Mechanisms of Cu(II) Adsorption onto Biochars Derived from Fallen and Non-Fallen Maple Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Biochar
2.3. Biochar Characterization
2.4. Batch Adsorption Studies
2.5. Adsorption Kinetic and Isotherm Studies
2.6. Analytical Determination of Cu2+ Concentration
3. Results and Discussion
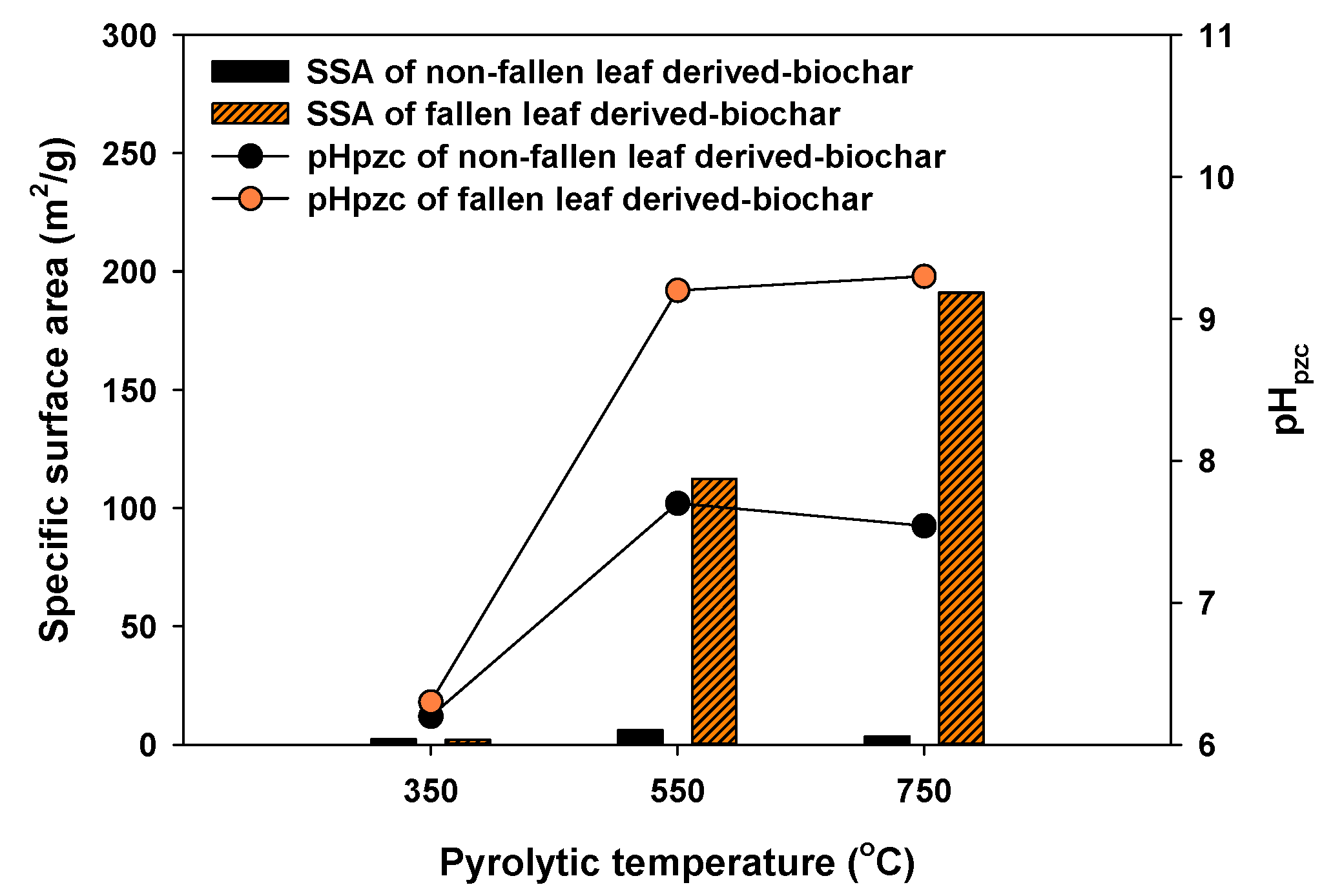
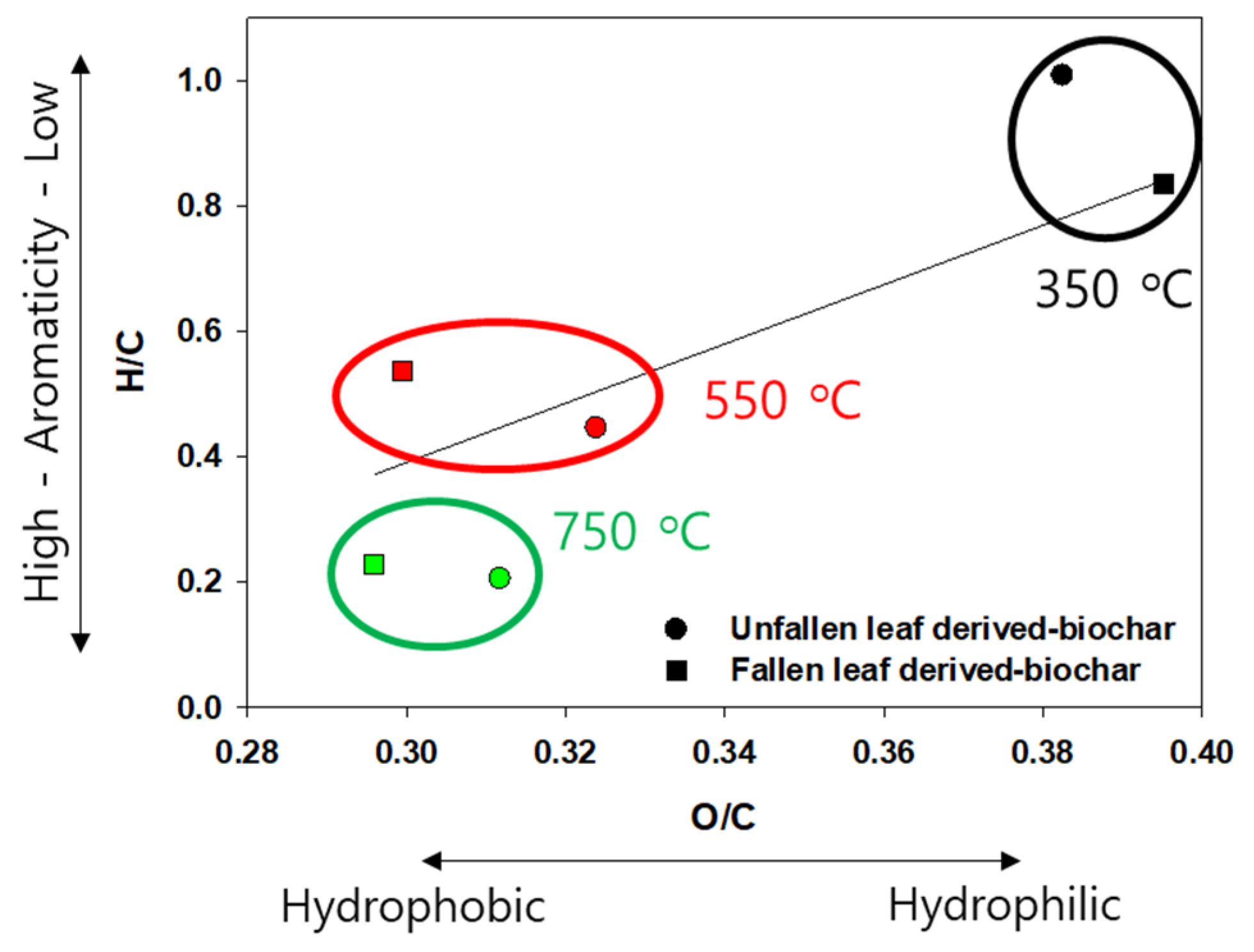
3.1. Physicochemical Properties of NF-BCs and F-BCs
3.2. Screening of NF-BCs and F-BCs for Cu(II) Adsorption
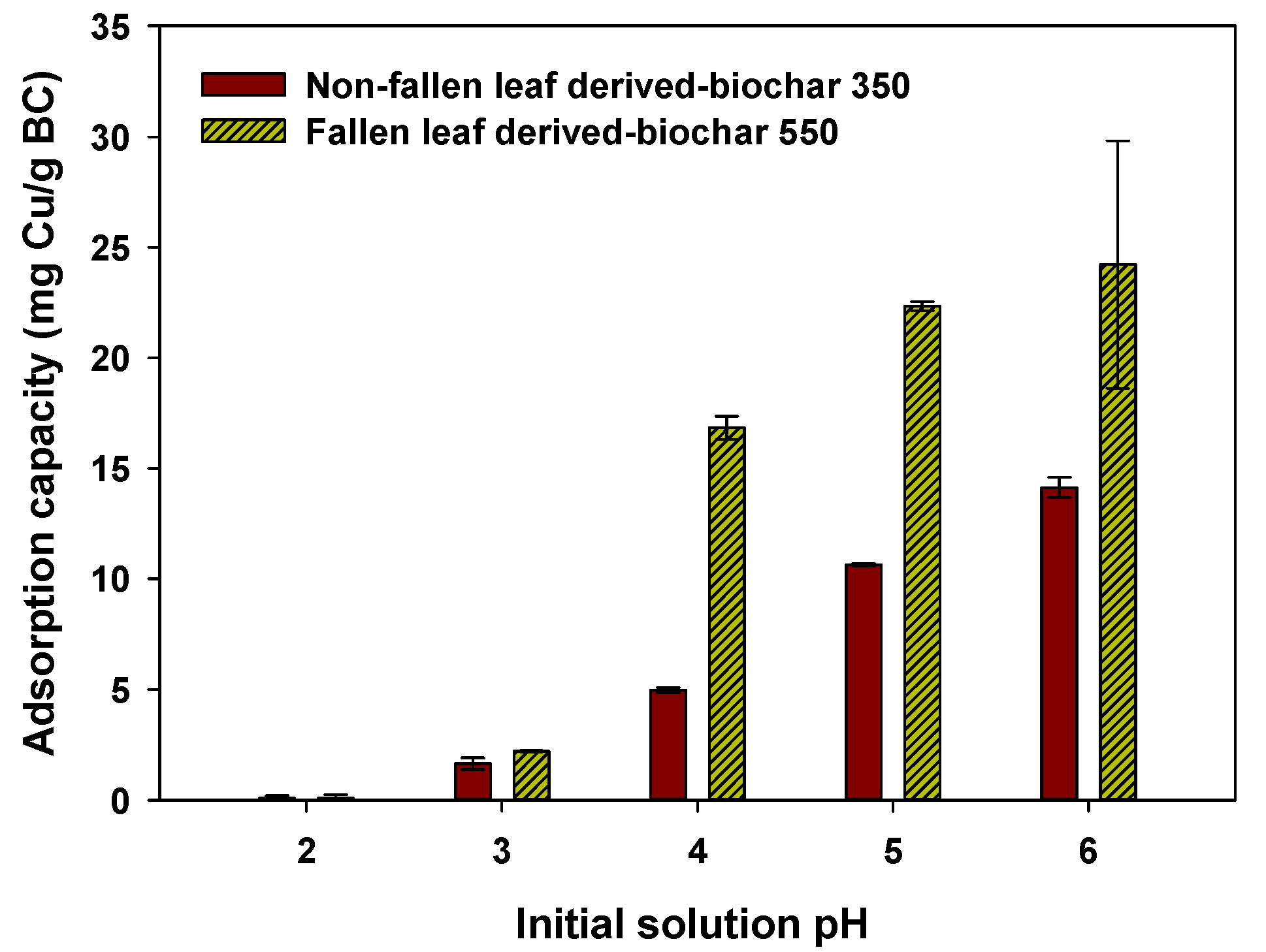
3.3. Influence of Solution pH on Cu(II) Adsorption onto NF-BC350 and F-BC550
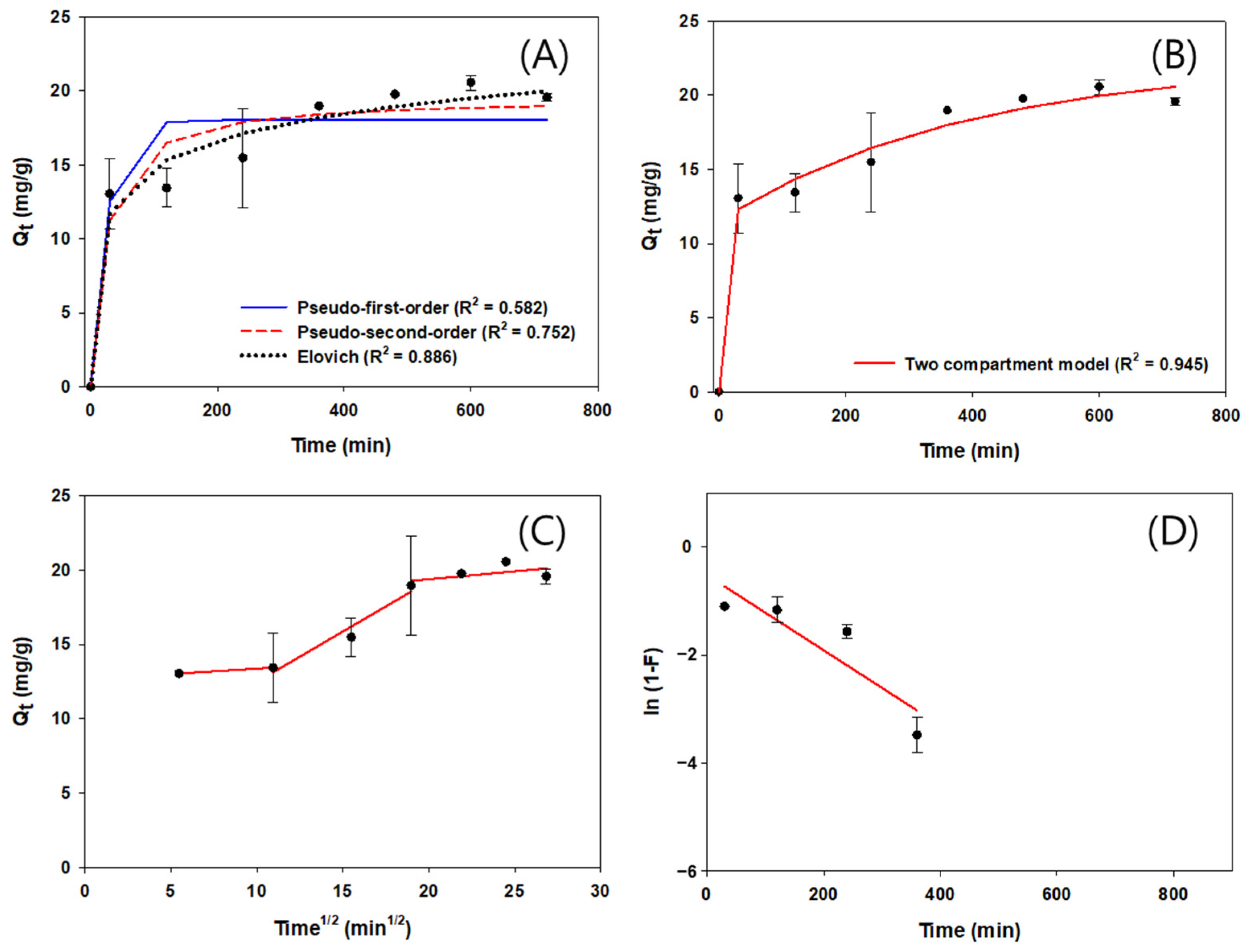
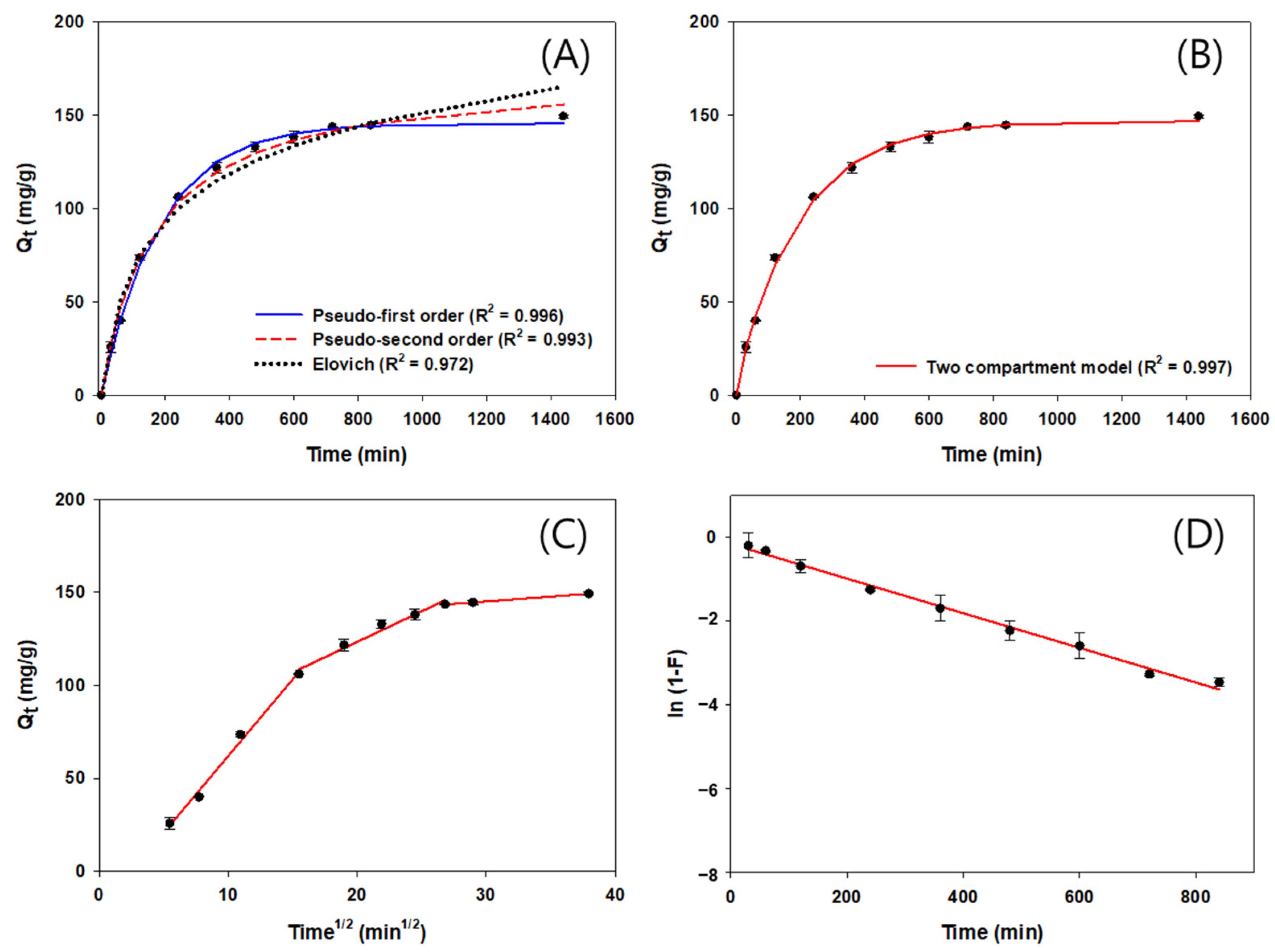
3.4. Adsorption Kinetics of NF-BCs and F-BCs
3.5. Adsorption Isotherms of NF-BCs and F-BCs
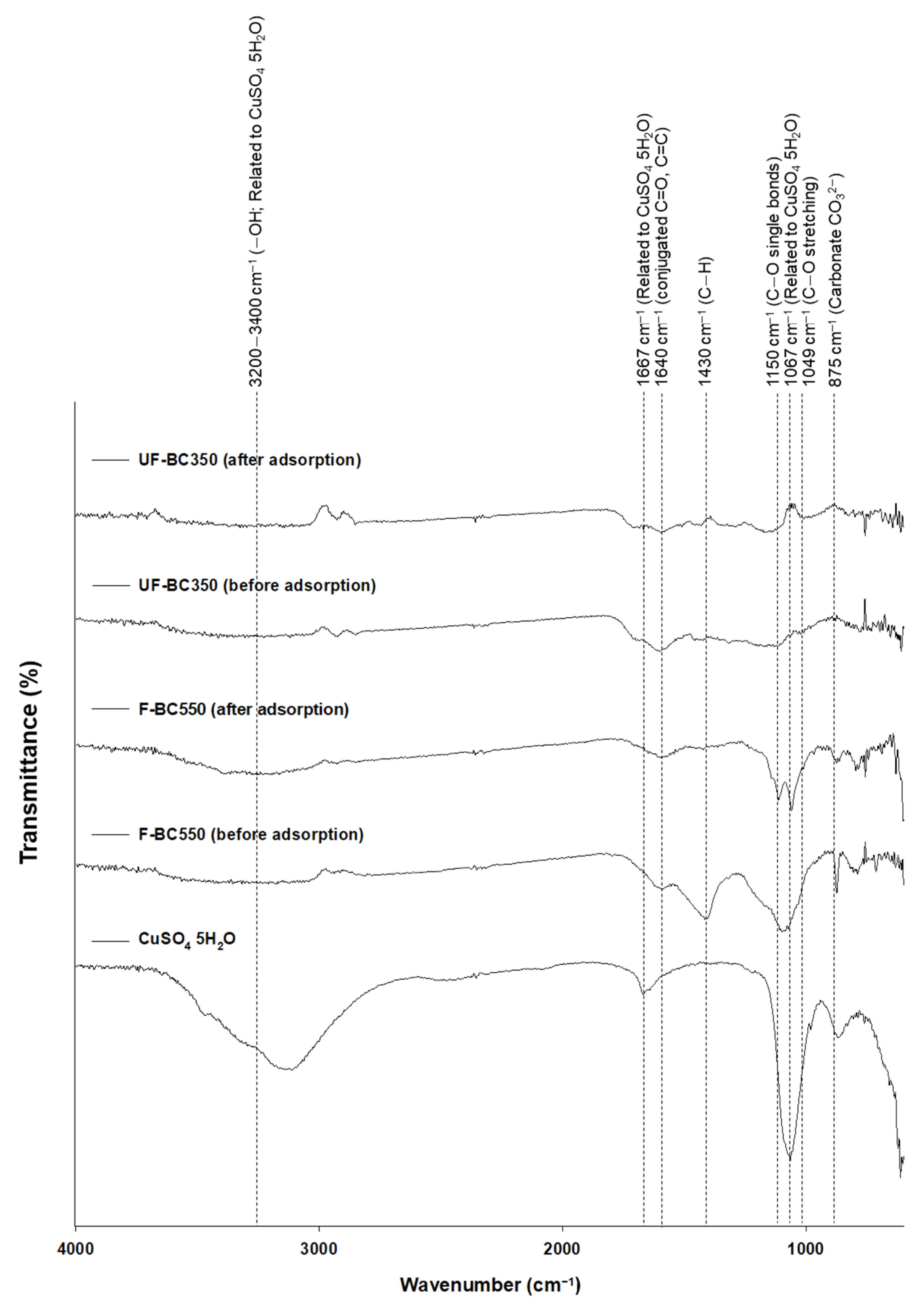
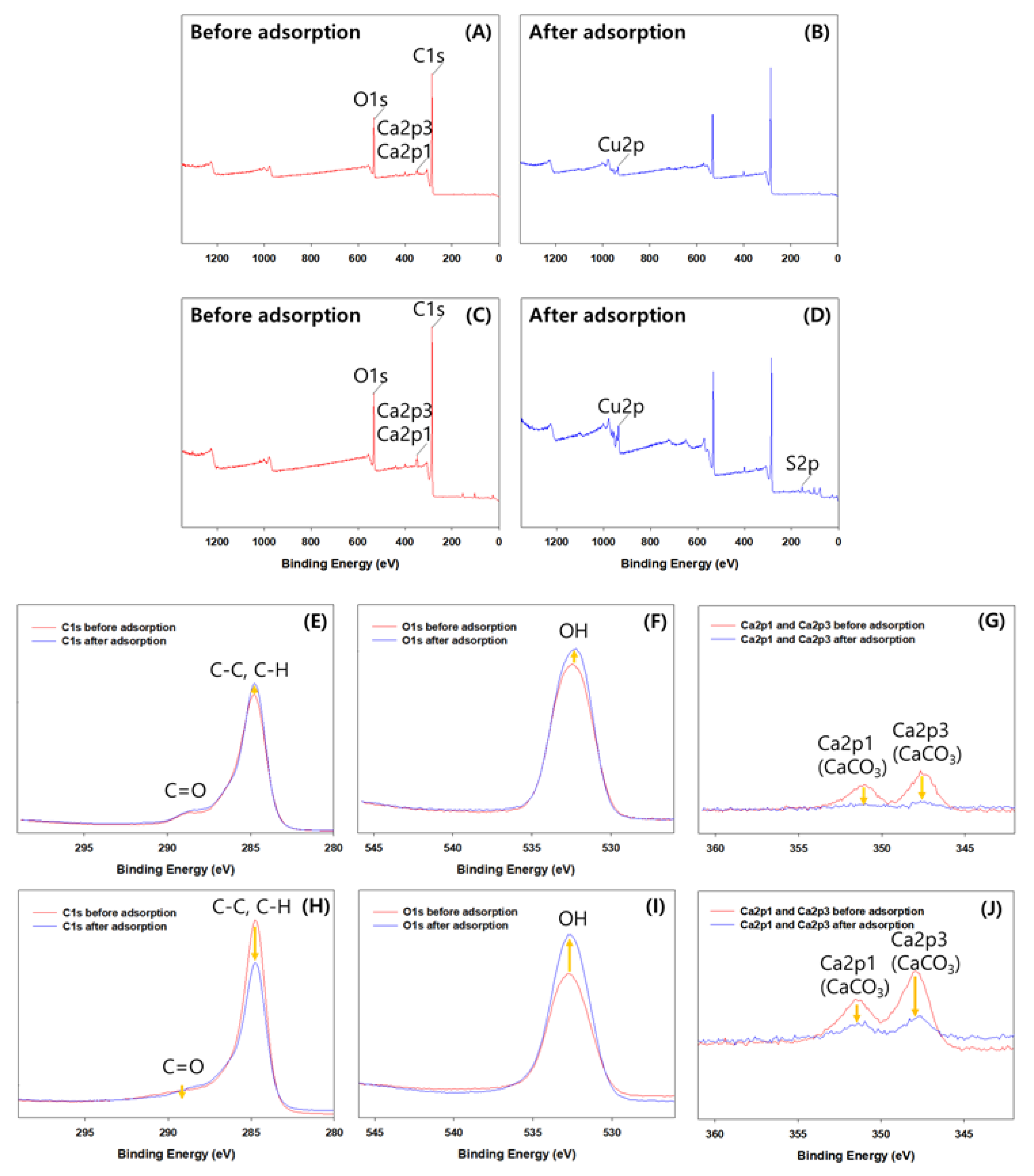
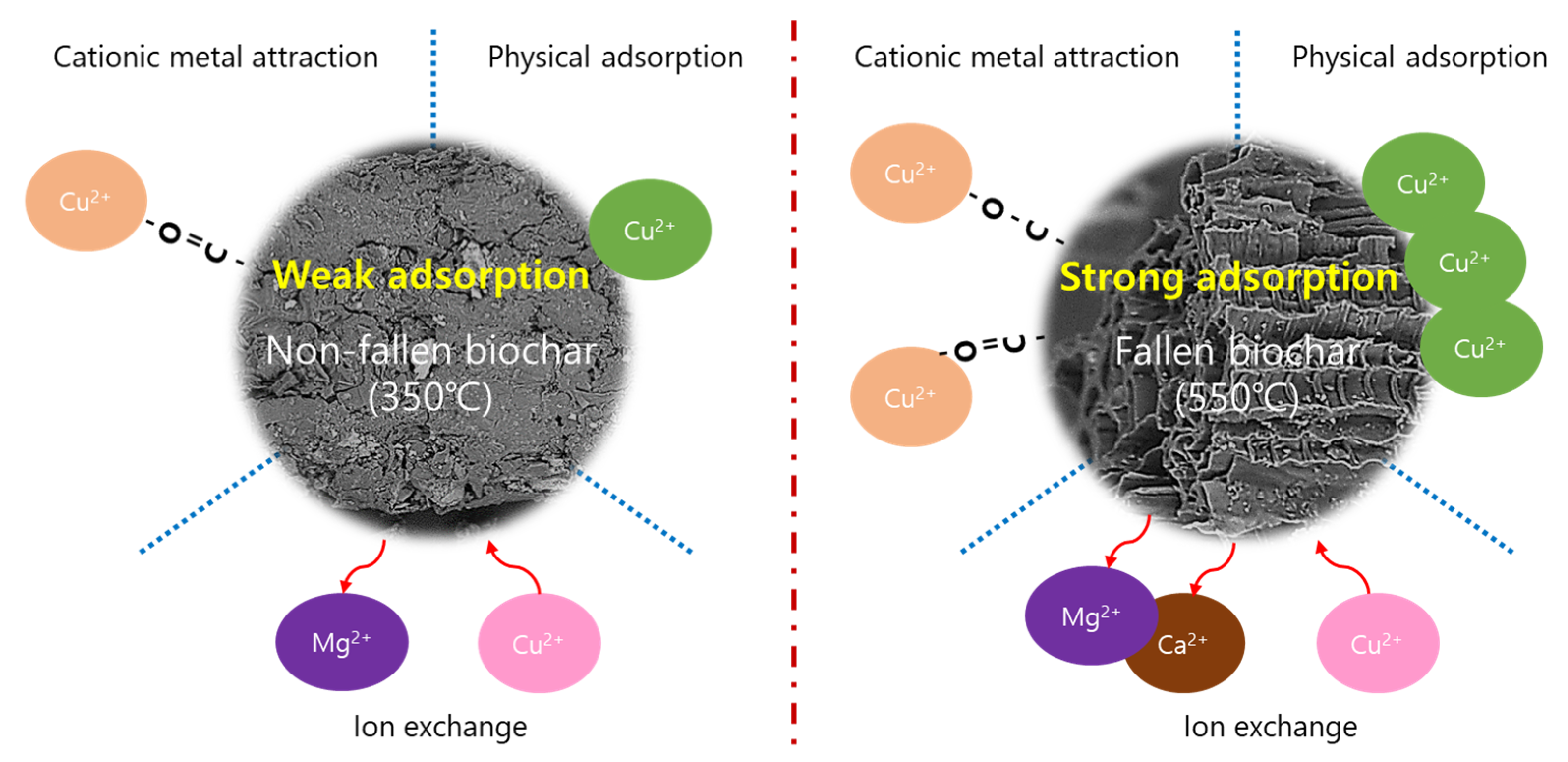
3.6. Mechanisms for Cu(II) Adsorption onto NF-BC350 and F-BC550
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Liu, H.; Liu, C.; Zhao, Z.; Han, W.; Yin, L.; Guo, A. Design and Analysis of Small Fallen Leaf Collection, Crushing, and Recycling Vehicle. Processes 2024, 12, 2011. [Google Scholar] [CrossRef]
- Tin, K.K.; Taweepreda, W.; Singh, A.; Wagri, N.K.; Kumar, A. Fallen Leaves to Sustainable Energy Solution: Review on Hydrogen Production. RSC Sustain. 2024, 2, 2751–2767. [Google Scholar] [CrossRef]
- Han, S.; Niu, X.; Wang, B.; Wang, Z.; Zhang, X.; Wang, Q.; Du, J.; Liu, P.; Liu, D.; Pan, F.; et al. Spatial-Temporal Patterns of Litterfall Mercury Concentration and Flux in Typical Vegetation in China. Ecosyst. Health Sustain. 2025, 11, 0288. [Google Scholar] [CrossRef]
- Noblet, C.; Besombes, J.L.; Lemire, M.; Pin, M.; Jaffrezo, J.L.; Favez, O.; Aujay-Plouzeau, R.; Dermigny, A.; Karoski, N.; Van Elsuve, D.; et al. Emission Factors and Chemical Characterization of Particulate Emissions from Garden Green Waste Burning. Sci. Total Environ. 2021, 798, 149367. [Google Scholar] [CrossRef]
- Kong, L.; Gong, L.; Wang, J. Removal of Methylene Blue from Wastewater Using Fallen Leaves as an Adsorbent. Desalination Water Treat. 2015, 53, 2489–2500. [Google Scholar] [CrossRef]
