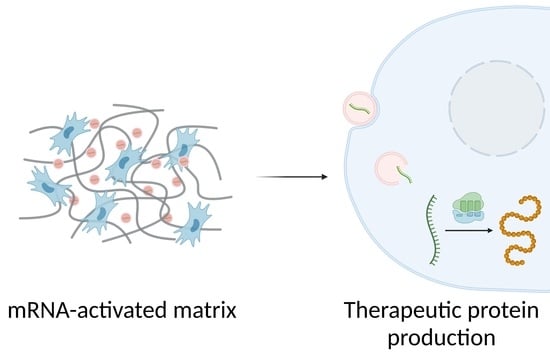
Matrices Activated with Messenger RNA
Abstract
1. Introduction
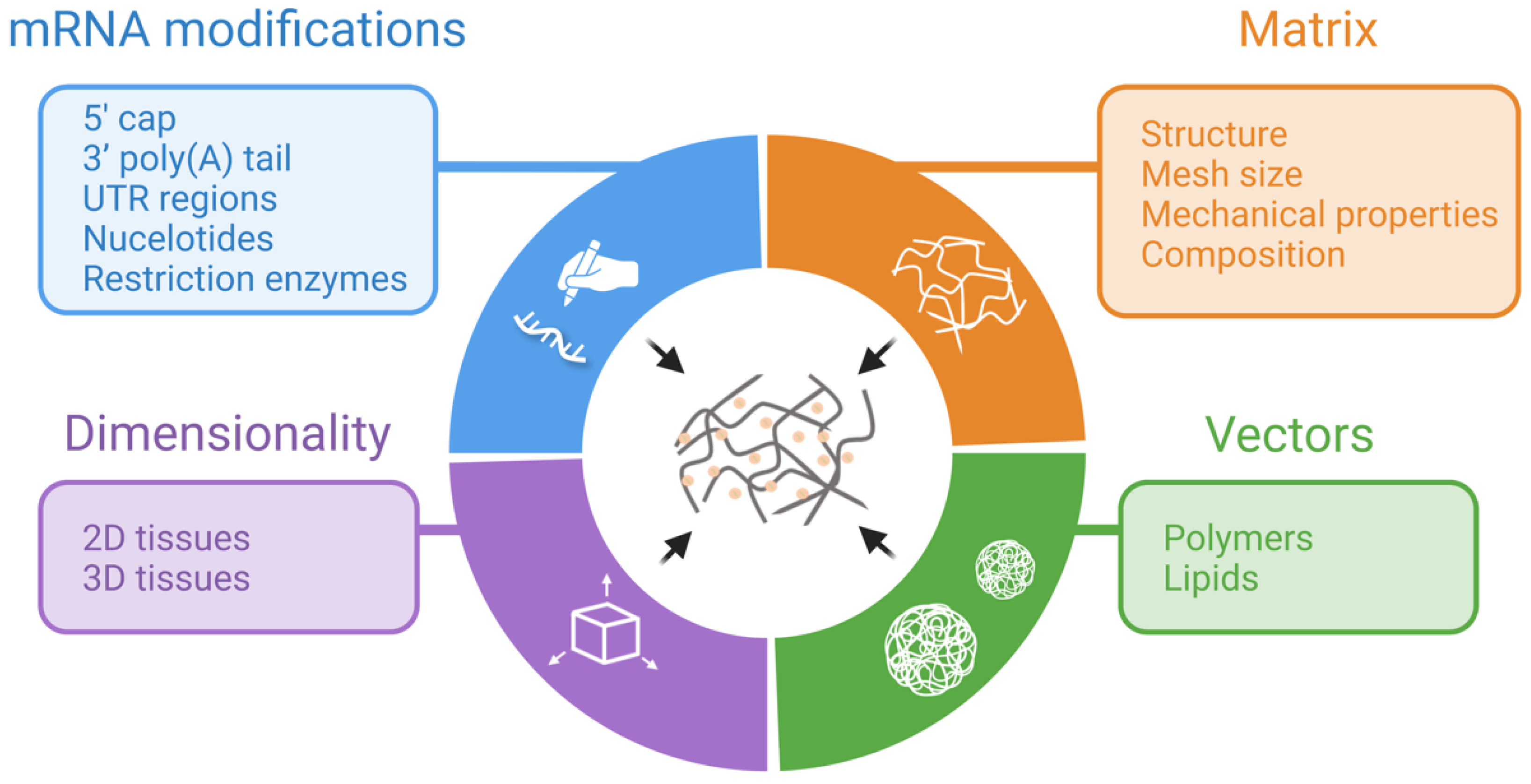
2. TAM Design Considerations
2.1. Differences between 2D and 3D Transfection
2.2. Composition and Mechanical Properties of Matrices
2.3. mRNA Modifications
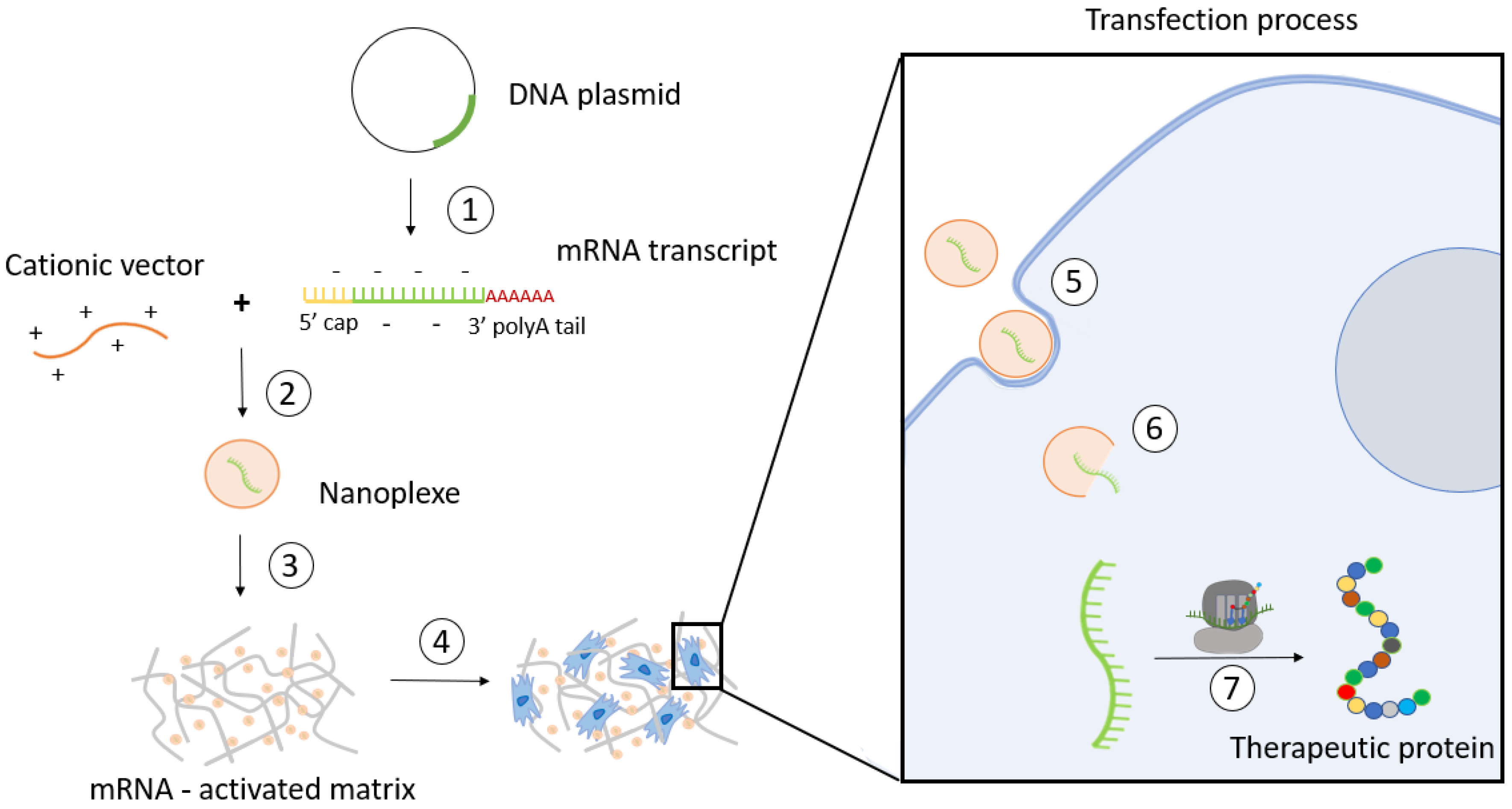
2.4. Nanosystems Employed in mRNA-Activated Matrices
3. Long-Term Stability of mRNA-Activated Matrices
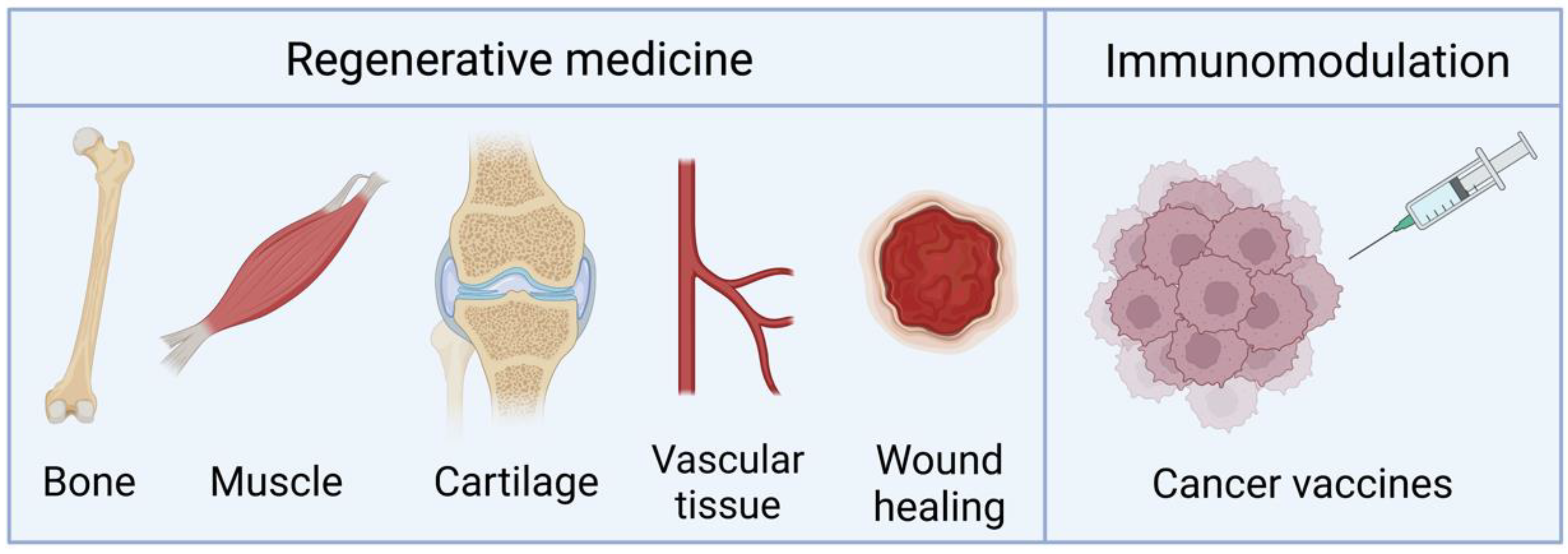
4. Applications
4.1. Bone Regeneration
4.2. Other Regenerative Applications
4.3. Vaccination and Immunomodulation
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef]
- Huang, G.; Li, F.; Zhao, X.; Ma, Y.; Li, Y.; Lin, M.; Jin, G.; Lu, T.J.; Genin, G.M.; Xu, F. Functional and Biomimetic Materials for Engineering of the Three-Dimensional Cell Microenvironment. Chem. Rev. 2017, 117, 12764–12850. [Google Scholar] [CrossRef] [PubMed]
- Aimin, C.; Chunlin, H.; Juliang, B.; Tinyin, Z.; Zhichao, D. Antibiotic loaded chitosan bar. An in vitro, in vivo study of a possible treatment for osteomyelitis. Clin. Orthop. Relat. Res. 1999, 366, 239–247. [Google Scholar] [CrossRef]
- Arcos, D.; Ragel, C.V.; Vallet-Regí, M. Bioactivity in glass/PMMA composites used as drug delivery system. Biomaterials 2001, 22, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A. Articular cartilage: Injuries and potential for healing. J. Orthop. Sports Phys. Ther. 1998, 28, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Weissman, D. mRNA transcript therapy. Expert Rev. Vaccines 2015, 14, 265–281. [Google Scholar] [CrossRef] [PubMed]
- De La Vega, R.E.; van Griensven, M.; Zhang, W.; Coenen, M.J.; Nagelli, C.V.; Panos, J.A.; Peniche Silva, C.J.; Geiger, J.; Plank, C.; Evans, C.H.; et al. Efficient healing of large osseous segmental defects using optimized chemically modified messenger RNA encoding BMP-2. Sci. Adv. 2022, 8, eabl6242. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhu, Y.Y.; Smiley, E.; Bonadio, J.; Rouleau, J.P.; Goldstein, S.A.; McCauley, L.K.; Davidson, B.L.; Roessler, B.J. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proc. Natl. Acad. Sci. USA 1996, 93, 5753–5758. [Google Scholar] [CrossRef]
- Wang, C.; Ma, L.; Gao, C. Design of gene-activated matrix for the repair of skin and cartilage. Polym. J. 2014, 46, 476–482. [Google Scholar] [CrossRef]
- Blume, P.; Driver, V.R.; Tallis, A.J.; Kirsner, R.S.; Kroeker, R.; Payne, W.G.; Wali, S.; Marston, W.; Dove, C.; Engler, R.L.; et al. Formulated collagen gel accelerates healing rate immediately after application in patients with diabetic neuropathic foot ulcers. Wound Repair Regen. 2011, 19, 302–308. [Google Scholar] [CrossRef]
- Mulder, G.; Tallis, A.J.; Marshall, V.T.; Mozingo, D.; Phillips, L.; Pierce, G.F.; Chandler, L.A.; Sosnowski, B.K. Treatment of nonhealing diabetic foot ulcers with a platelet-derived growth factor gene-activated matrix (GAM501): Results of a phase 1/2 trial. Wound Repair Regen. 2009, 17, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.; Chen, L.; Lv, Y. RNA-based scaffolds for bone regeneration: Application and mechanisms of mRNA, miRNA and siRNA. Theranostics 2020, 10, 3190–3205. [Google Scholar] [CrossRef]
- Hajj, K.A.; Whitehead, K.A. Tools for translation: Non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2017, 2, 17056. [Google Scholar] [CrossRef]
- Eskildsen, T.; Taipaleenmäki, H.; Stenvang, J.; Abdallah, B.M.; Ditzel, N.; Nossent, A.Y.; Bak, M.; Kauppinen, S.; Kassem, M. MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 6139–6144. [Google Scholar] [CrossRef] [PubMed]
