Improving Farming Practices for Sustainable Soil Use in the Humid Tropics and Rainforest Ecosystem Health
Abstract
:1. Introduction
2. Importance of Soil Physical Attributes to Sustainable Continuous Soil Use under Humid Tropical Soil Conditions
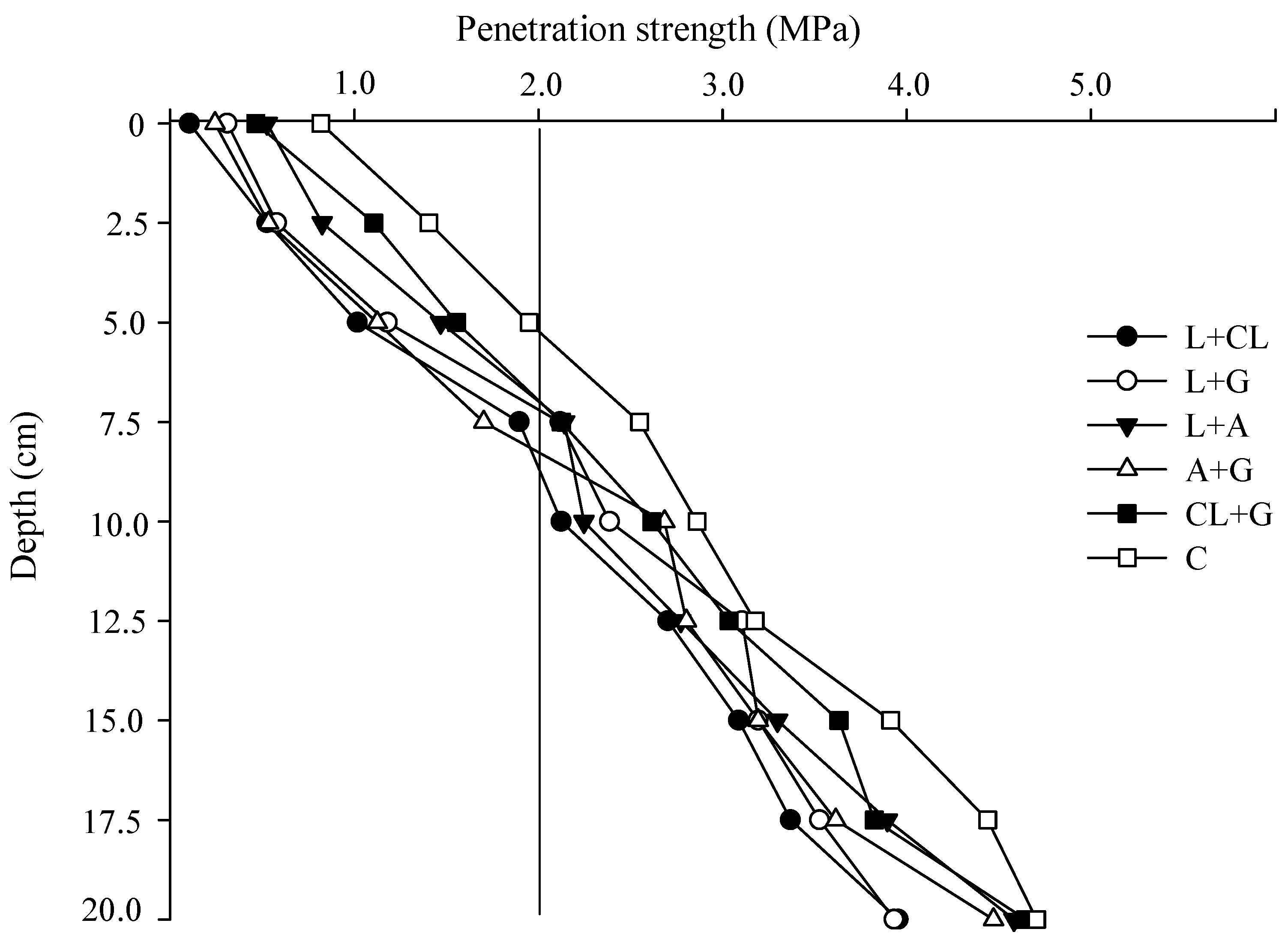
2.1. Soil Penetration Strength and Soil Rootability
2.2. Use of Leguminous Residues and Gypsum for Enhancing Tropical Soil Physical Properties
3. Importance and Dynamics of Soil Organic Matter for Soil Fertility and Land-Use Sustainability in the Humid Tropics
3.1. Relevance of Soil Organic Matter
3.2. Factors Influencing Organic Matter in Soils of the Humid Tropics
3.3. Anthropic Impacts on Amazonian SOM
3.4. Biochar and Indian Blacksoils
4. Soil Chemical Indicators, Availability, and Nutrient Use Efficiency in Humid Tropical Soil Affect Sustainability
4.1. Soil Chemical Indicators and Tropical Land Degradation
4.2. Nitrogen, Phosphorus, and Potassium Use Efficiency
5. Organic Management of Tropical Soil Taking Advantage of Local Resources to Overcome the Challenges of Sustainability
5.1. Tropical Environment and Conventional versus Organic Agriculture Systems
5.2. Alternative Local Resources for Organic Soil Management
5.3. Harm and Risks of Agrochemical Use in Tropical Agrosystems
6. Current and Future Developments
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). Report of the Council of FAO; FAO: Washington, DC, USA, 1988. [Google Scholar]
- Moura, E.G.; Sena, V.G.L.; Correa, M.S.; Aguiar, A.F.C. The Importance of an Alternative for Sustainability of Agriculture around the Periphery of the Amazon Rainforest. Recent Pat. Food Nutr. Agric. 2013, 5, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.C.F.; Freitas, I.C.; Carvalho, C.S.; Monroe, P.H.M.; Moura, E.G. Efficiency of an agrosystem designed for family farming in pre-Amazon region. Renew. Agric. Food Syst. 2011, 26, 24–30. [Google Scholar] [CrossRef]
- Stirling, G.R. Soil ecosystems management in sustainable agriculture. In Biological Control of Plant-Parasitic Nematodes; CABI: London, UK, 2014. [Google Scholar]
- Lu, Y.; Wang, R.; Zhang, Y.; Su, H.; Wang, P.; Jenkins, A.; Ferrier, R.C.; Bailey, M.; Squire, G. Ecosystem health towards sustainability. Ecosyst. Health Sustain. 2015, 1, 1–15. [Google Scholar] [CrossRef]
- Aweke, M.; Gelaw, R.; Singh, R.; Lal, R. Soil Quality indices for evaluating smallholder agricultural land uses in Notthern Ethiopia. Sustainability 2015, 7, 2322–2337. [Google Scholar]
- Viana, R.M.; Ferraz, J.B.S.; Neves Junior, A.F.; Vieira, G.; Pereira, B.F.F. Soil quality indicators for different restoration stages on Amazon rainforest. Soil Tillage Res. 2014, 140, 1–7. [Google Scholar] [CrossRef]
- Aguiar, A.C.F.; Candido, C.S.; Carvalho, C.S.; Monroe, P.H.M.; Moura, E.G. Organic matter fraction and pools of phosphorus as indicators of the impact of land use in the Amazonian periphery. Ecol. Indic. 2013, 30, 158–164. [Google Scholar] [CrossRef]
- Doré, T.; Makowski, D.; Malézieux, E.; Munier-Jolain, N.; Tchamitchian, M.; Tittonell, P. Facing up to the paradigm of ecological intensification in agronomy: Revisiting methods, concepts and knowlegde. Eur. J. Agron. 2011, 34, 197–210. [Google Scholar] [CrossRef]
