Emerging Trends in Advanced Translational Applications of Silver Nanoparticles: A Progressing Dawn of Nanotechnology
Abstract
1. Introduction
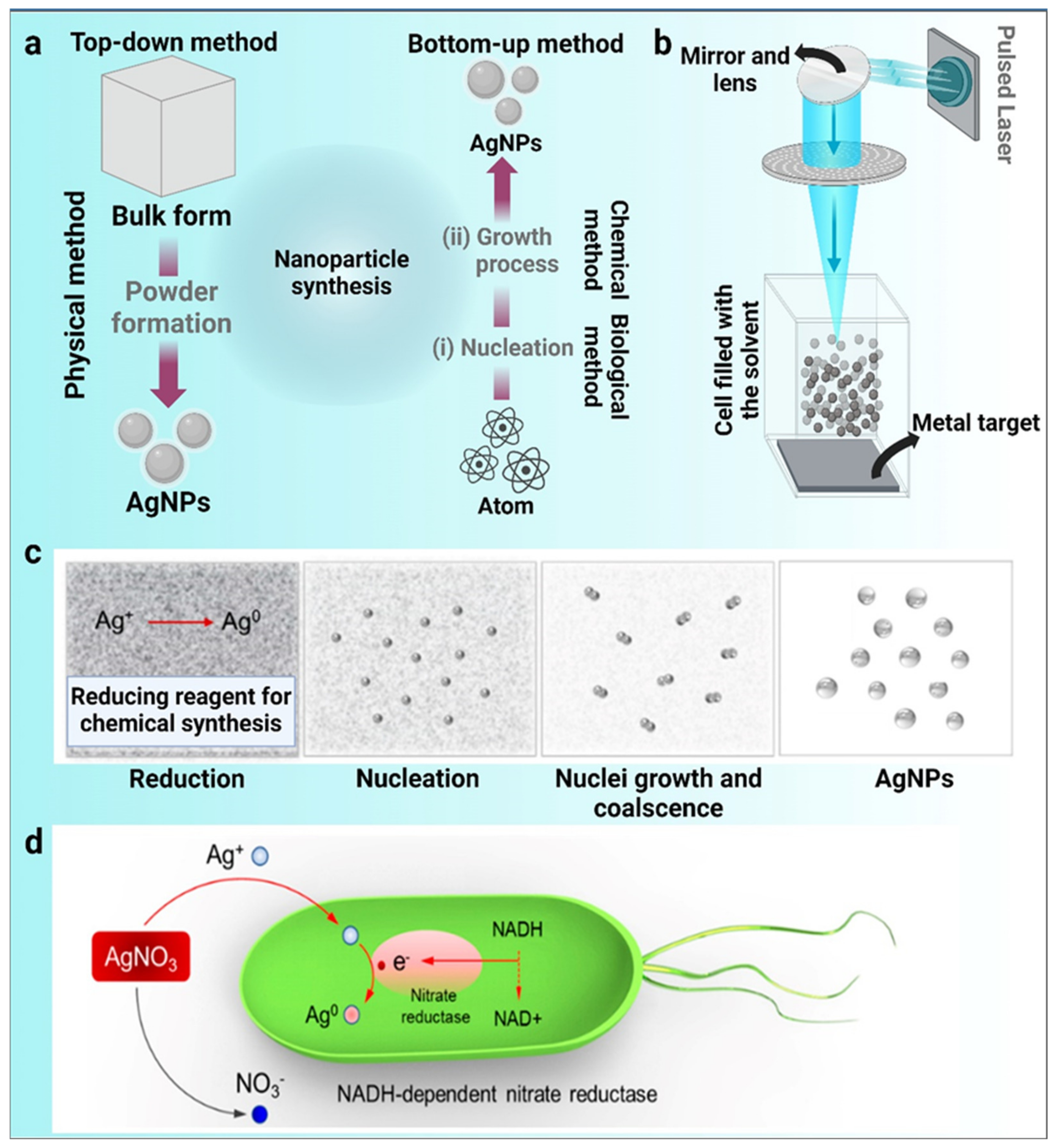
2. Traditional Techniques for the Synthesis of Metal Nanoparticles
2.1. Physical Method of Nanoparticle Synthesis
2.2. Chemical Method of Nanoparticle Synthesis
2.3. Biological Method of Nanoparticle Synthesis
3. Characterization of Nanoparticles
4. Applications of AgNPs
4.1. Medical Applications of AgNPs
4.1.1. AgNPs as Anti-Bacterial Agents
4.1.2. Implications of AgNPs in Catheters
4.1.3. AgNPs in the Detection of Alzheimer’s Disease
4.1.4. Dental Applications of AgNPs
4.1.5. Orthopedic Applications of AgNPs
4.1.6. Role of AgNPs in Wound Healing
4.1.7. AgNPs as an Anti-Inflammatory Substance
4.1.8. Anti-Fungal Properties of AgNPs
4.1.9. Anti-Plasmodial Properties of AgNPs
4.1.10. Anti-Cancer Possessions of AgNPs
4.1.11. Anti-Viral Properties of AgNPs
4.1.12. Anti-Diabetic Properties of AgNPs
| S.No. | Source of Synthesis | Size Range of AgNPs (nm) | Biomedical Applications | References |
|---|---|---|---|---|
| 1. | Cyanobacteria | 7 nm | Liver cancer, breast cancer | [3] |
| 2. | Cucumis melo L. (muskmelon) | 25 nm | Anticancer, antibacterial | [94] |
| 3. | Benincasa hispida | 26 ± 2 nm | Antibacterial, human cervical cancer | [95] |
| 4. | Lactobacillus brevis MSR104 | 45 nm | Antibacterial, anticancer, antioxidant | [96] |
| 5. | Spirulina platensis | 29 nm | Biofilm formation in urinary catheters | [97] |
| 6. | N,N,N′,N′-tetramethylethylenediamine | 100 nm | Catheter-related infections | [98] |
| 7. | Flavonoids, phenolic compounds, and glucose extract | 9.1 ± 0.4 nm | Catheters | [99] |
| 8. | Commercially purchased | 20–40 nm | Bone repair, antibacterial, wound healing | [100] |
| 9. | Rosa indica wichuriana hybrid leaf extract | 15–20 nm | Antifungal, anti-bacterial | [101] |
| 10. | Immobilization | 16 nm | Antibacterial | [102] |
| 11. | Chemically synthesized from NaBH4 | 5–500 nm | Biosensor for the detection of human chorionic gonadotropin | [103] |
| 12. | Tannic acid | 13,33,46 nm | Antiviral | [104] |
| 13. | Artemisia annua | 50 nm | Anti-malarial | [105] |
| 14. | Commercially purchased | 20–30 nm | Anti-Giardia | [106] |
| 15. | Indigofera oblongifolia | 10–30 nm | Anti-plasmodium | [107] |
| 16. | Commercially purchased | 7 ± 4 nm | Coating on appliances | [108] |
| 17. | Eucalyptus camaldulensis | 4–30 nm | Coated on braided silk, surgical | [109] |
| 18. | N. khasiana | 10–15 nm | Treatment of Alzheimer’s disease | [110] |
| 19. | Egg white | 9.5 ±2 nm to 30.2 ± 2 nm | Wound healing | [111] |
| 20. | Sodium borohydride | 15 nm | Orthopedics | [112] |
| 21. | Acacia nilotica or natural gum | 10–78 nm | Different cancer cell lines | [113] |
| 22. | Chitosan | 17–50 nm | Cancer cell line HepG2 | [114] |
4.2. AgNPs as an Odor-Controlling Agent in the Textile Industry

4.3. AgNPs in Cosmetics
5. Role of AgNPs in Agriculture
5.1. AgNPs in Crop Production
5.2. AgNPs as Herbicides
5.3. AgNPs as Pesticides
5.4. Improvement of Soil Quality through AgNPs
5.5. AgNPs for Controlling Viruses in Plants

6. Influence of AgNPs on the Food Industry
7. Toxicity-Based Investigations of AgNPs
8. Prospects
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Husain, S.; Sardar, M.; Fatma, T. Screening of cyanobacterial extracts for synthesis of silver nanoparticles. World J. Microbiol. Biotechnol. 2015, 31, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Afzal, B.; Yasin, D.; Husain, S.; Zaki, A.; Srivastava, P.; Kumar, R.; Fatma, T. Screening of cyanobacterial strains for the selenium nanoparticles synthesis and their anti-oxidant activity. Biocatal. Agric. Biotechnol. 2019, 21, 101307. [Google Scholar] [CrossRef]
- Husain, S.; Afreen, S.; Hemlata; Yasin, D.; Afzal, B.; Fatma, T. Corrigendum to “Cyanobacteria as a bioreactor for synthesis of silver nanoparticles-an effect of different reaction conditions on the size of nanoparticles and their dye decolorization ability” [Journal of Microbiological Methods 162 (2019) 77–82]. J. Microbiol. Methods 2020, 168, 105764. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Verma, S.K.; Yasin, D.; Hemlata; Rizvi, M.M.A.; Fatma, T. Facile green bio-fabricated silver nanoparticles from Microchaete infer dose-dependent antioxidant and anti-proliferative activity to mediate cellular apoptosis. Bioorganic Chem. 2021, 107, 104535. [Google Scholar] [CrossRef] [PubMed]
- Vilchis-Nestor, A.R.; Sánchez-Mendieta, V.; Camacho-López, M.A.; Gómez-Espinosa, R.M.; Camacho-López, M.A.; Arenas-Alatorre, J.A. Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater. Lett. 2008, 62, 3103–3105. [Google Scholar] [CrossRef]
- Husain, S.; Verma, S.K.; Hemlata; Azam, M.; Sardar, M.; Haq, Q.; Fatma, T. Antibacterial efficacy of facile cyanobacterial silver nanoparticles inferred by antioxidant mechanism. Mater. Sci. Eng. C 2021, 122, 111888. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Zhu, J.; Kou, Z.; Hu, P.; Liu, L.; Li, S.; Mu, S.; Huang, Y. Defect and pyridinic nitrogen engineering of carbon-based metal-free nanomaterial toward oxygen reduction. Nano Energy 2018, 52, 307–314. [Google Scholar] [CrossRef]
- Čubová, K.; Čuba, V. Synthesis of inorganic nanoparticles by ionizing radiation—A review. Radiat. Phys. Chem. 2020, 169, 108774. [Google Scholar] [CrossRef]
- Irshad, M.A.; Nawaz, R.; Rehman, M.Z.U.; Imran, M.; Ahmad, J.; Ahmad, S.; Inam, A.; Razzaq, A.; Rizwan, M.; Ali, S. Synthesis and characterization of titanium dioxide nanoparticles by chemical and green methods and their antifungal activities against wheat rust. Chemosphere 2020, 258, 127352. [Google Scholar] [CrossRef]
- García-Barrasa, J.; López-De-Luzuriaga, J.M.; Monge, M. Silver nanoparticles: Synthesis through chemical methods in solution and biomedical applications. Open Chem. 2010, 9, 7–19. [Google Scholar] [CrossRef]
- Howson, S.E.; Bolhuis, A.; Brabec, V.; Clarkson, G.J.; Malina, J.; Rodger, A.; Scott, P. Optically pure, water-stable metallo-helical ‘flexicate’ assemblies with antibiotic activity. Nat. Chem. 2011, 4, 31–36. [Google Scholar] [CrossRef]
- Mie, R.; Samsudin, M.W.; Din, L.B.; Ahmad, A.; Ibrahim, N.; Adnan, S.N.A. Synthesis of silver nanoparticles with antibacterial activity using the lichen Parmotrema praesorediosum. Int. J. Nanomed. 2013, 9, 121–127. [Google Scholar] [CrossRef]