- Trompeter, H.I.; Dreesen, J.; Hermann, E.; Iwaniuk, K.M.; Hafner, M.; Renwick, N.; Tuschl, T.; Wernet, P. MicroRNAs miR-26a, miR-26b, and miR-29b accelerate osteogenic differentiation of unrestricted somatic stem cells from human cord blood. BMC Genom. 2013, 14, 111. [Google Scholar] [CrossRef]
- Takayama, K.; Suzuki, A.; Manaka, T.; Taguchi, S.; Hashimoto, Y.; Imai, Y.; Wakitani, S.; Takaoka, K. RNA interference for noggin enhances the biological activity of bone morphogenetic proteins in vivo and in vitro. J. Bone Miner. Metab. 2009, 27, 402–411. [Google Scholar] [CrossRef]
- Manaka, T.; Suzuki, A.; Takayama, K.; Imai, Y.; Nakamura, H.; Takaoka, K. Local delivery of siRNA using a biodegradable polymer application to enhance BMP-induced bone formation. Biomaterials 2011, 32, 9642–9648. [Google Scholar] [CrossRef]
- Elangovan, S.; Khorsand, B.; Do, A.V.; Hong, L.; Dewerth, A.; Kormann, M.; Ross, R.D.; Sumner, D.R.; Allamargot, C.; Salem, A.K. Chemically modified RNA activated matrices enhance bone regeneration. J. Control. Release 2015, 218, 22–28. [Google Scholar] [CrossRef]
- Balmayor, E.R.; Geiger, J.P.; Aneja, M.K.; Berezhanskyy, T.; Utzinger, M.; Mykhaylyk, O.; Rudolph, C.; Plank, C. Chemically modified RNA induces osteogenesis of stem cells and human tissue explants as well as accelerates bone healing in rats. Biomaterials 2016, 87, 131–146. [Google Scholar] [CrossRef]
- Ledo, A.M.; Senra, A.; Rilo-Alvarez, H.; Borrajo, E.; Vidal, A.; Alonso, M.J.; Garcia-Fuentes, M. mRNA-activated matrices encoding transcription factors as primers of cell differentiation in tissue engineering. Biomaterials 2020, 247, 120016. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.; Liang, Z.; Lv, Y. Demineralized bone matrix scaffold modified with mRNA derived from osteogenically pre-differentiated MSCs improves bone repair. Mater. Sci. Eng. C. Mater. Biol. Appl. 2021, 119, 111601. [Google Scholar] [CrossRef] [PubMed]
- Lui, K.O.; Zangi, L.; Silva, E.A.; Bu, L.; Sahara, M.; Li, R.A.; Mooney, D.J.; Chien, K.R. Driving vascular endothelial cell fate of human multipotent Isl1+ heart progenitors with VEGF modified mRNA. Cell Res. 2013, 23, 1172–1186. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.A. RNA-based tools for nuclear reprogramming and lineage-conversion: Towards clinical applications. J. Cardiovasc. Transl. Res. 2013, 6, 956–968. [Google Scholar] [CrossRef]
- Joo, J.Y.; Park, G.Y.; An, S.S. Biocompatible and biodegradable fibrinogen microspheres for tumor-targeted doxorubicin delivery. Int. J. Nanomed. 2015, 10, 101–111. [Google Scholar]
- Yan, J.; Chen, R.; Zhang, H.; Bryers, J.D. Injectable Biodegradable Chitosan-Alginate 3D Porous Gel Scaffold for mRNA Vaccine Delivery. Macromol. Biosci. 2019, 19, e1800242. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, H.; Yan, J.; Bryers, J.D. Scaffold-mediated delivery for non-viral mRNA vaccines. Gene Ther. 2018, 25, 556–567. [Google Scholar] [CrossRef]
- Dastmalchi, F.; Karachi, A.; Mehkri, Y.; O’Malley, A.; Subramaniam, V.; Angelini, T.; Mitchell, D.; Rahman, M. IMMU-20. HYDROGEL-CXCL9 VACCINE RESULTS IN MRNA DELIVERY TO DENDRITIC CELLS AND POTENT ANTI-TUMOR RESPONSES IN GBM. Neuro Oncol. 2021, 23, vi96. [Google Scholar] [CrossRef]
- Dhaliwal, A.; Oshita, V.; Segura, T. Transfection in the third dimension. Integr. Biol. 2013, 5, 1206–1216. [Google Scholar] [CrossRef]