- Moura, E.G.; Marques, E.S.; Silva, T.M.B.; Piedade, A.; Aguiar, A.C.F. Interactions among leguminous trees, crops and weeds in a no-till alley cropping system. Int. J. Plant Prod. 2014, 8, 441–456. [Google Scholar]
- Lordan, J.; Pascual, M.; Fonseca, F.; Villar, J.M.; Rufat, J. Use of rice husk to enhance peach tree performance in soils with limiting physical properties. Soil Tillage Res. 2013, 129, 19–22. [Google Scholar] [CrossRef]
- Moura, E.G.; Sena, V.G.L.; Sousa, C.C.M.; Silva, F.R.; Coelho, M.J.A.; Macedo, V.R.A.; Aguiar, A.C.F. Enhancement of the rootability of a structurally fragile tropical soil using gypsum and leguminous residues to increase the maize yield. Soil Use Manag. 2016, 32, 118–126. [Google Scholar] [CrossRef]
- Mishra, A.K.; Aggarwal, P.; Bhattacharyya, R.; Das, T.K.; Sharma, A.R.; Singh, R. Least limiting water range for two conservation agriculture cropping systems in India. Soil Tillage Res. 2015, 150, 43–56. [Google Scholar] [CrossRef]
- Letey, J. Relationship between soil physical properties and crop production. Adv. Soil Sci. 1985, 1, 277–294. [Google Scholar]
- Moura, E.G.; Oliveira, A.K.C.; Pinheiro, K.M.; Aguiar, A.C.F. Management of a cohesive tropical soil enhance rootability and increase the efficiency of nitrogen and potassium use. Soil Use Manag. 2012, 28, 370–377. [Google Scholar] [CrossRef]
- Daniells, I.G. Hardsetting soils: A review. Soil Res. 2012, 50, 349–359. [Google Scholar] [CrossRef]
- Moura, E.G.; Serpa, S.S.; Santos, J.G.D.; Costa Sobrinho, J.R.S.; Aguiar, A.C.F. Nutrient use efficiency in alley cropping systems in the Amazonian periphery. Plant Soil 2010, 335, 363–371. [Google Scholar] [CrossRef]
- Duval, M.E.; Galantini, J.A.; Iglesias, J.O.; Canelo, S.; Martinez, J.M.; Wall, L. Analysis of organic fractions as indicators of soil quality under natural and cultivated systems. Soil Tillage Res. 2013, 131, 11–19. [Google Scholar] [CrossRef]
- Moura, E.G.; Aguiar, A.C.F.; Piedade, A.R.; Rousseau, G.X. The contribution of legume tree residues and macrofauna to the improvement of abiotic soil properties in the eastern Amazon. Appl. Soil Ecol. 2015, 86, 91–99. [Google Scholar] [CrossRef]
- Mulumba, L.N.; Lal, R. Mulching effects on selected soil physical properties. Soil Tillage Res. 2008, 98, 106–111. [Google Scholar] [CrossRef]
- Moura, E.G.; Monroe, P.H.M.; Coelho, M.J.A.; Costa Sobrinho, J.R.S.; Aguiar, A.C.F. Effectiveness of calcined rock phosphate and leucaena prunings as a source of nutrients for maize in a tropical soil. Biol. Agric. Hort. 2013, 29, 132–144. [Google Scholar] [CrossRef]
- Twum, E.K.A.; Nii-Annang, S. Impact of soil compaction on bulk density and root biomass of Quercus petraea L. at Reclaimed Post-Lignite Mining Site in Lusatia, Germany. Appl. Environ. Soil Sci. 2015. [Google Scholar] [CrossRef]
- Johan, A.; Hakansson, I. Response of different crops to soil compaction—Short-term effects in Swedish field experiments. Soil Tillage Res. 2014, 138, 56–63. [Google Scholar]
- Sumner, M.E. Gypsum improves subsoil root growth. In Proceedings of the International Symposium Root Reseacher and Aplications, Viena, Austria, 2–4 September 2009.
