Engineering Behavior and Characteristics of Water-Soluble Polymers: Implication on Soil Remediation and Enhanced Oil Recovery
Abstract
:1. Introduction
2. Literature Review
2.1. Utilization of Biopolymer in Soil Remediation and Enhanced Oil Recovery
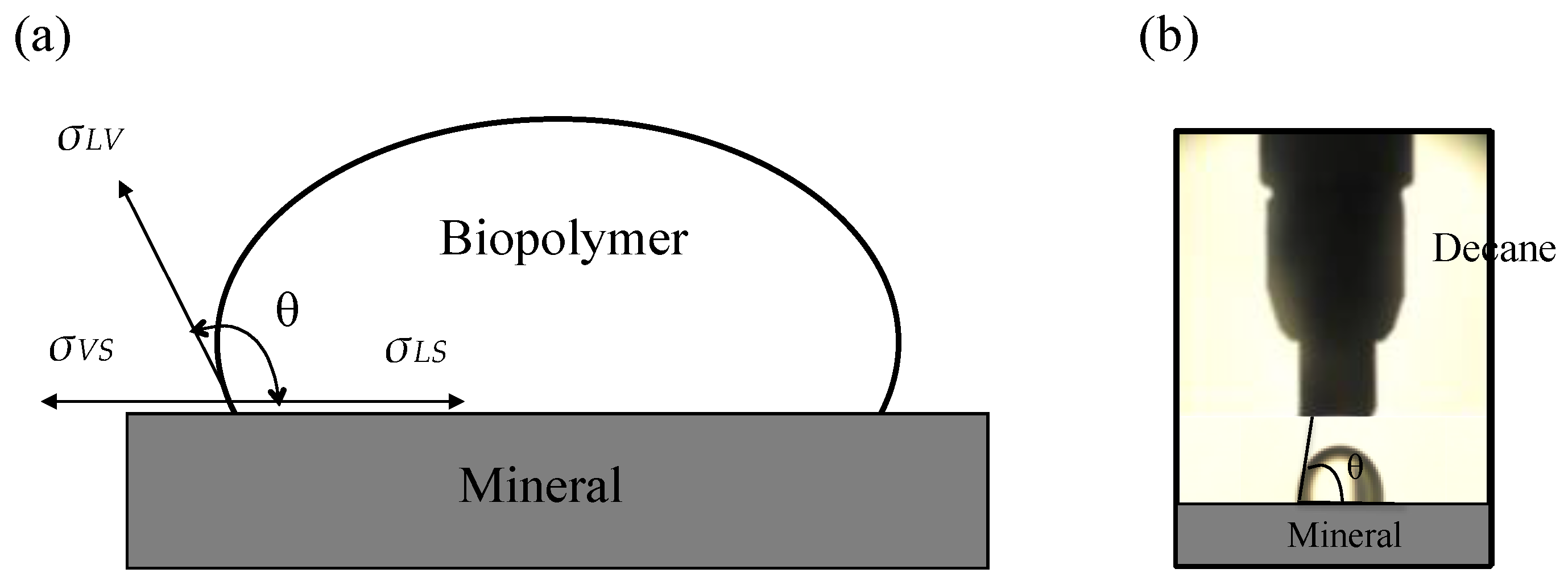
2.2. Capillary Pressure
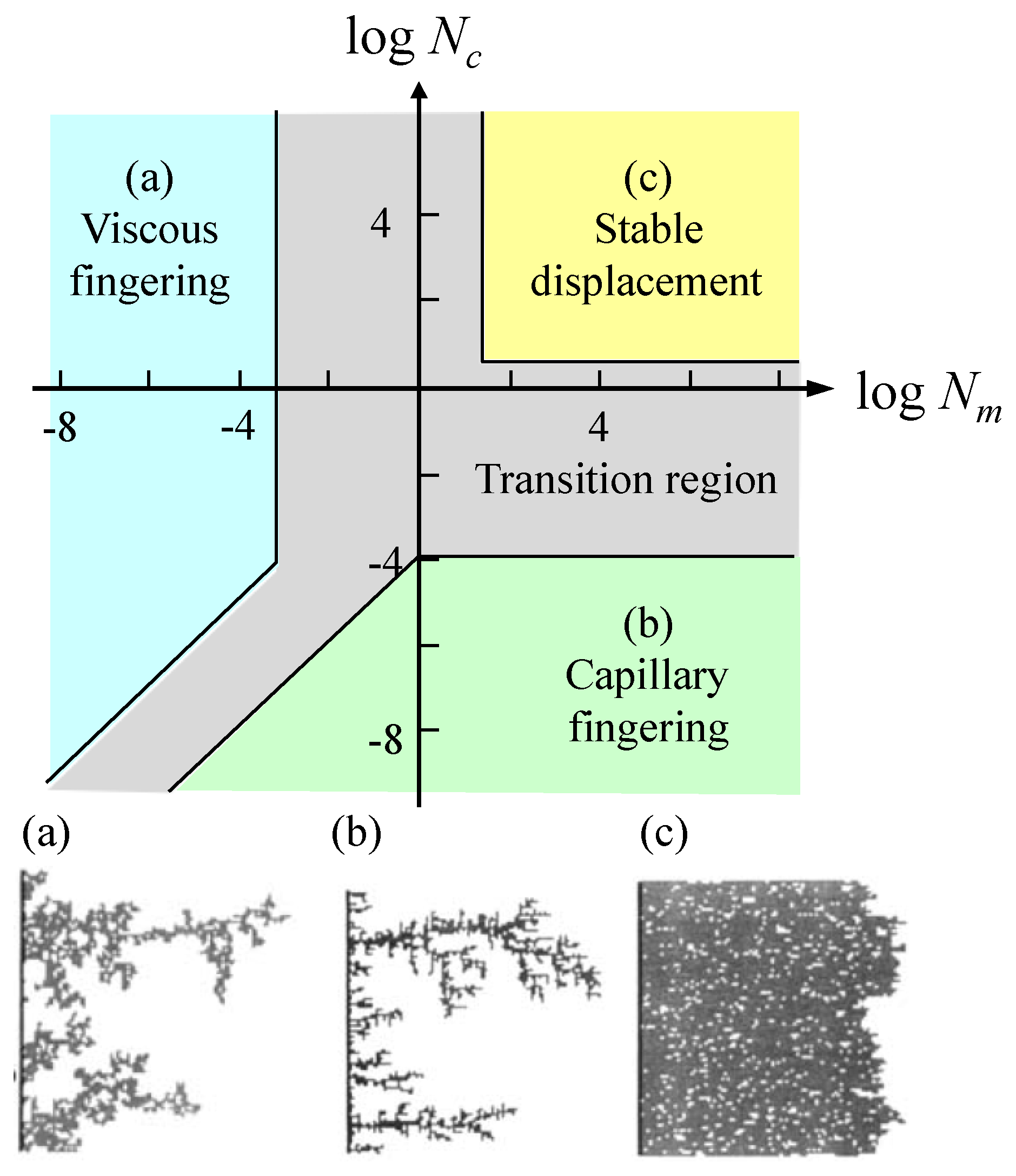
2.3. Multiphase Fluids Flow in Porous Media
3. Experimental Methods and Results
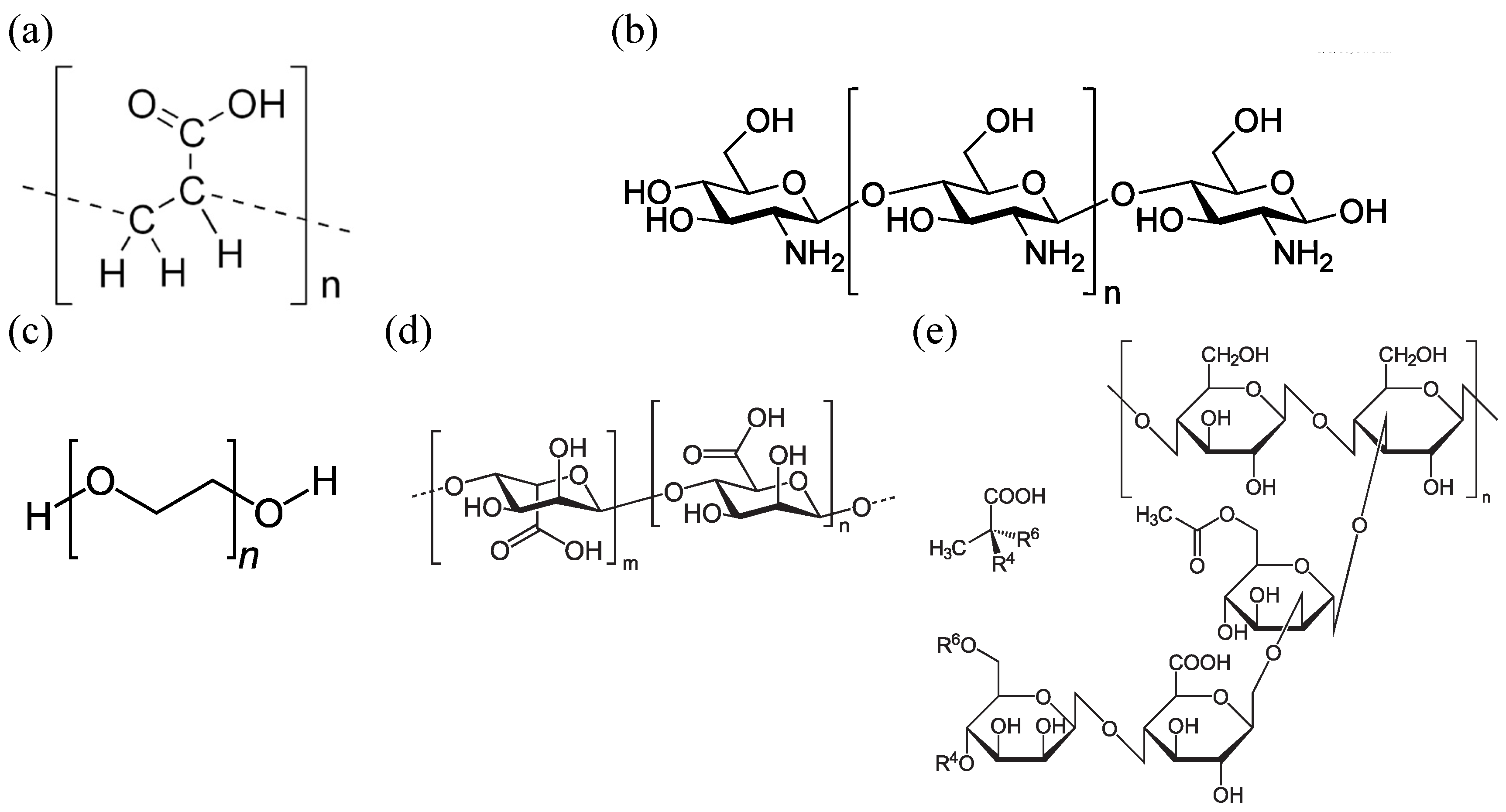
3.1. Material
3.2. Contact Angle of Biopolymer Solution