- Badieyan, Z.S.; Berezhanskyy, T.; Utzinger, M.; Aneja, M.K.; Emrich, D.; Erben, R.; Schüler, C.; Altpeter, P.; Ferizi, M.; Hasenpusch, G.; et al. Transcript-activated collagen matrix as sustained mRNA delivery system for bone regeneration. J. Control. Release 2016, 239, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Balmayor, E.R.; Geiger, J.P.; Koch, C.; Aneja, M.K.; van Griensven, M.; Rudolph, C.; Plank, C. Modified mRNA for BMP-2 in Combination with Biomaterials Serves as a Transcript-Activated Matrix for Effectively Inducing Osteogenic Pathways in Stem Cells. Stem Cells Dev. 2017, 26, 25–34. [Google Scholar] [CrossRef]
- Zaitseva, T.S.; Yang, G.; Dionyssiou, D.; Zamani, M.; Sawamura, S.; Yakubov, E.; Ferguson, J.; Hallett, R.L.; Fleischmann, D.; Paukshto, M.V.; et al. Delivery of hepatocyte growth factor mRNA from nanofibrillar scaffolds in a pig model of peripheral arterial disease. Regen. Med. 2020, 15, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Fayed, O.; van Griensven, M.; Birgani, Z.T.; Plank, C.; Balmayor, E.R. Transcript-Activated Coatings on Titanium Mediate Cellular Osteogenesis for Enhanced Osteointegration. Mol. Pharm. 2021, 18, 1121–1137. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Kormann, M.; Rosenecker, J.; Rudolph, C. Current prospects for mRNA gene delivery. Eur. J. Pharm. Biopharm. 2009, 71, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Steinle, H.; Ionescu, T.M.; Schenk, S.; Golombek, S.; Kunnakattu, S.J.; Özbek, M.T.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Incorporation of Synthetic mRNA in Injectable Chitosan-Alginate Hybrid Hydrogels for Local and Sustained Expression of Exogenous Proteins in Cells. Int. J. Mol. Sci. 2018, 19, 1313. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Perche, F.; Midoux, P.; Cabral, C.S.D.; Malard, V.; Correia, I.J.; EI-Hafci, H.; Petite, H.; Logeart-Avramoglou, D.; Pichon, C. In Vivo bone tissue induction by freeze-dried collagen-nanohydroxyapatite matrix loaded with BMP2/NS1 mRNAs lipopolyplexes. J. Control. Release 2021, 334, 188–200. [Google Scholar] [CrossRef]
- Schneider-Barthold, C.; Baganz, S.; Wilhelmi, M.; Scheper, T.; Pepelanova, I. Hydrogels based on collagen and fibrin-Frontiers and applications. BioNanoMaterials 2016, 17, 3–12. [Google Scholar] [CrossRef]
- Patel, S.; Athirasala, A.; Menezes, P.P.; Ashwanikumar, N.; Zou, T.; Sahay, G.; Bertassoni, L.E. Messenger RNA Delivery for Tissue Engineering and Regenerative Medicine Applications. Tissue Eng. Part A 2019, 25, 91–112. [Google Scholar] [CrossRef]
- Kundu, J.; Pati, F.; Shim, J.H.; Cho, D.W. 10-Rapid prototyping technology for bone regeneration. In Rapid Prototyping of Biomaterials; Narayan, R., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 254–284. [Google Scholar]
- Hesse, E.; Hefferan, T.E.; Tarara, J.E.; Haasper, C.; Meller, R.; Krettek, C.; Lu, L.; Yaszemski, M.J. Collagen type I hydrogel allows migration, proliferation, and osteogenic differentiation of rat bone marrow stromal cells. J. Biomed. Mater. Res. A 2010, 94, 442–449. [Google Scholar] [CrossRef]
- Almelkar, S.I.; Patwardhan, A.M.; Divate, S.A.; Agrawal, N.B.; Bhonde, R.R.; Chaukar, A.P. Fibrin matrix supports endothelial cell adhesion and migration in culture. OA Biol. 2014, 2, 5. [Google Scholar]
