In Situ Hyperspectral Remote Sensing for Monitoring of Alpine Trampled and Recultivated Species
Abstract
:1. Introduction
2. Study Area and Research Objects
3. Materials and Methods
- Identification of individual tufts of homogenous species through taking documentary photographs;
- Spectral properties using an ASD FieldSpec 3 (ASD Inc., Longmont, CO, USA) spectrometer using a fiber optic cable at an angle of 25° from a plant’s surface at a distance of about 1 m2 [32], as well as the ASD PlantProbe (ASD Inc., Longmont, CO, USA) in order to keep identical measurement conditions in all years [32]. Prior to each series of measurements, the spectrometer was calibrated using a white Spectralon tile (SG 33151 Zenith Lite Reflectance Target and calibration screen P/N A122634 Leaf clip). Thanks to the application of a probe, it was possible to precisely register the properties of individual species, eliminating the influence of the environment on the spectral reflectance curve. In total, 4115 × 25 spectrometric measurements were performed with the sets of 25 independent measurements automatically averaged and recorded as one out of 4115 measurements;
- Leaf chlorophyll content measurements using a Chlorophyll Content Meter CCM-200 (OptiScience, Inc. Opti-Sciences, Inc., Hudson, NH, USA) that measures the Chlorophyll Content Index (CCI).
- Chlorophyll fluorescence using the Plant Stress Meter fluorometer (PSM Mark II, Biomonitor, Stockholm, Sweden) and LeafClip applied to create a dark adaptation that terminates the photosynthesis processes. Measurements were also performed without dark adaptation. As the first stage, a measurement of leaves without adaptation to darkness (i.e., the Fv’/Fm’ fluorescence rate), then a second stage measurement with adaptation to darkness (a leaf fragment was put in the dark using a LeafClip, the Fv/Fm fluorescence rate); then, after about 30 min of being in the dark, a final measurement was performed that allowed all the chlorophyll pigments to be excited by a light impulse.
- Air thermodynamic temperature (ta) and leaf radiation temperature (ts) using the IRtec MiniRay 100 with an insert probe pyrometer (Eurotron Instruments S.p.A., Milan, Italy) in order to evaluate the water stress using the thermal infrared (TIR). Measurements were conducted in accordance with the 30:1 parity, which means that if the distance to the plant amounted to 30 units, the diameter from which information was gathered equaled to 1 unit. In practice, when measuring a single plant species, the measurement was performed at a distance of about 3 m from a studied tuft, with a circle whose diameter was about 10 cm that was equivalent to the canopy of a given species.
- Measuring energy balance of Absorbed Photosynthetically Active Radiation (APAR) and the fraction of APAR (fAPAR = APAR/PARo) [25] by measuring its components (PAR0—total radiation incoming to the canopy, PARc—canopy reflected PAR, PARt—PAR transmitted through the canopy, PARs—soil-reflected PAR) using a line ceptometer AccuPAR model 80 (Decagon Devices, Pullman, Washington, USA) [46].
3.1. Calculation of Remote Sensing Vegetation Indices
3.2. Statistical Analysis of Data
- Analysis of variance (ANOVA) The ANOVA analysis showed which ranges of the electromagnetic spectrum are statistically differentiated between polygons (trampled, referenced and recultivated) for each tested species and sites.
- The difference in remote sensing vegetation index values between the tested polygons for each of the three species was also checked. The procedure of statistical indicator analysis had the following structure:
- The ANOVA Kruskal-Wallis one-way analysis of variance by ranks was applied (due to the lack of normal data distribution) to analyze the species differences for each situation (trampled, reference and recultivated). This information was acquired through multiple comparisons (so-called post hoc comparisons)—in the present case, the Tukey’s HSD test was selected.
- The correlation of calculated remote sensing indices is based on spectral reflectance curves and biophysical variables. Because the distribution of both type of indicators: calculated from the spectral reflectance curve and biometric indicators (defining biophysical variables), was not normal then Spearman’s rank-order correlation coefficient was applied (Rs).
- Multivariate adaptive regression splines (MARS) were used to forecast the object condition based on remote sensing indices and biophysical variables (selected in the course of the prior statistical analysis) that showed a statistically significant relationship with the alpine sward species. Based on all the measurements conducted, a model for the Fv/Fm (n = 1600 cases) and CCI (n = 2400 cases) rates were created for the dominant alpine sward species (excluding the verification area, which in this case was the Red Peaks). The occurrence of interactions means that the influence of an independent variable (X1) on a dependent variable (Y) is different, depending on the level of the next independent variable (X2) or a series of successive independent variables. One may speak of an interaction or combination of two factors when the reaction of a studied feature at the level of one factor is not the same on all levels of the other factor [64]. A model was developed per parameter as well as the Generalized Cross Validation error (GCV), which demonstrates an error in cross-validation, i.e., proves whether a model matches real data. Moreover, the GCV parameter accounts for both the residual error and the extent of model complexity. In order to implement the research methodology for remote monitoring of alpine sward condition, e.g., hyperspectral imaging, the researchers attempted to verify the model in the Red Peaks area. And so, the rates modeled based on spectrometric data were compared with the rates determined in the field, using independent bioradiometric measurements, and then presented in the form of the coefficient of determination (R2), whereby the closer the rates to one, the more the model matches real data.