3.2.1. Material and Test Procedure
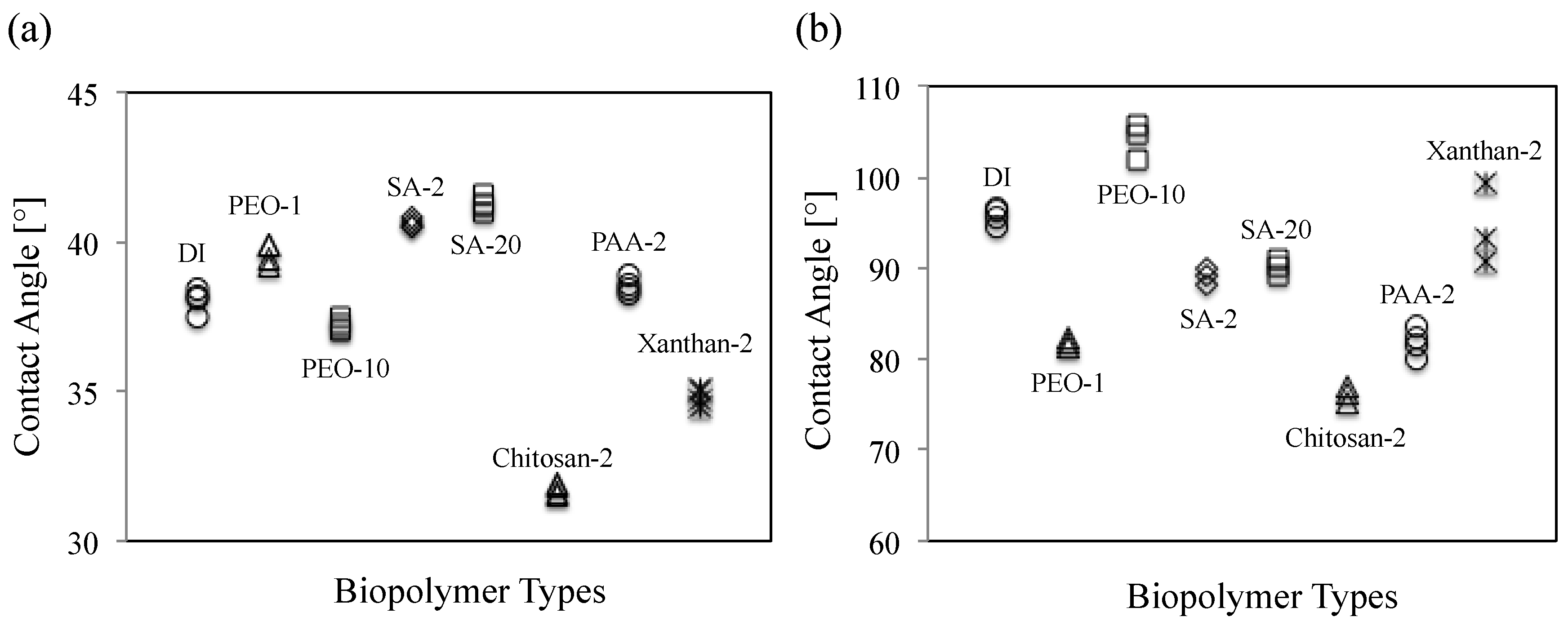
3.2.2. Result
3.3. Interfacial Tension and Surface Tension of Biopolymer Solution
3.3.1. Material and Test Procedure
3.3.2. Result
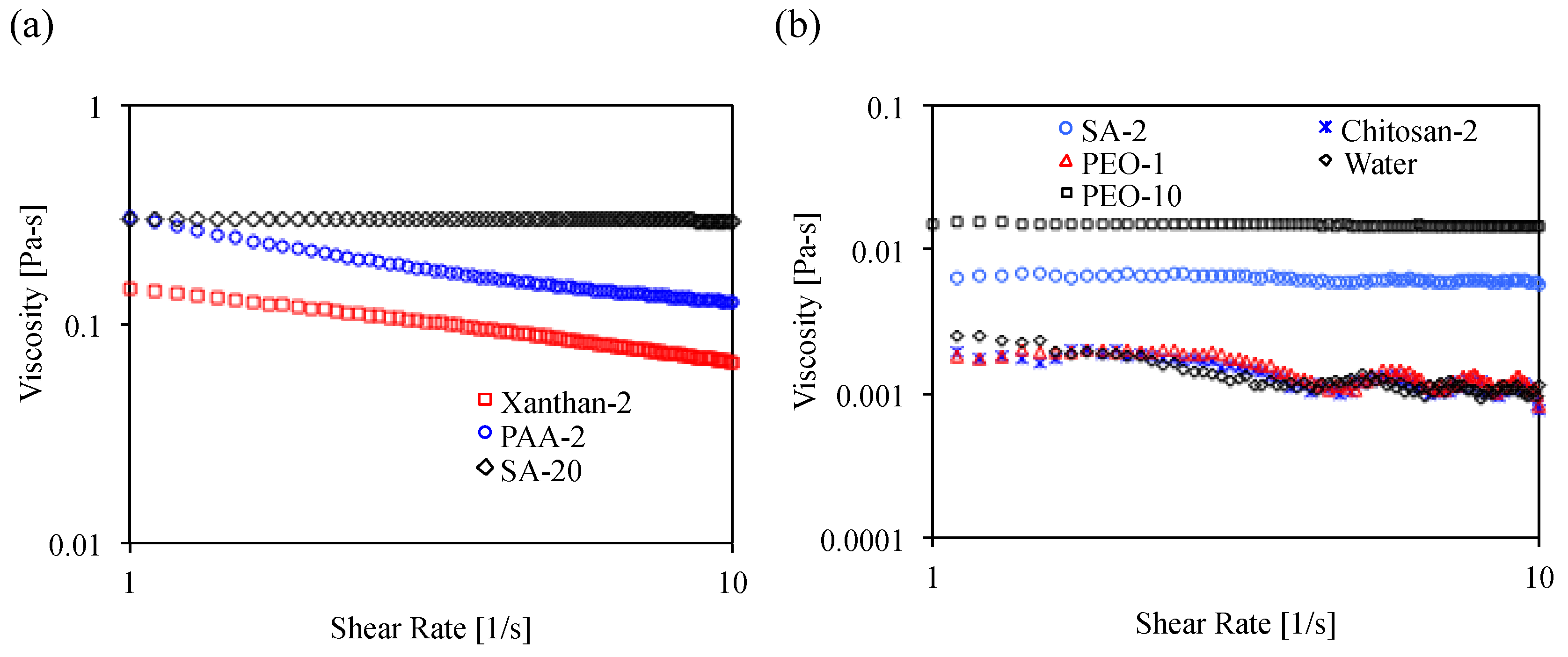
3.4. Viscosity of Biopolymer Solution
3.4.1. Material and Test Procedure
3.4.2. Result
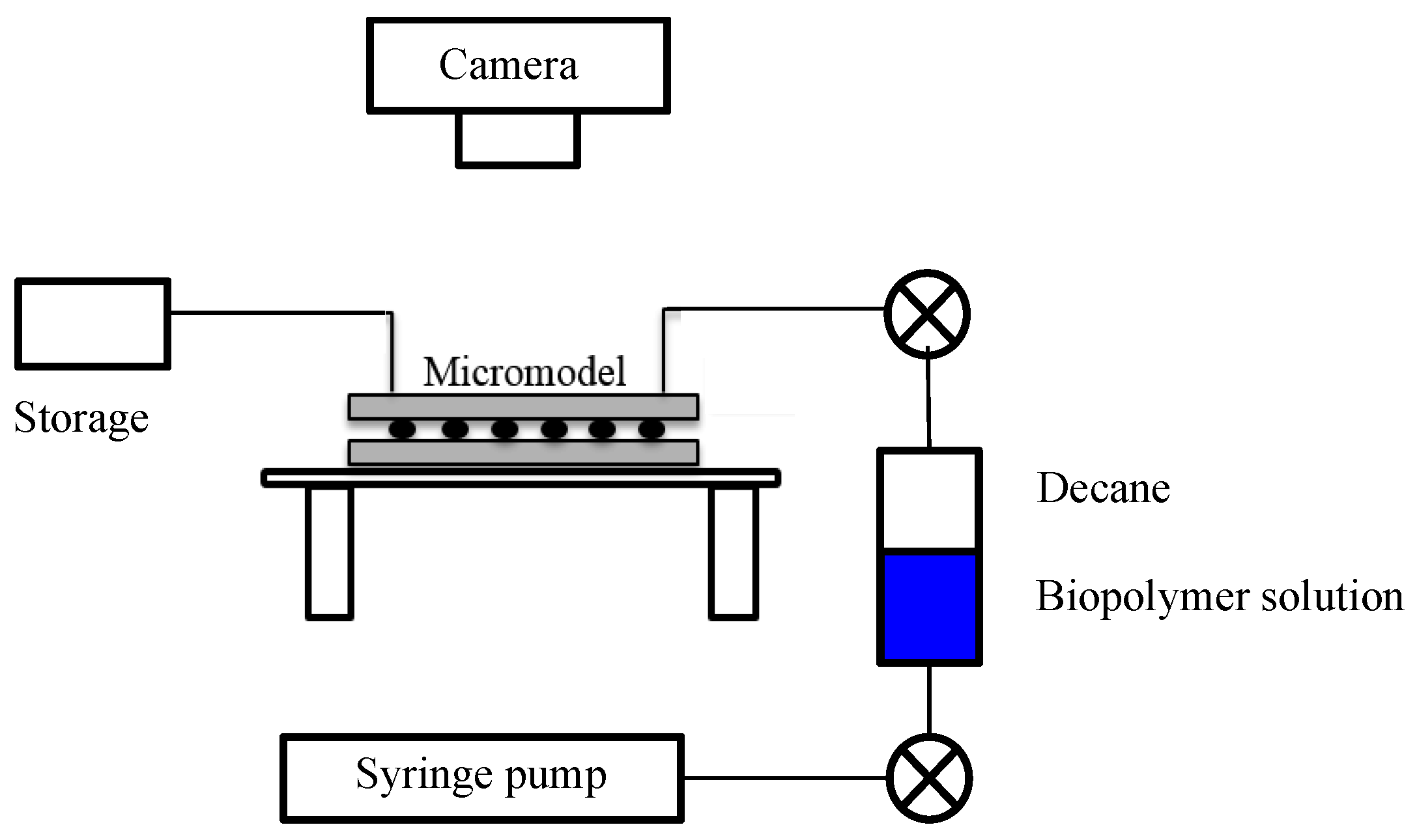
3.5. Micromodel Test
3.5.1. Apparatus and Materials
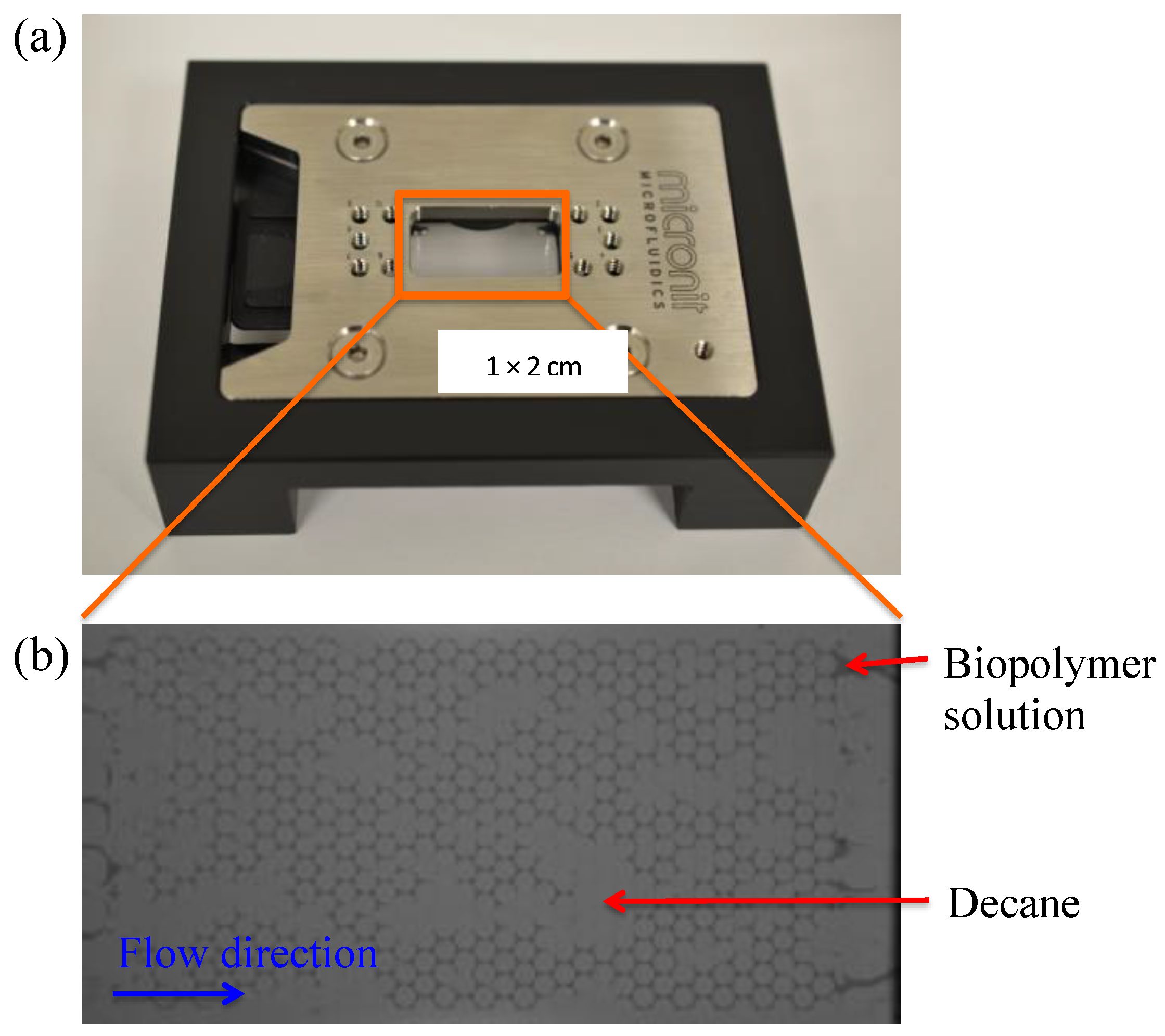
3.5.2. Micromodel
3.5.3. Experimental Procedure