- Mahato, R.; Qaiyum, M.A.; Samal, P.P.; Dutta, S.; Dey, B.; Dey, S. Exploring the Promising Potential of Fallen Bamboo Leaves (Bambusa bambos) for Efficient Removal of Crystal Violet from Wastewater. Int. J. Phytoremediat. 2023, 25, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tang, J.; Ma, H.; Li, Z.; Li, J.; Han, Q.; Chen, C. Research on the Adsorption Behaviour of Crystal Violet Simulated Wastewater on Fallen Willow Leaves. IOP Conf. Ser. Earth Environ. Sci. 2019, 267, 032092. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, Q.; Zhao, Y.; Peng, Y. Efficient Removal of Methyl Violet from Aqueous Solution by a Low-Cost Adsorbent—C. Camphora Fallen Leaves Powder. J. Dispers. Sci. Technol. 2017, 38, 1135–1141. [Google Scholar] [CrossRef]
- Jain, S.N.; Gogate, P.R. Adsorptive Removal of Acid Violet 17 Dye from Wastewater Using Biosorbent Obtained from NaOH and H2SO4 Activation of Fallen Leaves of Ficus Racemosa. J. Mol. Liq. 2017, 243, 132–143. [Google Scholar] [CrossRef]
- Jain, S.N.; Gogate, P.R. Acid Blue 113 Removal from Aqueous Solution Using Novel Biosorbent Based on NaOH Treated and Surfactant Modified Fallen Leaves of Prunus Dulcis. J. Environ. Chem. Eng. 2017, 5, 3384–3394. [Google Scholar] [CrossRef]
- Guo, D.; Li, Y.; Cui, B.; Hu, M.; Luo, S.; Ji, B.; Liu, Y. Natural Adsorption of Methylene Blue by Waste Fallen Leaves of Magnoliaceae and Its Repeated Thermal Regeneration for Reuse. J. Clean. Prod. 2020, 267, 121903. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, J.; Dai, G.; Wu, J.; Yan, H. Adsorption Characteristics of Pb(II) from Aqueous Solution onto a Natural Biosorbent, Fallen Cinnamomum camphora Leaves. Desalination 2010, 262, 174–182. [Google Scholar] [CrossRef]
- Lee, M.E.; Park, J.H.; Chung, J.W. Comparison of the Lead and Copper Adsorption Capacities of Plant Source Materials and Their Biochars. J. Environ. Manag. 2019, 236, 118–124. [Google Scholar] [CrossRef]
- Shi, J.; Fang, Z.; Zhao, Z.; Sun, T.; Liang, Z. Comparative Study on Pb(II), Cu(II), and Co(II) Ions Adsorption from Aqueous Solutions by Arborvitae Leaves. Desalination Water Treat. 2016, 57, 4732–4739. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Z.; Liang, Z.; Sun, T. Adsorption Characteristics of Pb(II) from Aqueous Solutions onto a Natural Biosorbent, Fallen Arborvitae Leaves. Water Sci. Technol. 2016, 73, 2422–2429. [Google Scholar] [CrossRef]