- Ali, K.; Ahmed, B.; Dwivedi, S.; Saquib, Q.; Al-Khedhairy, A.; Musarrat, J. Microwave Accelerated Green Synthesis of Stable Silver Nanoparticles with Eucalyptus globulus Leaf Extract and Their Antibacterial and Antibiofilm Activity on Clinical Isolates. PLoS ONE 2015, 10, e0131178. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Sepeur, S. Nanotechnology: Technical Basics and Applications; Vincentz Network GmbH & Co KG: Hanover, Germany, 2008. [Google Scholar]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-strain control of the activity in dealloyed core–shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of copper nanoparticles: An overview of the various methods. Korean J. Chem. Eng. 2014, 31, 1105–1109. [Google Scholar] [CrossRef]
- Egorova, E.; Revina, A. Synthesis of metallic nanoparticles in reverse micelles in the presence of quercetin. Colloids Surfaces A: Physicochem. Eng. Asp. 2000, 168, 87–96. [Google Scholar] [CrossRef]
- Matussin, S.; Harunsani, M.H.; Tan, A.L.; Khan, M.M. Plant-Extract-Mediated SnO2 Nanoparticles: Synthesis and Applications. ACS Sustain. Chem. Eng. 2020, 8, 3040–3054. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Chun-Ming, W.; Jin-Feng, W.; Hong-Cun, C.; Wen-Bin, S.; Guo-Zhong, Z.; Peng, Q. Effects of Er2O3 on Electrical Properties of the SnO2.CoO.Ta2O5 Varistor System. Chin. Phys. Lett. 2004, 21, 716–719. [Google Scholar] [CrossRef]
- Crewe, A.V. V Production of Electron Probes Using a Field Emission Source. In Progress in Optics; Elsevier: Amsterdam, The Netherlands, 1973; pp. 223–246. [Google Scholar] [CrossRef]
- Avoyan, A.; Rupprechter, G.; Eppler, A.S.; Somorjai, G.A. Fabrication and characterization of the Ag-based high-technology model nanocluster catalyst for ethylene epoxidation manufactured by electron beam lithography. Top. Catal. 2000, 10, 107–113. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Lincoln, F.; Sugiyama, K.; Hiraga, K. Structure refinement of (Al, Zn)49Mg32-type phases by single-crystal X-ray diffraction. Mater. Sci. Eng. A 2000, 294–296, 327–330. [Google Scholar] [CrossRef]
- Kailasa, S.K.; Park, T.-J.; Rohit, J.V.; Koduru, J.R. Antimicrobial activity of silver nanoparticles. In Nanoparticles Pharmacother; Elsevier: Amsterdam, The Netherlands, 2019; pp. 461–484. [Google Scholar] [CrossRef]
- Hashemi, Z.; Ebrahimzadeh, M.A.; Biparva, P.; Mortazavi-Derazkola, S.; Goli, H.R.; Sadeghian, F.; Kardan, M.; Rafiei, A. Biogenic Silver and Zero-Valent Iron Nanoparticles by Feijoa: Biosynthesis, Characterization, Cytotoxic, Antibacterial and Antioxidant Activities. Anti-Cancer Agents Med. Chem. 2020, 20, 1673–1687. [Google Scholar] [CrossRef]
- Verma, S.K.; Jha, E.; Panda, P.K.; Thirumurugan, A.; Patro, S.; Parashar, S.; Suar, M. Molecular insights to alkaline based bio-fabrication of silver nanoparticles for inverse cytotoxicity and enhanced antibacterial activity. Mater. Sci. Eng. C 2018, 92, 807–818. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Peng, X.; Li, Z.; Xiang, J.; Chen, Y.; Hao, K.; Wang, S.; Nie, D.; Cui, Y.; et al. Green synthesis of silver nanoparticles through oil: Promoting full-thickness cutaneous wound healing in methicillin-resistant Staphylococcus aureus infections. Front. Bioeng. Biotechnol. 2022, 10, 856651. [Google Scholar] [CrossRef]
- Shrivastava, S.; Bera, T.; Singh, S.K.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of Antiplatelet Properties of Silver Nanoparticles. ACS Nano 2009, 3, 1357–1364. [Google Scholar] [CrossRef]
- Abalkhil, T.A.; Alharbi, S.A.; Salmen, S.H.; Wainwright, M. Bactericidal activity of biosynthesized silver nanoparticles against human pathogenic bacteria. Biotechnol. Biotechnol. Equip. 2017, 31, 411–417. [Google Scholar] [CrossRef]
- Mohamed, H.E.A.; Afridi, S.; Khalil, A.T.; Zia, D.; Iqbal, J.; Ullah, I.; Shinwari, Z.K.; Maaza, M. Biosynthesis of silver nanoparticles from Hyphaene thebaica fruits and their in vitro pharmacognostic potential. Mater. Res. Express 2019, 6, 1050c9. [Google Scholar] [CrossRef]
- Jeon, J.; Lim, D.-K.; Nam, J.-M. Functional nanomaterial-based amplified bio-detection strategies. J. Mater. Chem. 2009, 19, 2107–2117. [Google Scholar] [CrossRef]
- Nazem, A.; Mansoori, G. Nanotechnology for Alzheimer’s disease detection and treatment. Insciences J. 2011, 1, 169–193. [Google Scholar] [CrossRef]
- Huang, C.-L.; Hsiao, I.-L.; Lin, H.-C.; Wang, C.-F.; Huang, Y.-J. Silver nanoparticles affect on gene expression of inflammatory and neurodegenerative responses in mouse brain neural cells. Environ. Res. 2015, 136, 253–263. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Weir, M.D.; Melo, M.A.S.; Zhou, X.; Xu, H.H. Nanotechnology strategies for antibacterial and remineralizing composites and adhesives to tackle dental caries. Nanomedicine 2015, 10, 627–641. [Google Scholar] [CrossRef]
- Vogel, K.; Westphal, N.; Salz, D.; Thiel, K.; Wittig, L.; Ciacchi, L.C.; Grunwald, I. Dental implants coated with a durable and antibacterial film. Surf. Innov. 2015, 3, 27–38. [Google Scholar] [CrossRef]
- Noronha, V.T.; Paula, A.J.; Durán, G.; Galembeck, A.; Cogo-Müller, K.; Franz-Montan, M.; Durán, N. Silver nanoparticles in dentistry. Dent. Mater. 2017, 33, 1110–1126. [Google Scholar] [CrossRef]
- Manikandan, A.; Meenatchi, B.; Vadivel, S.; Jaganathan, S.; Ladchumananandasivam, R.; Henini, M.; Maaza, M.; Aanand, J.S. Rare earth element (REE) lanthanum doped zinc oxide (La: ZnO) nanomaterials: Synthesis structural optical and antibacterial studies. J. Alloys Compd. 2017, 723, 1155–1161. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mukherjee, S.; Mishra, M. Nanoparticles used in dentistry: A review. J. Oral Biol. Craniofacial Res. 2018, 8, 58–67. [Google Scholar] [CrossRef]
- Nguyen, S.; Hiorth, M. Advanced drug delivery systems for local treatment of the oral cavity. Ther. Deliv. 2015, 6, 595–608. [Google Scholar] [CrossRef]
- de Castro, D.T.; Nascimento, C.D.; Alves, O.L.; Santos, E.D.S.; Agnelli, J.A.M.; dos Reis, A.C. Analysis of the oral microbiome on the surface of modified dental polymers. Arch. Oral Biol. 2018, 93, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Mahross, H.Z.; Baroudi, K. Effect of silver nanoparticles incorporation on viscoelastic properties of acrylic resin denture base material. Eur. J. Dent. 2015, 09, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Cochis, A.; Cazzola, M.; Tortello, M.; Scalia, A.; Spriano, S.; Rimondini, L. Cytocompatible and Anti-bacterial Adhesion Nanotextured Titanium Oxide Layer on Titanium Surfaces for Dental and Orthopedic Implants. Front. Bioeng. Biotechnol. 2019, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Odekerken, J.C.E.; Welting, T.J.M.; Arts, J.J.C.; Walenkamp, G.H.I.M.; Emans, P.J. Modern Orthopaedic Implant Coatings—Their Pro’s, Con’s and Evaluation Methods. In Methods, Modern Surface Engineering Treatments; InTech: London, UK, 2013. [Google Scholar] [CrossRef]