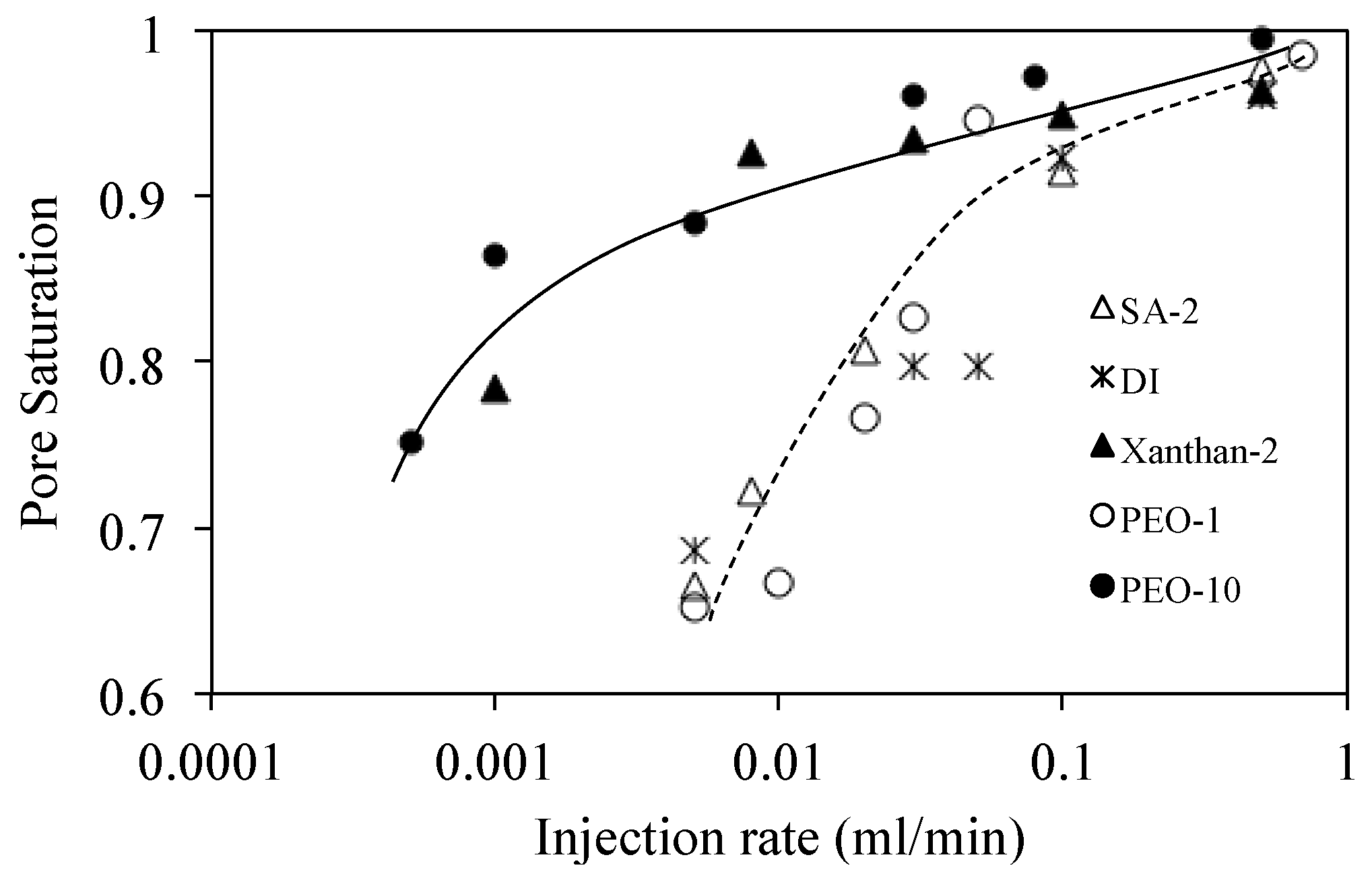
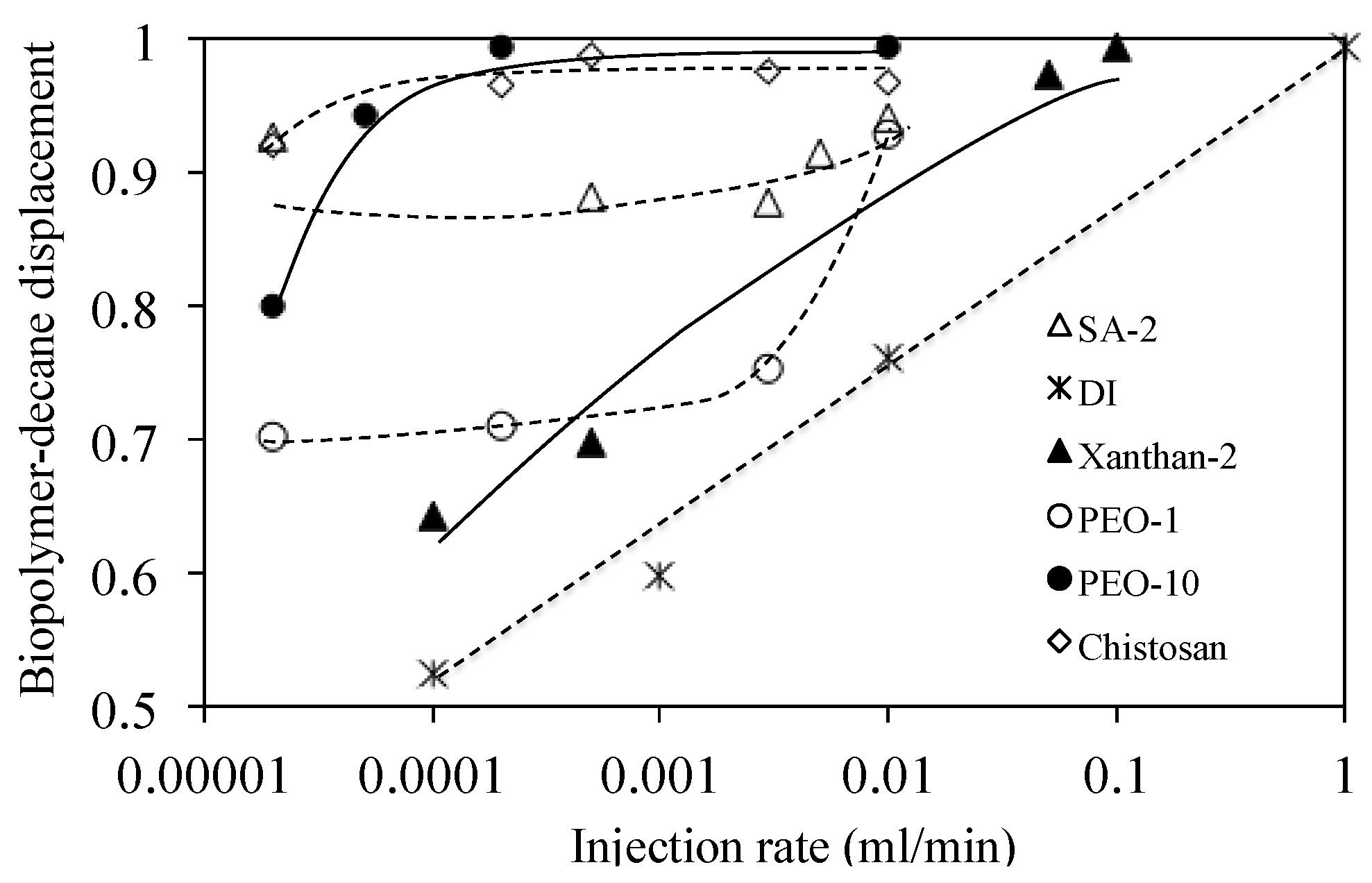
3.5.4. Result
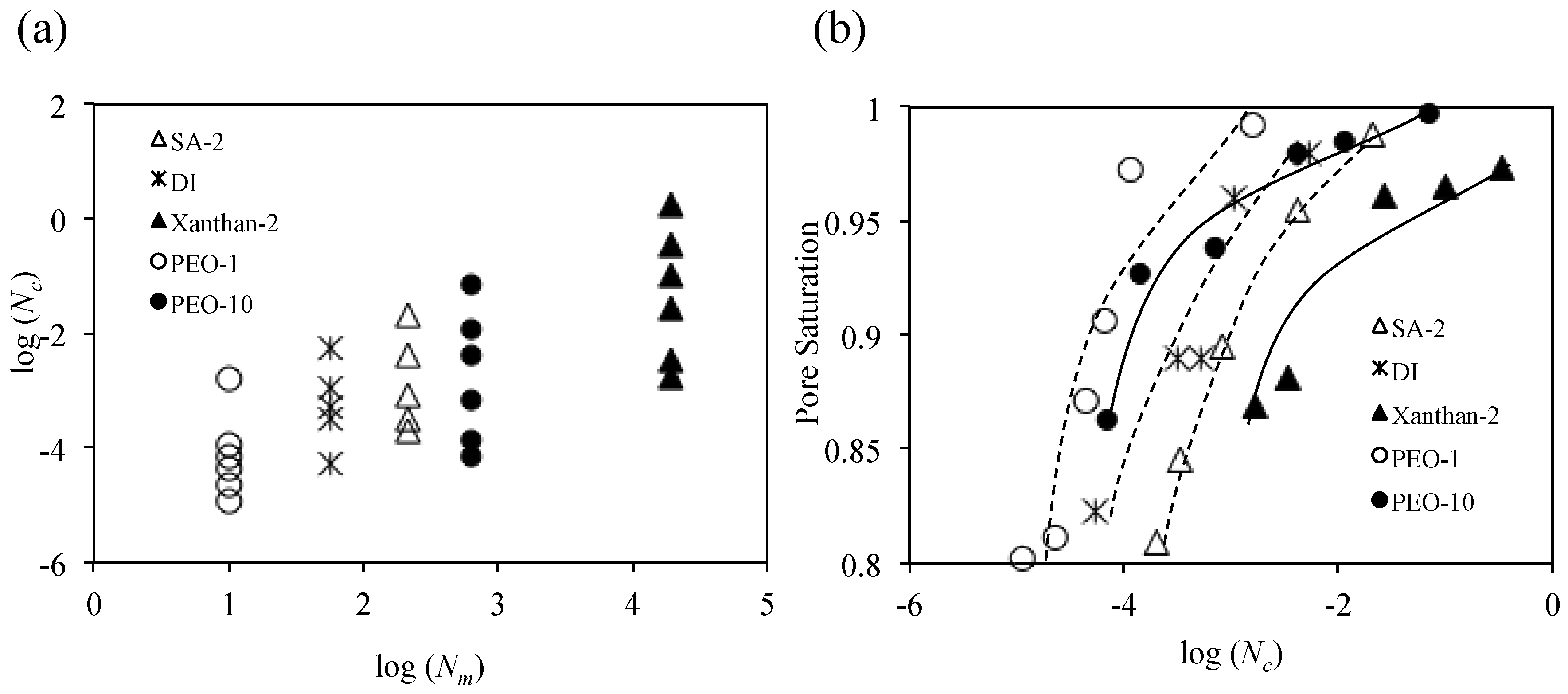
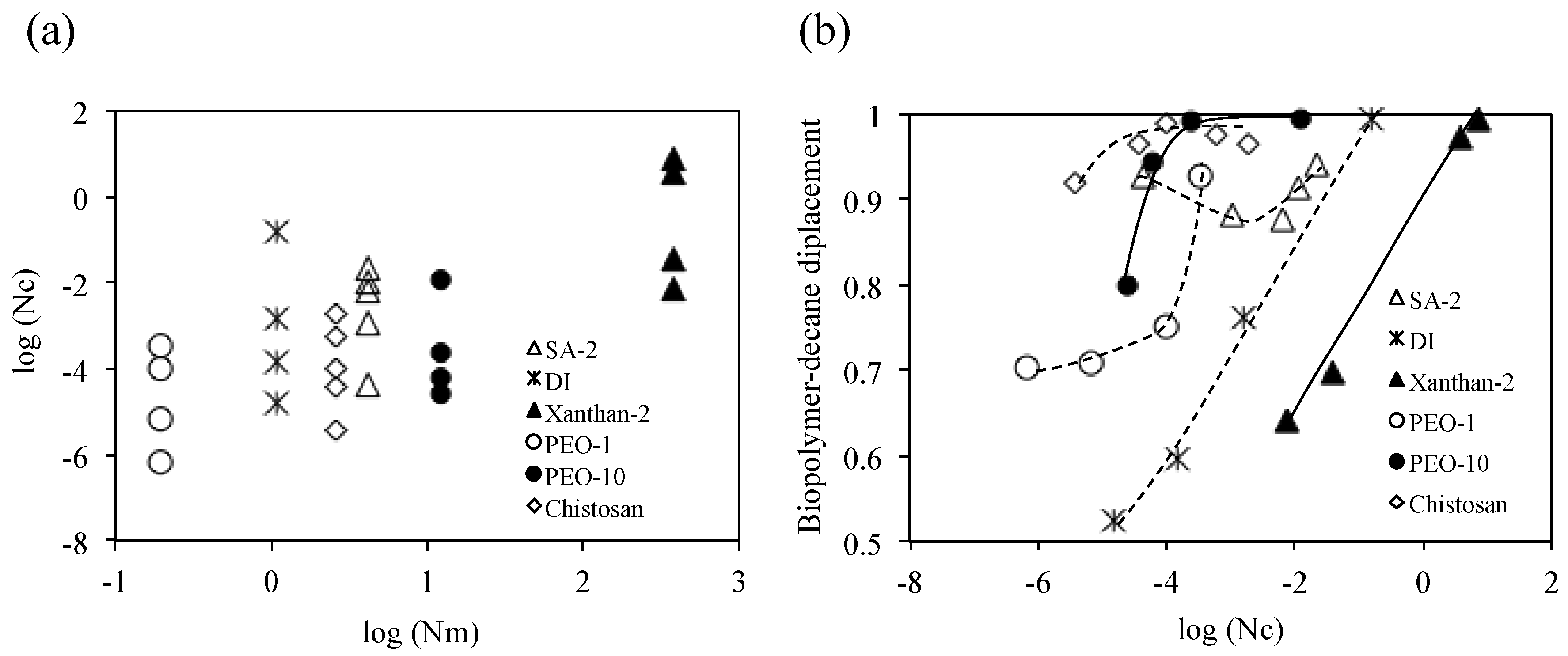
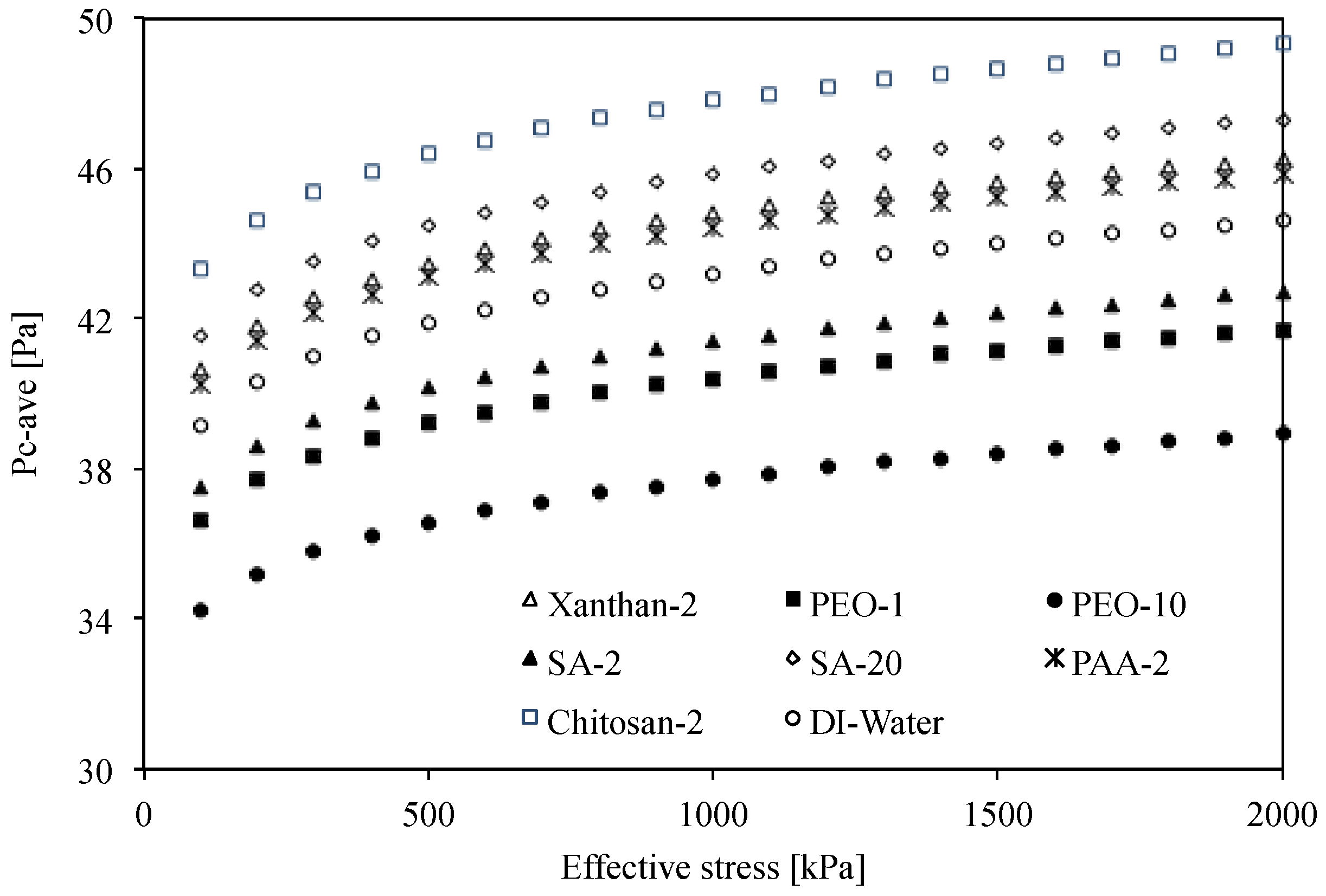
4. Analyses and Discussion
4.1. Viscous Number (Nm) and Capillary Number (Nc)
4.2. Effects of Effective Stress on Capillary Pressure
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mitchell, R.; Nevo, Z. Effect of bacterial polysaccharide accumulation on infiltration of water through sand. Appl. Microbiol. Biotechnol. 1964, 12, 219–223. [Google Scholar]
