Gelatin Matrix as Functional Biomaterial for Immobilization of Nanoparticles of Metal-Containing Compounds
Abstract
1. Introduction
- -
- Dispersion of previously created particles of a given substance in an array or a thin layer of gelatin;
- -
- The formation of particles of a given substance directly in a gelatin mass or a thin gelatin layer as a result of chemical transformations occurring with the participation of any other gelatin-immobilized chemicals.
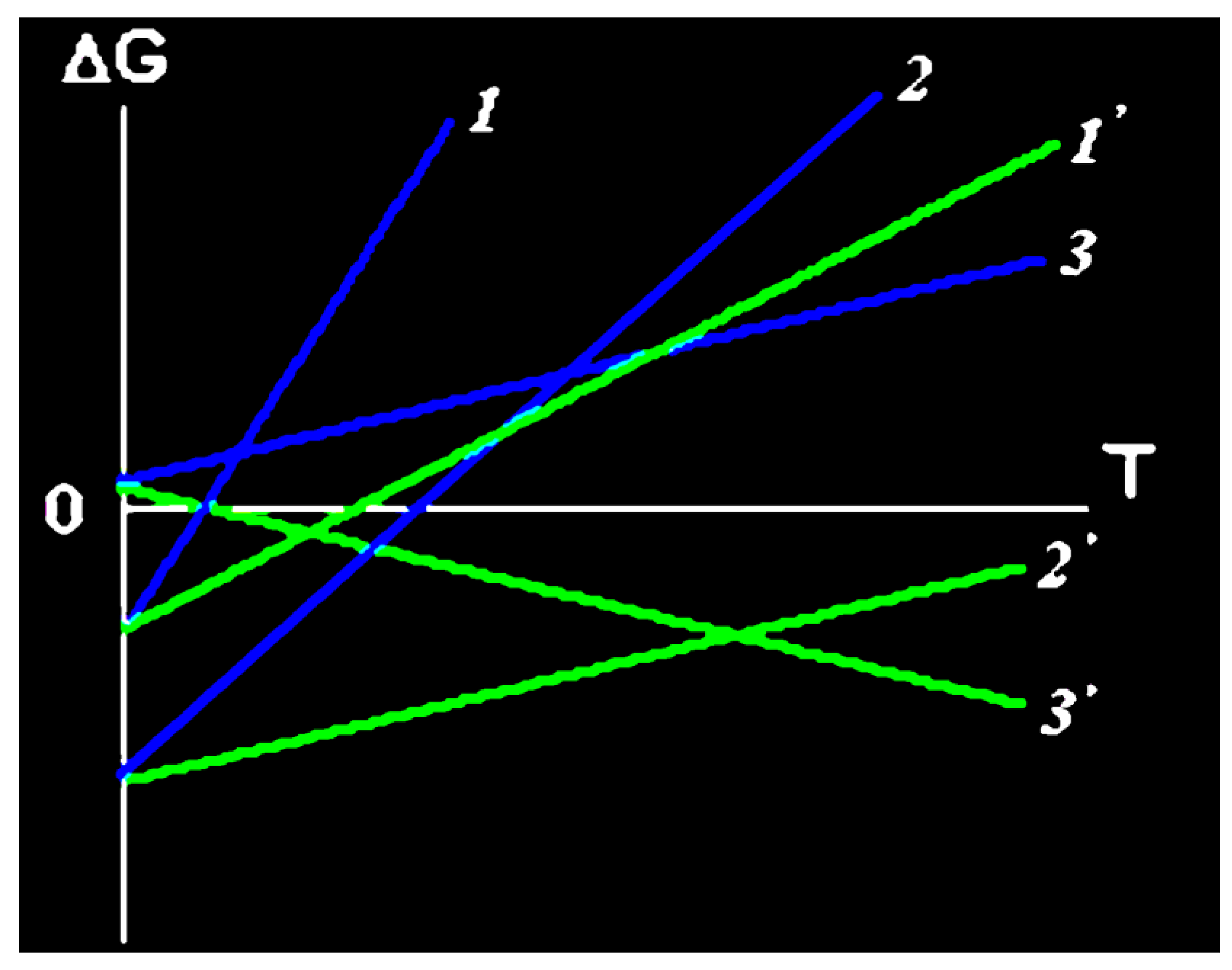
2. Physicochemistry of Gelatin as a High-Molecular Component of Immobilized Matrix Systems
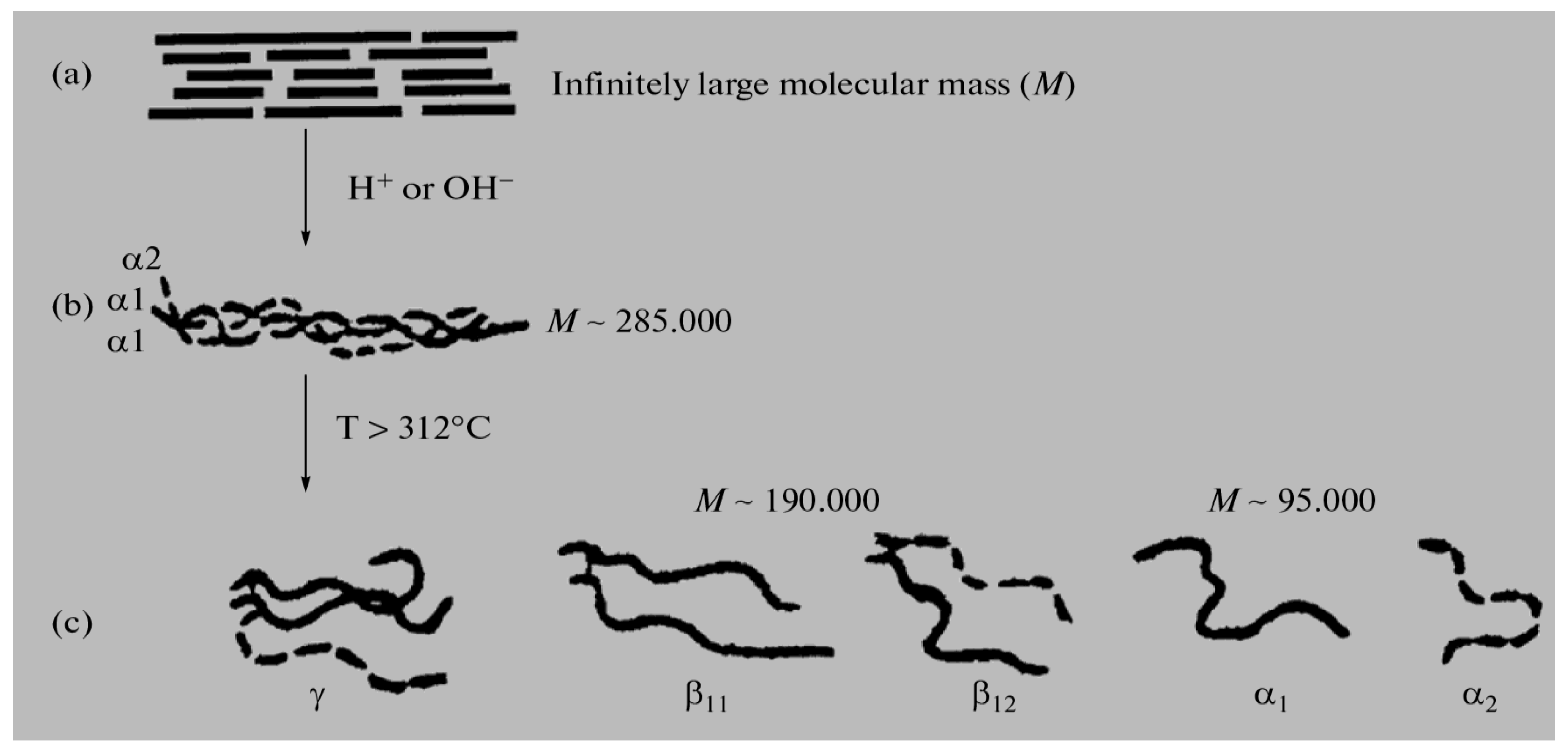
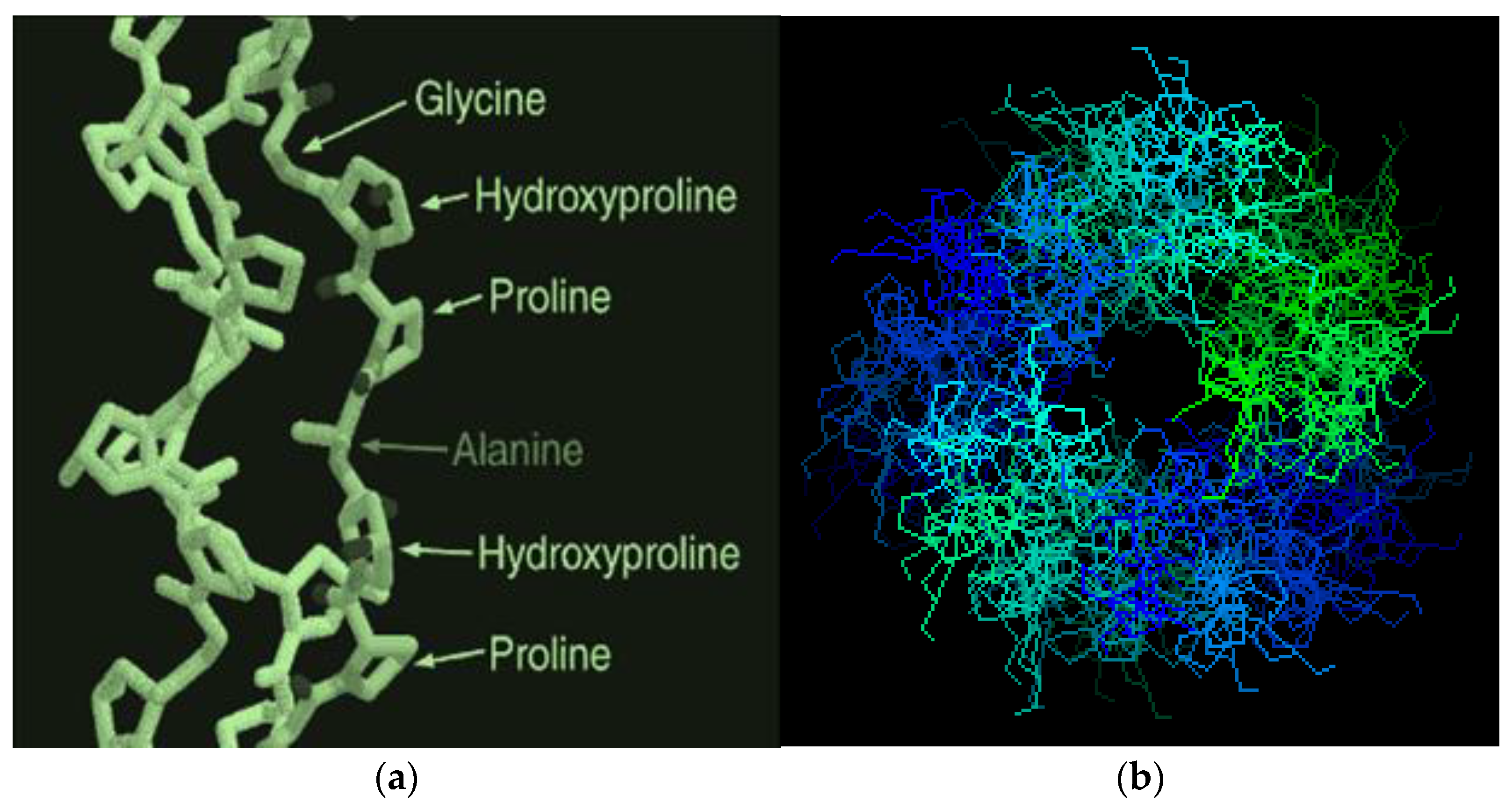
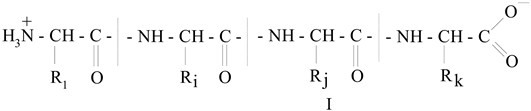 various radicals). The molecular mass (M) of these fractions is in a very wide range, from 15,000 to ~300,000 Daltons, so among them there are both low-molecular weight and high-molecular weight polypeptides [10,11,12,13,14,15]. In general, gelatin contains residues of 18 natural amino acids out of 20, with the exception of cystine and cysteine. Gelatin is often called a “biopolymer”, and although such a name is not entirely correct (since the concept of “polymer” in general and “biopolymer” in particular includes only those synthetic and natural high-molecular compounds for which a regularly repeating structural unit can be indicated, as, for example, in polypropylene (–CH2–CH2–CH2−)n or in natural rubber (–CH2–C(CH3)=CH2−)n, which is not the case for gelatin), we will also use this term from time to time. Gelatin is obtained from another natural protein that is part of various connective tissues of animals, namely collagen, by exposing it to either sulfuric, hydrochloric, orthophosphoric acids (the so-called acid gelatin) or an aqueous solution of calcium hydroxide (the so-called alkaline gelatin). In this regard, three levels of organization of polypeptide fractions associated with it and gelatin are distinguished (Figure 1). The structure and properties of both of these biopolymers have been repeatedly studied over the past few decades (see, in particular [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70]). The total number of works on this topic is at least several hundred, and in this article, it is not possible even to simply cite them in full. As a result of the studies, it was found that the macromolecules of these high-molecular polypeptides consist of three polypeptide chains with almost the same molecular mass (Figure 2a); moreover, two of them, as a rule, are almost identical to each other in the set and sequence of amino acids (the so-called α1-chains), while the third (the so-called α2-chain) in this respect differs from the two others [26,32,33]. Each of these chains contains over 1000 amino acid residues [19,50] and has M ~ 95,000 Daltons. The gelatin molecule is stabilized by the formation of covalent cross-links, both within the gelatin triple helix and between gelatin helices. During the transformation of collagen into gelatin, a polydisperse mixture is formed containing single (α1 and α2), double (β11 and β12) and triple (γ) polypeptide chains, which, unlike collagen macromolecules, are formed in the form of coils or clots (Figure 2b).
various radicals). The molecular mass (M) of these fractions is in a very wide range, from 15,000 to ~300,000 Daltons, so among them there are both low-molecular weight and high-molecular weight polypeptides [10,11,12,13,14,15]. In general, gelatin contains residues of 18 natural amino acids out of 20, with the exception of cystine and cysteine. Gelatin is often called a “biopolymer”, and although such a name is not entirely correct (since the concept of “polymer” in general and “biopolymer” in particular includes only those synthetic and natural high-molecular compounds for which a regularly repeating structural unit can be indicated, as, for example, in polypropylene (–CH2–CH2–CH2−)n or in natural rubber (–CH2–C(CH3)=CH2−)n, which is not the case for gelatin), we will also use this term from time to time. Gelatin is obtained from another natural protein that is part of various connective tissues of animals, namely collagen, by exposing it to either sulfuric, hydrochloric, orthophosphoric acids (the so-called acid gelatin) or an aqueous solution of calcium hydroxide (the so-called alkaline gelatin). In this regard, three levels of organization of polypeptide fractions associated with it and gelatin are distinguished (Figure 1). The structure and properties of both of these biopolymers have been repeatedly studied over the past few decades (see, in particular [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70]). The total number of works on this topic is at least several hundred, and in this article, it is not possible even to simply cite them in full. As a result of the studies, it was found that the macromolecules of these high-molecular polypeptides consist of three polypeptide chains with almost the same molecular mass (Figure 2a); moreover, two of them, as a rule, are almost identical to each other in the set and sequence of amino acids (the so-called α1-chains), while the third (the so-called α2-chain) in this respect differs from the two others [26,32,33]. Each of these chains contains over 1000 amino acid residues [19,50] and has M ~ 95,000 Daltons. The gelatin molecule is stabilized by the formation of covalent cross-links, both within the gelatin triple helix and between gelatin helices. During the transformation of collagen into gelatin, a polydisperse mixture is formed containing single (α1 and α2), double (β11 and β12) and triple (γ) polypeptide chains, which, unlike collagen macromolecules, are formed in the form of coils or clots (Figure 2b).3. Specific Features of Immobilization in a Gelatin Matrix
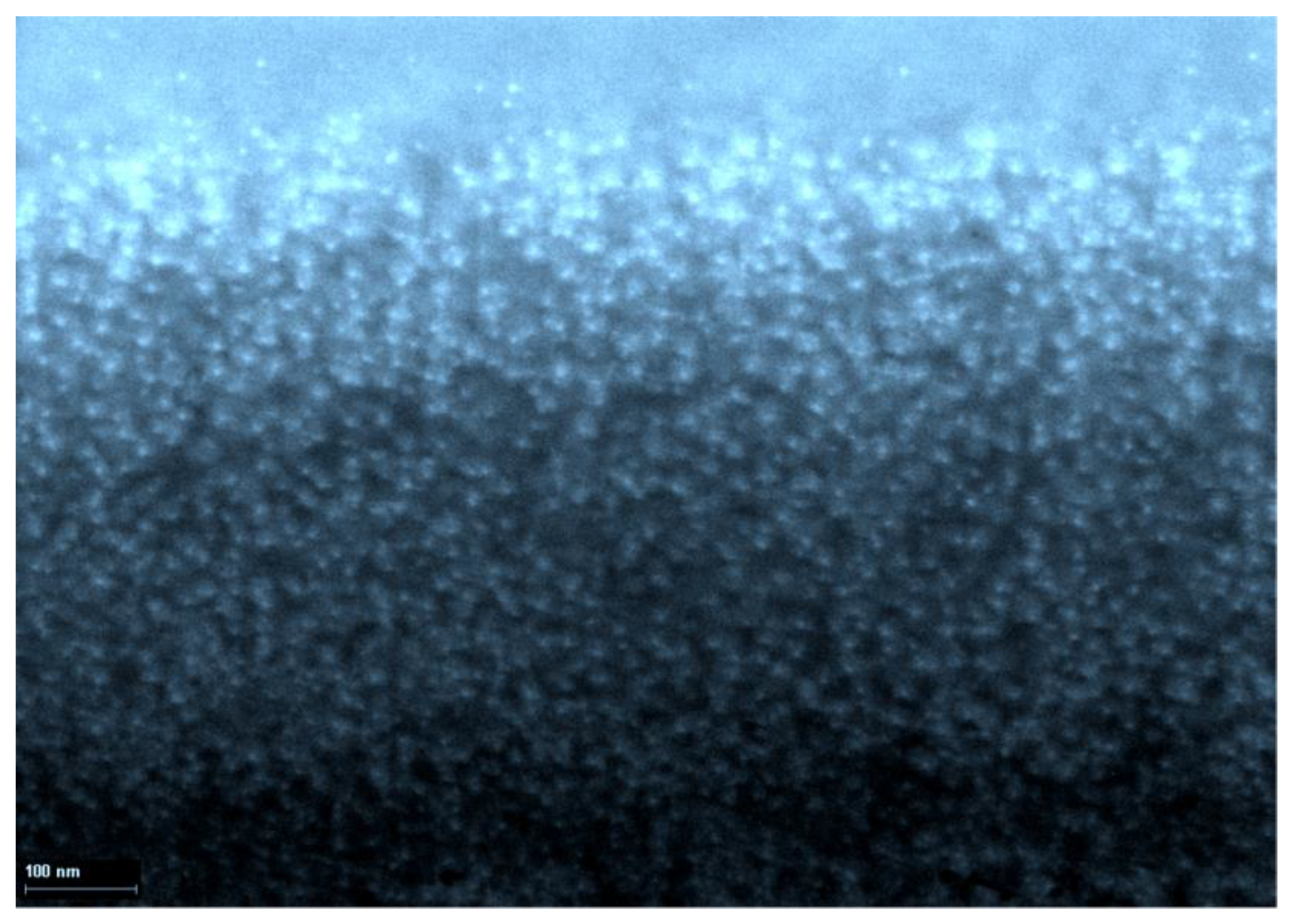
4. Immobilization of Nanoparticles of Elemental Metals in a Gelatin Matrix Using Redox Reactions
5. Immobilization of Nanoparticles of Metal Complexes in Reactions of Nucleophilic Substitution and Template Synthesis
6. Immobilization of Nanoparticles of Metal Hexacyanoferrates(II) in Reactions of Nucleophilic Substitution and Ionic Exchange
7. Possible Applications of Gelatin-Immobilized Systems Formed by Metal-Containing Chemical Compounds
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mees, C.E.K.; James, T.H. The Theory of the Photographic Process, 3rd ed.; McMillan Publishing Co.: New York, NY, USA; Collier McMillan Publishers: London, UK, 1967; 608p. [Google Scholar]
- James, T.H. The Theory of the Photographic Process, 4th ed.; McMillan Publishing Co.: New York, NY, USA; Collier McMillan Publishers: London, UK, 1977; 714p. [Google Scholar]
- Maddox, R.L. An Experiment with Gelatino-Bromide. Br. J. Photogr. 1871, 18, 422–423. [Google Scholar]
- Farbfotografie (3 Bände). Band 1: Alte Verfahren. Die Zeit der frühen Pioniere. Farbrasterfotografie. Die alten Kopierverfahren und Geräte für Papierbilder und Diapositive. Vom Ausbleichverfahren zum Silberfarbstoff-Bleichverfahren; Laterna Magica: München, Germany, 1981; 189p. (In German)