- Fan, R.; Yi, Q.; Xie, Y.; Xie, F.; Zhang, Q.; Luo, Z. Enhanced Adsorption and Recovery of Pb(II) from Aqueous Solution by Alkali-Treated Persimmon Fallen Leaves. J. Appl. Polym. Sci. 2016, 133, 43656. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.H.; Lee, J.W.; Jang, S.; Kim, J.E.; Kang, G.; Choi, Y.K. Adsorptive Performance of Rice Husk-Derived Biochar for Nodularin Cyanotoxin from Aqueous Solution: Isotherm, Kinetic, Regeneration, and Column Studies. J. Water Process Eng. 2025, 70, 106866. [Google Scholar] [CrossRef]
- Na, Y.; Weon, S.H.; Lee, G.W.; Kim, H.J.; Lee, S.H.; Kim, Y.H.; Kim, J.E.; Kang, G.; Park, S.; Choi, Y.K. Evaluation of Benzene Adsorption onto Grass-Derived Biochar and Comparison of Adsorption Capacity via RSM (Response Surface Methodology). J. Compos. Sci. 2024, 8, 132. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, Modification and Environmental Application of Biochar: A Review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Bartoli, M.; Giorcelli, M.; Jagdale, P.; Rovere, M.; Tagliaferro, A. A Review of Non-Soil Biochar Applications. Materials 2020, 13, 261. [Google Scholar] [CrossRef]
- Hassan, M.; Liu, Y.; Naidu, R.; Parikh, S.J.; Du, J.; Qi, F.; Willett, I.R. Influences of Feedstock Sources and Pyrolysis Temperature on the Properties of Biochar and Functionality as Adsorbents: A Meta-Analysis. Sci. Total Environ. 2020, 744, 140714. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Song, H.J.; Gurav, R.; Bhatia, S.K.; Lee, E.B.; Kim, H.J.; Yang, Y.H.; Kan, E.; Kim, H.H.; Lee, S.H.; Choi, Y.K. Treatment of Microcystin-LR Cyanotoxin Contaminated Water Using Kentucky Bluegrass-Derived Biochar. J. Water Process Eng. 2021, 41, 102054. [Google Scholar] [CrossRef]
- Kim, J.E.; Bhatia, S.K.; Song, H.J.; Yoo, E.; Jeon, H.J.; Yoon, J.Y.; Yang, Y.; Gurav, R.; Yang, Y.H.; Kim, H.J.; et al. Adsorptive Removal of Tetracycline from Aqueous Solution by Maple Leaf-Derived Biochar. Bioresour. Technol. 2020, 306, 123092. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Cao, Y.; Han, L. Characteristics of Tetracycline Adsorption by Cow Manure Biochar Prepared at Different Pyrolysis Temperatures. Bioresour. Technol. 2019, 285, 121348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tu, Y.J.; Duan, Y.P.; Liu, J.; Zhi, W.; Tang, Y.; Xiao, L.S.; Meng, L. Production of Biochar from Waste Sludge/Leaf for Fast and Efficient Removal of Diclofenac. J. Mol. Liq. 2020, 299, 112193. [Google Scholar] [CrossRef]