- Bate, B.; Zhao, Q.; Burns, S. Impact of organic coatings on frictional strength of organically modified clay. J. Geotech. Geoenviron. Eng. 2013, 140, 228–236. [Google Scholar] [CrossRef]
- Briscoe, W.H.; Klein, J. Friction and adhesion hysteresis between surfactant monolayers in water. J. Adhes. 2007, 83, 705–722. [Google Scholar] [CrossRef]
- Cabalar, A.F.; Canakci, H. Ground Improvement by Bacteria; Taylor and Francis Group: Norman, OK, USA, 2005; pp. 707–712. [Google Scholar]
- Kang, X.; Kang, G.C.; Bate, B. Measurement of Stiffness Anisotropy of Kaolinite Using Bender Element Tests in a Floating Wall Consolidometer. Geotechn. Test. J. 2014, 37, 869–883. [Google Scholar] [CrossRef]
- Kavazanjian, E., Jr.; Iglesias, E.; Karatas, I. Biopolymer soil stabilization for wind erosion control. In Proceedings of the 17th International Conference Soil Mechnics Geotechnical Engineering, Alexandria, Egypt, 5–9 October 2009; pp. 881–884.
- Martin, G.; Yen, T.; Karimi, S. Application of biopolymer technology in silty soil matrices to form impervious barriers. In Proceedings of the 7th Australia New Zealand Conference on Geomechanics: Geomechanics in a Changing World, Melbourne, Australia, 1–5 July 1996; p. 814.
- Nugent, R.; Zhang, G.; Gambrell, R. The Effect of Exopolymers and Void Ratio on the Erosional Resistance of Cohesive Sediments; In Proceedings of Geo-Frontiers 2011@ Advances in Geotechnical Engineering, Dallas, TX, USA, 13–16 March 2011; ASCE: Reston, VA, USA, 2011; pp. 1493–1502.