- Nora, D.D.; Amado, T.J.C.; Bortolotto, R.P.; Ferreira, A.O.; Reichardt, K.; Santi, A.L. Subsoil chemical amelioration and crop yields under continuous long-term no-till in a subtropical Oxisol. Afr. J. Agric. Res. 2014, 9, 3338–3349. [Google Scholar]
- Anikwe, M.A.N.; Eze, J.C.; Ibudialo, A.N. Influence of lime and gypsum application on soil properties and yield of cassava (Manihot esculenta Crantz.) in a degraded Ultisol in Agbani, Enugu Southeastern Nigeria. Soil Tillage Res. 2016, 158, 32–38. [Google Scholar] [CrossRef]
- Wuddivira, M.N.; Camps-Roach, G. Effects of organic matter and calcium on soil structural stability. Eur. J. Soil Sci. 2007, 58, 722–727. [Google Scholar] [CrossRef]
- Chaudhari, P.R.; Ahire, D.V.; Ahire, V.D.; Chkravarty, M.; Maity, S. Soil bulk density as related to soil texture, organic matter content and available total nutrients of Coimbatore soil. Int. J. Sci. Res. Publ. 2013, 3, 1–8. [Google Scholar]
- Blanco-canqui, H.; Shapiro, C.A.; Wortmann, C.S.; Drijber, R.A.; Mamo, M.; Shaver, T.M.; Ferguson, R.B. Soil organic carbon: The value to soil properties. J. Soil Water Conserv. 2013, 68, 129A–134A. [Google Scholar] [CrossRef]
- Manns, H.R.; Berg, A.A. Importance of soil organic carbon on surface soil water content variability among agricultural fields. J. Hydrol. 2014, 516, 297–303. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, G.L.; Yang, J.L.; Li, D.C.; Zhao, Y.G.; Liu, F.; Yang, R.M.; Yang, F. Organic matter controls of soil water retention in an alpine grassland and its significance for hydrological processes. J. Hydrol. 2014, 519, 3086–3093. [Google Scholar] [CrossRef]
- Zaffar, M.; Lu, S.G. Pore size distribution of clayey soils and its correlation with soil organic matter. Pedosphere 2015, 25, 240–249. [Google Scholar] [CrossRef]
- Blume, H.P.; Brümmer, G.W.; Fleige, H.; Horn, R.; Kandeler, E.; Kögel-Knabner, I.; Kretzschmar, R.; Stahr, K.; Wilke, B.M. Soil Organic Matter. In Scheffer/Schachtschabel Soil Science; Springer: Berlin, Germany; Heidelberg, Germany, 2016; pp. 55–86. [Google Scholar]
- Flores, J.C.M.; Realpe, I.B.; Montoya, J.C. Retention and availability of phosphorus associated with organic matter in a typic melanudands of Cauca Department, Colombia. Acta Agron. 2014, 62, 261–267. [Google Scholar]
- Hagvall, K.; Persson, P.; Karlsson, T. Speciation of aluminum in soils and stream waters: The importance of organic matter. Chem. Geol. 2015, 417, 32–43. [Google Scholar] [CrossRef]
- Dungait, J.A.J.; Hopkins, D.W.; Gregory, A.S.; Whitmore, A.P. Soil organic matter turnover is governed by accessibility not recalcitrance. Glob. Chang. Biol. 2012, 18, 1781–1796. [Google Scholar] [CrossRef]
- Whalen, J.K. Managing soil biota-mediated decomposition and nutrient mineralization in sustainable agroecosystems. Adv. Agric. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The microbial efficiency-matrix stabilization (mems) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Hinsinger, P.; Bengough, A.G.; Vetterlein, D.; Young, I. Rhizosphere: Biophysics, biogeochemistry and ecological relevance. Plant Soil 2009, 321, 117–152. [Google Scholar] [CrossRef]
- Jones, D.L.; Nguyen, C.; Finlay, R.D. Carbon flow in the rhizosphere: Carbon trading at the soil–root interface. Plant Soil 2009, 321, 5–33. [Google Scholar] [CrossRef]
- Lal, R. Challenges and opportunities in soil organic matter research. Eur. J. Soil Sci. 2009, 60, 158–169. [Google Scholar] [CrossRef]
- Gama-Rodrigues, A.C.; Sales, M.V.S.; Silva, P.S.D.; Comerford, N.B.; Cropper, W.P.; Gama-Rodrigues, E.F. An exploratory analysis of phosphorus transformations in tropical soils using structural equation modeling. Biogeochemistry 2014, 118, 453–469. [Google Scholar] [CrossRef]
- Cristancho, R.J.A.; Hanafi, M.M.; Syed Omar, S.R.; Rafii, M.Y. Aluminum speciation of amended acid tropical soil and its effects on plant root growth. J. Plant Nut. 2014, 37, 811–827. [Google Scholar] [CrossRef]
- Chave, J.; Navarrete, D.; Almeida, S. Regional and seasonal patterns of litterfall in tropical South America. Biogeosciences 2010, 7, 43–55. [Google Scholar] [CrossRef]
- Jones, D.L.; Hodge, A.; Kuzyakov, Y. Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 2004, 163, 459–480. [Google Scholar] [CrossRef]
- Souza Braz, A.M.; Fernandes, A.R.; Alleoni, L.R.F. Soil attributes after the conversion from forest to pasture in Amazon. Land Degrad. Dev. 2013, 24, 33–38. [Google Scholar] [CrossRef]
- Anderson-Teixeira, K.J.; Wang, M.M.H.; McGarvey, J.C.; LeBauer, D.S. Carbon dynamics of mature and regrowth tropical forests derived from a pantropical database (TropForC-db). Glob. Chang. Biol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Skinner, C.; Gattinger, A.; Muller, A.; Mäder, P.; Flieβbach, A.; Stolze, M.; Ruser, R.; Niggli, U. Greenhouse gas fluxes from agricultural soils under organic and non-organic management—A global meta-analysis. Sci. Total Environ. 2014, 468–469, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Craswell, E.T.; Lefroy, R.D.B. The role and function of organic matter in tropical soils. Nutri. Cycl. Agroecol. 2001, 61, 7–18. [Google Scholar] [CrossRef]