4. Results
4.1. Differentiation of Spectral Characteristics
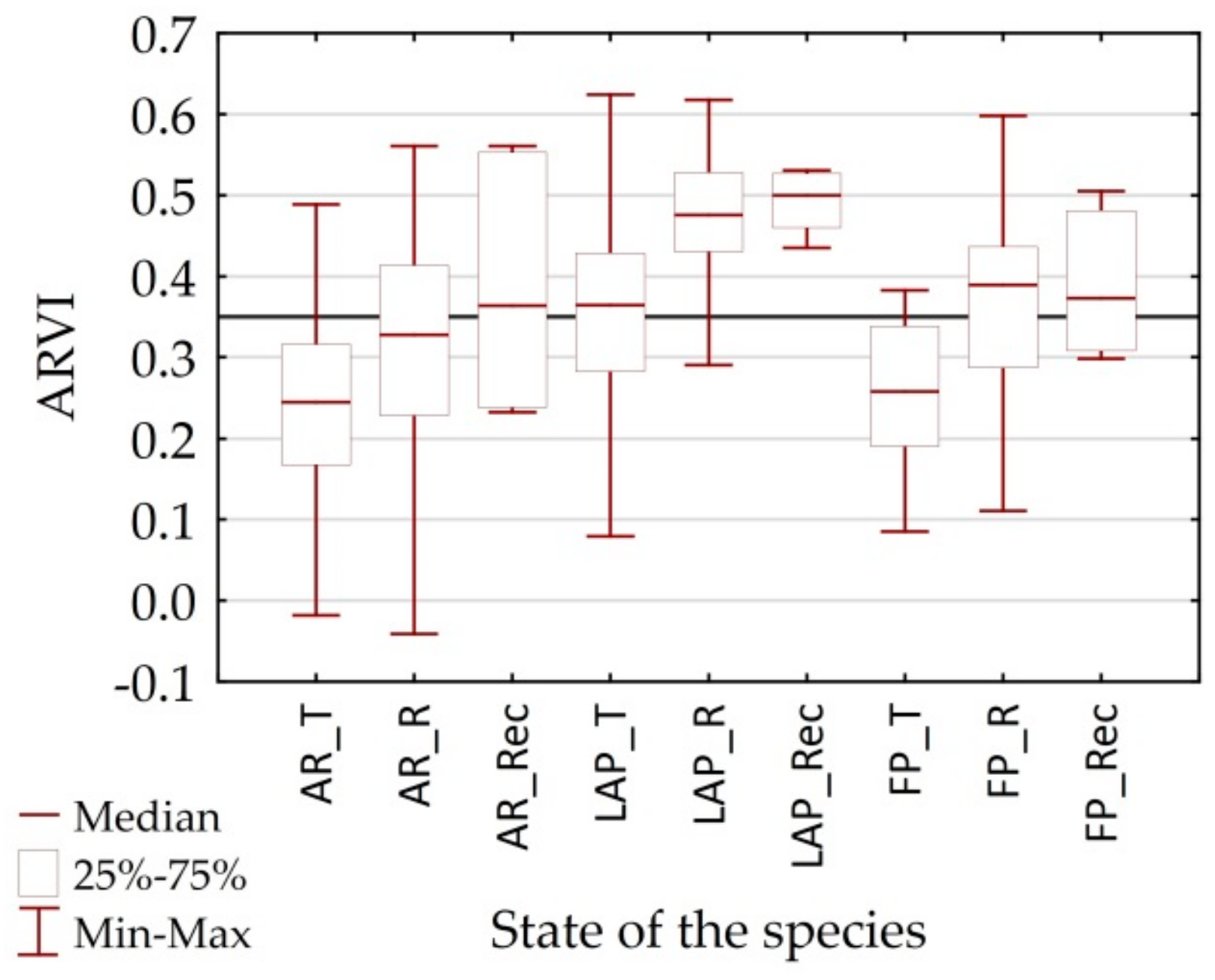
4.2. Utility of Remote Sensing Vegetation Indices
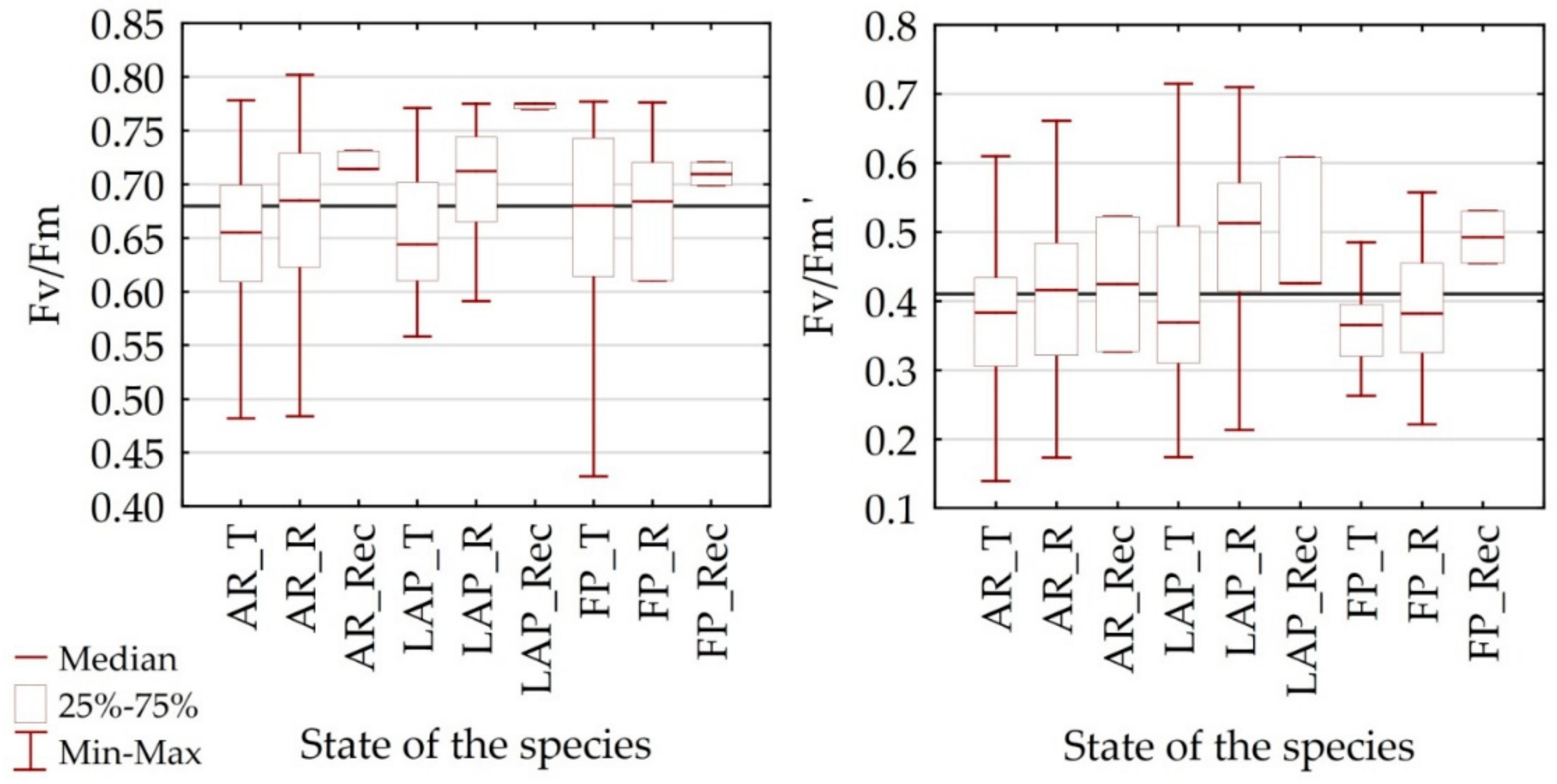
4.3. Estimation of the Chlorophyll Content Basing on Spectral Properties of Dominant Alpine Species
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Formula of the Indicator | Accuracy |
|---|---|
| Fv/Fm Agrostis rupestris = 0.627 + 1.92 × max(0; NDNI–0.171) + 1.93 × max(0; 0.171–NDNI) – 34.7 × max(0; CAI+1.93 × 10−3) + 8.14 × max(0; CAI+1.69 × 10−2) + 1.64 × max(0; RARSa –0.583) + 0.285 × max(0; 0.39–ARVI) – 0.137 × max(0; 2.12–G) + 6.74 × max(0; PRI+3.60 × 10−3) + 1.48 × max(0; PSRI–8.79 × 10−2) – 4.88 × max(0; 8.79 × 10−2-PSRI) – 1.41 × max(0; RARSa–0.493) – 0.266 × max(0; G – 2.48) + 1.57 × max(0; 1.21–SIPI) + 2.31 × max(0; NDWI–4.83 × 10−2) | Error GCV = 0.0020 |
| R2 = 0.71 | |
| RMSE = 8% | |
| Fv/Fm Luzula alpino-pilosa = 0.942 + 2.52 × max(0; SIPI – 1.02) + 4.45 × max(0; 1.02–SIPI) + 1.90 × max(0; NDNI–0.197) – 4.37 × max(0; 0.197–NDNI) – 4.12 × max(0; RARSa–0.567) – 4.20 × max(0; WBI–0.936) + 5.88 × max(0; 0.936–WBI) + 3.79 × max(0; NMDI– 0.549) – 0.919 × max(0; 0.549–NMDI) – 0.416 × max(0; 1.94–G) - 5.55 × max(0; ARVI – 0.436) + 2.03 × max(0; ARVI – 0.406) + 3.54 × max(0; ARVI – 0.461) + 1.67 × max(0; NDWI + 1.64 × 10−3) – 1.44 × max(0; ARVI–0.527) | Error GCV = 0.0014 |
| R2 = 0.83 | |
| RMSE = 6% | |
| Fv/Fm Festuca Picta = 0.512 + 0.845 × max(0; ARVI – 0.435) – 0.256 × max(0; 0.435–ARVI) – 5.64 × max(0; SIPI–1.07) + 2.74 × max(0; 1.07–SIPI) + 2.33 × max(0; PRI + 1.63 × 10−2) + 2.93 × max(0; SIPI – 1.01) – 2.80 × max(0; PRI + 2.39 × 10−2) – 2.22 × max(0; NDWI – 3.54 × 10−2) + 7.25 × max(0; CAI + 1.02 × 10−2) + 0.738 × max(0; NMDI –0.560) | Error GCV = 0.0001 |
| R2 = 0.96 | |
| RMSE = 2% | |
| CCI Agrostis rupestris = 26.0 – 593 × max(0; NDNI – 0.242) + 89.8 × max(0; 0.242–NDNI) + 283 × max(0; NDNI–0.186) + 351 × max(0; WBI–1.01) – 247 × max(0; 1.01–WBI) – 308 × max(0; 9.06 × 10−2–PSRI) – 63.4 × max(0; NMDI–0.519) – 73.6 × max(0; ARVI – 0.247) + 190 × max(0; ARVI–0.374) – 37.5 × max(0; G–1.65) – 18.7 × max(0; 1.65–G) + 286 × max(0; PRI + 4.09 × 10−2) + 145 × max(0; 0.549–RARSa) | Error GCV = 11.11 |
| R2 = 0.77 | |
| RMSE = 6% | |
| CCI Luzula alpino-pilosa = 7.16 + 1140 × max(0; NDWI–8.20 × 10−2) + 198 × max(0; 8.20 × 10−2–NDWI) + 31.2 × max(0; G–2.46) – 351 × max(0; NDNI – 0.212) – 143 × max(0; 0.212–NDNI) + 485 × max(0; NDWI + 4.82 × 10−2) – 391 × max(0; NMDI–0.539) – 89.2 × max(0; 0.540–NMDI) + 260 × max(0; NMDI – 0.582) – 644 × max(0; 2.53 × 10−2–PSRI) – 153 × max(0; NMDI – 0.569) + 112 × max(0; NDWI–5.37 × 10−2) – 2830 × max(0; NDWI–7.24 × 10−2) | Error GCV = 60.15 |
| R2 = 0.68 | |
| RMSE = 7% | |
| CCI Festuca Picta = 51.8 – 17.5 × max(0; G–2.64) – 14.0 × max(0; 2.64–G) – 86.8 × max(0; 0.589–NMDI) + 503 × max(0; PSRI – 1.77 × 10−2) – 188 × max(0; 1.77 × 10−2–PSRI) – 237 × max(0; SIPI–0.989) – 348 × max(0; –5.62 × 10−3–PRI) + 496 × max(0; NDNI – 0.205) + 144 × max(0; 0.205–NDNI) – 399 × max(0; NDWI – 3.53 × 10−2) + 327 × max(0; NMDI–0.551) – 249 × max(0; PRI + 2.73 × 10−2) | Error GCV = 6.38 |
| R2 = 0.93 | |
| RMSE = 4% |
References
- Kacperska, A. Reakcje roślin na abiotyczne czynniki stresowe. In Fizjologia Roślin; Kopcewicz, J., Lewak, S., Eds.; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2005; pp. 612–678. [Google Scholar]