- Engesæter, L.; Lie, S.A.; Espehaug, B.; Furnes, O.; Vollset, S.E.; Havelin, L.I. Antibiotic prophylaxis in total hip arthroplastyEffects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0–14 years in the Norwegian Arthroplasty Register. Acta Orthop. Scand. 2003, 74, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Albers, C.E.; Hofstetter, W.; Siebenrock, K.A.; Landmann, R.; Klenke, F.M. In vitro cytotoxicity of silver nanoparticles on osteoblasts and osteoclasts at antibacterial concentrations. Nanotoxicology 2011, 7, 30–36. [Google Scholar] [CrossRef]
- Pauksch, L.; Hartmann, S.; Rohnke, M.; Szalay, G.; Alt, V.; Schnettler, R.; Lips, K.S. Biocompatibility of silver nanoparticles and silver ions in primary human mesenchymal stem cells and osteoblasts. Acta Biomater. 2014, 10, 439–449. [Google Scholar] [CrossRef]
- Zielinska, E.; Tukaj, C.; Radomski, M.W.; Inkielewicz-Stepniak, I. Molecular Mechanism of Silver Nanoparticles-Induced Human Osteoblast Cell Death: Protective Effect of Inducible Nitric Oxide Synthase Inhibitor. PLoS ONE 2016, 11, e0164137. [Google Scholar] [CrossRef]
- Hackenberg, S.; Scherzed, A.; Kessler, M.; Hummel, S.; Technau, A.; Froelich, K.; Ginzkey, C.; Koehler, C.; Hagen, R.; Kleinsasser, N. Silver nanoparticles: Evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol. Lett. 2011, 201, 27–33. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Q.; Li, J.; Wang, S.; Wang, Y.; Li, B.; Yang, H. Identification of Rabbit Annulus Fibrosus-Derived Stem Cells. PLoS ONE 2014, 9, e108239. [Google Scholar] [CrossRef]
- Kim, I.; Lee, B.-T.; Kim, H.-A.; Kim, K.-W.; Kim, S.D.; Hwang, Y.-S. Citrate coated silver nanoparticles change heavy metal toxicities and bioaccumulation of Daphnia magna. Chemosphere 2016, 143, 99–105. [Google Scholar] [CrossRef]
- Asha, A.B.; Ounkaew, A.; Peng, Y.-Y.; Gholipour, M.R.; Ishihara, K.; Liu, Y.; Narain, R. Bioinspired antifouling and antibacterial polymer coating with intrinsic self-healing property. Biomater. Sci. 2022, 11, 128–139. [Google Scholar] [CrossRef]
- Xin, Q.; Rotchell, J.M.; Cheng, J.; Yi, J.; Zhang, Q. Silver nanoparticles affect the neural development of zebrafish embryos. J. Appl. Toxicol. 2015, 35, 1481–1492. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Shen, W.; Gurunathan, S. Silver Nanoparticle-Mediated Cellular Responses in Various Cell Lines: An in Vitro Model. Int. J. Mol. Sci. 2016, 17, 1603. [Google Scholar] [CrossRef]
- Ewald, A.; Glückermann, S.K.; Thull, R.; Gbureck, U. Antimicrobial titanium/silver PVD coatings on titanium. Biomed. Eng. Online 2006, 5, 22. [Google Scholar] [CrossRef]
- Wu, P.; Xie, R.; Imlay, K.; Shang, J.K. Visible-Light-Induced Bactericidal Activity of Titanium Dioxide Codoped with Nitrogen and Silver. Environ. Sci. Technol. 2010, 44, 6992–6997. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Huang, C.-Y.; Chen, C.-C. A clinical experience of treating exfoliative wounds using nanocrystalline silver-containing dressings (Acticoat®). Burns 2007, 33, 793–797. [Google Scholar] [CrossRef]
- Asz, J.; Asz, D.; Moushey, R.; Seigel, J.; Mallory, S.B.; Foglia, R.P. Treatment of toxic epidermal necrolysis in a pediatric patient with a nanocrystalline silver dressing. J. Pediatr. Surg. 2006, 41, e9–e12. [Google Scholar] [CrossRef]
- Yang, C.-W.O.; Hung, S.-I.; Juo, C.-G.; Lin, Y.-P.; Fang, W.-H.; Lu, I.-H.; Chen, S.-T.; Chen, Y.-T. HLA-B*1502–bound peptides: Implications for the pathogenesis of carbamazepine-induced Stevens-Johnson syndrome. J. Allergy Clin. Immunol. 2007, 120, 870–877. [Google Scholar] [CrossRef]
- Vlachou, E.; Chipp, E.; Shale, E.; Wilson, Y.T.; Papini, R.; Moiemen, N.S. The safety of nanocrystalline silver dressings on burns: A study of systemic silver absorption. Burns 2007, 33, 979–985. [Google Scholar] [CrossRef]
- Sibbald, R.G.; Elliott, J.; Ayello, E.A.; Somayaji, R. Optimizing the Moisture Management Tightrope with Wound Bed Preparation 2015©. Adv. Ski. Wound Care 2015, 28, 466–476. [Google Scholar] [CrossRef]
- Nadworny, P.L.; Wang, J.; Tredget, E.E.; Burrell, R.E. Anti-inflammatory activity of nanocrystalline silver in a porcine contact dermatitis model. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Larese, F.F.; D’Agostin, F.; Crosera, M.; Adami, G.; Renzi, N.; Bovenzi, M.; Maina, G. Human skin penetration of silver nanoparticles through intact and damaged skin. Toxicology 2009, 255, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, T.; Nigusse, T.; Dhanaraju, M.D. Silver Nanoparticles as Real Topical Bullets for Wound Healing. J. Am. Coll. Clin. Wound Spéc. 2011, 3, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.L.; Churg, A. A Model of Tobacco Smoke-Induced Airflow Obstruction in the Guinea Pig. Chest 2002, 121, 188S–191S. [Google Scholar] [CrossRef] [PubMed]
- Gardea-Torresdey, J.; Hejazi, M.; Tiemann, K.; Parsons, J.; Duarte-Gardea, M.; Henning, J. Use of hop (Humulus lupulus) agricultural by-products for the reduction of aqueous lead(II) environmental health hazards. J. Hazard. Mater. 2002, 91, 95–112. [Google Scholar] [CrossRef]
- Logeswari, P.; Silambarasan, S.; Abraham, J. Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. J. Saudi Chem. Soc. 2015, 19, 311–317. [Google Scholar] [CrossRef]
- Yuan, Y.-G.; Peng, Q.-L.; Gurunathan, S. Effects of Silver Nanoparticles on Multiple Drug-Resistant Strains of Staphylococcus aureus and Pseudomonas aeruginosa from Mastitis-Infected Goats: An Alternative Approach for Antimicrobial Therapy. Int. J. Mol. Sci. 2017, 18, 569. [Google Scholar] [CrossRef]
- Dos Santos, C.A.; Seckler, M.M.; Ingle, A.P.; Gupta, I.; Galdiero, S.; Galdiero, M.; Gade, A.; Rai, M. Silver Nanoparticles: Therapeutical Uses, Toxicity, and Safety Issues. J. Pharm. Sci. 2014, 103, 1931–1944. [Google Scholar] [CrossRef]
- Panáček, A.; Kolář, M.; Večeřová, R.; Prucek, R.; Soukupová, J.; Kryštof, V.; Hamal, P.; Zbořil, R.; Kvítek, L. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials 2009, 30, 6333–6340. [Google Scholar] [CrossRef]
- Pereira, L.; Dias, N.; Carvalho, J.; Fernandes, S.; Santos, C.; Lima, N. Synthesis, characterization and antifungal activity of chemically and fungal-produced silver nanoparticles against Trichophyton rubrum. J. Appl. Microbiol. 2014, 117, 1601–1613. [Google Scholar] [CrossRef]
- Xia, Z.-K.; Ma, Q.-H.; Li, S.-Y.; Zhang, D.-Q.; Cong, L.; Tian, Y.-L.; Yang, R.-Y. The antifungal effect of silver nanoparticles on Trichosporon asahii. J. Microbiol. Immunol. Infect. 2014, 49, 182–188. [Google Scholar] [CrossRef]
- Balakumaran, M.; Ramachandran, R.; Kalaichelvan, P. Exploitation of endophytic fungus, Guignardia mangiferae for extracellular synthesis of silver nanoparticles and their in vitro biological activities. Microbiol. Res. 2015, 178, 9–17. [Google Scholar] [CrossRef]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Soni, N.; Prakash, S. Efficacy of fungus mediated silver and gold nanoparticles against Aedes aegypti larvae. Parasitol. Res. 2012, 110, 175–184. [Google Scholar] [CrossRef]