- Park, J.H.; Wang, J.J.; Xiao, R.; Wang, M.; Lee, Y.H.; Kang, S.W.; Seo, D.C. Characteristics of Adsorption Behavior of Potentially Toxic Metals by Biochar Derived from Fallen Leaves (Platanus) and Its Mechanism. Sustain. Chem. Pharm. 2022, 29, 100776. [Google Scholar] [CrossRef]
- Lee, M.E.; Park, J.H.; Chung, J.W. Adsorption of Pb(II) and Cu(II) by Ginkgo-Leaf-Derived Biochar Produced under Various Carbonization Temperatures and Times. Int. J. Environ. Res. Public. Health 2017, 14, 1528. [Google Scholar] [CrossRef]
- Thangagiri, B.; Sakthivel, A.; Jeyasubramanian, K.; Seenivasan, S.; Raja, J.D. Modeling and Optimization of Removal of Pb(II) in Aqueous Solutions by Biochar Derived from Neem Leaves Using Central Composite Design of Response Surface Methodology. Biomass Convers. Biorefin. 2024, 14, 16019–16034. [Google Scholar] [CrossRef]
- Tan, R.; Li, K.; Sun, Y.; Fan, X.; Shen, Z.; Tang, L. Sustainable Management of Campus Fallen Leaves through Low-Temperature Pyrolysis and Application in Pb Immobilization. J. Environ. Sci. 2024, 139, 281–292. [Google Scholar] [CrossRef]
- Choi, Y.K.; Gurav, R.; Kim, H.J.; Yang, Y.H.; Bhatia, S.K. Evaluation for Simultaneous Removal of Anionic and Cationic Dyes onto Maple Leaf-Derived Biochar Using Response Surface Methodology. Appl. Sci. 2020, 10, 2982. [Google Scholar] [CrossRef]
- Ji, B.; Wang, J.; Song, H.; Chen, W. Removal of Methylene Blue from Aqueous Solutions Using Biochar Derived from a Fallen Leaf by Slow Pyrolysis: Behavior and Mechanism. J. Environ. Chem. Eng. 2019, 7, 103036. [Google Scholar] [CrossRef]
- Ji, B.; Zhu, L.; Song, H.; Chen, W.; Guo, S.; Chen, F. Adsorption of Methylene Blue onto Novel Biochars Prepared from Magnolia Grandiflora Linn Fallen Leaves at Three Pyrolysis Temperatures. Water Air Soil Pollut. 2019, 230, 281. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.; Kang, X.; Song, J.; Guo, H.; Zhang, Q. Insight into Atrazine Removal by Fallen Leaf Biochar Prepared at Different Pyrolysis Temperatures: Batch Experiments, Column Adsorption and DFT Calculations. Environ. Pollut. 2023, 317, 120832. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan-Nejad, Z.; Jung, M.C. Remediation of Multi-Metal Contaminated Soil Using Biochars from Rice Husk and Maple Leaves. J. Mater. Cycles Waste Manag. 2019, 21, 457–468. [Google Scholar] [CrossRef]