- Falińska, K. Ekologia Roślin; Wydawnictwo Naukowe PWN: Warszawa, Poland, 1997; p. 456. ISBN 83-01-12065-7. [Google Scholar]
- Kelly, C.L.; Pickering, C.M.; Buckley, R.C. Impacts of tourism on threatened plant taxa and communities in Australia. Ecol. Manag. Restor. 2003, 4, 37–44. [Google Scholar] [CrossRef]
- Sørensen, L.I.; Mikola, J.; Kytöviita, M.-M.; Olofsson, J. Trampling and Spatial Heterogeneity Explain Decomposer Abundances in a Sub-Arctic Grassland Subjected to Simulated Reindeer Grazing. Ecosystems 2009, 12, 830–842. [Google Scholar] [CrossRef]
- Sun, D.; Liddle, M.J. Plant morphological characteristics and resistance to simulated trampling. Environ. Manag. 1993, 17, 511–521. [Google Scholar] [CrossRef]
- Sunohara, Y.; Ikeda, H.; Tsukagoshi, S.; Murata, Y.; Sakurai, N.; Noma, Y. Effects of trampling on morphology and ethylene production in asiatic plantain. Weed Sci. 2002, 50, 479–484. [Google Scholar] [CrossRef]
- Striker, G.G.; Mollard, F.P.O.; Grimoldi, A.A.; León, R.J.C.; Insausti, P. Trampling enhances the dominance of graminoids over forbs in flooded grassland mesocosms. Appl. Veg. Sci. 2011, 14, 95–106. [Google Scholar] [CrossRef]
- Symonides, E. Ochrona Przyrody; Wydawnictwa Uniwersytetu Warszawskiego: Warszawa, Poland, 2007. [Google Scholar]
- Wall, D.H. Global change tipping points: Above- and below-ground biotic interactions in a low diversity ecosystem. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 2291–2306. [Google Scholar] [CrossRef] [PubMed]
- Kłos, A.; Ziembik, Z.; Rajfur, M.; Dołhańczuk-Śródka, A.; Bochenek, Z.; Bjerke, J.W.; Tømmervik, H.; Zagajewski, B.; Ziółkowski, D.; Jerz, D.; et al. Using moss and lichens in biomonitoring of heavy-metal contamination of forest areas in southern and north-eastern Poland. Sci. Total Environ. 2018, 627, 438–449. [Google Scholar] [CrossRef]
- Ustin, S.L.; Roberts, D.A.; Gamon, J.A.; Asner, G.P.; Green, R.O. Using imaging spectroscopy to study ecosystem processes and properties. Bioscience 2004, 54, 523–533. [Google Scholar] [CrossRef]
- Xue, J.; Su, B. Significant Remote Sensing Vegetation Indices: A Review of Developments and Applications. J. Sens. 2017, 2017, 1353691. [Google Scholar] [CrossRef]
- Tyystjärvi, E.; Koski, A.; Keränen, M.; Nevalainen, O. The Kautsky Curve Is a Built-in Barcode. Biophys. J. 1999, 77, 1159–1167. [Google Scholar] [CrossRef] [Green Version]
- Cendrero-Mateo, M.d.P. Chlorophyll Fluorescence Response to Water and Nitrogen Deficit; The University of Arizona: Tucson, AZ, USA, 2013; Available online: https://repository.arizona.edu/handle/10150/312504 (accessed on 27 May 2019).
- Kalaji, H.M.; Bąba, W.; Gediga, K.; Goltsev, V.; Samborska, I.A.; Cetner, M.D.; Dimitrova, S.; Piszcz, U.; Bielecki, K.; Karmowska, K.; et al. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth. Res. 2018, 136, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef]
- Johnson, G.N.; Young, A.J.; Scholes, J.D.; Horton, P. The dissipation of excess excitation energy in British plant species. Plant Cell Environ. 1993, 16, 673–679. [Google Scholar] [CrossRef]
- Drozak, A.; Romanowska, E. Acclimation of mesophyll and bundle sheath chloroplasts of maize to different irradiances during growth. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 1539–1546. [Google Scholar] [CrossRef] [Green Version]
- Rapacz, M. Chlorophyll a fluorescence transient during freezing and recovery in winter wheat. Photosynthetica 2007, 45, 409–418. [Google Scholar] [CrossRef]