- Yoshikawa, T.; Naito, Y.; Kishi, A.; Tomii, T.; Kaneko, T.; Iinuma, S.; Ichikawa, H.; Yasuda, M.; Takahashi, S.; Kondo, M. Role of active oxygen, lipid peroxidation, and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in rats. Gut 1993, 34, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Hove, A.O.; Nilson, V.; Loknes, J. Visualization of xanthan flood behavior in core samples by means of x-ray tomography. SPE Reserv. Eng. 1990, 5, 475–480. [Google Scholar] [CrossRef]
- Philips, J.C.; Miller, J.W.; Wernau, W.C.; Tate, B.E.; Auerbach, M.H. A high-pyruvate xanthan for eor. Soc. Pet. Eng. J. 1985, 25, 594–602. [Google Scholar] [CrossRef]
- Pollock, T.J.; Thorne, L. Xanthomonas campestris strain for production of xanthan gum. Google Patents US5279961 A, 1994. [Google Scholar]
- Sandiford, B.B. Laboratory and field studies of water floods using polymer solutions to increase oil recoveries. J. Pet. Technol. 1964, 16, 917–922. [Google Scholar] [CrossRef]
- Strom, T.E.; Paul, J.M.; Phelps, C.H.; Sampath, K. A New Biopolymer for High-Temperature Profile Control: Part 1-Laboratory Testing. Soc. Pet. Eng. J. 1991, 6, 360–364. [Google Scholar] [CrossRef]
- Blokker, N.C.M. Analysis of Alginate-like Exopolysaccharides for the Application in Enhanced Oil Recovery. Master’s Thesis, Delft University of Technology, Delft, The Netherlands, 2014. [Google Scholar]
- Bailey, S.A.; Bryant, R.S.; Duncan, K.E. Use of Biocatalysts for Triggering Biopolymer Gelants; Society of Petroleum Engineers: Tulsa, OK, USA, 2000. [Google Scholar]
- Kosaric, N. Biosurfactants and their application for soil bioremediation. Food Technol. Biotechno. 2001, 39, 295–304. [Google Scholar]
- Leveratto, M.A.; Lauri, J.; Sanz, C.; Sigal, J.; Farouq Ali, S.M. EOR Polymer Screening For An Oil Field With High Salinity Brines. Soc. Pet. Eng. J. 1996, 4, 73–81. [Google Scholar] [CrossRef]
- Stepp, A.K.; Bailey, S.A.; Bryant, R.S.; Evans, D.B. Alternative Methods for Permeability Modification Using Biotechnology; Society of Petroleum Engineers: Denver, CA, USA, 1996. [Google Scholar]
- Meuser, H. Soil Remediation and Rehabilitation; Springer: Dordrecht, Germany, 2010; Volume 23, pp. 1–2. [Google Scholar]
- Swartjes, F.A. Dealing with Contaminated Sites; Springer: Dordrecht, Germany, 2011. [Google Scholar]
- Mulligan, C.; Eftekhari, F. Remediation with surfactant foam of pcp-contaminated soil. Eng. Geol. 2003, 70, 269–279. [Google Scholar] [CrossRef]
- Chai, J.C.; Miura, N. Field vapor extraction and long-term monitoring at a pce contaminated site. J. Hazard. Mat. 2004, 110, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; DiGiulio, D.C.; Wilson, J.T. In-situ air injection, soil vacuum extraction aqnd enhanced biodegradation: A case study in a jp-4 jet fuel contaminated site. Environ. Prog. 1997, 16, 570–576. [Google Scholar] [CrossRef]
- Hall, B.L.; Lachmar, T.E.; Dupont, R.R. Field monitoring and performance evaluation of a field-scale in-well aeration system at a gasoline-contaminated site. J. Hazard. Mater 2001, 82, 197–212. [Google Scholar] [CrossRef]
- Tse, K.K.C.; Lo, S.L.; Wang, J.W.H. Pilot study of in-situ thermal treatment for the remediation of pentachlorophenol-contaminated aquifers. Environ. Sci. Technol. 2001, 35, 4910–4915. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.; Dahlstrom, J.D.; Esposito, P.; Everett, G.; Peterson, G.; Traver, R.P. Soil washing/soil flushing. In Innovative Site Remediation Technology; Anderson, W.C., Ed.; WASTECH (American Academy of Engineers): Annapolis, MD, USA, 1993. [Google Scholar]
- Khatami, H.R.; O’Kelly, B.C. Improving mechanical properties of sand using biopolymers. J. Geotech. Geoenviron. Eng. 2013, 139, 1402–1406. [Google Scholar] [CrossRef]
- Lee, M.; Chung, S.; Kang, D.; Choi, S.; Kim, M. Surfactant enhanced in-site soil flushing pilot test for the soil and groundwater remediation in an oil contaminated site. J. Korean Soc. Soil Groundw. Environ. 2002, 7, 77–86. [Google Scholar]
- Rouse, J.D.; Sabatini, D.A.; Brown, R.E.; Harwel, L.J.H. Evaluation of ethoxylated alkylsulfate surfactants for use in subsurface remediation. Water Environ. Res. 1996, 68, 162–168. [Google Scholar] [CrossRef]
- Sabatini, D.A.; Knox, R.C. Transport and Remediation of Subsurface Contaminants: Colloidal, Interfacial and Surfactant Phenomena; ACS: Washington, DC, USA, 1992; p. 834. [Google Scholar]
- Jafvert, C.T. Surfactants/Cosolvents, Technology Evaluation Report TE-96-02; Groundwater Remediation Technologies Analysis Center: Pittsburgh, PA, USA, 1996; p. 45. [Google Scholar]
- Jawitz, J.W.; Annable, M.D.; Rao, P.S.C.; Rhue, R.D. Field implementation of a winsor typesurfactant/alcohol mixture for in situ solubilization of a complex lnapl as a single-phase microemulsion. Environ. Sci. Technol. 1998, 32, 523–530. [Google Scholar] [CrossRef]
- Lee, M.; Kang, H.; Do, W. Application of nonionic surfactant-enhanced in situ flushing to a diesel contaminated site. Water Res. 2005, 39, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.N.; Yong, B.R.; Gibbs, B.F. Surfactant-enhanced remediation of contaminated soil: A review. Eng. Geol. 2001, 60, 371–380. [Google Scholar] [CrossRef]
- Park, S.K.; Bielefeldt, A.R. Non-ionic surfactant flushing of pentachlorophenol from napl-contaminated soil. Water Res. 2005, 39, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.T.; Kao, C.M.; Yeh, T.Y.; Liang, S.H.; Chien, H.Y. Application of surfactant enhanced permanganate oxidation and biodegradation of trichloroethylene in groundwater. J. Hazard. Mater. 2009, 161, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhu, L. Enhanced soil flushing of phenanthrene by anionic-nonionic mixed surfactant. Water Res. 2008, 42, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Lake, L.W.; Johns, R.; Rossen, B.; Pope, G. Fundamentals of enhanced oil recovery; Society of Petroleum Engineers: Richardson, TX, USA, 2014. [Google Scholar]
- Hong, C.H.; Jang, C.H.; Choi, H.J. Turbulent drag reduction with polymers in rotating disk flow. Polymers 2015, 7, 1279–1298. [Google Scholar] [CrossRef]
- Sorbie, K.S. Polymer-Improved Oil Recovery; Blackie & Son: Glasgow, UK, 1991; p. 359. [Google Scholar]
- Delamaide, E.; Zaitoun, A.; Gérard, R.; Tabary, T. Pelican lake field: First successful application of polymer flooding in a heavy-oil reservoir. SPE Reserv. Elval. Eng. 2013, 17, 340–354. [Google Scholar]
- Moawad, T.; Elhomadhi, E.; Gawish, A. Novel promising, high viscosifier, cheap, available and environmental friendley biopolymer (polymtea) for different applications at reservoir conditions under investigation. Part a: Polymer properties. In Processing of The Seventh Egyptian-Syrian Conference in Chemical and Petroleum Engineering, Suez, Egypt, 29–31 October 2007.