- Koshofer, G. Farbfotografie (3 Bände). Band 2: Moderne Verfahren. Zeitalter der chromogenen Entwicklung. Bilder vom Dia und Negativ. Maskenverfahren. Das farbige Sofortbild; Laterna Magica: München, Germany, 1981; 240p. (In German) [Google Scholar]
- Koshofer, G. Farbfotografie (3 Bände). Band 3: Lexikon der Verfahren, Geräte und Materialien. Das System der Verfahren. Chronik der Farbfotografie; Laterna Magica: München, Germany, 1981; 152p. (In German) [Google Scholar]
- Kirillov, N.I. Osnovy Protsessov Obrabotki Kinofotomaterialov (Fundamentals of Processing Film and Photo Materials); Iskusstvo: Moscow, Russia, 1977; 478p. (In Russian) [Google Scholar]
- Yashtold-Govorko, V.A. Fotos”yemka i Obrabotka (Photographic Shooting and Processing); Iskusstvo: Moscow, Russia, 1977; 344p. (In Russian) [Google Scholar]
- Red’ko, A.V. Osnovy Chyorno-Belykh i Tsvetnykh Fotoprotsessov (Fundamentals of Black-and-White and Color Photoprocesses); Iskusstvo: Moscow, Russia, 1990; 256p. (In Russian) [Google Scholar]
- Larry, D.; Vedrines, M. Photographic Gelatin, Proceedings of the Fourth IAG Conference (1983); Ammann-Brass, H., Pouradier, J., Eds.; Internationale Arbeitsgemeinschaft fur Photogelatine: Fribourg, Switzerland, 1985; pp. 35–54. [Google Scholar]
- Chen, X.; Peng, B. Photographic Gelatin, Proceedings of the Fourth IAG Conference (1983); Ammann-Brass, H., Pouradier, J., Eds.; Internationale Arbeitsgemeinschaft fur Photogelatine: Fribourg, Switzerland, 1985; pp. 55–64. [Google Scholar]
- Beutel, J. Photographic Gelatin, Proceedings of the Fourth IAG Conference (1983); Ammann-Brass, H., Pouradier, J., Eds.; Internationale Arbeitsgemeinschaft fur Photogelatine: Fribourg, Switzerland, 1985; pp. 65–78. [Google Scholar]
- Aoyagi, S. Photographic Gelatin, Proceedings of the Fourth IAG Conference (1983); Ammann-Brass, H., Pouradier, J., Eds.; Internationale Arbeitsgemeinschaft fur Photogelatine: Fribourg, Switzerland, 1985; pp. 79–94. [Google Scholar]
- Rich, A.; Crick, F.H.C. The structure of collagen. Nature 1955, 176, 915–916. [Google Scholar] [CrossRef] [PubMed]
- Cowan, P.M.; McGavin, S.; North, A.C.T. The polypeptide chain configuration of collagen. Nature 1955, 176, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Boedker, H.; Doty, P. A Study of Gelatin Molecules, Aggregates and Gels. J. Phys. Chem. 1954, 58, 968–983. [Google Scholar] [CrossRef]
- Ramachandran, G.N.; Kartha, G. Structure of Collagen. Nature 1955, 176, 593–597. [Google Scholar] [CrossRef]
- Veis, A.; Anesey, J.; Cohen, J. The long range reorganization of gelatin to the collagen structure. Arch. Biochem. Biophys. 1961, 94, 20–31. [Google Scholar] [CrossRef]
- Groome, R.J.; Clegg, F.G. Photographic Gelatin; Focal Press: London, UK, 1965; p. 35. [Google Scholar]
- Elsdale, T.; Bard, J. Collagen substrata for studies on cell behavior. J. Cell Biol. 1972, 54, 626–637. [Google Scholar] [CrossRef]
- Hulmes, D.J.S.; Miller, A.; Parry, D.A.D.; Piez, K.A.; Woodhead-Galloway, J. Analysis of the primary structure of collagen for the origins of molecular packing. J. Mol. Biol. 1973, 79, 137–148. [Google Scholar] [CrossRef]
- Miller, E.J. Biochemical characteristics and biological significance of the genetically-distinct collagens. Mol. Cell. Biochem. 1976, 13, 165–192. [Google Scholar] [CrossRef]
- Ramachadran, G.N. Treatise on Collagen; Academic Press: New York, NY, USA, 1967; p. 187. [Google Scholar]
- Kronman, J.H.; Goldman, M.; Habib, C.M.; Mengel, L. Electron microscopic evaluation of altered collagen structure induced by N-monochloroglycine (GK-101). J. Dent. Res. 1977, 56, 1539–1545. [Google Scholar] [CrossRef]
- Kurata, H.; Sakaoku, K. The investigation of the structure of collagen in growth process by X-ray analysis and electron microscopy. Biochim. Biophys. Acta 1984, 791, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Kung, C.E.; Feairheller, S.E.; Brown, E.M. An energetic evaluation of a “Smith” collagen microfibril model. J. Protein Chem. 1991, 10, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Fridman, R.; Fuerst, T.R.; Bird, R.E.; Hoyhtya, M.; Oelkuct, M.; Kraus, S.; Komarck, D.; Liotta, L.A.; Berman, M.L.; Stetler-Stevenson, J. Domain structure of human 72-kDa gelatinase/type IV collagenase. Characterization of proteolytic activity and identification of the tissue inhibitor of metalloproteinase-2 (TIMP-2) binding regions. J. Biol. Chem. 1992, 267, 15398–15405. [Google Scholar] [CrossRef] [PubMed]
- Bányai, L.; Tordai, H.; Patthy, L. Structure and Domain-Domain Interactions of the Gelatin-binding Site of Human 72-Kilodalton Type IV Collagenase (Gelatinase A, Matrix Metalloproteinase 2). J. Biol. Chem. 1996, 271, 12003–12008. [Google Scholar] [CrossRef]
- Pickford, A.R.; Potts, J.R.; Bright, J.R.; Han, I.; Campbell, I.D. Solution structure of a type 2 module from fibronectin: Implications for the structure and function of the gelatin-binding domain. Structure 1997, 5, 359–370. [Google Scholar] [CrossRef]
- Tordai, H.; Patthy, L. The gelatin-binding site of the second type-II domain of gelatinase A/MMP-2. Eur. J. Biochem. 1999, 259, 513–518. [Google Scholar] [CrossRef]
- Caldararu, H.; Timmins, G.S.; Gilbert, B.C. The structure of gelatin–water/oil microemulsion sols and gels. An EPR spin-probe and spin-labelling study. Phys. Chem. Chem. Phys. 1999, 1, 5689–5697. [Google Scholar] [CrossRef]
- Phillips, G.O.; Williams, P.A. (Eds.) Handbook of Hydrocolloids; Woodhead Publishing: London, UK, 2000; 450p. [Google Scholar]
- Lin, W.; Yan, L.; Mu, C.; Li, W.; Zhang, M.; Zhu, O. Effect of pH on gelatin self-association investigated by laser light scattering and atomic force microscopy. Polym. Inter. 2002, 51, 233–236. [Google Scholar] [CrossRef]
- Trexler, M.; Briknarova, K.; Gehrmann, M.; Llinas, M.; Patthy, L. Peptide Ligands for the Fibronectin Type II Modules of Matrix Metalloproteinase 2 (MMP-2). J. Biol. Chem. 2003, 278, 12241–12246. [Google Scholar] [CrossRef]
- Gutsmann, T.; Fantner, G.E.; Venturoni, M.; Ekani-Nkodo, A.; Thompson, J.B.; Kindt, J.H.; Morse, D.E.; Fygenson, D.K.; Hansma, P.K. Evidence that collagen fibrils in tendons are inhomogeneously structured in a tubelike manner. Biophys. J. 2003, 84, 2593–2598. [Google Scholar] [CrossRef]
- Gehrmann, M.L.; Douglas, J.T.; Banyai, L.; Tordai, H.; Patthy, L.; Llinas, M. Modular Autonomy, Ligand Specificity, and Functional Cooperativity of the Three In-tandem Fibronectin Type II Repeats from Human Matrix Metalloproteinase 2. J. Biol. Chem. 2004, 279, 46921–46929. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, B.; Persikov, A.V. Molecular structure of the collagen triple helix. Adv. Protein Chem. 2005, 70, 301–339. [Google Scholar] [CrossRef]
- Bella, J.; Liu, J.; Kramer, R.; Brodsky, B.; Berman, H.M. Conformational effects of Gly-X-Gly interruptions in the collagen triple helix. J. Mol Biol. 2006, 362, 298–311. [Google Scholar] [CrossRef]