- De Boeck, H.J.; Bassin, S.; Verlinden, M.; Zeiter, M.; Hiltbrunner, E. Simulated heat waves affected alpine grassland only in combination with drought. New Phytol. 2016, 209, 531–541. [Google Scholar] [CrossRef]
- Lang, M.; Lichtenthaler, H.K.; Sowinska, M.; Heisel, F.; Miehe, J.A. Demonstrain water and temperature stress in the photosynthetic apparatus by fluorescence imaging. In Proceedings of the International Colloqium Photosynthesis and Remote Sensing, Montpellier, Paris, 28–30 August 1995; EARSeL: Montpellier, France, 1995; pp. 29–36. [Google Scholar]
- Zagajewski, B.; Tømmervik, H.; Bjerke, J.; Raczko, E.; Bochenek, Z.; Kłos, A.; Jarocińska, A.; Lavender, S.; Ziółkowski, D. Intraspecific Differences in Spectral Reflectance Curves as Indicators of Reduced Vitality in High-Arctic Plants. Remote Sens. 2017, 9, 1289. [Google Scholar] [CrossRef]
- Mishra, K.B.; Iannacone, R.; Petrozza, A.; Mishra, A.; Armentano, N.; La Vecchia, G.; Trtílek, M.; Cellini, F.; Nedbal, L. Engineered drought tolerance in tomato plants is reflected in chlorophyll fluorescence emission. Plant Sci. 2012, 182, 79–86. [Google Scholar] [CrossRef]
- Din, M.; Zheng, W.; Rashid, M.; Wang, S.; Shi, Z. Evaluating hyperspectral vegetation indices for leaf area index estimation of Oryza sativa L. at diverse phenological stages at diverse phenological stages. Front. Plant Sci. 2017, 8, 820. [Google Scholar] [CrossRef]
- Inoue, Y.; Guerif, M.; Baret, F.; Skidmore, A.; Gitelson, A.; Schlerf, M.; Darvishzadeh, R.; Olioso, A. Simple and robust methods for remote sensing of canopy chlorophyll content: A comparative analysis of hyperspectral data for different types of vegetation. Plant Cell Environ. 2016, 39, 2609–2623. [Google Scholar] [CrossRef] [PubMed]
- Jacquemoud, S.; Verhoef, W.; Baret, F.; Bacour, C.; Zarco-Tejada, P.J.; Asner, G.P.; François, C.; Ustin, S.L. PROSPECT + SAIL models: A review of use for vegetation characterization. Remote Sens. Environ. 2009, 113, S56–S66. [Google Scholar] [CrossRef]
- Tian, J.; Wang, S.; Zhang, L.; Wu, T.; She, X.; Jiang, H. Evaluating different vegetation index for estimating lai of winter wheat using hyperspectral remote sensing data. In Proceedings of the 2015 7th Workshop on Hyperspectral Image and Signal Processing: Evolution in Remote Sensing (WHISPERS), Tokyo, Japan, 2–5 June 2015; pp. 1–4. [Google Scholar] [CrossRef]
- Inoue, Y.; Peñuelas, J.; Miyata, A.; Mano, M. Normalized difference spectral indices for estimating photosynthetic efficiency and capacity at a canopy scale derived from hyperspectral and CO2 flux measurements in rice. Remote Sens. Environ. 2008, 112, 156–172. [Google Scholar] [CrossRef]
- Nawar, S.; Buddenbaum, H.; Hill, J.; Kozak, J.; Mouazen, A.M. Estimating the soil clay content and organic matter by means of different calibration methods of vis-NIR diffuse reflectance spectroscopy. Soil Tillage Res. 2016, 155, 510–522. [Google Scholar] [CrossRef] [Green Version]
- Ishii, J.; Lu, S.; Funakoshi, S.; Shimizu, Y.; Omasa, K.; Washitani, I. Mapping potential habitats of threatened plant species in a moist tall grassland using hyperspectral imagery. Biodivers. Conserv. 2009, 18, 2521–2535. [Google Scholar] [CrossRef]
- Moreno, J.; Alonso, L.; Delegido, J.; Rivera, J.P.; Ruiz-Verdú, A.; Sabater, N.; Tenjo, C.; Verrelst, J.; Vicent, J. FLEX (Fluorescence Explorer) mission: Observation fluorescence as a new remote sensing technique to study the global terrestrial vegetation state. Revista de Telede. [S.l.] 2014, 41, 111–119. [Google Scholar] [CrossRef]