- Zhang, W.; De La Vega, R.E.; Coenen, M.J.; Müller, S.A.; Peniche Silva, C.J.; Aneja, M.K.; Plank, C.; van Griensven, M.; Evans, C.H.; Balmayor, E.R. An Improved, Chemically Modified RNA Encoding BMP-2 Enhances Osteogenesis In Vitro and In Vivo. Tissue Eng. Part A 2019, 25, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Oude Egberink, R.; Zegelaar, H.M.; El Boujnouni, N.; Versteeg, E.M.M.; Daamen, W.F.; Brock, R. Biomaterial-Mediated Protein Expression Induced by Peptide-mRNA Nanoparticles Embedded in Lyophilized Collagen Scaffolds. Pharmaceutics 2022, 14, 1619. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Duan, H.; Xu, L.; Witman, N.; Yan, B.; Yu, Z.; Wang, H.; Tan, Y.; Lin, L.; Li, D.; et al. BMP-2 and VEGF-A modRNAs in collagen scaffold synergistically drive bone repair through osteogenic and angiogenic pathways. Commun. Biol. 2021, 4, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Khorsand, B.; Elangovan, S.; Hong, L.; Dewerth, A.; Kormann, M.S.D.; Salem, A.K. A Comparative Study of the Bone Regenerative Effect of Chemically Modified RNA Encoding BMP-2 or BMP-9. AAPS J. 2017, 19, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Khorsand, B.; Elangovan, S.; Hong, L.; Kormann, M.S.D.; Salem, A.K. A bioactive collagen membrane that enhances bone regeneration. J. Biomed. Mater. Res. B. Appl. Biomater. 2019, 107, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Chen, T.; Song, Y.; Feng, C.; Chen, H.; Zhang, Q.; Chen, G.; Zhu, X. mRNA Delivery by a pH-Responsive DNA Nano-Hydrogel. Small 2021, 17, e2101224. [Google Scholar] [CrossRef]
- Zaitseva, T.S.; Alcazar, C.; Zamani, M.; Hou, L.; Sawamura, S.; Yakubov, E.; Hopkins, M.; Woo, Y.J.; Paukshto, M.V.; Huang, N.F. Aligned Nanofibrillar Scaffolds for Controlled Delivery of Modified mRNA. Tissue Eng. Part A 2019, 25, 121–130. [Google Scholar] [CrossRef]
- Houchin-Ray, T.; Swift, L.A.; Jang, J.; Shea, L.D. Patterned PLG substrates for localized DNA delivery and directed neurite extension. Biomaterials 2007, 28, 2603–2611. [Google Scholar] [CrossRef]
- Huang, N.F.; Patel, S.; Thakar, R.G.; Wu, J.; Hsiao, B.S.; Chu, B.; Lee, R.J.; Li, S. Myotube assembly on nanofibrous and micropatterned polymers. Nano Lett. 2006, 6, 537–542. [Google Scholar] [CrossRef]
- Downing, T.L.; Soto, J.; Morez, C.; Houssin, T.; Fritz, A.; Yuan, F.; Chu, J.; Patel, S.; Schaffer, D.V.; Li, S. Biophysical regulation of epigenetic state and cell reprogramming. Nat. Mater. 2013, 12, 1154–1162. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Haugh, M.G.; Murphy, C.M.; McKiernan, R.C.; Altenbuchner, C.; O’Brien, F.J. Crosslinking and Mechanical Properties Significantly Influence Cell Attachment, Proliferation, and Migration Within Collagen Glycosaminoglycan Scaffolds. Tissue Eng. Part A 2011, 17, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Liu, M.; Chen, G.; Xu, Z.; Lv, Y. Demineralized Bone Scaffolds with Tunable Matrix Stiffness for Efficient Bone Integration. ACS Appl. Mater. Interfaces 2018, 10, 27669–27680. [Google Scholar] [CrossRef] [PubMed]
- Keeney, M.; Onyiah, S.; Zhang, Z.; Tong, X.; Han, L.H.; Yang, F. Modulating polymer chemistry to enhance non-viral gene delivery inside hydrogels with tunable matrix stiffness. Biomaterials 2013, 34, 9657–9665. [Google Scholar] [CrossRef] [PubMed]
- Ledo, A.M.; Vining, K.H.; Alonso, M.J.; Garcia-Fuentes, M.; Mooney, D.J. Extracellular matrix mechanics regulate transfection and SOX9-directed differentiation of mesenchymal stem cells. Acta Biomater. 2020, 110, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Williams, J.K.; Yoo, J.J.; Atala, A. Chapter 59-Regenerative Medicine Approaches for Tissue Engineered Heart Valves. In Principles of Regenerative Medicine, 3rd ed.; Atala, A., Lanza, R., Mikos, A.G., Nerem, R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 1041–1058. [Google Scholar]