- Derakhshan Nejad, Z.; Jung, M.C. The Effects of Biochar and Inorganic Amendments on Soil Remediation in the Presence of Hyperaccumulator Plant. Int. J. Energy Environ. Eng. 2017, 8, 317–329. [Google Scholar] [CrossRef]
- Derakhshan Nejad, Z.; Kim, J.W.; Jung, M.C. Reclamation of Arsenic Contaminated Soils around Mining Site Using Solidification/Stabilization Combined with Revegetation. Geosci. J. 2017, 21, 385–396. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kan, E. Effects of Pyrolysis Temperature on the Physicochemical Properties of Alfalfa-Derived Biochar for the Adsorption of Bisphenol A and Sulfamethoxazole in Water. Chemosphere 2019, 218, 741–748. [Google Scholar] [CrossRef]
- Wu, K.; Liu, Y.; Liu, J.; Ren, Y.; Liu, Y.; Sun, F.; Abozeid, A.; Tang, Z.; Mu, L. Comparison of Seasonally Adaptive Metabolic Response Strategies of Two Acer Species. Forests 2022, 13, 2141. [Google Scholar] [CrossRef]
- Qian, T.; Wang, Y.; Fan, T.; Fang, G.; Zhou, D. A New Insight into the Immobilization Mechanism of Zn on Biochar: The Role of Anions Dissolved from Ash. Sci. Rep. 2016, 6, 33630. [Google Scholar] [CrossRef]
- Usevičiūtė, L.; Baltrėnaitė-Gedienė, E. Dependence of Pyrolysis Temperature and Lignocellulosic Physical-Chemical Properties of Biochar on Its Wettability. Biomass Convers. Biorefin. 2021, 11, 2775–2793. [Google Scholar] [CrossRef]
- Bandara, T.; Xu, J.; Potter, I.D.; Franks, A.; Chathurika, J.B.A.J.; Tang, C. Mechanisms for the Removal of Cd(II) and Cu(II) from Aqueous Solution and Mine Water by Biochars Derived from Agricultural Wastes. Chemosphere 2020, 254, 126745. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, J.Y.; Cho, T.S.; Choi, J.W. Influence of Pyrolysis Temperature on Physicochemical Properties of Biochar Obtained from the Fast Pyrolysis of Pitch Pine (Pinus rigida). Bioresour. Technol. 