- Santhoshkumar, T.; Rahuman, A.A.; Rajakumar, G.; Marimuthu, S.; Bagavan, A.; Jayaseelan, C.; Zahir, A.A.; Elango, G.; Kamaraj, C. Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol. Res. 2011, 108, 693–702. [Google Scholar] [CrossRef]
- Marimuthu, S.; Rahuman, A.A.; Rajakumar, G.; Santhoshkumar, T.; Kirthi, A.V.; Jayaseelan, C.; Bagavan, A.; Zahir, A.A.; Elango, G.; Kamaraj, C. Evaluation of green synthesized silver nanoparticles against parasites. Parasitol. Res. 2011, 108, 1541–1549. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Rahuman, A.A.; Rajakumar, G.; Kirthi, A.V.; Santhoshkumar, T.; Marimuthu, S.; Bagavan, A.; Kamaraj, C.; Zahir, A.A.; Elango, G. Synthesis of pediculocidal and larvicidal silver nanoparticles by leaf extract from heartleaf moonseed plant, Tinospora cordifolia Miers. Parasitol. Res. 2011, 109, 185–194. [Google Scholar] [CrossRef]
- Prabakar, K.; Jebanesan, A. Larvicidal efficacy of some Cucurbitacious plant leaf extracts against Culex quinquefasciatus (Say). Bioresour. Technol. 2004, 95, 113–114. [Google Scholar] [CrossRef]
- Murugan, K.; Nataraj, D.; Madhiyazhagan, P.; Sujitha, V.; Chandramohan, B.; Panneerselvam, C.; Dinesh, D.; Chandirasekar, R.; Kovendan, K.; Suresh, U.; et al. Carbon and silver nanoparticles in the fight against the filariasis vector Culex quinquefasciatus: Genotoxicity and impact on behavioral traits of non-target aquatic organisms. Parasitol. Res. 2016, 115, 1071–1083. [Google Scholar] [CrossRef]
- Suman, T.; Rajasree, S.R.; Kanchana, A.; Elizabeth, S.B. Biosynthesis, characterization and cytotoxic effect of plant mediated silver nanoparticles using Morinda citrifolia root extract. Colloids Surf. B Biointerfaces 2013, 106, 74–78. [Google Scholar] [CrossRef]
- Raghunandan, D.; Ravishankar, B.; Sharanbasava, G.; Mahesh, D.B.; Harsoor, V.; Yalagatti, M.S.; Bhagawanraju, M.; Venkataraman, A. Anti-cancer studies of noble metal nanoparticles synthesized using different plant extracts. Cancer Nanotechnol. 2011, 2, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Das, M.P.; Velusamy, P. Sesbania grandiflora leaf extract mediated green synthesis of antibacterial silver nanoparticles against selected human pathogens. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 104, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Suriyakalaa, U.; Antony, J.J.; Suganya, S.; Siva, D.; Sukirtha, R.; Kamalakkannan, S.; Pichiah, P.T.; Achiraman, S. Hepatocurative activity of biosynthesized silver nanoparticles fabricated using Andrographis paniculata. Colloids Surf. B Biointerfaces 2013, 102, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ayala-Nuñez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnology 2010, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Liang, J.; Dong, N.; Lu, J.; Fu, Y.; Fang, L.; Xiao, S.; Han, H. Glutathione-Capped Ag2S Nanoclusters Inhibit Coronavirus Proliferation through Blockage of Viral RNA Synthesis and Budding. ACS Appl. Mater. Interfaces 2018, 10, 4369–4378. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnology 2011, 9, 30. [Google Scholar] [CrossRef]
- Swarnalatha, L.; Rachela, S.; Ranjan, P.; Baradwaj, P. Evaluation of invitro antidiabetic activity of Sphaeranthus amaranthoides silver nanoparticles. Int. J. Nanomater. Biostruct. 2012, 2, 25–29. [Google Scholar]
- Pickup, J.C.; Zhi, Z.-L.; Khan, F.; Saxl, T.; Birch, D. Nanomedicine and its potential in diabetes research and practice. Diabetes/Metabolism Res. Rev. 2008, 24, 604–610. [Google Scholar] [CrossRef]
- Balan, K.; Qing, W.; Wang, Y.; Liu, X.; Palvannan, T.; Wang, Y.; Ma, F.; Zhang, Y. Antidiabetic activity of silver nanoparticles from green synthesis using Lonicera japonica leaf extract. RSC Adv. 2016, 6, 40162–40168. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Bai, Y.; Li, W.; Li, X.; Xing, X.; Wang, C.; Gao, L.; Yogi, M.; Swamy, M.K.; et al. Anticancer and Antibacterial Activities of Silver Nanoparticles (AgNPs) Synthesized from Cucumis melo L. J. Nanosci. Nanotechnol. 2020, 20, 4143–4151. [Google Scholar] [CrossRef]
- Soliman, W.; Khan, S.; Rizvi, S.; Moin, A.; Elsewedy, H.; Abulila, A.; Shehata, T. Therapeutic Applications of Biostable Silver Nanoparticles Synthesized Using Peel Extract of Benincasa hispida: Antibacterial and Anticancer Activities. Nanomaterials 2020, 10, 1954. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Mehwish, H.M.; Zhang, H.; Ashraf, M.; Fang, H.; Zeng, X.; Wu, Y.; Khurshid, M.; Zhao, L.; He, Z. Antibacterial and antioxidant activity of exopolysaccharide mediated silver nanoparticle synthesized by Lactobacillus brevis isolated from Chinese koumiss. Colloids Surf. B Biointerfaces 2020, 186, 110734. [Google Scholar] [CrossRef]
- LewisOscar, F.; Nithya, C.; Vismaya, S.; Arunkumar, M.; Pugazhendhi, A.; Nguyen-Tri, P.; Alharbi, S.A.; Alharbi, N.S.; Thajuddin, N. In vitro analysis of green fabricated silver nanoparticles (AgNPs) against Pseudomonas aeruginosa PA14 biofilm formation, their application on urinary catheter. Prog. Org. Coat. 2021, 151, 106058. [Google Scholar] [CrossRef]
- Roe, D.; Karandikar, B.; Bonn-Savage, N.; Gibbins, B.; Roullet, J.-B. Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J. Antimicrob. Chemother. 2008, 61, 869–876. [Google Scholar] [CrossRef]
- Chutrakulwong, F.; Thamaphat, K.; Limsuwan, P. Photo-irradiation induced green synthesis of highly stable silver nanoparticles using durian rind biomass: Effects of light intensity, exposure time and pH on silver nanoparticles formation. J. Phys. Commun. 2020, 4, 095015. [Google Scholar] [CrossRef]
- Rao, S.-Q.; Zhang, R.-Y.; Chen, R.; Gao, Y.-J.; Gao, L.; Yang, Z.-Q. Nanoarchitectonics for enhanced antibacterial activity with Lactobacillus buchneri S-layer proteins-coated silver nanoparticles. J. Hazard. Mater. 2022, 426, 128029. [Google Scholar] [CrossRef]
- Manjumeena, R.; Duraibabu, D.; Sudha, J.; Kalaichelvan, P. Biogenic nanosilver incorporated reverse osmosis membrane for antibacterial and antifungal activities against selected pathogenic strains: An enhanced eco-friendly water disinfection approach. J. Environ. Sci. Health Part A 2014, 49, 1125–1133. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Pratsinis, S.E. Antibacterial Activity of Nanosilver Ions and Particles. Environ. Sci. Technol. 2010, 44, 5649–5654. [Google Scholar] [CrossRef]
- Xia, N.; Chen, Z.; Liu, Y.; Ren, H.; Liu, L. Peptide aptamer-based biosensor for the detection of human chorionic gonadotropin by converting silver nanoparticles-based colorimetric assay into sensitive electrochemical analysis. Sens. Actuators B Chem. 2017, 243, 784–791. [Google Scholar] [CrossRef]