- Jeon, O.; Ryu, S.H.; Chung, J.H.; Kim, B.S. Control of basic fibroblast growth factor release from fibrin gel with heparin and concentrations of fibrinogen and thrombin. J. Control. Release 2005, 105, 249–259. [Google Scholar] [CrossRef]
- Gandhi, J.K.; Knudsen, T.; Hill, M.; Roy, B.; Bachman, L.; Pfannkoch-Andrews, C.; Schmidt, K.N.; Metko, M.M.; Ackerman, M.J.; Resch, Z.; et al. Human Fibrinogen for Maintenance and Differentiation of Induced Pluripotent Stem Cells in Two Dimensions and Three Dimensions. Stem Cells Transl. Med. 2019, 8, 512–521. [Google Scholar] [CrossRef]
- Horasawa, N.; Yamashita, T.; Uehara, S.; Udagawa, N. High-performance scaffolds on titanium surfaces: Osteoblast differentiation and mineralization promoted by a globular fibrinogen layer through cell-autonomous BMP signaling. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 46, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Rahim, M.; Ng, Q.; Segura, T. Hyaluronic acid and fibrin hydrogels with concentrated DNA/PEI polyplexes for local gene delivery. J. Control. Release 2011, 153, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Lauer-Fields, J.; Schmoekel, H.G.; Metters, A.T.; Weber, F.E.; Fields, G.B.; Hubbell, J.A. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proc. Natl. Acad. Sci. USA 2003, 100, 5413–5418. [Google Scholar] [CrossRef] [PubMed]
- Utzinger, M.; Jarzebinska, A.; Haag, N.; Schweizer, M.; Winter, G.; Dohmen, C.; Rudolph, C.; Plank, C. cmRNA/lipoplex encapsulation in PLGA microspheres enables transfection via calcium phosphate cement (CPC)/PLGA composites. J. Control. Release 2017, 249, 143–149. [Google Scholar] [CrossRef]
- Sanz-Horta, R.; Matesanz, A.; Jorcano, J.L.; Velasco, D.; Acedo, P.; Gallardo, A.; Reinecke, H.; Elvira, C. Preparation and Characterization of Plasma-Derived Fibrin Hydrogels Modified by Alginate di-Aldehyde. Int. J. Mol. Sci. 2022, 23, 4296. [Google Scholar] [CrossRef]
- Martina, M.; Hutmacher, D.W. Biodegradable polymers applied in tissue engineering research: A review. Polym. Int. 2007, 56, 145–157. [Google Scholar] [CrossRef]
- Arun, Y.; Ghosh, R.; Domb, A.J. Biodegradable Hydrophobic Injectable Polymers for Drug Delivery and Regenerative Medicine. Adv. Funct. Mater. 2021, 31, 2010284. [Google Scholar] [CrossRef]
- Khalil, A.S.; Yu, X.; Umhoefer, J.M.; Chamberlain, C.S.; Wildenauer, L.A.; Diarra, G.M.; Hacker, T.A.; Murphy, W.L. Single-dose mRNA therapy via biomaterial-mediated sequestration of overexpressed proteins. Sci. Adv. 2020, 6, eaba2422. [Google Scholar] [CrossRef]
- Jemielity, J.; Fowler, T.; Zuberek, J.; Stepinski, J.; Lewdorowicz, M.; Niedzwiecka, A.; Stolarski, R.; Darzynkiewicz, E.; Rhoads, R.E. Novel “anti-reverse” cap analogs with superior translational properties. RNA 2003, 9, 1108–1122. [Google Scholar] [CrossRef] [PubMed]
- Leppek, K.; Das, R.; Barna, M. Functional 5’ UTR mRNA structures in eukaryotic translation regulation and how to find them. Nat. Rev. Mol. Cell Biol. 2018, 19, 158–174. [Google Scholar] [CrossRef]
- Holtkamp, S.; Kreiter, S.; Selmi, A.; Simon, P.; Koslowski, M.; Huber, C.; Türeci, O.; Sahin, U. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood 2006, 108, 4009–4017. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Kormann, M.S.D.; Hasenpusch, G.; Aneja, M.K.; Nica, G.; Flemmer, A.W.; Herber-Jonat, S.; Huppmann, M.; Mays, L.E.; Illenyi, M.; Schams, A.; et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat. Biotechnol. 2011, 29, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.; Manos, P.D.; Ahfeldt, T.; Loh, Y.; Li, H.; Lau, F.; Ebina, W.; Mandal, P.K.; Smith, Z.D.; Meissner, A.; et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010, 7, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Tang, X.; Chen, Y.; Chen, K.; Fan, N.; Xiao, W.; Zheng, Q.; Li, G.; Teng, Y.; Wu, M.; et al. mRNA-based therapeutics: Powerful and versatile tools to combat diseases. Signal Transduct. Target Ther. 2022, 7, 166. [Google Scholar] [CrossRef]