- Zech, W.; Senesi, N.; Guggenberger, G.; Kaiser, K.; Lehmann, J.; Miano, T.M.; Miltner, A.; Schroth, G. Factors controlling humification and mineralization of soil organic matter in the tropics. Geoderma 1997, 79, 117–161. [Google Scholar] [CrossRef]
- Labrière, N.; Locatelli, B.; Laumonier, Y.; Freycon, V.; Bernoux, M. Soil erosion in the humid tropics: A systematic quantitative review. Agric. Ecosyst. Environ. 2015, 203, 127–139. [Google Scholar] [CrossRef]
- Bonal, D.; Burban, B.; Stahl, C.; Wagner, F.; Hérault, B. The response of tropical rainforests to drought—Lessons from recent research and future prospects. Ann. For. Sci. 2016, 73, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Dias, T.; Oakley, S.; Alarcón-Gutiérrez, E.; Ziarelli, F.; Trindade, H.; Martins-Loução, M.A.; Sheppard, L.; Ostle, N.; Cruz, C. N-driven changes in a plant community affect leaf-litter traits and may delay organic matter decomposition in a Mediterranean maquis. Soil Biol. Biochem. 2013, 58, 163–171. [Google Scholar] [CrossRef]
- Qiao, N.; Xu, X.; Hu, Y.; Blagodatskaya, E.; Liu, Y.; Schaefer, D.; Kuzyakov, Y. Carbon and nitrogen additions induce distinct priming effects along an organic-matter decay continuum. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Talbot, J.M.; Yelle, D.J.; Nowick, J.; Treseder, K.K. Litter decay rates are determined by lignin chemistry. Biogeochemistry 2012, 108, 279–295. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, K. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Hättenschwiler, S.; Vitousek, P.M. The role of polyphenols in terrestrial ecosystem nutrient cycling. TREE 2000, 15, 238–243. [Google Scholar] [CrossRef]
- Russell, A.E.; Raich, J.W. Rapidly growing tropical trees mobilize remarkable amounts of nitrogen, in ways that differ surprisingly among species. Proc. Nat. Acad. Sci. USA 2012, 109, 10398–10402. [Google Scholar] [CrossRef] [PubMed]
- Vesterdal, L.; Clarke, N.; Sigurdsson, B.D.; Gundersen, P. Do tree species influence soil carbon stocks in temperate and boreal forests? For. Ecol. Manag. 2013, 309, 4–18. [Google Scholar] [CrossRef]
- Gentile, R.; Vanlauwe, B.; Six, J. Litter quality impacts short- but not long-term soil carbon dynamics in soil aggregate fractions. Ecol. Appl. 2011, 21, 695–703. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Coq, S.; Barantal, S.; Handa, I.T. Leaf traits and decomposition in tropical rainforests: Revisiting some commonly held views and towards a new hypothesis. New Phytol. 2011, 189, 950–965. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Germer, S.; Zimmermann, A.; Neill, C.; Krusche, A.V.; Elsenbeer, H. Disproportionate single-species contribution to canopy-soil nutrient flux in an Amazonian rainforest. For. Ecol. Manag. 2012, 267, 40–49. [Google Scholar] [CrossRef]
- Austin, A.T.; Vivanco, L.; Gonzáles-Arzac, A.; Pérez, L.I. There’s no place like home? An exploration of the mechanisms behind plant litter–decomposer affinity in terrestrial ecosystems. New Phytol. 2014, 204, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.A.; Congdon, R.A.; Shoo, L.P.; Valdez-Ramirez, V.; Williams, S.E. Spatial variability in litterfall, litter standing crop and litter quality in a tropical rain forest region. Biotropica 2014, 46, 378–386. [Google Scholar] [CrossRef]
- Desjardins, T.; Barros, E.; Sarrazin, M.; Girardin, C.; Mariotti, A. Effects of forest conversion to pasture on soil carbon content and dynamics in Brazilian Amazonia. Agric. Ecosyst. Environ. 2004, 103, 365–373. [Google Scholar] [CrossRef]
- Feller, C.; Beare, M.H. Physical control of soil organic matter dynamics in the tropics. Geoderma 1997, 79, 69–116. [Google Scholar] [CrossRef]
- Feng, W.; Plante, A.F.; Aufdenkampe, A.K.; Six, J. Soil organic matter stability in organo-mineral complexes as a function of increasing C loading. Soil Biol. Biochem. 2014, 69, 398–405. [Google Scholar] [CrossRef]
- Wattel-Koekkoek, E.J.W.; Buurman, P.; van der Plicht, J.; Wattel, E.; van Breemen, M. Mean residence time of soil organic matter associated with kaolinite and smectite. Eur. J. Soil Sci. 2003, 54, 269–278. [Google Scholar] [CrossRef]
- Chaopricha, N.T.; Marín-Spiotta, E. Soil burial contributes to deep soil organic carbon storage. Soil Biol. Biochem. 2014, 69, 251–264. [Google Scholar] [CrossRef]
- Stone, M.M.; Plante, A.F. Relating the biological stability of soil organic matter to energy availability in deep tropical soil profiles. Soil Biol. Biochem. 2015, 90, 162–171. [Google Scholar] [CrossRef]
- Padmanabhan, E.; Eswaran, H.; Reich, P.F. Soil carbon stocks in Sarawak, Malaysia. Sci. Total Environ. 2013, 465, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Couwenberg, J. Greenhouse gas emissions from managed peat soils: Is the IPCC reporting guidance realistic. Mires Peat 2011, 8, 1–10. [Google Scholar]
- Page, S.E.; Rieley, J.O.; Banks, C.J. Global and regional importance of the tropical peatland carbon pool. Glob. Chang. Biol. 2011, 17, 798–818. [Google Scholar] [CrossRef]
- Cochrane, M.A. Fire science for rainforests. Nature 2003, 421, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Couwenberg, J. Emission Factors for Managed Peat Soils. An Analysis of IPCC Default Values. Wetlands International, 2009. Available online: http://www.imcg.net/media/download_gallery/climate/couwenberg_2009a.pdf (accessed on 20 September 2015).