- Orlowski, P.; Tomaszewska, E.; Gniadek, M.; Baska, P.; Nowakowska, J.; Sokolowska, J.; Nowak, Z.; Donten, M.; Celichowski, G.; Grobelny, J.; et al. Tannic Acid Modified Silver Nanoparticles Show Antiviral Activity in Herpes Simplex Virus Type 2 Infection. PLoS ONE 2014, 9, e104113. [Google Scholar] [CrossRef]
- Avitabile, E.; Senes, N.; D’Avino, C.; Tsamesidis, I.; Pinna, A.; Medici, S.; Pantaleo, A. The potential antimalarial efficacy of hemocompatible silver nanoparticles from Artemisia species against P. falciparum parasite. PLoS ONE 2020, 15, e0238532. [Google Scholar] [CrossRef]
- Idan, E.; Ardalan, N. Introducing Silver Nanoparticles as Anti-Giardial inExperimentally Infected Mice: Therapy versus Toxicity. Syst. Rev. Pharm. 2020, 11, 701–708. [Google Scholar]
- Al-Quraishy, S.; Murshed, M.; Delic, D.; Al-Shaebi, E.M.; Qasem, M.A.; Mares, M.M.; Dkhil, M.A. Plasmodium chabaudi-infected mice spleen response to synthesized silver nanoparticles from Indigofera oblongifolia extract. Lett. Appl. Microbiol. 2020, 71, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.; Sokolonski, A.; Fonseca, M.; Stanisic, D.; Araújo, D.; Azevedo, V.; Portela, R.; Tasic, L. Applications of Silver Nanoparticles in Dentistry: Advances and Technological Innovation. Int. J. Mol. Sci. 2021, 22, 2485. [Google Scholar] [CrossRef] [PubMed]
- Syukri, D.M.; Nwabor, O.F.; Singh, S.; Ontong, J.C.; Wunnoo, S.; Paosen, S.; Munah, S.; Voravuthikunchai, S.P. Antibacterial-coated silk surgical sutures by ex situ deposition of silver nanoparticles synthesized with Eucalyptus camaldulensis eradicates infections. J. Microbiol. Methods 2020, 174, 105955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Hu, Y. Green synthesis of silver nanoparticles and their preventive effect in deficits in recognition and spatial memory in sporadic Alzheimer’s rat model. Colloids Surf. A Physicochem. Eng. Asp. 2020, 605, 125288. [Google Scholar] [CrossRef]
- Chen, H.; Lan, G.; Ran, L.; Xiao, Y.; Yu, K.; Lu, B.; Dai, F.; Wu, D.; Lu, F. A novel wound dressing based on a Konjac glucomannan/silver nanoparticle composite sponge effectively kills bacteria and accelerates wound healing. Carbohydr. Polym. 2018, 183, 70–80. [Google Scholar] [CrossRef]
- Kemah, B.; Uzer, G.; Turhan, Y.; Özturan, B.; Kılıç, B.; Gültepe, B.S.; Ceyran, A.B.; Ertürk, S.; Aksoylu, B.; Şenaydın, Ö.; et al. Effects of Local Application of Nano-silver on Osteomyelitis and Soft Tissue Infections: An Experimental Study in Rats. J. Bone Jt. Infect. 2018, 3, 43–49. [Google Scholar] [CrossRef]
- Yeasmin, S.; Datta, H.K.; Chaudhuri, S.; Malik, D.; Bandyopadhyay, A. In-vitro anti-cancer activity of shape controlled silver nanoparticles (AgNPs) in various organ specific cell lines. J. Mol. Liq. 2017, 242, 757–766. [Google Scholar] [CrossRef]
- Priya, K.; Vijayakumar, M.; Janani, B. Chitosan-mediated synthesis of biogenic silver nanoparticles (AgNPs), nanoparticle characterisation and in vitro assessment of anticancer activity in human hepatocellular carcinoma HepG2 cells. Int. J. Biol. Macromol. 2020, 149, 844–852. [Google Scholar] [CrossRef]
- Yoosaf, K.; Ipe, B.I.; Suresh, C.H.; Thomas, K.G. In Situ Synthesis of Metal Nanoparticles and Selective Naked-Eye Detection of Lead Ions from Aqueous Media. J. Phys. Chem. C 2007, 111, 12839–12847. [Google Scholar] [CrossRef]
- Kossyrev, P.A.; Yin, A.; Cloutier, S.G.; Cardimona, D.A.; Huang, D.; Alsing, P.M.; Xu, J.M. Electric Field Tuning of Plasmonic Response of Nanodot Array in Liquid Crystal Matrix. Nano Lett. 2005, 5, 1978–1981. [Google Scholar] [CrossRef]
- Zhang, H.; Tao, X.; Yu, T.; Wang, S. Conductive knitted fabric as large-strain gauge under high temperature. Sens. Actuators A Phys. 2006, 126, 129–140. [Google Scholar] [CrossRef]
- Sergeev, G.B.; Shabatina, T.I. Cryochemistry of nanometals. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313–314, 18–22. [Google Scholar] [CrossRef]
- Sudrik, S.G.; Sharma, J.; Chavan, V.B.; Chaki, N.K.; Sonawane, H.R.; Vijayamohanan, K.P. Wolff Rearrangement of α-Diazoketones Using in Situ Generated Silver Nanoclusters as Electron Mediators. Org. Lett. 2006, 8, 1089–1092. [Google Scholar] [CrossRef]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Duan, S.-S.; Ouyang, Y.-S.; Chen, Y.-B. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Biometals 2011, 24, 135–141. [Google Scholar] [CrossRef]
- Petrus, E.; Tinakumari, S.; Chai, L.; Ubong, A.; Tunung, R.; Elexson, N.; Chai, L.; Son, R. A study on the minimum inhibitory concentration and minimum bactericidal concentration of nano colloidal silver on food-borne pathogens. Int. Food Res. J. 2011, 18, 55–66. [Google Scholar]
- Zarei, M.; Jamnejad, A.; Khajehali, E. Antibacterial Effect of Silver Nanoparticles Against Four Foodborne Pathogens. Jundishapur J. Microbiol. 2014, 7, e8720. [Google Scholar] [CrossRef]
- Islam, S.U.; Butola, B.; Verma, D. Facile synthesis of chitosan-silver nanoparticles onto linen for antibacterial activity and free-radical scavenging textiles. Int. J. Biol. Macromol. 2019, 133, 1134–1141. [Google Scholar] [CrossRef]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 168–171. [Google Scholar] [CrossRef]
- Wijnhoven, S.W.; Peijnenburg, W.J.; Herberts, C.A.; Hagens, W.I.; Oomen, A.G.; Heugens, E.H.; Roszek, B.; Bisschops, J.; Gosens, I.; Van De Meent, D.; et al. Nano-silver—A review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 2009, 3, 109–138. [Google Scholar] [CrossRef]
- Seung, W.; Gupta, M.K.; Lee, K.Y.; Shin, K.-S.; Lee, J.-H.; Kim, T.Y.; Kim, S.; Lin, J.; Kim, J.H.; Kim, S.-W. Nanopatterned Textile-Based Wearable Triboelectric Nanogenerator. ACS Nano 2015, 9, 3501–3509. [Google Scholar] [CrossRef] [PubMed]
- Kerem, E.; Hirawat, S.; Armoni, S.; Yaakov, Y.; Shoseyov, D.; Cohen, M.; Nissim-Rafinia, M.; Blau, H.; Rivlin, J.; Aviram, M.; et al. Effectiveness of PTC124 treatment of cystic fibrosis caused by nonsense mutations: A prospective phase II trial. Lancet 2008, 372, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.S.; Cho, H.S.; Rha, D.S.; Kim, J.M.; Park, J.D.; Choi, B.S.; Lim, R.; Chang, H.K.; Chung, Y.H.; et al. Twenty-Eight-Day Oral Toxicity, Genotoxicity, and Gender-Related Tissue Distribution of Silver Nanoparticles in Sprague-Dawley Rats. Inhal. Toxicol. 2008, 20, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Noorbakhsh, F.; Rezaie, S.; Shahverdi, A. Antifungal Effects of Silver Nanoparticle alone and with Combination of Antifungal Drug on Dermatophyte Pathogen Trichophyton Rubrum. In Proceedings of the 2011 International Conference on Bioscience, Biochemistry and Bioinformatics, Singapore, 26–28 February 2011; Volume 5, pp. 364–367. [Google Scholar]