- Uchida, S.; Itaka, K.; Chen, Q.; Osada, K.; Ishii, T.; Shibata, M.; Harada-Shiba, M.; Kataoka, K. PEGylated polyplex with optimized PEG shielding enhances gene introduction in lungs by minimizing inflammatory responses. Mol. Ther. 2012, 20, 1196–1203. [Google Scholar] [CrossRef]
- Godbey, W.T.; Wu, K.K.; Mikos, A.G. Poly(ethylenimine) and its role in gene delivery. J. Control. Release 1999, 60, 149–160. [Google Scholar] [CrossRef]
- Bettinger, T.; Carlisle, R.C.; Read, M.L.; Ogris, M.; Seymour, L.W. Peptide-mediated RNA delivery: A novel approach for enhanced transfection of primary and post-mitotic cells. Nucleic Acids Res. 2001, 29, 3882–3891. [Google Scholar] [CrossRef]
- Elangovan, S.; D’Mello, S.R.; Hong, L.; Ross, R.D.; Allamargot, C.; Dawson, D.V.; Stanford, C.M.; Johnson, G.K.; Sumner, D.R.; Salem, A.K. The enhancement of bone regeneration by gene activated matrix encoding for platelet derived growth factor. Biomaterials 2014, 35, 737–747. [Google Scholar] [CrossRef]
- Ferkol, T.; Perales, J.C.; Eckman, E.; Kaetzel, C.S.; Hanson, R.W.; Davis, P.B. Gene transfer into the airway epithelium of animals by targeting the polymeric immunoglobulin receptor. J. Clin. Investig. 1995, 95, 493–502. [Google Scholar] [CrossRef]
- Wagner, E.; Cotten, M.; Foisner, R.; Birnstiel, M.L. Transferrin-polycation-DNA complexes: The effect of polycations on the structure of the complex and DNA delivery to cells. Proc. Natl. Acad. Sci. USA 1991, 88, 4255–4259. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhang, S.; Jiang, H.; Zhao, B.; Lv, H. Lipoplex morphologies and their influences on transfection efficiency in gene delivery. J. Control. Release 2007, 123, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ishida, T.; Okada, Y.; Oku, N.; Kiwada, H. Increased gene expression by cationic liposomes (TFL-3) in lung metastases following intravenous injection. Biol. Pharm. Bull. 2005, 28, 701–706. [Google Scholar] [CrossRef]
- Persano, S.; Guevara, M.L.; Li, Z.; Mai, J.; Ferrari, M.; Pompa, P.P.; Shen, H. Lipopolyplex potentiates anti-tumor immunity of mRNA-based vaccination. Biomaterials 2017, 125, 81–89. [Google Scholar] [CrossRef]
- Su, X.; Fricke, J.; Kavanagh, D.G.; Irvine, D.J. In vitro and in vivo mRNA delivery using lipid-enveloped pH-responsive polymer nanoparticles. Mol. Pharm. 2011, 8, 774–787. [Google Scholar] [CrossRef]
- Mével, M.; Neveu, C.; Gonçalves, C.; Yaouanc, J.; Pichon, C.; Jaffrès, P.; Midoux, P. Novel neutral imidazole-lipophosphoramides for transfection assays. Chem. Commun. 2008, 27, 3124–3126. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Han, D.; Cai, C.; Tang, X. An overview of liposome lyophilization and its future potential. J. Control. Release 2010, 142, 299–311. [Google Scholar] [CrossRef]
- Mesquida, P.; Kohl, D.; Andriotis, O.G.; Thurner, P.J.; Duer, M.; Bansode, S.; Schitter, G. Evaluation of surface charge shift of collagen fibrils exposed to glutaraldehyde. Sci. Rep. 2018, 8, 10126. [Google Scholar] [CrossRef] [PubMed]
- Reckhenrich, A.K.; Hopfner, U.; Krötz, F.; Zhang, Z.; Koch, C.; Kremer, M.; Machens, H.-G.; Plank, C.; Egaña, J.T. Bioactivation of dermal scaffolds with a non-viral copolymerprotected gene vector. Biomaterials 2011, 32, 1996–2003. [Google Scholar] [CrossRef]