2012, 118, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Batista, E.M.C.C.; Shultz, J.; Matos, T.T.S.; Fornari, M.R.; Ferreira, T.M.; Szpoganicz, B.; De Freitas, R.A.; Mangrich, A.S. Effect of Surface and Porosity of Biochar on Water Holding Capacity Aiming Indirectly at Preservation of the Amazon Biome. Sci. Rep. 2018, 8, 10677. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Liao, B.; Lin, L.; Qiu, W.; Song, Z. Adsorption of Cu(II) and Cd(II) from Aqueous Solutions by Ferromanganese Binary Oxide–Biochar Composites. Sci. Total Environ. 2018, 615, 115–122. [Google Scholar] [CrossRef]
- Cibati, A.; Foereid, B.; Bissessur, A.; Hapca, S. Assessment of Miscanthus × giganteus Derived Biochar as Copper and Zinc Adsorbent: Study of the Effect of Pyrolysis Temperature, PH and Hydrogen Peroxide Modification. J. Clean. Prod. 2017, 162, 1285–1296. [Google Scholar] [CrossRef]
- Liu, G.; Song, Y.; Sheng, H.; Ye, M.; Stedtfeld, R.D.; Bian, Y.; Gu, C.; Jiang, X.; Wang, F. Adsorption Kinetics of 2,2′,4,4′-Tetrabromodiphenyl Ether (BDE-47) on Maize Straw-Derived Biochars. Pedosphere 2019, 29, 721–729. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Chen, L.; Chen, Y.; Lehmann, J.; McBride, M.B.; Hay, A.G. Adsorption of Copper and Zinc by Biochars Produced from Pyrolysis of Hardwood and Corn Straw in Aqueous Solution. Bioresour. Technol. 2011, 102, 8877–8884. [Google Scholar] [CrossRef]
- Adeyemo, A.; Adebowale, K.O.; Olu-Owolabi, B.I. Adsorption of Copper by Biochar. Int. Res. J. Pure Appl. Chem. 2014, 4, 727–736. [Google Scholar] [CrossRef]
- Wang, Q.; Lai, Z.; Mu, J.; Chu, D.; Zang, X. Converting Industrial Waste Cork to Biochar as Cu(II) Adsorbent via Slow Pyrolysis. Waste Manag. 2020, 105, 102–109. [Google Scholar] [CrossRef]
- Mahdi, Z.; Yu, Q.J.; El Hanandeh, A. Investigation of the Kinetics and Mechanisms of Nickel and Copper Ions Adsorption from Aqueous Solutions by Date Seed Derived Biochar. J. Environ. Chem. Eng. 2018, 6, 1171–1181. [Google Scholar] [CrossRef]
- Park, J.H.; Wang, J.J.; Kim, S.H.; Cho, J.S.; Kang, S.W.; Delaune, R.D.; Han, K.J.; Seo, D.C. Recycling of Rice Straw through Pyrolysis and Its Adsorption Behaviors for Cu and Zn Ions in Aqueous Solution. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 330–337. [Google Scholar] [CrossRef]