- Strasser, S.; Zink, A.; Janko, M.; Heckl, W.M.; Thalhammer, S. Structural investigations on native collagen type I fibrils using AFM. Biochem. Biophys. Res. Commun. 2007, 354, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, B.; Thiagarajan, G.; Madhan, B.; Kar, K. Triple-helical peptides: An approach to collagen conformation, stability, and self-association. Biopolymers 2008, 89, 345–353. [Google Scholar] [CrossRef]
- Okuyama, K. Revisiting the molecular structure of collagen. Connect Tissue Res. 2008, 49, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Alexander, S.; Schacht, V.; Coussens, L.M.; von Andrian, U.H.; van Rheenen, J.; Deryugina, E.; Friedl, P. Collagen-based cell migration models in vitro and in vivo. Semin. Cell Dev. Biol. 2009, 20, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Bella, J. A new method for describing the helical conformation of collagen: Dependence of the triple helical twist on amino acid sequence. J. Struct. Biol. 2010, 170, 377–391. [Google Scholar] [CrossRef]
- Okuyama, K.; Miyama, K.; Mizuno, K.; Bächinger, H.P. Crystal structure of (Gly-Pro-Hyp)(9): Implications for the collagen molecular model. Biopolymers 2012, 97, 607–616. [Google Scholar] [CrossRef]
- Adzhubei, A.A.; Sternberg, M.J.E.; Makarov, A.A. Polyproline-II helix in proteins: Structure and function. J. Mol. Biol. 2013, 425, 2100–2132. [Google Scholar] [CrossRef]
- Walters, B.D.; Stegemann, J.P. Strategies for directing the structure and function of three-dimensional collagen biomaterials across length scales. Acta Biomater. 2014, 10, 1488–1501. [Google Scholar] [CrossRef] [PubMed]
- Bella, J. Collagen structure: New tricks from a very old dog. Biochem. J. 2016, 473, 1001–1025. [Google Scholar] [CrossRef] [PubMed]
- Price, J.C.; Roach, P.; El Haj, A.J. Liquid crystalline ordered collagen substrates for applications in tissue engineering. ACS Biomater. Sci. Eng. 2016, 2, 625–633. [Google Scholar] [CrossRef]
- Bella, J.; Hulmes, D.J.S. Fibrillar collagens. In Fibrous Proteins: Structures and Mechanisms; Parry, A.D., Squire, J.M., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 457–490. [Google Scholar] [CrossRef]
- Darvish, D.M. Collagen fibril formation in vitro: From origin to opportunities. Mater. Today Bio 2022, 15, 100322. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Sol–gel technology and template synthesis in thin gelatin films. J. Sol Gel Sci. Technol. 2014, 72, 314–327. [Google Scholar] [CrossRef]
- Engel, J. Investigation of the denaturation and renaturation of soluble collagen by light scattering. Arch. Biochem. Biophys. 1962, 97, 150–156. [Google Scholar] [CrossRef]
- Veis, A.; Drake, M.P. The Introduction of Intramolecular Covalent Cross-linkages into Ichthyocol Tropocollagen with Monofunctional Aldehydes. J. Biol. Chem. 1963, 238, 2003–2011. [Google Scholar] [CrossRef]
- Drake, M.P.; Veis, A. Interchain Interactions in Collagen-Fold Formation. I. The Kinetics of Renaturation of γ-Gelatin. Biochemistry 1964, 3, 135–145. [Google Scholar] [CrossRef]
- Yuan, L.; Veis, A. The self-assembly of collagen molecules. Biopolymers 1973, 12, 1437–1442. [Google Scholar] [CrossRef]
- Olsen, A.K. Evidence of Structure in Gelatin Gels. J. Phys. Chem. 1932, 36, 529–535. [Google Scholar] [CrossRef]
- Hauschka, P.V.; Harrington, W.F. Collagen structure in solution. III. Effect of cross-links on thermal stability and refolding kinetics. Biochemistry 1970, 9, 3734–3745. [Google Scholar] [CrossRef]
- Bianchi, E.; Conio, I.; Ciferri, A.; Puett, D.; Rajagh, L. The Role of pH, Temperature, Salt Type, and Salt Concentration on the Stability of the Crystalline, Helical, and Randomly Coiled Forms of Collagen. J. Biol. Chem. 1967, 242, 1361–1369. [Google Scholar] [CrossRef]
- Kenchington, A.W.; Ward, A.G. The titration curve of gelatin. Biochem. J. 1954, 58, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, S.E.; Houck, R.C.; Dittmar, C. The Sorption of Soluble Dyes by Gelatin. J. Phys. Chem. 1942, 46, 158–176. [Google Scholar] [CrossRef]
- Hofmeister, F. Zur Lehre von der Wirkung der Salze. Arch. Exp. Pathol. Pharmakol. 1888, 24, 247–260. (In German) [Google Scholar] [CrossRef]
- Zhang, Y.; Cremer, P.S. Interactions between macromolecules and ions: The Hofmeister series. Curr. Opinion Chem. Biol. 2006, 10, 658–663. [Google Scholar] [CrossRef]
- Gustavson, K.H. The Chemistry and Reactivity of Collagen; Academic Press: New York, NY, USA, 1956; pp. 171–172. [Google Scholar]
- Bianchi, E.; Conio, G.; Ciferri, A. The helix–coil transformation for tropocollagen solutions and its relationship to transformations involving the crystalline form of the protein. Biopolymers 1966, 4, 957–970. [Google Scholar] [CrossRef]
- Green, A.; Levenson, G.I.P. Emulsion Swelling During Washing, Etc. J. Photogr. Sci. 1974, 22, 194–198. [Google Scholar] [CrossRef]
- Mi, S.; Chen, B.; Wright, B.; Connon, C.J. Plastic compression of a collagen gel forms a much improved scaffold for ocular surface tissue engineering over conventional collagen gels. J. Biomed. Mater. Res. 2010, 95A, 447–453. [Google Scholar] [CrossRef]
- Micol, L.A.; Ananta, M.; Engelhardt, E.M.; Mudera, V.C.; Brown, R.A.; Hubbell, J.A.; Frey, P. High-density collagen gel tubes as a matrix for primary human bladder smooth muscle cells. Biomaterials 2011, 32, 1543–1548. [Google Scholar] [CrossRef]
- Marelli, B.; Ghezzi, C.E.; Zhang, Y.L.; Rouiller, I.; Barralet, J.E.; Nazhat, S.N. Fibril formation pH controls intrafibrillar collagen biomineralization invitro and invivo. Biomaterials 2015, 37, 252–259. [Google Scholar] [CrossRef]
- Hu, K.; Shi, H.; Zhu, J.; Deng, D.; Zhou, G.; Zhang, W.; Cao, Y.; Liu, W. Compressed collagen gel as the scaffold for skin engineering. Biomed. Microdevices 2010, 12, 627–635. [Google Scholar] [CrossRef]
- Zhou, L.; Hinerman, J.M.; Blaszczyk, M.; Miller, J.L.C.; Conrady, D.G.; Barrow, A.D.; Chirgadze, D.Y.; Bihan, D.; Farndale, R.W.; Herr, A.B. Structural basis for collagen recognition by the immune receptor OSCAR. Blood 2016, 127, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Andrée, L.; Oude Egberink, R.; Dodemont, J.; Hassani Besheli, N.; Yang, F.; Brock, R.; Leeuwenburgh, S.C.G. Gelatin Nanoparticles for Complexation and Enhanced Cellular Delivery of mRNA. Nanomaterials 2022, 12, 3423. [Google Scholar] [CrossRef] [PubMed]
- Lowry, T.M. The uniqueness of hydrogen. J. Soc. Chem. Ind. 1923, 42, 43–47. [Google Scholar] [CrossRef]
- Brönsted, J.N. Einige Bemerkungen über den Begriff der Säuren und Basen [Some observations about the concept of acids and bases]. Rec. Trav. Chim. Pays-Bas. 1923, 42, 718–728. (In German) [Google Scholar] [CrossRef]
- Paik, S.-H. Understanding the Relationship Among Arrhenius, Brønsted–Lowry, and Lewis Theories. J. Chem. Educ. 2015, 92, 1484–1489. [Google Scholar] [CrossRef]
- Lofrano, G.; Meric, S.; Balci, G.; Orhon, D. Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: A review. Sci. Total Environ. 2013, 461–462, 265–281. [Google Scholar] [CrossRef]
- Pomogailo, A.D. Polimernye Immobilizovannye Metallokompleksnye Katalizatory (Polymer Immobilized Metal Complex Catalysts); Nauka: Moscow, Russia, 1988; pp. 10–20. ISBN 5-02-001390-0. (In Russian) [Google Scholar]