- Scherer, F.; Schillinger, U.; Putz, U.; Stemberger, A.; Plank, C. Nonviral vector loaded collagen sponges for sustained gene delivery in vitro and in vivo. J. Gene Med. 2002, 4, 634–643. [Google Scholar] [CrossRef]
- Zhang, L.; Glasser, F.P. Critical examination of drying damage to cement pastes. Adv. Cem. Res. 2000, 12, 79–88. [Google Scholar] [CrossRef]
- Xu, D.; Zhao, Y.; Wang, J.; He, J.; Weng, Y.; Luo, J. Smads, p38 and ERK1/2 are involved in BMP9-induced osteogenic differentiation of C3H10T1/2 mesenchymal stem cells. BMB Rep. 2012, 45, 247–252. [Google Scholar] [CrossRef]
- Karam, M.; Daoud, G. mRNA vaccines: Past, present, future. Asian J. Pharm. Sci. 2022, 17, 491–522. [Google Scholar] [CrossRef] [PubMed]
- McNamara, M.A.; Nair, S.K.; Holl, E.K. RNA-Based Vaccines in Cancer Immunotherapy. J. Immunol. Res. 2015, 2015, 794528. [Google Scholar] [CrossRef]
- Fotin-Mleczek, M.; Duchardt, K.M.; Lorenz, C.; Pfeiffer, R.; Ojkić-Zrna, S.; Probst, J.; Kallen, K.J. Messenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activity. J. Immunother. 2011, 34, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Peng, K.; Qiu, L.; Li, M.; Ruan, J.; He, L.; Yuan, Z. Hitchhiking on Controlled-Release Drug Delivery Systems: Opportunities and Challenges for Cancer Vaccines. Front. Pharmacol. 2021, 12, 679602. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, A.N.; Irvine, D.J. Inverse opal hydrogel-collagen composite scaffolds as a supportive microenvironment for immune cell migration. J. Biomed. Mater. Res. A 2008, 85, 815–828. [Google Scholar] [CrossRef]

| Application | Matrix Composition | Encoded Protein | mRNA Modifications | Transfection Reagents | Development | Publication Year |
|---|---|---|---|---|---|---|
| Bone regeneration | Collagen scaffold | BMP-2 | ARCA | PEI | In vitro and in vivo (rat critical-sized calvarial bone defect model) | 2015 [20] |
| s2U (0.25) m5C (0.25) or Ψ (1.0) m5C (1.0) | ||||||
| Poly(A) - 120 | ||||||
| Bone regeneration | Collagen scaffold | BMP-7 | Cap 1 structure | PepFect14 or Lipofectamine™ MessengerMAX | In vitro | 2022 [44] |
| Sequence modifications | ||||||
| Bone regeneration | Collagen scaffold | BMP-2 or BMP-9 | ARCA | PEI | In vitro and in vivo (rat critical-sized calvarial defect model) | 2017 [46] |
| Ψ (1.0) m5C (1.0) | ||||||
| Poly(A) - 120 | ||||||
| Bone regeneration | Collagen fibre matrix | BMP-2 and VEGF-A | m1Ψ (1.0) | Lipofectamine™ MessengerMAX | In vitro and in vivo (rat critical-sized calvarial defect model) | 2021 [45] |
| Poly(A) tail | ||||||
| Bone regeneration | Collagen-nanohydroxyapatite matrix | BMP-2 and NS1 | Poly(A) tail | Lipopolyplexes His-lPEI/Lip100 or Lipofectamine™ MessengerMax | In vitro and in vivo (mouse ectopic model) | 2021 [37] |
| Bone regeneration | Collagen sponge scaffold | BMP-2 | Cap structure | Proprietary lipid/DPPC/cholesterol/DMG-PEG or PEI | In vitro and in vivo (rat non-critical femoral bone defect model) | 2016 [31] |
| s2U m5C | ||||||
| Poly(A) - 200 | ||||||
| Bone healing | Collagen sponge scaffold | BMP-2 | TISU sequence | Proprietary lipid/DPPC/cholesterol/DMG-PEG | In vivo (rat critical-sized femoral osteotomies defect) | 2022 [9] |
| 5IU (0.35) 5IC (0.075) | ||||||
| Bone healing | Collagen sponge scaffold | BMP-2 | ARCA | Proprietary lipid/DPPC/cholesterol/DMG-PEG | In vitro and in vivo (rat critical-sized femoral defect) | 2019 [43] |
| TISU sequence | ||||||
| 5IU (0.35) 5IC (0.075) or s2U (0.25) m5C (0.25) | ||||||
| Poly(A) - 120 | ||||||
| Poly(A) tail | ||||||