- Page, S.E.; Siegert, F.; Rieley, J.O. The amount of carbon released from peat and forest fires in Indonesia during 1997. Nature 2002, 420, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Hooijer, A.; Page, S.; Canadell, J.G. Current and future CO2 emissions from drained peatlands in Southeast Asia. Biogeosciences 2010, 7, 1505–1514. [Google Scholar] [CrossRef]
- Nogueira, E.M.; Yanai, A.M.; Fonseca, F.O.R.; Fearnside, P.M. Carbon stock loss from deforestation through 2013 in Brazilian Amazonia. Glob. Chang. Biol. 2015, 21, 1271–1292. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Science and Technology (MCT). Brazilian Inventory of Anthropogenic Emissions by Sources and Removals by Sinks of Greenhouse Gases Not Controlled by the Montreal Protocol. In Brazil’s Initial National Communication; MCT: Brasilia, Brazil, 2004. Available online: http://www.mct.gov.br/upd_blob/0005/5163.pdf (accessed on 13 December 2003). [Google Scholar]
- Fujisaki, K.; Perrin, A.S.; Desjardins, T.; Mernoux, M.; Balbino, L.C.; Brossard, M. From forest to cropland and pasture systems: A critical review of soil organic carbon stocks changes in Amazonia. Glob. Chang. Biol. 2015, 21, 2773–2786. [Google Scholar] [CrossRef] [PubMed]
- Fearnside, P.M.; Imbrozio Barbosa, R.; Alencastro Graça, P.M.L. Burning of secondary forest in Amazonia: Biomass, burning efficiency and charcoal formation during land preparation for agriculture in Apiau´, Roraima, Brazil. For. Ecol. Manag. 2007, 242, 678–687. [Google Scholar] [CrossRef]
- Giovannini, C.; Lucchesi, S.; Giachetti, M. Effects of heating on some chemical parameters related to soil fertility and plant growth. Soil Sci. 1990, 149, 344–350. [Google Scholar] [CrossRef]
- Raison, R.; Woods, P.; Jakobsen, B.; Bary, G. Soil temperatures during and following low-intensity prescribed burning in a Eucalyptus passiflora forest. Soil Res. 1986, 24, 33–47. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Benscoter, B.; Page, S.; Rein, G.; van der Werf, G.; Watts, A. Global vulnerability of peatlands to fire and carbon loss. Nat. Geosci. 2015, 8, 11–14. [Google Scholar] [CrossRef]
- Guimarães, D.V.; Gonzaga, M.I.S.; Silva, T.O.; Silva, T.L.; Silva Dias, N.; Matias, M.I.S. Soil organic matter pools and carbon fractions in soil under different land uses. Soil Tillage Res. 2013, 126, 177–182. [Google Scholar] [CrossRef]
- Rosenstock, T.S.; Tully, K.L.; Arias-Navarro, C.; Naufeldt, H.; Butterbach-Bahl, K.; Verchot, L.V. Agroforestry with N2-fixing trees: Sustainable development’s friend or foe? Curr. Opin. Environ. Sustain. 2014, 6, 15–21. [Google Scholar] [CrossRef]
- Prescott, C.E. Litter decomposition: What controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 2010, 101, 133–149. [Google Scholar] [CrossRef]
- Chaer, G.M.; Resende, A.S.; Campello, E.F.C.; Faria, S.M.; Boddey, R.M.; Schmidt, S. Nitrogen-fixing legume tree species for the reclamation of severely degraded lands in Brazil. Tree Physiol. 2011, 31, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.F.S.; Cerri, C.C.; Volkoff, B.; Andreux, F.; Chauvel, A. Consequences of clearing and tillage on the soil of a natural Amazonian ecosystem. For. Ecol. Manag. 1991, 38, 273–282. [Google Scholar] [CrossRef]
- Bowling, D.R.; Pataki, D.E.; Randerson, J.T. Carbon isotopes in terrestrial ecosystem pools and CO2 fluxes. New Phytol. 2008, 171, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Rouw, A.; Soulileuth, B.; Huon, S. Stable carbon isotope ratios in soil and vegetation shift with cultivation practices (Northern Laos). Agric. Ecosyst. Environ. 2015, 200, 161–168. [Google Scholar] [CrossRef]
- Guan, X.K.; Turner, N.C.; Song, L.; Gu, Y.J.; Wang, T.C.; Li, F.M. Soil carbon sequestration by three perennial legume pastures is greater in deeper soil layers than in the surface soil. Biogeosciences 2016, 13, 527–534. [Google Scholar] [CrossRef]
- Silva, J.E.; Resck, D.V.S.; Corazza, E.J.; Vivaldi, L. Carbon storage in clayey Oxisol cultivated pastures in the “Cerrado” region, Brazil. Agric. Ecosyst. Environ. 2004, 103, 357–363. [Google Scholar] [CrossRef]
- Schedlbauer, J.L.; Kavanagh, K.L. Soil carbon dynamics in a chronosequence of secondary forests in northeastern Costa Rica. For. Ecol. Manag. 2008, 255, 1326–1335. [Google Scholar] [CrossRef]
- Marín-Spiotta, E.; Sharma, S. Carbon storage in successional and plantation forest soils: A tropical analysis. Glob. Ecol. Biogeogr. 2013, 22, 105–117. [Google Scholar] [CrossRef]
- Fialho, R.C.; Zinn, Y.L. Changes in soil organic carbon under Eucalyptus plantations in Brazil: A comparative analysis. Land Degrad. Dev. 2014, 25, 428–437. [Google Scholar] [CrossRef]
- Glaser, B.; Birk, J.J. State of the scientific knowledge on properties and genesis of Anthropogenic Dark Earths in Central Amazonia (terra preta de Índio). Geochim. Cosmochim. Acta 2012, 82, 39–51. [Google Scholar] [CrossRef]
- Lima, A.B.; Cannavan, F.S.; Navarrete, A.A. Amazonian Dark Earth and plant species from the Amazon region contribute to shape rhizosphere bacterial communities. Microb. Ecol. 2014, 69, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Novotny, E.; Hayes, M.B.; Azevedo, E.; Bonagamba, T. Characterisation of black carbon-rich samples by 13C solid-state nuclear magnetic resonance. Naturwissenschaften 2006, 93, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Ding, W.; Zhang, J.; Li, Y.; Luo, J.; Bolan, N.; Xie, Z. Biochar suppressed the decomposition of organic carbon in a cultivated sandy loam soil: A negative priming effect. Soil Biol. Biochem. 2014, 76, 12–21. [Google Scholar] [CrossRef]