- Pomogailo, A.D. Catalysis by Polymer-Immobilized Metal Complexes; CRC Press: London, UK, 2020; pp. 14–24. [Google Scholar] [CrossRef]
- Yermakov, Y.I. Supported Catalysts Obtained by Interaction of Organometallic Compounds of Transition Elements with Oxide Supports. Catal. Rev. Sci. Eng. 1976, 13, 77–120. [Google Scholar] [CrossRef]
- Ballard, D.G.H. Transition metal alkyl compounds as polymerization catalysts. J. Polym. Sci. Polym. Chem. Ed. 1975, 13, 2191–2212. [Google Scholar] [CrossRef]
- Mathur, N.K.; Williams, R.E. Organic Syntheses Using Polymeric Supports, Polymeric Reagents, and Polymeric Catalysts. J. Macromol. Sci. C 1976, 15, 117–142. [Google Scholar] [CrossRef]
- Hartley, E.R. Supported Metal Complexes. A New Generation of Catalysis; Reidel Publishing: Dordrecht, Germany, 1985; 318p. [Google Scholar]
- Mikhailov, O.V. Complex formation processes in 3d-metal hexacyanoferrate(II) gelatin-immobilised matrices. Russ. Chem. Revs. 1995, 64, 657–673. [Google Scholar] [CrossRef]
- Kirschning, A.; Monenschein, H.; Wittenberg, R. Functionalized Polymers-Emerging Versatile Tools for Solution-Phase Chemistry and Automated Parallel Synthesis. Angew. Chem. Int. Ed. 2001, 40, 650–679. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Kondakov, A.V.; Krikunenko, R.I. Image Intensification in Silver Halide Photographic Materials for Detection of High-Energy Radiation by Reprecipitation of Elemental Silver. High Energy Chem. 2005, 39, 324–329. [Google Scholar] [CrossRef]
- Naumkina, N.I.; Mikhailov, O.V.; Kondakov, A.V.; Lygina, T.Z. On a New Phase of Elemental Silver, Appearing on Its “Reprecipitation” in Ag–Gelatin-Immobilized Matrix Systems. Russ. J. Gen. Chem. 2008, 78, 1650–1654. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Naumkina, N.I. Novel modification of elemental silver formed in Ag4[Fe(CN)6]-gelatin-immobilized matrix Implants. Cent. Eur. J. Chem. 2010, 8, 448–452. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Synthesis of Ag nanoparticles under a contact of water solution with silver(I)chloride biopolymer matrix. J. Mol. Liq. 2019, 291, 111354. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Mikhailova, E.O. Elemental Silver Nanoparticles: Biosynthesis and Bio Applications. Materials 2019, 12, 3177. [Google Scholar] [CrossRef] [PubMed]
- Korzun, G.M.; Rakhmanov, S.K.; Belenkov, V.V.; Khvalyuk, V.N.; Vrublevsky, A.V. Enhancement of black-and-white silver image in redox processing II. Zh. Nauch. Priklad. Fotogr. 1991, 36, 366–370. (In Russian) [Google Scholar]
- Rakhmanov, S.K.; Belenkov, V.V.; Korzun, G.M. A photographic process based on the redox dispersion of silver. Zh. Nauch. Priklad. Fotogr. 1999, 44, 44–52. (In Russian) [Google Scholar]
- Branitskii, G.A.; Stashonok, V.D.; Sergeeva, O.V.; Sviridov, V.V. Photographic images based on the colloidal silver particles. Zh. Nauch. Priklad. Fotogr. 1999, 44, 1–10. (In Russian) [Google Scholar]
- Bokshits, Y.V.; Shevchenko, G.P.; Ponyavina, A.N.; Rakhmanov, S.K. Formation of silver and copper nanoparticles upon the reduction of their poorly soluble precursors in aqueous solution. Colloid J. 2004, 66, 517–522. [Google Scholar] [CrossRef]
- Kaliaha, A.E.; Sergeyeva, O.V.; Stashonok, V.D.; Rakhmanov, S.K. Optical properties of photosensitive layers containing nanosized silver particles. In Physics, Chemistry and Applications of Nanostructures—Reviews and Short Notes to Nanomeeting 2005; World Scientific: Singapore, 2005; pp. 382–385. [Google Scholar]
- Sharabanov, A.A.; Kalent’yev, V.K.; Mikhailov, O.V. «Pereosazhdeniye» elementnogo serebra v Ag-zhelatin-immobilizovannykh matrichnykh implantatakh s ispol’zovaniyem rastvorov, soderzhashchikh anion [BH4]– i kompozitsii N,N’-etilendiamintetraatsetatnykh kompleksov [Cu(II), Ni(II), Sn(II)], [Cu(II), Ni(II), Co(II)] i [Cu(II), Ni(II), Fe(II)]. ("Reprecipitation" of elemental silver in Ag-gelatin-immobilized matrix implants using solutions containing the [BH4]– anion and compositions of N,N-ethylenediaminetetraacetate complexes [Cu(II), Ni(II), Sn(II)], [Cu(II), Ni(II), Co(II)] and [Cu(II), Ni(II), Fe(II)].). Izv. VUZov Ser. Khimiya I Khimicheskaya Tekhnologiya 2009, 52, 20–23. (In Russian) [Google Scholar]
- Kalent’yev, V.K.; Mikhailov, O.V. Usileniye izobrazheniy na AgHal-radiograficheskikh materialakh «pereosazhdeniyem» elementnogo serebra v rastvore, soderzhashchem kompleks M(II) s N,N’-etilendiamintetraatsetatom (M = Fe, Co) i anion [BH4]−. (Enhancement of images on AgHal radiographic materials by “reprecipitation” of elemental silver in a solution containing a complex of M(II) with N,N’-ethylenediaminetetraacetate (M = Fe, Co) and anion [BH4]−). Izv. VUZov Ser. Khimiya I Khimicheskaya Tekhnologiya 2010, 53, 54–58. (In Russian) [Google Scholar]
- Sviridov, V.V.; Kondrat’ev, V.A. Fotograficheskiye protsessy s besserebryanym fizicheskim proyavleniyem (Photographic processes with silver-free physical development). Uspekhi Nauchnoi Fotogr. 1978, 19, 43–64. (In Russian) [Google Scholar]
- Mikhailov, O.V. The theory of heterogeneous complexation on immobilized matrices of hexacyanoferrates(II) 3d-elements. Soviet J. Coord. Chem. 1992, 18, 1008–1017. [Google Scholar]
- Mikhailov, O.V.; Polovnyak, V.K. Photography without Silver: Non-Silver Photographic Images Obtained from Metalorganic Complexes Having Strong Absorption. J. Imaging Sci. 1991, 35, 258–262. [Google Scholar]
- Mikhailov, O.V. Synthesis of Gelatin-Immobilized Hexacyanoferrates of p-, d- and f-Metals. Russ. J. Gen. Chem. 1998, 68, 827–828. [Google Scholar]
- Mikhailov, O.V.; Polovnyak, V.K. The reaction of Ni2[Fe(CN)6] with dithiooxamide in nickel(II)hexacyanoferrate(II) matrices immobilized in thin gelatin layers. Russ. J. Inorg. Chem. 1990, 35, 1169–1173. [Google Scholar]
- Mikhailov, O.V. Enzyme-assisted matrix isolation of novel dithiooxamide complexes of nickel(II). Indian J. Chem. A 1991, 30, 252–254. [Google Scholar]
- Mikhailov, O.V.; Polovnyak, V.K. Complexation reactions on the immobilized matrices: Complexes of iron(III)hexacyanoferrate(II) and some N,O and N,S-containing ligands formed in thin gelatin layer. Indian J. Chem. A 1992, 31, 54–57. [Google Scholar]
- Mikhailov, O.V. Complex formation in the Cu2[Fe(CN)6]-dithiooxamide system in copper(II) hexacyanoferrate(II) immobilized in thin gelatin layer. Russ. J. Inorg. Chem. 1992, 37, 172–174. [Google Scholar]
- Mikhailov, O.V. From novel complexing conditions to novel coordination compounds of nickel(II) with dithiooxamide and its bulky analogues. Transit. Metal Chem. 1996, 21, 363–369. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Complex Formation between Fe(II) and 8-Mercaptoquinoline and Some of Its 5-Substituted Derivatives on Iron(III) Hexacyanoferrate(II) Gelatin-Immobilized Matrices. Russ. J. Inorg. Chem. 1996, 41, 1437–1441. [Google Scholar]
- Tatarintseva, T.B.; Mikhailov, O.V. Copper(II) Complex Formation with 1,1-Dialkyl-3-Benzoylthioureas in Gelatin-Immobilized Copper(II) Hexacyanoferrate(II) Matrix Systems. Russ. J. Inorg. Chem. 1997, 42, 218–222. [Google Scholar]