| Bone regeneration | Perforated collagen membranes | BMP-9 | ARCA | PEI | In vitro and in vivo (rat critical-sized calvarial defect model) | 2019 [47] |
| Ψ (1.0) m5C (1.0) | ||||||
| Poly(A) - 120 | ||||||
| Bone regeneration | Demineralized bone matrix scaffold | Oi-mRNA | None | PEI | In vitro and in vivo (rat critical-sized calvarial defect model) | 2021 [23] |
| Bone healing | Fibrin gel | BMP-2 | ARCA | Proprietary lipid/DOPE/cholesterol/DMPE-PEG | In vitro and in vivo (non-critical rat femur bone defect model) | 2016 [21] |
| s2U (0.25) m5C (0.25) | ||||||
| Bone regeneration | Fibrin gel or micro-macro biphasic calcium phosphate (MBCP) ceramic granules | BMP-2 | ARCA | DreamFect™ Gold | In vitro | 2017 [32] |
| s2U (0.25) m5C (0.25) | ||||||
| Poly(A) tail | ||||||
| Bone healing | PLGA microspheres in calcium phosphate cements | Reporter proteins | s2U m5C | Proprietary lipid/DOPE/cholesterol/DMG-PEG | In vitro | 2017 [66] |
| Poly(A) - 200 | ||||||
| Ortho-regeneration | Poly-D,L-lactic acid (PDLLA), fibrin or fibrinogen coating | BMP-2 | ARCA | Proprietary lipid/DPPC/cholesterol/DMG-PEG | In vitro | 2021 [34] |
| 5IU (0.35) 5IC (0.075) | ||||||
| Poly(A) - 200 | ||||||
| Tissue engineering | Chitosan-alginate hybrid hydrogels | Reporter proteins | ARCA | GenaxxoFect™ reagent | In vitro | 2018 [36] |
| Ψ (1.0) m5C (1.0) | ||||||
| mRNA delivery | DNA nano-hydrogel | Reporter proteins | m7G cap | None | In vitro | 2021 [48] |
| Poly (A) tail | ||||||
| Chondrogenesis and myogenesis | Fibrin gel | SOX-9 or MYOD | ARCA | 3DfectIN™ | In vitro | 2020 [22] |
| Kozak consensus sequence | ||||||
| alpha-globin 3′ UTR terminating | ||||||
| Vascular regeneration | Matrigel™ | VEGF-A | ARCA | Lipofectamine™ RNAiMAX | In vitro and in vivo (NOD/SCID mice) | 2013 [24] |
| Ψ m5C | ||||||
| Poly(A) tail | ||||||
| Vascular regeneration | Parallel-aligned nanofibrillar collagen scaffolds | HGF | Cap 1 structure | Lipofectamine™ Messenger Max | In vitro and in vivo (porcine peripheral arterial disease model) | 2020 [33] |
| Ψ m5C | ||||||
| Poly(A) - 175 | ||||||
| Wound healing | Mineral-coated microparticles (MCMs) | bFGF | ARCA | Lipofectamine™ Messenger Max | In vitro and in vivo (murine model of diabetic ulcers) | 2020 [70] |
| Ψ m5C | ||||||
| Poly(A) tail | ||||||
| Vaccine | pHEMA scaffold | Reporter proteins | m7G cap | Lipofectamine™ Messenger Max or Stemfect™ or in vivo-jetPEI™ or Poly (β-amino ester)) | In vitro and in vivo (mouse subcutaneous implant model) | 2018 [28] |
| Poly(A) tail | ||||||
| Vaccine | Chitosan-alginate 3D porous gel | OVA | m7G cap | Stemfect™ | In vitro and in vivo (murine model) | 2018 [27] |
| poly(A) tail | ||||||
| Vaccine | Hydrogel | Tumour proteins | None | Nanoparticles | In vitro and in vivo (murine glioblastoma multiforme model) | 2021 [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Campelo, R.; Garcia-Fuentes, M. Matrices Activated with Messenger RNA. J. Funct. Biomater. 2023, 14, 48. https://doi.org/10.3390/jfb14010048
Martinez-Campelo R, Garcia-Fuentes M. Matrices Activated with Messenger RNA. Journal of Functional Biomaterials. 2023; 14(1):48. https://doi.org/10.3390/jfb14010048
Chicago/Turabian StyleMartinez-Campelo, Raquel, and Marcos Garcia-Fuentes. 2023. "Matrices Activated with Messenger RNA" Journal of Functional Biomaterials 14, no. 1: 48. https://doi.org/10.3390/jfb14010048
APA StyleMartinez-Campelo, R., & Garcia-Fuentes, M. (2023). Matrices Activated with Messenger RNA. Journal of Functional Biomaterials, 14(1), 48. https://doi.org/10.3390/jfb14010048