- Ameloot, N.; Graber, E.R.; Verheijen, F.G.A.; Neve, S. Interactions between biochar stability and soil organisms: Review and research needs. Eur. J. Soil Sci. 2013, 64, 379–390. [Google Scholar] [CrossRef]
- Smith, P. Soil carbon sequestration and biochar as negative emission technologies. Glob. Chang. Biol. 2016, 22, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. Review of biochar and use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- Novotny, E.H.; Hayes, M.H.B.; Madari, B.E. Lessons from the Terra Preta de Índios of the Amazon region for the utilization of charcoal for soil amendment. J. Braz. Chem. Soc. 2009, 20, 1003–1010. [Google Scholar] [CrossRef]
- Novotny, E.H.; Maia, C.M.B.F.; Carvalho, M.T.M.; Madari, B.E. Biochar: Pyrogenic carbon for agricultural use—A critical review. Rev. Bras. Cienc. Solo 2015, 39, 321–344. [Google Scholar] [CrossRef]
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef]
- Agegnehu, G.; Bass, A.M.; Nelson, P.M.; Muirhead, B.; Wright, G.; Bird, M.I. Biochar and biochar-compost as soil amendments: Effects on peanut yield, soil properties and greenhouse gas emissions in tropical North Queensland, Australia. Agric. Ecosyst. Environ. 2015, 213, 72–85. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.-X.; Wu, W.-X. Evaluation of Biochar Effects on Nitrogen Retention and Leaching in Multi-Layered Soil Columns. Water Air Soil Pollut. 2010, 213, 47–55. [Google Scholar] [CrossRef]
- Nelson, N.O.; Agudelo, S.C.; Yuan, W.; Gan, J. Nitrogen and Phosphorus Availability in Biochar-Amended Soils. Soil Sci. 2011, 176, 218–226. [Google Scholar] [CrossRef]
- Borchard, N.; Ladd, B.; Eschemann, S.; Hegenberg, D.; Möseler, B.M.; Amelung, W. Black carbon and soil properties at historical charcoal production sites in Germany. Geoderma 2014, 223–224, 236–242. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M.; Kammann, C.; Mueller, C. Review of biochar and soil nitrogen dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef]
- Sousa, A.M.B.; Soares Santos, R.R.; Gehring, C. Charcoal in Amazonian paddy soil—Nutrient availability, rice growth and methane emissions. J. Plant Nutr. Soil Sci. 2013, 177, 39–47. [Google Scholar] [CrossRef]
- Xu, G.; Lv, Y.; Sun, J.; Shao, H.; Wei, L. Recent advances in biochar applications in agricultural soils: Benefits and environmental implications. CLEAN Soil Air Water 2012, 40, 1093–1098. [Google Scholar] [CrossRef]
- Zhang, A.; Cui, L.; Pan, G. Effect of biochar amendment on yield and methane and nitrous oxide emissions from a rice paddy from Tai Lake plain, China. Agric. Ecosyst. Environ. 2010, 139, 469–475. [Google Scholar] [CrossRef]
- Gurwick, N.P.; Moore, L.A.; Kelly, C.; Elias, P. A Systematic review of biochar research, with a focus on its stability in situ and its promise as a climate mitigation strategy. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shen, J.; Li, Y.; Su, Y.; Ge, T.; Jones, D.L.; Wu, J. Effects of biochar amendment on the net greenhouse gas emission and greenhouse gas intensity in a Chinese double rice cropping system. Eur. J. Soil Biol. 2014, 65, 30–39. [Google Scholar] [CrossRef]
- LeCroy, C.; Masiello, C.A.; Rudgers, J.A.; Hockaday, W.C.; Silberg, J.J. Nitrogen, biochar, and mycorrhizae: Alteration of the symbiosis and oxidation of the char surface. Soil Biol. Biochem. 2013, 58, 248–254. [Google Scholar] [CrossRef]
- Mia, S.; van Groenigen, J.W.; van de Voorde, T.F.J.; Orama, N.J.; Bezemer, T.M.; Mommer, L.; Jeffery, S. Biochar application rate affects biological nitrogen fixation in red clover conditional on potassium availability. Agric. Ecosyst. Environ. 2014, 191, 83–91. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Deluca, T.H.; Jones, D.L. Biochar application reduces nodulation but increases nitrogenase activity in clover. Plant Soil 2013, 366, 83–92. [Google Scholar] [CrossRef]
- Warnock, D.; Lehmann, J.; Kuyper, T.; Rillig, M. Mycorrhizal responses to biochar in soil—Concepts and mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockadayd, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Murphy, D.V.; Abbott, L.K. Biochars influence seed germination and early growth of seedlings. Plant Soil 2012, 353, 273–287. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Ponge, J.F.; Topoliantz, S.; Ballof, S. Ingestion of charcoal by the Amazonian earthworm Pontoscolex corethrurus: A potential for tropical soil fertility. Soil Biol. Biochem. 2006, 38, 2008–2009. [Google Scholar] [CrossRef]
- Dube, O.P. Linking fire and climate: Interactions with land use, vegetation, and soil. Curr. Opin. Environ. Sustain. 2009, 1, 161–169. [Google Scholar] [CrossRef]
- Styger, E.; Rakodrondamasi, H.M.; Pfeffer, M.J.; Fernandes, E.C.M.; Bates, D.M. Influence of slash-and-burn farming practices on fallow succession and land degradation in the rainforest region of Madagascar. Agric. Ecosyst. Environ. 2007, 119, 257–269. [Google Scholar] [CrossRef]
- Alencar, A.A.C.; Brando, P.M.; Asner, G.P.; Putz, F.E. Landscape fragmentation, severe drought and the new amazon forest fire regime. Ecol. Appl. 2015, 25, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char Sequestration in Terrestrial Ecosystems—A Review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 395–419. [Google Scholar] [CrossRef]