- Wang, H.; Gao, B.; Wang, S.; Fang, J.; Xue, Y.; Yang, K. Removal of Pb(II), Cu(II), and Cd(II) from Aqueous Solutions by Biochar Derived from KMnO4 Treated Hickory Wood. Bioresour. Technol. 2015, 197, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Kooh, M.R.R.; Dahri, M.K.; Lim, L.B.L. Removal of Methyl Violet 2B Dye from Aqueous Solution Using Nepenthes Rafflesiana Pitcher and Leaves. Appl. Water Sci. 2017, 7, 3859–3868. [Google Scholar] [CrossRef]
- Lee, J.W.; Han, J.; Choi, Y.K.; Park, S.; Lee, S.H. Reswellable Alginate/Activated Carbon/Carboxymethyl Cellulose Hydrogel Beads for Ibuprofen Adsorption from Aqueous Solutions. Int. J. Biol. Macromol. 2023, 249, 126053. [Google Scholar] [CrossRef]
- Firmansyah, M.L.; Nurisca, A.P.; Shafiyah, B.C.A.; Samendawai, B.; Adila, F.P.; Rahadi, M.A.B.; Sutanto, L.G.; Azzahra, R.M.; Adzijah, J.N.; Wardhana, B.Y.; et al. Synthesis of Silica Nanosphere for the Recovery of Gold from Aqueous Solution. AIP Conf. Proc. 2024, 3047, 050007. [Google Scholar]
- Song, Z.; Lian, F.; Yu, Z.; Zhu, L.; Xing, B.; Qiu, W. Synthesis and Characterization of a Novel MnOx-Loaded Biochar and Its Adsorption Properties for Cu2+ in Aqueous Solution. Chem. Eng. J. 2014, 242, 36–42. [Google Scholar] [CrossRef]
- Otero, M.; Rozada, F.; Morán, A.; Calvo, L.F.; García, A.I. Removal of Heavy Metals from Aqueous Solution by Sewage Sludge Based Sorbents: Competitive Effects. Desalination 2009, 239, 46–57. [Google Scholar] [CrossRef]
- Tong, X.J.; Li, J.Y.; Yuan, J.H.; Xu, R.K. Adsorption of Cu(II) by Biochars Generated from Three Crop Straws. Chem. Eng. J. 2011, 172, 828–834. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L.; Wang, H.; Yu, H.; Gao, B. Removal of Cu, Zn, and Cd from Aqueous Solutions by the Dairy Manure-Derived Biochar. Environ. Sci. Pollut. Res. 2013, 20, 358–368. [Google Scholar] [CrossRef]
- Jiang, J.; Peng, Y.; Yuan, M.; Hong, Z.; Wang, D.; Xu, R. Rice Straw-Derived Biochar Properties and Functions as Cu(II) and Cyromazine Sorbents as Influenced by Pyrolysis Temperature. Pedosphere 2015, 25, 781–789. [Google Scholar] [CrossRef]
- He, P.; Yu, Q.; Zhang, H.; Shao, L.; Lü, F. Removal of Copper (II) by Biochar Mediated by Dissolved Organic Matter. Sci. Rep. 2017, 7, 7091. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, R.; Patel, A.K.; Nguyen, T.B.; Singhania, R.R.; Chen, C.W.; Dong, C. Di Adsorption of Copper (II) in Aqueous Solution Using Biochars Derived from Ascophyllum Nodosum Seaweed. Bioresour. Technol. 2021, 328, 124829. [Google Scholar] [CrossRef] [PubMed]