- Mikhailov, O.V. From novel complexing conditions to novel coordination compounds of copper(II) with dithiooxamide and its bulky analogues. Transit. Metal Chem. 1997, 22, 535–540. [Google Scholar] [CrossRef]
- Shigapova, L.S.; Mikhailov, O.V.; Khamitova, A.I. Complex Formation of Dioxouranium(VI) with 8-Hydroxyquinoline and 8-Mercaptoquinoline in Gelatin-immobilized (UO2)2[Fe(CN)6] Matrices. Russ. J. Gen. Chem. 1997, 67, 1935–1936. [Google Scholar]
- Mikhailov, O.V. Complexation of Cobalt(III) with 8-Mercaptoquinoline and its 5-Bromo and 5-Thiomethyl-substituted Derivatives in the KCo[Fe(CN)6]-Gelatin-Immobilized Matrix. Russ. J. Coord. Chem. 1997, 23, 850–855. [Google Scholar]
- Mikhailov, O.V.; Tatarintseva, T.B. Complex Formation of 5-Chloro- and 5-Bromo-8-mercaptoquinolines with Gelatin-supported Cu2[Fe(CN)6]. Russ. J. Gen. Chem. 1998, 68, 73–77. [Google Scholar]
- Mikhailov, O.V.; Kazymova, M.A. Novel coordination compounds of nickel(II) and copper(II) with N,N’-diphenylthiooxamide; formation of complexed metalhexacyanoferrate(II) gelatin-immobilized matrix systems. Transit. Metal Chem. 1998, 23, 195–199. [Google Scholar] [CrossRef]
- Mikhailov, O.V. The complexing into metal-containing gelatin-immobilized matrices as novel perspective method of coordination polymer synthesis. Unchelated coordination polymers of nickel(II) and copper(II) with dithiooxamide. Phosphorus Sulfur Silicon Relat. Elem. 1997, 126, 129–136. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Kazymova, M.A. Iron(II)-8-mercaptoquinoline, iron(II)-5-chloro-8-mercaptoquinoline and iron(II)-5-bromo-8-mercaptoquinoline complexing in the iron(III)hexacyanoferrate(II) gelatin-immobilized matrix systems. Transit. Metal Chem. 1998, 23, 497–500. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Complex Formation of Uranium(VI) with 8-Quinolinol and Its 5-Halo Derivatives in Gelatin-Immobilized Uranyl Ferrocyanide Systems. Radiochemistry 1998, 40, 326–332. [Google Scholar]
- Mikhailov, O.V.; Tatarintseva, T.B. Complexing between copper(II) and bulky substituted 3-benzoylthioureas in copper(II) hexacyanoferrate(II) gelatin-immobilized matrix systems. Transit. Metal Chem. 1999, 24, 218–223. [Google Scholar] [CrossRef]
- Kazymova, M.A.; Mikhailov, O.V. Cobalt(III)-dithiooxamide, cobalt (III)-N,N’-diphenylthiooxamide and cobalt(III)-N,N’-diphenyldithiooxamide complexing in KCoFe(CN)6]-gelatin-immobilized matrices. Transit. Metal Chem. 1999, 24, 517–524. [Google Scholar] [CrossRef]
- Tatarintseva, T.B.; Mikhailov, O.V.; Sopin, V.F. Copper(II)-1,2-bis(thiocarbamoyl)hydrazine and copper(II)-(1-carbamoyl-2-thiocarbamoyl)hydrazine complexing in copper(II) hexacyanoferrate(II) gelatin-immobilized matrix systems. Transit. Metal Chem. 2000, 25, 32–36. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Tatarintseva, T.B.; Gorbunova, T.S.; Sopin, V.F. Copper(II)-8-mercaptoquinoline, copper(II)-5,8-dimercaptoquinoline and copper(II)-5-thiomethyl-8-mercaptoquinoline complexing in a copper(II)hexacyanoferrate(II) gelatin-immobilized matrix. Transit. Metal Chem. 2000, 25, 45–51. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Cobalt (III)-8-mercaptoquinoline complexing in cobalt(III) hexacyanoferrate(II) gelatin-immobilized matrix systems. Transit. Metal Chem. 2000, 25, 341–343. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Sopin, V.F. Cobalt(II)-8-mercaptoquinoline complexing in cobalt(II)hexacyanoferrate(II) gelatin-immobilized matrix materials. Transit. Metal Chem. 2002, 27, 159–162. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Complexation of Nickel(II) with Dioximes in Ni2[Fe(CN)6] Gelatin-Immobilized Matrix Implants. Russ. J. Coord. Chem. 2002, 28, 352–357. [Google Scholar] [CrossRef]
- Tatarintseva, T.B.; Mikhailov, O.V.; Brus’ko, V.V.; Zabirov, N.G. M(II)-N-diisoproxythiophosphorylthiobenzamide complexing processes in M2[Fe(CN)6]-gelatin-immobilized matrices (M = Co, Ni, Cu). Transit. Metal Chem. 2002, 27, 423–428. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Complexation in Binary Pb(II)–Dithiooxamide System in Pb2[Fe(CN)6] Gelatin-Immobilized Matrices. Russ. J. Coord. Chem. 2003, 29, 276–280. [Google Scholar] [CrossRef]
- Khamitova, A.I.; Mikhailov, O.V. Mild Template Synthesis of Co(III)-Dithiooxamide-Glyoxal in Gelatin-immobilized KCoFe(CN)6 Matrix Systems. Russ. J. Gen. Chem. 1997, 67, 1913–1920. [Google Scholar]
- Khamitova, A.I.; Mikhailov, O.V. Mild template synthesis of nickel (II) and copper(II) chelates with an (N,N,S,S)-tetradentate ligand in metal hexacyanoferrate(II) immobilized matrix systems. Mendeleev Commun. 1998, 3, 96–97. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Khamitova, A.I.; Shigapova, L.S.; Busygina, T.E. Soft template synthesis of (2,8-dithio-3,7-diaza-5-oxanonandithioamide-1,9)nickel(II) and (2,7-dithio-3,6-diazaoctadien-3,5-dithioamide-1,8)nickel(II) using a nickel(II)hexacyanoferrate(II) gelatin-immobilized matrix. Transit. Metal Chem. 1999, 24, 503–510. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Khamitova, A.I. Low-temperaturic template synthesis of macrocyclic cobalt(III) chelates with (N,N,S,S)-donor atomic ligands in the cobalt(II)-dithiooxamide-formaldehyde and cobalt(II)-dithiooxamide-glyoxal systems in the Co2[Fe(CN)6]-gelatin-immobilized matrices. Transit. Metal Chem. 2000, 25, 26–31. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Soft template synthesis in copper(II)-dithiooxamide-methanal, copper(II)-dithiooxamide-ethanal and copper(II)-dithiooxamide-propanone triple systems in a copper(II)hexacyanoferrate(II) gelatin-immobilized matrix. Transit. Metal Chem. 2000, 25, 552–558. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Khamitova, A.I.; Morozov, V.I. Template Synthesis of Cobalt(II,III), Nickel(II) and Copper(II) Macrocyclic Compounds with 2,8-Dithio-3,7-diaza-5-oxanonandithioamide-1,9 in Gelatin-Immobilized Matrix. Heterocycl. Commun. 2000, 6, 137–142. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Kazymova, M.A.; Shumilova, T.A.; Vafina, L.R. Ni(II)- and Cu(II)-Containing Heterocyclic Compounds with 4,4′,6-trimethyI-2,8-dithio-3,7-diazanonen-6-dithioamide-l,9 Obtained in Gelatin-Immobilized Matrices in the Template Synthesis Process. Heterocyclic Commun. 2000, 6, 357–362. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Low-Temperature Template Synthesis of Nickel-Containing Heterocyclic Compound with 2,8-Dithio-3,5,7-triazanonandithioamide-1,9 in Gelatin-Immobilized Matrix. Heterocycl. Commun. 2001, 7, 79–82. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Khamitova, A.I.; Morozov, V.I. Mild template synthesis of (2,7-dithio-3,6-diazaoctadien-3,5-dithioamide-1,8)nickel(II) and (2,7-dithio-3,6-diazaoctadien-3,5-dithioamide-1,8)copper(II) in the Ni2[Fe(CN)6]- and Cu2[Fe(CN)6]-gelatin-immobilized matrix systems. Int. J. Inorg. Mater. 2001, 3, 161–167. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Low-temperature template synthesis of (2,8-dithio-3,7-diaza-5-oxanonandithioamide-1,9) nickel(II), (2,8-dithio-3,7-diaza-4,6-dimethyl-5-oxanonandithioamide-1,9)nickel(II) and (4,4′,6-trimethyl-2,8-dithio-3,7-diazanonen-6-dithioamide-1,9)nickel(II) in thin films of nanostructured Ni2[Fe(CN)6]-gelatin-immobilized matrix materials. Int. J. Inorg. Mater. 2001, 3, 1053–1061. [Google Scholar] [CrossRef]