- Steiner, C.; Teixeira, W.; Zech, W. Slash and Char: An Alternative to Slash and Burn Practiced in the Amazon Basin. In Amazonian Dark Earths: Explorations in Space and Time; Glaser, B., Woods, W., Eds.; Springer: Heidelberg, Germany; Berlin, Germany, 2004; pp. 183–193. [Google Scholar]
- Turcios, M.M.; Jaramillo, M.M.A.; Vale, J.E.F.; Fearnside, P.M.; Barbosa, R.I. Soil charcoal as long-term pyrogenic carbon storage in Amazonian seasonal forests. Glob. Chang. Biol. 2016, 22, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, E.J.B.N.; Vasconcellos, R.L.F.; Bini, D.; Miyauchi, M.Y.H.; Santos, C.A.; Alves, P.R.L.; Paula, A.M.; Nakatani, A.S.; Pereira, J.M.; Nogueira, M.A. Soil health: Looking for suitable indicators. What should be considered to assess the effects of use and management on soil health? Sci. Agric. 2013, 70, 274–289. [Google Scholar] [CrossRef]
- Oliver, D.P.; Bramley, R.G.V.; Riches, D.; Porter, I.; Edwards, J. Soil physical and chemical properties as indicators of soil quality in Australian viticulture. Aust. J. Grape Wine Res. 2013, 19, 29–139. [Google Scholar] [CrossRef]
- Doran, J.W.; Parkin, T.B. Defining and assessing soil quality. In Defining Soil Quality for a Sustainable Environment. Soil Science Society of America; Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; American Society of Agronomy: Madison, WI, USA, 1994; pp. 3–21. [Google Scholar]
- Karlen, D.L.; Andrews, S.S.; Doran, J.W. Soil quality: Current concepts and applications. Adv. Agron. 2001, 74, 1–40. [Google Scholar]
- Cerri, D.G.P.; Magalhães, P.S.G. Correlation of physical and chemical attributes of soil with sugarcane yield. Pesqui. Agropecu. Bras. 2012, 47, 613–620. [Google Scholar] [CrossRef]
- Montanari, R.; Passos e Carvalho, M.; Silva Junior, C.A.; Rodrigues Corrêa, A.; Dalchiavon, F.C.; Paz González, A. Relations between the yield of bean (Phaseolus vulgaris L.) and chemical attributes of an Acrustox under no-tillage. J. Soil Sci. Plant Nutr. 2013, 13, 367–379. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, Y.; Xie, X.; Li, X.; Zhang, X.; Shen, X. Effect of annual variation in soil pH on available soil nutrients in pear orchards. Acta Ecol. Sin. 2011, 31, 212–216. [Google Scholar] [CrossRef]
- Jiao, F.; Shi, X.-R.; Han, F.-P.; Yuan, Z.-Y. Increasing aridity, temperature and soil pH induce soil C-N-P imbalance in grasslands. Nature 2016, 6, 1–9. [Google Scholar]
- Stark, S.; Männistö, M.K.; Eskelinen, A. Nutrient availability and pH jointly constrain microbial extracellular enzyme activities in nutrient-poor tundra soils. Plant Soil 2014, 383, 373–385. [Google Scholar] [CrossRef]
- Zhao, C.; Fu, S.; Mathew, R.P.; Lawrence, K.S.; Feng, Y. Soil microbial community structure and activity in a 100-year-old fertilization and crop rotation experiment. J. Plant Ecol. 2015, 1–10. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bååth, E. Contrasting Soil pH Effects on Fungal and Bacterial Growth Suggest Functional Redundancy in Carbon Mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Busari, M.A.; Salako, F.K. Effect of tillage, poultry manure and NPK fertilizer on soil chemical properties and maize yield on an Alfisol at Abeokuta, south-western Nigeria. Niger. J. Soil Sci. 2013, 23, 206–218. [Google Scholar]
- Nduwumuremyi, A.; Ruganzu, V.; Mugwe, J.N.; Rusanganwa, A.C. Effects of unburned lime on soil pH and base cations in acidic soil. ISRN Soil Sci. 2013. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2011. [Google Scholar]
- Fageria, N.; Baligar, V. Enhancing nitrogen use efficiency in crop plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar]
- Lynch, J.P. Root phenes for enhanced soil exploration and phosphorus acquisition: Tools for future crops. Plant Physiol. 2011, 156, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Kochian, L.V. Rooting for more phosphorus. Nature 2012, 488, 466–467. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Post, W.M. Phosphorus transformation as a function of pedogenesis: A synthesis of soil phosphorus data using Hedley fractionation method. Biogeosciences 2011, 8, 2907–2916. [Google Scholar] [CrossRef]
- Braos, L.B.; da Cruz, M.C.P.; Ferreira, M.E.; Kuhnen, F. Organic phosphorus fractions in soil fertilized with cattle manure. Rev. Bras. Cienc. Solo 2015, 39, 140–150. [Google Scholar] [CrossRef]
- Richardson, A.E.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, F.A.; Smith, S.E.; Harvey, P.R.; Ryan, M.H. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 2011, 349, 121–156. [Google Scholar] [CrossRef]
- Rodrigues, M.; Pavinato, P.S.; Withers, P.J.; Teles, A.P.; Herrera, W.F. Legacy phosphorus and no tillage agriculture in tropical oxisols of the Brazilian savanna. Sci. Total Environ. 2016, 15, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Pinto, F.A.; de Souza, E.D.; Paulino, H.B.; Curi, N.; Carneiro, M.A.C. P-sorption and desorption in Savanna Brazilian soils as a support for phosphorus fertilizer management. Ciênc. Agrotecnol. 2013, 37, 521–530. [Google Scholar] [CrossRef]
- Drinkwater, L.E.; Snapp, S.S. Nutrients in Agroecosystems: Rethinking the Management Paradigm. Adv. Agron. 2007, 92, 163–186. [Google Scholar]
- Sustainable Agricultural Systems in the 21st Century. Available online: http://dels.nas.edu/resources/static-assets/materials-based-on-reports/reports-in-brief/Systems-Ag-Report-Brief.pdf (accessed on 15 December 2015).