- Nasser, F.; Lynch, I. Updating traditional regulatory tests for use with novel materials: Nanomaterial toxicity testing with Daphnia magna. Saf. Sci. 2019, 118, 497–504. [Google Scholar] [CrossRef]
- Kokura, S.; Handa, O.; Takagi, T.; Ishikawa, T.; Naito, Y.; Yoshikawa, T. Silver nanoparticles as a safe preservative for use in cosmetics. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 570–574. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, G.; Meng, H.; Li, L. Effect of Exercise-induced Sweating on facial sebum, stratum corneum hydration, and skin surface pH in normal population. Ski. Res. Technol. 2012, 19, e312–e317. [Google Scholar] [CrossRef]
- Wiechers, J.W.; Musee, N. Engineered Inorganic Nanoparticles and Cosmetics: Facts, Issues, Knowledge Gaps and Challenges. J. Biomed. Nanotechnol. 2010, 6, 408–431. [Google Scholar] [CrossRef]
- Gajbhiye, S.; Sakharwade, S. Silver Nanoparticles in Cosmetics. J. Cosmet. Dermatol. Sci. Appl. 2016, 06, 48–53. [Google Scholar] [CrossRef]
- Lohani, A.; Verma, A.; Joshi, H.; Yadav, N.; Karki, N. Nanotechnology-Based Cosmeceuticals. ISRN Dermatol. 2014, 2014, 843687. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Jain, J.; Arora, S.; Rajwade, J.M.; Omray, P.; Khandelwal, S.; Paknikar, K.M. Silver Nanoparticles in Therapeutics: Development of an Antimicrobial Gel Formulation for Topical Use. Mol. Pharm. 2009, 6, 1388–1401. [Google Scholar] [CrossRef]
- El-Temsah, Y.S.; Joner, E.J. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ. Toxicol. 2010, 27, 42–49. [Google Scholar] [CrossRef]
- Nejatzadeh-Barandozi, F.; Darvishzadeh, F.; Aminkhani, A. Effect of nano silver and silver nitrate on seed yield of (Ocimum basilicum L.). Org. Med. Chem. Lett. 2014, 4, 11. [Google Scholar] [CrossRef]
- Byczyńska, A. Improvement of postharvest quality of cut tulip “White Parrot” by nano silver. World Sci. News. 2017, 83, 224–228. [Google Scholar]
- Thuesombat, P.; Hannongbua, S.; Akasit, S.; Chadchawan, S. Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol. Environ. Saf. 2014, 104, 302–309. [Google Scholar] [CrossRef]
- Mehrian, S.K.; De Lima, R. Nanoparticles cyto and genotoxicity in plants: Mechanisms and abnormalities. Environ. Nanotechnol. Monit. Manag. 2016, 6, 184–193. [Google Scholar] [CrossRef]
- Shams, A.S.; Abd El-Rahman, H.M.; El-Ramady, H.R. Evaluation of integrated nutrient management practices for lettuce production under drip irrigation system. J. Appl. Sci. Res. 2013, 9, 2223–2231. [Google Scholar]
- Hatami, M.; Hatamzadeh, A.; Ghasemnezhad, M.; Sajidi, R.H. Variations of the Phytochemical Compounds in Rose-scented Geranium Plant Exposed to Nanosilver Particles. J. Essent. Oil Bear. Plants 2016, 19, 1747–1753. [Google Scholar] [CrossRef]
- Samaras, A.; Efthimiou, K.; Roumeliotis, E.; Karaoglanidis, G. Biocontrol potential and plant-growth-promoting effects of Bacillus amyloliquefaciens MBI 600 against Fusarium oxysporum f. sp. radicis-lycopersici on tomato. Acta Hortic. 2018, 1207, 139–146. [Google Scholar] [CrossRef]
- Da Costa, M.V.J.; Sharma, P.K. Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 2016, 54, 110–119. [Google Scholar] [CrossRef]
- Homaee, M.B.; Ehsanpour, A.A. Silver nanoparticles and silver ions: Oxidative stress responses and toxicity in potato (Solanum tuberosum L) grown in vitro. Hortic. Environ. Biotechnol. 2016, 57, 544–553. [Google Scholar] [CrossRef]
- Sharma, P.; Bhatt, D.; Zaidi, M.G.H.; Saradhi, P.P.; Khanna, P.K.; Arora, S. Silver Nanoparticle-Mediated Enhancement in Growth and Antioxidant Status of Brassica juncea. Appl. Biochem. Biotechnol. 2012, 167, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Gusev, A.; Ko, A.; Shi, H.; Bhatia, G.; Chung, W.; Penninx, B.W.J.H.; Jansen, R.; de Geus, E.J.C.; Boomsma, D.I.; Wright, F.A.; et al. Integrative approaches for large-scale transcriptome-wide association studies. Nat. Genet. 2016, 48, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.U.; Yasur, J.; Loke, K.S.; Dutta, D. Effect of synthetic and biosynthesized silver nanoparticles on growth, physiology and oxidative stress of water hyacinth: Eichhornia crassipes (Mart) Solms. Acta Physiol. Plant. 2016, 38, 58. [Google Scholar] [CrossRef]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Kah, M.; Beulke, S.; Tiede, K.; Hofmann, T. Nanopesticides: State of Knowledge, Environmental Fate, and Exposure Modeling. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1823–1867. [Google Scholar] [CrossRef]
- Lamsal, K.; Kim, S.W.; Jung, J.H.; Kim, Y.S.; Kim, K.S.; Lee, Y.S. Application of Silver Nanoparticles for the Control of Colletotrichum Species In Vitro and Pepper Anthracnose Disease in Field. Mycobiology 2011, 39, 194–199. [Google Scholar] [CrossRef]
- Marimuthu, S.; Rahuman, A.A.; Jayaseelan, C.; Kirthi, A.V.; Santhoshkumar, T.; Velayutham, K.; Bagavan, A.; Kamaraj, C.; Elango, G.; Iyappan, M.; et al. Acaricidal activity of synthesized titanium dioxide nanoparticles using Calotropis gigantea against Rhipicephalus microplus and Haemaphysalis bispinosa. Asian Pac. J. Trop. Med. 2013, 6, 682–688. [Google Scholar] [CrossRef]
- Zahir, A.A.; Rahuman, A.A. Evaluation of different extracts and synthesised silver nanoparticles from leaves of Euphorbia prostrata against Haemaphysalis bispinosa and Hippobosca maculata. Vet. Parasitol. 2012, 187, 511–520. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Salem, N.M.; Albanna, L.S.; Awwad, A.M. Green synthesis of sulfur nanoparticles using Punica granatum peels and the effects on the growth of tomato by foliar spray applications. Environ. Nanotechnol. Monit. Manag. 2016, 6, 83–87. [Google Scholar] [CrossRef]
- Ghidan, A.; Al-Antary, T.; Awwad, A.; Ghidan, O.; Araj, S.; Ateyyat, M. Comparison of different green synthesized nanomaterials on green peach aphid as aphicidal potential. Fresenius Environ. Bull. 2018, 27, 7009–7016. [Google Scholar]
- Höglund, S. Some Electron Microscopic Studies on the Satellite Tobacco Necrosis Virus and its IgG-antibody. J. Gen. Virol. 1968, 2, 427–436. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Babu, R.S.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf. B Biointerfaces 2008, 65, 150–153. [Google Scholar] [CrossRef]
- Pugazhenthiran, N.; Anandan, S.; Kathiravan, G.; Prakash, N.K.U.; Crawford, S.; AshokKumar, M. Microbial synthesis of silver nanoparticles by Bacillus sp. J. Nanopart. Res. 2009, 11, 1811–1815. [Google Scholar] [CrossRef]