- Kazymova, M.A.; Mikhailov, O.V.; Shumilova, T.A.; Solovieva, S.E.; Mannafov, T.G. Soft template synthesis of macrocyclic copper(II) chelates with 3,9-dithio-4,8-diaza-6-oxaundekandithioamide-1,11 in a Cu2[Fe(CN)6]-gelatin-immobilized matrix. Transit. Metal Chem. 2003, 28, 592–594. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Mild template synthesis of a copper(II)-containing macrocyclic compound with 4,4,6-trimethyl-2,3,7,8-tetraazanonen-6-dithiohydrazide-1,9 in a gelatin-immbolized matrix. Transit. Metal Chem. 2003, 28, 665–667. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Kazymova, M.A.; Shumilova, T.A.; Solovieva, S.S. Template synthesis in M(II)-thiocarbohydrazide- diacetyl triple system (M = Ni, Cu) in a metal(II)hexacyanoferrate(II) gelatin-immobilized matrix. Transit. Metal Chem. 2004, 29, 732–736. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Khamitova, A.I.; Kazymova, M.A. Soft template synthesis of cobalt(III) chelates with 2,8-dithio-3,7-diaza-5-oxanonandithioamide-1,9 and with 2,7-dithio-3,6-diazaoctadien-3,5-dithioamide-1,8 into cobalt(III) hexacyanoferrate(II) gelatin-immobilized matrix materials. Transit. Metal Chem. 2005, 30, 22–26. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Kazymova, M.A.; Shumilova, T.A.; Chmutova, G.A.; Solovieva, S.E. Template synthesis in the nickel(II)-thiocarbohydrazide-propanone triple system. Transit. Metal Chem. 2005, 30, 299–304. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Kazymova, M.A.; Shumilova, T.A. Mild template synthesis of copper(II)-containing macrocyclic compounds in the Cu(II)-1,2-diaminoethanedithione-1,2-ethanedione-1,2 and Cu(II)-1,2-diaminoethanedithione-1,2- buhanedione-2,3 triple systems into Cu2[Fe(CN)6]-gelatin-immobilized implants. Transit. Metal Chem. 2007, 32, 1056–1060. [Google Scholar] [CrossRef]
- Kazymova, M.A.; Mikhailov, O.V. Soft template synthesis in the cobalt(III)–1,2-diaminoethane-1,2-dithione–propanone triple system on a K[CoFe(CN)6]-gelatin-immobilized matrix. Transit. Metal Chem. 2008, 33, 523–527. [Google Scholar] [CrossRef]
- Chachkov, D.V.; Mikhailov, O.V. Mild template synthesis in the iron(III)-ethanedithioamide-1,2-formaldehyde triple system on a K[Fe2(CN)6]-gelatin-immobilized matrix. J. Coord. Chem. 2009, 62, 1058–1066. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Chachkov, D.V. Self-assembly of supramolecular Ni(II) and Cu(II) metalmacrocyclic compounds with tetraazamacrocyclic ligand into a gelatin-immobilized matrix. J. Coord. Chem. 2010, 63, 4309–4318. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Kazymova, M.A.; Chachkov, D.V. On Template Synthesis in the Ternary System Ni(II)–Thiosemicarbazide–Diacetyl. Russ. J. Inorg. Chem. 2014, 59, 60–64. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Substitution reactions and template synthesis in the metal hexacyanoferrate(II) gelatin-immobilized matrix systems. Russ. J. Coord. Chem. 2000, 26, 702–713. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Reactions of nucleophilic, electrophilic substitution and template synthesis in the metal-hexacyanoferrate(II) gelatin-immobilized matrix. Revs. Inorg. Chem. 2003, 23, 37–74. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Gelatin-Immobilized Metalcomplexes: Synthesis and Employment. J. Coord. Chem. 2008, 61, 1333–1384. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Synthesis of 3d-element metal-macrocyclic chelates into polypeptide biopolymer medium and their molecular structures. Inorg. Chim. Acta 2013, 394, 664–684. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Kazymova, M.A.; Chachkov, D.V. Self-assembly and quantum-chemical design of macrotricyclic and macrotetracyclic metalchelates of 3d-elements formed in the gelatin-immobilized matrix. Russ. Chem. Bull. 2015, 64, 1757–1771. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Molecular structure design and soft template synthesis of aza-, oxaaza- and thiaazamacrocyclic metal chelates in the gelatin matrix. Arab. J. Chem. 2017, 10, 47–67. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Polycyclic 3d-metalchelates formed owing to inner-sphere transmutations in the gelatin matrix: Synthesis and structures. Revs. Inorg. Chem. 2017, 37, 71–94. [Google Scholar] [CrossRef]
- Gafarov, M.P.; Mikhailov, O.V.; Yusupov, R.A. Lead(II) Sulfide. Synthesis in Lead (II) Tetraoxophosphate(V) Gelatin-immobilized Matrix Implantates and Sorption Activity Toward Silver(I) Ions. Russ. J. Gen. Chem. 2003, 73, 1183–1187. [Google Scholar] [CrossRef]
- Gafarov, M.P.; Mikhailov, O.V. Reaction of Gelatin-immobilized Lead(II) Hexacyanoferrate(II) with Dithioxamide. Russ. J. Gen. Chem. 2004, 74, 960–961. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Electron Microscopical Study of Lead(II) Sulfide Implanted into a Glassy Biopolymer Matrix. Glass Phys. Chem. 2017, 43, 191–193. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Achievements in the Synthesis of Elemental Silver Nanoparticles with Various Geometric Forms. Curr. Nanosci. 2018, 15, 112–128. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Constable, E.C. What’s in a Name?—A Short History of Coordination Chemistry from Then to Now. Chemistry 2019, 1, 126–163. [Google Scholar] [CrossRef]
- Tananaev, I.V.; Seifer, G.B.; Kharitonov, Y.A.; Kuznetsov, V.G.; Korol’kov, A.P. Khimiya Ferrotsianidov. [Chemistry of Ferrocyanides]; Nauka: Moscow, Russia, 1971; 320p. (In Russian) [Google Scholar]
- Dunbar, K.R.; Heintz, R.A. Chemistry of transition metal cyanide compounds: Modern perspectives. Progr. Inorg. Chem. 1996, 45, 283–391. [Google Scholar] [CrossRef]
- Uemura, T.; Kitagawa, S. Prussian blue nanoparticles protected by poly(vinylpyrrolidone). J. Am. Chem. Soc. 2003, 125, 7814–7815. [Google Scholar] [CrossRef]
- Uemura, T.; Ohba, M.; Kitagawa, S. Size and surface effects of Prussian blue nanoparticles protected by organic polymers. Inorg. Chem. 2004, 43, 7339–7345. [Google Scholar] [CrossRef]
- Qian, L.; Yanf, X. Preparation of Cobalt hexacyanoferrate nanowires using carbon nanotubes as templates. Talanta 2006, 69, 957–962. [Google Scholar] [CrossRef]
- Abbaspour, A.; Khajehzadeh, A.; Ghaffarinejad, A. Electrocatalytic oxidation and determination of hydrazine on nickel hexacyanoferrate nanoparticles-modified carbon ceramic electrode. J. Electroanal. Chem. 2009, 631, 52–57. [Google Scholar] [CrossRef]
- Berrettoni, M.; Giorgetti, M.; Zamponi, S.; Conti, P.; Ranganathan, D.; Zanotto, A.; Maria Luisa Saladino, M.L.; Caponetti, E. Synthesis and Characterization of Nanostructured Cobalt Hexacyanoferrate. J. Phys. Chem. C 2010, 114, 6401–6407. [Google Scholar] [CrossRef]