- Kycko, M.; Zagajewski, B.; Kozłowska, A. Variability in spectral characteristics of trampled high-mountain grasslands. Misc. Geogr. 2014, 18, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Kycko, M.; Zagajewski, B.; Zwijacz-Kozica, M.; Cierniewski, J.; Romanowska, E.; Orłowska, K.; Ochtyra, A.; Jarocińska, A. Assessment of Hyperspectral Remote Sensing for Analyzing the Impact of Human Trampling on Alpine Swards. Mt. Res. Dev. 2017, 37, 66–74. [Google Scholar] [CrossRef]
- Kycko, M.; Zagajewski, B.; Lavender, S.; Romanowska, E.; Zwijacz-Kozica, M. The Impact of Tourist Traffic on the Condition and Cell Structures of Alpine Swards. Remote Sens. 2018, 10, 220. [Google Scholar] [CrossRef]
- Zagajewski, B.; Kycko, M.; Tømmervik, H.; Bochenek, Z.; Wojtuń, B.; Bjerke, J.W.; Kłos, A. Feasibility of hyperspectral vegetation indices for the detection of chlorophyll concentration in three High Arctic plants: Salix polaris, Bistorta vivipara, and Dryas octopetala. Acta Soc. Bot. Pol. 2018, 87, 3604. [Google Scholar] [CrossRef]
- Piękoś-Mirek, H.; Mirek, Z. Zbiorowiska roślinne. In Przyroda Tatrzańskiego Parku Narodowego; Mirek, Z., Ed.; Wydawnictwa Tatrzańskiego Parku Narodowego: Zakopane, Poland, 1996; pp. 237–275. [Google Scholar]
- Matuszkiewicz, W. Przewodnik do Oznaczania Zbiorowisk Roślinnych Polski; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2001. [Google Scholar]
- Kozłowska, A.; Plit, J. Mapa roślinności wysokogórskiej Tatr (od Krzyżnego do Przełęczy Kondrackiej) w skali 1:10,000 i 1:20,000. In Przemiany środowiska przyrodniczego Tatr; Borowiec, W., Kotarba, A., Kownacki, A., Krzan, Z., Mirek, Z., Eds.; Tatrzański Park Narodowy: Zakopane, Poland, 2002; pp. 197–201. [Google Scholar]
- Skiba, S.; Drewnik, M.; Szymański, W.; Skiba, M. Monitoring przyrodniczy i proba rekultywacji pokrywy glebowej i szaty roślinnej w sąsiedztwie szlaku turystycznego w rejonie wzmożonego ruchu na odcinku Kasprowy Wierch–Beskid. In Nauka Tatrom, tom III–Człowiek i Środowisko; Chrobak, A., Zwijacz-Kozica, T., Eds.; Tatrzański Park Narodowy, Polskie Towarzystwo Przyjaciół Nauk o Ziemi—Oddział Krakowski: Zakopane, Poland, 2015; pp. 119–126. [Google Scholar]
- Kupková, L.; Červená, L.; Suchá, R.; Jakešová, L.; Zagajewski, B.; Březina, S.; Albrechtová, J. Classification of Tundra Vegetation in the Krkonoše Mts. National Park Using APEX, AISA Dual and Sentinel-2A Data. Eur. J. Remote Sens. 2017, 50, 29–46. [Google Scholar] [CrossRef]
- Grau, J.; Kremer, B.P.; Rambold, G.; Triebel, D. Trawy. Leksykon przyrodniczy; GeoCenter: Warszawa, Poland, 1984. [Google Scholar]
- Górski, P. Piargowy charakter muraw wysokogórskich z Senecio carniolicus Willd. w Tatrach (Karpaty Zachodnie). Roczniki AR Pozn. 2002, 347, 51–60. [Google Scholar]
- Radwańska-Paryska, Z.; Paryski, W.H. Wielka encyklopedia tatrzańska; Wydawnictwo Górskie: Poronin, Poland, 2004. [Google Scholar]
- Mirek, Z.; Piękoś-Mirek, H. Trawy gór. In Księga Polskich Traw; Frey, L., Ed.; Polska Akademia Nauk: Kraków, Poland, 2007; pp. 203–228. [Google Scholar]
- Pawłowska, S. Świat roślinny Tatr. In Tatrzański Park Narodowy; Szafer, W., Ed.; Polska Akademia Nauk. Zakład Ochrony Przyrody, Wydawnictwa popularnonaukowe: Kraków, Poland, 1955; pp. 132–180. [Google Scholar]
- AccuPAR Model PAR-80. Operator’s Manual. Version 3.4; Detection Devices: Pullman, WA, USA, 2001.