| Biochars | Mineral Content (%) | |||||
|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | Fe | |
| NF-BC350 | 3.26 | 0.00 | 0.12 | 1.71 | 0.62 | 0.04 |
| NF-BC550 | 3.04 | 0.00 | 0.19 | 3.68 | 1.58 | 0.09 |
| NF-BC750 | 2.62 | 0.55 | 0.10 | 3.21 | 1.82 | 0.10 |
| F-BC350 | 1.15 | 0.04 | 0.10 | 3.51 | 0.25 | 0.14 |
| F-BC550 | 1.14 | 0.07 | 0.13 | 3.70 | 0.48 | 0.17 |
| F-BC750 | 0.74 | 0.07 | 0.26 | 6.30 | 0.46 | 0.35 |
| Property | NF-BC350 | F-BC550 |
|---|---|---|
| Cu(II) adsorption capacity (mg Cu/g BC) * | 18.8 | 147.3 |
| Specific surface area (m2/g) | 2.31 | 112.3 |
| Main peak in XRD | MgCO3 | CaCO3 |
| Main functional groups | C=O, C=C, –OH | CO32−, C–O, C–H, C=O, C=C |
| Optimal solution pH | pH 6 | pH 6 |
| pHpzc | 6.2 | 9.2 |
| Major minerals | Ca, Mg | Ca, Mg |
| Best-fitted kinetic model | Two-compartment first-order | Two-compartment first-order |
| Best-fitted isotherm model | Langmuir | Langmuir |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, K.B.; Park, S.; Kim, Y.J.; Lee, G.W.; Jo, J.W.; Kim, J.H.; Kim, J.E.; Kang, G.; Lee, S.H.; Kim, H.J.; et al. Mechanisms of Cu(II) Adsorption onto Biochars Derived from Fallen and Non-Fallen Maple Leaves. Sustainability 2025, 17, 4233. https://doi.org/10.3390/su17094233
Oh KB, Park S, Kim YJ, Lee GW, Jo JW, Kim JH, Kim JE, Kang G, Lee SH, Kim HJ, et al. Mechanisms of Cu(II) Adsorption onto Biochars Derived from Fallen and Non-Fallen Maple Leaves. Sustainability. 2025; 17(9):4233. https://doi.org/10.3390/su17094233
Chicago/Turabian StyleOh, Kyung Bin, Saerom Park, Ye Jin Kim, Gyu Won Lee, Jeong Wook Jo, Jae Hun Kim, Ji Eun Kim, Gwangnam Kang, Sang Hyun Lee, Hyung Joo Kim, and et al. 2025. "Mechanisms of Cu(II) Adsorption onto Biochars Derived from Fallen and Non-Fallen Maple Leaves" Sustainability 17, no. 9: 4233. https://doi.org/10.3390/su17094233
APA StyleOh, K. B., Park, S., Kim, Y. J., Lee, G. W., Jo, J. W., Kim, J. H., Kim, J. E., Kang, G., Lee, S. H., Kim, H. J., & Choi, Y.-K. (2025). Mechanisms of Cu(II) Adsorption onto Biochars Derived from Fallen and Non-Fallen Maple Leaves. Sustainability, 17(9), 4233. https://doi.org/10.3390/su17094233







