudon, M. Airborne Cladosporium fungal spores and climate change in France. Aerobiologia 2016, 32, 53–68. [Google Scholar] [CrossRef]
- Blachowicz, A.; Chiang, A.J.; Romsdahl, J.; Kalkum, M.; Wang, C.C.; Venkateswaran, K. Proteomic characterization of Aspergillus fumigatus isolated from air and surfaces of the International Space Station. Fungal Genet. Biol. 2019, 124, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Météo France. Available online: http://www.meteofrance.fr/climat- passe-et-futur/bilans-climatiques/bilan-2018/bilan-climatique-de-l-annee-2018 (accessed on 4 March 2019).
- Kowalski, M.; Pastuszka, J.S. Effect of ambient air temperature and solar radiation on changes in bacterial and fungal aerosols concentration in the urban environment. Ann. Agric. Environ. Med. 2018, 25, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Des chiffres et des céréales—Édition 2018. Available online: https://publications.passioncereales.fr/chiffres-cereales-2018 (accessed on 4 March 2019).
- Nicolaisen, M.; West, J.S.; Sapkota, R.; Canning, G.G.; Schoen, C.; Justesen, A.F. Fungal communities including plant pathogens in near surface air are similar across northwestern Europe. Front. Microbiol. 2017, 8, 1729. [Google Scholar] [CrossRef] [PubMed]
- Enquête sur les principals grandes cultures. Available online: http://agreste.agriculture.gouv.fr/IMG/file/dossier8_cultures_panorama.pdf (accessed on 4 March 2019).
- Sadyś, M.; Kennedy, R.; West, J.S. Potential impact of climate change on fungal distributions: Analysis of 2 years of contrasting weather in the UK. Aerobiologia 2016, 32, 127–137. [Google Scholar] [CrossRef]
- Petrie, G.A. Alternaria brassicicola on imported garden crucifer seed, a potential threat to rapeseed production in Western Canada. Can. Plant Dis. Surv. 1974, 54, 1–4. [Google Scholar]
- Mitakakis, T.Z.; Clift, A.; McGee, P.A. The effect of local cropping activities and weather on the airborne concentration of allergenic Alternaria spores in rural Australia. Grana 2001, 40, 230–239. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarda-Estève, R.; Baisnée, D.; Guinot, B.; Sodeau, J.; O’Connor, D.; Belmonte, J.; Besancenot, J.-P.; Petit, J.-E.; Thibaudon, M.; Oliver, G.; et al. Variability and Geographical Origin of Five Years Airborne Fungal Spore Concentrations Measured at Saclay, France from 2014 to 2018. Remote Sens. 2019, 11, 1671. https://doi.org/10.3390/rs11141671
Sarda-Estève R, Baisnée D, Guinot B, Sodeau J, O’Connor D, Belmonte J, Besancenot J-P, Petit J-E, Thibaudon M, Oliver G, et al. Variability and Geographical Origin of Five Years Airborne Fungal Spore Concentrations Measured at Saclay, France from 2014 to 2018. Remote Sensing. 2019; 11(14):1671. https://doi.org/10.3390/rs11141671
Chicago/Turabian StyleSarda-Estève, Roland, Dominique Baisnée, Benjamin Guinot, John Sodeau, David O’Connor, Jordina Belmonte, Jean-Pierre Besancenot, Jean-Eudes Petit, Michel Thibaudon, Gilles Oliver, and et al. 2019. "Variability and Geographical Origin of Five Years Airborne Fungal Spore Concentrations Measured at Saclay, France from 2014 to 2018" Remote Sensing 11, no. 14: 1671. https://doi.org/10.3390/rs11141671
APA StyleSarda-Estève, R., Baisnée, D., Guinot, B., Sodeau, J., O’Connor, D., Belmonte, J., Besancenot, J.-P., Petit, J.-E., Thibaudon, M., Oliver, G., Sindt, C., & Gros, V. (2019). Variability and Geographical Origin of Five Years Airborne Fungal Spore Concentrations Measured at Saclay, France from 2014 to 2018. Remote Sensing, 11(14), 1671. https://doi.org/10.3390/rs11141671