- Kaufman, Y.J.; Tanre, D. Atmospherically resistant vegetation index (ARVI) for EOS-MODIS. IEEE Trans. Geosci. Remote Sens. 1992, 30, 261–270. [Google Scholar] [CrossRef]
- Wang, L.; Qu, J.J. NMDI: A normalized multi-band drought index for monitoring soil and vegetation moisture with satellite remote sensing. Geophys. Res. Lett. 2007, 34, L20405. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, H.-L.; Shen, S. The application of normalized multi-band drought index (NMDI) method in cropland drought monitoring. In Proceedings of the SPIE 7472, Remote Sensing for Agriculture Ecosystems, and Hydrology XI, 74721Q, Berlin, Germany, 18 September 2009. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Bejron, A.; Miller, J.R. Stress detection in crops with hyperspectral remote sensing and physical simulation models. In Proceedings of the Airborne Imaging Spectroscopy Workshop, Bruges, Belgium, 8 October 2004. [Google Scholar]
- Chappelle, E.W.; Kim, M.S.; McMurtrey, J.E. Ratio analysis of reflectance spectra (RARS): An algorithm for the remote estimation of the concentrations of chlorophyll A, chlorophyll B, and carotenoids in soybean leaves. Remote Sens. Environ. 1992, 39, 239–247. [Google Scholar] [CrossRef]
- Serrano, L.; Peñuelas, J.; Ustin, S.L. Remote sensing of nitrogen and lignin in Mediterranean vegetation from AVIRIS data. Remote Sens. Environ. 2002, 81, 355–364. [Google Scholar] [CrossRef]
- Gamon, J.A.; Peñuelas, J.; Field, C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Peñuelas, J.; Baret, F.; Filella, I. Semi-empirical indices to assess carotenoids/chlorophyll-a ratio from leaf spectral reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-destructive optical detection of pigment changes during leaf senescence and fruit ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Daughtry, C.; Walthall, C.; Kim, M.; Brown de Colstoun, E.; McMurtrey, J. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Nagler, P.L.; Inoue, Y.; Glenn, E.; Russ, A.; Daughtry, C.S. Cellulose absorption index (CAI) to quantify mixed soil–plant litter scenes. Remote Sens. Environ. 2003, 87, 310–325. [Google Scholar] [CrossRef]
- Penuelas, J.; Pinol, J.; Ogaya, R.; Filella, I. Estimation of plant water concentration by the reflectance Water Index WI (R900/R970). Int. J. Remote Sens. 1997, 18, 2869–2875. [Google Scholar] [CrossRef]
- Gao, B.C. Normalized Difference Water Index for remote sensing of vegetation liquid water from space. Proc. SPIE 1995, 2480, 225–236. [Google Scholar]
- Gao, B.C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- StatSoft. StatSoft Manual, Internetowy Podręcznik Statystyki; Statistica: Krakow, Poland, 2012. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Lehmann, E.L.; Romano, J.P. Testing Statistical Hypotheses, 3rd ed.; Springer: New York, NY, USA, 2005. [Google Scholar]
- PQStat Software, Podręcznik Użytkownika PQStat 1.6.2. 2016. Available online: http://manuals.pqstat.pl (accessed on 27 May 2019).
- Balcerkiewicz, S. Roślinność wysokogórska Doliny Pięciu Stawów Polskich w Tatrach i jej przemiany antropogeniczne; Wydawnictwo Naukowe UAM: Poznań, Poland, 1984. [Google Scholar]
- Oprządek, M. Changes in vegetation structure along four tourist trails from Kasprowy Wierch, Tatra Mountains. J. Environ. Tour. Anal. 2014, 2, 75–82. [Google Scholar]
- Górski, P. Roślinność piargowa towarzysząca szlakom turystycznym w obszarach górskich po polskiej stronie Karpat; Akademia Rolnicza im. Augusta Cieszkowskiego: Poznań, Poland, 2007. [Google Scholar]
- Ruban, A.V.; Horton, P.; Young, A.J. Aggregation of higher plant xanthophylls: Differences in absorption spectra and in the dependency on solvent polarity. J. Photochem. Photobiol. B Biol. 1993, 21, 229–234. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Adams, W.W., III; Demming-Adams, B.; Logan, B.A.; Barker, D.H.; Osmond, C.B. Rapid changes in xantophyll cycledependent energy dissipation and photosystem II efficiency in two vines, Stephania japonica and Smilax australias, growing in the understory of an open Eucalyptus forest. Plant Cell Environ. 1999, 22, 125–136. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, R.R. Determination of total caretonoids and chlorophyll a and b in the leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 603, 591–592. [Google Scholar] [CrossRef]
- Plummer, S.E.; North, P.R.J.; Briggs, S.A. The angular vegetatio index: An atmospherically resistant index for the second along track scanning radiometer (ATSR-2). In Proceedings of the 6th Symposium on Physical Measurements and Spectral Signatures in Remote Sensing CNES, Toulouse, France, 17–21 January 1994; pp. 717–722. [Google Scholar]
- North, P.R. Estimation of fAPAR, LAI, and vegetation fractional cover from ATSR-2 imagery. Remote Sens. Environ. 2002, 80, 114–121. [Google Scholar] [CrossRef]
- Datt, B. Red edge shifts for detecting phonological change stress symptoms in evergreen eucalyptus forests. In Proceedings of the 10th Australasian Remote Sensing and Photogrammetry Conference, Adelaide, Australia, 21–25 August 2000; pp. 863–874. [Google Scholar]
- Carter, G.A. Ratios of leaf reflectance in narrow wavebands as indicators of plant stress. Ratios of leaf reflectances in narrow wavebands as indicators of plant stress. Int. J. Remote Sens. 1994, 15, 697–703. [Google Scholar] [CrossRef]
- Aldakheel, Y.Y.; Danson, F.M. Spectral reflectance of dehudrating leaves: Measurements and modeling. Int. J. Remote Sens. 1997, 18, 3683–3690. [Google Scholar] [CrossRef]