- Xie, J.; Lee, J.Y.; Wang, D.I.C.; Ting, Y.P. Silver Nanoplates: From Biological to Biomimetic Synthesis. ACS Nano 2007, 1, 429–439. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jagan, E.; Ramachandran, R.; Abirami, S.; Mohan, N.; Kalaichelvan, P. Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. plant growth metabolism. Process. Biochem. 2012, 47, 651–658. [Google Scholar] [CrossRef]
- Fu, L.; Wang, Z.; Dhankher, O.P.; Xing, B. Nanotechnology as a new sustainable approach for controlling crop diseases and increasing agricultural production. J. Exp. Bot. 2020, 71, 507–519. [Google Scholar] [CrossRef]
- Pérez-De-Luque, A. Interaction of Nanomaterials with Plants: What Do We Need for Real Applications in Agriculture? Front. Environ. Sci. 2017, 5, 12. [Google Scholar] [CrossRef]
- An, C.; Sun, C.; Li, N.; Huang, B.; Jiang, J.; Shen, Y.; Wang, C.; Zhao, X.; Cui, B.; Wang, C.; et al. Nanomaterials and nanotechnology for the delivery of agrochemicals: Strategies towards sustainable agriculture. J. Nanobiotechnology 2022, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Kalaichelvan, P.T.; Venkatesan, R. Mycobased Synthesis of Silver Nanoparticles and Their Incorporation into Sodium Alginate Films for Vegetable and Fruit Preservation. J. Agric. Food Chem. 2009, 57, 6246–6252. [Google Scholar] [CrossRef] [PubMed]
- de Azeredo, H.M.C. Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Effect of nanocomposite packaging containing Ag and ZnO on inactivation of Lactobacillus plantarum in orange juice. Food Control 2011, 22, 408–413. [Google Scholar] [CrossRef]
- Fernandez, A.; Picouet, P.; Lloret, E. Reduction of the Spoilage-Related Microflora in Absorbent Pads by Silver Nanotechnology during Modified Atmosphere Packaging of Beef Meat. J. Food Prot. 2010, 73, 2263–2269. [Google Scholar] [CrossRef]
- Babu, M.; Devi, V.; Ramakritinan, C.; Umarani, R.; Taredahalli, N.; Kumaraguru, A. Application of Biosynthesized Silver Nanoparticles in Agricultural and Marine Pest Control. Curr. Nanosci. 2013, 10, 374–381. [Google Scholar] [CrossRef]
- Mansoor, S.; Zahoor, I.; Baba, T.R.; Padder, S.A.; Bhat, Z.A.; Koul, A.M.; Jiang, L. Fabrication of Silver Nanoparticles Against Fungal Pathogens. Front. Nanotechnol. 2021, 3, 679358. [Google Scholar] [CrossRef]
- Gosavi, V.C.; Daspute, A.A.; Patil, A.; Gangurde, A.; Wagh, S.G.; Sherkhane, A.; Deshmukh, V.A. Synthesis of green nanobiofertilizer using silver nanoparticles of Allium cepa extract Short title: Green nanofertilizer from Allium cepa. Int. J. Chem. Stud. 2020, 8, 1690–1694. [Google Scholar] [CrossRef]
- Fouda, M.M.; Abdelsalam, N.R.; El-Naggar, M.E.; Zaitoun, A.F.; Salim, B.M.; Bin-Jumah, M.; Allam, A.; Abo-Marzoka, S.A.; Kandil, E.E. Impact of high throughput green synthesized silver nanoparticles on agronomic traits of onion. Int. J. Biol. Macromol. 2020, 149, 1304–1317. [Google Scholar] [CrossRef]
- Sadak, M.S. Impact of silver nanoparticles on plant growth, some biochemical aspects, and yield of fenugreek plant (Trigonella foenum-graecum). Bull. Natl. Res. Cent. 2019, 43, 38. [Google Scholar] [CrossRef]
- Kumar, P.; Pahal, V.; Gupta, A.; Vadhan, R.; Chandra, H.; Dubey, R.C. Effect of silver nanoparticles and Bacillus cereus LPR2 on the growth of Zea mays. Sci. Rep. 2020, 10, 20409. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, B.R.; Singh, A.; Keswani, C.; Naqvi, A.H.; Singh, H.B. Biofabricated Silver Nanoparticles Act as a Strong Fungicide against Bipolaris sorokiniana Causing Spot Blotch Disease in Wheat. PLoS ONE 2014, 9, e97881. [Google Scholar] [CrossRef]
- Chandhru, M.; Logesh, R.; Rani, S.K.; Ahmed, N.; Vasimalai, N. One-pot green route synthesis of silver nanoparticles from jack fruit seeds and their antibacterial activities with escherichia coli and salmonella bacteria. Biocatal. Agric. Biotechnol. 2019, 20, 101241. [Google Scholar] [CrossRef]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Evaluation of nanocomposite packaging containing Ag and ZnO on shelf life of fresh orange juice. Innov. Food Sci. Emerg. Technol. 2010, 11, 742–748. [Google Scholar] [CrossRef]
- Chen, P.; Yin, L.; El-Seedi, H.R.; Zou, X.; Guo, Z. Green reduction of silver nanoparticles for cadmium detection in food using surface-enhanced Raman spectroscopy coupled multivariate calibration. Food Chem. 2022, 394, 133481. [Google Scholar] [CrossRef]
- Noshad, A.; Hetherington, C.; Iqbal, M. Impact of AgNPs on Seed Germination and Seedling Growth: A Focus Study on Its Antibacterial Potential against Clavibacter michiganensis subsp. michiganensis Infection in Solanum lycopersicum. J. Nanomater. 2019, 2019, 6316094. [Google Scholar] [CrossRef]
- Pallavi, N.; Mehta, C.M.; Srivastava, R.; Arora, S.; Sharma, A.K. Impact assessment of silver nanoparticles on plant growth and soil bacterial diversity. 3 Biotech 2016, 6, 254. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Hatata, M.M.; Al-Huqail, A.A.; Ibrahim, M.M. Preparation, characterization of silver phyto nanoparticles and their impact on growth potential of Lupinus termis L. seedlings. Saudi J. Biol. Sci. 2018, 25, 313–319. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Shweta; Upadhyay, N.; Singh, J.; Liu, S.; Singh, V.P.; Prasad, S.M.; Chauhan, D.; Tripathi, D.K.; Sharma, S. Differential Phytotoxic Impact of Plant Mediated Silver Nanoparticles (AgNPs) and Silver Nitrate (AgNO3) on Brassica sp. Front. Plant Sci. 2017, 8, 1501. [Google Scholar] [CrossRef]
- Mehmood, A.; Murtaza, G. Impact of biosynthesized silver nanoparticles on protein and carbohydrate contents in seeds of Pisum sativum L. Crop Breed. Appl. Biotechnol. 2017, 17, 334–340. [Google Scholar] [CrossRef]
- Zhai, Y.; Hunting, E.R.; Wouters, M.; Peijnenburg, W.J.G.M.; Vijver, M.G. Silver Nanoparticles, Ions, and Shape Governing Soil Microbial Functional Diversity: Nano Shapes Micro. Front. Microbiol. 2016, 7, 1123. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Yousef, N.M.H.; Nafady, N.A. Application of Biosynthesized Silver Nanoparticles for the Control of Land Snail Eobania vermiculata and Some Plant Pathogenic Fungi. J. Nanomater. 2015, 2015, 218904. [Google Scholar] [CrossRef]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.H.; Jung, J.H.; Kim, S.S.; Yoon, J.-U.; Park, J.D.; Choi, B.S.; Chung, Y.H.; Kwon, I.H.; Jeong, J.; Han, B.S.; et al. Twenty-Eight-Day Inhalation Toxicity Study of Silver Nanoparticles in Sprague-Dawley Rats. Inhal. Toxicol. 2007, 19, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, Z.; Zara, J.N.; Hsu, C.; Soofer, D.E.; Lee, K.S.; Siu, R.K.; Miller, L.S.; Zhang, X.; Carpenter, D.; et al. The antimicrobial and osteoinductive properties of silver nanoparticle/poly (dl-lactic-co-glycolic acid)-coated stainless steel. Biomaterials 2012, 33, 8745–8756. [Google Scholar] [CrossRef]