- Jung, J.C.; Zhang, K.; Chon, B.H.; Choi, H.J. Rheology and polymer characteristics of partially hydrolyzed polyacrylamide for enhanced heavy oil recovery. J. Appl. Polymer Sci. 2013, 127, 4833. [Google Scholar] [CrossRef]
- Jang, H.Y.; Zhang, K.; Chon, B.H.; Choi, H.J. Enhanced oil recovery performance and viscosity characteristics of polysaccharide xanthan gum solution. J. Ind. Eng. Chem. 2015, 21, 741–745. [Google Scholar] [CrossRef]
- Cho, D.H.; Chae, H.J.; Kim, E.Y. Synthesis and characterization of a novel extracellular polysaccharide by rhodotorula glutinis. Appl. Biochem. Biotechnol. 2001, 98, 183–193. [Google Scholar] [CrossRef]
- Akit, R.J.; Cooper, D.G.; Neufield, R.J. Effects of oil reservoir conditions on the production of water-insoluble levan by bacillus licheniformis. Geomicrobiol. J. 1989, 7, 115–165. [Google Scholar]
- Canella, W.J.; Huh, C.; Seright, R.S. Prediction of xanthan rheology in porous media. In Processing of SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 2–5 October, 1988; Society of Petroleum Engineers: Richardson, TX, USA, 1988. [Google Scholar]
- Kim, D.S.; Fogler, H.S. The effects of exopolymers on cell morphology and culturability of l. Mesenteroides during starvation. Appl. Microbiol. Biotechnol. 1999, 52, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Sandford, P.A. Exocellular microbial polysaccharides. Adv. Carbohydr. Chem. Biochem. 1979, 36, 265–312. [Google Scholar] [PubMed]
- Bear, J. Dynamics of Fluids in Porous Media; Dover Publications Inc.: New York, NY, USA, 1972. [Google Scholar]
- Lenormand, R. Liquids in porous media. J. Phys. Condens. Matter 1990, 2, 79. [Google Scholar] [CrossRef]
- Lenormand, R.; Touboul, E.; Zarcone, C. Numerical models and experiments on immiscible displacements in porous media. J. Fluid Mech. 1988, 189, 165–187. [Google Scholar] [CrossRef]
- Buchgraber, M. An enhanced oil recovery micromodel study with associative and conventional polymers. Master’s Thesis, University of Leoben, Austria, 2008; pp. 16–30. [Google Scholar]
- Daripa, P.; Pasa, G. On capillary slowdown of viscous fingering in immiscible displacement in porous media. Transp. Porous Media 2008, 75, 1–16. [Google Scholar] [CrossRef]
- Lenormand, R.; Zarcone, C.; Sarr, A. Mechanisms of the displacement of one fluid by another in a network of capillary ducts. J. Fluid Mech. 1983, 135, 337–353. [Google Scholar] [CrossRef]
- Goosen, M.F.A. Applications of Chitin and Chitosan; CRC: Boca Raton, FL, USA, 1996; pp. 132–139. [Google Scholar]
- Woodmansey, A. Chitosan Treatment of Sediment Laden Wate-Washington State I-90 Issaquah Project; Federal Highway Administration: Washington, DC, USA, 2012. [Google Scholar]
- Kahovec, J.; Fox, R.B.; Hatada, K. Nomenclature of regular single-strand organic polymers. Pure Appl. Chem. 2002, 74, 1921–1956. [Google Scholar] [CrossRef]
- Orwoll, R.A.; Yong, C.S. Poly (Acrylic Acid); Oxford University Press, Inc: Oxford, UK, 1999; pp. 252–253. [Google Scholar]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces, 6th ed.; Wiley: New York, NY, USA, 1997. [Google Scholar]
- Ferer, M.; Bromhal, G.S.; Smith, D.H. Pore-level modeling of carbon dioxide sequestration in brine fields. J. Energy Environ. Res. 2002, 2, 120–132. [Google Scholar]
- Espinoza, D.N.; Santamarina, J.C. Water-CO2-mineral systems: Interfacial tension, contact angle, and diffusion-implications to CO2 geological storage. Water Resour. Res. 2010, 46, W07537. [Google Scholar] [CrossRef]
- Burland, J.B. On the compressibility and shear strength of natural clays. Geotechnique 1990, 40, 329–378. [Google Scholar] [CrossRef]
- Lambe, T.W.; Whitman, R.V. Soil Mechanics; Wiley: New York, NY, USA, 1969. [Google Scholar]
- Mitchell, J.K.; Soga, K. Fundamentals of Soil Behavior; Wiley: New York, NY, USA, 2005. [Google Scholar]
- Santamarina, J.C.; Klein, K.A.; Fam, M.A. Soils and Waves: Particulate Materials Behavior, Characterization and Process Monitoring; Wiley: New York, NY, USA, 2001; pp. 250–280. [Google Scholar]
- Skempton, A.W. The consolidation of clays by gravitational compaction. Q. J. Geol. Soc. 1970, 125, 373–411. [Google Scholar] [CrossRef]

| PEO-1 | PEO-10 | SA-2 | SA-20 | Xanthan-2 | PAA-2 | Chitosan-2 | |
|---|---|---|---|---|---|---|---|
| Concentration (g/L) | 1 | 10 | 2 | 20 | 2 | 2 | 2 |
| Soil Properties | Sand | Silt | Kaolinite | Illite | Montmorillonite |
|---|---|---|---|---|---|
| Ss (m2/g) | 0.044 | 0.045–1.0 | 10.0–20.0 | 65–100 | 300–780 |
| ρparticle (t/m3) | 2.655–2.659 | 2.798 | 2.65 | 2.6–2.9 | 2.35 |
| ρbulk (t/m3) | 1.586–2.083 | 1.3 | 7.69 | - | - |
| e100 (at 100 Kpa) | 0.35–0.85 | 0.6–0.8 | 0.9–1.1 | 2.0–3.0 | 2.5–4.0 |
| Cc | <0.02 | 0.02–0.09 | 0.2–0.4 | 0.5–1.1 | 1.0–2.0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, S.C.; Bate, B.; Hu, J.W.; Jung, J. Engineering Behavior and Characteristics of Water-Soluble Polymers: Implication on Soil Remediation and Enhanced Oil Recovery. Sustainability 2016, 8, 205. https://doi.org/10.3390/su8030205
Cao SC, Bate B, Hu JW, Jung J. Engineering Behavior and Characteristics of Water-Soluble Polymers: Implication on Soil Remediation and Enhanced Oil Recovery. Sustainability. 2016; 8(3):205. https://doi.org/10.3390/su8030205
Chicago/Turabian StyleCao, Shuang Cindy, Bate Bate, Jong Wan Hu, and Jongwon Jung. 2016. "Engineering Behavior and Characteristics of Water-Soluble Polymers: Implication on Soil Remediation and Enhanced Oil Recovery" Sustainability 8, no. 3: 205. https://doi.org/10.3390/su8030205
APA StyleCao, S. C., Bate, B., Hu, J. W., & Jung, J. (2016). Engineering Behavior and Characteristics of Water-Soluble Polymers: Implication on Soil Remediation and Enhanced Oil Recovery. Sustainability, 8(3), 205. https://doi.org/10.3390/su8030205