- Dawson, T.P.; Curran, P.J. A new technique for interpolating the reflectance red edge position. Technical note. Int. J. Remote Sens. 1998, 11, 2133–2139. [Google Scholar] [CrossRef]
- Tian, Q.; Tong, Q.; Pu, R.; Guo, X.; Zhao, C. Spectroscopic determination of wheat water status using 1650–1850 nm spectral absorption features. Int. J. Remote Sens. 2001, 22, 2329–2338. [Google Scholar] [CrossRef]
- Fourty, T.; Baret, F. On spectral estimates of fresh leaf biochemistry. Int. J. Remote Sens. 1998, 19, 1283–1297. [Google Scholar] [CrossRef]
- Hoerig, B.; Kuehn, F.; Oschuetz, F.; Lehmann, F. Hyperspectral remote sensing to detect hydrocarbons. Int. J. Remote Sens. 2001, 22, 1413–1422. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Rodriquez-Perez, J.R.; Riano, D.; Carlisle, E.; Ustin, S.; Smart, D.R. Evaluation of hyperspectral reflectance index to detect grapevine water status in vineyards. Am. J. Enol. Vit. 2007, 58, 302–317. [Google Scholar]
- Zhang, C.; Filella, I.; Garbulsky, M.F.; Peñuelas, J. Affecting Factors and Recent Improvements of the Photochemical Reflectance Index (PRI) for Remotely Sensing Foliar, Canopy and Ecosystemic Radiation-Use Efficiencies. Remote Sens. 2016, 8, 677. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll Fluorescence Analysis: A Guide to Good Practice and Understanding Some New Applications. J. Exp. Bot. 2013, 13, 3983–3998. [Google Scholar] [CrossRef]
- Perks, M.; Monaghan, S.; O’Reilly, C.; Osborne, B.; Mitchell, D. Chlorophyll Fluorescence Characteristics, Performance and Survival of Freshly Lifted and Cold Stored Douglas Fir Seedlings. Ann. For. Sci. 2001, 58, 225–235. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [Green Version]
- Kalaji, H.M.; Carpentier, R.; Allakhverdiev, S.I.; Bosa, K. Fluorescence parameters as early indicators of light stress in barley. J. Photochem. Photobiol. B Biol. 2012, 112, 1–6. [Google Scholar] [CrossRef]
- Berkhout, J.; Jans, W.; Jongenans, J.; Kooijman, A.; Raaphorst, S.; Steingrover, E. Monitoring Photosynthesis in Evergreen Mediterranean Frest in Order to Assess the Scientific and Operational Potential of the LEAF Instrument; Number 94-03 in NRSP-2; BCRS: Delft, The Netherlands, 1996. [Google Scholar]
- Costa, A.C.; Rezende-Silva, S.L.; Megguer, C.A.; Moura, L.M.F.; Rosa, M.; Silva, A.A. The effect of irradiance and water restriction on photosynthesis in young jatobá-do-cerrado (Hymenaea stigonocarpa) plants. Photosynthetica 2015, 53, 118–127. [Google Scholar] [CrossRef]
- Kobayashi, T.; Hori, Y.; Nomoto, N. Effects of trampling and vegetation removal on species diversity and micro-environment under different shade conditions. J. Veg. Sci. 1997, 8, 873–880. [Google Scholar] [CrossRef]
- Öquist, G.; Wass, R. A portable, microprocessor operated instrument for measuring chlorophyll fluorescence kinetics in stress physiology. Physiol. Plant. 1988, 73, 211–217. [Google Scholar] [CrossRef]
- Devi Prasad, P.V.; Potluri, S.D.P. Influence of proline and hydroxyproline on salt-stressed axillary bud cultures of two varieties of potato (Solanum tuberosum). Vitr. Cell. Dev. Biol. Plant 1996, 32, 47–50. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.-O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant. 2015, 153, 284–298. [Google Scholar] [CrossRef]
- Tan, C.W.; Huang, W.J.; Jin, X.L.; Wang, J.C.; Tong, L.; Wang, J.H.; Guo, W.S. Monitoring the chlorophyll fluorescence parameter Fv/Fm in compact corn based on different hyperspectral vegetation indices. Guang Pu Xue Yu Guang Pu Fen Xi 2012, 32, 1287–1291. [Google Scholar]
- Rossini, M.; Fava, F.; Cogliati, S.; Meroni, M.; Marchesi, A.; Panigada, C.; Giardino, C.; Busetto, L.; Migliavacca, M.; Amaducci, S.; et al. Assessing canopy PRI from airborne imagery to map water stress in maize. ISPRS J. Photogramm. Remote Sens. 2013, 86, 168–177. [Google Scholar] [CrossRef]
- Panigada, C.; Rossini, M.; Meroni, M.; Cilia, C.; Busetto, L.; Amaducci, S.; Boschetti, M.; Cogliati, S.; Picchi, V.; Pinto, F.; et al. Fluorescence, PRI and canopy temperature for water stress detection in cereal crops. Int. J. Appl. Earth Obs. Geoinf. 2014, 30, 167–178. [Google Scholar] [CrossRef]
- Ač, A.; Malenovský, Z.; Hanuš, J.; Tomášková, I.; Urban, O.; Marek, M.V. Near-distance imaging spectroscopy investigating chlorophyll fluorescence and photosynthetic activity of grassland in the daily course. Funct. Plant Biol. 2009, 36, 1006–1015. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Preece, C.; Filella, I.; Farré-Armengol, G.; Peñuelas, J. Assessment of the response of photosynthetic activity of mediterranean evergreen oaks to enhanced drought stress and recovery by using PRI and R690/R630. Forests 2017, 8, 386. [Google Scholar] [CrossRef]
- Ohashi, Y.; Nakayama, N.; Saneoka, H.; Fujita, K. Effects of drought stress on photosynthetic gas exchange, chlorophyll fluorescence and stem diameter of soybean plants. Biol. Plant. 2006, 50, 138–141. [Google Scholar] [CrossRef]
- Guóth, A.; Tari, I.; Gallé, Á.; Csiszár, J.; Pécsváradi, A.; Cseuz, L.; Erdei, L. Comparison of the Drought Stress Responses of Tolerant and Sensitive Wheat Cultivars During Grain Filling: Changes in Flag Leaf Photosynthetic Activity, ABA Levels, and Grain Yield. J. Plant Growth Regul. 2009, 28, 167–176. [Google Scholar] [CrossRef]
- Nawar, S.; Buddenbaum, H.; Hill, J. Digital Mapping of Soil Properties Using Multivariate Statistical Analysis and ASTER Data in an Arid Region. Remote Sens. 2015, 7, 1181–1205. [Google Scholar] [CrossRef] [Green Version]








| Application | Index | Name | Formula | Source |
|---|---|---|---|---|
| General condition of vegetation | ARVI | Atmospherically Resistant Vegetation Index | ARVI = {[R860 − (2* R650 − R470)]/[ R860 + (R650 − R470)]} | [47] |