- Srinonate, A.; Banlunara, W.; Maneewattanapinyo, P.; Thammacharoen, C.; Ekgasit, S.; Kaewamatawong, T. Acute toxicity study of nanosilver particles in tilapia (Oreochromis niloticus): Pathological changes, particle bioaccumulation and metallothionien protein expression. Thai J. Vet. Med. 2015, 45, 81–89. [Google Scholar]
- Sung, J.H.; Ji, J.H.; Park, J.D.; Yoon, J.U.; Kim, D.S.; Jeon, K.S.; Song, M.Y.; Jeong, J.; Han, B.S.; Han, J.H.; et al. Subchronic Inhalation Toxicity of Silver Nanoparticles. Toxicol. Sci. 2008, 108, 452–461. [Google Scholar] [CrossRef]
- Tang, J.; Xiong, L.; Zhou, G.; Wang, S.; Wang, J.; Liu, L.; Li, J.; Yuan, F.; Lu, S.; Wan, Z.; et al. Silver Nanoparticles Crossing Through and Distribution in the Blood-Brain Barrier In Vitro. J. Nanosci. Nanotechnol. 2010, 10, 6313–6317. [Google Scholar] [CrossRef]
- Vandebriel, R.J.; Tonk, E.C.; De La Fonteyne-Blankestijn, L.J.; Gremmer, E.R.; Verharen, H.W.; Van Der Ven, L.T.; Van Loveren, H.; De Jong, W.H. Immunotoxicity of silver nanoparticles in an intravenous 28-day repeated-dose toxicity study in rats. Part. Fibre Toxicol. 2014, 11, 21. [Google Scholar] [CrossRef]
- Li, W.W.-Y.; Lu, G.; Pang, C.-P.; Lam, D.S.-C.; Yew, D.T. The Eyes of Anencephalic Babies: A Morphological and Immunohistochemical Evaluation. Int. J. Neurosci. 2007, 117, 121–134. [Google Scholar] [CrossRef]
- AshaRrani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Panda, P.K.; Kumari, P.; Patel, P.; Samal, S.K.; Mishra, S.; Tambuwala, M.M.; Dutt, A.; Hilscherová, K.; Mishra, Y.K.; Varma, R.S.; et al. Molecular nanoinformatics approach assessing the biocompatibility of biogenic silver nanoparticles with channelized intrinsic steatosis and apoptosis. Green Chem. 2021, 24, 1190–1210. [Google Scholar] [CrossRef]
- Murphy, M.; Ting, K.; Zhang, X.; Soo, C.; Zheng, Z. Current Development of Silver Nanoparticle Preparation, Investigation, and Application in the Field of Medicine. J. Nanomater. 2015, 2015, 696918. [Google Scholar] [CrossRef]
- Hussain, S.M.; Hess, K.L.; Gearhart, J.M.; Geiss, K.T.; Schlager, J.J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. Vitr. 2005, 19, 975–983. [Google Scholar] [CrossRef]
- Sahu, S.C.; Zheng, J.; Graham, L.; Chen, L.; Ihrie, J.; Yourick, J.J.; Sprando, R.L. Comparative cytotoxicity of nanosilver in human liver HepG2 and colon Caco2 cells in culture. J. Appl. Toxicol. 2014, 34, 1155–1166. [Google Scholar] [CrossRef]
- Taghyan, S.A.; El Messiry, H.; El Zainy, M.A. Evaluation of the toxic effect of silver nanoparticles and the possible protective effect of ascorbic acid on the parotid glands of albino rats: An in vivo study. Toxicol. Ind. Health 2020, 36, 446–453. [Google Scholar] [CrossRef]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique Cellular Interaction of Silver Nanoparticles: Size-Dependent Generation of Reactive Oxygen Species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef]
- Kumar, N.; Palmer, G.R.; Shah, V.; Walker, V.K. The Effect of Silver Nanoparticles on Seasonal Change in Arctic Tundra Bacterial and Fungal Assemblages. PLoS ONE 2014, 9, e99953. [Google Scholar] [CrossRef]
- Völker, C.; Gräf, T.; Schneider, I.; Oetken, M.; Oehlmann, J. Combined effects of silver nanoparticles and 17α-ethinylestradiol on the freshwater mudsnail Potamopyrgus antipodarum. Environ. Sci. Pollut. Res. 2014, 21, 10661–10670. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef]
- Quevedo, A.C.; Lynch, I.; Valsami-Jones, E. Silver nanoparticle induced toxicity and cell death mechanisms in embryonic zebrafish cells. Nanoscale 2021, 13, 6142–6161. [Google Scholar] [CrossRef] [PubMed]

| S. No. | Source of Synthesis | Size Range of AgNPs (nm) | Agriculture and Food Industry Applications | References |
|---|---|---|---|---|
| 1. | Shewanella algae bangaramma | 5–30 nm | Pest control | [171] |
| 2. | β-1, 3 glucan | 32–46 nm | Nanofertlizer | [172] |
| 3. | Allium cepa | 10–30 nm | Nanofertilizer | [173] |
| 4. | Rice starch | 8 nm | Effect on agronomic traits of onion | [174] |
| 5. | Tri-sodium citrate | 20 nm | Effect on growth, some biochemical aspects, and yield of Trigonella foenumgraecum | [175] |
| 6. | Tagetes erecta | 60 nm | Effect on the growth of Zea mays | [176] |
| 7. | Serratia sp. BHU-S4 | 10–20 nm | Effect against spot blotch disease in wheat | [177] |
| 8. | Jack fruit seeds | 20–30 nm | Antibacterial activity against food-borne bacteria | [178] |
| 9. | Commercially purchased | 10 nm | For increasing the shelf life of fresh orange juice | [179] |
| 10. | Electrostatic adsorption method | 10 nm | Ant mildew and in the storage of rice | [180] |
| 11. | Fungal extract | 3–20 and 4–20 nm | Impact on Seed Germination and Seedling Growth | [181] |
| 12. | Chemical reduction | 30 and 40 nm | Effect on plant growth and soil bacterial diversity | [182] |
| 13. | Coriandrum sativum leaves extract | 20–80 nm | Effect on the growth potential of Lupinus termis L. seedlings | [183] |
| 14. | Aloe vera leaves | 47 nm | Phytotoxic impact on Brassica sp. | [184] |
| 15. | Berberis lycium | 54 nm | Impact on protein and carbohydrate contents in seeds of Pisum sativum | [185] |
| 16. | Commercially purchased | 50–80 nm | Soil improvement | [186] |
| 17. | Raphanus sativus var. aegyptiacus | 2–100 nm | Control of land snail Eobania vermiculata and pathogenic fungi on plants | [187] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husain, S.; Nandi, A.; Simnani, F.Z.; Saha, U.; Ghosh, A.; Sinha, A.; Sahay, A.; Samal, S.K.; Panda, P.K.; Verma, S.K. Emerging Trends in Advanced Translational Applications of Silver Nanoparticles: A Progressing Dawn of Nanotechnology. J. Funct. Biomater. 2023, 14, 47. https://doi.org/10.3390/jfb14010047
Husain S, Nandi A, Simnani FZ, Saha U, Ghosh A, Sinha A, Sahay A, Samal SK, Panda PK, Verma SK. Emerging Trends in Advanced Translational Applications of Silver Nanoparticles: A Progressing Dawn of Nanotechnology. Journal of Functional Biomaterials. 2023; 14(1):47. https://doi.org/10.3390/jfb14010047
Chicago/Turabian StyleHusain, Shaheen, Aditya Nandi, Faizan Zarreen Simnani, Utsa Saha, Aishee Ghosh, Adrija Sinha, Aarya Sahay, Shailesh Kumar Samal, Pritam Kumar Panda, and Suresh K. Verma. 2023. "Emerging Trends in Advanced Translational Applications of Silver Nanoparticles: A Progressing Dawn of Nanotechnology" Journal of Functional Biomaterials 14, no. 1: 47. https://doi.org/10.3390/jfb14010047
APA StyleHusain, S., Nandi, A., Simnani, F. Z., Saha, U., Ghosh, A., Sinha, A., Sahay, A., Samal, S. K., Panda, P. K., & Verma, S. K. (2023). Emerging Trends in Advanced Translational Applications of Silver Nanoparticles: A Progressing Dawn of Nanotechnology. Journal of Functional Biomaterials, 14(1), 47. https://doi.org/10.3390/jfb14010047