- Jassal, V.; Shanker, U.; Shankar, S. Synthesis, Characterization and Applications of Nano-structured Metal Hexacyanoferrates: A Review. J. Environ. Anal. Chem. 2015, 2, 128. [Google Scholar] [CrossRef]
- Barton, G.B.; Hepworth, J.I.; McClenahan, E.D.; Moore, R.L.; van Tuyl, H.H. Chemical Processing Wastes. Recovering Fission Products. Ind. Eng. Chem. 1958, 50, 212–216. [Google Scholar] [CrossRef]
- Kourim, V.; Rais, I.; Steiskal, J. Exchange properties of complex cyanides—II. Ion exchange of alkali metals on zinc ferrocyanide. J. Inorg. Nucl. Chem. 1964, 26, 1761–1763. [Google Scholar] [CrossRef]
- Haas, P.A. A Review of Information on Ferrocyanide Solids for Removal of Cesium from Solutions. Separ. Sci. Technol. 1993, 28, 2479–2506. [Google Scholar] [CrossRef]
- Hariula, R.; Lehto, J.; Tusa, E.H.A. Industrial scale removal of cesium with hexacyanoferrate exchanger-process development. Nucl. Technol. 1994, 107, 272–278. [Google Scholar] [CrossRef]
- Rigamonti, R. Struttura e costituzione chimica di alcuni ferrocianuri. Gazz. Chim. Ital. 1938, 68, 803–816. (In Italian) [Google Scholar]
- Ovcharenko, V.I.; Sagdeev, R.Z. Molecular ferromagnets. Russ. Chem. Revs. 1999, 68, 345–364. [Google Scholar] [CrossRef]
- Tatarintseva, T.B.; Mikhailov, O.V.; Naumkina, N.I.; Lygina, T.Z. Electrophilic Substitution Co(II) → M(II) (M = Mn, Ni, Cu, Zn, Cd) in Co2[Fe(CN)6] Gelatin-Immobilized Matrices. Russ. J. Coord. Chem. 2003, 29, 115–122. [Google Scholar] [CrossRef]
- Tatarintseva, T.B.; Mikhailov, O.V.; Naumkina, N.I. Complexation in Binary Cd2[Fe(CN)6]–M(II) Systems (M = Mn, Co, Ni, Cu, Zn) in Gelatin-Immobilized Cadmium(II) Hexacyanoferrates(II). Russ. J. Coord. Chem. 2003, 29, 327–334. [Google Scholar] [CrossRef]
- Tatarintseva, T.B.; Mikhailov, O.V.; Kolgina, V.A. Chemisorption of M(II) Ions (M = Co, Ni, Zn, and Cd) by Gelatin-Immobilized Copper(II) Hexacyanoferrate(II). Russ. J. Phys. Chem. A 2003, 77, 822–824. [Google Scholar]
- Mikhailov, O.V.; Tatarintseva, T.B.; Naumkina, N.I.; Yusupov, R.A. Electrophilic Substitution Ni(II) → M(II) in Ni2[Fe(CN)6] Gelatin-immobilized Matrix Materials. Russ. J. Gen. Chem. 2003, 73, 847–854. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Tatarintseva, T.B.; Naumkina, N.I.; Kolgina, V.A. Electrophilic Substitution Mn(II) → M(II) (M = Co, Ni, Cu, Zn, Cd) in Gelatin-Immobilized Mn2[Fe(CN)6]. Russ. J. Coord. Chem. 2003, 29, 630–635. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Tatarintseva, T.B. Sorption of d-Element Ions by Nickel(II) Hexaferrocyanide Immobilized in Gelatin. Russ. J. Phys. Chem. A 2003, 77, 1491–1493. [Google Scholar]
- Mikhailov, O.V.; Tatarintseva, T.B. Chemisorption of M(II) ions (M = Co, Ni, Cu, Zn, and Cd) by Gelatin-Immobilized Fe(III) Hexaferrocyanide(II). Russ. J. Phys. Chem. A 2003, 77, 1996–1998. [Google Scholar]
- Tatarintseva, T.B.; Mikhailov, O.V.; Naumkina, N.I.; Lygina, T.Z. Electrophilic Copper(II) → Metal(II) Substitution in Gelatin-Immobilized Copper(II) Hexacyanoferrate(II) Matrix Implants. Russ. J. Gen. Chem. 2004, 74, 7–12. [Google Scholar] [CrossRef]
- Tatarintseva, T.B.; Mikhailov, O.V. Chemisorption of d-Element Ions by Gelatin-Immobilized Zn2[Fe(CN)6]. Russ. J. Phys. Chem. A 2004, 78, 67–69. [Google Scholar]
- Tatarintseva, T.B.; Mikhailov, O.V. Chemisorption of Fe(III) Ions by Gelatin-Immobilized Hexacyanoferrates(II) of the M2[Fe(CN)6] Type. Russ. J. Phys. Chem. A 2004, 78, 269–271. [Google Scholar]
- Tatarintseva, T.B.; Mikhailov, O.V. Chemisorption of d-Element Ions on Gelatin-Immobilized Mn2[Fe(CN)6]. Russ. J. Phys. Chem. A 2004, 78, 955–958. [Google Scholar]
- Tatarintseva, T.B.; Mikhailov, O.V.; Naumkina, N.I.; Lygina, T.Z. Ion-Exchange Processes M(II) → Fe(III) and Fe(III) → M(II) in Metal Hexacyanoferrate(II) Gelatin-Immobilized Matrices (M = Mn, Co, Ni, Cu, Zn, Cd). Russ. J. Coord. Chem. 2004, 30, 639–647. [Google Scholar] [CrossRef]
- Tatarintseva, T.B.; Mikhailov, O.V.; Lygina, T.Z. The Chemisorption of M(II) Ions (M = Mn, Ni, Cu, Zn, and Cd) by Gelatin-Immobilized Cobalt (II) Hexacyanoferrate(II). Russ. J. Phys. Chem. A 2004, 78, 1463–1466. [Google Scholar]
- Tatarintseva, T.B.; Mikhailov, O.V. Chemisorption of M(II) Ions (M = Mn, Co, Ni, Cu, Zn) on Gelatin-Immobilized Cd2[Fe(CN)6] Complex. Russ. J. Phys. Chem. A 2004, 78, 1818–1821. [Google Scholar]
- Tatarintseva, T.B.; Mikhailov, O.V.; Naumkina, N.I.; Lygina, T.Z. Complexation of Zn2[Fe(CN)6] with M2+ (M = Mn, Co, Ni, Cu, Cd) in Zn2[Fe(CN)6] gelatin-immobilized matrices. Russ. J. Coord. Chem. 2005, 31, 101–107. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Immobilization of (dd)heteronuclear hexacyanoferrates(II) in a gelatin matrix. Russ. Chem. Bull. 2008, 57, 8–17. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Electrophilic substitution in the d-metal hexacyanoferrate(II) gelatin-immobilized matrix systems. Revs. Inorg. Chem. 2018, 38, 103–126. [Google Scholar] [CrossRef]
- Nishimoto, M.; Sakamoto, R.; Mizuta, S.; Yoshinaka, R. Identification and characterization of molecular species of collagen in ordinary muscle and skin of the Japanese flounder (Paralichthys olivaceus). Food Chem. 2005, 90, 151–156. [Google Scholar] [CrossRef]
- Desai, M.P.; Labhasetwar, V.; Amidon, G.L.; Levy, R.J. Gastrointestinal uptake of biodegradable microparticles: Effect of particle size. Pharm. Res. 1996, 13, 1838–1845. [Google Scholar] [CrossRef]
- Esposito, E.; Cortesi, R.; Nastruzzi, C. Gelatin microspheres: Influence of preparation parameters and thermal treatment on chemico-physical and biopharmaceutical properties. Biomaterials 1996, 7, 2009–2020. [Google Scholar] [CrossRef]
- Vandelli, M.; Romagnoli, M.; Monti, A.; Gozzi, M.; Guerra, P.; Rivasi, F.; Forni, F. Microwave treated gelatin microspheres as drug delivery system. J. Control. Release 2004, 96, 67–84. [Google Scholar] [CrossRef]
- Way, D.V.; Nele, M.; Pinto, J.C. Preparation of gelatin beads treated with glucose and glycerol. Polímeros 2018, 28, 468–476. [Google Scholar] [CrossRef]
- Sivakumar, M.; Panduranga, R.K. Preparation, characterization and in vitro release of gentamicin from coralline hydroxyapatite-gelatin composite microspheres. Biomaterials 2002, 23, 3175–3181. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Kim, H.E.; Salih, V. Stimulation of Osteoblast Responses to Biomimetic Nanocomposites of Gelatin-Hydroxyapatite for Tissue Engineering Scaffolds. Biomaterials 2005, 26, 5221–5230. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Knowles, J.C.; Kim, H.E. Porous scaffolds of gelatin-hydroxyapatite nanocomposites obtained by biomimetic approach: Characterization and antibiotic drug release. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.; Chen, L.; Guo, Y.; Shi, J. Formation of nano-hydroxyapatite in gelatin droplets and the resulting porous composite microspheres. J. Inorg. Biochem. 2007, 101, 686–691. [Google Scholar] [CrossRef]
- Landi, E.; Valentini, F.; Tampieri, A. Porous hydroxyapatite/gelatine scaffolds with ice-designed channel-like porosity for biomedical applications. Acta Biomater. 2008, 4, 1620–1626. [Google Scholar] [CrossRef]