- Meena, S.K.; Rakshit, A.; Meena, V.S. Effect of seed bio-priming and N doses under varied soil type on nitrogen use efficiency (NUE) of wheat (Triticum aestivum L.) under greenhouse conditions. Biocatal. Agric. Biotech. 2016, 6, 68–75. [Google Scholar] [CrossRef]
- Kolahchi, Z.; Jalali, M. Effect of water quality on the leaching of potassium from sandy soil. J. Arid Environ. 2007, 68, 624–639. [Google Scholar] [CrossRef]
- Gehring, C.; Moura, E.G.; Santos, R.R.S.; Aguiar, A.C.F.; Sousa, A.M.B.; Boddey, R.M. Ecological intensification of rice production in the lowlands of Amazonia—Options for smallholder rice producers. Eur. J. Agro. 2013, 46, 25–33. [Google Scholar] [CrossRef]
- Gomiero, T.; Pimentel, D.; Paoletti, M.G. Environmental impact of different agricultural management practices: conventional vs. organic agriculture. Crit. Rev. Plant Sci. 2011, 30, 95–124. [Google Scholar] [CrossRef]
- Niggli, U. Sustainability of organic food production: Challenges and innovations. Proc. Nutr. Soc. 2015, 74, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Comadira, G.; Rasool, B.; Karpinska, B.; Morris, J.; Verrall, S.R.; Hedley, P.E.; Foyer, C.H.; Hancock, R.D. Nitrogen deficiency in barley (Hordeum vulgare) seedlings induces molecular and metabolic adjustments that trigger aphid resistance. J. Exp. Bot. 2015, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Altieri, M.A.; Ponti, L.; Nicholls, C.I. Biodiversity and insect pests: Key issues for sustainable management. In Soil Fertility, Biodiversity and Pest Management; Gurr, G.M., Wratten, S.D., Snyder, W.E., Read, D.M.Y., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2012; pp. 72–84. [Google Scholar]
- Sampaio, L.R.; Araújo, J.R.G.; Sousa, E.H.S.; Ferraz Júnior, A.S.L.; Araujo, A.M.S. Cultivo de abóbora, suplementada com biofertilizante, em aléias de leguminosas arbóreas. Hortic. Bras. 2015, 33, 40–44. [Google Scholar] [CrossRef]
- Mondego, J.M. Resistência Induzida em Milho à Spodoptera Frugiperda (J.E. Smith, 1797) (Lepidoptera: Noctuidae). Ph.D. Thesis, Universidade Federal da Paraíba, Areia, Paraíba, 2014. [Google Scholar]
- Crowder, D.; Reganold, J. Financial competitiveness of organic agriculture on a global scale. Proc. Natl. Acad. Sci. USA 2015. [Google Scholar] [CrossRef] [PubMed]
- Sands, D.C.; Pilgeram, A.; Morris, C.E. Development and marketing of perennial grains with benefits for human health and nutrition. In Perennial Crops for Food Security Proceedings of the FAO Expert Workshop; Batello, C., Wade, L., Cox, S., Pogna, N., Bozzini, A., Choptiany, J., Eds.; FAO: Rome, Italy, 2013; pp. 208–220. [Google Scholar]
- Muhammed, D.; Serkan, A. Intercropping of legumes with cereal crops in particular with the perennials to enhance forage yields and quality. In Perennial Crops for Food Security Proceedings of the FAO Expert Workshop; Batello, C., Wade, L., Cox, S., Pogna, N., Bozzini, A., Choptiany, J., Eds.; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013; pp. 221–228. [Google Scholar]
- Tsimbiri, P.F.; Moturi, W.N.; Sawe, J.; Henley, P.; Bend, J.R. Health impact of pesticides on residents and horticultural workers in the Lake Naivasha Region, Kenya. Occup. Dis. Environ. Med. 2015, 3, 24–34. [Google Scholar] [CrossRef]
- Gan, Y.; Hamel, C.; O’Donovan, J.T.; Cutforth, H.; Zentner, R.P.; Campbell, C.A.; Niu, Y.; Poppy, L. Diversifying crop rotations with pulses enhances system productivity. Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- Parolin, P.; Bresch, C.; Poncet, C.; Desneux, N. Introducing the term ‘Biocontrol Plants’ for integrated pest management. Sci. Agric. 2014, 71, 77–80. [Google Scholar] [CrossRef]
- Khalil, M.A.; Al Assiuty, A.L.; van Straalen, N.M.; Al Assiuty, B.A. Changes in soil oribatid communities associated with conversion from conventional to organic agriculture. Exp. Appl. Acarol. 2015, 67, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nobre, C.P.; Ferraz Júnior, A.S.L.; Goto, B.T.; Berbara, R.L.L.; Noqueira, M.D.C. Fungos micorrízicos arbusculares em sistema de aléias no Estado do Maranhão, Brasil. Acta Amazon. 2010, 40, 641–646. [Google Scholar] [CrossRef]
- Oliveira, N.N.F.C. Efeito de um Sistema de Cultivo em Aléias em Diferentes Consórcios de Leguminosas Arbóreas Sobre Grupos de Artrópodes. Master’s Thesis, Universidade Estadual do Maranhão, São Luís, Brazil, 2013. [Google Scholar]
- Restrictions on Genetically Modified Organisms. Available online: https://www.loc.gov/law/help/restrictions-on-gmos/restrictions-on-gmos.pdf (accessed on 15 December 2015).
| Uncovered Soil | Covered Soil | |||
|---|---|---|---|---|
| Year I | Year II | Year I | Year II | |
| NRE (kg·kg−1) | 26.51 b 1 | 11.51 b | 47.86 a | 41.04 a |
| PRE (kg·kg−1) | 2.93 b | 0.97 b | 13.49 a | 24.0 a |
| NAE (kg·ha−1) | 8.21 b | 1.83 b | 21.22 a | 14.09 a |
| PAE (kg·ha−1) | 6.16 b | 1. 14 b | 15.92 a | 14.09 a |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moura, E.G.d.; Gehring, C.; Braun, H.; Ferraz Junior, A.D.S.L.; Reis, F.D.O.; Aguiar, A.D.C.F. Improving Farming Practices for Sustainable Soil Use in the Humid Tropics and Rainforest Ecosystem Health. Sustainability 2016, 8, 841. https://doi.org/10.3390/su8090841
Moura EGd, Gehring C, Braun H, Ferraz Junior ADSL, Reis FDO, Aguiar ADCF. Improving Farming Practices for Sustainable Soil Use in the Humid Tropics and Rainforest Ecosystem Health. Sustainability. 2016; 8(9):841. https://doi.org/10.3390/su8090841
Chicago/Turabian StyleMoura, Emanoel Gomes de, Christoph Gehring, Heder Braun, Altamiro De Souza Lima Ferraz Junior, Fabricio De Oliveira Reis, and Alana Das Chagas Ferreira Aguiar. 2016. "Improving Farming Practices for Sustainable Soil Use in the Humid Tropics and Rainforest Ecosystem Health" Sustainability 8, no. 9: 841. https://doi.org/10.3390/su8090841
APA StyleMoura, E. G. d., Gehring, C., Braun, H., Ferraz Junior, A. D. S. L., Reis, F. D. O., & Aguiar, A. D. C. F. (2016). Improving Farming Practices for Sustainable Soil Use in the Humid Tropics and Rainforest Ecosystem Health. Sustainability, 8(9), 841. https://doi.org/10.3390/su8090841