| NMDI | Normalized Multi-band Drought Index | NMDI = {[R860 − (R1640 − R2130)]/[R860 + (R1640 − R2130)]} | [48,49] | |
| Amount of photosynthetic active pigments | GI | Greenness Index | GI = R554/R677 | [50] |
| RARSa | Ratio analysis of reflectance spectra algorithm chlorophyll a | RARSa = R675/R700 | [51] | |
| Canopy nitrogen | NDNI | Normalized Difference Nitrogen Index | NDNI = [[LOG(1/R1510) − LOG(1/R1680)]/[LOG(1/R1510) + LOG(1/R1680)] | [52] |
| Light use efficiency | PRI | Photochemical Reflectance Index | PRI = (R531 − R570)/(R531 + R570) | [53] |
| SIPI | Structure Insensitive Pigment Index | SIPI = (R800 − R445)/(R800 − R680) | [54] | |
| Dry or senescent carbon | PSRI | Plant Senescence Reflectance Index | PSRI = (R680 − R500)/R750 | [55] |
| CAI | Cellulose Absorption Index | CAI = [[0.5*(R2000 + R2200)] − R2100 | [56,57] | |
| Canopy water content | WBI | Water Band Index | WBI = R970/R900 | [58] |
| NDWI | Normalized Difference Water Index | NDWI = (R857 − R1241)/(R857 + R1241) | [59,60] |
| Spectral Ranges | Width of the Range (nm) | Description of the Plant Characteristics |
|---|---|---|
| 448–514 | 26 | The amount of photosynthetically active pigments |
| 531–707 | 176 | |
| 1385–1556 | 171 | Vegetation cellular structures |
| 1801–1835 | 34 | Water content, dry matter content, absorption of proteins and nitrogen compounds |
| 1845–1862 | 17 | |
| 1879–2500 | 621 |
| Species | Festuca picta | Luzula alpino-pilosa | Agrostis rupestris | |||
|---|---|---|---|---|---|---|
| State | T | R | T | R | T | R |
| CCI | ARVI [0.80], WBI [0.70], CAI [−0.65], NDWI [0.60], NDNI [0.50] | NMDI [0.84], PRI [0.78], NDWI [0.76], PSRI [−0.76], ARVI [0.61], WBI [0.56] | CAI [−0.73], WBI [−0.45] | - | PRI [0.75], WBI [0.71], PSRI [−0.65] | NDNI [0.60], PRI [0.54] |
| fAPAR | NDWI [0.52], PRI [−0.51] | NMDI [−0.74], CAI [0.54], SIPI [0.53], PSRI [0.52] | - | ARVI [0.74], RARSa [−0.72] | PRI [−0.55] | - |
| Fv/Fm | - | PRI [0.65], WBI [0.51], NMDI [0.51] | RARSa [0.53], GI [−0.45] | SIPI [−0.58] | - | NMDI [0.70], NDWI [0.61], |
| Fv’/Fm’ | - | PRI [0.60] | ARVI [−0.51] | - | CAI [0.51] | - |
| ts-ta | CAI [0.89], ARVI [−0.71], WBI [−0.70], NDWI [−0.60] | CAI [0.77], NMDI [−0.75], RARSa [0.61], SIPI [0.56], NDWI [−0.55] | WBI [−0.65], RARSa [0.57] | NDWI [0.92], WBI [0.88], RARSa [−0.70], GI [0.53] | PSRI [0.64], SIPI [0.62], WBI [0.50] | NMDI [−0.70], NDWI [0.57], PSRI [−0.55] |
| Predictors | Accuracy | ||
|---|---|---|---|
| GCV | R2 | RMSE | |
| Fv/Fm Agrostis rupestris: NDNI; CAI; RARSa; ARVI; GI; PRI; PSRI; SIPI; NDWI | 0.002 | 0.71 | 8% |
| Fv/Fm Luzula alpino-pilosa: SIPI; NDNI; RARSa; WBI; NMDI; ARVI; NDWI | 0.0014 | 0.83 | 6% |
| Fv/Fm Festuca picta: ARVI; SIPI; PRI; NDWI; NMDI; CAI | 0.0001 | 0.96 | 2% |
| CCI Agrostis rupestris: NDNI; WBI; PSRI; ARVI; GI; RARSa; PRI; NMDI | 11.11 | 0.77 | 6% |
| CCI Luzula alpino-pilosa: NDWI; GI; NDNI; NDMI; PSRI; | 60.15 | 0.68 | 7% |
| CCI Festuca Picta: NMDI; GI; PSRI; SIPI; NDNI; NDWI; PRI | 6.38 | 0.93 | 4% |
| This Research | Literature | |||
|---|---|---|---|---|
| Range of the Spectrum (nm) from Statistically Significant Spectral Curves | Wavelength (nm) from Statistically Significant Indices | Wavelength (nm) | Application | Source of Information |
| 448–514 | 470, 500 | 463 | analysis of b-carotene absorption | [68] |
| 470 | analysis of the absorption of total carotenoids | [68] | ||
| 31–1556 | 531, 554, 570, 650, 675, 677, 680, 700, 750, 800, 857, 860, 900, 970, 1241, 1510 | 530–630 | analysis of chlorophyll content | [69] |
| 650 | chlorosis analysis | [70] | ||
| 663.2 | analysis of absorption of chlorophyll-a | [71] | ||
| 646.8 | analysis of absorption of chlorophyll-b | [71] | ||
| 670 | soil effect normalization and AVI analysis, bands for the analysis of small amounts of chlorophyll | [69,72,73] | ||
| 680 | analysis of chlorophyll absorption | [74] | ||
| 695 | analysis of plant stress PSI (760/695 nm) | [75] | ||
| 1450 | analysis of water absorption in leaves | [76] | ||
| 1510 | analysis of the absorption of proteins and nitrogen compounds in conifers | [77] | ||
| 1801–1835, 1845–1862, 1879–2500 | 1640, 1680, 2000, 2100, 2130, 2200 | 1650–1850 | analysis of water content in cereals (wheat) | [78] |
| 1870 | analysis of dry matter content | [79] | ||
| 1910 | plant turgor analysis (water content) | [79] | ||
| 2160 | analysis of dry matter content | [79] | ||
| 2180 | analysis of the absorption of proteins and nitrogen compounds | [77] | ||
| 2310 | analysis of dry leaves, absorption of hydrocarbons | [79,80] | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kycko, M.; Zagajewski, B.; Lavender, S.; Dabija, A. In Situ Hyperspectral Remote Sensing for Monitoring of Alpine Trampled and Recultivated Species. Remote Sens. 2019, 11, 1296. https://doi.org/10.3390/rs11111296
Kycko M, Zagajewski B, Lavender S, Dabija A. In Situ Hyperspectral Remote Sensing for Monitoring of Alpine Trampled and Recultivated Species. Remote Sensing. 2019; 11(11):1296. https://doi.org/10.3390/rs11111296
Chicago/Turabian StyleKycko, Marlena, Bogdan Zagajewski, Samantha Lavender, and Anca Dabija. 2019. "In Situ Hyperspectral Remote Sensing for Monitoring of Alpine Trampled and Recultivated Species" Remote Sensing 11, no. 11: 1296. https://doi.org/10.3390/rs11111296
APA StyleKycko, M., Zagajewski, B., Lavender, S., & Dabija, A. (2019). In Situ Hyperspectral Remote Sensing for Monitoring of Alpine Trampled and Recultivated Species. Remote Sensing, 11(11), 1296. https://doi.org/10.3390/rs11111296