- Dressler, M.; Dombrowski, F.; Simon, U.; Börnstein, J.; Hodoroaba, V.; Feigl, M.; Grunow, S.; Gildenhaar, R.; Neumann, M. Influence of gelatin coatings on compressive strength of porous hydroxyapatite ceramics. J. Eur. Ceramic Soc. 2011, 31, 523–529. [Google Scholar] [CrossRef]
- Perez, R.A.; Del Valle, S.; Altankov, G.; Ginebra, M.P. Porous hydroxyapatite and gelatin/hydroxyapatite microspheres obtained by calcium phosphate cement emulsion. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 97, 156–166. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Traykova, T.; Planell, J.A. Calcium phosphate cements as bone drug delivery systems: A review. J. Control. Release 2006, 113, 102–110. [Google Scholar] [CrossRef]
- Habraken, W.J.; de Jonge, L.T.; Wolke, J.G.; Yubao, L.; Mikos, A.G.; Jansen, J.A. Introduction of gelatin microspheres into an injectable calcium phosphate cement. J. Biomed. Mater. Res. A 2008, 87, 643–655. [Google Scholar] [CrossRef]
- Habraken, W.J.; Boerman, O.C.; Wolke, J.G.; Mikos, A.G.; Jansen, J.A. In vitro growth factor release from injectable calcium phosphate cements containing gelatin microspheres. J. Biomed. Mater. Res. A 2009, 91, 614–622. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Ge, B. Calcium phosphate cement with bmp-2-loaded gelatin microspheres enhances bone healing in osteoporosis: A pilot study. Clin. Orthop. Relat. Res. 2010, 468, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Walboomers, X.F.; Habraken, W.J.; Zhang, Z.; Li, Y.; Grijpma, D.W.; Mikos, A.G.; Wolke, J.G.; Jansen, J.A. Injectable calcium phosphate cement with plga, gelatin and ptmc microspheres in a rabbit femoral defect. Acta Biomater. 2011, 7, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Park, K. Biomaterials for Cancer Therapeutics: Diagnosis, Prevention and Therapy; Woodhead Publishing: Sawston, UK, 2013; 542p, ISBN 0857096648. [Google Scholar]
- Park, K. Biomaterials for Cancer Therapeutics: Evolution and Innovation, 2nd ed.; Woodhead Publishing: Sawston, UK, 2020; 782p, ISBN 9780081029831. [Google Scholar]
- Gilbert, J.A.; Simpson, A.E.; Rudnick, D.E.; Geroski, D.H.; Aaberg, T.M., Jr.; Edelhauser, H.F. Transscleral permeability and intraocular concentrations of cisplatin from a collagen matrix. J. Control. Release 2003, 89, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M.; Tabata, Y.; Kariya, M.; Hosseinkhani, H.; Suzuki, A.; Fukuhara, K.; Mandai, M.; Takakura, K.; Fujii, S. In vivo anti-tumor effect of dual release of cisplatin and adriamycin from biodegradable gelatin hydrogel. J. Control. Release 2005, 103, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M.; Tabata, Y.; Kariya, M.; Hosseinkhani, H.; Suzuki, A.; Fukuhara, K.; Mandai, M.; Nanbu, K.; Takakura, K.; Fujii, S. In vivo anti-tumor effect through the controlled release of cisplatin from biodegradable gelatin hydrogel. J. Control. Release 2003, 92, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.L.; Su, W.Y.; Yen, K.C.; Yang, K.C.; Lin, F.H. The use of biotinylated-egf-modified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation. Biomaterials 2009, 30, 3476–3485. [Google Scholar] [CrossRef] [PubMed]
- Nezhad-Mokhtari, P.; Arsalani, N.; Javanbakht, S.; Shaabani, A. Development of gelatin microsphere encapsulated Cu-based metal-organic framework nanohybrid for the methotrexate delivery. J. Drug Deliv. Sci. Technol. 2019, 50, 174–180. [Google Scholar] [CrossRef]
- Mariod, A.A.; Adam, H.F. Review: Gelatin, source, extraction and industrial applications. Acta Sci. Pol. Technol. Aliment. 2013, 12, 135–147. [Google Scholar]
- Wheeler, D.L.; Dunn, E.F.; Harari, P.M. Understanding resistance to egfr inhibitors-impact on future treatment strategies. Nat. Rev. Clin. Oncol. 2010, 7, 493–507. [Google Scholar] [CrossRef]
- Sakr, M.A.; Sakthivel, K.; Hossain, T.; Shin, S.R.; Siddiqua, S.; Kim, J.; Kim, K. Recent trends in gelatin methacryloyl nanocomposite hydrogels for tissue engineering. J. Biomed. Mater. Res. Part A 2022, 110, 708–724. [Google Scholar] [CrossRef]
- Jeznach, O.; Kołbuk, D.; Reich, T.; Sajkiewicz, P. Immobilization of Gelatin on Fibers for Tissue Engineering Applications: A Comparative Study of Three Aliphatic Polyesters. Polymers 2022, 14, 4154. [Google Scholar] [CrossRef]
- Shao, Y.; Liao, Z.; Gao, B.; He, B. Emerging 3D Printing Strategies for Enzyme Immobilization: Materials, Methods, and Applications. ACS Omega 2022, 7, 11530–11543. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, O.V. New Method for Preparation of Samples for Measurement of Electronic Absorption Spectra of Difficult Soluble Inorganic and Metalorganic Compounds. Appl. Spectrosc. 1992, 46, 1240–1244. [Google Scholar]
- Mikhailov, O.V. Gelatin-immobilized Matrices as a New Material for Recording Absorption Spectra of Chemical Compounds. J. Anal. Chem. 1999, 54, 320–323. [Google Scholar]
- Markova, B.; Nazarova, D.; Sharlandjiev, P. Control of the spectral position of dichromated gelatin reflection holograms. Appl. Opt. 2011, 50, 5534–5537. [Google Scholar] [CrossRef] [PubMed]
- Ganzherli, N.M.; Gulyaev, S.N.; Maurer, I.A. The effect of UV radiation on the properties of diffraction gratings based on dichromated gelatin. Tech. Phys. Lett. 2016, 42, 988–989. [Google Scholar] [CrossRef]
- Ganzherli, N.M.; Gulyaev, S.N.; Maurer, I.A. Properties of holographic structures on dichromated gelatin subjected to ultraviolet radiation. J. Opt. Technol. 2017, 84, 617–620. [Google Scholar] [CrossRef]
- Ganzherli, N.M.; Gulyaev, S.N.; Maurer, I.A.; Khazvalieva, D.R. The transfer of a holographic structure from dihromated gelatine layers on a polymethylmethacrylate substrate. Opt. Spectrosc. 2018, 124, 408–411. [Google Scholar] [CrossRef]
- Calixto, S.; Ganzherli, N.; Gulyaev, S.; Figueroa-Gerstenmaier, S. Gelatin as a Photosensitive Material. Molecules 2018, 23, 2064. [Google Scholar] [CrossRef]
- Asal, M.; Ozen, O.; Sahinler, M.; Polatoglu, I. Recent developments in enzyme, DNA and immuno-based biosensors. Sensors 2018, 18, 1924. [Google Scholar] [CrossRef]
- Guan, Y.; Huang, Y.; Li, T. Applications of Gelatin in Biosensors: Recent Trends and Progress. Biosensors 2022, 12, 670. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailov, O.V. Gelatin Matrix as Functional Biomaterial for Immobilization of Nanoparticles of Metal-Containing Compounds. J. Funct. Biomater. 2023, 14, 92. https://doi.org/10.3390/jfb14020092
Mikhailov OV. Gelatin Matrix as Functional Biomaterial for Immobilization of Nanoparticles of Metal-Containing Compounds. Journal of Functional Biomaterials. 2023; 14(2):92. https://doi.org/10.3390/jfb14020092
Chicago/Turabian StyleMikhailov, Oleg V. 2023. "Gelatin Matrix as Functional Biomaterial for Immobilization of Nanoparticles of Metal-Containing Compounds" Journal of Functional Biomaterials 14, no. 2: 92. https://doi.org/10.3390/jfb14020092
APA StyleMikhailov, O. V. (2023). Gelatin Matrix as Functional Biomaterial for Immobilization of Nanoparticles of Metal-Containing Compounds. Journal of Functional Biomaterials, 14(2), 92. https://doi.org/10.3390/jfb14020092






